Abstract
Prostate cancer antigen 3 (PCA3) is a novel urine-based prostate cancer biomarker that has recently been studied extensively for the prediction of prostate biopsy results and treatment outcomes. Numerous studies have demonstrated that urinary PCA3 scores are predictive of prostate cancer detection on both initial and repeat biopsy. There is conflicting evidence on the relationship between PCA3 with aggressive tumor features and treatment outcomes. This article reviews the current evidence on PCA3 as a marker for prostate cancer detection and prognosis.
Key words: Prostate cancer, Prostate cancer antigen 3, Prostatectomy, Risk assessment
The prostate cancer antigen 3 (PCA3), or DD3, gene was initially found to be overexpressed in prostate cancer tissue by Bussemakers and colleagues in 1999.1 By contrast, DD3 expression was not found in normal brain, breast, bladder, colon, duodenum, heart, liver, lung, ovary, pancreas, placenta, seminal vesicles, skeletal muscle, skin, spinal cord, spleen, or testis. DD3 expression was also not detected in bladder, breast, cervix, endometrium, kidney, ovary, or testis tumors.
In a follow-up study, de Kok and colleagues examined DD3 messenger RNA (mRNA) copy numbers in numerous normal and tumor tissue types using quantitative reverse transcription polymerase chain reaction.2 Among 22 normal tissue types, only kidney (insignificant levels) and prostate had detectable DD3 expression. Similarly, they found no detectable DD3 expression in 39 nonprostate malignancies. Finally, they showed no significant difference in DD3 mRNA between normal prostate and prostate tissue with benign prostatic hyperplasia, but a 34-fold median increased expression of DD3 in prostate cancer. These combined findings suggested that DD3 expression may be used as a tissue marker for prostate cancer with excellent specificity.
Subsequent scientific advances paved the way for PCA3 mRNA to be measured not only in tissue samples but also in urine.3 Over time, the process of obtaining and measuring PCA3 in urine has been considerably streamlined,4 facilitating an increasing number of studies on its potential utility in prostate cancer screening and prognostication.5 An automated PCA3 urinary assay is commercially available in Europe and is seeking approval from the US Food and Drug Administration.
PCA3 and Prostate Biopsy
Prostate biopsy remains the gold standard to confirm a histological diagnosis of prostate cancer. Unfortunately, due to limitations in existing prostate cancer screening protocols, in most series < 50% of prostate biopsies performed for current indications reveal prostate cancer. This large number of negative biopsy results leads to added avoidable cost, as well as unnecessary anxiety and pain. Although prostate biopsy has not been shown to be associated with excess mortality,6 there remains the potential for significant morbidity, including increasing reports of serious infectious complications.7 It is noteworthy that 38% of Medicare participants with a negative prostate biopsy had repeat biopsy by 5 years8; thus, these same drawbacks also extend to the repeat biopsy setting. These issues have triggered a considerable amount of investigation into alternate markers such as PCA3 with greater specificity for prostate cancer that might help to reduce the number of unnecessary biopsies.
One of the initial clinical studies on PCA3 and prostate biopsy outcomes was reported by Marks and colleagues,9 as previously reviewed.10 Briefly, in 233 US men undergoing repeat biopsy, they found improved performance of PCA3 compared with prostate-specific antigen (PSA) for prostate cancer detection. Haese and coauthors validated these results in a larger population of European men undergoing repeat biopsy.11 In this study, biopsy was positive in 39% of men with a PCA3 score ≥ 35, versus 22% with a PCA3 score <35 (P=.0001). In addition, PCA3 outperformed free/total prostate-specific antigen ratio (% of PSA) for prostate cancer detection, and was also associated with tumor grade, the percentage of positive cores, clinical stage, and significant disease for those diagnosed with prostate cancer.
More recently, Aubin and colleagues examined PCA3 in 1072 men from the placebo arm of the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial, all of whom had a negative biopsy result within 6 months of enrollment in the trial, followed by protocol-mandated biopsies at year 2 and year 4 of the study.12 They found a significantly higher PCA3 at both year 2 (P = .0013) and year 4 (P = .005) among men with a positive biopsy result at year 4. Thus, this study showed a significant relationship between PCA3 and future prostate cancer risk. A significant association between PCA3 and high-grade disease was also observed, but not with the number of positive cores or percentage of core involvement with tumor.
PCA3 has also been evaluated for use in the initial prostate biopsy setting. For example, de la Taille and colleagues examined PCA3 in 516 men from a European multicenter trial with PSA 2.5 to 10 ng/mL undergoing first biopsy.13 PCA3 outperformed PSA on receiver operating characteristic curve (ROC) analysis for the prediction of positive biopsy results (area under the curve [AUC] 0.76 PCA3 vs 0.577 PSA; P < .001), and did not appear to be affected by prostate volume. Moreover, higher PCA3 scores were significantly associated with a biopsy Gleason score ≥ 7, > 33% positive cores, and significant cancer (based on the Epstein criteria).
Hessels and associates examined PCA3 in 336 men undergoing prostate biopsy for a PSA > 3 ng/mL, abnormal digital rectal examination (DRE), and/or positive family history.14 They found that the median PCA3 was significantly higher in those with positive versus negative biopsy (50 vs 18; P < .0001). The AUC was 0.72 for overall prostate cancer detection compared with 0.65 for total PSA. As in prior studies, they did not find a significant relationship between PCA3 and prostate volume. However, they also found no significant difference in PCA3 between those with a Gleason score < 7 versus ≥ 7 (P = .622).
The relationship between PCA3 and biopsy outcome has also been studied in diverse populations from around the world. For example, in an Asian population, Ochiai and colleagues reported on 105 men undergoing initial or repeat biopsy for an elevated PSA or abnormal DRE, of which 38 (36%) were diagnosed with prostate cancer.15 The median PCA3 was significantly higher in those with a positive biopsy result compared with those with a negative biopsy result (59.5 vs 14.2; P < .0001).
Adam and colleagues reported on 105 consecutive South African men referred for biopsy, of which 81.9% were first biopsies.16 Although PCA3 was significantly higher in men with positive biopsy (P = .003), PSA outperformed PCA3 on ROC analysis (0.844 vs 0.705). Unlike PSA, PCA3 was not associated with prostate volume as in prior studies; nevertheless, there was also no association between PCA3 and Gleason score in this population.
Finally, Roobol and associates examined a subset of men from a randomized prostate cancer screening trial undergoing biopsy for a PSA level ≥ 3 ng/mL, PCA3 score ≥ 10, or both indications,17 as previously reviewed.10 Overall, they reported an AUC of 0.635 for PCA3 and 0.581 for PSA, although this may reflect the fact that this population was prescreened with PSA.
PCA3 and Treatment Outcomes
Numerous studies have examined the relationship between PCA3 with pathologic features at radical prostatectomy. For example, Whitman and colleagues reported a significant relationship between PCA3 with adverse tumor features, including the risk of extracapsular extension in 72 men undergoing radical prostatectomy.18
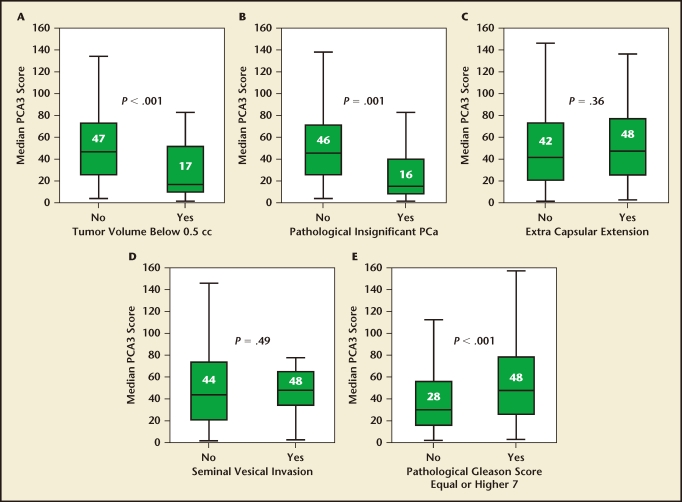
Auprich and coauthors reported on PCA3 and prostatectomy outcomes in 305 men with newly diagnosed prostate cancer treated at three institutions (Figure 1).19 The median PCA3 score was significantly lower in patients with a tumor volume < 0.5 cc (median 17 vs 47 in those with higher tumor volume; P < .001) and in those with insignificant disease (median 16 vs 46 for those with significant disease; P = .001). PCA3 maintained a significant inverse association with both tumor volume < 0.5 and insignificant prostate cancer in a multivariable model adjusting for PSA, biopsy Gleason score, and the number of positive cores. However, interestingly, they found no significant relationship between PCA3 with adverse tumor features such as extracapsular extension or seminal vesicle invasion. The authors proposed that this “nonlinear” relationship might be related to differential shedding of tumor cells related to the degree of glandular differentiation.
Figure 1.
Box plots of median prostate cancer antigen 3 (PCA3) assay scores comparing men with and without (A) tumor volume < 0.5 mL; (B) pathologically insignificant prostate cancer (PCa); (C) extracapsular extension; (D) seminal vesicle invasion; and (E) pathologic Gleason score ≥ 7. Reproduced with permission from European Urology, Volume 59, Auprich M et al, “Critical assessment of preoperative urinary prostate cancer antigen 3 on the accuracy of prostate cancer screening,” pp. 96–105, Copyright 2011, with permission from Elsevier.19
In the study by Hessels and colleagues just described, 70 of the men diagnosed with prostate cancer on biopsy subsequently elected radical prostatectomy.14 In this group, PCA3 was not associated with tumor volume (P = .680), insignificant versus significant cancer (P = .496), Gleason score (P = .199), or extracapsular extension (P = .765).
Vlaeminck-Guillem and colleagues tested PCA3 in 102 men undergoing radical prostatectomy, and found a significant correlation with tumor volume but not with pathologic stage, prostatectomy Gleason score, or upgrading. 20 They did demonstrate a significantly higher mean PCA3 in multifocal disease (88 vs 46; P = .007), which they suggested might have implications for men considering focal therapy.
Similarly, Ploussard and associates recently studied 106 men with low-risk disease (PSA < 10 ng/mL, clinical stage T1c–T2a, biopsy Gleason 6) undergoing prostatectomy to determine whether PCA3 might help identify potential candidates for active surveillance (AS).21 On multivariate analysis, a PCA3 score > 25 was associated with a 5.37-fold increased odds (P = .01) of tumor volume > 0.5 cc and 12.74-fold increased odds (P = .003) of “significant” prostate cancer, although the sample sizes were small. Based on these data, the authors concluded that PCA3 might be useful in the selection of patients for AS.
Nevertheless, very limited data are available on the utility of PCA3 among men actually enrolled in AS. Tosoian and colleagues examined PCA3 in 294 men from the Johns Hopkins AS program, and found no significant difference in PCA3 between those who did and those who did not progress on repeat surveillance biopsy (P > .131).22 In this study, the AUC was only 0.589 to predict short-term biopsy progression using PCA3. Further studies are necessary to examine PCA3 for the prediction of more long-term treatment outcomes.
Future Directions
The field of prostate cancer is moving away from reliance on a single marker toward more integrated risk-assessment tools. Several studies have examined PCA3 in conjunction with other urinary markers, suggesting a future role as part of a multiplex predictive panel.23–25 Others have incorporated PCA3 along with clinical data and serum markers into validated nomograms and risk calculators for enhanced risk stratification.26 Preliminary studies in external populations have found encouraging results through the incorporation of PCA3 into these multivariable predictive tools,27 and additional confirmatory studies are warranted.
Conclusions
PCA3 is a relatively new urinary marker with demonstrated utility for the prediction of biopsy outcome in numerous studies from diverse populations. Its use may help enhance the specificity of screening and reduce unnecessary biopsies. PCA3 may also play an important role as part of a multivariable risk-assessment tool with other clinical, serum, and/or urinary markers. Nevertheless, it remains unclear whether the use of PCA3 would help to reduce the overdetection and overtreatment of indolent disease because its association with adverse prognostic features is more controversial. Additional studies on the relationship between PCA3 and long-term disease-specific outcomes will help to clarify these issues.
Main Points.
Prostate biopsy remains the gold standard to confirm a histological diagnosis of prostate cancer, although < 50% of prostate biopsies performed for current indications reveal prostate cancer. This large number of negative biopsy results leads to added avoidable cost, as well as unnecessary anxiety and pain.
The field of prostate cancer is moving away from reliance on a single marker toward more integrated risk-assessment tools. Several studies have examined prostate cancer antigen 3 (PCA3), a relatively new urinary marker, in conjunction with other urinary markers, suggesting a future role as part of a multiplex predictive panel. Its use may help enhance the specificity of screening and reduce unnecessary biopsies.
The process of obtaining and measuring PCA3 in urine has been considerably streamlined, facilitating an increasing number of studies on its potential use in prostate cancer screening.
It remains unclear whether the use of PCA3 would help to reduce the overdetection and overtreatment of indolent disease because its association with adverse prognostic features is more controversial. Additional studies on the relationship between PCA3 and long-term disease-specific outcomes will help to clarify these issues.
References
- 1.Bussemakers MJ, van Bokhoven A, Verhaegh GW, et al. DD3: a new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res. 1999;59:5975–5979. [PubMed] [Google Scholar]
- 2.de Kok JB, Verhaegh GW, Roelofs RW, et al. DD3(PCA3), a very sensitive and specific marker to detect prostate tumors. Cancer Res. 2002;62:2695–2698. [PubMed] [Google Scholar]
- 3.Hessels D, Klein Gunnewiek JM, van Oort I, et al. DD3(PCA3)-based molecular urine analysis for the diagnosis of prostate cancer. Eur Urol. 2003;44:8–15. doi: 10.1016/s0302-2838(03)00201-x. discussion 15–16. [DOI] [PubMed] [Google Scholar]
- 4.van Gils MP, Cornel EB, Hessels D, et al. Molecular PCA3 diagnostics on prostatic fluid. Prostate. 2007;67:881–887. doi: 10.1002/pros.20564. [DOI] [PubMed] [Google Scholar]
- 5.Durand X, Moutereau S, Xylinas E, de la Taille A. Progensa™ PCA3 test for prostate cancer. Expert Rev Mol Diagn. 2011;11:137–144. doi: 10.1586/erm.10.122. [DOI] [PubMed] [Google Scholar]
- 6.Carlsson SV, Holmberg E, Moss SM, et al. No excess mortality after prostate biopsy: results from the European Randomized Study of Screening for Prostate Cancer. BJU Int. 2011;107:1912–1917. doi: 10.1111/j.1464-410X.2010.09712.x. [DOI] [PubMed] [Google Scholar]
- 7.Loeb S, Carter HB, Berndt SI, et al. Complications following prostate biopsy: data from SEER-Medicare. J Urol. 2011;186:1830–1834. doi: 10.1016/j.juro.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welch HG, Fisher ES, Gottlieb DJ, Barry MJ. Detection of prostate cancer via biopsy in the Medicare-SEER population during the PSA era. J Natl Cancer Inst. 2007;99:1395–1400. doi: 10.1093/jnci/djm119. [DOI] [PubMed] [Google Scholar]
- 9.Marks LS, Fradet Y, Deras IL, et al. PCA3 molecular urine assay for prostate cancer in men undergoing repeat biopsy. Urology. 2007;69:532–535. doi: 10.1016/j.urology.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Loeb S, Partin AW. PCA3 urinary biomarker for prostate cancer. Rev Urol. 2010;12:e205–e206. [PMC free article] [PubMed] [Google Scholar]
- 11.Haese A, de la Taille A, van Poppel H, et al. Clinical utility of the PCA3 urine assay in European men scheduled for repeat biopsy. Eur Urol. 2008;54:1081–1088. doi: 10.1016/j.eururo.2008.06.071. [DOI] [PubMed] [Google Scholar]
- 12.Aubin SM, Reid J, Sarno MJ, et al. PCA3 molecular urine test for predicting repeat prostate biopsy outcome in populations at risk: validation in the placebo arm of the dutasteride REDUCE trial. J Urol. 2010;184:1947–1952. doi: 10.1016/j.juro.2010.06.098. [DOI] [PubMed] [Google Scholar]
- 13.de la Taille A, Irani J, Graefen M, et al. Clinical evaluation of the PCA3 assay in guiding initial biopsy decisions. J Urol. 2011;185:2119–2125. doi: 10.1016/j.juro.2011.01.075. [DOI] [PubMed] [Google Scholar]
- 14.Hessels D, van Gils MP, van Hooij O, et al. Predictive value of PCA3 in urinary sediments in determining clinico-pathological characteristics of prostate cancer. Prostate. 2010;70:10–16. doi: 10.1002/pros.21032. [DOI] [PubMed] [Google Scholar]
- 15.Ochiai A, Okihara K, Kamoi K, et al. Prostate cancer gene 3 urine assay for prostate cancer in Japanese men undergoing prostate biopsy. Int J Urol. 2011;18:200–205. doi: 10.1111/j.1442-2042.2010.02711.x. [DOI] [PubMed] [Google Scholar]
- 16.Adam A, Engelbrecht MJ, Bornman MS, et al. The role of the PCA3 assay in predicting prostate biopsy outcome in a South African setting. BJU Int. 2011;108:1728–1733. doi: 10.1111/j.1464-410X.2011.10202.x. [DOI] [PubMed] [Google Scholar]
- 17.Roobol MJ, Schröder FH, van Leeuwen P, et al. Performance of the prostate cancer antigen 3 (PCA3) gene and prostate-specific antigen in prescreened men: exploring the value of PCA3 for a first-line diagnostic test. Eur Urol. 2010;58:475–481. doi: 10.1016/j.eururo.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 18.Whitman EJ, Groskopf J, Ali A, et al. PCA3 score before radical prostatectomy predicts extracapsular extension and tumor volume. J Urol. 2008;180:1975–1978. doi: 10.1016/j.juro.2008.07.060. discussion 1978–1979. [DOI] [PubMed] [Google Scholar]
- 19.Auprich M, Chun FK, Ward JF, et al. Critical assessment of preoperative urinary prostate cancer antigen 3 on the accuracy of prostate cancer staging. Eur Urol. 2011;59:96–105. doi: 10.1016/j.eururo.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 20.Vlaeminck-Guillem V, Devonec M, Colombel M, et al. Urinary PCA3 score predicts prostate cancer multifocality. J Urol. 2011;185:1234–1239. doi: 10.1016/j.juro.2010.11.072. [DOI] [PubMed] [Google Scholar]
- 21.Ploussard G, Durand X, Xylinas E, et al. Prostate cancer antigen 3 score accurately predicts tumour volume and might help in selecting prostate cancer patients for active surveillance. Eur Urol. 2011;59:422–429. doi: 10.1016/j.eururo.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 22.Tosoian JJ, Loeb S, Kettermann A, et al. Accuracy of PCA3 measurement in predicting short-term biopsy progression in an active surveillance program. J Urol. 2010;183:534–538. doi: 10.1016/j.juro.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Ouyang B, Bracken B, Burke B, et al. A duplex quantitative polymerase chain reaction assay based on quantification of alpha-methylacyl-CoA racemase transcripts and prostate cancer antigen 3 in urine sediments improved diagnostic accuracy for prostate cancer. J Urol. 2009;181:2508–2513. doi: 10.1016/j.juro.2009.01.110. discussion 2513–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laxman B, Morris DS, Yu J, et al. A first-generation multiplex biomarker analysis of urine for the early detection of prostate cancer. Cancer Res. 2008;68:645–649. doi: 10.1158/0008-5472.CAN-07-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rigau M, Ortega I, Mir MC, et al. AThree-Gene panel on urine increases PSA specificity in the detection of prostate cancer. Prostate. 2011;71:1736–1745. doi: 10.1002/pros.21390. [DOI] [PubMed] [Google Scholar]
- 26.Chun FK, de la Taille A, van Poppel H, et al. Prostate cancer gene 3 (PCA3): development and internal validation of a novel biopsy nomogram. Eur Urol. 2009;56:659–667. doi: 10.1016/j.eururo.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 27.Perdonà S, Cavadas V, Di Lorenzo G, et al. Prostate cancer detection in the “grey area” of prostate-specific antigen below 10 ng/ml: headto- head comparison of the updated PCPT calculator and Chun’s nomogram, two risk estimators incorporating prostate cancer antigen 3. Eur Urol. 2011;59:81–87. doi: 10.1016/j.eururo.2010.09.036. [DOI] [PubMed] [Google Scholar]