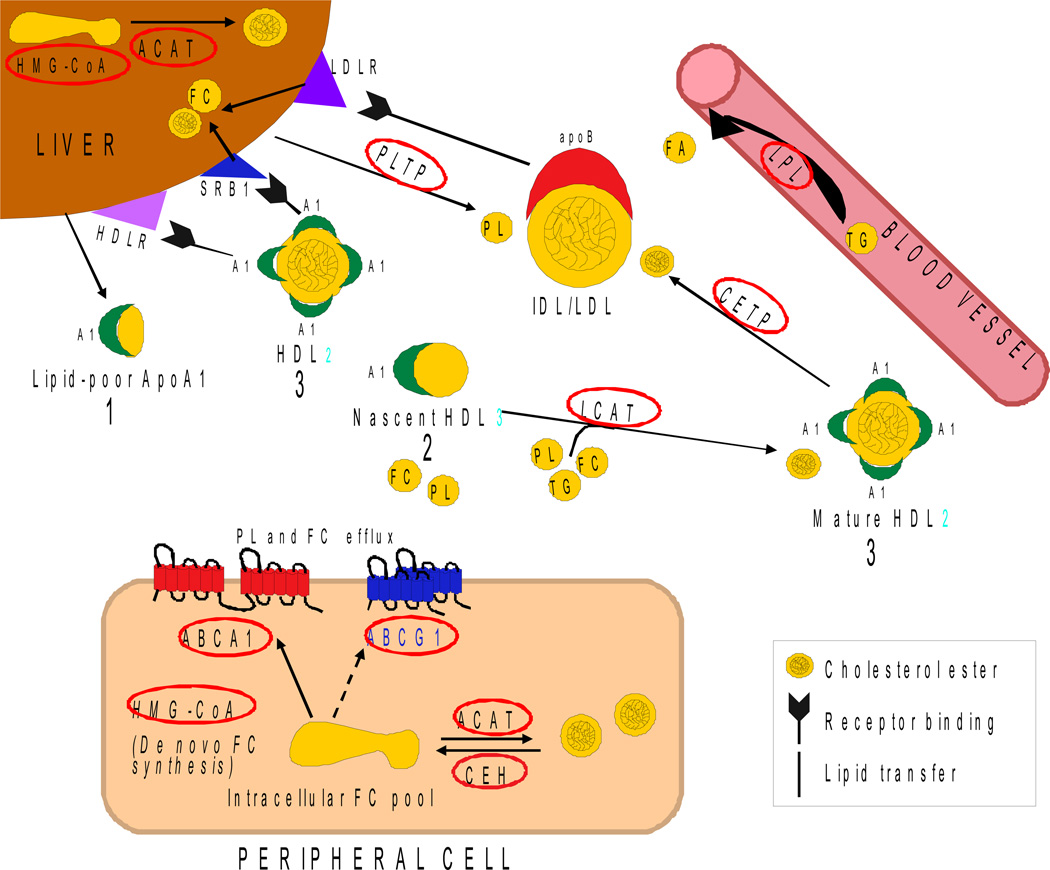
Lipoprotein remodelling in the periphery has many component processes and is extremely complex [1–3]. Perhaps the best peripheral model for the proposed process of lipoprotein biogenesis in the brain is an adaptation of apoAI-HDL mediated reverse cholesterol transport (RCT) (Figure 1, Tables 1 and 2 provide abbreviations and functions for the various participants in this process). Figure 1 is not meant to be comprehensive but to simplify the portion of RCT remodelling that emphasizes key enzymes and transporters known to be present and active in the brain (summarized in Table 2). For further simplicity, apoAI is presented as the primary apolipoprotein although apoE and other apolipoproteins are also present on various lipoprotein intermediates. In this abbreviated process, apoAI plays the role proposed for apoE in the brain (Figure 4). Lipid-poor apoAI particles are secreted by the liver and travel to peripheral tissue to take-up excess FC (and some PL) released by ABCA1 and ABCG1 to become nascent HDL3. These particles then acquire additional PL, FC and TG via the enzyme LCAT to become mature HDL2. This acquired cholesterol (FC and CE) is transported back to the liver, either directly by HDL2 or by transfer of CE via CETP to larger particles, primarily IDL and LDL. At the liver, HDL2 is taken-up by HDLR and SRB1, and IDL/LDL are taken-up by LDLR as apoB is a ligand for these receptors. Thus, the original nascent particles undergo an extensive series of modifications and interconversions in the interstitial space and the plasma. The result is an array of lipoprotein particles with different apolipoprotein and lipid compositions that can bind to specific receptors and perform a variety of functions. Our hypothesis is that a similar process occurs within brain via glia-secreted lipid-poor apoE particles undergoing remodelling to become mature central nervous system (CNS) lipoproteins (Figure 4).
Figure 1. ApoAI-mediated reverse cholesterol transport.
Lipid-poor particles are secreted by the liver, take up lipid in the periphery and are cleared by the liver. See description in text and Tables 1 and 2 (Adapted from [2], see also [1, 3]).
Table 1.
| Lipids |
| TG= Triglyceride |
| FA= Free Fatty Acid |
| PL= Phospholipid |
| FC= Free Cholesterol |
| CE= Cholesterol Ester |
| Lipoproteins: |
| HDL2 = high density2 |
| HDL3 = high density3 |
| IDL = intermediate density |
| LDL = low density |
| Receptors: |
| LDLR = LDL receptor |
| SR-B1 = scavenger receptor BI |
| HDLR = HDL receptor |
| Apolipoproteins: |
| apoAl |
| apoB |
Table 2.
| Enzymes/transporters potentially involved in apoAI-lipoprotein remodelling during reverse cholesterol transport (identified by a red circle) |
| LPL = lipoprotein lipase. Hydrolyses circulating TG from circulating chylomicrons and VLDL to FAA for uptake by surrounding tissue |
| LCAT: Lecithin-cholesterol acyl transferase. Esterification of CE from PL + FC |
| ACAT: Acyl-CoA cholesterol acyl transferase. Esterification of intracellular CE from FC + FFA |
| PLTP: Phospholipid transfer protein. Transfers PL between cells and lipoproteins |
| CETP = CE transfer protein |
| CEH = Cholesterol ester hydrolase |
| ABC transporters: ATP binding cassette. Promotes efflux of FC from cells to lipoproteins |
| HMG-CoA reductase: 3-Hydroxy-3 methly-glutaryl co-enzyme A reductase. De nova synthesis of FC from mevalonic acid pathway |
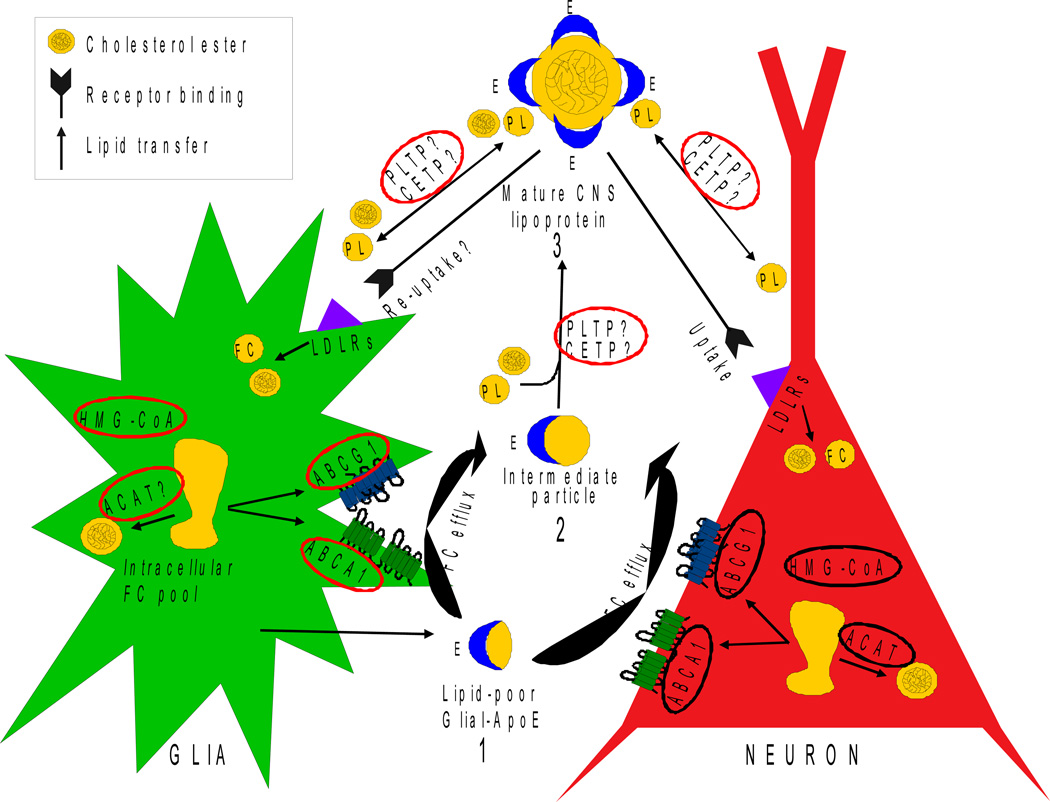
Figure 4. Hypothesis for lipoprotein remodelling in the parenchyma of the brain.
1) Nascent apoE-containing particles are secreted by glial cells. 2) ABCA1 in glia and neurons promotes FC efflux to the nascent particles, followed by additional FC efflux by ABCG1. The result is an intermediate particle(s). 3) Intermediate particle(s) become mature CNS lipoproteins by a poorly understood process that includes acquiring a CE core and additional PL.
Lipoproteins
In the aqueous environment of the blood and interstitial space/parenchyma, neutral lipids circulate packaged as lipoproteins. Lipoproteins are composed of a PL and FC shell surrounding a TG and CE core. Lipoproteins are stabilized by surface apolipoproteins that also serve as cofactors for enzymatic reactions and ligands for lipoprotein receptors. Plasma lipoproteins can be separated by size and density into four major classes that vary in their apolipoprotein composition and core TG/CE content: chylomicrons, very low density lipoproteins (VLDL), low density lipoproteins (LDL), and high density lipoproteins (HDL). The soluble apolipoprotein gene family, which includes apoE and apoAI, encode proteins with amphipathic α-helices at the N-terminus that contains the receptor binding domain and at the C-terminus that allow the apolipoproteins to attach to the lipoprotein, providing stability at the water-lipid interface [4].
ApoAI
ApoA1 is a 28-kDa apolipoprotein associated with the HDL family of lipoproteins, as well as chylomicrons. While it is not a ligand for the LDLR-family, it does bind HDLR and SR-B1. Like apoAII, apoE, apoCI, CII, and CIII, it is also an exchangeable apolipoprotein and can be transferred from one lipoprotein to another. As discussed above, apoAI plays a major role in reverse cholesterol transport.
ApoE
ApoE is a 35-kDa glycoprotein that circulates in the plasma associated with several classes of lipoproteins including chylomicron remnants, VLDL and a subset of HDL. ApoE-containing lipoproteins are bound and internalised via receptor mediated endocytosis by a number of receptors in the LDL receptor (LDLR) family including LDLR, LDL receptor-related protein (LRP), VLDL receptor (VLDLR), and LR8/apoE receptor-2 (apoER2) (for review [5]). ApoE plays a major role in the transport of lipoproteins in the bloodstream, where it participates in the delivery and clearance of plasma cholesterol. Human apoE has 299 amino acids and three major isoforms: E2 (Cys112Cys158), E3 (Cys112Arg158), and E4 (Arg112Arg158). These single amino acid changes result in functional differences between the apoE isoforms, including their relative affinities for both LDL receptors and lipoprotein subtypes (for review [6]). As a result of these isoform-specific differences, apoE4 is associated with an increased risk for heart disease, as well as an increased risk for Alzheimer’s disease (AD).
Lipoproteins in the CNS
This topic is covered in depth in a paper in this volume entitled “Formation and Function of Apolipoprotein E-containing Lipoproteins in the Nervous System” by Jean E. Vance and Hideki Hayashi.
In addition to the plasma, lipoproteins are present in other body fluids such as the interstitial space in the brain and the cerebro spinal fluid (CSF) (for review [7]). While the synthesis and processing of peripheral lipoproteins are well characterized, little is known about lipoprotein remodelling within the brain. These unique CNS lipoproteins exist in the enclosed environment of the brain, separate from peripheral lipoproteins. For example, labelled cholesterol injected IP or IV in the periphery does NOT appear in the brain [8, 9].
ApoE
ApoE expression in the brain is exceeded only by its expression in the liver. As another example of the separation of peripheral lipid metabolism from that within the brain, liver transplantation that changes the apoE isoform of the recipient to that of the donor occurs in the periphery, but NOT in the brain [10]. In the brain, astrocytes and microglia are the primary apoE-lipoprotein secreting cells, although neuronal expression can occur under specific conditions such as injury [11]. ApoE is considered the only functional apolipoprotein in the brain. While apoJ is also expressed by neurons and glia, it does not appear to have a role in extracellular lipid transport as it can not support the production of a lipoprotein [12]. In addition, the only known receptor for apoJ, megalin (gp330, also known as LRP-2), is not expressed by neurons or glia [13].
Glial lipoproteins
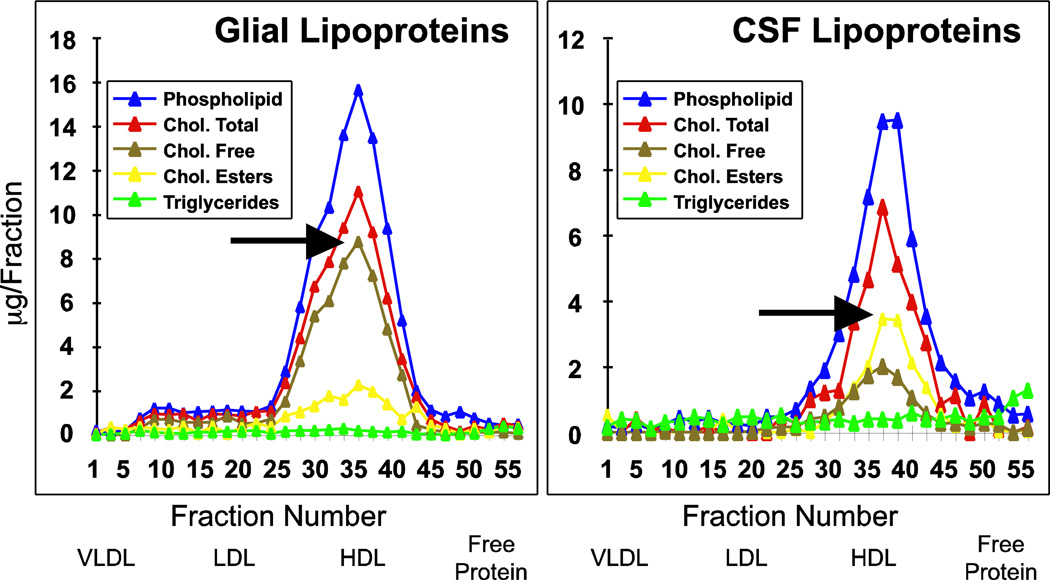
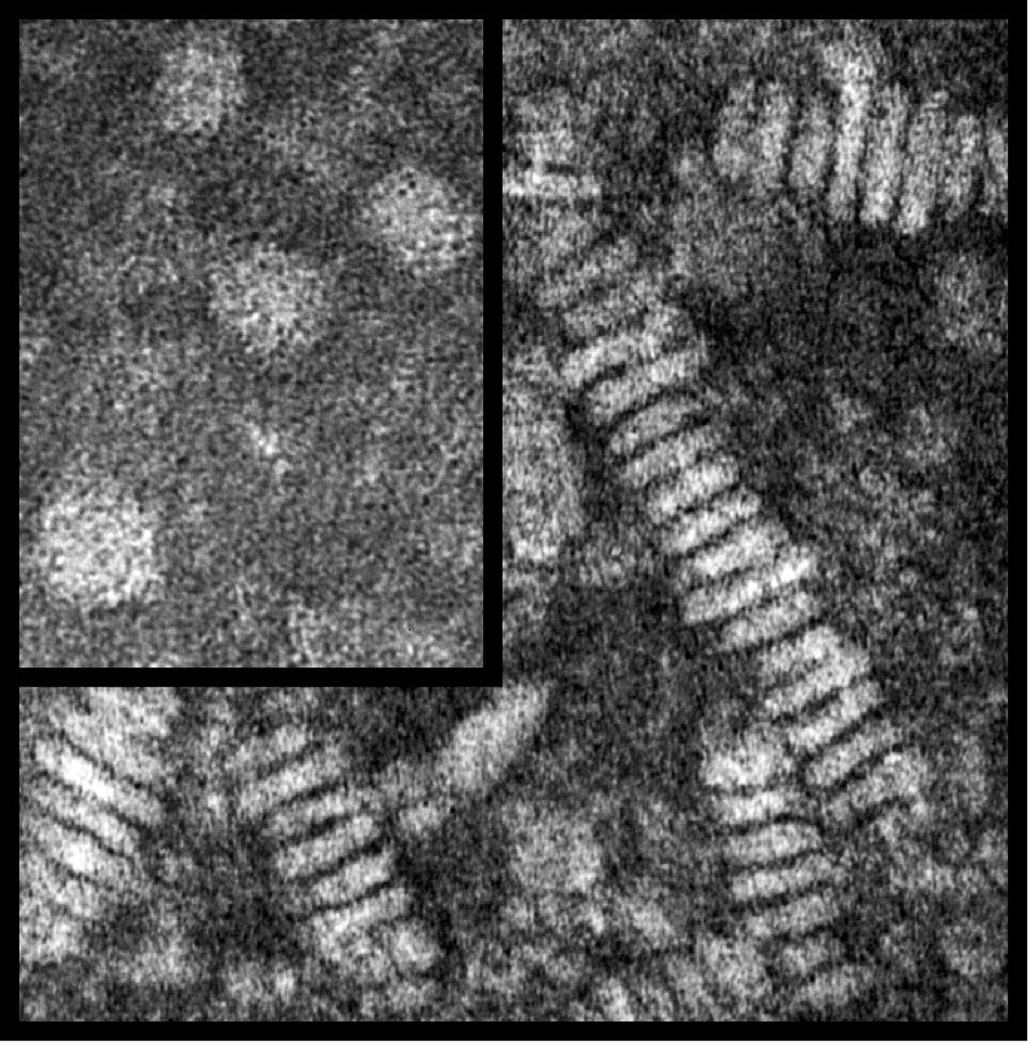
Glia secrete nascent lipoprotein particles composed of PL, FC (arrow, Figure 2) and apoE (data not shown). Because of the lack of core lipids (CE or TG), these apoE-containing particles are discoidal (Figure 3). Recent evidence suggest that these nascent particles incorporate cholesterol first from ABCA1, followed by ABCG1, a process occurring via both glia and neurons, as both cell types express these transporters [14, 15] (Figure 4). Intermediate apoE-containing lipoproteins can deliver their contents to the surrounding cells via endocytosis by the variety of LDLRs expressed by neurons and glia (for review [16]).
Figure 2. Lipid distribution in human CSF and rat glial lipoproteins.
50ml of CSF or serum-free glial conditioned media were concentrated to 1ml and fractionated by size exclusion chromatography with tandem Superose-6 columns, and the resulting fractions analysed for lipid. Adapted from [17].
Figure 3. Negative stained electron micrographs of lipoproteins isolated by gel chromatography.
A concentrated aliquot (0.5mg/ml of protein) from CSF (inset) and astrocyte media was placed on a carbon coated EM grid and negatively stained with 1% uranyl acetate. Magnification 500,000 X. Adapted from [17].
CSF lipoproteins
Lipoproteins are also present in the CSF. CSF is produced by the choroid plexus, contains non-resorbed products derived from the interstitial space of the brain, the likely source of CSF lipoprotein, and, to a lesser degree, the plasma. CSF lipoproteins appear to be the diameter (7–15nM) and density of plasma HDL, contain apoE (data not shown) and the core lipid CE (arrow, Figure 2), and are spherical (inset, Figure 3) [17].
Lipoproteins and lipid transport
Based on Figure 1, we hypothesize that lipoprotein remodelling occurs in the parenchyma of the brain, maintaining lipid homeostasis by adapting to clearance or delivery of lipid, depending primarily on the demand of neurons (Figure 4). To date, this process remains basically unknown. Lipid homeostasis, particularly cholesterol homeostasis, is critical to the environment surrounding neurons and thus needs to be tightly regulated. The perhaps obvious function for glial-lipoproteins would be to deliver lipid from glia to neurons via the LDLR family members for the maintenance and adaptation of the vast network of membranes that comprise the synaptic connections of a dendritic tree. While neurons can synthesize cholesterol via the HMG-CoA reductase pathway [18], apoE-containing glial lipoproteins are important in balancing supply and clearance [19–22]. Evidence demonstrates that nascent glial lipoproteins secreted as discs acquire FC from ABCA1 and ABCG1 (for review [23]). These intermediate particles must acquire additional PL and a CE core, becoming spherical before reaching the CSF (Figure 3). This process of developing a “mature” CNS lipoproteins would appear to require CETP and PLTP to transfer CE as well as PL [24–27]. In addition, there may also be a non-CETP cholesterol ester transport activity (CETA) in the brain. Finally, LCAT, possibly secreted by glial cells [28, 29], may also enhance the CE content of intermediate particles [30].
CNS Lipoproteins: What do we need to know next?
Lipoprotein remodelling in the brain
The actual step-by-step process of nascent particles becoming intermediate and then mature lipoproteins in the brain remains unclear. The only known genetic risk factor in AD, and the only known lipid/cholesterol carrier in the CNS, is apoE. However, little is known about apoE isoform-specific effects on the composition and processing of glial-lipoproteins. This leaves fundamental questions about lipoprotein remodelling in the brain unanswered. For example, we do not know the composition of intermediate particles that allow the nascent, glial-apoE lipoproteins to mature, or the effect of apoE isoform on this developing lipoprotein profile. Once the roles of apoE receptors and enzymatic activities are determined, we need to understand how these interactions change in response to an environment that is lipid poor or lipid rich. The role of glia in lipid homeostasis, beyond secreting the nascent particles, is also unknown. Is it possible that the glia act as the “liver” of the brain, playing a major role in regulating lipid distribution by its influx and efflux of particularly cholesterol?
Future directions for the role of CNS lipoproteins
1) Once the biogenesis of gliallipoproteins is understood, its potential role AD can be investigated. For example, the interaction between amyloid precursor protein (APP) and this now completed pathway can be dissected. In addition, both the effect of amyloid-beta peptide (Aβ) on this lipoprotein profile and the effect of this lipoprotein profile on APP processing can be studied. 2) One of the next challenges for this model is to incorporate endothelial cells, which will add several dimensions to the process of lipoprotein remodelling. Additional enzymes that can modulate lipoprotein composition will be added in the form of endothelial and lipoprotein lipases. Initially, the role of the blood brain barrier (BBB) could be probed in vitro (for review [31]). 3) In general, lipid diffusion between vessels and the parenchyma of the brain will have some level of bi-directionality, even in the absence of specific transporters or enzymatic activities. This adds another level of complexity when interpreting lipid levels and maintenance of homeostasis. While the primary clearance mechanism of presumably cholesterol-rich lipoproteins from the parenchyma is to the CSF, diffusion via the BBB is also possible, for example the diffusion of small apoA1-containing particles into the brain.
The application of principles learned from peripheral, particularly vascular, changes induced by lipid fluctuations may provide established methods, reagents and models for treatment to induce the ideal lipid environment in the parenchyma of the brain. Understanding lipoprotein biogenesis within the brain, the exchange of lipid between neurons and glia, the capacity of these cells to synthesize and store lipid, and the role of diffusion to the CSF and transport across the BBB would fill a critical gap in our current knowledge. Importantly, understanding this process will potentially contribute new targets to manage the development of AD, based on the connection of apoE and cholesterol to risk for this disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Duffy D, Rader DJ. Emerging therapies targeting high-density lipoprotein metabolism and reverse cholesterol transport. Circulation. 2006;113:1140–1150. doi: 10.1161/CIRCULATIONAHA.105.593855. [DOI] [PubMed] [Google Scholar]
- 2.Chang TY, Chang CC, Ohgami N, Yamauchi Y. Cholesterol sensing, trafficking, and esterification. Annu Rev Cell Dev Biol. 2006;22:129–157. doi: 10.1146/annurev.cellbio.22.010305.104656. [DOI] [PubMed] [Google Scholar]
- 3.Brewer HB, Jr, Santamarina-Fojo S. New insights into the role of the adenosine triphosphate-binding cassette transporters in high-density lipoprotein metabolism and reverse cholesterol transport. Am J Cardiol. 2003;91:3E–11E. doi: 10.1016/s0002-9149(02)03382-9. [DOI] [PubMed] [Google Scholar]
- 4.Li WH, Tanimura M, Luo CC, Datta S, Chan L. The apolipoprotein multigene family: biosynthesis, structure, structure-function relationships, and evolution. J. Lipid Res. 1998;29:245–571. [PubMed] [Google Scholar]
- 5.Herz J, Willnow TE. Functions of the LDL receptor gene family. Ann N Y Acad Sci. 1994;737:14–19. doi: 10.1111/j.1749-6632.1994.tb44298.x. [DOI] [PubMed] [Google Scholar]
- 6.Weisgraber KH. Apolipoprotein E: structure-function relationships. Advances in protein chemistry. 1994;45:249–302. doi: 10.1016/s0065-3233(08)60642-7. [DOI] [PubMed] [Google Scholar]
- 7.Ladu MJ, Reardon C, Van Eldik L, Fagan AM, Bu G, Holtzman D, Getz GS. Lipoproteins in the central nervous system. Ann N Y Acad Sci. 2000;903:167–175. doi: 10.1111/j.1749-6632.2000.tb06365.x. [DOI] [PubMed] [Google Scholar]
- 8.Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr Opin Lipidol. 2001;12:105–112. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Jurevics H, Morell P. Cholesterol for synthesis of myelin is made locally, not imported into brain. J Neurochem. 1995;64:895–901. doi: 10.1046/j.1471-4159.1995.64020895.x. [DOI] [PubMed] [Google Scholar]
- 10.Linton MF, Gish R, Hubl ST, Butler E, Esquivel C, Bry WI, Boyles JK, Wardell MR, Young SG. Phenotypes of apolipoprotein B and apolipoprotein E after liver transplantation. J. Clin. Invest. 1991;88:270–281. doi: 10.1172/JCI115288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, Huang Y. Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J Neurosci. 2006;26:4985–4994. doi: 10.1523/JNEUROSCI.5476-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fagan AM, Holtzman DM, Munson G, Mathur T, Schneider D, Chang LK, Getz GS, Reardon CA, Lukens J, Shah JA, LaDu MJ. Unique lipoproteins secreted by primary astrocytes from wild type, apoE (−/−), and human apoE transgenic mice. J Biol Chem. 1999;274:30001–30007. doi: 10.1074/jbc.274.42.30001. [DOI] [PubMed] [Google Scholar]
- 13.Kounnas MZ, Moir RD, Rebeck GW, Bush AI, Argraves WS, Tanzi RE, Hyman BT, Strickland DK. LDL-receptor-related protein, a multifunctional apoE receptor, binds secreted β-amyloid precursor protein and mediates its degradation. Cell. 1995;82:331–340. doi: 10.1016/0092-8674(95)90320-8. [DOI] [PubMed] [Google Scholar]
- 14.Tarr PT, Edwards PA. ABCG1 and ABCG4 are coexpressed in neurons and astrocytes of the CNS and regulate cholesterol homeostasis through SREBP-2. J Lipid Res. 2008;49:169–182. doi: 10.1194/jlr.M700364-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Wang N, Yvan-Charvet L, Lutjohann D, Mulder M, Vanmierlo T, Kim TW, Tall AR, Wang N, Yvan-Charvet L, Lutjohann D, Mulder M, Vanmierlo T, Kim T-W, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cholesterol and desmosterol efflux to HDL and regulate sterol accumulation in the brain. FASEB Journal. 2008;22:1073–1082. doi: 10.1096/fj.07-9944com. [DOI] [PubMed] [Google Scholar]
- 16.Herz J, Bock HH. Lipoprotein receptors in the nervous system. Annu Rev Biochem. 2002;71(2002):405–434. doi: 10.1146/annurev.biochem.71.110601.135342. [DOI] [PubMed] [Google Scholar]
- 17.LaDu MJ, Gilligan SM, Lukens JR, Cabana VG, Reardon CA, Van Eldik LJ, Holtzman DM. Nascent astrocyte particles differ from lipoproteins in CSF. J Neurochem. 1998;70:2070–2081. doi: 10.1046/j.1471-4159.1998.70052070.x. [DOI] [PubMed] [Google Scholar]
- 18.Pooler AM, Xi SC, Wurtman RJ, Pooler AM, Xi SC, Wurtman RJ. The 3-hydroxy-3-methylglutaryl co-enzyme A reductase inhibitor pravastatin enhances neurite outgrowth in hippocampal neurons.[erratum appears in J Neurochem. 2006 Aug;98(3):992] Journal of Neurochemistry. 2006;97:716–723. doi: 10.1111/j.1471-4159.2006.03763.x. [DOI] [PubMed] [Google Scholar]
- 19.Pfrieger FW, Pfrieger FW. Outsourcing in the brain: do neurons depend on cholesterol delivery by astrocytes? Bioessays. 2003;25:72–78. doi: 10.1002/bies.10195. [see comment] [DOI] [PubMed] [Google Scholar]
- 20.Funfschilling U, Saher G, Xiao L, Mobius W, Nave KA, Funfschilling U, Saher G, Xiao L, Mobius W, Nave K-A. Survival of adult neurons lacking cholesterol synthesis in vivo. BMC Neuroscience. 2007;8:1. doi: 10.1186/1471-2202-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dietschy JM, Turley SD, Dietschy JM, Turley SD. Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. Journal of Lipid Research. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Langan TJ, Iimori Y, White G, Volpe JJ. Regulation of sterol synthesis and of 3- hydroxy-3-methylglutaryl coenzyme A reductase by lipoproteins in glial cells in primary. Journal of Neuroscience Research. 1987;17:361–366. doi: 10.1002/jnr.490170406. [DOI] [PubMed] [Google Scholar]
- 23.Kim WS, Weickert CS, Garner B. Role of ATP-binding cassette transporters in brain lipid transport and neurological disease. J Neurochem. 2008;104:1145–1166. doi: 10.1111/j.1471-4159.2007.05099.x. [DOI] [PubMed] [Google Scholar]
- 24.Albers JJ, Tollefson JH, Wolfbauer G, Albright RE., Jr Cholesteryl ester transfer protein in human brain. Int J Clin Lab Res. 1992;21:264–266. doi: 10.1007/BF02591657. [DOI] [PubMed] [Google Scholar]
- 25.Lagrost L, Athias A, Gambert P, Lallemant C. Comparative study of phospholipid transfer activities mediated by cholesteryl ester transfer protein and phospholipid transfer protein. Journal of Lipid Research. 1994;35:825–835. [PubMed] [Google Scholar]
- 26.Oram JF, Wolfbauer G, Vaughan AM, Tang C, Albers JJ, Oram JF, Wolfbauer G, Vaughan AM, Tang C, Albers JJ. Phospholipid transfer protein interacts with and stabilizes ATP-binding cassette transporter A1 and enhances cholesterol efflux from cells. Journal of Biological Chemistry. 2003;278:52379–52385. doi: 10.1074/jbc.M310695200. [DOI] [PubMed] [Google Scholar]
- 27.Oram JF, Wolfbauer G, Tang C, Davidson WS, Albers JJ, Oram JF, Wolfbauer G, Tang C, Davidson WS, Albers JJ. An amphipathic helical region of the N-terminal barrel of phospholipid transfer protein is critical for ABCA1-dependent cholesterol efflux. Journal of Biological Chemistry. 2008;283:11541–11549. doi: 10.1074/jbc.M800117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirsch-Reinshagen V, Donkin J, Stukas S, Chan J, Wilkinson A, Fan J, Parks JS, Kuivenhoven JA, Lutjohann D, Pritchard H, Wellington CL. LCAT synthesized by primary astrocytes esterifies cholesterol on glia-derived lipoproteins. J Lipid Res. 2009;50:885–893. doi: 10.1194/jlr.M800584-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collet X, Francone O, Besnard F, Fielding CJ. Secretion of lecithin:cholesterol acyltransferase by brain neuroglial cell lines. Biochem Biophys Res Commun. 1999;258:73–76. doi: 10.1006/bbrc.1999.0601. [DOI] [PubMed] [Google Scholar]
- 30.Amaducci L, Antuono P, Bartolini L, De Medio GE, Inzitari D, Porcellati G. A possible mechanism for cholesteryl ester formation during demyelination: lecithin:cholesterol acyltransferase (LCAT) activity in rat brain. Neurochemical Research. 1978;3:725–731. doi: 10.1007/BF00965995. [DOI] [PubMed] [Google Scholar]
- 31.Deane R, Zlokovic BV. Role of the blood-brain barrier in the pathogenesis of Alzheimer's disease. Curr Alzheimer Res. 2007;4:191–197. doi: 10.2174/156720507780362245. [DOI] [PubMed] [Google Scholar]