Abstract
Objectives
To compare health-related quality of life (HRQOL) outcomes in patients treated with carotid artery stenting (CAS) versus carotid endarterectomy (CEA).
Background
In CREST, the largest randomized trial of carotid revascularization to date, there was no significant difference in the primary composite endpoint but rates of stroke and MI differed between CAS and CEA. To help guide individualized clinical decision-making, we compared HRQOL among patients enrolled in CREST. We also performed exploratory analyses to evaluate the association between periprocedural complications and HRQOL.
Methods
We measured HRQOL at baseline, and after 2-weeks, 1-month, and 1-year among 2502 patients randomized to either CAS or CEA in CREST. HRQOL was assessed using the Medical Outcomes Study Short-Form 36 (SF-36) and 6 disease-specific scales designed to study HRQOL in patients undergoing carotid revascularization.
Results
At both 2-weeks and 1-month, CAS patients had better outcomes for multiple components of the SF-36, with large differences for role physical function, pain, and the physical component summary scale (all p<0.01). On the disease-specific scales, CAS patients reported less difficulty with driving, eating/swallowing, neck pain, and headaches but more difficulty with walking and leg pain (all p<0.05). However, by 1 year there were no differences in any HRQOL measure between CAS and CEA. In the exploratory analyses, periprocedural stroke was associated with poorer 1-year HRQOL across all SF-36 domains, but periprocedural MI or cranial nerve palsy were not.
Conclusions
Among patients undergoing carotid revascularization, CAS is associated with better HRQOL during the early recovery period as compared with CEA—particularly with regard to physical limitations and pain—but these differences diminish over time and are not evident after 1-year. Although CAS and CEA are associated with similar overall HRQOL at 1-year, event-specific analyses confirm that stroke has a greater and more sustained impact on HRQOL than MI.
Keywords: carotid stenosis, quality of life, carotid stenting, carotid endarterectomy, stroke
Carotid endarterectomy (CEA) plus medical management of modifiable risk factors is an established approach for primary and secondary stroke prevention for patients with significant carotid atherosclerosis (1–4). Some patients, however, are considered poor candidates for surgical revascularization due to anatomic complexity or medical comorbidities, and adverse outcomes occur more frequently in these individuals (5). Carotid artery stenting (CAS) was therefore developed as a less invasive option for carotid revascularization. The results of clinical trials of CAS have varied with several finding acceptable rates of safety and efficacy (6–11), but others reporting higher rates of adverse events as compared with CEA (12–14). As a result, the U.S. Food and Drug Administration continues to restrict the indications for CAS to patients at high surgical risk.
The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) recently compared CAS and CEA in patients at low risk of surgical complications and found no difference in the primary composite endpoint of stroke, myocardial infarction (MI), or death during the periprocedural period, or ipsilateral stroke within 4 years (15). Individual endpoints, however, varied between treatment groups with patients assigned to CAS having higher rates of stroke and those assigned to CEA having higher rates of MI. These differences in risk of periprocedural stroke and MI between the two treatment groups in CREST have led to considerable debate regarding the optimal treatment strategy for patients undergoing carotid revascularization (16–19).
In light of this ongoing controversy, evaluation of health-related quality of life (HRQOL) may help further inform individualized clinical decision-making for patients undergoing carotid revascularization. Prior studies have suggested less impairment during the early recovery period after CAS as compared with CEA, but these differences were brief and limited to highly sensitive, disease-specific outcomes and physical role limitations (20,21). Moreover, these findings were based on non-randomized studies or small randomized trials that enrolled highly selected patients. To address these gaps in knowledge, we performed a prospectively-planned analysis of HRQOL among patients randomized to CAS versus CEA in CREST. In addition, we performed exploratory analyses to evaluate the association between periprocedural complications and HRQOL during 1-year follow-up.
METHODS
Trial Design
Details of the CREST design and primary outcomes have been described previously (15,22). In brief, CREST was a randomized trial of CAS versus CEA in both symptomatic and asymptomatic adult patients with significant carotid stenosis by ultrasound, computed tomography, magnetic resonance imaging, or conventional angiography. Exclusion criteria were prior severe stroke, atrial fibrillation, unstable angina, or acute MI within the past 30 days. Clinical and anatomical suitability for either revascularization approach was required, after which patients were enrolled and treated by certified operators (based on adequate procedural volume and low complication rates) at 117 centers in the U.S. and Canada (23).
Risk factor modification and aspirin were recommended for all patients, and CEA was performed according to published guidelines. Patients undergoing CAS received the Rx Acculink stent and Rx Accunet embolic protection device whenever feasible (Abbott Vascular Solutions, Santa Clara, CA). Anticoagulation was administered according to local practice, and thienopyridine therapy was recommended for a minimum of 4 weeks following the procedure. Neurologic evaluation at scheduled intervals, including the use of standardized stroke assessment measures, was performed in all patients. Cardiac biomarkers and electrocardiograms were obtained in all patients before and after the index procedure and following signs or symptoms of cardiac ischemia. Approval was obtained from the Human Studies Committee at each enrolling site, and all patients provided written informed consent prior to participation.
Data Definitions
The periprocedural period was defined as the time from randomization through 30 days after revascularization (or 36 days after randomization when the procedure was not performed within 30 days of randomization). Stroke was defined as an acute neurologic event with focal findings consistent with cerebral ischemia that lasted for 24 hours or more. MI was defined as the presence of elevated cardiac biomarkers at least twice the upper limit of normal at the site’s hospital laboratory plus either: 1) electrocardiographic changes consistent with coronary ischemia, or 2) symptoms of a coronary event. Cranial nerve palsy was defined as evidence of new cranial nerve injury on either the post-procedure or 1-month neurologic assessment.
Health Status Assessment
HRQOL was assessed using standardized questionnaires at baseline, and at 2-weeks and 1-month after the procedure and 1-year after randomization in all patients. The baseline and 1-year questionnaires were administered in written fashion, whereas the 2-week and 1-month assessments were performed by telephone by a single, trained interviewer using the same questionnaires. Overall health status was assessed using the Medical Outcomes Study Short-Form 36 (SF-36) (24). The SF-36 is a commonly-used health survey which assesses 8 dimensions of health status (physical functioning, physical role limitations, bodily pain index, vitality, general health, social functioning, emotional role limitations, and mental health) and has been validated in patients with cardiovascular disease, stroke, and in the general population (24–27). Scores for the SF-36 range from 0–100, with higher scores indicating better health status; a difference of 5–10 points is considered a clinically important change for an individual (smaller differences may be important for group comparisons) (28). In addition, the SF-36 provides summary scales for overall physical and mental health, which are standardized to a population mean of 50 and a standard deviation of 10, and for which individual differences of 2.5–5 points are considered clinically meaningful.
In addition to the SF-36, six disease-specific modified Likert scales designed specifically for comparison of CAS versus CEA were used to evaluate aspects of functional status and symptoms that may be impacted by one or both of the treatments (21,22). The first 3 questions assessed the level of difficulty each patient experienced with walking, eating/swallowing, and driving (1=no difficulty at all, 2=mild difficulty, 3=moderate difficulty, 4=severe difficulty, 5=unable to perform this activity). The next 3 questions evaluated how often patients were bothered by headaches, neck pain, and leg pain during the previous week (1=not at all bothered, 2=bothered a little bit, 3=moderately bothered, 4=bothered quite a bit, 5=extremely bothered). Two additional questions asked patients to rate their level of pain (0–10 scale in which 0=no pain and 10=worst possible pain) and to estimate the number of times pain medications were needed over the past week.
Statistical Approach
All primary analyses of HRQOL were performed on an intention-to-treat basis. Patients who died during the study were included in the analyses of HRQOL outcomes up until the time of death. Primary findings were based on raw data, and sensitivity analyses were performed using multiple imputation to estimate missing HRQOL scores for surviving patients (29). Covariates used in the multiple imputation models included all of the available HRQOL scores, treatment assignment, and baseline clinical and demographic characteristics. An on-treatment analysis was also performed comparing patients who underwent CAS versus those who underwent CEA (regardless of initial treatment assignment). Health status scores were compared between the CAS and CEA groups using analysis of covariance (ANCOVA) for continuous variables and ordinal logistic regression for categorical variables, adjusting for symptomatic status and baseline scores. In addition, to account for the effect of ascertainment bias (in case patients with more severe periprocedural stroke or MI were unable to provide adequate health status data), these analyses were repeated after imputing “worst case scores” to patients with periprocedural events who had missing health status data during follow-up.
For the exploratory analyses of the impact of clinical events on HRQOL, only periprocedural events were considered since there was not a systematic attempt to capture late MI, and rates of late stroke and late MI were extremely low and similar between treatment groups during longer-term follow-up. For each HRQOL outcome, we used multiple linear regression to estimate the independent change associated with the events of interest (stroke, MI, cranial nerve palsy) while adjusting for age, sex, diabetes, history of cardiovascular disease, and symptomatic status at randomization.
For all analyses, a p-value <0.05 was considered statistically significant; no adjustments were performed for multiple comparisons. All analyses were performed using SAS software version 9.1 (SAS Institute, Cary, NC).
RESULTS
Patient Population and Key Clinical Outcomes
Between December 2000 and July 2008, 2502 patients were randomized to either CAS (n=1262) or CEA (n=1240). Mean age was 69 years, 65% of patients were male, the overwhelming majority of patients had at least 1 cardiovascular risk factor, and >85% of carotid stenoses were at least 70% in severity. Overall, 47% of patients were asymptomatic. As previously reported, rates of the primary composite endpoint were similar for CAS and CEA (7.2% and 6.8%, respectively, p=0.51) (15). Periprocedural stroke was more common with CAS (4.1% versus 2.3%, p=0.01), and periprocedural MI was more common with CEA (2.3% vs. 1.1%, p=0.03). Cranial nerve palsy was noted in 0.3% and 4.7% of the CAS and CEA patients, respectively (p<0.01).
HRQOL Outcomes
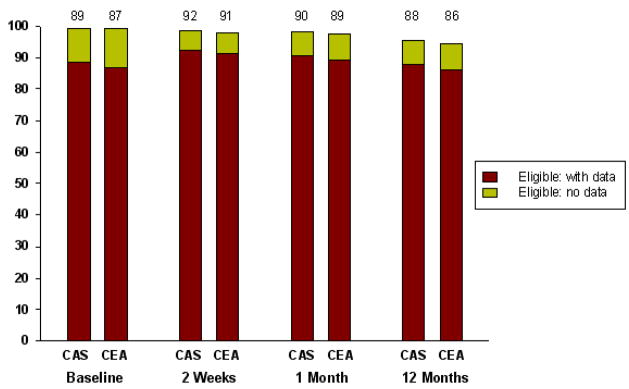
The CAS and CEA groups had similar response rates for the HRQOL assessments both at baseline and during follow-up (85–90% among surviving patients at all timepoints; Figure 1). Results from the analyses of the general health status outcomes are summarized in Table 1 and Figure 2.
Figure 1. Completeness of Data.
Available health status data in patients randomized to CAS versus CEA. Percentages listed above each bar indicate the proportion of surviving patients with health status scores available at that timepoint. CAS indicates carotid artery stent; CEA, carotid endarterectomy.
Table 1.
Baseline Health Status Scores and Differences Between CAS versus CEA During Follow-up, After Adjusting for Symptomatic Status and Baseline Scores
| SF-36 Subscale (raw data) | Mean Baseline Scores (± SD) | Mean Difference During Follow-up (CAS-CEA) (95% CI) | |||
|---|---|---|---|---|---|
| CAS | CEA | 2-weeks | 1-month | 12-months | |
| Physical function | 61.5 ± 29.2 | 63.5 ± 28.3 | 2.2 (0.6 to 3.9) ** | 2.3 (0.5 to 4.1) * | 0.8 (−1.2 to 2.9) |
| Role-physical | 44.6 ± 43.3 | 46.4 ± 43.8 | 8.7 (5.7 to 11.7) ** | 8.3 (5.1 to 11.6) ** | 2.0 (−1.7 to 5.6) |
| Vitality | 54.8 ± 23.2 | 55.6 ± 22.9 | 3.5 (1.7 to 5.3) ** | 0.9 (−0.7 to 2.5) | 0 (−1.7 to 1.7) |
| Pain index | 64.2 ± 27.7 | 66.0 ± 26.7 | 3.0 (1.0 to 5.0) ** | 1.4 (−0.5 to 3.2) | 0.2 (−1.9 to 2.2) |
| Social function | 73.0 ± 27.3 | 73.8 ± 27.2 | 3.3 (1.3 to 5.3) ** | 2.5 (0.6 to 4.5) * | 1.2 (−0.9 to 3.2) |
| General health | 62.0 ± 21.1 | 62.8 ± 20.7 | −0.9 (−2.3 to 0.5) | 0 (−1.3 to 1.4) | 0.1 (−1.4 to 1.7) |
| Role-emotional | 69.1 ± 40.7 | 69.2 ± 41.4 | 0.4 (−2.9 to 3.7) | 0.9 (−2.2 to 4.0) | −1.1 (−4.4 to 2.2) |
| Mental health | 74.1 ± 19.3 | 74.1 ± 19.3 | 1.1 (−0.4 to 2.5) | 0.5 (−0.8 to 1.8) | 0.4 (−0.9 to 1.8) |
|
| |||||
| Physical component summary | 39.9 ± 11.6 | 40.8 ± 11.2 | 1.6 (0.9 to 2.3) ** | 1.4 (0.7 to 2.1) ** | 0.4 (−0.5 to 1.2) |
| Mental component summary | 50.4 ± 10.9 | 50.3 ± 10.8 | 0.5 (−0.3 to 1.4) | 0.2 (−0.6 to 1.0) | 0 (−0.8 to 0.8) |
CAS indicates carotid artery stenting; CEA, carotid endarterectomy; CI, confidence interval; SD, standard deviation; and SF-36, Medical Outcomes Study Short-Form 36.
indicates p≤0.05 for the comparison of CAS vs. CEA.
indicates p≤0.01 for the comparison of CAS vs. CEA.
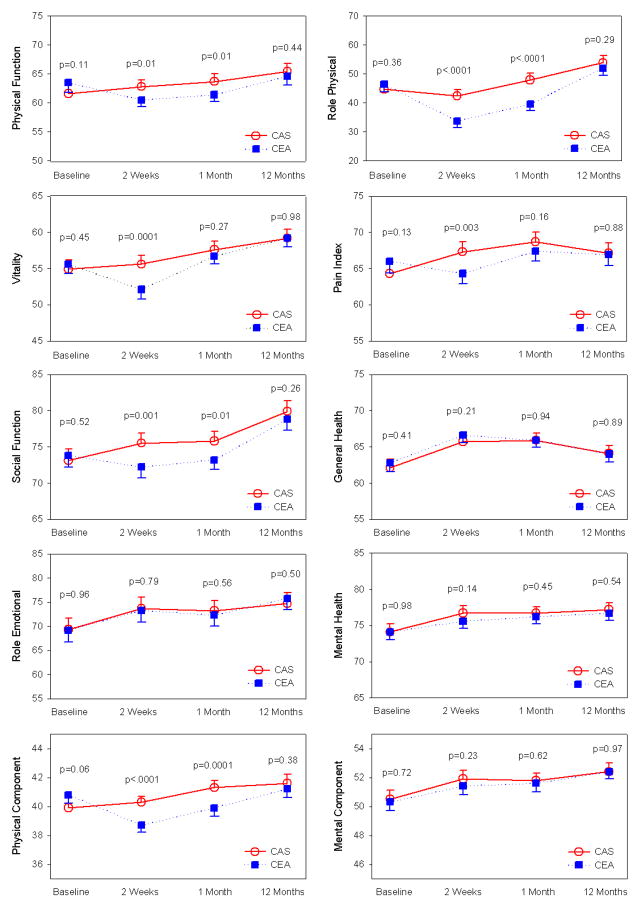
Figure 2. Generic Health Status During Follow-Up.
Trend in SF-36 scores from baseline to 1-year. Higher scores indicate better quality-of-life; significant differences in scores (p≤0.05) were noted in 5 of 8 subscales and the physical component summary scale at the 2-week follow-up visit, and in 3 of 8 subscales and the physical component summary scale at the 1-month follow-up visit. All differences between groups had resolved by 1-year. Plotted values at each timepoint represent least-squares means and associated 95% confidence intervals derived from the analysis of covariance. CAS indicates carotid artery stenting; CEA, carotid endarterectomy; SF-36, Medical Outcomes Study Short-Form 36.
At baseline, all SF-36 subscale scores were similar for the 2 groups. Compared with the CEA group, CAS patients had better scores at 2-weeks for 5 of the 8 SF-36 subscales (all p≤0.01), with the role physical subscale demonstrating the greatest difference between treatment groups. By 1-month follow-up, only 3 of the 8 subscales had better scores in the CAS group, and there were no significant differences for any of the SF-36 subscales at 1-year. Findings were unchanged when multiple imputation was used to account for missing data (see Supplementary Appendix, Table 1), when the analyses were repeated according to treatment received (Supplementary Appendix, Table 2), or when using “worst case scores” for missing health status data among patients experiencing periprocedural events (Supplementary Appendix, Tables 3 and 4).
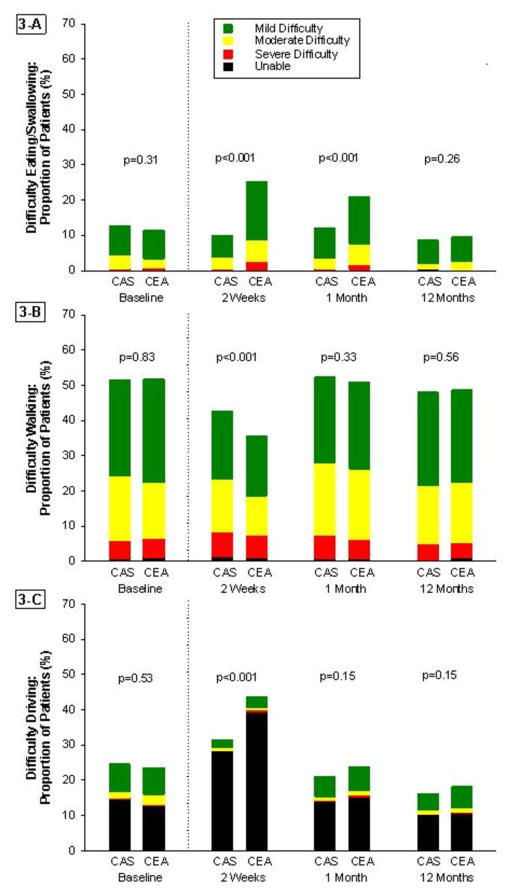
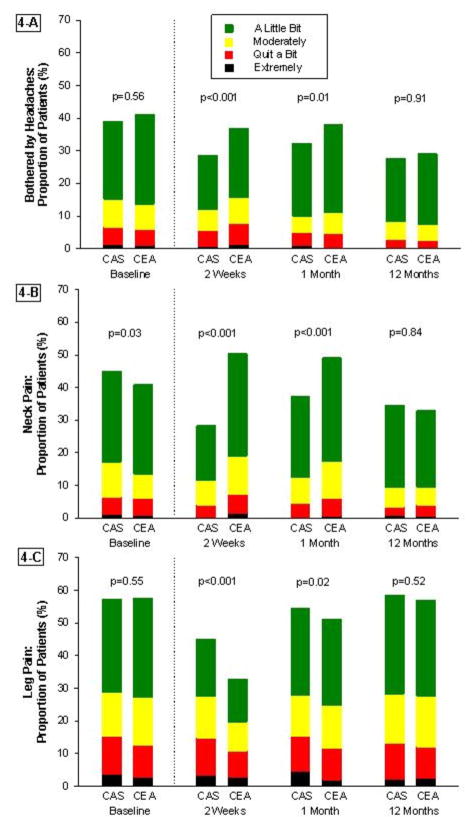
Results for the disease-specific Likert scales are summarized in Figures 3 and 4. At 2-weeks, CAS patients reported less difficulty eating or swallowing, less difficulty driving, and less impairment from headaches and neck pain as compared with CEA patients. However, CAS patients also reported more difficulty walking and more impairment from leg pain. By the 1-month follow-up visit, CAS patients continued to experience less difficulty eating or swallowing and less impairment from headaches and neck pain, but more limitations from leg pain than those in the CEA group. There were no significant differences in any of the other disease-specific measures at 1-month, and no differences between the groups for any measure by the 12-month assessment. These results were unchanged when multiple imputation was used to account for missing data (Supplementary Appendix, Figures 1 and 2), or for the on-treatment analysis (Supplementary Appendix, Figures 3 and 4).
Figure 3. Disease-Specific Functional Limitations.
Trend in modified Likert scores from baseline to 1-year for (A) difficulty eating or swallowing, (B) difficulty walking, and (C) difficulty driving. CAS indicated carotid artery stenting; CEA, carotid endarterectomy.
Figure 4. Disease-Specific Pain Scales.
Trend in modified Likert scores from baseline to 1-year for (A) headaches, (B) neck pain, and (C) leg pain. CAS indicates carotid artery stenting; CEA, carotid endarterectomy.
For ratings of overall pain on a 0–10 scale, CAS and CEA patients reported similar scores at baseline (mean score 3.1 versus 3.0, p=0.23). At the 2-week assessment, CAS patients reported significantly lower pain scores than CEA patients (mean 2.9 versus 3.1, p<0.01) but these differences were no longer present by the 1-month (mean 3.0 versus 3.1, p=0.16) and 12-month (mean 3.0 versus 3.0, p=0.86) assessments. Similarly, CAS patients reported less need for pain medications during the week preceding the 2-week assessment (OR 0.64 versus CEA patients, CI 0.54–0.76, p<0.01), but there was no difference between the groups at the 1-month (OR 1.01, CI 0.85–1.21, p=0.90) or 12-month (OR 1.06, CI 0.88–1.27, p=0.57) evaluations.
Impact of Periprocedural Stroke and MI Events on HRQOL
The results of the exploratory analyses to estimate the impact of periprocedural events on 1-year health status domains are summarized in Table 2. Patients who had a periprocedural stroke reported worse HRQOL scores at 1-year for 7 of 8 domains of the SF-36 when compared with patients who had no periprocedural events. In contrast, periprocedural MI was associated with worse general health perception at 1-year, but no differences in any other health status domains. Cranial nerve palsy was not associated with a sustained impact on HRQOL.
Table 2.
Impact of Periprocedural Events on Health Status Scores at 1-Year Follow-up
| SF-36 Subscale | Stroke (any) | Myocardial Infarction | Cranial Nerve Palsy | |||
|---|---|---|---|---|---|---|
| Mean Difference * (95% CI) | p-value | Mean Difference * (95% CI) | p-value | Mean Difference * (95% CI) | p-value | |
| Physical function | −14.2 (−20.7 to −7.8) | <0.001 | −5.8 (−14.5 to 3.0) | 0.196 | 1.5 (−4.7 to 7.6) | 0.643 |
| Role-physical | −23.3 (−34.4 to −12.1) | <0.001 | −2.4 (−17.6 to 12.7) | 0.752 | 3.9 (−6.7 to 14.6) | 0.471 |
| Vitality | −11.0 (−16.3 to −5.7) | <0.001 | −4.6 (−11.8 to 2.5) | 0.205 | 4.6 (−0.5 to 9.7) | 0.075 |
| Pain index | −7.6 (−14.1 to −1.1) | 0.021 | 7.0 (−1.8 to 15.8) | 0.120 | −1.3 (−7.5 to 5.0) | 0.692 |
| General health | −4.3 (−9.1 to 0.5) | 0.080 | −7.8 (−14.3 to −1.2) | 0.020 | 1.9 (−2.7 to 6.5) | 0.429 |
| Social function | −7.0 (−13.5, −0.6) | 0.033 | −2.4 (−11.1 to 6.4) | 0.598 | 3.2 (−3.0 to 9.4) | 0.307 |
| Role-emotional | −25.1 (−35.4 to −14.7) | <0.001 | 0 (−14.0 to 14.0) | 0.999 | 0.8 (−9.1 to 10.6) | 0.881 |
| Mental health | −5.5 (−9.8 to −1.2) | 0.013 | 3.0 (−2.8 to 8.9) | 0.308 | 3.0 (−1.1 to 7.1) | 0.157 |
|
| ||||||
| Physical component summary | −4.4 (−7.0 to −1.8) | 0.001 | −2.0 (−5.6 to 1.5) | 0.260 | 0.1 (−2.4 to 2.6) | 0.939 |
| Mental component summary | −4.1 (−6.6 to −1.7) | 0.001 | 0.5 (−2.9 to 3.8) | 0.787 | 1.4 (−1.0 to 3.7) | 0.263 |
CI indicates confidence interval; SF-36 indicates Medical Outcomes Study Short-Form 36.
Mean difference between patients with or without each periprocedural event after adjustment for age, sex, diabetes, prior coronary bypass surgery, history of cardiovascular disease, and symptomatic status prior to carotid revascularization.
DISCUSSION
In this pre-specified substudy of CREST, we found that patients undergoing CAS had better health-related quality of life during the first month after carotid revascularization relative to patients undergoing CEA. These benefits were most pronounced for measures of overall physical function and pain. In addition, disease-specific measures demonstrated that limitations related to ambulation and leg discomfort were more common after CAS, whereas limitations related to eating and neck discomfort were more common after CEA. All of these differences between CAS and CEA were modest in magnitude and were no longer present at 1-year follow-up.
Exploratory analyses of the impact of periprocedural events on health status revealed a strong and consistent impairment of HRQOL at 1-year among those patients who experienced a periprocedural stroke when compared with those who did not. For most scales, these differences exceeded values generally considered to be clinically meaningful. In contrast, there was minimal or no long-term impairment in health status among patients who had a periprocedural MI or cranial nerve palsy. To date, this is the largest study comparing recovery patterns among patients randomized to either CAS or CEA and the first study to directly evaluate the impact of periprocedural events on HRQOL after carotid revascularization.
These results are consistent with previous results from one nonrandomized evaluation of CAS and CEA (20), and are also similar to findings from the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) randomized clinical trial (6). In the quality of life substudy of SAPPHIRE, patients reported fewer symptoms and less impairment of physical function at the 2-week visit when undergoing CAS vs. CEA, but these differences were no longer apparent at 1-month (21). The present analysis from CREST demonstrated somewhat greater functional status benefits of CAS over CEA at 2 weeks, and in contrast to SAPPHIRE, these differences were largely maintained at the 1-month follow-up visit for both generic and disease-specific assessments.
There are several potential explanations for the differences between the CREST and SAPPHIRE results. First, CREST enrolled nearly 8 times as many patients as SAPPHIRE, and the additional quality of life differences in CREST may simply reflect its greater statistical power. Alternatively, the differences between trials may be explained by differences in patient populations, as patients in the SAPPHIRE trial had a greater burden of comorbidity due to the enrollment requirement of high surgical risk. As a result, the healthier patient population in CREST may have experienced a more benign recovery period which would allow differences in health status after CAS versus CEA to be more readily detected. Regardless of the underlying mechanisms, HRQOL outcomes were similar for the two treatment groups during longer-term follow-up for both the CREST and SAPPHIRE populations.
In addition to the prespecified analyses comparing CAS versus CEA, we performed post-hoc analyses to explore in greater depth the impact of early events on late health status. These exploratory analyses demonstrated that health status at 1-year was influenced by the occurrence of periprocedural stroke, but not by either MI or cranial nerve palsy. These findings are not particularly surprising. Most studies of stroke have found persistent disability in 15–30% of surviving patients (30,31), which would be expected to result in impaired physical function, role function, and general health perception. In contrast, after the initial short term recovery phase, patients with MI generally have health status that is similar to the general population unless the MI is large and associated with clinical heart failure or a protracted recovery period (32,33). Notwithstanding the results of our analysis, MI should not be construed as a benign event as it has been associated with a poor long-term prognosis in multiple settings (34–36).
On the other hand, the lack of association between cranial nerve palsy and quality of life was somewhat unexpected as ~5% have been reported to be persistent after CEA in previous studies (with rates ranging from 3% to 23%) (37). The effects of cranial nerve injury can be quite variable, however, ranging from complete facial palsy to mild paresthesias of the tongue. It is also possible that the SF-36 was insensitive to the degree of disability and HRQOL impairment caused by cranial nerve palsies in the CREST population.
It may seem counterintuitive that overall health status and quality of life did not differ after CAS or CEA despite the higher rate of stroke after CAS and the significant impact of stroke on 1-year HRQOL in the study population. It is important to note, however, that the vast majority of patients (>95% in both treatment groups) did not experience a stroke—thus limiting the impact of such events on any between group measures of HRQOL. Indeed, a trial powered to detect a 0.5 point difference in role physical function (the expected between-group difference based on the CREST results) would require randomization of more than 200,000 patients.
Although 1-year HRQOL did not differ between the CAS and CEA groups, some patients may favor one or the other approach to carotid revascularization according to their individual values and preferences. Given the greater impact of stroke on late health status and the fact that stroke prevention is the principal indication for carotid revascularization, many patients may prefer CEA over CAS, because CEA minimizes the risk of such events. On the other hand, patients at very low risk of periprocedural stroke (e.g., younger, asymptomatic patients) may consider the more rapid recovery and lesser health status impairment during the first month after revascularization to be a compelling argument for CAS.
Limitations
As with any clinical trial, the results of our study may not be generalizable to all patients who are candidates for carotid revascularization. Nonetheless, the large number of sites and operators included in CREST suggests that our results should apply to many other centers and operators who are able to meet the volume and training criteria required for CREST certification (15,23). In addition, ~10% of CREST patients did not receive their assigned revascularization procedure, mainly related to patients who were enrolled based on non-invasive carotid imaging and were subsequently found to be anatomically unsuitable for CAS— many of whom then underwent CEA. The effect of such treatment crossovers would be expected to dilute any true treatment differences, however, and the similarity of our intention-to-treat and on-treatment results suggests that the extent of bias introduced was small. Finally, some quality of life data were missing at each follow-up timepoint. Nonetheless, the response rates were quite high considering the patient population, and it is reassuring that the results were unchanged in analyses incorporating multiply imputed data.
Conclusions
In summary, among patients with clinical indications and anatomy suitable for either surgical or percutaneous revascularization, CAS was associated with better health-related quality of life during the early recovery period as compared with CEA. These differences were less pronounced at 1-month than at 2-weeks and were no longer present after 1-year. Although stroke was more common after CAS than CEA and was associated with clinically important health status impairment throughout follow-up, the small absolute difference in event rates did not result in a detectable difference in long-term HRQOL between the 2 treatments. These health status data, in conjunction with evidence (from CREST and other trials) regarding the absolute and relative risk of important clinical events, should help to better inform patients and clinicians regarding the risks and benefits of CAS versus CEA and thus help guide patient-centered decision making.
Supplementary Material
Acknowledgments
Financial Support: Supported by the National Institute of Neurological Disorders and Stroke (NINDS) and the NIH (R01 NS 038384) with supplemental funding from Abbott Vascular Solutions (formerly Guidant), including donations of Accunet and Acculink systems equivalent to ~15% of the total study cost for CREST centers in Canada and to CREST centers in the U.S. that were at Veterans Affairs sites.
ABBREVIATIONS
- ANCOVA
analysis of covariance
- CAS
carotid artery stenting
- CEA
carotid endarterectomy
- CI
confidence interval
- CREST
Carotid Revascularization Endarterectomy versus Stenting Trial
- HRQOL
health-related quality of life
- MI
myocardial infarction
- OR
odds ratio
- SAPPHIRE
Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy
- SF-36
Medical Outcomes Study Short-Form 36
Footnotes
ClinicalTrials.gov number NCT00004732
Conflicts of Interest: Dr. Cohen has received research support from Boston Scientific, Abbott Vascular, Medtronic, Edwards Lifesciences, MedRad, Merck/Schering-Plough, and Eli Lilly-Daiichi Sankyo; he reports serving as a consultant to Schering-Plough, Eli Lilly, Medtronic, and Cordis; and he has served on the speakers’ bureau for Eli Lilly and The Medicines Company. Dr. Stolker has served on the speakers’ bureau for AstraZeneca. Dr. Magnuson has received research support from Eli-Lilly-Daiichi Sankyo, Sanofi-Aventis, and Bristol Myers Squibb; and she has received honoraria from Sanofi-Aventis. Dr. Aronow has served on the speakers’ bureau/advisory board for Medtronic. Dr. Goldstein has served as consultant/advisory board member for Abbott, ACT-1 Trial Clinical Oversight Committee. Dr. Roubin has received royalties from Abbott Vascular, Inc. and Cook, Inc. Dr. Howard has served as consultant/advisory board member for Bayer Healthcare and is a member of the ARRIVE Executive Committee. The other authors have no conflicts to report regarding this analysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Circulation. 2006;113:e409–49. [PubMed] [Google Scholar]
- 2.Zarins CK. Carotid endarterectomy: the gold standard. J Endovasc Surg. 1996;3:10–5. doi: 10.1177/152660289600300106. [DOI] [PubMed] [Google Scholar]
- 3.Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1991;325:445–53. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 4.Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995;273:1421–8. [PubMed] [Google Scholar]
- 5.Wennberg DE, Lucas FL, Birkmeyer JD, Bredenberg CE, Fisher ES. Variation in carotid endarterectomy mortality in the Medicare population: trial hospitals, volume, and patient characteristics. JAMA. 1998;279:1278–81. doi: 10.1001/jama.279.16.1278. [DOI] [PubMed] [Google Scholar]
- 6.Yadav JS, Wholey MH, Kuntz RE, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493–501. doi: 10.1056/NEJMoa040127. [DOI] [PubMed] [Google Scholar]
- 7.Gray WA, Hopkins LN, Yadav S, et al. Protected carotid stenting in high-surgical-risk patients: the ARCHeR results. J Vasc Surg. 2006;44:258–68. doi: 10.1016/j.jvs.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 8.Hopkins LN, Myla S, Grube E, et al. Carotid artery revascularization in high surgical risk patients with the NexStent and the Filterwire EX/EZ: 1-year results in the CABERNET trial. Catheter Cardiovasc Interv. 2008;71:950–60. doi: 10.1002/ccd.21564. [DOI] [PubMed] [Google Scholar]
- 9.Iyer SS, White CJ, Hopkins LN, et al. Carotid artery revascularization in high-surgical-risk patients using the Carotid WALLSTENT and FilterWire EX/EZ: 1-year outcomes in the BEACH Pivotal Group. J Am Coll Cardiol. 2008;51:427–34. doi: 10.1016/j.jacc.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 10.Marine LA, Rubin BG, Reddy R, Sanchez LA, Parodi JC, Sicard GA. Treatment of asymptomatic carotid artery disease: similar early outcomes after carotid stenting for high-risk patients and endarterectomy for standard-risk patients. J Vasc Surg. 2006;43:953–8. doi: 10.1016/j.jvs.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Ederle J, Bonati LH, Dobson J, et al. Endovascular treatment with angioplasty or stenting versus endarterectomy in patients with carotid artery stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): long-term follow-up of a randomised trial. Lancet Neurol. 2009;8:898–907. doi: 10.1016/S1474-4422(09)70228-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mas JL, Chatellier G, Beyssen B, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660–71. doi: 10.1056/NEJMoa061752. [DOI] [PubMed] [Google Scholar]
- 13.Ringleb PA, Allenberg J, Bruckmann H, et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239–47. doi: 10.1016/S0140-6736(06)69122-8. [DOI] [PubMed] [Google Scholar]
- 14.Ederle J, Dobson J, Featherstone RL, et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet. 2010;375:985–97. doi: 10.1016/S0140-6736(10)60239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brott TG, Hobson RW, 2nd, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis SM, Donnan GA. Carotid-artery stenting in stroke prevention. N Engl J Med. 2010;363:80–2. doi: 10.1056/NEJMe1005220. [DOI] [PubMed] [Google Scholar]
- 17.Rothwell PM. Carotid stenting: more risky than endarterectomy and often no better than medical treatment alone. Lancet. 2010;375:957–9. doi: 10.1016/S0140-6736(10)60404-7. [DOI] [PubMed] [Google Scholar]
- 18.Roffi M, Sievert H, Gray WA, et al. Carotid artery stenting versus surgery: adequate comparisons? Lancet Neurol. 2010;9:339–41. doi: 10.1016/S1474-4422(10)70027-2. [DOI] [PubMed] [Google Scholar]
- 19.Brown MM, Mas JL, Ringleb PA, Hacke W. Carotid artery stenting versus surgery: adequate comparisons? Triallists’ reply. Lancet Neurol. 2010;9:341–2. doi: 10.1016/S1474-4422(10)70027-2. [DOI] [PubMed] [Google Scholar]
- 20.Carotid Revascularization Using Endarterectomy or Stenting Systems (CaRESS) phase I clinical trial: 1-year results. J Vasc Surg. 2005;42:213–9. doi: 10.1016/j.jvs.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Stolker JM, Mahoney EM, Safley DM, Pomposelli FB, Jr, Yadav JS, Cohen DJ. Health-related quality of life following carotid stenting versus endarterectomy: results from the SAPPHIRE (Stenting and Angioplasty with Protection in Patients at HIgh Risk for Endarterectomy) trial. JACC Cardiovasc Interv. 2010;3:515–23. doi: 10.1016/j.jcin.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Hobson RW., 2nd CREST (Carotid Revascularization Endarterectomy versus Stent Trial): background, design, and current status. Semin Vasc Surg. 2000;13:139–43. [PubMed] [Google Scholar]
- 23.Hopkins LN, Roubin GS, Chakhtoura EY, et al. The Carotid Revascularization Endarterectomy versus Stenting Trial: credentialing of interventionalists and final results of lead-in phase. J Stroke Cerebrovasc Dis. 2010;19:153–62. doi: 10.1016/j.jstrokecerebrovasdis.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 25.Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305:160–4. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Failde I, Ramos I. Validity and reliability of the SF-36 Health Survey Questionnaire in patients with coronary artery disease. J Clin Epidemiol. 2000;53:359–65. doi: 10.1016/s0895-4356(99)00175-4. [DOI] [PubMed] [Google Scholar]
- 27.Kiebzak GM, Pierson LM, Campbell M, Cook JW. Use of the SF36 general health status survey to document health-related quality of life in patients with coronary artery disease: effect of disease and response to coronary artery bypass graft surgery. Heart Lung. 2002;31:207–13. doi: 10.1067/mhl.2002.124299. [DOI] [PubMed] [Google Scholar]
- 28.Cohen J. Statistical Power Analysis for the Behavioural Sciences. 2. New Jersey: Lawrence Earlbaum; 1988. [Google Scholar]
- 29.Taylor JM, Cooper KL, Wei JT, Sarma AV, Raghunathan TE, Heeringa SG. Use of multiple imputation to correct for nonresponse bias in a survey of urologic symptoms among African-American men. Am J Epidemiol. 2002;156:774–82. doi: 10.1093/aje/kwf110. [DOI] [PubMed] [Google Scholar]
- 30.Carod-Artal FJ, Egido JA. Quality of life after stroke: the importance of a good recovery. Cerebrovasc Dis. 2009;27 (Suppl 1):204–14. doi: 10.1159/000200461. [DOI] [PubMed] [Google Scholar]
- 31.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 32.Stewart AL, Greenfield S, Hays RD, et al. Functional status and well-being of patients with chronic conditions. Results from the Medical Outcomes Study. JAMA. 1989;262:907–13. [PubMed] [Google Scholar]
- 33.Mortensen OS, Madsen JK, Haghfelt T, et al. Health related quality of life after conservative or invasive treatment of inducible postinfarction ischaemia. DANAMI study group. Heart. 2000;84:535–40. doi: 10.1136/heart.84.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kushner FG, Hand M, Smith SC, Jr, et al. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention. Circulation. 2009;120:2271–306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- 35.Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2004;110:e340–437. [PubMed] [Google Scholar]
- 36.Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2007;50:e159–241. doi: 10.1016/j.jacc.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Cunningham EJ, Bond R, Mayberg MR, Warlow CP, Rothwell PM. Risk of persistent cranial nerve injury after carotid endarterectomy. J Neurosurg. 2004;101:445–8. doi: 10.3171/jns.2004.101.3.0445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.