Abstract
In many types of cancer, the expression of the immunoregulatory protein B7-H3 has been associated with poor prognosis. Previously, we observed a link between B7-H3 and tumor cell migration and invasion, and in present work we have investigated the role of B7-H3 in chemoresistance in breast cancer. We observed that silencing of B7-H3, via stable shRNA or transient siRNA transfection, increased the sensitivity of multiple human breast cancer cell lines to paclitaxel as a result of enhanced drug-induced apoptosis. Overexpression of B7-H3 made the cancer cells more resistant to the drug. Next, we investigated the mechanisms behind B7-H3 mediated paclitaxel resistance, and found that the level of Stat3 Tyr705 phosphorylation was decreased in B7-H3 knockdown cells, along with the expression of its direct downstream targets Mcl-1 and Survivin. The phosphorylation of Jak2, an upstream molecule of Stat3, was also significantly decreased. In contrast, reexpression of B7-H3 in B7-H3 knockdown and low B7-H3- expressing cells increased the phosphorylation of Jak2 and Stat3. In vivo animal experiments showed that B7-H3 knock down tumors displayed a slower growth rate than the control xenografts. Importantly, paclitaxel treatment showed a strong anti-tumor activity in the mice with B7-H3 knockdown tumors, but only a marginal effect in the control group. Taken together, our data demonstrate that in breast cancer cells B7-H3 induces paclitaxel resistance, at least partially by interfering with Jak2/Stat3 pathway. These results provide novel insight into the function of B7-H3 and encourage the design and testing of approaches targeting this protein and its partners.
Keywords: Breast cancer, paclitaxel, B7-H3, apoptosis, Stat3
Introduction
Breast cancer is the most common type of cancer as well as the most common cause of cancer-related death in women. Once breast cancer becomes metastatic, it is difficult to cure because it is often resistant to commonly used chemotherapeutics, such as taxanes, which are among the most effective chemotherapeutic agents against this tumor type. Hence, if the sensitivity to taxane-based chemotherapy could be increased, it would represent an important step in improving the clinical management of this disease. B7-H3, a transmembrane protein with immunoglobulin-like structure (1), is known to have immunoregulatory properties with both inhibitory and stimulatory effects on the activation of T-cells (2–5). Although the B7-H3 protein is found in many human tissues, it is overexpressed in several types of human cancers. Notably, immunohistochemical reports have shown a correlation between high expression of B7-H3 and poor outcome in patients with breast cancer (6), clear cell renal cell carcinoma (7), urothelial cell carcinoma (8), prostate cancer (9), non-small-cell lung cancer (10), and pancreatic cancer (11). Whereas these studies have focused on the immunoregulatory function of B7-H3, our previous in vitro studies in a non-immunological system, demonstrated that the knockdown of B7-H3 expression resulted in a robust inhibition of tumor cell migration and invasion, indicating a role of B7-H3 in these processes (12). Since metastasis is closely related to chemoresistance, we focused the present study on the role of B7-H3 in the sensitivity of metastatic breast cancer cells to paclitaxel and the possible underlying mechanisms. We found that silencing of B7-H3 sensitized the MDA-MB-231, MDA-MB-435 and MDA-MB-436 tumor cell lines to paclitaxel whereas B7-H3 overexpression led to increased resistance to this drug. Moreover, we discovered that knockdown of B7-H3 abrogated the phosphorylation of Stat3 on Tyr705 through inactivation of Jak2, and led to downregulation of the direct target genes of Stat3: Mcl-1 and to a lesser extent Survivin. In contrast, overexpression of B7-H3 increased the phosphophorylation of Jak2 and Stat3, indicating that Jak2/Stat3 pathway contributes to B7-H3 mediated paclitaxel resistance. These novel findings have important implications for the therapeutic strategy for treating paclitaxel-refractory cancers.
Material and Methods
Reagents
Anti-human B7-H3 antibody and anti-Survivin was purchased from R&D Systems, Inc (Minneapolis, MN). Antibodies against Stat3, p-Stat3Tyr705, p-Stat3Ser727, Jak2, p-Jak1Tyr1022/1023, p-Jak2Tyr1007/1008, p-SrcTyr416, Mcl-1 and PARP were from Cell Signaling Technology (Danvers, MA). Antibody against β-actin and Tryphostins AG490 were from Sigma-Aldrich Chemical Co (St. Louis, MO). The horseradish peroxidase conjugated secondary anti-mouse, anti-rabbit, and anti-goat antibodies were from Bio-Rad Laboratories, Inc (Hercules, CA). Paclitaxel was from Mead Johnson Inc. (Princeton, NJ).
Cells and cell culture
Cell lines MDA-MB-231, MDA-MB-435, MDA-MB-436, BT474, BT20, MCF-7 and SKBR3 were purchased from the American Type Culture Collection (ATCC) (Manassas, VA). No further authentication was performed by the authors. These cells were cultured in DMEM/F12 medium (Mediatech, Inc., Manassas, VA) supplemented with a 10% fetal bovine serum (FBS, Atlanta Biologicals Inc., Lawrenceville, GA) and 1% penicillin–streptomycin (Gibco, Paisley, UK) at 37°C under an atmosphere of 5% CO2. The non-transformed immortalized human mammary epithelial cells HMEC were cultured as previously described (13), which were generously provided by Drs. Rajeev Samant and Robert Weinberg and no further authentication was performed.
Generation of stable cell lines
HuSH 29mer shRNA constructs against B7-H3 (shB7-H3) and control plasmid pRS non-targeted TR30003 (TR33) were purchased from Origene Technologies, Inc (Rockville, MD) The sequences of shB 7-H3 were as follows: shRNA-1, 5 ’–TTCAGCCTGGCACAGCTCAACCTCATCTG-3 ’ ; shRNA-2, 5’–TCGTGTGCTGGAGAAAGATCAAACAGAGC-3’. The non-targeted control sequence was: 5’-GCACTACCAGAGCTAACTCAGATAGTACT -3’. Either B7-H3 shRNA construct or control vector were transfected into MDA-MB-231 and MDA-MB-435 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocols, followed by selection with 1 µg/ml puromycin for 2 weeks. Antibiotic-resistant clones were isolated in medium with 0.5 µg/ml puromycin. RT-PCR and immunoblotting were performed to confirm the knockdown of mRNA and protein of B7-H3 in those transfectants. To generate B7-H3 overexpession stable cell lines, cDNAs for full-length human B7H3 (NM_001024736) were amplified by PCR using the following primer sequences: Forward (5’-TCACTCGAGCCCTGAGTCCCAGAGTCGGC-3’); Reverse (5’- ACTGAATTCGGTTGTGGGTGGTCTGTTCAT-3’); and then the full-length cDNA was cloned into xho I and EcoR I linearized plasmid vector pIRES2-EGFP (Clontech, Mountain View, CA). The control vector pIRES2-EGFP and human B7-H3 expression vector pIRES2-B7-H3 were transfected in MDA-MB-231 cells using Lipofectamine 2000 (Invitrogen). Stable clones were selected in medium containing 1.8 mg/mL Geneticin (Invitrogen). The expression of B7-H3 was determined by Western blot analysis.
si-RNA experiments
siRNA targeting human B7-H3 and the control scrambled siRNA were purchased from Sigma-Aldrich. Transfection was performed using Lipofectamine 2000 (Invitrogen).Twenty-four h after transfection, cells treated with 20 nmol/L paclitaxel for another 48 h were used for Western blot analysis and apoptosis-specific ELISA detection.
In vitro growth inhibition
Cells (1 × 104 cells) were initially plated in triplicate in 96-well culture plates. Twenty-four h later, the medium was replaced with fresh medium with or without paclitaxel and incubated for indicated time. Cell viability was determined using CellTiter 96 Aqueous One Solution Cell Proliferation Assay Kit (Promega, Madison, WI).
Annexin V-FITC Staining
Cells (3 × 105 cells) were grown in triplicate in 60 mm dishes with exposure of 20 nmol/L paclitaxel for 0, 48, and 72 h respectively. And then cells were harvested and processed as described in the Annexin V-FITC Apoptosis Detection Kit I manual (BD Transduction Laboratories, BD Biosciences, (San Jose, CA) and analyzed by flow cytometry (BD LSR II).
TUNEL assay
Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end labeling (TUNEL) assay was performed using recombinant terminal transferase (TdT) and biotin-16-dUTP (Roche Diagnostics GmbH, Mannheim, Germany). After treatment with with 0, 10 and 20 nmol/L paclitaxel for 72 h, cells were processed following manufacturer’s protocol and analyzed by flow cytometer (BD Biosciences). Each experiment was repeated three times.
Quantification of apoptosis by ELISA kit
An apoptosis ELISA kit (Roche Diagnostics Co. Indianapolis, IN) was used to quantitatively measure cytoplasmic histone-associated DNA fragments. After treatment with 0 and 20 nmol/L paclitaxel for 72 h, cells were analyzed following manufacturer’s protocol. Each experiment was repeated three times.
Western blot analysis
Western blotting was performed on whole cell extracts prepared by lysing cells in 50 mmol/L Tris-HCl (pH8.0), 150 mmol/L NaCl, 1% Triton-X 100, 10 mmol/L EDTA, 5 mmol/L NaF, 5 mmol/L sodium pyrophosphate, 1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L Na3VO4, and protease inhibitor cocktail (Sigma-Aldrich) for 20 min on ice. The proteins were separated by SDS-PAGE and then electrotransferred onto nitrocellulose membrane (Bio-rad). Membranes were probed with indicated antibodies following the manufacturer’s protocol, and immunoreactive bands were visualized using ECL Western Blotting Substrate (Pierce Biotechnology, Inc. Rockford, IL).
In vivo studies
Four groups of 5–6 female Balb/c nude mice, bred at the nude rodent facility at the Norwegian Radium Hospital were used. The animals were maintained under specific pathogen-free conditions, and food and water were supplied ad libitum. Animal experiments were performed according to protocols approved by the animal care and use committee and were in compliance with the guidelines on animal welfare of the Norwegian National Committee for Animal Experiments. When the animals were 6–8 weeks of age, 5×106 cells (MDA-MB-435 shB7-H3 or MDA-MB-435 TR33 cells) in 0.2 ml PBS were injected s.c. in both flanks of nude mice. For therapy experiments, a stock solution of paclitaxel in ethanol (6 mg/ml) was dissolved in PBS and a single dose of 10 mg/kg of the drug was injected i.v into the tail vein when the mean tumor diameter was 5–6 mm (day 0). The control mice received only the solvent. Tumor diameters were measured one to two times per week. Tumor volume was calculated by the formula 0.5 × length × width2 and growth curves constructed, and the data presented as mean of two independent experiments ± standard error of the mean (SEM).
Immunohistochemistry
The tumor xenografts were removed on day 7 after treatment and usedfor immunhistochemical studies. Four µm sections were stained using the Dako EnVision™ + System (K4007 and K4011) (Dako A/S, Glostrup, Denmark). The sections were stained towards B7-H3 using a goat anti-human polyclonal antibody (R&D System). For the clinical breast carcinoma samples 5 micron sections were cut and incubated with the antibody against B7-H3 (R&D System) using The Dako Envision system (K4007) (Dako). Positive and negative controls were included. The slides were observed under an Olympus BX51 microscope at 10 and 40 × magnification.
Statistical analysis
Statistical evaluation for experimental data analysis was determined with an unpaired student’s t-test. A statistical difference of P <0.05 was considered significant.
Results
B7-H3 expression in breast cancer cell lines and clinical specimen
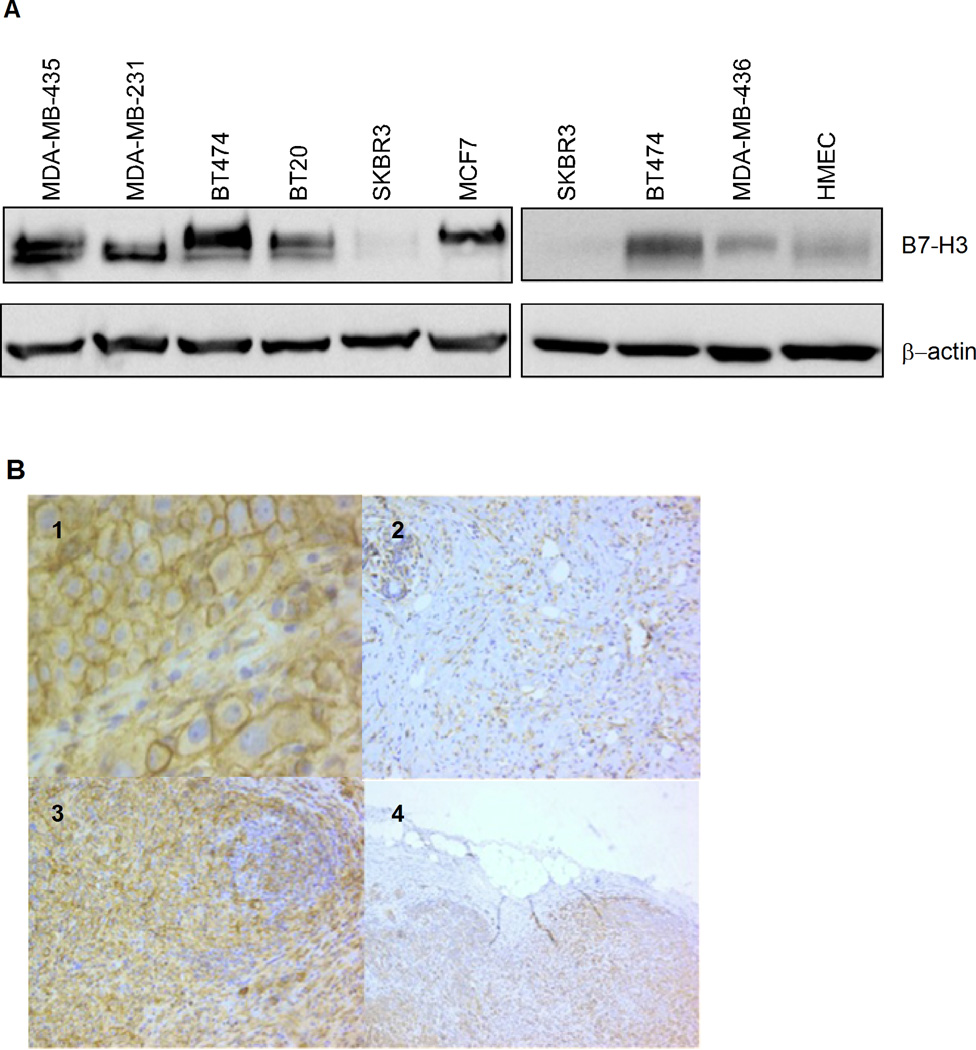
The expression of B7-H3 in human breast cancer tissues from primary tumors and lymph node metastasis has recently been demonstrated (6). Here, we tested a panel of breast cell lines, including immortalized “normal” human mammary epithelial cells HMEC, transformed luminal cells BT474, MCF7 and SKBR3, basal A cells BT20, and basal B cells MDA-MB-231, MDA-MB-435 and MDA-MB-436 (Fig. 1A). As assessed by Western blotting, the B7-H3 protein was present in all the tested breast cancer cell lines except SKBR3, and compared to the non-transformed breast epithelial HMEC cells, the protein levels were elevated. We also included samples from breast cancer patients, and as shown in Fig. 1B panels 1–4, the immunohistochemical evaluation revealed that all tumor cells in ductal and lobular carcinomas and in a lymph node metastasis showed strong membrane and cytoplasmatic staining. Tumor-near stromal cells were also stained, but generally much weaker than the tumor cells, both in primary tumors and in metastases.
Figure 1.
B7-H3 expression in breast cancer cell lines and clinical specimen. A, protein expression of B7-H3 was analysed by Western blotting in a panel of human breast cell lines as indicated. β-actin levels served as loading control. B, Immunorstaining of B7-H3 in primary breast carcinomas. 1: ductal carcinoma with strong membranous and cytoplasmic staining. 2: lobular carcinoma. Single cells in a collagen rich stroma showed diffuse cytoplasmic staining. Note the normal glandular structures in the upper left corner being moderately stained. 3: ductal breast carcinoma and lymphoid infiltrate. Note the intense cytoplasmic staining of the epithelial cells. 4: lymph node metastasis from ductal carcinoma: All lymphoid tissue is replaced with carcinoma cells with diffuse cytoplasmic staining. (Magnification: panel 1 400 ×, panel 2–4 100×).
Silencing of B7-H3 enhances paclitaxel-induced cytotoxicity in cancer cells
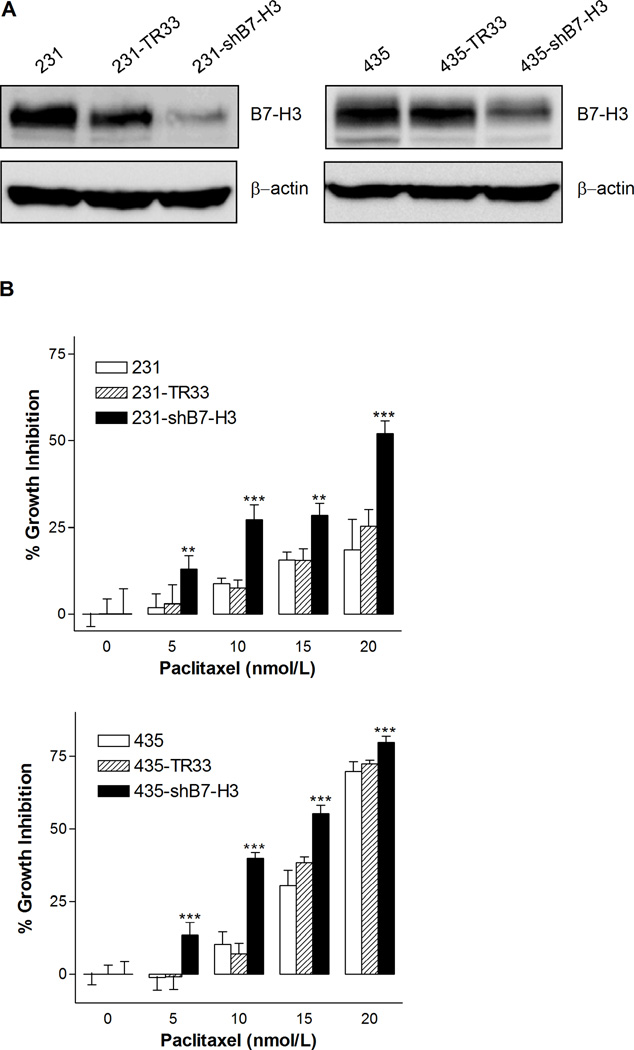
To study the possible role of B7-H3 in affecting the sensitivity of breast cancer cells to paclitaxel, we used shRNA to create two stable B7-H3 knockdown cell variants derived from the MDA-MB-231 and MDA-MB-435 cell lines. Compared to the parental and the non-target shRNA transfected control cell variants MDA-MB-231-TR33 (231-TR33) and MDA-MB-435-TR33 (435-TR33), their corresponding B7-H3 knockdown cell variants MDA-MB-231-shB7-H3 (231-shB7-H3) and MDA-MB-435-shB7-H3 (435-shB7-H3) expressed very low levels of B7-H3, demonstrating an effective knockdown of the B7-H3 protein (Fig. 2A).
Figure 2.
B7-H3 silencing increased the sensitivity to paclitaxel in breast cancer cells. A, Western blot analysis demonstrating the B7-H3 protein level of MDA-MB-231 (left) and MDA-MB-435 cells (right), expressing either non-target vector control TR33 or shB7-H3 and their corresponding parental cells. β-actin levels served as loading control. B, Cell viability of 231, 231-TR33, 231-shB7-H3 (top), 435, 435-TR33, and 435-shB7-H3 cell variants (bottom) following treatment with paclitaxel at various concentrations for 72 h was assessed using MTS assay. Data are presented as the percentage of cell growth inhibition measured in paclitaxel treated cells compared to untreated cells. The p value shows the difference between paclitaxel-treated B7-H3 knockdown cells and parental cells. Columns, mean of three independent experiments performed in triplicates; bars, S.E.; ** p< 0.01; *** p < 0.001.
Upon treatment with various concentrations of paclitaxel for 72 h, a dose-dependent inhibition of cell growth was observed in both MDA-MB-231 and MDA-MB-435 cells (Fig. 2B), and the B7-H3 knockdown cells were about two-fold more sensitive to paclitaxel compared to parental and control cells. In MDA-MB-231 cell variants, the inhibition of cell growth was 52 % after exposure to 20 nmol/L paclitaxel in the B7-H3 knockdown cells compared to 18 % and 25 % in parental and control cells. In the MDA-MB-435 variants, 15 nmol/L paclitaxel induced inhibition of cell growth by 55 %, 31 % and 38 % in the B7-H3 knockdown, parental and control cells, respectively. Statistical analysis shows that the differences between B7-H3 knockdown and control cells were significant in both the MDA-MB-231 and MDA-MB-435 cell lines. These results indicate that B7-H3 plays a role in tumor cell resistance to paclitaxel. There were no clear differences between the parental and vector control cells with respect to paclitaxel responsiveness, hence we did not include the parental cell lines in the further biochemical and molecular studies.
B7-H3 plays a critical role in cancer cell resistance to paclitaxel-induced apoptosis
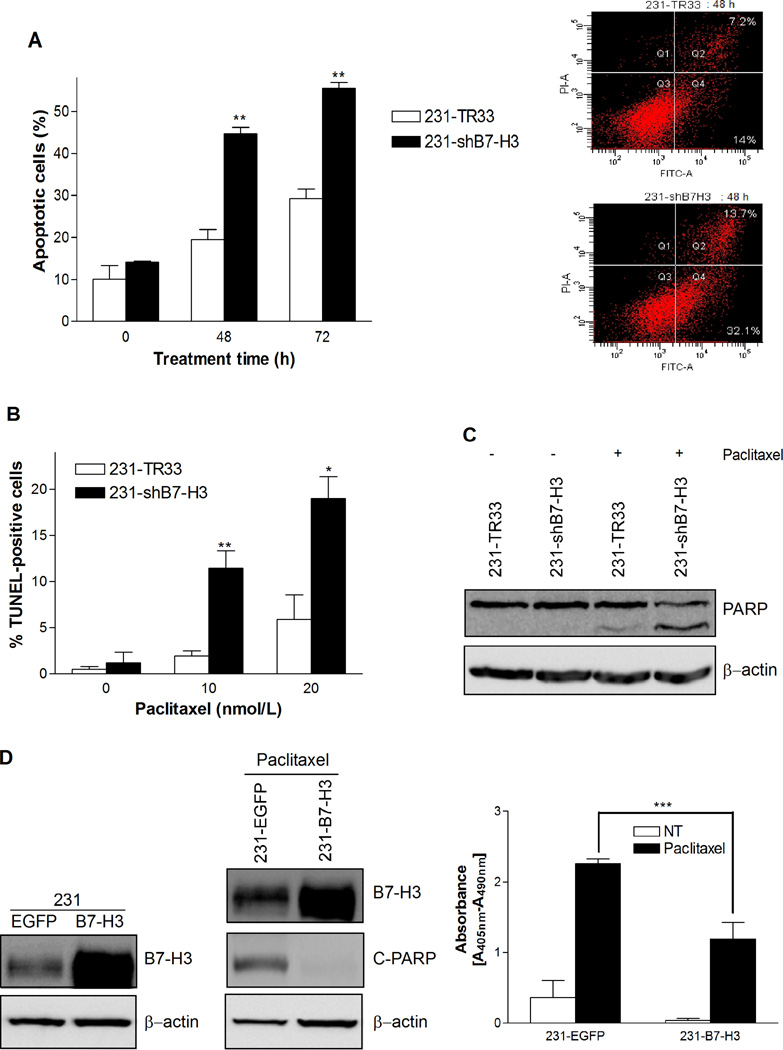
Paclitaxel is known to exert its cytotoxic effect through induction of apoptosis (14), and hence we investigated whether the increased paclitaxel cytotoxicity observed in B7-H3 knockdown cells could be related to effects on apoptosis. The extent of apoptosis was investigated by measuring the amount of Annexin-V stained cells, a marker for early stage apoptosis, in MDAMB-231 and MDA-MB-435 cells, and in MDA-MB-231 cells also for the amount of TUNEL positive cells, which reflects late stage apoptosis. In the Annexin-V assay, the response to 20 nmol/L paclitaxel was time dependent with an increase in the amount Annexin-V positive cells detected at 48 and 72 h. The MDA-MB-231 B7-H3 knockdown cells were more sensitive to paclitaxel-induced apoptosis than the control cells, with approximately two fold difference in the percentage of apoptototic cells at both 48 h and 72 h (Fig. 3A). Similar results were observed for the MDA-MB-435 cell variants (Supplementary Fig. S1A). As shown in Figure 3B, the late stage apoptotic response was dose dependent in MDA-MB-231 cells, showing increased DNA fragmentation with drug concentration, and the 231-shB7-H3 cells were significantly more susceptible to paclitaxel-induced apoptosis compared to the vector control cells, as the amount of TUNEL positive cells were 11 % versus 2 % at 48 h and 18 % versus 6 % at 72 h. These results were confirmed by investigating the cleavage of poly (ADP-ribose) polymerase (PARP), another marker of apoptosis. Both MDA-MB-231 and MDA-MB-435 B7-H3 knockdown cells had increased PARP cleavage compared to the control cells upon treatment with 20 nmol/L paclitaxel for 72 h (Fig. 3C and Supplementary Fig. S1B). To validate the specificity of the effects observed in B7-H3 knockdown cells, we confirmed the results with another stable cell clone 231-sh1-h9 (Supplementary Fig. S1C and D), which was constructed using a different B7-H3-targeting sequence shRNA-1 (see M & M). In addition, we transiently transfected B7-H3 siRNA, targeting a different sequence than the shRNAs, into another breast cancer cell line MDA-MB-436 (Supplementary Fig. S1E). The apoptosis-specific ELISA detection revealed that paclitaxel treatment induced more DNA fragmentation in B7-H3 siRNA transfected cells compared to in control cells. Together, these results clearly demonstrate that the silencing of the B7-H3 protein either by shB7-H3 or siB7-H3 makes the cells more prone to paclitaxel-induced apoptosis. To elucidate further the effect of B7-H3 on paclitaxel resistance, we generated stable B7-H3 overexpressing cells. Figure 3D, left, demonstrates that the B7-H3 overexpressing MDAMB-231 cells had higher levels of B7-H3 protein compared to the control EGFP cells. Upon treatment with 20nmol/L paclitaxel for 72 h we observed dramatically decreased amounts of cleaved-PARP (Fig. 3D, middle) and significantly lower levels of DNA fragmentation in 231-B7-H3 than in control 231-EGFP cells (Fig. 3D, right). This indicates that overexpression of B7-H3 is sufficient to induce paclitaxel resistance and further support an important role of B7-H3 in cancer cell resistance to paclitaxel.
Figure 3.
B7-H3 silencing sensitizes breast cancer cells to paclitaxel-induced apoptosis. A, The percentage of Annexin V- FITC stained cells was increased in B7-H3 knockdown population. 231-TR33 and 231-shB7-H3 cells were treated with 20 nmol/L paclitaxel for 0, 48, and 72 h, respectively, and apoptosis was examined by Annexin V-FITC staining and flow cytometry. Left: The percentage of apoptotic cells; Right: Flow cytometry diagram at 48 h. B, The percentage of TUNEL-positive cells was increased in B7-H3 knockdown population. Cells were treated with 0, 10 and 20 nmol/L paclitaxel for 72 h, and apoptosis was examined by TUNEL assay and flow cytometry. C, PARP cleavage was increased in B7-H3 knockdown cells, as detected by Western blotting following treatment with 20 nmol/L paclitaxel for 72 h. β-actin was used as a loading control. D, B7-H3 overexpressing MDA-MB-231 cell varaiants were more resistance to pacilitaxel-induced apoptosis. Left: B7-H3 overexpressing 231-B7-H3 cells expressed higher level of B7-H3, compared to the 231-EGFP control cells as detected by Western blot analysis; Middle: B7-H3 overexpressing cells showed decreased level of cleaved-PARP compared to the control cells upon treatment with 20nmol/L paclitaxel for 72 h; Right: apoptosis-specific ELISA detection revealed that the level of cytoplasmic histone-associated DNA fragments in paclitaxel treated 231-B7H3 cells was significantly lower than in control 231-EGFP cells. Bars, S.E.; * p< 0.05; ** p< 0.01; *** p < 0.001.
B7-H3 regulates the activation of Jak2/Stat3 pathway and its downstream anti-apoptotic molecules
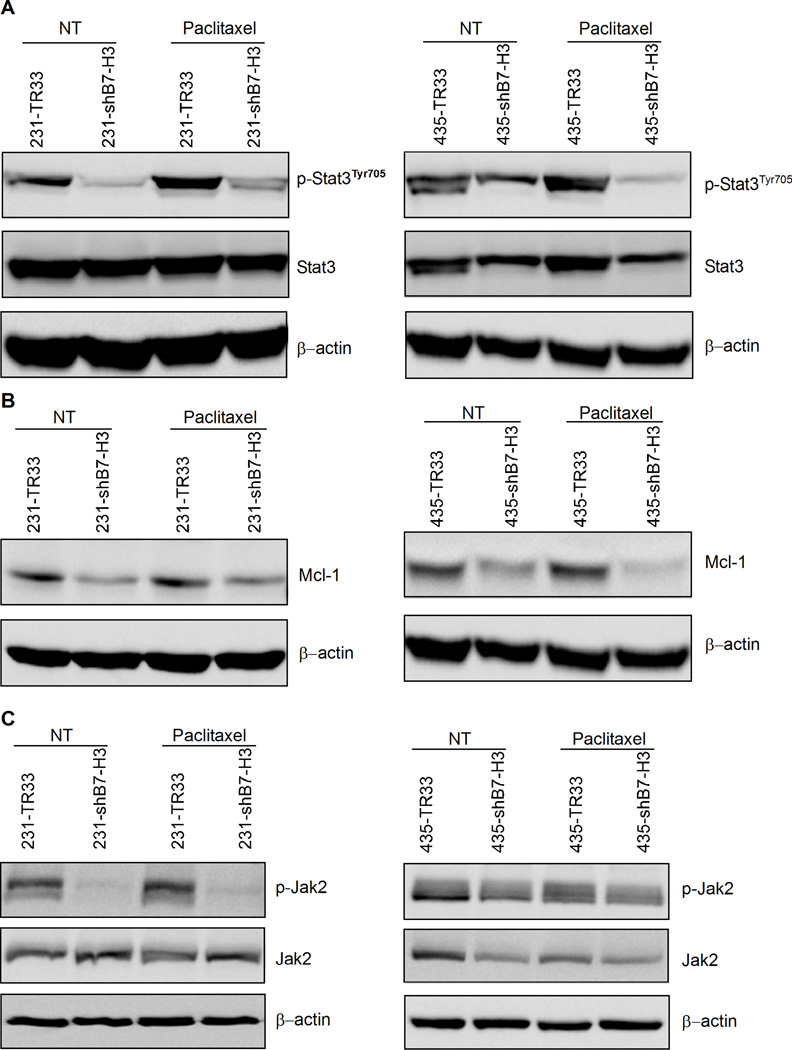
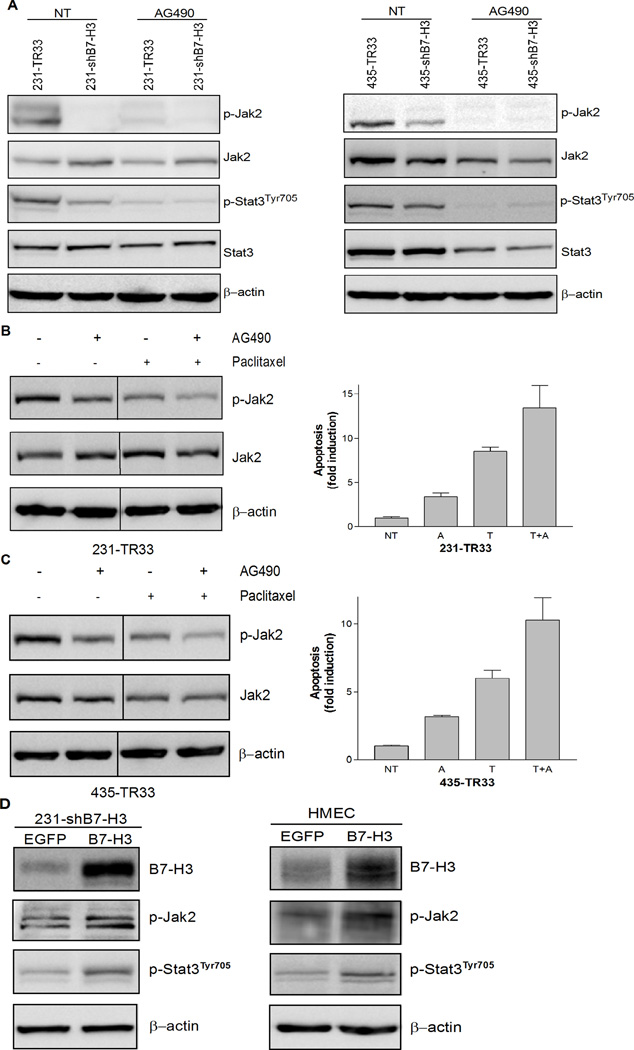
Since we observed chemo-sensitization accompanied by an increase in apoptosis in paclitaxel-treated B7-H3 knockdown cells, we studied whether the effects of B7-H3 could be related to molecules and signaling pathways known to be involved in the apoptotic response. Stat3, a transcription factor often constitutively activated in breast cancer cells, has previously been reported to lead to drug resistance (15) through the upregulation of antiapoptotic factors such as Mcl-1, Bcl-xL, Bcl-2, and Survivin (16). As seen in Fig. 4A, the silencing of B7-H3 induced a dramatic reduction in the phosphorylation level of Stat3, an indicator of Stat3 activation, both in untreated and paclitaxel-treated cells. Comparable results were obtained in transient siRNA B7-H3 transfected MDA-MB-436 cells (Supplementary Fig. S2A), targeting a different sequence than the shRNAs. Furthermore, Mcl-1 was dramatically repressed in B7-H3 silenced cells with reduced Stat3 phosphorylation (Fig. 4B). Survivin, another direct target of Stat3, was also, to a lesser extent, reduced in B7-H3 silenced cells (Supplementary Fig. S3). To elucidate whether the effects of B7-H3 on Stat3 was direct or indirect, we examined whether molecular kinases upstream of Stat3, such as Jak1, Jak2 and Src, were affected by the silencing of B7-H3. We observed a significant decline in the phosphorylation of Jak2 in both 231- and 435-shB7-H3 cells (Fig. 4C) and similar results were also observed in the B7-H3 shRNA transfected 231-sh1-h9 and 231-sh1-g10 cell variants (Supplementary Fig. S2B). In addition, the phosphorylation of Src appeared to be reduced in B7-H3 knockdown cells (Supplementary Fig. S4), indicating the possibility that Src may be partially involved in the B7-H3 mediated effects, whereas there was no obvious change in Jak1 phosphorylation in these cells. When we treated the cells with the Jak2 selective inhibitor AG490 for 24 h, at concentrations similar to what has been used in previous studies (17), the phosphorylation level of Jak2 was almost abolished followed by a dramatic inhibition of tyrosine phosphorylation of Stat3 in both B7-H3 knockdown cells and control cells (Fig. 5A). This indicates that the effect of B7-H3 on Stat3 occurs through Jak2. To investigate the involvement of Jak2/Stat3 pathway in the observed anti-apoptotic function of B7-H3, we tested the effect of AG490 in combination with paclitaxel on B7-H3 expressing cells, 231-TR33 and 435-TR33 (Fig. 5B and 5C). Interestingly, we observed that the combination showed a synergistic effect in enhancement of paclitaxel-induced apoptosis, compared with each agent alone. In summary, these results indicate that the silencing of B7-H3 reduces the phosphorylation of Jak2, which leads to reduced phosphorylation of Stat3, which in turn leads to decreased expression of the anti-apoptotic proteins, Mcl-1 and Survivin.
Figure 4.
B7-H3 regulated the phosphorylation of Stat3 and the expression of Mcl-1 at least partially through Jak2 in breast cancer cells. Cells were treated with 20 nmol/L paclitaxel (72 h) or left untreated. Whole cell lysates were probed with indicated antibodies with β-actin as a loading control. A, B7-H3 silencing suppressed Stat3 phosphorylation in 231 (left) or 435 (right) cells. B, B7-H3 silencing downregulated Mcl-1 expression in 231 (left) or 435 (right) cells. C, The levels of phosphorylated Jak2 were decreased in B7-H3 knockdown cells.
Figure 5.
The involvement of Jak2/Stat3 pathway in mediating the antiapoptotic effect of B7-H3 A, AG490 treatment almost abolished the phosphorylation level of Jak2 and led to reduced levels of tyrosine phosphorylation of Stat3 in both B7-H3 knockdown cells and control cells. MDA-MB-231 and MDA-MB-435 cell variants were treated with 50 µmol/L or 100 µmol/L AG490, respectively, for 24 h and Western blot analysis was performed to examine the protein levels of phospho- and total Jak2 and Stat3 with β-actin as a loading control. B and C, The combination treatment of AG490 and paclitaxel reduced the phosphorylation of Jak2 to a lesser extent (left) and enhanced the extent of apoptosis (right) in B7-H3 expressing cells, compared with the cells treated with AG490 or paclitaxel alone. 231-TR33 and 435-TR33 were treated with 5 µmol/L or 10 µmol/L AG490 respectively in the absence or presence of 20 nmol/L paclitaxel or left untreated. Whole cell lysates were made 72 h following treatment and western blot analysis and apoptosis-specific ELISA detection were performed as previously described. D, Overexpression of B7-H3 increased the phosphorylation level of both Jak2 and Stat3. B7-H3 expression vector B7-H3 and control vector EGFP were transiently transfected into 231-shB7-H3 (left) or HMEC cells (right) respectively for 48 h, and whole cell lysates were probed with indicated antibodies using β-actin as a loading control.
To validate this conclusion, we examined whether overexpression of B7-H3 is sufficient to cause Jak2/Stat3 activation. We transiently transfected B7-H3 into B7-H3 knockdown cells 231-shB7-H3 (Fig. 5D, left) and B7-H3 low expressing HMEC cells (Fig. 5D, right). Noteworthy, the results show that the phosphorylation level of both Jak2 and Stat3 increased with the B7-H3 expression, further confirming an important role of B7-H3 in regulating the Jak2/Stat3 signaling pathway.
Silencing of B7-H3 enhances cancer cell sensitivity to paclitaxel in a xenograft mouse model
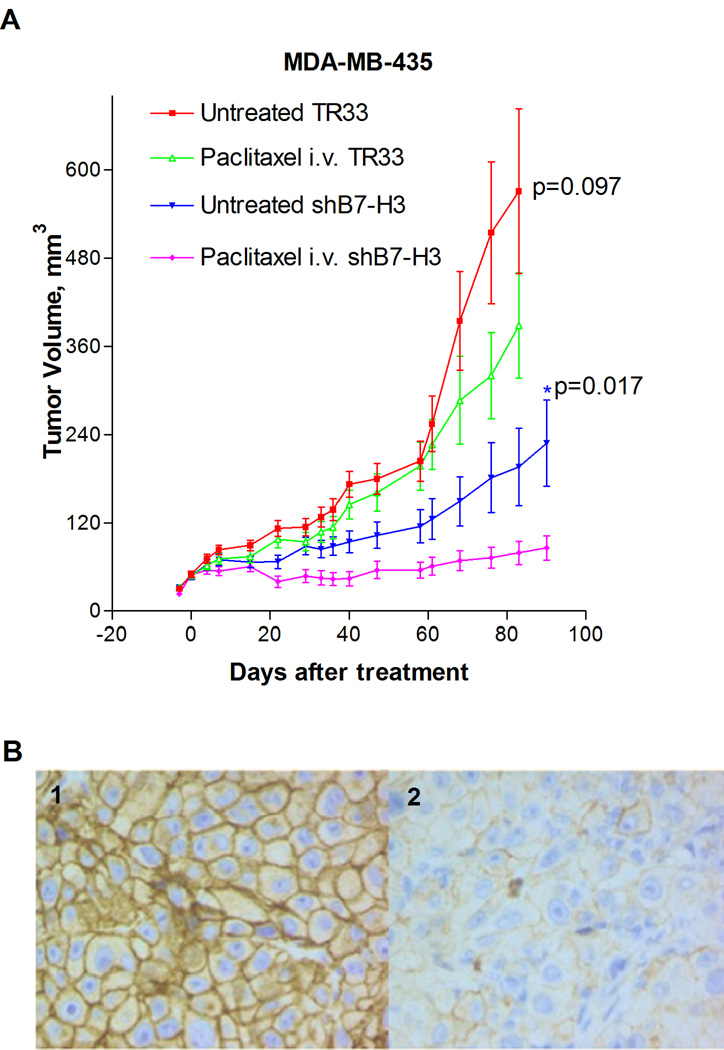
The in vitro experiments with the MDA-MB-231 and MDA-MB-435 cells showed that the cytotoxic effect of paclitaxel was enhanced in cells with silenced B7-H3 and reduced in cells with overexpression of B7-H3. Hence, we examined whether this could be observed also in vivo. MDA-MB-435 B7-H3 knockdown and control cells were injected s.c in nude mice, and the animals were treated with paclitaxel when the tumors had reached a mean diameter of 5–6 mm. As demonstrated in Figure 6A, the growth rate was reduced by the knockdown in B7-H3 alone, and whereas paclitaxel had a marginal effect on the growth of MDA-MB-435 TR33 tumors, it showed a strong anti-tumor effect in the mice carrying MDA-MB-435 shB7-H3 xenografts. Thus the difference in tumor volume assessed at each time point became statistically significant from day 22 (all the p-values at each time point after day 22 were lower than 0.03). The knockdown of the shB7-H3 xenografts was confirmed by immunochemical staining seven day post treatment, and whereas the level of B7-H3 expression retained low in the shB7-H3 tumors, the TR33 control tumors showed strong staining (Fig. 6B, panels 1–2). Clearly, these in vivo results strongly support the effects observed in vitro that B7-H3 plays a critical role in paclitaxel responsiveness of breast cancer cells.
Figure 6.
Effects of B7-H3 knock-down in MDA-MB-435 xenografts. A, Growth curves of breast cancer xenografts in nude mice established by s.c injection of 5×106 MDA-MB-435-shB7-H3 or MDA-MB-435-TR33 cells in both flanks of nude mice treated with paclitaxel (10 mg/kg) or solvent. The drug/solvent was injected i.v. into the tail vein at day 0 when the mean tumor diameter was 5–6 mm, and the tumor diameter was measured 1–2 times per week. Each group consisted of × 5–6 animals and the data is presented as mean of two independent experiments ± standard error of the mean (SEM). B, Immunohistochemical staining of: 1: xenograft from a paclitaxel treated vector control mouse showing distinct membranous immunoreactivity for B7-H3. 2: xenograft from a treated B7-H3 knock down mouse showing weak B7-H3 expression. (Magnification: panels 1–2 400×). Bars, S.E.; * p< 0.05
Discussion
In this study, we examined the role of B7-H3 in paclitaxel resistance in several metastatic breast cancer cell lines. B7-H3 shRNA induced knock down of the B7-H3 protein in these cells resulted in increased sensitivity to the drug, whereas B7-H3 overexpressing cells were less sensitive to paclitaxel-induced apoptosis. Our findings demonstrate that targeting B7-H3 could counteract cellular resistance to paclitaxel. Furthermore, in attempts to elucidate the mechanisms underlying the observed effects we obtained evidence for B7-H3 regulating key genes in the Jak2/Stat3 pathway.
Stat3 is a cytoplasmic transcription factor that regulates cellular differentiation, proliferation, and survival (18–20). It is activated through phosphorylation by various tyrosine kinases, such as Janus activated kinases (Jak) and Src family kinases and high activity has been shown to predict intrinsic chemotherapeutic drug resistance due to the upregulation of the antiapoptotic factors Mcl-1, Bcl-xL, Bcl-2, and Survivin (15, 21, 22). Interestingly, here we found that downregulation of B7-H3 reduced phosphorylation of both Stat3 and its upstream activator Jak2 while overexpression of B7-H3 activated Jak2/Stat3 signaling. This may explain why the B7-H3 knock-down cells became more prone to paclitaxel-induced apoptosis, and the findings are in accordance with reports showing induction of apoptosis following a blockade of Stat3 signaling in multiple malignancies (23–31). Based on these findings, we investigated the effects of B7-H3 knockdown on Stat3-regulated genes involved in apoptosis. Mcl-1 is a direct target gene transcriptionally activated by Stat3 (32, 33) and its downregulation has been shown to promote apoptosis in numerous human cancer cells (34–36). In our study, reduced expression of Mcl-1 occurred in parallel with increased apoptosis in B7-H3 knockdown cells treated with paclitaxel. The expression of Survivin, a member of the IAP family of antiapoptotic proteins, was also reduced upon silencing of B7-H3. In a previous study it was found that overexpression of Survivin is associated with resistance to paclitaxel induced apoptosis in breast cancer cells (37). Blockage of phosphorylation of Jak2 by its specific inhibitor AG490 resulted in more dramatic reduction on Stat3 phosphorylation in B7-H3 expressing cells compared to B7-H3 knockdown cells, indicating that Jak2 plays a major role in mediating the effect of B7-H3 on Stat3.
Importantly, the results on paclitaxel sensitivity in vitro were confirmed in our animal model. The growth rate of established B7-H3 knockdown xenografts was slower than that of vector control tumors, but still these tumors were significantly inhibited upon paclitaxel treatment, whereas the growth of vector control tumors were only marginally affected. This is an interesting observation as it is well established that fast-growing tumors generally respond better to chemotherapy than more slowly growing counterparts (38, 39). The data indicates that silencing of B7-H3 induces in parallel reduced proliferation and enhances the apoptosis induced by paclitaxel. Immunohistochemical analysis of the xenograft tissue confirmed that the tumors originating from shB7-H3 cells retained low expression levels of the protein, whereas the transfection control tumors showed strong B7-H3 staining.
The human MDA-MB-435 cell line used in our study, was originally described as of breast cancer origin, whereas gene expression array studies indicated the cells to originate from malignant melanoma (40). However, subsequent evidence suggests that MDA-MB-435 is in fact a breast cancer cell line (41, 42). Importantly, in our study, the results obtained with MDA-MB-435 cells were closely similar to those with MDA-MB-231 and MDA-MB-436 breast cancer cells. In summary, our study investigating the role of B7-H3 in drug resistance demonstrates that the protein confers resistance to paclitaxel both in vitro and in vivo by reducing the sensitivity of breast cancer cells to apoptosis, mediated via the Jak2/Stat3 pathway. Furthermore, in contrast to previous reports focusing on the immuno-regulatory effects of B7-H3, our data demonstrates that it plays an important role in determining the resistance to paclitaxel via non-immune mechanisms. These findings provide new insight into the role of B7-H3 in cancer and may have important implications in the development of targeted therapeutics for overcoming paclitaxel resistance. The clinical relevance of our results in breast cancer is illustrated by the recent findings of Arigami et al on high levels of B7-H3 expression in primary tumors and metastases (6). In separate experiments we have showed that knocking down B7-H3 also increased breast cancer cell sensitivity to cisplatin (not shown), thus the effects may be extrapolated to other chemotherapeutic compounds a possibility being investigated in ongoing studies.
Supplementary Material
Acknowledgment
We thank Ms. Indrejit Dybsjord and Ms. Ellen Hellesylt for technical assistance, Dr. Rajeev Samant and Dr. Robert Weinberg for HMEC cells, and Ms. Amy Brown for editorial assistance.
Grant Support:
This work was supported by the following grants: The Vincent F. Kilborn, Jr. Cancer Research Foundation (M. Tan), National Institutes of Health grant 1RO1CA149646 (M. Tan), Radiumhospitalets Legater Project 334003 (M. Tan and O. Fodstad), Research Council of Norway, The Norwegian Cancer Society, and The Blix Foundation (O. Fodstad).
Abbreviations List
- Jak2
Janus activated kinase-2
- Stat3
Signal transducer and activator of transcription 3
- Mcl-1
myeloid cell leukemia sequence 1
- PARP
poly (ADP-ribose) polymerase
- TUNEL
Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end labeling
- TdT
terminal transferase
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Steinberger P, Majdic O, Derdak SV, Pfistershammer K, Kirchberger S, Klauser C, et al. Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J Immunol. 2004;172:2352–2359. doi: 10.4049/jimmunol.172.4.2352. [DOI] [PubMed] [Google Scholar]
- 2.Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 3.Leitner J, Klauser C, Pickl WF, Stockl J, Majdic O, Bardet AF, et al. B7-H3 is a potent inhibitor of human T-cell activation: No evidence for B7-H3 and TREML2 interaction. Eur J Immunol. 2009;39:1754–1764. doi: 10.1002/eji.200839028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4:899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- 5.Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci U S A. 2003;100:10388–10392. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arigami T, Narita N, Mizuno R, Nguyen L, Ye X, Chung A, et al. B7-h3 ligand expression by primary breast cancer and associated with regional nodal metastasis. Annals of surgery. 252:1044–1051. doi: 10.1097/SLA.0b013e3181f1939d. [DOI] [PubMed] [Google Scholar]
- 7.Crispen PL, Sheinin Y, Roth TJ, Lohse CM, Kuntz SM, Frigola X, et al. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res. 2008;14:5150–5157. doi: 10.1158/1078-0432.CCR-08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boorjian SA, Sheinin Y, Crispen PL, Farmer SA, Lohse CM, Kuntz SM, et al. T-cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clin Cancer Res. 2008;14:4800–4808. doi: 10.1158/1078-0432.CCR-08-0731. [DOI] [PubMed] [Google Scholar]
- 9.Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, et al. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci U S A. 2007;104:19458–19463. doi: 10.1073/pnas.0709802104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Y, Wang Y, Zhao J, Gu M, Giscombe R, Lefvert AK, et al. B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung cancer (Amsterdam, Netherlands) 2006;53:143–151. doi: 10.1016/j.lungcan.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Yamato I, Sho M, Nomi T, Akahori T, Shimada K, Hotta K, et al. Clinical importance of B7-H3 expression in human pancreatic cancer. British journal of cancer. 2009;101:1709–1716. doi: 10.1038/sj.bjc.6605375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen YW, Tekle C, Fodstad O. The immunoregulatory protein human B7H3 is a tumor-associated antigen that regulates tumor cell migration and invasion. Curr Cancer Drug Targets. 2008;8:404–413. doi: 10.2174/156800908785133141. [DOI] [PubMed] [Google Scholar]
- 13.Elenbaas B, Spirio L, Koerner F, Fleming MD, Zimonjic DB, Donaher JL, et al. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes & development. 2001;15:50–65. doi: 10.1101/gad.828901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan M, Yu D. Molecular mechanisms of erbB2-mediated breast cancer chemoresistance. Advances in experimental medicine and biology. 2007;608:119–129. doi: 10.1007/978-0-387-74039-3_9. [DOI] [PubMed] [Google Scholar]
- 15.Barre B, Vigneron A, Perkins N, Roninson IB, Gamelin E, Coqueret O. The STAT3 oncogene as a predictive marker of drug resistance. Trends Mol Med. 2007;13:4–11. doi: 10.1016/j.molmed.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Al Zaid Siddiquee K, Turkson J. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res. 2008;18:254–267. doi: 10.1038/cr.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burke WM, Jin X, Lin HJ, Huang M, Liu R, Reynolds RK, et al. Inhibition of constitutively active Stat3 suppresses growth of human ovarian and breast cancer cells. Oncogene. 2001;20:7925–7934. doi: 10.1038/sj.onc.1204990. [DOI] [PubMed] [Google Scholar]
- 18.Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 19.Levy DE, Inghirami G. STAT3: a multifaceted oncogene. Proc Natl Acad Sci U S A. 2006;103:10151–10152. doi: 10.1073/pnas.0604042103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlessinger K, Levy DE. Malignant transformation but not normal cell growth depends on signal transducer and activator of transcription 3. Cancer Res. 2005;65:5828–5834. doi: 10.1158/0008-5472.CAN-05-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gariboldi MB, Ravizza R, Molteni R, Osella D, Gabano E, Monti E. Inhibition of Stat3 increases doxorubicin sensitivity in a human metastatic breast cancer cell line. Cancer Lett. 2007;258:181–188. doi: 10.1016/j.canlet.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Gritsko T, Williams A, Turkson J, Kaneko S, Bowman T, Huang M, et al. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res. 2006;12:11–19. doi: 10.1158/1078-0432.CCR-04-1752. [DOI] [PubMed] [Google Scholar]
- 23.Aoki Y, Feldman GM, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003;101:1535–1542. doi: 10.1182/blood-2002-07-2130. [DOI] [PubMed] [Google Scholar]
- 24.Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 25.Epling-Burnette PK, Liu JH, Catlett-Falcone R, Turkson J, Oshiro M, Kothapalli R, et al. Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J Clin Invest. 2001;107:351–362. doi: 10.1172/JCI9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grandis JR, Drenning SD, Chakraborty A, Zhou MY, Zeng Q, Pitt AS, et al. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor- mediated cell growth In vitro. J Clin Invest. 1998;102:1385–1392. doi: 10.1172/JCI3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grandis JR, Drenning SD, Zeng Q, Watkins SC, Melhem MF, Endo S, et al. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc Natl Acad Sci U S A. 2000;97:4227–4232. doi: 10.1073/pnas.97.8.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mora LB, Buettner R, Seigne J, Diaz J, Ahmad N, Garcia R, et al. Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 2002;62:6659–6666. [PubMed] [Google Scholar]
- 29.Niu G, Bowman T, Huang M, Shivers S, Reintgen D, Daud A, et al. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene. 2002;21:7001–7010. doi: 10.1038/sj.onc.1205859. [DOI] [PubMed] [Google Scholar]
- 30.Song L, Turkson J, Karras JG, Jove R, Haura EB. Activation of Stat3 by receptor tyrosine kinases and cytokines regulates survival in human non-small cell carcinoma cells. Oncogene. 2003;22:4150–4165. doi: 10.1038/sj.onc.1206479. [DOI] [PubMed] [Google Scholar]
- 31.Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 32.Hsieh FC, Cheng G, Lin J. Evaluation of potential Stat3-regulated genes in human breast cancer. Biochem Biophys Res Commun. 2005;335:292–299. doi: 10.1016/j.bbrc.2005.07.075. [DOI] [PubMed] [Google Scholar]
- 33.Jing N, Tweardy DJ. Targeting Stat3 in cancer therapy. Anticancer Drugs. 2005;16:601–607. doi: 10.1097/00001813-200507000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Andersson Y, Juell S, Fodstad O. Downregulation of the antiapoptotic MCL-1 protein and apoptosis in MA-11 breast cancer cells induced by an anti-epidermal growth factor receptor- Pseudomonas exotoxin a immunotoxin. Int J Cancer. 2004;112:475–483. doi: 10.1002/ijc.20371. [DOI] [PubMed] [Google Scholar]
- 35.Chetoui N, Sylla K, Gagnon-Houde JV, Alcaide-Loridan C, Charron D, Al-Daccak R, et al. Down-regulation of mcl-1 by small interfering RNA sensitizes resistant melanoma cells to fas-mediated apoptosis. Mol Cancer Res. 2008;6:42–52. doi: 10.1158/1541-7786.MCR-07-0080. [DOI] [PubMed] [Google Scholar]
- 36.Wei SH, Dong K, Lin F, Wang X, Li B, Shen JJ, et al. Inducing apoptosis and enhancing chemosensitivity to gemcitabine via RNA interference targeting Mcl-1 gene in pancreatic carcinoma cell. Cancer Chemother Pharmacol. 2008;62:1055–1064. doi: 10.1007/s00280-008-0697-7. [DOI] [PubMed] [Google Scholar]
- 37.Lu J, Tan M, Huang WC, Li P, Guo H, Tseng LM, et al. Mitotic deregulation by survivin in ErbB2-overexpressing breast cancer cells contributes to Taxol resistance. Clin Cancer Res. 2009;15:1326–1334. doi: 10.1158/1078-0432.CCR-08-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arriola E, Moreno A, Varela M, Serra JM, Falo C, Benito E, et al. Predictive value of HER-2 and Topoisomerase IIalpha in response to primary doxorubicin in breast cancer. Eur J Cancer. 2006;42:2954–2960. doi: 10.1016/j.ejca.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 39.Dougherty MK, Schumaker LM, Jordan VC, Welshons WV, Curran EM, Ellis MJ, et al. Estrogen receptor expression and sensitivity to paclitaxel in breast cancer. Cancer Biol Ther. 2004;3:460–467. doi: 10.4161/cbt.3.5.810. [DOI] [PubMed] [Google Scholar]
- 40.Ross DT, Scherf U, Eisen MB, Perou CM, Rees C, Spellman P, et al. Systematic variation in gene expression patterns in human cancer cell lines. Nat Genet. 2000;24:227–235. doi: 10.1038/73432. [DOI] [PubMed] [Google Scholar]
- 41.Sellappan S, Grijalva R, Zhou X, Yang W, Eli MB, Mills GB, et al. Lineage infidelity of MDA-MB-435 cells: expression of melanocyte proteins in a breast cancer cell line. Cancer Res. 2004;64:3479–3485. doi: 10.1158/0008-5472.CAN-3299-2. [DOI] [PubMed] [Google Scholar]
- 42.Chambers AF. MDA-MB-435 and M14 cell lines: identical but not M14 melanoma? Cancer Res. 2009;69:5292–5293. doi: 10.1158/0008-5472.CAN-09-1528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.