Abstract
Background
Fixed-dose combinations of candesartan 32 mg and hydrochlorothiazide (HCTZ) have been shown to be effective in clinical trials. Upon market entry we conducted a noninterventional study to document the safety and effectiveness of this fixed-dose combination in an unselected population in primary care and to compare blood pressure (BP) values obtained during office measurement (OBPM) with ambulatory blood pressure measurement (ABPM).
Methods
CHILI CU Soon was a prospective, noninterventional, noncontrolled, open-label, multicenter study with a follow-up of at least 10 weeks. High-risk patients aged ≥18 years with previously uncontrolled hypertension were started on candesartan 32 mg in a fixed-dose combination with either 12.5 mg or 25 mg HCTZ. OBPM and ABPM reduction and adverse events were documented.
Results
A total of 4131 patients (52.8% male) with a mean age of 63.0 ± 11.0 years were included. BP was 162.1 ± 14.8/94.7 ± 9.2 mmHg during office visits at baseline. After 10 weeks of candesartan 32 mg/12.5 mg or 25 mg HCTZ, mean BP had lowered to 131.7 ± 10.5/80.0 ± 6.6 mmHg (P < 0.0001 for both comparisons). BP reduction was comparable irrespective of prior or concomitant medication. In patients for whom physicians regarded an ABPM to be necessary (because of suspected noncontrol over 24 hours), ABP at baseline was 158.2/93.7 mmHg during the day and 141.8/85.2 mmHg during the night. At the last visit, BP had significantly reduced to 133.6/80.0 mmHg and 121.0/72.3 mmHg, respectively, resulting in 20.8% being normotensive over 24 hours (<130/80 mmHg). The correlation between OBPM and ABPM was good (r = 0.589 for systolic BP and r = 0.389 for diastolic BP during the day). Of those who were normotensive upon OBPM, 35.1% had high ABPM during the day, 49.3% were nondippers, and 3.4% were inverted dippers. Forty-nine adverse events (1.19%) were reported, of which seven (0.17%) were regarded as serious.
Conclusion
Candesartan 32 mg in a fixed-dose combination with either 12.5 mg or 25 mg HCTZ is safe and effective for further BP lowering irrespective of prior antihypertensive drug class not being able to control BP.
Keywords: ambulatory blood pressure, office blood pressure, normalization, response
Background
Fixed-dose combinations of candesartan 32 mg and hydrochlorothiazide (HCTZ) have been shown to be effective in clinical trials.1,2 Mean reductions in systolic blood pressure (SBP) and diastolic blood pressure (DBP) were significantly greater with candesartan 32 mg/HCTZ 25 mg (21/14 mmHg) than with candesartan 32 mg (13/9 mmHg) or HCTZ 25 mg alone (12/8 mmHg).2 The addition of 12.5 mg HCTZ or 25 mg HCTZ to 32 mg candesartan resulted in a further BP reduction by 13.0/8.8 mmHg in the HCTZ 12.5 mg group and by 15.5/10.0 mmHg in the HCTZ 25 mg group in a study by Bönner.3 At the same time, adverse events (AEs) were scarce, with about 1% serious AEs when candesartan combination therapy including HCTZ was considered.2,3
Upon market entry we aimed to conduct a non interventional study to document the safety and effectiveness of this fixed-dose combination in an unselected population in primary care. These non interventional studies complement the findings of prior controlled trials including typical patient groups in clinical practice and reflecting current treatment approaches and include patients not enrolled into prior trials because of high age, substantial cardiovascular risks, or concomitant medication.4
Within this context we considered it to be of considerable interest to compare the results on the effectiveness of office blood pressure measurement (OBPM) with data obtained during ambulatory blood pressure measurement (ABPM). This is of relevance because several studies have demonstrated that BP reduction achieved in non interventional studies is higher than that observed in randomized clinical trials. Further, ABP is more closely related to cardiovascular morbidity and target organ damage and may therefore have a greater prognostic value.5,6 In patients with uncontrolled hypertension, Salles et al7 showed that OBPM had no prognostic value, whereas ABPM correlated to cardiovascular morbidity and mortality.
Patients and methods
CHILI CU Soon was a prospective, noninterventional, noncontrolled, open-label, multi center study with a follow-up of at least 10 weeks. It was conducted by 1111 primary care physicians, internists, cardiologists, or diabetologists throughout Germany. The study was registered at the Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM) and the Kassenärztliche Bundesvereinigung (KBV) in accordance with section 67(6) of the medicinal law. Applicable data protection Acts were respected. Participating physicians received remuneration for the documentation of patients, which was in accordance with the Gebührenordnung für Ärzte (GOÄ). Ethical approval was obtained prior to commencement of the study by the Freiburg Ethics Commission International, Germany, on March 23, 2009. Written informed consent was obtained from all patients.
Patients
High-risk patients aged at least 18 years were eligible for inclusion when a treatment decision had been made to start candesartan 32 mg plus HCTZ 12.5 mg or candesartan 32 mg plus HCTZ 25 mg due to arterial hypertension. Further inclusion criteria were uncontrolled BP (≥140/90 mmHg or ≥130/80 mmHg in patients with metabolic syndrome or diabetes), on prior antihypertensive therapy for at least 8 weeks, the presence of additional cardiovascular risk factors (eg, diabetes, dyslipidemia), and compliance with the prescribing information of Blopress® 32 mg PLUS 12.5 mg or Blopress 32 mg forte PLUS 25 mg (Takeda Pharma GmbH, Aachen, Germany). In case of an insufficient BP control at the first follow-up visit (at least 6 weeks after inclusion), physicians were allowed to increase the dose of HCTZ to 25 mg (Blopress 32 mg forte PLUS 25 mg). Any concomitant medication was allowed as necessary.
Objectives
The primary objective was to document a change in BP with the introduction of candesartan cilexetil 32 mg and HCTZ 12.5/25 mg. Secondary objectives were (1) to document the proportion of patients who reach the target BP or are responders (DBP < 90 or reduction by ≥10 mmHg) using OBPM, (2) to determine the change in BP stratified according to prior/concomitant therapies, and (3) to collect data on tolerability and drug safety in routine clinical practice.
Variables
Three visits were scheduled throughout a 10-week follow-up. At the first visit (enrollment), patient data, medical history, BP values, laboratory values, and previous and concomitant pharmacotherapy were documented. At the first follow-up visit (after 5–8 weeks), BP, concomitant pharmacotherapy, safety, and tolerability were assessed. At the last visit (>10 weeks after inclusion and at least 4 weeks after the first interim visit), BP, weight, body mass index, waist circumference, laboratory tests, prior and concomitant pharmacotherapy, and safety/tolerability were documented.
Definitions
Normal OBP was <140/90 mmHg for nondiabetic patients and <130/80 mmHg for diabetic patients or those with metabolic syndrome. Diastolic responders were defined at a DBP <90 mmHg or a reduction of at least 10 mmHg vs baseline. Systolic responders were defined at a SDP <140 mmHg or a reduction of at least 20 mmHg vs baseline.
Normal ABP values during the day were <135/85 mmHg and <120/70 mmHg during the night. Normal ABP values over 24 hours were <130/80 mmHg. Normal dippers were those with a reduction of ≥10% and <20% of the daytime mean during the night. Nondippers were those with a reduction between ≥0% and <10% of the daytime mean. Inverted dippers were those with a reduction of <0% of the mean during daytime or an increase at night. Extreme dipping was defined as a reduction at night that exceeded 20% of the values during the day.
Statistics
The case report forms were collected by the clinical research organization Factum GmbH, Offenbach, Germany, entered into a validated application based on ColdFusion MX 6.1 (Adobe Systems Incorporated, Seattle, WA), and saved on an SQL Server 2003 (Microsoft, Redmond, WA). Case report forms were checked for consistency and a subset of the forms verified with the source data (8%).
Regarding safety, the trial was adequately sized (n = 5000) in order to identify rare AEs, ie, those that may not have been detected in previous clinical studies (incidence 1:1000), with a probability of >95%.
The statistical analysis was performed descriptively and was interpreted in an explorative way. Comparisons were made for a number of variables and analyzed using descriptive statistics. Differences were calculated in patients with values at baseline and follow-up (per protocol), for both the OBPM group and the ABPM group. The last documented visit was regarded to be the follow-up value in case only the interim visit was documented. Data were analyzed using IBM® SPSS® Statistics 18 (IBM Corporation, Somer, NY). The tests applied are indicated in the legends of the tables and figures.
Results
Between June 2009 and December 2009 a total of 4131 patients were included (safety population), of which 4130 were available for the analysis (efficacy population). The mean age of all patients was 63.0 ± 11.0 years and 52.8% were male (Table 1). Frequent comorbid disease conditions were diabetes (51.3%), coronary artery disease (43.5%), angina pectoris (26.2%), heart failure (25.1%), and prior stroke (23.5%).
Table 1.
Baseline characteristics of the study population
| Variables | Patients with OBPM | ABPM and OBPM | ||
|---|---|---|---|---|
|
|
|
|||
| No. available | % | No. available | % | |
| Males (%) | 4130 | 52.8 | 351 | 57.5 |
| Age (years ± SD) | 4128 | 63.0 ± 11.0 | 351 | 62.1 ± 10.0 |
| Body mass index (kg/m2 ± SD) | 4098 | 29.6 ± 5.3 | 350 | 30.3 ± 7.1 |
| Waist circumference (cm ± SD) | 3253 | 103.6 ± 14.2 | 318 | 104.5 ± 13.1 |
| Smokers (%) | 4102 | 20.3 | 351 | 25.9 |
| Comorbid disease conditions | ||||
| Diabetes (%) | 3601 | 51.3 | 241 | 53.5 |
| Coronary artery disease (%) | 3669 | 48.9 | 248 | 60.9 |
| Angina pectoris (%) | 3418 | 26.2 | 239 | 40.2 |
| Heart failure (%) | 3443 | 25.1 | 244 | 34.8 |
| Stroke/TIA (%) | 3322 | 23.5 | 232 | 33.1 |
| Myocardial infarction (%) | 3462 | 22.5 | 236 | 27.5 |
| Peripheral arterial disease (%) | 3371 | 15.4 | 232 | 20.3 |
| Renal insufficiency (%) | 3348 | 13.1 | 231 | 19.0 |
| Neuropathy (%) | 3322 | 10.5 | 233 | 16.3 |
| Retinopathy (%) | 3324 | 9.8 | 231 | 19.9 |
| Atrial fibrillation (%) | 3352 | 9.8 | 233 | 16.7 |
Abbreviations: ABPM, ambulatory blood pressure measurement; OBPM, office blood pressure measurement; SD, standard deviation; TIA, transient ischemic attack.
Blood pressure reduction with OBPM
BP at baseline was 162.1 ± 14.8/94.7 ± 9.2 mmHg (Table 2), which meant that 31.3% had mild, 48.4% moderate, and 18.8% severe hypertension. After about 10 weeks of candesartan 32 mg/12.5 mg or 25 mg HCTZ treatment, mean BP had lowered to 131.7 ± 10.5/80.0 ± 6.6 mmHg (P < 0.0001 for both comparisons). A total of 91.0% were diastolic and 77.2% systolic responders, resulting in 31.2% normalization of those without diabetes or metabolic syndrome (<140/90 mmHg) and 8.6% for those with diabetes or metabolic syndrome (<130/80 mmHg). BP reduction was as effective in the total as in subgroups of patients defined by prior or concomitant medication (Table 3).
Table 2.
Blood pressure values at baseline and follow-up
| Variables | All patients with OBPM | ||
|---|---|---|---|
|
|
|||
| Baseline | Last visit (LOCF) | P-value | |
| OBPM | |||
| SBP (mmHg) | 162.1 ± 14.8 | 131.7 ± 10.5 | <0.0001 |
| DBP (mmHg) | 94.7 ± 9.2 | 80.0 ± 6.6 | <0.0001 |
| Severity of hypertension (%) | <0.0001 | ||
| <140 mmHg and <90 mmHg (%)a | 1.5 | 74.0 | |
| 140–159 mmHg or 90–99 mmHg (%) | 31.3 | 23.0 | |
| 160–179 mmHg or 100–109 mmHg (%) | 48.4 | 2.7 | |
| ≥180 mmHg or ≥110 mmHg (%) | 18.8 | 0.3 | |
| Normalization/response (%) | |||
| <130 mmHg and <80 mmHg (%)b | 0.2 | 27.0 | <0.0001 |
| <140 mmHg and <90 mmHg (%)c | 2.5 | 37.0 | <0.0001 |
| DBP <90 mmHg or Δ ≥ 10 mmHg (%) | 91.0 | ||
| SBP < 140 mmHg or Δ ≥ 20 mmHg (%) | 77.2 | ||
Notes: For all patients;
for patients with diabetes or metabolic syndrome;
for patients without diabetes or metabolic syndrome.
Abbreviations: DBP, diastolic blood pressure; LOCF, last observation carried forward; OBPM, office blood pressure measurement; SBP, systolic blood pressure.
Table 3.
Change in blood pressure (OBPM, n = 4130) stratified according to prior visit/concomitant therapies
| SBP | DBP | |||
|---|---|---|---|---|
|
|
|
|||
| ΔSBP | P-value vs baseline | ΔDBP | P-value vs baseline | |
| Prior but discontinued therapies | ||||
| ACE inhibitors | −30.7 ± 14.8 | <0.0001 | −15.4 ± 9.6 | <0.0001 |
| Angiotensin receptor blockers | −27.9 ± 14.8 | <0.0001 | −13.2 ± 9.7 | <0.0001 |
| Beta blockers | −31.3 ± 16.3 | <0.0001 | −15.5 ± 10.3 | <0.0001 |
| CCBs | −31.2 ± 15.7 | <0.0001 | −15.1 ± 10.5 | <0.0001 |
| Diuretics | −30.6 ± 16.1 | <0.0001 | −15.2 ± 10.0 | <0.0001 |
| None | −30.1 ± 17.5 | <0.0001 | −16.3 ± 11.8 | <0.0001 |
| Prior and continued therapies | ||||
| ACE inhibitors | −30.4 ± 15.4 | <0.0001 | −14.3 ± 8.9 | <0.0001 |
| Angiotensin receptor blockers | −25.3 ± 25.8 | <0.0001 | −9.0 ± 14.8 | <0.0001 |
| Beta blockers | −30.2 ± 16.2 | <0.0001 | −14.3 ± 10.6 | <0.0001 |
| CCBs | −30.6 ± 16.4 | <0.0001 | −14.2 ± 10.6 | <0.0001 |
| Diuretics | −29.9 ± 16.9 | <0.0001 | −13.3 ± 11.2 | <0.0001 |
Abbreviations: ACE, angiotensin-converting enzyme; CCB, calcium channel blocker; DBP, diastolic blood pressure; OBPM, office blood pressure measurement; SBP, systolic blood pressure.
Comparison of OBPM and ABPM values
Physicians regarded ABPM to be necessary in 351 patients (because of suspected noncontrol over 24 hours). These patients were more likely to be male (57.5% vs 52.8%) and smokers (25.9% vs 20.3%) and had a considerably higher burden of comorbid disease conditions such as coronary artery disease (60.9% vs 48.9%), angina pectoris (40.2% vs 26.2%), heart failure (34.8% vs 25.1%), and retinopathy (16.7% vs 9.8%) (Table 1).
In these patients, BP at baseline was 158.2 ± 14.4/93.7 ± 10.0 mmHg during the day and 141.8 ± 16.9/85.2 ± 10.5 mmHg during the night (Table 4). At the last visit, BP was significantly reduced to 133.6 ± 10.0/80.0 ± 6.6 mmHg and 121.0 ± 12.2/72.3 ± 7.4 mmHg, respectively, resulting in 20.8% being normotensive over 24 hours (<130/80 mmHg).
Table 4.
Blood pressure values at baseline and follow-up
| Variables | Patients with ABPM | ||
|---|---|---|---|
|
|
|||
| Baseline | Last visit (LOCF) | P-value | |
| ABPM day | |||
| Systolic blood pressure (mmHg) | 158.2 ± 14.4 | 133.6 ± 10.6 | <0.0001 |
| Diastolic blood pressure (mmHg) | 93.7 ± 10.0 | 80.0 ± 6.6 | <0.0001 |
| ABPM night | |||
| Systolic blood pressure (mmHg) | 141.8 ± 16.9 | 121.0 ± 12.2 | <0.0001 |
| Diastolic blood pressure (mmHg) | 85.2 ± 10.5 | 72.3 ± 7.4 | <0.0001 |
| ABPM 24 hours mean | |||
| Systolic blood pressure (mmHg) | 151.7 ± 13.7 | 128.9 ± 10.2 | <0.0001 |
| Diastolic blood pressure (mmHg) | 90.4 ± 9.0 | 77.5 ± 6.4 | <0.0001 |
| Severity of hypertension | <0.0001 | ||
| <135 mmHg and <85 mmHg (%) | 0.9 | 53.0 | |
| 135–146 mmHg or 85–89 mmHg (%) | 6.0 | 33.6 | |
| 147–156 mmHg or 90–95 mmHg (%) | 29.3 | 10.3 | |
| ≥157 mmHg or ≥96 mmHg (%) | 63.8 | 3.1 | |
Abbreviations: ABPM, ambulatory blood pressure measurement; LOCF, last observation carried forward.
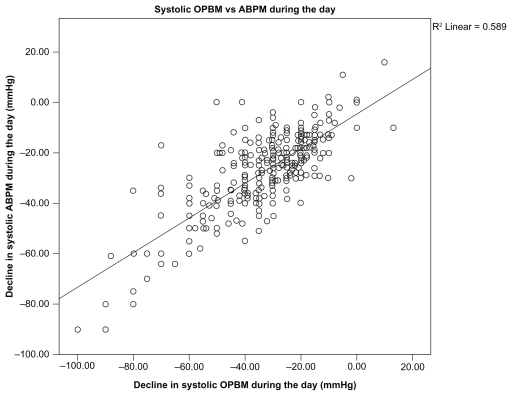
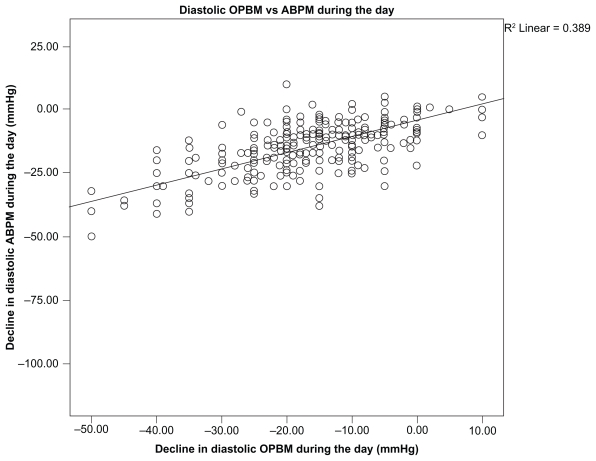
The correlation between OBPM and ABPM was good with r = 0.589 for SBP (Figure 1) and r = 0.389 for DBP during the day (Figure 2).
Figure 1.
Systolic OPBM vs ABPM during the day.
Abbreviations: ABPM, ambulatory blood pressure measurement; OBPM, office blood pressure measurement.
Figure 2.
Diastolic OPBM vs ABPM during the day.
Abbreviations: ABPM, ambulatory blood pressure measurement; OBPM, office blood pressure measurement.
Of those who were normotensive upon OBPM (<140/90 mmHg), 35.1% had high ABPM during the day, 49.8% were nondippers, and 3.4% were inverted dippers (Table 5). Of those who were hypertensive during their office visit, 21.1% had a normal BP during ABPM at daytime and 7.1% at nighttime. Again, there was a larger subset whose BP pattern was compatible with a nondipping or inverted dipping pattern.
Table 5.
Comparison of OBPM and ABPM blood pressure values
| OBPM | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| <140/90 mmHg | ≥140 mmHg or ≥90 mmHg | Total | ||||
|
|
|
|
||||
| n | % | n | % | n | % | |
| ABPM day | ||||||
| Normal (<135/85 mmHg) | 190 | 64.9 | 27 | 21.1 | 225 | 52.0 |
| High (≥135 or ≥85 mmHg) | 107 | 35.1 | 101 | 78.9 | 208 | 48.0 |
| Total | 305 | 100.0 | 128 | 100.0 | 433 | 100.0 |
| ABPM 24 hours | ||||||
| Normal (<130/80 mmHg) | 79 | 26.7 | 9 | 7.1 | 88 | 20.8 |
| High (≥130 mmHg or ≥80 mmHg) | 217 | 73.3 | 118 | 92.9 | 335 | 79.2 |
| Total | 296 | 100.0 | 127 | 100.0 | 423 | 100.0 |
| ABPM dipping | ||||||
| Normal dippera | 119 | 40.3 | 48 | 38.7 | 167 | 39.9 |
| Nondipperb | 147 | 49.8 | 64 | 51.6 | 211 | 50.4 |
| Inverted dipperc | 10 | 3.4 | 7 | 5.7 | 17 | 4.1 |
| Extreme dipperd | 19 | 6.4 | 5 | 4.0 | 24 | 5.7 |
| Total | 295 | 100.0 | 124 | 100.0 | 419 | 100.0 |
Notes: Normal dipper (reduction ≥10% and <20% of the daytime mean);
nondipper (reduction between ≥0% and <10% of the daytime mean);
inverted dipper (reduction of <0% of the mean during daytime or an increase at night);
extreme dipper (reduction at night that exceeded 20% of the values during the day).
Abbreviations: ABPM, ambulatory blood pressure measurement; OBPM, office blood pressure measurement.
Number of patients with (serious) adverse events
The mean serum potassium value was 4.41 ± 0.55 at baseline and 4.36 ± 0.63 at follow-up. This was a statistically significant reduction of the mean serum potassium (P = 0.013). This was reflected in 10.6% with hyperkalemia at baseline and only 7.8% during follow-up. Reductions were also significant in the subgroup of patients with only candesartan as a renin-angiotensin system blocker but not in those receiving two different renin-angiotensin system blocking agents. A direct comparison, however, yielded no significant results (P = 0.476). During the course of the observation, 49 AEs were reported in patients receiving a fixed combination of 32 mg candesartan with either 12.5 mg HCTZ or 25 mg HCTZ (n = 49/4131; 1.19%). Of these, seven (0.17%) were regarded as serious. Most AEs were related to the nervous system (n = 13; 0.31%) or cardiac disorders (n = 12; n = 0.29%). Details are displayed in Table 6 ( MedDRA® Primary System Organ Classes, Northrop Grummon Corporation, California, US).
Table 6.
Number of patients with AEs or SAEs during survey and AEs coded by MedDRA® Version 11.1 (safety population, n = 4131)
| Type of adverse event | n | % |
|---|---|---|
| No AE | 4082 | 98.81 |
| Any AE | 49 | 1.19 |
| Serious AE | 7 | 0.17 |
| Not serious | 42 | 1.02 |
| MedDRA® Primary System Organ Class AEs | ||
| Nervous system disorders | 13 | 0.31 |
| Cardiac disorders | 12 | 0.29 |
| Investigations | 5 | 0.12 |
| Skin and subcutaneous tissue disorders | 4 | 0.10 |
| General disorders and administration site conditions | 3 | 0.07 |
| Vascular disorders | 3 | 0.07 |
| Musculoskeletal and connective tissue disorders | 3 | 0.07 |
| Gastrointestinal disorders | 2 | 0.05 |
| Renal and urinary disorders | 2 | 0.05 |
| MedDRA® Primary System Organ Class SAEs | ||
| Nervous system disorders | 3 | 0.07 |
| Vascular disorders | 1 | 0.02 |
| Renal and urinary disorders | 1 | 0.02 |
| Metabolism and nutrition disorders | 1 | 0.02 |
| Cardiac disorders | 1 | 0.02 |
Abbreviations: AE, adverse event; SAE, serious adverse event.
Discussion
CHILI CU Soon demonstrated that 32 mg candesartan in combination with 12.5 mg HCTZ or 25 mg HCTZ is safe and effective at lowering BP in patients who are uncontrolled on prior antihypertensive therapy. Patients were at high cardiovascular risk, as exemplified by the high prevalence of diabetes, angina pectoris/coronary artery disease, and heart failure. After about 10 weeks of treatment, mean BP had lowered by 30.4/14.7 mmHg from a baseline value of 162.1 ± 14.8/94.7 ± 9.2 mmHg. In a patient subgroup at risk, ABPM was performed and indicated that about 40% had a normal dipping pattern, whereas about 60% were nondippers or even inverted dippers.
Effectiveness in clinical practice in the context of recent controlled trials
The extent of BP reduction with candesartan/HCTZ depends on BP at baseline and the dose used. A variety of combinations with different doses of up to 32 mg candesartan and up to 25 mg HCTZ has been tested and found to be effective in clinical trials.1,2,8,9 Uen et al,1 for example, demonstrated that replacing previously ineffective antihypertensive drugs with candesartan/HCTZ in patients with uncontrolled arterial hypertension significantly reduced BP and markers of ischemic stress such as ST-segment depression. In respect of the doses used in the present study, Edes2 observed mean reductions in SBP and DBP that were significantly greater with candesartan 32 mg/HCTZ 25 mg (21/14 mmHg) than with candesartan 32 mg (13/9 mmHg) or HCTZ 25 mg alone (12/8 mmHg) or placebo (4/3 mmHg) (P < 0.001 for all comparisons). The proportion of patients with controlled BP (SBP < 140 mmHg and DBP < 90 mmHg) at the end of this study was also significantly greater in the candesartan 32 mg/HCTZ 25 mg group (63%) than in the other treatment groups (P < 0.001 for all comparisons). Bönner3 investigated the efficacy of candesartan 32 mg in combination with HCTZ 12.5 mg or 25 mg in patients who were not optimally controlled using candesartan monotherapy. Mean BP (153/97 mmHg at baseline) was further reduced by 13.0/8.8 mmHg in the fixed combination with the HCTZ 12.5 mg group and by 15.5/10.0 mmHg in the fixed combination with HCTZ 25 mg group (P < 0.01 for all between-treatment comparisons). Against this background the results of the present noninterventional trial deserve to be noted, with a mean BP reduction of 30.4/14.7 mmHg. BP reduction was consistent and similar across all subgroups of patients defined by prior but discontinued therapies, concomitant therapies, and cardiovascular risk at baseline.
Comparison of OBPM and ABPM
BP readings obtained by OBPM and ABPM were quite similar at baseline (162.1 ± 14.8/94.7 ± 9.2 mmHg vs 158.2 ± 14.4/93.7 ± 10.0 mmHg) and at follow-up (131.7 ± 10.5/80.0 ± 6.6 mmHg vs 133.6 ± 10.6/80.0 ± 6.6 mmHg). There was also a high degree of correlation between OBPM and ABPM (r = 0.589 for SBP and r = 0.389 for DBP) during the day in our study, which mirrors previous analyses that reported correlations coefficients of 0.41 for DBP (Mengden et al)10 and 0.73 for DBP and 0.64 for SBP (Head et al).11 Although the study by Mengden et al10 was a randomized controlled trial, the study by Head et al11 was a prospective cohort study that was biased toward those being referred for ambulatory assessment.
A high proportion of at-risk patients had normal BP readings during OBPM but a nondipping or even inverted dipping BP pattern at night. This may have been because of the noninterventional study type in which patients were scheduled only for ABPM when it was considered to be reasonable by the treating physician. In fact, about 60% of patients were documented to have either nondipping or inverted dipping of BP, suggesting that a tailored intervention (eg, bedtime medication) would be beneficial in those patients. On the other hand, the results clearly illustrate that achieving a normal BP during OBPM does not necessarily mean satisfactory BP control over 24 hours, reinforcing previous calls for a more comprehensive work-up of hypertensive patients, including ABPM.12
Safety and tolerability
Candesartan/HCTZ is generally well tolerated in patients with mild to moderate hypertension. Combined data from five randomized, double-blind, placebo-controlled clinical trials indicated that AEs during candesartan/HCTZ therapy are uncommon and that few were serious.13 The AE profile of candesartan 32 mg in combination with 12.5 mg or 25 mg HCTZ, in particular, is likewise safe.2,3 Bönner3 reported about 1% serious AEs when candesartan combination therapy including HCTZ was considered. For metabolic parameters, a slight increase of serum ureate and serum creatinine was observed with the fixed combinations, whereas other parameters were essentially unchanged. Edes2 reported a rate of serious AE for the fixed-dose combination that was even lower compared with placebo (0.2% vs 3.1%), with overall AE rate ranging between 23% and 25% for placebo, HCTZ, candesartan, and their combination. The present trial reassures that the high-dose fixed combination of candesartan and HCTZ is well tolerated, with 1.2% of patients having AEs and 0.2% having serious AEs, a proportion that is lower than the rates previously reported from randomized trials but about comparable with recent data from primary care.14 This is also consistent with findings that reported systematically lower AE rates in noninterventional studies than in randomized controlled trials, because of the lessened observation and reporting.
Limitations
Observational studies in primary care, including typical patient groups and reflecting current treatment approaches, are useful for complementing the findings of randomized controlled trials.4 The present results have to be considered against the background of potential limitations, however. First, the study was not controlled and therefore the role of a placebo effect or the withdrawal of antihypertensive agents is unknown. Second, in the absence of a randomization procedure, the influence of unknown biases, eg, through patient selection, cannot be ruled out. Third, because of the concurrent documentation of a 12.5 mg and 25 mg HCTZ combination with 32 mg candesartan and their addition to, or substitution for, other medications, bias cannot be ruled out. Fourth, because ABPM was not mandatory, the number of patients with both OBPM and ABPM is limited to about 25% of all patients. Because ABPM is usually performed in clinical practice in patients whose BP is difficult to control, ABPM may not be completely representative for the total OBPM population.12 This may explain the high proportion of nondipping patients.
Conclusion
Candesartan 32 mg in a fixed-dose combination with either 12.5 mg or 25 mg HCTZ is safe and effective for BP lowering in patients at high cardiovascular risk, irrespective of prior antihypertensive drug class not being able to control BP.
Acknowledgments
This noninterventional study was conducted by Takeda Pharma GmbH, Aachen, Germany. We would like to thank the participating physicians for their assistance and all patients observed during the study. Special gratitude goes to the clinical research organization Factum GmbH for data processing and conducting the statistical analyses. Takeda Pharma GmbH (Reinhold Hübner) designed the study. Thomas Mengden and Peter Bramlage explored the data and requested statistical analyses from the Factum GmbH (responsible statistician Dr Michael Vornkahl). Peter Bramlage wrote the first draft of the manuscript. Thomas Mengden and Reinhold Hübner revised the manuscript for important intellectual content. All authors reviewed and approved the final manuscript.
Footnotes
Disclosure
Thomas Mengden and Peter Bramlage have received research support and honoraria for medical consulting. Reinhold Hübner is an employee of the sponsor, Takeda Pharma GmbH.
References
- 1.Uen S, Un I, Fimmers R, Vetter H, Mengden T. Effect of candesartan cilexetil with hydrochlorothiazide on blood pressure and ST-segment depression in patients with arterial hypertension. Dtsch Med Wochenschr. 2007;132(3):81–86. doi: 10.1055/s-2007-959292. [DOI] [PubMed] [Google Scholar]
- 2.Edes I. Combination therapy with candesartan cilexetil 32 mg and hydrochlorothiazide 25 mg provides the full additive antihypertensive effect of the components: a randomized, double-blind, parallel-group study in primary care. Clin Drug Investig. 2009;29(5):293–304. doi: 10.2165/00044011-200929050-00002. [DOI] [PubMed] [Google Scholar]
- 3.Bönner G. Antihypertensive efficacy and tolerability of candesartan-hydrochlorothiazide 32/12.5 mg and 32/25 mg in patients not optimally controlled with candesartan monotherapy. Blood Press. 2008;17(Suppl 2):22–30. doi: 10.1080/08038020802519220. [DOI] [PubMed] [Google Scholar]
- 4.Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342(25):1887–1892. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46(1):156–161. doi: 10.1161/01.HYP.0000170138.56903.7a. [DOI] [PubMed] [Google Scholar]
- 6.Clement DL, De Buyzere ML, De Bacquer DA, et al. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348(24):2407–2415. doi: 10.1056/NEJMoa022273. [DOI] [PubMed] [Google Scholar]
- 7.Salles GF, Cardoso CR, Muxfeldt ES. Prognostic influence of office and ambulatory blood pressures in resistant hypertension. Arch Intern Med. 2008 Nov 24;168(21):2340–2346. doi: 10.1001/archinte.168.21.2340. [DOI] [PubMed] [Google Scholar]
- 8.Bramlage P, Schonrock E, Odoj P, Wolf WP, Funken C. Importance of a fixed combination of AT1-receptor blockade and hydrochlorothiazide for blood pressure lowering in cardiac risk patients. A postmarketing surveillance study with Candesartan/HCTZ. MMW Fortschr Med. 2008;149(Suppl 4):172–181. [PubMed] [Google Scholar]
- 9.Azizi M, Nisse-Durgeat S French Collaborative Group. Comparison of the antihypertensive effects of the candesartan 8 mg hydrochlorothiazide 12.5 mg combination vs the valsartan 80 mg hydrochlorothiazide 12.5 mg combination in patients with essential hypertension resistant to monotherapy [abstract no. P2.367] J Hypertens. 2004;22(Suppl 2):S254–255. [Google Scholar]
- 10.Mengden T, Binswanger B, Weisser B, Vetter W. An evaluation of self-measured blood pressure in a study with a calcium-channel antagonist versus a beta-blocker. Am J Hypertens. 1992;5(3):154–160. doi: 10.1093/ajh/5.3.154. [DOI] [PubMed] [Google Scholar]
- 11.Head GA, Mihailidou AS, Duggan KA, et al. Definition of ambulatory blood pressure targets for diagnosis and treatment of hypertension in relation to clinic blood pressure: prospective cohort study. BMJ. 2010;340:c1104. doi: 10.1136/bmj.c1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luders S, Franz IW, Hilgers KF, et al. Twenty-four hour ambulatory blood pressure monitoring. Dtsch Med Wochenschr. 2005;130(46):2664–2668. doi: 10.1055/s-2005-922054. [DOI] [PubMed] [Google Scholar]
- 13.Belcher G, Hubner R, George M, Elmfeldt D, Lunde H. Candesartan cilexetil: safety and tolerability in healthy volunteers and patients with hypertension. J Hum Hypertens. 1997 Sep;11(Suppl 2):S85–89. [PubMed] [Google Scholar]
- 14.Bönner G, Landers B, Bramlage P. Candesartan cilexitil/hydrochlorothiazide combination treatment versus high-dose candesartan cilexetil monotherapy in patients with mild to moderate cardiovascular risk (CHILI Triple T) Vasc Health Risk Manag. 2011;7:85–95. doi: 10.2147/VHRM.S17004. [DOI] [PMC free article] [PubMed] [Google Scholar]