Abstract
Molecular classification of cancers has been significantly improved patient outcomes through the implementation of treatment protocols tailored to the abnormalities present in each patient's cancer cells. Breast cancer represents the poster child with marked improvements in outcome occurring due to the implementation of targeted therapies for estrogen receptor or human epidermal growth factor receptor-2 positive breast cancers. Important subtypes with characteristic molecular features as potential therapeutic targets are likely to exist for all tumor lineages including hepatocellular carcinoma (HCC) but have yet to be discovered and validated as targets. Because each tumor accumulates hundreds or thousands of genomic and epigenetic alterations of critical genes, it is challenging to identify and validate candidate tumor aberrations as therapeutic targets or biomarkers that predict prognosis or response to therapy. Therefore, there is an urgent need to devise new experimental and analytical strategies to overcome this problem. Systems biology approaches integrating multiple data sets and technologies analyzing patient tissues holds great promise for the identification of novel therapeutic targets and linked predictive biomarkers allowing implementation of personalized medicine for HCC patients.
Keywords: Oligonucleotide array sequence analysis, Gene expression profiling, Hepatocellular carcinoma, Genomics, Systems biology, Proteomics
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers in the world, accounting for an estimated 600,000 deaths annually [1]. Although much is known about both the cellular changes that lead to HCC and the etiological agents (i.e., hepatitis B and C infections, alcohol) responsible for the majority of cases, the molecular pathogenesis of HCC is not well understood [2-4]. Moreover, the severity of HCC, the lack of useful diagnostic markers and effective treatment strategies, and the clinical heterogeneity have rendered the disease a formidable challenge in oncology [4,5]. Patients with HCC have a highly variable clinical course [3,6], indicating that HCC comprises several biologically distinct subgroups providing an opportunity for improved classification, identification of novel targets and improved outcomes.
Several clinical classification systems, including the Cancer of the Liver Italian Program, the Barcelona Clinic Liver Cancer system, the Chinese University Prognostic Index, and the Japanese Integrated Staging schema, have been developed and are currently in use [7-10]. However, clinical and pathological diagnosis and classification of HCC remain unreliable in predicting patient survival and response to therapy. The prognostic variability likely reflects a molecular heterogeneity that has not been appreciated from methods traditionally used to characterize HCC combined with a lack of a deep mechanistic understanding of the molecular mechanisms driving disease initiation and progression. Improving the classification of HCC patients into groups with homogeneous prognosis, as well as a more comprehensive understanding of the underlying biology of HCC development at the molecular level, would improve the application of currently available treatment modalities and offer the possibility of new treatment strategies.
Because of the complex nature of cancers such as HCC that are highly heterogeneous at molecular, cellular, tissue, organism, and population levels, conventional "reductionist approaches," which investigate a single gene or protein at a time, are likely to provide only limited insight into the pathological and biological characteristics. Moreover, the rapid advance of technologies that collect large amounts of data from cancer patients or tissues presents another challenge in interpretation and development of core insights into these complex systems. Systems biology, generally regarded as the "comprehensive approach," has been developed to address these issues by blending high-throughput data collection, computational and mathematical modeling, and generation of new hypotheses from emergent properties [11,12]. Emergent properties are those that are not intuitively obvious in the absence of robust and usually mathematical models. In systems biology, large networks describing the regulation of entire genomes, metabolic pathways, or signal transduction pathways are analyzed in their totality at different levels of biological organization. Thus, this approach has been used for generating new hypotheses rather than testing existing hypotheses.
One of the most exciting developments in recent years has been the clinical validation of targeted drugs that inhibit the action of pathogenic gene products such as protein kinases and proteinases [13]. Treatment with these targeted drugs has proven more efficient than conventional therapies in altering the natural history of the disease and reducing mortality for various cancers, including HCC [14-16]. However, molecular characterization of HCC aimed at identifying driver oncogenes (potential therapeutic targets) has lagged in comparison to other cancers. To improve treatment options and reduce mortality for HCC, therefore, it is crucial to develop treatment strategies that can be applied in the near future while improving our understanding of hepatocarcinogenesis.
DNA Copy-Number Alterations in HCC Genome
Since the discovery of aneuploidy, copy number aberrations and genetic rearrangements in cancer [17], cytogenetic approaches have been used extensively to uncover the chromosomal basis for genetic alterations in cancer. The comparative genomic hybridization (CGH) technique was developed in the early 1990s and was the first genomic tool to provide a genome-wide characterization of copy-number changes in cancer [18]. With improvements in microscope and labeling technologies, CGH has become a frequently used tool to examine DNA copy-number changes in cancer and to identify altered expression and function of genes residing within the affected region of the genome. Such genomic loci are believed to harbor either tumor suppressor genes or oncogenes in loci with decreased and increased copy numbers, respectively. Despite limited spatial resolution of CGH mapping, approximately 10 megabasepair (Mbp) for low copy-number gains and losses and close to 2 Mbp for high copy-number amplifications, this technology has uncovered many candidate loci for tumor suppressor genes and oncogenes in HCC. Identification of genomic loci with copy-number aberrations combined with the capacity to identify the genes residing in these loci led to a better understanding of the development of these cancers. For example, increased copy number of the 8q24 region has been reported in many studies, and the most potent oncogene residing in 8q24 is MYC [19-22]. CGH data revealed that gains of chromosomal material were most prevalent in (besides 8q) 1q, 6p, and 17q, and losses were most frequent in 8p, 16q, 4q, and 17p [19,22].
Sensitivity in detecting copy-number variations improved significantly with the emergence of microarray-based technology; in array-based CGH, arrays of genomic sequences such as BAC clones and oligonucleotides replaced metaphase chromosomes as hybridization targets [23]. Coupled with improved annotation of genome sequence data, these technologies are facilitating identification of new genomic loci that are associated with cancer progression.
Microarray-Based Technologies
The genetic or epigenetic basis of complex diseases such as cancer remained largely indefinable until completion of the human genome project and the arrival of new microarray-based technologies that have enabled investigators to describe genetic variation across the entire genome. Completion of the human genome sequence was a crucial prerequisite for cataloging our genetic makeup. However, comprehension of the sequence data alone is not sufficient to decipher the complex physiological processes in play during tumor development.
Microarrays are the technologies most frequently used now to collect data on a global scale from any biological system of interest. Recent advances in our knowledge of the chemistry of oligonucleotides and the availability of genome information helped us to miniaturize northern blots to measure thousands of gene expressions simultaneously. The microarray technology for gene expression is a quantitative assessment of the relative amount of the specific mRNA that is directly related to the biological activity of that particular gene. The amount of the mRNA transcript present in tissues can be measured indirectly after hybridization of a complementary labeled cDNA with a complementary probe that has been previously deposited on its solid surfaces. This array contains a known set of gene sequences (genome). The intensity of each labeled cDNA directly reflects the expression level of its corresponding gene. Microarray assays allow massive parallel data acquisition and analysis. Although parallelism greatly increases the speed of data collection, the massive resulting dataset presents daunting challenges to processing and interpretation.
Microarray-based gene expression profiling studies in a variety of cancers have discovered consistent gene expression patterns associated with pathological or clinical phenotypes, and have identified subtypes of cancer previously undisclosed with conventional technologies [24-26]. This new technology has been used successfully to predict clinical outcomes and survival rates and to identify potential therapeutic targets and prognostic marker genes [27-29].
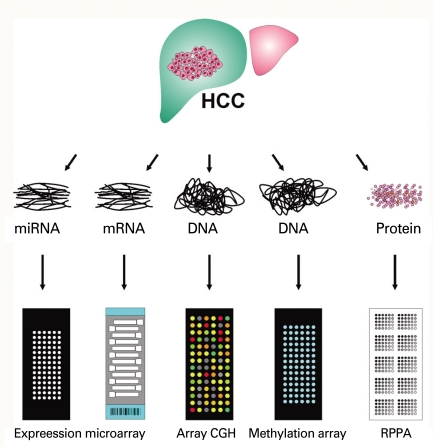
Application of this technology is not limited to collection of gene expression data from cells or tissues, but extends to identification of DNA copy numbers in cancer genomes, methylation status of gene promoters, single nucleotide polymorphisms associated with cancer risk, protein arrays, and even re-sequencing of whole genomes (Fig. 1). Array-based CGH, in which arrays of genomic sequences are used as hybridization targets, was quickly established as a substitute for conventional CGH [30,31]. The biggest advantage of array-based CGH is the ability to perform copy-number analyses with much higher resolution than was ever possible with conventional CGH, which used metaphase chromosomes as hybridization targets.
Fig. 1.
Applications of microarray-based technology. HCC, hepatocellular carcinoma; CGH, genomic hybridization; RPPA, reversephase protein array.
Protein microarrays have been developed by adopting the knowledge and technical innovations that have made DNA microarrays possible. The technical aspects of miniaturizing traditional methods, such as western blotting and protein dotting onto nitrocellulose or nylon membranes, were quickly adapted to protein microarray technology. The two approaches for producing protein microarrays are forward-phase protein array (FPPA) and reverse-phase protein array (RPPA). In a forward-phase array, antibodies are immobilized on the surface of slides and each array is incubated with one test sample such as a tissue lysate or serum sample; multiple protein features such as expression and phosphorylation from that sample are measured simultaneously. In contrast, the RPPA format immobilizes an individual tissue lysate in each array spot, and thus an array comprises thousands of different patient samples. Each array is then incubated with one antibody, and a protein feature is measured and directly compared across multiple samples. FPPA (antibody array) is particularly ill suited for tissue-based analysis since it requires a substantial amount of tissue lysate for incubation, thus RPPA (tissue lysate array) is better choice of platform in cancer research [32-35].
Next Generation Sequencing
During the last few years, there have been remarkable advances in DNA sequencing technologies, with the emergence and rapid evolution of massive parallel sequencing or 2nd generation sequencing. These technologies have dramatically reduced both cost-per-base and time of these analyses, making it possible to determine the nucleotide sequence of the human genome [36]. They provide unprecedented opportunities to examine every nucleotide sequence of the DNA from cancer cells and to compare it to that of normal cells to identify the genetic changes that occur during cancer development. Many different platforms have been developed by different companies: Life Technologies (sequencing by Oligonucleotide Ligation and Detection or SOLiD, Carlsbad, CA), Illumina (Genome Analyzer II, San Diego, CA), Roche Applied Science (454 Genome Sequencer FLX System, Indianapolis, IN), and Helicos BioSciences (HeliScope™ Single Molecule Sequencer, Cambridge, MA).
Gene Expression Profiling of HCC
Conventional approaches to the prognostic classification of HCC largely rely on single or multiple clinicopathological variables such as severity of liver impairment or characteristics of the tumor (i.e., size, number of nodules, vascular invasion, distant metastasis, and tumor differentiation grade). However, the utility of existing prognostic factors is limited because they measure tumor differentiation and bulk but do not otherwise characterize and/or measure underlying biological properties that likely dictate clinical outcomes and responses to targeted therapies.
In previous studies [26,37-40], an unbiased analytical approach applied to gene expression data from human HCC identified distinct subtypes of HCC significantly associated with patient survival. These findings suggest that gene expression profiling signatures accurately reflect biological and clinical differences between subtypes of HCC and would be highly valuable in determining patient prognosis. The current clinical challenge is to identify patients who do not derive much benefit from conventional therapies and to offer alternative treatments. If key (or master) regulators (genes, pathways, and/or networks) driving the biology of the tumor can be identified, they might lend themselves to therapeutic exploitation. In this context, it is not enough to rely entirely on gene expression signatures that are indicative of prognosis, since the profiles may fall short of explaining at the molecular level what drives the prognostic difference between subtypes of tumors.
Integration of Multiple Data Sets: Cross-species and Cross-platforms
Although the process of cancer development in humans has differences from that in mice, the similarities are particularly striking [41,42], leading many investigators to exploit the mouse as a model organism for the study of this complex disease. Previous studies provide clues on how to extend gene expression profiling studies beyond the current general practice of collecting massive data from human cancers. In an effort to identify the mouse HCC models that best mimic the human disease, gene expression data from patients were integrated with those from mouse HCC [43]. Gene expression patterns of mouse HCC were obtained from several HCC mouse models. Orthologous human and mouse genes from both datasets were selected before gene expression data were integrated. In analysis of integrated data, gene expression patterns of HCC developed in Myc, E2f1, and Myc/E2f1 transgenic mouse models had the greatest similarity with those of the longer surviving group of humans with HCC, while the expression patterns of HCC in the Myc/Tgfa transgenic mouse model were most similar to those of the poor survival group of humans with HCC. These results suggest that these two classes of mouse models might most closely recapitulate the molecular patterns of the two subclasses of human HCC.
Recent studies demonstrated that Sav1 and Mst1/2 knockout in liver leads to development of HCC [44-47], strongly indicating that MST1/2 and SAV1 are important tumor suppressors in liver. In future studies, therefore, it will be necessary to cross-compare well-defined molecular signatures of these mouse models to those from human HCC to determine the clinical relevance of inactivation of MST1/2 and SAV1 in human HCC. We anticipate that unique molecular identities of each subclass of HCC uncovered by comparative analysis of a genome-wide survey of gene expression from human and animal models will provide new therapeutic strategies to maximize the efficacy of treatments.
Cancer cells do not invent new pathways. They evolved from normal cells by using pre-existing pathways in different ways or by combining components of these pathways in a way that effectively drives tumorigenesis. By mapping and refining pathway maps in developing or normally functioning liver, gene expression profiling studies might provide insight into the connectivity of these pathways in HCC. In a previous study, the gene expression signature unique to rat fetal liver progenitor cells was integrated with those from human HCC in an attempt to determine the fraction of human HCC that shares gene expression patterns with liver progenitor cells [40]. This approach identified a novel subtype of HCC that may arise from hepatic progenitor cells. This new subtype accounts for around 20% of HCC cases examined in this study and is associated with extremely poor prognosis.
Previous studies in diffuse large B-cell lymphoma and T-cell acute lymphoblastic leukemia indicated that the cellular origins of a tumor largely dictate the clinical outcome [24,48], since mitogenic, motogenic, and morphogenic responses as well as the propensity for apoptosis may vary at different stages of normal differentiation. Genes involved in an invasive phenotype (MMP1, PLAUR, TIMP1, CD44, and VIL2) were strongly expressed in the subtype with hepatic progenitor cell features and may account for the extremely poor prognosis. This subtype showed marked activation of AP-1 complex, which is essential for normal hepatogenesis during embryonic development and critical for initiation of HCC development in mice [49,50]. Cancer cells arise from normal cells following accumulation of genetic alterations. One of the most important consequences of this process is the resurrection of pre-existing but dormant signaling pathways that were active during embryonic development [51]. Thus, this finding supports the growing appreciation that signaling pathways that control vertebrate embryonic development are also important in human carcinogenesis.
Searching for Therapeutic Targets
Many studies clearly demonstrated the gene expression signature as a utility that can classify tumors and provide prognostic information [5,26,40,43,52-54]. The current research focus has shifted toward identifying genetic determinants that are components of specific regulatory pathways altered in cancers, potentially leading to the discovery of novel therapeutic targets [4,55-57]. However, selection of relevant candidate genes for further studies from lengthy gene lists generated by gene expression profiling studies is a significant challenge due to the many confounding factors embedded in the gene expression profile data from human cancers. Moreover, since the gene expression profile from patients is only a "snapshot" of gene-to-gene interactions that lacks information on interactive time-dependent changes during tumorigenesis, it is difficult to discriminate the genes (drivers) that drive the tumorigenic process from genes (passengers) whose expression patterns simply reflect loss of organ function and/or degree of differentiation of the cancer cells.
As already discussed, CGH, and more recently array-based CGH analyses, have identified a number of recurrent regions of DNA copy-number changes in many cancers, including frequent DNA copy-number gains at 1q, 8q, and 20q, and losses at 1p, 4q, 8p, 13q, 16q, and 17p in HCC [4]. Some of these genomic loci contain well-characterized and/or putative oncogenes and tumor suppressor genes. Moreover, a number of genes in these regions have been linked to disease pathogenesis and clinical behavior. For example, associations of DNA copy-number aberrations with prognosis have been found for a variety of tumor types, including prostate cancer, breast cancer, gastric cancer, multiple myeloma, lymphomas, and HCC [57-62]. However, some amplified or deleted regions are large, and many of the genes residing in recurrent regions appeared to be silent in their expression in normal tissues as well as in tumors. Moreover, functional validation of genes residing in these loci is impractical when confronted with hundreds of candidate genomic loci. Therefore, there is an inevitable need for development of a new strategy that can overcome the limitations of gene expression data and array-based CGH data.
In a recent study in HCC, investigators tested the possibility that integrating gene expression and gene copy-number data from the same patient cohort would help identify potential driver genes [63]. The results clearly demonstrate that gene copy-number data provided extra prognostic relevance compared to when only gene expression data were available. Integrative analysis of gene expression and gene copy-number data also uncovered 50 potential drivers that are activated by recurrent gene amplifications in HCC and show an association with aggressive tumor types.
Alterations of expression patterns and genomic copy numbers of thousands of genes are fundamental properties of cancer cells. Since the application of high-throughput genomic technologies based on microarray, mass spectrometry, and 2nd generation sequencers for the analyses of cancer inevitably generates many false-positive results, it is almost impossible to select reasonable numbers of candidate genes to be further evaluated as therapeutic targets and/or biomarkers for diagnosis and prognosis. Therefore, it is important to cross-compare and integrate two or more genomic scale datasets (i.e., coding and noncoding gene expression and array-based CGH data or promoter methylation) independently collected from the same patient cohort.
Integration of Multiple-omics
While gene expression and copy-number profiling can provide important information on somatic genetic events during tumor progression, they are unable to provide an effective recapitulation of fluctuating protein-based signaling events that are the direct executors of cellular function. RPPA is a newly developed high-throughput functional proteomic technology [32-35] that provides quantitative analysis of the differential expression of signaling proteins. Moreover, the phosphorylation status of proteins can be detected and measured using specific anti-phospho-protein antibodies. Through the use of these phospho-specific antibodies, it is possible to evaluate the state of entire portions of a signaling pathway by looking at dozens of kinase substrates at the same time through multiplexed phospho-specific antibody analysis.
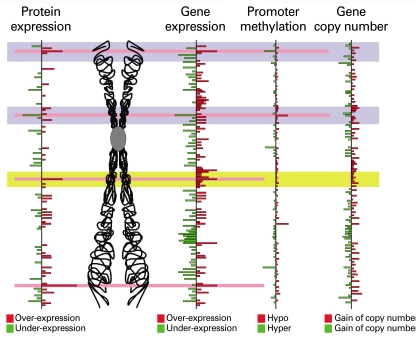
With RPPA, all samples are spotted at the same time and analyzed with a single antibody, making this method ideally suited for analysis of large numbers of specimens. However, its assessment of signaling pathways is limited by the number of available antibodies, which is far smaller than the number of gene probes in expression microarrays. This limitation of these datasets can be overcome by integrating datasets together during analysis (Fig. 2). Integration of genomic and proteomic data will undoubtedly enhance our understanding of tumor progression by increasing the dimensionality of molecular features. Moreover, identification of driver or contributor genes can be greatly accelerated by integration of more than one dimension of genomic information systems.
Fig. 2.
Integration of genomics and proteomics. Genomics data (expression, promoter methylation, and copy number of genes) or proteomics data (expression and posttranslational modification of proteins) alone provide too many candidate driver genes or proteins. Integrating these independently generated data from the same specimens greatly enhances the probability of identifying true driver genes or therapeutic targets.
Epilogue
The anticipated benefits of the systems biology approach in HCC, which has extremely heterogeneous properties (definite but various etiologies, diverse residual liver function, and heterogeneous tumor biology) would be more reliable determination of prognosis than conventional staging systems, optimized prediction of response to respective treatments, and novel identification of better therapeutic targets. In contrast to other solid tumors, many HCC develop in patients with considerably impaired organ function, which inhibits appropriate therapy and ultimately shortens survival. Therefore, it is crucial to develop a prognosis prediction system that is able to differentiate as well as integrate the impacts of liver function and tumor burden. Several distinct treatment options have been applied to HCC patients [64]: liver transplantation, resection, local ablation, transcatheter intraarterial chemoembolization, and novel targeted therapy sorafenib. Moreover, the indications for respective therapies frequently overlap, and which therapy is optimal is still being debated. Therefore, it will be necessary to develop discrimination systems that would identify the best treatment for an individual patient. Systems biology approaches with genomic and proteomics tools hold promise for personalized medicine in the management of HCC.
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Boix L, Sala M, Llovet JM. Focus on hepatocellular carcinoma. Cancer Cell. 2004;5:215–219. doi: 10.1016/s1535-6108(04)00058-3. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 4.Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- 5.Lee JS, Thorgeirsson SS. Genome-scale profiling of gene expression in hepatocellular carcinoma: classification, survival prediction, and identification of therapeutic targets. Gastroenterology. 2004;127(5 Suppl 1):S51–S55. doi: 10.1053/j.gastro.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–67. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 7.Calvet X, Bruix J, Ginés P, Bru C, Solé M, Vilana R, et al. Prognostic factors of hepatocellular carcinoma in the west: a multivariate analysis in 206 patients. Hepatology. 1990;12(4 Pt 1):753–760. doi: 10.1002/hep.1840120422. [DOI] [PubMed] [Google Scholar]
- 8.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 9.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score) J Gastroenterol. 2003;38:207–215. doi: 10.1007/s005350300038. [DOI] [PubMed] [Google Scholar]
- 10.Leung TW, Tang AM, Zee B, Lau WY, Lai PB, Leung KL, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760–1769. doi: 10.1002/cncr.10384. [DOI] [PubMed] [Google Scholar]
- 11.Faratian D, Bown JL, Smith VA, Langdon SP, Harrison DJ. Cancer systems biology. Methods Mol Biol. 2010;662:245–263. doi: 10.1007/978-1-60761-800-3_12. [DOI] [PubMed] [Google Scholar]
- 12.Kreeger PK, Lauffenburger DA. Cancer systems biology: a network modeling perspective. Carcinogenesis. 2010;31:2–8. doi: 10.1093/carcin/bgp261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sebolt-Leopold JS, English JM. Mechanisms of drug inhibition of signalling molecules. Nature. 2006;441:457–462. doi: 10.1038/nature04874. [DOI] [PubMed] [Google Scholar]
- 14.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 15.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 16.Abou-Alfa GK, Johnson P, Knox JJ, Capanu M, Davidenko I, Lacava J, et al. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA. 2010;304:2154–2160. doi: 10.1001/jama.2010.1672. [DOI] [PubMed] [Google Scholar]
- 17.Nowell PC, Hungerford DA. Chromosome studies on normal and leukemic human leukocytes. J Natl Cancer Inst. 1960;25:85–109. [PubMed] [Google Scholar]
- 18.Kallioniemi A, Kallioniemi OP, Sudar D, Rutovitz D, Gray JW, Waldman F, et al. Comparative genomic hybridization for molecular cytogenetic analysis of solid tumors. Science. 1992;258:818–821. doi: 10.1126/science.1359641. [DOI] [PubMed] [Google Scholar]
- 19.Moinzadeh P, Breuhahn K, Stützer H, Schirmacher P. Chromosome alterations in human hepatocellular carcinomas correlate with aetiology and histological grade: results of an explorative CGH meta-analysis. Br J Cancer. 2005;92:935–941. doi: 10.1038/sj.bjc.6602448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kusano N, Shiraishi K, Kubo K, Oga A, Okita K, Sasaki K. Genetic aberrations detected by comparative genomic hybridization in hepatocellular carcinomas: their relationship to clinicopathological features. Hepatology. 1999;29:1858–1862. doi: 10.1002/hep.510290636. [DOI] [PubMed] [Google Scholar]
- 21.Zimonjic DB, Keck CL, Thorgeirsson SS, Popescu NC. Novel recurrent genetic imbalances in human hepatocellular carcinoma cell lines identified by comparative genomic hybridization. Hepatology. 1999;29:1208–1214. doi: 10.1002/hep.510290410. [DOI] [PubMed] [Google Scholar]
- 22.Poon TC, Wong N, Lai PB, Rattray M, Johnson PJ, Sung JJ. A tumor progression model for hepatocellular carcinoma: bioinformatic analysis of genomic data. Gastroenterology. 2006;131:1262–1270. doi: 10.1053/j.gastro.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Pinkel D, Albertson DG. Comparative genomic hybridization. Annu Rev Genomics Hum Genet. 2005;6:331–354. doi: 10.1146/annurev.genom.6.080604.162140. [DOI] [PubMed] [Google Scholar]
- 24.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 25.Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406:536–540. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- 26.Lee JS, Chu IS, Heo J, Calvisi DF, Sun Z, Roskams T, et al. Classification and prediction of survival in hepatocellular carcinoma by gene expression profiling. Hepatology. 2004;40:667–676. doi: 10.1002/hep.20375. [DOI] [PubMed] [Google Scholar]
- 27.Beer DG, Kardia SL, Huang CC, Giordano TJ, Levin AM, Misek DE, et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nat Med. 2002;8:816–824. doi: 10.1038/nm733. [DOI] [PubMed] [Google Scholar]
- 28.van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 29.van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 30.Pinkel D, Segraves R, Sudar D, Clark S, Poole I, Kowbel D, et al. High resolution analysis of DNA copy number variation using comparative genomic hybridization to microarrays. Nat Genet. 1998;20:207–211. doi: 10.1038/2524. [DOI] [PubMed] [Google Scholar]
- 31.Pollack JR, Perou CM, Alizadeh AA, Eisen MB, Pergamenschikov A, Williams CF, et al. Genome-wide analysis of DNA copy-number changes using cDNA microarrays. Nat Genet. 1999;23:41–46. doi: 10.1038/12640. [DOI] [PubMed] [Google Scholar]
- 32.Sheehan KM, Calvert VS, Kay EW, Lu Y, Fishman D, Espina V, et al. Use of reverse phase protein microarrays and reference standard development for molecular network analysis of metastatic ovarian carcinoma. Mol Cell Proteomics. 2005;4:346–355. doi: 10.1074/mcp.T500003-MCP200. [DOI] [PubMed] [Google Scholar]
- 33.Espina V, Mehta AI, Winters ME, Calvert V, Wulfkuhle J, Petricoin EF, 3rd, et al. Protein microarrays: molecular profiling technologies for clinical specimens. Proteomics. 2003;3:2091–2100. doi: 10.1002/pmic.200300592. [DOI] [PubMed] [Google Scholar]
- 34.Haab BB. Antibody arrays in cancer research. Mol Cell Proteomics. 2005;4:377–383. doi: 10.1074/mcp.M500010-MCP200. [DOI] [PubMed] [Google Scholar]
- 35.Liotta LA, Espina V, Mehta AI, Calvert V, Rosenblatt K, Geho D, et al. Protein microarrays: meeting analytical challenges for clinical applications. Cancer Cell. 2003;3:317–325. doi: 10.1016/s1535-6108(03)00086-2. [DOI] [PubMed] [Google Scholar]
- 36.Davey JW, Hohenlohe PA, Etter PD, Boone JQ, Catchen JM, Blaxter ML. Genome-wide genetic marker discovery and genotyping using next-generation sequencing. Nat Rev Genet. 2011;12:499–510. doi: 10.1038/nrg3012. [DOI] [PubMed] [Google Scholar]
- 37.Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woo HG, Park ES, Cheon JH, Kim JH, Lee JS, Park BJ, et al. Gene expression-based recurrence prediction of hepatitis B virus-related human hepatocellular carcinoma. Clin Cancer Res. 2008;14:2056–2064. doi: 10.1158/1078-0432.CCR-07-1473. [DOI] [PubMed] [Google Scholar]
- 39.Ye QH, Qin LX, Forgues M, He P, Kim JW, Peng AC, et al. Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nat Med. 2003;9:416–423. doi: 10.1038/nm843. [DOI] [PubMed] [Google Scholar]
- 40.Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12:410–416. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- 41.Van Dyke T, Jacks T. Cancer modeling in the modern era: progress and challenges. Cell. 2002;108:135–144. doi: 10.1016/s0092-8674(02)00621-9. [DOI] [PubMed] [Google Scholar]
- 42.Klausner RD. Studying cancer in the mouse. Oncogene. 1999;18:5249–5252. doi: 10.1038/sj.onc.1203089. [DOI] [PubMed] [Google Scholar]
- 43.Lee JS, Chu IS, Mikaelyan A, Calvisi DF, Heo J, Reddy JK, et al. Application of comparative functional genomics to identify best-fit mouse models to study human cancer. Nat Genet. 2004;36:1306–1311. doi: 10.1038/ng1481. [DOI] [PubMed] [Google Scholar]
- 44.Lu L, Li Y, Kim SM, Bossuyt W, Liu P, Qiu Q, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci U S A. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee KP, Lee JH, Kim TS, Kim TH, Park HD, Byun JS, et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:8248–8253. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song H, Mak KK, Topol L, Yun K, Hu J, Garrett L, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci U S A. 2010;107:1431–1436. doi: 10.1073/pnas.0911409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferrando AA, Neuberg DS, Staunton J, Loh ML, Huard C, Raimondi SC, et al. Gene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemia. Cancer Cell. 2002;1:75–87. doi: 10.1016/s1535-6108(02)00018-1. [DOI] [PubMed] [Google Scholar]
- 49.Eferl R, Ricci R, Kenner L, Zenz R, David JP, Rath M, et al. Liver tumor development. c-Jun antagonizes the proapoptotic activity of p53. Cell. 2003;112:181–192. doi: 10.1016/s0092-8674(03)00042-4. [DOI] [PubMed] [Google Scholar]
- 50.Hilberg F, Aguzzi A, Howells N, Wagner EF. c-jun is essential for normal mouse development and hepatogenesis. Nature. 1993;365:179–181. doi: 10.1038/365179a0. [DOI] [PubMed] [Google Scholar]
- 51.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 52.Lee JS, Thorgeirsson SS. Functional and genomic implications of global gene expression profiles in cell lines from human hepatocellular cancer. Hepatology. 2002;35:1134–1143. doi: 10.1053/jhep.2002.33165. [DOI] [PubMed] [Google Scholar]
- 53.Lee JS, Grisham JW, Thorgeirsson SS. Comparative functional genomics for identifying models of human cancer. Carcinogenesis. 2005;26:1013–1020. doi: 10.1093/carcin/bgi030. [DOI] [PubMed] [Google Scholar]
- 54.Lee JS, Thorgeirsson SS. Genetic profiling of human hepatocellular carcinoma. Semin Liver Dis. 2005;25:125–132. doi: 10.1055/s-2005-871192. [DOI] [PubMed] [Google Scholar]
- 55.Thorgeirsson SS, Lee JS, Grisham JW. Functional genomics of hepatocellular carcinoma. Hepatology. 2006;43(2 Suppl 1):S145–S150. doi: 10.1002/hep.21063. [DOI] [PubMed] [Google Scholar]
- 56.Thorgeirsson SS, Lee JS, Grisham JW. Molecular prognostication of liver cancer: end of the beginning. J Hepatol. 2006;44:798–805. doi: 10.1016/j.jhep.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 57.Thorgeirsson SS. Genomic decoding of hepatocellular carcinoma. Gastroenterology. 2006;131:1344–1346. doi: 10.1053/j.gastro.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 58.Paris PL, Andaya A, Fridlyand J, Jain AN, Weinberg V, Kowbel D, et al. Whole genome scanning identifies genotypes associated with recurrence and metastasis in prostate tumors. Hum Mol Genet. 2004;13:1303–1313. doi: 10.1093/hmg/ddh155. [DOI] [PubMed] [Google Scholar]
- 59.Chin K, de Solorzano CO, Knowles D, Jones A, Chou W, Rodriguez EG, et al. In situ analyses of genome instability in breast cancer. Nat Genet. 2004;36:984–988. doi: 10.1038/ng1409. [DOI] [PubMed] [Google Scholar]
- 60.Weiss MM, Kuipers EJ, Postma C, Snijders AM, Pinkel D, Meuwissen SG, et al. Genomic alterations in primary gastric adenocarcinomas correlate with clinicopathological characteristics and survival. Cell Oncol. 2004;26:307–317. doi: 10.1155/2004/454238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carrasco DR, Tonon G, Huang Y, Zhang Y, Sinha R, Feng B, et al. High-resolution genomic profiles define distinct clinico-pathogenetic subgroups of multiple myeloma patients. Cancer Cell. 2006;9:313–325. doi: 10.1016/j.ccr.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 62.Rubio-Moscardo F, Climent J, Siebert R, Piris MA, Martin-Subero JI, Nieländer I, et al. Mantle-cell lymphoma genotypes identified with CGH to BAC microarrays define a leukemic subgroup of disease and predict patient outcome. Blood. 2005;105:4445–4454. doi: 10.1182/blood-2004-10-3907. [DOI] [PubMed] [Google Scholar]
- 63.Woo HG, Park ES, Lee JS, Lee YH, Ishikawa T, Kim YJ, et al. Identification of potential driver genes in human liver carcinoma by genomewide screening. Cancer Res. 2009;69:4059–4066. doi: 10.1158/0008-5472.CAN-09-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bruix J, Sherman M Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]