Abstract
With an explosion of available treatments for metastatic renal cell carcinoma (mRCC) in recent years, it is important to recognize that approved targeted therapies fall broadly into only two mechanistic categories. The first category, vascular endothelial growth factor (VEGF)-directed therapies, includes sunitinib, pazopanib, sorafenib and bevacizumab. The second category includes inhibitors of the mammalian target of rapamycin (mTOR), namely everolimus and temsirolimus. A pivotal trial of ever-olimus supports use of the agent in patients with mRCC refractory to VEGF- tyrosine kinase inhibitors (TKI) therapy, while pivotal data for temsirolimus supports use in poor-prognosis patients as first-line therapy. Multiple reviews exist to delineate the laboratory and clinical development of mTOR inhibitors. This paper will outline the future applications of these therapies. It will explore ongoing trials evaluating combinations of mTOR inhibitors with other targeted therapies, along with sequencing strategies and biomarker discovery efforts. The application of mTOR inhibitors in unique populations is also described.
Keywords: mTOR, Everolimus, Temsirolimus, Deferolimus, INTORACT, TORAVA, BeST, Biomarkers, Clinical trials
Introduction
Therapy for metastatic renal cell carcinoma (mRCC) has undergone a dramatic evolution in recent years. After the approval of interleukin-2 (IL-2) in 1992, an extended period ensued without any major therapeutic advances [1]. Although IL-2 yielded durable responses in a small subset of patients, the overwhelming majority obtained little benefit [2]. Nearly a decade after the approval of IL-2, Motzer et al. suggested that interferon-α (IFN-α) should be used as a comparator in further trials of novel therapies for mRCC [3]. Similar to IL-2, the data for IFN-α indicated only a modest clinical benefit, with meta-analysis data suggesting a median overall survival (OS) of 13 months.
Within the past 5 years, an explosion in drug development has occurred in the domain of mRCC, resulting in a dramatic improvement in outcomes. However, each of the six agents approved by the United States Food and Drug Administration (FDA) over this period of time can be divided into one of two mechanistic categories. In early 2007, phase III data for the vascular endothelial growth factor-tyrosine kinase inhibitors (VEGF-TKIs) sunitinib and sorafenib were published, preceded by their approvals [4–7]. The approvals of pazopanib (also a VEGF-TKI) and bevacizumab (a VEGF-directed monoclonal antibody) ensued, following report of progression-free survival (PFS) benefit in pivotal trials evaluating these agents [8–10]. Notably, despite their characterization, VEGF-TKIs possess mechanisms that extend far beyond inhibition of the VEGF receptor (VEGFR) family of molecules, including inhibition of cell membrane receptors including platelet-derived growth factor receptor (PDGFR), KIT, fms-like tyrosine kinase receptor-3 (FLT3), and Raf.
In addition to VEGF-directed therapies, inhibition of the mammalian target of rapamycin (mTOR) has also been shown to represent a clinically viable anticancer strategy in mRCC. Two agents, everolimus and temsirolimus, are currently approved for the treatment of this disease [11, 12]. Everolimus was compared to placebo in a randomized, phase III RECORD-1 study including 410 patients with clear-cell mRCC that had progressed on sunitinib, sorafenib, or both [11]. The study met its primary endpoint, demonstrating an improvement in PFS of 2.1 months (4.0 versus 1.9 months, P<0.0001). In contrast, the phase III study of temsirolimus compared the single agent to temsirolimus with IFN-α or IFN-α alone in patients with previously untreated, poor-prognosis mRCC [12]. Therapy with single-agent temsirolimus led to an improvement in OS as compared to IFN-α (10.9 versus 7.3 months, P<0.008). Other mTOR inhibitors (i.e., deferolimus) are currently in clinical development, as are numerous combined inhibitors that dually target upstream moieties (i.e., BEZ235, BGT225) [13, 14].
There are multiple reviews that summarize the clinical development and current indications of temsirolimus and everolimus in the context of mRCC [15–20]. However, the current review will focus on novel applications of mTOR inhibitors. Ongoing efforts exploring combination therapy, sequencing strategies, and biomarkers are described (see Tables 1 and 2). Furthermore, current strategies to investigate the utility of mTOR inhibitors in specific populations are discussed.
Table 1.
Planned and ongoing trials of everolimus in renal cell carcinoma
| NCI Identifier | Title |
|---|---|
| Combinations | |
| NCT01037257 [75] | A safety study of LBH589 (Panobinostat) and RAD001 (Everolimus) to stabilize kidney cancer |
| NCT01198158 [76] | Everolimus with or without bevacizumab in treating patients with advanced kidney cancer that progressed after first-line therapy |
| NCT01239342 [77] | MK2206 or everolimus in treating patients with refractory kidney cancer |
| NCT01218555 [78] | Study of everolimus (RAD001) in combination with lenalidomide in patients with advanced solid malignancies enriched for renal cell carcinoma |
| NCT01115803 [79] | A study of LY2584702 with erlotinib or everolimus in patients with solid tumors |
| NCT00303732 [80] | Vatalanib and everolimus in treating patients with advanced solid tumors |
| NCT00651482 [81] | Treatment of refractory metastatic renal cell carcinoma with bevacizumab and RADOO1 |
| NCT00422344 [82] | A study of RAD001 and sunitinib in metastatic renal cell carcinoma |
| NCT01034631 [83] | BNC105P in combination with everolimus/following everolimus for progressive Metastatic clear cell renal cell carcinoma |
| NCT00384969 [84] | Sorafenib and RAD001 renal cell carcinoma |
| NCT00719264 [85] | Safety and efficacy of bevacizumab plus RAD001 versus interferon alfa-2a and bevacizumab in adult patients with kidney cancer (L2201) |
| NCT00331409 [86] | Everolimus and imatinib mesylate in treating patients with metastatic or unresectable kidney cancer |
| NCT00655655 [87] | Everolimus and vatalanib in treating patients with advanced solid tumors |
| NCT00392821 [88] | Dosing and effectiveness study of sorafenib and RAD001 in the treatment of patients with advanced kidney cancer |
| NCT00323739 [89] | Bevacizumab (Avastin) and RAD001(Everolimus)in the treatment of advanced clear cell renal carcinoma |
| NCT00985374 [90] | A multiple ascending dose study of the mTOR inhibitor (RAD001) in combination with R1507 in patients with advanced solid tumors |
| NCT01184326 [91] | Pazopanib and everolimus in patients with advanced solid tumors and previously treated kidney cancer |
| NCT00788060 [92] | A phase Ib study of Rad001 and sutent to treat renal cell carcinoma (Rad/Sutent) |
| NCT01136733 [93] | A study of E7080 alone, and in combination with everolimus in subjects with unresectable advanced or metastatic renal cell carcinoma following one prior Vascular Endothelial Growth Factor (VEGF)-targeted treatment |
| NCT00448149 [94] | Phase I/II trial of RAD001 plus nexavar in patients with kidney cancer |
| Sequencing | |
| NCT00903175 [95] | Efficacy and safety comparison of RAD001 versus sunitinib in the first-line and second-line treatment of patients with metastatic renal cell carcinoma |
| NCT01217931 [38] | Sequential two-agent assessment in renal cell carcinoma therapy |
| Neo-adjuvant/adjuvant therapy | |
| NCT01107509 [47] | Pilot study of neo-adjuvant everolimus to treat advanced renal cell carcinoma—analysis of biomarkers |
| NCT01120249 [43] | Everolimus in treating patients with kidney cancer who have undergone surgery |
| NCT00831480 [96] | Everolimus(RAD001) for advanced Renal Cell Carcinoma(RCC) before kidney removal |
| Biomarker discovery/imaging | |
| NCT01028638 [74] | VEGF imaging before and during everolimus treatment for renal cell carcinoma |
| NCT00529802 [66] | Exploratory study evaluating fluorodeoxyglucose—position emission tomography as a predictive marker for therapy with RAD001 in metastatic renal cell cancer |
| NCT00827359 [62] | Biomarker trial of everolimus in patients with advanced renal cell carcinoma |
| Unique populations | |
| NCT01152801 [57] | Safety of RAD001 in Chinese patients with metastatic renal cell cancer |
| NCT01206764 [56] | A trial of everolimis in patients with advanced renal cell carcinoma (EVERMORE) |
| Non-clear cell | |
| NCT00830895 [52] | RAD001 for non-clear cell Renal Cell Carcinoma (RCC) |
| NCT00688753 [53] | RAPTOR: RAD001 as monotherapy in the treatment of advanced papillary renal cell tumors program in Europe (RAPTOR/LFR08) |
| NCT01185366 [97] | Everolimus versus sunitinib in non-clear cell renal cell carcinoma |
| NCT01108445 [51] | Phase II study of afinitor vs. sutent in patients with metastatic non-clear cell renal cell carcinoma (ASPEN) |
Table 2.
Planned and ongoing trials of temsirolimus in renal cell carcinoma
| NCI Identifier | Title |
|---|---|
| Combinations | |
| NCT00700258 [98] | Registry for temsirolimus and sunitinib treated patients with metastatic Renal Cell Carcinoma (mRCC), Mantle Cell Lymphoma (MCL), and Gastro-Intestinal Stroma Tumor (GIST) [STAR-TOR] |
| NCT00112840 [99] | CCI-779 and bevacizumab in treating patients with metastatic or unresectable kidney cancer |
| NCT00782275 [100] | Avastin and temsirolimus following tyrosine kinase inhibitor failure in patients with advanced renal cell carcinoma |
| NCT00563147 [101] | A phase 1b, open-label, dose-finding study to evaluate the safety of tivozanib (AV-951) in combination with temsirolimus in subjects with metastatic renal cell carcinoma |
| NCT00417677 [102] | A study combining treatment with temsirolimus and sunitinib for subjects with advanced renal cell carcinoma |
| NCT00631371 [29] | Study comparing bevacizumab+temsirolimus vs. bevacizumab+interferon-alfa in advanced renal cell carcinoma subjects (INTORACT) |
| NCT00065468 [103] | Study evaluating interferon and CCI-779 in advanced Renal Cell Carcinoma (ARCC) |
| NCT00378703 [104] | Bevacizumab, sorafenib, and temsirolimus in treating patients with metastatic kidney cancer |
| NCT00619268 [105] | Combination of temsirolimus and bevacizumab in patient with metastatic renal cell carcinoma (TORAVA) |
| NCT01079286 [106] | Study of nelfinavir and temsirolimus in patients with advanced cancers (I-NET) |
| NCT00112476 [107] | Temsirolimus and bryostatin 1 in treating patients with unresectable or metastatic solid tumors |
| NCT00600496 [108] | A phase I, open-label, multi-center study to assess the safety, tolerability and pharmacokinetics of AZD6244 (ARRY-142886) |
| NCT01198184 [109] | RO4929097 and temsirolimus in treating patients with advanced solid tumors |
| NCT01122615 [110] | Sunitinib plus temsirolimus in patients with Renal Cell Cancer (RCC) |
| NCT01155258 [111] | Temsirolimus and vinorelbine ditartrate in treating patients with unresectable or metastatic solid tumors |
| NCT00659568 [112] | Metformin and temsirolimus in treating patients with metastatic or unresectable solid tumor or lymphoma |
| Sequencing | |
| NCT00474786 [39] | Temsirolimus versus sorafenib as second-line therapy in patients with advanced RCC who have failed first-line sunitinib |
| Biomarker discovery/imaging | |
| NCT01246817 [67] | Temsirolimus-RCC-imaging |
| NCT01224288 [73] | Renal Cell Carcinoma (RCC) scramble |
| NCT00538772 [113] | An exploratory correlative study of biomarkers in patients with metastatic renal cell carcinoma who have progressed after sunitinib therapya |
| Unique populations | |
| NCT00494091 [58] | Study evaluating the safety, efficacy & pharmacokinetics of temsirolimus(CCI-779) in subjects with advanced renal cell carcinoma |
| Non-clear cell | |
| NCT00979966 [50] | Study in non-clear cell renal carcinoma (Ncc-RCC) temsirolimus versus sunitinib |
Study withdrawn prior to patient enrollment
Combination therapy
At present, numerous combinations of everolimus and temsirolimus with other cytotoxic agents are being explored. Data is available from only a fraction of these studies to date. The combination of sunitinib and temsirolimus was explored in a phase I clinical trial; however, two dose-limiting toxicities (DLTs) were observed among three patients within the first cohort receiving sunitinib at 25 mg daily and temsirolimus at 15 mg weekly [21]. As a consequence, this study was terminated. Sorafenib and temsirolimus in combination have been explored in several malignancies outside of RCC, including glioblastoma, melanoma, and hepatocellular carcinoma [22–24]. Phase I data for the combination suggested significant palmar-plantar erythrodysesthesias using full doses of both, although there was no pharmacokinetic interaction [25]. The randomized, phase II BeST study explores four permutations of bevacizumab, sorafenib and temsirolimus (including sorafenib with temsirolimus) in patients with mRCC [26]. The study is anticipated to enroll a total of 360 patients with a primary completion date in May of 2012.
The combination of bevacizumab and temsirolimus has recently received a great deal of attention. The phase I component of a phase I/II trial utilizing this regimen identified a recommended dose of temsirolimus 25 mg weekly and bevacizumab 10 mg/kg every 2 weeks [27]. DLTs incurred with the combination at these doses included hypertriglyceridemia and mucositis. Early data from the phase II component of this study were somewhat encouraging, with 4 patients (16%) experiencing a partial response (PR) and 18 patients (72%) with stable disease (SD) [28]. Enthusiasm for this regimen has been tempered by the recent results of the TORAVA study. In this trial, 171 patients were randomized in a 2:1:1 fashion to bevacizumab/temsirolimus, sunitinib or bevacizumab/IFN-α. Response rates were 25%, 24% and 34%, respectively. Non-progression rate at 48 weeks (the primary endpoint of the trial) was 43.2%, 47.6%, and 65.9%, respectively. The experimental arm (bevacizumab/temsirolimus) was accompanied by significant rates of toxicity, with upwards of 40% of patients discontinuing therapy for this reason. A specific focus on grade ≥3 events suggested a relatively high rate of colonic fistulas and hemorrhage. Moving forward, two large trials will further prospectively assess the combination with bevacizumab—the aforementioned BeST study an the phase III INTORACT trial [29, 30]. INTORACT will randomize 800 patients to either bevacizumab/temsirolimus or bevacizumab/IFN-α [29]. While the results of INTORACT are eagerly anticipated, the experience garnered from TORAVA underscores the importance of examining regimens in the phase II setting prior to embarking on larger phase III efforts.
Most recently, data has emerged exploring the combination of temsirolimus with the novel VEGF-TKI tivozanib (AV-951). Tivozanib has affinity for VEGFR-1, -2, and -3, and has been assessed in a randomized discontinuation study enrolling 272 patients with all types of mRCC histology [31]. Approximately 73% of the patients in this study had received prior nephrectomy, and 46% had received prior systemic therapy. Even with this degree of prior treatment, treatment with tivozanib elicited an overall response rate(RR) of 25.4% and a median progression-free survival(PFS) of 11.8 months. PFS was similar among patients who were treatment naïve and among patients who had received prior therapy. The combination of tivozanib and temsirolimus was explored in a phase I study including patients with mRCC and a clear-cell component, with no more than one prior VEGF-directed therapy and no prior mTOR inhibitor therapy [32]. Tivozanib was administered once daily for 3 weeks with a 1 week break thereafter, and temsirolimus was administered weekly. The maximally tolerated dose (MTD) for tivozanib and temsirolimus were 1.5 mg daily and 25 mg weekly, respectively. Of 14 evaluable patients, 2 had confirmed PR and 8 had SD in excess of 10 weeks. Encouraging clinical activity with this combination will likely prompt further study.
As with temsirolimus, everolimus has been paired with a number of emerging targeted therapies. The combination of sorafenib with temsirolimus was explored in a cohort of 18 patients. The combination appeared to be relatively well tolerated, with the MTD comprised of standard doses of both drugs (i.e., sorafenib 400 mg twice daily and everolimus 10 mg daily) [33]. Several concerning toxicities were highlighted, however: DLTs in this trial included pneumonitis, pulmonary embolism, and thrombocytopenia. The combination of sunitinib and everolimus has also been explored in a phase I trial enrolling 20 patients with mRCC (notably, 7 patients with non-clear cell histology were included) [34]. The recommended phase II dose was 20 mg of everolimus weekly in combination with 37.5 mg of sunitinib daily. A total of 5 patients were noted to have PR, and among these were 2 patients with papillary RCC and 1 patient with chromophobe RCC. Moving forward, it will be interesting to characterize the activity of this regimen in larger cohorts of non-clear cell patients.
Like bevacizumab with temsirolimus, the combination of bevacizumab with everolimus appears to be well tolerated. A phase I study of the combination identified a recommended phase II dose of bevacizumab at 10 mg/kg every 2 weeks with everolimus at 10 mg daily [35]. A follow-up phase II study employing this regimen included 80 patients with mRCC. Treatment-naïve patients in this cohort had a median PFS of 9.1 months, as compared to 7.1 months in pre-treated patients. The overall response rate (ORR) in treatment-naïve patients was 30%. The rates of grade 3/4 proteinuria were considerable (25%), but the rates of toxicity were otherwise reasonable [36]. The recently completed RECORD-2 study will further compare bevacizumab/everolimus to bevacizumab/IFN-α in 360 patients with mRCC [37].
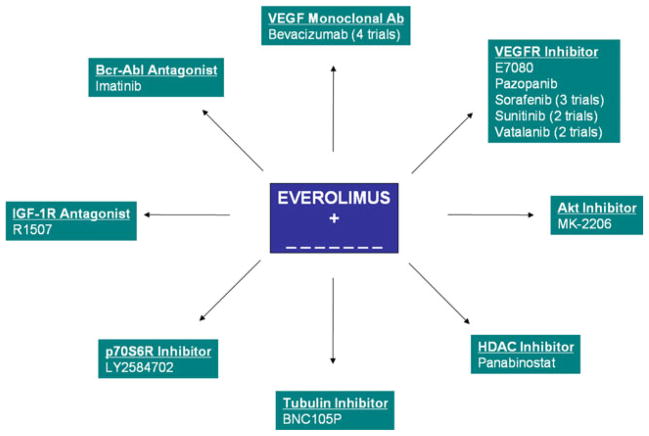
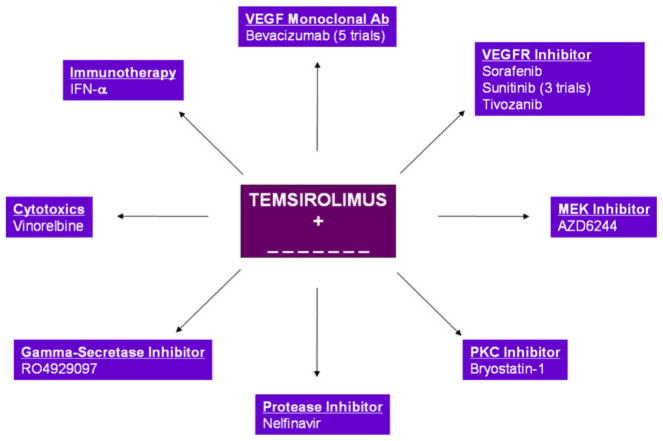
Outside of VEGF-directed therapies, there are a plethora of early clinical trials exploring combinations of everolimus and temsirolimus with novel targeted therapies, cytotoxic agents, and immunotherapy. Combination studies including patients with mRCC and incorporating everolimus and temsirolimus are depicted in Figs. 1 and 2. Limited data are available from these efforts at this point.
Fig. 1.
Planned and ongoing combination therapy trials incorporating everolimus
Fig. 2.
Planned and ongoing combination therapy trials incorporating temsirolimus
Optimizing the sequence of therapy
There are limited examples of trials incorporating mTOR inhibitors that employ a direct, sequential design (i.e., A→B versus B→A). One such study is RECORD-3, a randomized, phase II effort comparing sunitinib to everolimus in patients with treatment-naïve mRCC. At the time of progression on either first-line therapy, patients are crossed over to the other treatment arm. The primary outcome measure in the study is PFS after first-line therapy, with secondary endpoints including PFS after second-line therapy, OS, and objective response rates during each line of therapy. Employing a non-inferiority design, the study will accrue a total of 390 patients with an anticipated completion date in April of 2013. Results of this study have the potential to alter the paradigm established by the RECORD-1 trial [11]. If the sequence of everolimus followed by sunitinib is found to be non-inferior, up-front therapy with an mTOR inhibitor could be considered.
A second study that may identify an optimal sequence of targeted therapy is being coordinated by the MD Anderson Cancer Center [38]. The Sequential Two-agent Assessment in Renal Cell Carcinoma Therapy (START) trial will enroll a total of 240 patients and will randomize the patients to various permutations of pazopanib, bevacizumab and everolimus. The primary endpoint of the study is time to overall treatment failure, which is measured from the randomization date to the date of second disease progression.
The data for everolimus derived from RECORD-1 support therapy with the agent after either sunitinib or sorafenib, or both [11]. As such, there is a lack of clarity regarding whether mTOR inhibition is a superior strategy for second-line therapy. Although not a “sequencing” trial in its truest form, a randomized comparison between temsirolimus and sorafenib may aid in resolving this clinical dilemma [39]. The study assesses patients who have specifically failed first-line therapy with sunitinib, and is powered to compare the PFS associated with each agent. The study will enroll a total of 480 patients, with an estimated completion date in May of 2011.
Neo-adjuvant and adjuvant therapy
At present, there is no evidence to support use of targeted agents as adjuvant therapy for patients with localized RCC following nephrectomy. However, several trials are underway to explore this indication. The Eastern Cooperative Oncology Group (ECOG) has recently completed a trial (ECOG 2805) comparing 1 year of adjuvant sunitinib, sorafenib and placebo in 1,923 patients with localized RCC [40]. The study is powered to assess disease-free survival (DFS) in the three treatment groups. In contrast to ECOG 2805, the Sunitinib-Treatment of Renal Adjuvant Cancer (S-TRAC) study will randomize 600 patients to either sunitinib or placebo, while the SORCE trial will randomize 1,656 patients to receive either sorafenib or placebo [41, 42]. While trials of VEGF-TKIs as adjuvant therapy are abundant, there are more limited efforts exploring mTOR inhibitors in this context. The EVEREST trial, led by the Southwest Oncology Group (SWOG), will randomize 1,218 patients to either everolimus or placebo [43]. Eligibility requirements include RCC histology (excluding collecting duct and medullary carcinoma), negative surgical margins, and intermediate high-risk or very high-risk disease. Patients will receive a total of nine six-week cycles of therapy, with a primary end-point of recurrence-free survival (RFS). Correlative studies accompanying EVEREST will assess nephrectomy specimens for moieties along the AKT/mTOR signaling cascade, and will further assess steady-state trough concentrations of everolimus.
Neo-adjuvant trials offer a prime opportunity to ascertain the biologic effects of targeted therapies. Akin to the adjuvant setting, multiple studies have been established to explore neo-adjuvant therapy with VEGF-TKIs [44–46]. However, relatively few neo-adjuvant trials of mTOR inhibitors have been pursued to date. A neoadjuvant study of everolimus will enroll 20 patients with localized clear cell RCC (T2–4, or any stage with N1–2 disease) or metastatic clear-cell RCC [47]. Both blood and tissue biomarkers will be assessed in this study.
Non-clear cell RCC
Retrospective analyses suggest limited activity of VEGF-TKIs in non-clear cell renal cell carcinoma. As one example, Choueiri et al. assessed 53 patients with metastatic papillary or renal cell carcinoma treated in either France or the United States with either sunitinib or sorafenib [48]. The ORR for the entire cohort was only 10%; median OS was 19.6 months. Of 12 patients with chromophobe mRCC, 3 patients (25%) achieved PR. Only 2 (4.8%) of 41 patients with papillary mRCC achieved a response—notably, both had been treated with sunitinib. Plimack et al. reported a prospective experience assessing sunitinib in 23 patients with papillary mRCC [49]. The primary endpoint in this study was RR and PFS. Median PFS was a sobering 1.6 months (95%CI: 1.3–12 months), and no responses were observed. SD was observed as a best response in 8 patients, and the median OS in the cohort was 10.8 months.
The pivotal trial of temsirolimus (comparing temsirolimus with or without IFN-α to IFN-α alone) was unique in inclusion of non-clear cell patients. Of the 626 patients enrolled into the study, 124 (20%) had a non-clear cell histology [12]. A total of 73 non-clear cell mRCC patients received either temsirolimus or IFN-α, and analysis of this subset with respect to OS favored temsirolimus therapy. These compelling data have prompted further exploration of mTOR inhibitors in patients with papillary and chromophobe histologies. A prospective, randomized phase II study conducted by the Central European Society for Anticancer Drug Research will treat 108 patients with non-clear cell mRCC with either sunitinib or temsirolimus at standard doses [50]. The primary endpoint of the study is time to progression.
There is also considerable interest in exploring the role of everolimus in the same setting. The Phase II ASPEN study will randomize patients with non-clear cell RCC to receive either everolimus or sunitinib [51]. The study will also accrue a total of 108 patients, with a primary endpoint of PFS. Unique secondary outcome measures in this study include a comparison of antitumor activity to a historical cohort comprised of similar patients treated with IFN-α. A straightforward single-arm, phase II study of everolimus in non-clear cell mRCC is ongoing in Korea, and is anticipated to enroll 48 patients [52]. Specific to patients with papillary mRCC, the RAPTOR study will explore everolimus in a single-arm, phase II design [53]. The primary endpoint of the study is PFS at 6 months, with an anticipated enrollment of 60 patients.
Specific populations
Available datasets for a range of targeted therapies have suggested specific risk: benefit profiles that vary with geographic distribution and/or race. For instance, in a pivotal trial in non-small cell lung cancer (NSCLC), it was suggested that Asian origin (among limited other clinico-pathologic criteria) was associated with improved OS with erlotinib therapy [54]. As another prominent example, hypersensitivity reactions associated with cetuximab therapy have been noted to occur in specific territories within the United States [55]. Observations such as these have prompted exploration of targeted therapies for renal cell carcinoma in underrepresented populations. Two trials of everolimus fall into this category. The EVERMORE trial is an open-label phase II study that will assess patients with mRCC (any histology) who may have received cytokine therapy, but have not previously received VEGF-directed therapies or mTOR inhibitors [56]. The study will accrue a total of 110 patients from centers located in Africa, the Middle East, Southeast Asia and Russia, and explores a primary endpoint of PFS. A separate phase Ib trial of everolimus will be conducted in China [57]. The study will enroll patients who have progressed on VEGF-TKI therapy; prior cytokine therapy is also permitted. A total of 60 patients are anticipated to accrue to this effort. With respect to temsirolimus, one study is exploring two dose-levels of the agent in Korean, Japanese, or Chinese patients with mRCC, irrespective of histology or prior therapy [58]. A small cohort of Japanese patients with mRCC (n=6) will be treated with temsirolimus at a weekly dose of 20 mg intravenous. The remainder of the cohort (to total 80 patients) will receive temsirolimus at standard doses (i.e., 25 mg intravenous weekly).
Biomarker discovery
To date, the majority of efforts to characterize biomarkers predicting clinical benefit from mTOR inhibitors are retrospective. Few of these studies have yielded salient biomarkers. One notable exception emerges from the pivotal trial of temsirolimus. In this study, it was noted that an elevated LDH was associated with an improved OS with temsirolimus therapy (P<0.002) as compared to IFN-α [59]. In contrast, patients with a normal LDH did not obtain a survival benefit relative to patients treated with IFN-α. Other efforts to correlate biomarkers to clinical outcome with mTOR inhibitor therapy have been somewhat disappointing. For example, baseline levels PTEN and HIF-1α were also assessed in the phase III evaluation of temsirolimus [60]. However, neither demonstrated correlation with response; PFS and OS were improved with temsirolimus therapy irrespective of the levels of these markers.
Given the limitations of biomarker development using retrospective datasets, the research community has often cited the need for prospective efforts powered to assess novel biomarkers [61]. Unfortunately, few examples of biomarker-driven trial designs (as they pertain to mTOR inhibitor therapy) exist. A novel phase II biomarker-driven study of everolimus (coordinated by the Beth Israel Deaconess Medical Center) is planned [62]. This study would enroll 40 patients with mRCC, and requires the presence of metastatic lesions that are amenable to biopsy. The primary objective of the study is to prospectively validate phosphorylated-Akt and -S6 as biomarkers of everolimus response. Secondary objectives in this study include not only clinical response, but assessment of a panel of novel biomarkers including phosphorylated PRAS40, phosphorylated TSC, and elF4E. It is critical that the scientific community lend support to translational efforts such as this, which will certainly amass valuable information regarding putative biomarkers of mTOR inhibitor efficacy.
Outside of characterizing molecular mediators, novel imaging techniques may provide unique insights into subsets of patients with mRCC that derive benefit from mTOR inhibitors. Varying reports exist regarding the potential predictive capabilities of positron emission tomography (PET) in patients treated with mTOR inhibitors. In vivo preclinical studies suggest that fluorodeoxyglucose (FDG) uptake can be used to define the optimal biologic dose of everolimus therapy [63]. Supporting this, a study of eight patients with NSCLC treated with everolimus suggested marked reductions of 18F-FDG uptake within just days of initiating therapy, suggesting that this may be an early effect of everolimus treatment [64]. These encouraging reports are tempered by others; for instance, an assessment of 34 cancer patients treated with rapamycin analogues suggested that changes in 18F-FDG PET may be indicative of changes in Akt activation, but was not necessarily predictive of therapeutic response [65].
In the setting of mRCC, a recently completed study at the University of Chicago assessed a cohort of 60 patients receiving everolimus with 18F-FDG-PET [66]. Enrolled patients were refractory to sunitinib and/or sorafenib. The principal aim of the study was to determine if high uptake on PET at an 8-week interval was correlated with the extent of tumor shrinkage. Results from this study are eagerly awaited. A second study centered in the Netherlands will assess 51 mRCC patients receiving temsirolimus with standard 18F-FDG-PET, as well as 18F-fluoro-L-thymidine (18F-FLT)-PET [67]. Patients must have progressed on at least one prior antiangiogenic agent. 18F-FLT-PET has been developed as a modality to image the extent of tumor cell proliferation, and therefore may be highly useful in interpreting the activity of largely cytostatic agents such as temsirolimus [68–72].
Several other novel imaging modalities are under evaluation for mRCC patients receiving mTOR inhibitor therapy. The RCC Scramble study, a companion to the aforementioned START trial, will assess patients receiving sequential targeted therapies (including everolimus) with dynamic contrast-enhanced computed tomography (DCE-CT) [38, 73]. DCE-CT provides an estimate of blood flow to tumor tissue; as a consequence, the modality may offer greater biological insight into the efficacy of mTOR inhibitor therapy. A second study centered in the Netherlands will assess VEGF production in 14 patients with mRCC receiving everolimus using 89Zr-labelled bevacizumab [74]. The primary outcome measure in the study is correlation between 89Zr- bevacizumab uptake on baseline scan and on-treatment scans performed at 2 and 6 weeks. Novel imaging studies such as these may ultimately offer a substitute for algorithms such as RECIST, which often cannot account for antitumor activity reflected in tumor necrosis and cavitation.
Conclusions
The multitude of described and ongoing trials (Tables 1 and 2) [75–113] suggests that the clinical development strategy for everolimus and temsirolimus in mRCC extend far beyond their current indications. While these efforts are to be applauded, the research community will ultimately be challenged to prioritize them in the coming years. For instance, how does the necessity of large combination therapy trials (i.e., RECORD-2 and INTORACT) compare with the necessity to evaluate novel mTOR inhibitors, such as deferolimus? Do sequencing strategies or biomarker discovery serve as the ideal manner in which to resolve current areas of equipoise in therapeutic assignment? Will large, comparative trials be necessary to juxtapose the effect of combined inhibitors (i.e., BEZ235) against currently available agents? While the number of clinical dilemmas in RCC therapy is limitless, the availability of appropriate study patients is not. In the future, the research community will need to unite to develop a cohesive strategy to optimize use of available agents that move the efforts in renal cell carcinoma forward and identify the optimal strategies on behalf of our patients.
Acknowledgments
Dr. Pal’s efforts are supported by the National Institutes of Health (NIH) Loan Repayment Plan (LRP), the CBCRP 15IB-0140 (California Breast Cancer Research Program Junior IDEA Award) and NIH K12 2K12CA001727-16A1.
Footnotes
Conflict of interest statement Dr. Pal receives honoraria from Pfizer, Novartis, GSK and Sanofi-Aventis, consulting fees from Genentech and Novartis. Dr. Figlin receives consulting fees from Onyx, GSK and Pfizer, and receives research support from Novartis, Pfizer and GSK.
Contributor Information
Sumanta Kumar Pal, Email: spal@coh.org, Division of Genitourinary Malignancies, Department of Medical Oncology & Experimental Therapeutics, City of Hope Comprehensive Cancer Center, Los Angeles, CA, USA.
Robert A. Figlin, Email: robert.figlin@cshs.org, Division of Hematology Oncology, Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA. Academic Program Development, Samuel Oschin Comprehensive Cancer Institute, Los Angeles, CA, USA. David Geffen School of Medicine at UCLA, 8700 Beverly Blvd., AC 1042-B, North Tower, Los Angeles, CA 90048, USA
References
- 1. [last accessed December 2, 2010];The Pharma Letter. 1992 May; Available at: http://www.thepharmaletter.com/file/45036/chirons-il-2-approved-in-usa.html.
- 2.Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13(3):688–696. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20(1):289–296. doi: 10.1200/jco.20.1.289. [DOI] [PubMed] [Google Scholar]
- 4. [last accessed March 24, 2010];FDA Approval Letter for Sorafenib. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2005/021923ltr.pdf.
- 5. [last accessed March 24, 2010];FDA Approval Letter for Sunitinib. Available at http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2006/021968s000ltr.pdf.
- 6.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM the TARGET Study Group. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 8.Escudier B, Bellmunt J, Negrier S, Bajetta E, Melichar B, Bracarda S, Ravaud A, Golding S, Jethwa S, Sneller V. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol. 2010;28(13):2144–2150. doi: 10.1200/jco.2009.26.7849. [DOI] [PubMed] [Google Scholar]
- 9.Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Archer L, Atkins JN, Picus J, Czaykowski P, Dutcher J, Small EJ. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol. 2010;28(13):2137–2143. doi: 10.1200/jco.2009.26.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA, Kavina A, Zarba JJ, Chen M, McCann L, Pandite L, Roychowdhury DF, Hawkins RE. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061–1068. doi: 10.1200/jco.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 11.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grunwald V, Thompson JA, Figlin RA, Hollaender N, Urbanowitz G, Berg WJ, Kay A, Lebwohl D, Ravaud A. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 12.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IGH, Barbarash O, Gokmen E, O’Toole T, Lustgarten S, Moore L, Motzer RJ the Global ARCC Trial. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 13.Burris H, Rodon J, Sharma S, Herbst RS, Tabernero J, Infante JR, Silva A, Demanse D, Hackl W, Baselga J. First-inhuman phase I study of the oral PI3K inhibitor BEZ235 in patients (pts) with advanced solid tumors. J Clin Oncol. 2010;28(15s):abstr 3005. [Google Scholar]
- 14.Pal SK, Figlin RA, Reckamp KL. The role of targeting mammalian target of rapamycin in lung cancer. Clin Lung Cancer. 2008;9(6):340–345. doi: 10.3816/CLC.2008.n.049. [DOI] [PubMed] [Google Scholar]
- 15.Eimer C, Gerullis H, Heuck C, Otto T. mTOR inhibition in advanced renal cell carcinoma: which criteria should be used to evaluate therapeutic outcome? Anticancer Drugs. 2011;22(1):18–23. doi: 10.1097/CAD.0b013e3283407dde. [DOI] [PubMed] [Google Scholar]
- 16.Gerullis H, Ecke TH, Eimer C, Heuck CJ, Otto T. mTOR-inhibition in metastatic renal cell carcinoma. Focus on temsirolimus: a review. Minerva Urol Nefrol. 2010;62(4):411–423. R19101845. [PubMed] [Google Scholar]
- 17.Pal SK, Figlin RA. Treatment options in metastatic renal cell carcinoma: focus on mTOR inhibitors. Clin Med Insights Oncol. 2010;4:43–53. doi: 10.4137/cmo.s1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinto Marin A, Redondo Sanchez A, Espinosa Arranz E, Zamora Aunon P, Castelo Fernandez B, Gonzalez Baron M. mTOR pathway inhibition in renal cell carcinoma. Urol Oncol. 2010 doi: 10.1016/j.urolonc.2009.11.008. S1078-1439(09)00361-5. [DOI] [PubMed] [Google Scholar]
- 19.Hudes GR. Targeting mTOR in renal cell carcinoma. Cancer. 2009;115(10 Suppl):2313–2320. doi: 10.1002/cncr.24239. [DOI] [PubMed] [Google Scholar]
- 20.Rini BI. Update on the use of mTOR inhibitors in renal cell carcinoma. Clin Adv Hematol Oncol. 2008;6(10):722–724. [PubMed] [Google Scholar]
- 21.Fischer P, Patel P, Carducci MA, McDermott DF, Hudes GR, Lubiniecki GM, Gelder MS, Senico P, Curiel RE, Motzer RJ. Phase I study combining treatment with temsirolimus and sunitinib malate in patients with advanced renal cell carcinoma. J Clin Oncol. 2008 May 20;26(suppl):abstr 16020. doi: 10.3816/CGC.2009.n.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelley RK, Nimeiri HS, Vergo MT, Bergsland EK, Ko AH, Munster PN, Reinert A, Mulcahy MF, Benson AB, Venook AP. A phase I trial of the combination of temsirolimus (TEM) and sorafenib (SOR) in advanced hepatocellular carcinoma (HCC) ASCO Meet Abstr. 2010;28(15_suppl):TPS213. [Google Scholar]
- 23.Kim KB, Davies MA, Papadopoulos NE, Bedikian AY, Hwu W, Woodard K, Washington EW, Dancey JE, Wright J, Hwu P. Phase I/II study of the combination of sorafenib and temsirolimus in patients with metastatic melanoma. ASCO Meet Abstr. 2009;27(15S):9026. [Google Scholar]
- 24.Wen PY, Cloughesy T, Kuhn J, Lamborn K, Abrey LE, Lieberman F, Robins HI, Wright J, Prados MD, Gilbert M. Phase I/II study of sorafenib and temsirolimus for patients with recurrent glioblastoma (GBM) (NABTC 05–02) ASCO Meet Abstr. 2009;27(15S):2006. [Google Scholar]
- 25.Patnaik A, Ricart A, Cooper J, Papadopoulos K, Beeram M, Mita C, Mita MM, Hufnagel D, Izbicka E, Tolcher AW National Cancer I. A phase I, pharmacokinetic and pharmacodynamic study of sorafenib (S), a multi-targeted kinase inhibitor in combination with temsirolimus (T), an mTOR inhibitor in patients with advanced solid malignancies. ASCO Meet Abstr. 2007;25(18_suppl):3512. [Google Scholar]
- 26.Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, Redman BG, Margolin KA, Merchan JR, Wilding G, Ginsberg MS, Bacik J, Kim ST, Baum CM, Michaelson MD. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295(21):2516–2524. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 27.Merchan JR, Liu G, Fitch T, Picus J, Qin R, Pitot HC, Maples W, Erlichman C. Phase I/II trial of CCI-779 and bevacizumab in stage IV renal cell carcinoma: phase I safety and activity results. J Clin Oncol. 2007;27(15s):abstr 5039. [Google Scholar]
- 28.Merchan JR, Pitot HC, Qin R, Liu G, Fitch TR, Picus J, Maples WJ, Erlichman C. Phase I/II trial of CCI 779 and bevacizumab in advanced renal cell carcinoma (RCC): safety and activity in RTKI refractory RCC patients. J Clin Oncol (Meet Abstr) 2009;27(15S):5039. [Google Scholar]
- 29. [last accessed December 4, 2010]; NCT00631371: study comparing bevacizumab + temsirolimus vs. bevacizumab + interferon-alfa in advanced renal cell carcinoma subjects (INTORACT) Available at: http://www.clinicaltrials.gov.
- 30. [Accessed December 22, 2009]; NCT00378703: the BeST trial: a randomized phase II study of VEGF, RAF kinase, and mTOR Combination Targeted Therapy (CTT) with bevacizumab, sorafenib and temsirolimus in advanced renal cell carcinoma [BeST] Available at: http://www.clinicaltrials.gov.
- 31.Bhargava P, Esteves B, Al-Adhami M, Nosov D, Lipatov ON, Lyulko AA, Anischenko AA, Chacko RT, Doval D, Slichenmyer WJ. Activity of tivozanib (AV-951) in patients with renal cell carcinoma (RCC): subgroup analysis from a phase II randomized discontinuation trial (RDT) J Clin Oncol. 2010;28(15s):abstr 4599. doi: 10.1200/JCO.2011.35.3524. [DOI] [PubMed] [Google Scholar]
- 32.Kabbinavar FF, Srinivas S, Hauke RJ, Amato RJ, Esteves B, Dhillon R, Cotreau MM, Al-Adhami M, Bhargava P, Fishman MN. Combination of tivozanib (AV-951) and temsirolimus in patients with renal cell carcinoma: preliminary results from a phase 1 trial. Presented at the 2010 Kidney Cancer Symposium; October 2, 2010; Chicago, IL. 2010. p. Abstr 49. [Google Scholar]
- 33.Cen P, Daleiden A, Doshi G, Amato R. A phase I study of everolimus plus sorafenib in patients with metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2009;27:abstr e1605. [Google Scholar]
- 34.Kroog GS, Feldman DR, Kondagunta GV, Ginsberg MS, Fischer PM, Trinos MJ, Patil S, Ishill NM, Motzer RJ. Phase I trial of RAD001 (everolimus) plus sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(15s):abstr 5037. [Google Scholar]
- 35.Zafar Y, Bendell J, Lager J, Yu D, George D, Nixon A, Petros W, Beci R, Arrowood C, Hurwitz H. Preliminary results of a phase I study of bevacizumab (BV) in combination with everolimus (E) in patients with advanced solid tumors. J Clin Oncol. 2006;24(18s):abstr 3097. [Google Scholar]
- 36.Hainsworth JD, Spigel DR, Burris HA, Waterhouse D, Clark BL, Whorf R. Phase II trial of bevacizumab and everolimus in patients with advanced renal cell carcinoma. J Clin Oncol. 2010;28(13):2131–2136. doi: 10.1200/JCO.2009.26.3152. [DOI] [PubMed] [Google Scholar]
- 37. [Accessed December 22, 2009]; NCT00719264: a randomized, open-label, multi-center phase II study to compare bevacizumab plus RAD001 versus interferon alfa-2a plus bevacizumab for the first-line treatment of patients with metastatic clear cell carcinoma of the kidney. Available at: http://www.clinicaltrials.gov.
- 38. [last accessed December 4, 2010]; NCT01217931: sequential two-agent assessment in renal cell carcinoma therapy. Available at: http://www.clinicaltrials.gov.
- 39. [last accessed December 4, 2010]; NCT00474786: temsirolimus versus sorafenib as second-line therapy in patients with advanced RCC who have failed first-line sunitinib. Available at: http://www.clinicaltrials.gov.
- 40. [last accessed December 10, 2010]; NCT00326898: sunitinib or sorafenib in treating patients with kidney cancer that was removed by surgery. Available at: http://www.clinicaltrials.gov.
- 41. [last accessed December 10, 2010]; NCT00375674: a clinical trial comparing efficacy and safety of sunitinib versus placebo for thetreatment of patients at high risk of recurrent renal cell cancer (S-TRAC) Available at: http://www.clinicaltrials.gov.
- 42. [last accessed December 10, 2010]; NCT00492258: sorafenib in treating patients at risk of relapse after undergoing surgery to remove kidney cancer. Available at: http://www.clinicaltrials.gov.
- 43. [last accessed December 4, 2010]; NCT01120249: everolimus in treating patients with kidney cancer who have undergone surgery. Available at: http://www.clinicaltrials.gov.
- 44.Cowey CL, Amin C, Pruthi RS, Wallen EM, Nielsen ME, Grigson G, Watkins C, Nance KV, Crane J, Jalkut M, Moore DT, Kim WY, Godley PA, Whang YE, Fielding JR, Rathmell WK. Neoadjuvant clinical trial with sorafenib for patients with stage II or higher renal cell carcinoma. J Clin Oncol. 2010;28(9):1502–1507. doi: 10.1200/JCO.2009.24.7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas AA, Rini BI, Lane BR, Garcia J, Dreicer R, Klein EA, Novick AC, Campbell SC. Response of the primary tumor to neoadjuvant sunitinib in patients with advanced renal cell carcinoma. J Urol. 2009;181(2):518–523. doi: 10.1016/j.juro.2008.10.001. discussion 523. [DOI] [PubMed] [Google Scholar]
- 46.Hellenthal NJ, Underwood W, Penetrante R, Litwin A, Zhang S, Wilding GE, Teh BT, Kim HL. Prospective clinical trial of preoperative sunitinib in patients with renal cell carcinoma. J Urol. 2010;184(3):859–864. doi: 10.1016/j.juro.2010.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. [last accessed December 4, 2010]; NCT01107509: pilot study of neo-adjuvant everolimus to treat advanced renal cell carcinoma—analysis of biomarkers. Available at: http://www.clinicaltrials.gov.
- 48.Choueiri TK, Plantade A, Elson P, Negrier S, Ravaud A, Oudard S, Zhou M, Rini BI, Bukowski RM, Escudier B. Efficacy of sunitinib and sorafenib in metastatic papillary and chromo-phobe renal cell carcinoma. J Clin Oncol. 2008;26(1):127–131. doi: 10.1200/JCO.2007.13.3223. [DOI] [PubMed] [Google Scholar]
- 49.Plimack ER, Jonasch E, Bekele BN, Qiao W, Ng CS, Tannir NM. Sunitinib in papillary renal cell carcinoma (pRCC): results from a single-arm phase II study. J Clin Oncol. 2010;28(15s):abstr 4604. [Google Scholar]
- 50. [last accessed December 4, 2010]; NCT00979966: study in Non-clear cell Renal Carcinoma (Ncc-RCC) temsirolimus versus sunitinib. Available at: http://www.clinicaltrials.gov.
- 51. [last accessed December 4, 2010]; NCT01108445: phase II study of afinitor vs. sutent in patients with metastatic non-clear cell renal cell carcinoma (ASPEN) Available at: http://www.clinicaltrials.gov.
- 52. [last accessed December 4, 2010]; NCT00830895: RAD001 for non-clear cell Renal Cell Carcinoma (RCC) Available at: http://www.clinicaltrials.gov.
- 53. [last accessed December 4, 2010]; NCT00688753: RAPTOR: RAD001 as monotherapy in the treatment of advanced papillary renal cell tumors program in Europe (RAPTOR/LFR08) Available at: http://www.clinicaltrials.gov.
- 54.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabarbara P, Seymour L. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 55.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, Murphy BA, Satinover SM, Hosen J, Mauro D, Slebos RJ, Zhou Q, Gold D, Hatley T, Hicklin DJ, Platts-Mills TA. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358(11):1109–1117. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. [last accessed December 4, 2010]; NCT01206764: a trial of everolimis in patients with advanced renal cell carcinoma (EVERMORE) Available at: http://www.clinicaltrials.gov.
- 57. [last accessed December 4, 2010]; NCT01152801: safety of RAD001 in Chinese patients with metastatic renal cell cancer. Available at: http://www.clinicaltrials.gov.
- 58. [last accessed December 4, 2010]; NCT00494091: study evaluating the safety, efficacy & pharma-cokinetics of temsirolimus(CCI-779) in subjects with advanced renal cell carcinoma. Available at: http://www.clinicaltrials.gov.
- 59.Armstrong AJ, George DJ, Halabi S. Serum lactate dehydrogenase (LDH) as a biomarker for survival with mTOR inhibition in patients with metastatic renal cell carcinoma (RCC) J Clin Oncol. 2010;28(15s):abstr 4631. doi: 10.1200/JCO.2011.40.9631. [DOI] [PubMed] [Google Scholar]
- 60.Figlin RA, Pd S, McDermott D, Dutcher JP, Berkenblit A, Thiele A, Krygowski M, Strahs A, Feingold J, Boni J, Hudes G. Analysis of PTEN and HIF-1alpha and correlation with efficacy in patients with advanced renal cell carcinoma treated with temsirolimus versus interferon-alpha. Cancer. 2009;115(16):3651–3660. doi: 10.1002/cncr.24438. [DOI] [PubMed] [Google Scholar]
- 61.Sargent DJ, Conley BA, Allegra C, Collette L. Clinical trial designs for predictive marker validation in cancer treatment trials. J Clin Oncol. 2005;23(9):2020–2027. doi: 10.1200/jco.2005.01.112. [DOI] [PubMed] [Google Scholar]
- 62. [last accessed December 4, 2010]; NCT00827359: biomarker trial of everolimus in patients with advanced renal cell carcinoma. Available at: http://www.clinicaltrials.gov.
- 63.Cejka D, Kuntner C, Preusser M, Fritzer-Szekeres M, Fueger BJ, Strommer S, Werzowa J, Fuereder T, Wanek T, Zsebedics M, Mueller M, Langer O, Wacheck V. FDG uptake is a surrogate marker for defining the optimal biological dose of the mTOR inhibitor everolimus in vivo. Br J Cancer. 2009;100(11):1739–1745. doi: 10.1038/sj.bjc.6605076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nogova L, Boellaard R, Kobe C, Hoetjes N, Zander T, Gross SH, Dimitrijevic S, Pellas T, Eschner W, Schmidt K, Bangard C, Hayes W, Thomas RK, Dietlein M, Giaccone G, Hoekstra OS, Lammertsma AA, Wolf J. Downregulation of 18F-FDG uptake in PET as an early pharmacodynamic effect in treatment of non-small cell lung cancer with the mTOR inhibitor everolimus. J Nucl Med. 2009;50(11):1815–1819. doi: 10.2967/jnumed.109.065367. [DOI] [PubMed] [Google Scholar]
- 65.Ma WW, Jacene H, Song D, Vilardell F, Messersmith WA, Laheru D, Wahl R, Endres C, Jimeno A, Pomper MG, Hidalgo M. [18F]Fluorodeoxyglucose positron emission tomography correlates with AKT pathway activity but is not predictive of clinical outcome during mTOR inhibitor therapy. J Clin Oncol. 2009;27(16):2697–2704. doi: 10.1200/JCO.2008.18.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. [last accessed December 4, 2010]; NCT00529802: exploratory study evaluating fluorodeoxyglucose—position emission tomography as a predictive marker for therapy with RAD001 in metastatic renal cell cancer. Available at: http://www.clinicaltrials.gov.
- 67. [last accessed December 4, 2010]; NCT01246817: temsirolimus-RCC-imaging. Available at: http://www.clinicaltrials.gov.
- 68.Frings V, de Langen AJ, Smit EF, van Velden FH, Hoekstra OS, van Tinteren H, Boellaard R. Repeatability of metabolically active volume measurements with 18F-FDG and 18F-FLT PET in non-small cell lung cancer. J Nucl Med. 2010;51(12):1870–1877. doi: 10.2967/jnumed.110.077255. [DOI] [PubMed] [Google Scholar]
- 69.Yang W, Zhang Y, Fu Z, Yu J, Sun X, Mu D, Han A. Imaging of proliferation with 18F-FLT PET/CT versus 18F-FDG PET/CT in non-small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2010;37(7):1291–1299. doi: 10.1007/s00259-010-1412-6. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto Y, Nishiyama Y, Kimura N, Ishikawa S, Okuda M, Bandoh S, Kanaji N, Asakura M, Ohkawa M. Comparison of (18)F-FLT PET and (18)F-FDG PET for preoperative staging in non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2008;35(2):236–245. doi: 10.1007/s00259-007-0613-0. [DOI] [PubMed] [Google Scholar]
- 71.Saga T, Kawashima H, Araki N, Takahashi JA, Nakashima Y, Higashi T, Oya N, Mukai T, Hojo M, Hashimoto N, Manabe T, Hiraoka M, Togashi K. Evaluation of primary brain tumors with FLT-PET: usefulness and limitations. Clin Nucl Med. 2006;31(12):774–780. doi: 10.1097/01.rlu.0000246820.14892.d2. [DOI] [PubMed] [Google Scholar]
- 72.Chen W, Cloughesy T, Kamdar N, Satyamurthy N, Bergsneider M, Liau L, Mischel P, Czernin J, Phelps ME, Silverman DH. Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. J Nucl Med. 2005;46(6):945–952. [PubMed] [Google Scholar]
- 73. [last accessed December 4, 2010]; NCT01224288: Renal Cell Carcinoma (RCC) scramble. Available at: http://www.clinicaltrials.gov.
- 74. [last accessed December 4, 2010]; NCT01028638: VEGF imaging before and during everolimus treatment for renal cell carcinoma (Everolimage) Available at: http://www.clinicaltrials.gov.
- 75. [last accessed December 4, 2010];NCT01037257: a safety study of LBH589 (Panobinostat) and RAD001 (Everolimus) to stabilize kidney cancer. Available at: http://www.clinicaltrials.gov.
- 76. [last accessed December 4, 2010]; NCT01198158: everolimus with or without bevacizumab in treating patients with advanced kidney cancer that progressed after first-line therapy. Available at: http://www.clinicaltrials.gov.
- 77. [last accessed December 4, 2010]; NCT01239342: MK2206 or everolimus in treating patients with refractory kidney cancer. Available at: http://www.clinicaltrials.gov.
- 78. [last accessed December 4, 2010]; NCT01218555: study of everolimus (RAD001) in combination with lenalidomide in patients with advanced solid malignancies enriched for renal cell carcinoma. Available at: http://www.clinicaltrials.gov.
- 79. [last accessed December 4, 2010]; NCT01115803: a study of LY2584702 with erlotinib or everolimus in patients with solid tumors. Available at: http://www.clinicaltrials.gov.
- 80. [last accessed December 4, 2010]; NCT00303732: vatalanib and everolimus in treating patients with advanced solid tumors. Available at: http://www.clinicaltrials.gov.
- 81. [last accessed December 4, 2010]; NCT00651482: treatment of refractory metastatic renal cell carcinoma with bevacizumab and RADOO1. Available at: http://www.clinicaltrials.gov.
- 82. [last accessed December 4, 2010]; NCT00422344: a study of RAD001 and sunitinib in metastatic renal cell carcinoma. Available at: http://www.clinicaltrials.gov.
- 83. [last accessed December 4, 2010]; NCT01034631: BNC105P in combination with everolimus/following everolimus for progressive metastatic clear cell renal cell carcinoma. Available at: http://www.clinicaltrials.gov.
- 84. [last accessed December 4, 2010]; NCT00384969: sorafenib and RAD001 renal cell carcinoma. Available at: http://www.clinicaltrials.gov.
- 85. [last accessed December 4, 2010]; NCT00719264: safety and efficacy of bevacizumab plus RAD001 versus interferon alfa-2a and bevacizumab in adult patients with kidney cancer (L2201) Available at: http://www.clinicaltrials.gov.
- 86. [last accessed December 4, 2010]; NCT00331409: everolimus and imatinib mesylate in treating patients with metastatic or unresectable kidney cancer. Available at: http://www.clinicaltrials.gov.
- 87. [last accessed December 4, 2010]; NCT00655655: everolimus and vatalanib in treating patients with advanced solid tumors. Available at: http://www.clinicaltrials.gov.
- 88. [last accessed December 4, 2010]; NCT00392821: dosing and effectiveness study of sorafenib and RAD001 in the treatment of patients with advanced kidney cancer. Available at: http://www.clinicaltrials.gov.
- 89. [last accessed December 4, 2010]; NCT00323739: bevacizumab (Avastin) and RAD001(Everolimus) in the treatment of advanced clear cell renal carcinoma. Available at: http://www.clinicaltrials.gov.
- 90. [last accessed December 4, 2010]; NCT00985374: a multiple ascending dose study of the mTOR inhibitor (RAD001) in combination with R1507 in patients with advanced solid tumors. Available at: http://www.clinicaltrials.gov.
- 91. [last accessed December 4, 2010]; NCT01184326: pazopanib and everolimus in patients with advanced solid tumors and previously treated kidney cancer. Available at: http://www.clinicaltrials.gov.
- 92. [last accessed December 4, 2010]; NCT00788060: a phase Ib study of Rad001 and sutent to treat renal cell carcinoma (Rad/Sutent) Available at: http://www.clinicaltrials.gov.
- 93. [last accessed December 4, 2010]; NCT01136733: a study of E7080 alone, and in combination with everolimus in subjects with unresectable advanced or metastatic renal cell carcinoma following one prior Vascular Endothelial Growth Factor (VEGF)-targeted treatment. Available at: http://www.clinicaltrials.gov.
- 94. [last accessed December 4, 2010]; NCT00448149: phase I/II trial of RAD001 plus nexavar in patients with kidney cancer. Available at: http://www.clinicaltrials.gov.
- 95. [last accessed June 23, 2009]; NCT00903175: efficacy and safety comparison of RAD001 versus sunitinib in the first-line and second-line treatment of patients with metastatic renal cell carcinoma. Available at: http://www.ClinicalTrials.gov.
- 96. [last accessed December 4, 2010]; NCT00831480: everolimus(RAD001) for advanced Renal Cell Carcinoma(RCC) before kidney removal. Available at: http://www.clinicaltrials.gov.
- 97. [last accessed December 4, 2010]; NCT01185366: everolimus versus sunitinib in non-clear cell renal cell carcinoma. Available at: http://www.clinicaltrials.gov.
- 98. [last accessed December 4, 2010]; NCT00700258: registry for temsirolimus and sunitinib treated patients with Metastatic Renal Cell Carcinoma (mRCC), Mantle Cell Lymphoma (MCL), and Gastro-Intestinal Stroma Tumor (GIST) [STAR-TOR] Available at: http://www.clinicaltrials.gov.
- 99. [last accessed December 4, 2010]; NCT00112840: CCI-779 and bevacizumab in treating patients with metastatic or unresectable kidney cancer. Available at: http://www.clinicaltrials.gov.
- 100. [last accessed December 4, 2010]; NCT00782275: avastin and temsirolimus following tyrosine kinase inhibitor failure in patients with advanced renal cell carcinoma. Available at: http://www.clinicaltrials.gov.
- 101. [last accessed December 4, 2010]; NCT00563147: a phase 1b, open-label, dose-finding study to evaluate the safety of tivozanib (AV-951) in combination with temsirolimus in subjects with metastatic renal cell carcinoma. Available at: http://www.clinicaltrials.gov.
- 102. [last accessed December 4, 2010]; NCT00417677: a study combining treatment with temsirolimus and sunitinib for subjects with advanced renal cell carcinoma. Available at: http://www.clinicaltrials.gov.
- 103. [last accessed December 4, 2010]; NCT00065468: study evaluating interferon and CCI-779 in Advanced Renal Cell Carcinoma (ARCC) Available at: http://www.clinicaltrials.gov.
- 104. [last accessed December 4, 2010]; NCT00378703: bevacizumab, sorafenib, and temsirolimus in treating patients with metastatic kidney cancer. Available at: http://www.clinicaltrials.gov.
- 105. [last accessed December 4, 2010]; NCT00619268: combination of temsirolimus and bevacizumab in patient with metastatic renal cell carcinoma (TORAVA) Available at: http://www.clinicaltrials.gov.
- 106. [last accessed December 4, 2010]; NCT01079286: study of nelfinavir and temsirolimus in patients with advanced cancers (I-NET) Available at: http://www.clinicaltrials.gov.
- 107. [last accessed December 4, 2010]; NCT00112476: temsirolimus and bryostatin 1 in treating patients with unresectable or metastatic solid tumors. Available at: http://www.clinicaltrials.gov.
- 108. [last accessed December 4, 2010]; NCT00600496: a phase I, open-label, multi-center study to assess the safety, tolerability and pharmacokinetics of AZD6244 (ARRY-142886) Available at: http://www.clinicaltrials.gov.
- 109. [last accessed December 4, 2010]; NCT01198184: RO4929097 and temsirolimus in treating patients with advanced solid tumors. Available at: http://www.clinicaltrials.gov.
- 110. [last accessed December 4, 2010]; NCT01122615: sunitinib plus temsirolimus in patients with Renal Cell Cancer (RCC) Available at: http://www.clinicaltrials.gov.
- 111. [last accessed December 4, 2010]; NCT01155258: temsirolimus and vinorelbine ditartrate in treating patients with unresectable or metastatic solid tumors. Available at: http://www.clinicaltrials.gov.
- 112. [last accessed December 4, 2010]; NCT00659568: metformin and temsirolimus in treating patients with metastatic or unresectable solid tumor or lymphoma. Available at: http://www.clinicaltrials.gov.
- 113. [last accessed December 4, 2010]; NCT00538772: an exploratory correlative study of biomarkers in patients with metastatic renal cell carcinoma who have progressed after sunitinib therapy. Available at: http://www.clinicaltrials.gov.