Abstract
Purpose
The aims of this study are to find out whether the sequence of chemotherapeutic regimens including second- and third-line taxane and irinotecan influences the survival of patients with unresectable gastric carcinoma and to identify clinical characteristics of patients with improved response.
Materials and Methods
Fifty gastric carcinoma patients who were treated by third-line sequential chemotherapy between November 2004 and July 2010 were enrolled in this study. Their overall survival (OS) and time to progression (TTP) were set up as primary and secondary end points. For the sequence of chemotherapy regimen, two arms were used. Arm A was defined as 5-fluorouracil (5-FU)+cisplatin (FP) or folinic acid, 5-FU and oxaliplati (FOLFOX), followed by folinic acid, 5-FU and irinotecan (FOLFIRI), and paclitaxel or docetaxel plus 5-FU, with or without epirubicin. Arm B was defined as FP or FOLFOX, followed by paclitaxel or docetaxel plus 5-FU, and FOLFIRI.
Results
The median OS of all patients was 16.0 months (95% confidence interval, 13.6 to 18.3 months), which is longer than historical control of patients who did not receive third-line chemotherapy. The sequence of second and third-line regimen, including irinotecan and taxane, did not present significant difference in OS or TTP after failure of 5-FU with platinum chemotherapy. In survival analysis of patients' clinicopathologic characteristics, poor prognosis was shown in patients with poorly differentiated histologic features, elevated serum carcinoembryonic level, and shorter TTP of first line chemotherapy.
Conclusion
It is possible for patients to respond differently to chemotherapy due to differences in clinical features and underlying gene expression profiles. Development of individualized chemotherapy regimens based on gene expression profiles is warranted.
Keywords: Stomach neoplasms, Salvage therapy, Prognosis, Oxaliplatin, Irinotecan, Taxane
Introduction
Gastric cancer is the fourth most common malignancy worldwide [1], and most common malignancy in Korea [2]. Although survival time has been lengthened, the life expectancy of advanced disease still remains within one year.
For patients with advanced gastric carcinoma or recurrent cancer, the response rate (RR) for first-line chemotherapy ranges from 33-55%, and the survival time following chemotherapy is superior to that of patients given best supportive care [3]. Despite poorer survival benefit and tolerance of second-line chemotherapy, about 20% of patients with failure of first-line chemotherapy receive second-line chemotherapy [4]. Asian studies have reported a RR of second-line chemotherapy as 5-25% and progression-free survival (PFS) of 5.2-8.8 months [5,6]. Many clinicians consider second-line chemotherapy after failure of first-line chemotherapy for patients with advanced gastric carcinoma.
There is a controversy about the benefit of sequential salvage therapy further to second-line. However, with prolonged survival time of patients and tolerable toxicity of drugs, there has been renewed interest in chemotherapy after failure of second-line treatments. It has been our empiric observation that some patients maintain good performance after failure of second-line chemotherapy, and live longer with salvage chemotherapy than other patients with supportive care. However, few studies have been conducted on third-line and sequential therapy.
The aim of the present study was to clarify the relationship between the sequence of second- and third-line chemotherapy regimen including taxane and irinotecan with survival of patients, and to identify clinical characteristics of patients with better response to salvage chemotherapy than others.
Materials and Methods
1. Patients
We retrospectively evaluated the medical records of advanced or recurred gastric carcinoma patients who were diagnosed and treated at Gangnam Severance Hospital between November 2004 and July 2010. Patients who met the following criteria were eligible: histologically proven advanced gastric adenocarcinoma with metastatic or recurrent disease; received third-line chemotherapy at Gangnam Severance Hospital using folinic acid, 5-fluorouracil (5-FU) and oxaliplatin (FOLFOX) or 5-FU+cisplatin (FP), folinic acid, 5-FU and irinotecan (FOLFIRI), paclitaxel or docetaxel with 5-FU, with or without epirubicin; measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) or a non-measurable lesion, assessed using either computed tomography (CT) or magnetic resonance imaging; 18-75 years of age; Eastern Cooperative Oncology Group (ECOG) performance status≤2; life expectancy of at least 3 months; and adequate hematologic function, evident as absolute neutrophil count≥1500/µL, platelet count≥100,000/µL, hepatic function (total bilirubin≤2 times the upper normal limit [UNL], serum transaminase level≤2 times the UNL) and renal function (serum creatine≤1.5 times the UNL). Exclusion criteria were the presence of other severe medical illness, central nervous system metastasis, another active malignancy, or history of anaphylaxis to drugs. The protocols were reviewed and approved by Yonsei University Health System Institutional Review Board.
Clinical information included gender, age at diagnosis, ECOG performance status, pathologic differentiation, Lauren classification, coexisting peritoneal or hepatic metastases, date of chemotherapy, and number of cycles. In addition, laboratory data including hemoglobin, serum albumin, carcinoembryonic antigen (CEA), and cancer antigen (CA) 19-9 were also reviewed. Tumor responses were evaluated according to the RECIST guidelines.
2. Treatment
Sequential chemotherapy was defined as chemotherapy using more than two different chemotherapeutic regimens performed consecutively. Patients were assigned to either arm A or arm B, according to the sequence of three chemotherapeutic regimens. Arm A comprised FOLFOX or FP for first-line chemotherapy; FOLFIRI for second-line chemotherapy; and docetaxel or paclitaxel, leucovorin, and 5-FU (with or without epirubicin) for third-line chemotherapy. In arm B, patients were treated with FOLFOX or FP for first-line chemotherapy; docetaxel or paclitaxel, leucovorin, and FU with or without epirubicin for second-line chemotherapy; and FOLFIRI for third-line chemotherapy.
1) FOLFOX chemotherapy
Patients received oxaliplatin (100 mg/m2 in 500 mL normal saline) or 5% dextrose water over 2 hours followed by leucovorin (100 mg/m2) over 2 hours on day 1 and continuous infusion of 5-FU 1,000 mg/m2/day for 2 days. The cycle was repeated every two weeks.
2) FP chemotherapy
Cisplatin (70 mg/m2 in 500 mL normal saline) or 5% dextrose water was intravenously administered over 2 hours on day 1 followed by continuous infusion of 5-FU (1,000 mg/m2/day) for 2 days. The cycle was repeated every three weeks.
3) FOLFIRI chemotherapy
Irinotecan (150 mg/m2 in 500 mL normal saline) was intravenously administered over 2 hours on followed by leucovorin (100 mg/m2 over 2 hours) on day 1 and 5-FU (1,000 mg/m2/day) as a continuous infusion for 2 days. The cycle was repeated every two weeks.
4) Docetaxel or paclitaxel, leucovorin, and 5-FU chemotherapy, with or without epirubicin
Docetaxel (75 mg/m2 in 500 mL normal saline) or paclitaxel (175 mg/m2 in 500 mL normal saline) was intravenously administered over 2 hours followed by leucovorin (100 mg/m2 over 2 hours) on day 1 and 5-FU (1,000 mg/m2/day) as a continuous infusion for 2 days. The cycle was repeated every 3 weeks. For some patients, epirubicin (50 mg/m2 in 100 mL normal saline) was intravenously administered over 30 minutes on day 2.
5) Efficacy
Response was assessed after two cycles of chemotherapy and when disease progression was clinically suspected. A measurable lesion was defined as a lesion with the longest diameter ≥ 10 mm in any dimension assessed by spiral CT imaging. Tumor response was evaluated according to the RECIST guidelines as follows: complete response (CR) if all target lesions disappeared, partial response (PR) if the sum of diameters of the target lesions decreased by at least 30%, progressive disease (PD) if the sum of the longest diameters of the target lesions increased by at least 20% and one or more new lesions appeared, and stable disease (SD) for responses that were neither PR nor PD. The assessment was done by CT scan. The RR was defined as proportion of the patients with responses of CR or PR out of all patients.
3. Statistical analyses
Overall survival (OS) was defined as the interval from the first date of chemotherapy to death or the last follow-up date. Time to progression (TTP) was defined as the time from the first date of chemotherapy to the time of progression of the disease or the last follow-up date. The Kaplan-Meier method was used for survival analysis, and survival curves were compared assessed using the log-rank test. Following variables were included in univariate analysis: age at diagnosis, gender, performance status by ECOG criteria, pathologic differentiation, coexisting peritoneal or hepatic metastases, type of chemotherapeutic regimen, hemoglobin, serum albumin, CEA, CA19-9, and TTP of first- or second-line chemotherapy. For variables with a probability p-value≤0.3 in univariate analysis, multivariate analysis was also performed using the Cox's proportional hazard regression model (two-sided). All statistical tests were two-sided, with p-values≤0.05 considered significant.
Results
1. Baseline characteristics
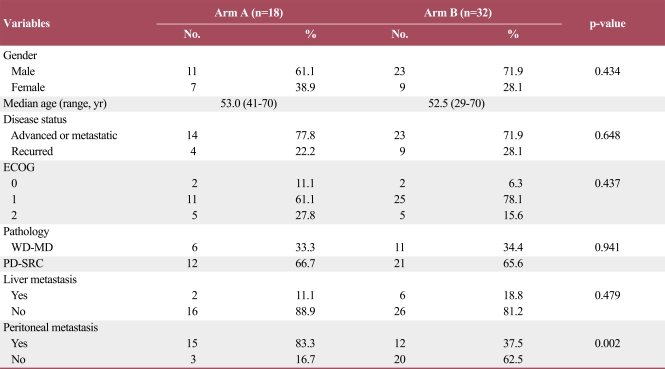
Between November 2004 and July 2010, 50 patients with gastric carcinoma were treated with a sequential chemotherapy protocol, based only on FOLFOX or FP, FOLFIRI, and paclitaxel or docetaxel, at Gangnam Severance Hospital. The baseline characteristics of the patients are presented in Table 1. The median age was 52.5 years (range, 29 to 70 years), and 34 patients (68%) were male. Forty subjects (80%) had good performance status (ECOG 0-1). Seven patients had diffuse type, 13 patients had interstitial type, and the other two patients had mixed type adenocarcinoma. No significant difference except for peritoneal carcinomatosis was observed.
Table 1.
Baseline characteristics of the patients
ECOG, Eastern Cooperative Oncology Group; WD-MD, adenocarcinoma, well differentiated and adenocarcinoma, moderate differentiated; PDSRC, adenocarcinoma, poorly differentiated and signet ring cell carcinoma. a)p-values from Pearson's χ2 test, except for age (Kruskal-Walis test).
2. Treatment outcomes
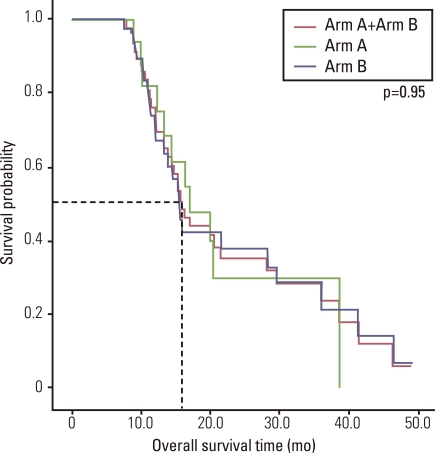
Fifty patients received median fourth-line chemotherapy (range, 3 to 10), and a median of 18 cycles (range, 4 to 60 cycles) were administered. Median OS of all patients was 16.0 months (95% confidence interval [CI], 13.6 to 18.3 months) (Fig. 1), and median TTP was 5.0 months (95% CI, 3.9 to 6.1 months) in first-line chemotherapy, 2.4 months (95% CI, 1.6 to 3.2 months) in second-line chemotherapy, and 2.5 months (95% CI, 1.8 to 3.3 months) in third-line chemotherapy.
Fig. 1.
Overall survival by treatment Arm.
The median OS time of arm A and arm B was 17.1 months (95% CI, 10.7 to 23.6 months), and 15.4 months (range, 13.8 to 17.1 months), respectively. The difference was not significant (p=0.950) (Fig. 1).
TTP of arm A and arm B with first-line chemotherapy was 5.5 months (95% CI, 1.2 to 9.8 months) and 4.8 months (95% CI, 3.8 to 5.8 months), respectively (p=0.064). For the second-line chemotherapy, TTP of arm A and arm B was 2.7 months and 2.2 months, respectively (p=0.850). Each TTP of the two groups were 2.2 months in arm A and 2.7 months in arm B (p=0.297). These data did not show significant differences.
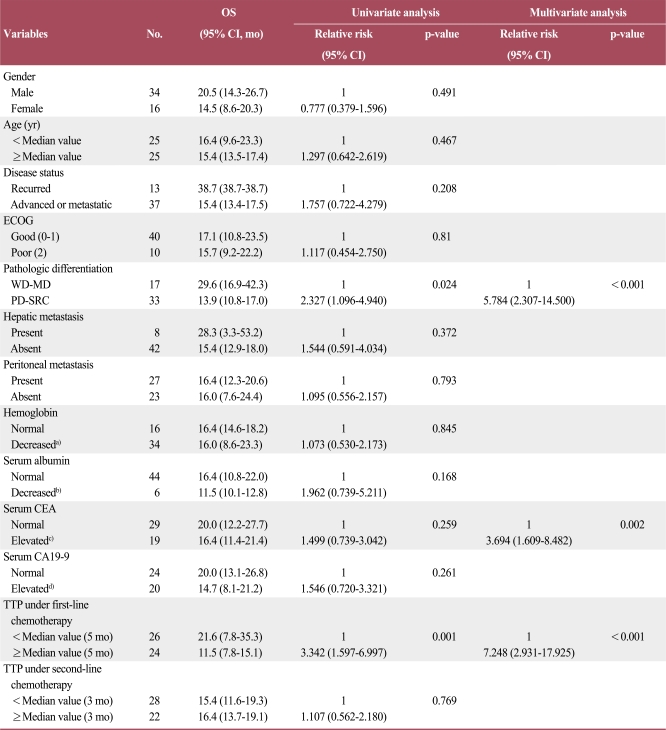
In addition, we analyzed clinicopathologic characteristics of the patients. Patients with poorly-differentiated adenocarcinoma or signet ring cell feature showed poorer prognosis than others. Furthermore, elevated CEA level and shorter TTP of first-line chemotherapy were significantly related with shorter survival times (Table 2).
Table 2.
Survival analysis according to baseline characteristics
OS, overall survival; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; WD-MD, adenocarcinoma, well differentiated and adenocarcinoma, moderate differentiated; PD-SRC, adenocarcinoma, poorly differentiated and signet ring cell carcinoma; CEA, carcinoembryonic antigen; CA, cancer antigen; TTP, time to progression. a)Hemoglobin≤13 g/dL in men, ≤12 g/dL in woman, b)Serum albumin≤3.4 g/dL, c)Serum CEA≥5 ng/mL, d)Serum CA19-9≥35 U/mL.
3. Efficacy
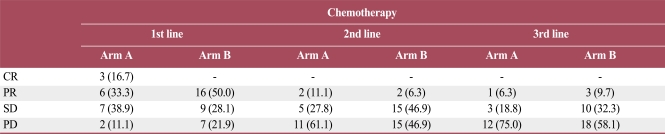
The RR was 50% for first-line chemotherapy, 8% for second-line chemotherapy, and 8.5% for third-line chemotherapy. RR of first-line chemotherapy was 50% in both arms. In second-line chemotherapy, RR was 11.1% in arm A and 6.3% in arm B. In third-line chemotherapy, RR was 6.3% in arm A and 9.7% in arm B. The disease control rate (DCR) which was determined by the disease status (CR, PR, or SD) controlled by treatment, of first-line chemotherapy was 88.9% in arm A and 78.1% in arm B (p=0.342). In second-line chemotherapy, DCR of arm A was 38.9%, and that of arm B was 53.1% (p=0.333). In third-line chemotherapy, DCR was 25% in arm A and 41.9% in arm B (p=0.252). These results showed no significant differences (Table 3).
Table 3.
Response to sequential chemotherapy of the two groups
Values are presented as number (%). CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
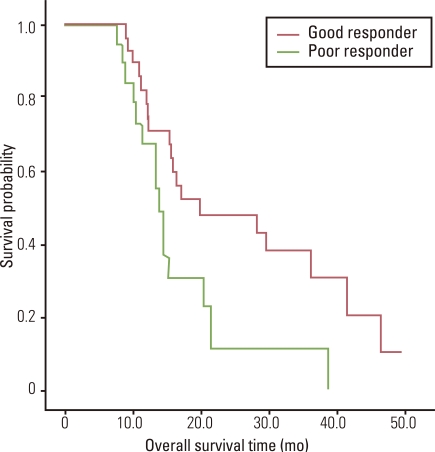
Twenty-one patients did not show any response (PR or CR) to any of the three sequential chemotherapies. They were grouped as poor-responders. The other 29 patients were classified as good-responders. The median OS was 20.0 months (95% CI, 2.4 to 37.5 months) in the good-responders and 13.9 months (95% CI, 12.0 to 15.8 months) in the poor-responders. The difference was significant (p=0.028) (Fig. 2).
Fig. 2.
Overall survival by response to chemotherapy.
Discussion
It has generally been accepted that palliative chemotherapy can significantly prolong survival of patients with advanced gastric carcinoma, longer than best supportive care [7]. Platinum-based chemotherapy is widely-used as first-line chemotherapy for advanced gastric cancer. Given the reported tolerance and survival benefits of second-line chemotherapy [4], studies have sought to identify the benefit and adequate regimen of second-line chemotherapy.
The current study evaluated two groups of gastric cancer patients who received third-line chemotherapy. Previous studies reported the median PFS of 2.5-3.3 months and the median OS of 5.3-8.7 months in gastric cancer patients who received second-line chemotherapy [6,8-10]. In this study, the median PFS was 2.4 months (95% CI, 1.6 to 3.2 months) for patients treated with second-line chemotherapy, similar to the previous report. The median OS was 16.0 months (95% CI, 13.6 to 18.3 months), and 32.0% of patients survived after 2 years. These times were slightly longer than that of historical control. We suggest that this is due to sequential treatment of the patients.
This study included a restricted population with a performance status that permitted the use of third-line chemotherapy after failure of second-line chemotherapy. Therefore, this result was subject to limitation in comparison with other survival data of patients without third-line chemotherapy. Nevertheless, based on 2.5 months of median TTP in third-line chemotherapy, the authors suggest that third-line chemotherapy would provide a survival benefit for patients with favorable performance status.
In many phase II clinical trials of second-line chemotherapy for advanced gastric cancer, RR was about 20.8%, and DCR was about 51% [4]. In the present study, RR for second-line chemotherapy was 8% and DCR was 48%. Considering only third-line chemotherapy, RR was 8.5% and DCR 36.2%.
There is still little information to support the provision of a second-line chemotherapeutic regimen after failure of platinum-based first-line chemotherapy.
Satoh et al. [11] reported that sequential chemotherapy appears to be both a feasible and effective treatment for advanced gastric cancer. Their sequential protocol was S-1 based chemotherapy for first-line chemotherapy, a low dose irinotecan/cisplatin for second-line chemotherapy, and paclitaxel for third-line chemotherapy. Thirty-two gastric cancer patients showed relatively long median survival (17.4 months; 95% CI, 323 to 723 days). This result shows that a consecutive use of chemotherapy has a substantial impact on OS of gastric cancer patients. These facts also demonstrate that second- and third-line chemotherapy is necessary to treat advanced gastric cancer.
Many clinicians consider the FOLFIRI regimen and paclitaxel or docetaxel with 5-FU regimen as second-line chemotherapy after failure of platinum-based first line chemotherapy [4]. Numerous physicians suggested that FOLFIRI is tolerable and has a modest effect as a second-line chemotherapy for gastric carcinoma. In an Asian study, the median PFS was 3.2 months, and the median OS was 9.1 months [12]. In another study, RR was 18.2%, median OS was 5.1 months, and median TTP was 2.3 months [13]. On the other hand, the tolerance and effectiveness of taxane-based chemotherapy apparent in several studies (median TTP, 2.6-3.9 months; median OS, 7.2 to 10.1 months) warrants its use as second-line chemotherapy in advanced gastric carcinoma [10,14].
Kim et al. [15] reported that overall RR and DCR for patients with docetaxel/cisplatin followed by FOLFIRI and patients with FOLFIRI followed by a docetaxel/cisplatin regimen showed no significant difference. However, to the best of our knowledge, survival benefits according to sequence of regimens of second-line and third-line chemotherapy after failure of first line chemotherapy have not been reported.
In this study, we analyzed 50 patients treated with either FOLFOX or FP chemotherapy followed by FOLFIRI, and paclitaxel or docetaxel, or FOLFOX or FP chemotherapy followed by paclitaxel or docetaxel, and FOLFIRI regimen. The median OS and TTP showed no difference regardless of sequence. However, we found some patients responded better to chemotherapy, and their OS was longer than the others. Previous investigators suggested that low hemoglobin level [8,9], low serum albumin level [16,17], high CEA level [17], poor performance status [8-10,16,17], shorter TTP of first-line chemotherapy [9,17], shorter TTP of second-line chemotherapy [16], and poor differentiation of tumor [10,16] are poor prognostic factors for patients with gastric carcinoma under sequential chemotherapy. We analyzed the previously reported factors and found some differences in survival according to histologic differentiation, elevated CEA level, and TTP of first line chemotherapy in this study.
There has been a controversy regarding the influence of the histological feature of signet ring cell on the prognosis of gastric carcinoma. Some researchers reported poorer prognosis of advanced gastric carcinoma with signet ring cell histology [16,18], while better prognosis of early gastric carcinoma with signet ring cell histology has been reported [19]. In this study, gastric adenocarcinoma with poorly differentiated or signet ring cell features had poorer response to sequential chemotherapy, and had shorter median OS than well or moderately differentiated ones. Concerning CEA level, some investigators reported the relationship of serum CEA level with tumor invasion [20], lymph node metastasis, and higher recurrence rate [21].
Herein, we suggest that differences in gene and protein expressions are other prognostic values that should be taken into account. In a recent study, the regulation of different pathways in histologically different carcinomas was reported [22]. We suppose that there are some genetic differences in diffuse gastric adenocarcinoma and that the different genetic defect influences a poorer response to sequential chemotherapy. Furthermore, CEA is a widely used tumor marker, and the CEA gene has been widely-used as a tumor-specific promoter and target of treatment over the last decade [23].
We propose that the difference in response could also be attributed to the differences in gene expression profiles between the two groups. In recent years, it has become clear that a pharmacogenic approach is a potential tool for optimizing treatment for several human tumors [24]. The ability of genetic polymorphisms to influence pharmacogenetics of 5-FU, platinum derivatives, anthracyclines, irinotecan, and docetaxel in gastric cancer has been reported. The use of multiple gene analyses in the development of individualized gastric cancer chemotherapies could be helpful in selecting patients who are more likely to benefit better to a particular therapy. The possible prediction of cancer outcome from gene expression classifiers, sets of genes, or signatures associated with prognosis together with classification rules has been suggested [25].
Limitations of this study include the retrospective study design and small patient pool. Nevertheless, the results of this study provides evidence that third-line chemotherapy, including FOLFOX or FP, FOLFIRI, paclitaxel or docetaxel regimen, helps to improve OS of patients, regardless of the sequence of use of taxane and irinotecan. In addition, OS seems to be poorly affected by poorly differentiated or signet ring cell feature of adenocarcinoma, elevated CEA level, and shorter TTP of first-line chemotherapy.
Conclusion
Different chemotherapy-responses to locally advanced or metastatic gastric carcinoma treated with second and third-line chemotherapy using irinotecan or taxane could be attributed not to sequence of chemotherapeutic regimen, but to patients' different clinical features underlying different gene expression profiles. Development of individualized chemotherapy-based regimens based on multiple gene analysis is warranted. Future studies will be required to investigate the impact of genetic difference on response to sequential chemotherapy.
Acknowledgments
This study was supported by research fund from Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (No. 2010-0024248).
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Park EC, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat. 2011;43:1–11. doi: 10.4143/crt.2011.43.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ajani JA. Evolving chemotherapy for advanced gastric cancer. Oncologist. 2005;10(Suppl 3):49–58. doi: 10.1634/theoncologist.10-90003-49. [DOI] [PubMed] [Google Scholar]
- 4.Wilson D, Hiller L, Geh JI. Review of second-line chemotherapy for advanced gastric adenocarcinoma. Clin Oncol (R Coll Radiol) 2005;17:81–90. doi: 10.1016/j.clon.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Moon YW, Rha SY, Jeung HC, Kim C, Hong MH, Chang H, et al. Outcomes of multiple salvage chemotherapy for advanced gastric cancer: implications for clinical practice and trial design. Cancer Chemother Pharmacol. 2010;66:797–805. doi: 10.1007/s00280-010-1295-z. [DOI] [PubMed] [Google Scholar]
- 6.Jeong J, Jeung HC, Rha SY, Im CK, Shin SJ, Ahn JB, et al. Phase II study of combination chemotherapy of 5-fluorouracil, low-dose leucovorin, and oxaliplatin (FLOX regimen) in pretreated advanced gastric cancer. Ann Oncol. 2008;19:1135–1140. doi: 10.1093/annonc/mdn013. [DOI] [PubMed] [Google Scholar]
- 7.Glimelius B, Ekström K, Hoffman K, Graf W, Sjödén PO, Haglund U, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8:163–168. doi: 10.1023/a:1008243606668. [DOI] [PubMed] [Google Scholar]
- 8.Ji SH, Lim do H, Yi SY, Kim HS, Jun HJ, Kim KH, et al. A retrospective analysis of secondline chemotherapy in patients with advanced gastric cancer. BMC Cancer. 2009;9:110. doi: 10.1186/1471-2407-9-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanagavel D, Pokataev IA, Fedyanin MY, Tryakin AA, Bazin IS, Narimanov MN, et al. A prognostic model in patients treated for metastatic gastric cancer with second-line chemotherapy. Ann Oncol. 2010;21:1779–1785. doi: 10.1093/annonc/mdq032. [DOI] [PubMed] [Google Scholar]
- 10.Jo JC, Lee JL, Ryu MH, Sym SJ, Lee SS, Chang HM, et al. Docetaxel monotherapy as a second-line treatment after failure of fluoropyrimidine and platinum in advanced gastric cancer: experience of 154 patients with prognostic factor analysis. Jpn J Clin Oncol. 2007;37:936–941. doi: 10.1093/jjco/hym123. [DOI] [PubMed] [Google Scholar]
- 11.Satoh S, Kawashima K, Matsumoto S, Hasegawa S, Okabe H, Nomura A, et al. Retrospective evaluation of sequential outpatient chemotherapy for advanced gastric cancer. Chemotherapy. 2007;53:226–232. doi: 10.1159/000100865. [DOI] [PubMed] [Google Scholar]
- 12.Seo MD, Lee KW, Lim JH, Yi HG, Kim DY, Oh DY, et al. Irinotecan combined with 5-fluorouracil and leucovorin as second-line chemotherapy for metastatic or relapsed gastric cancer. Jpn J Clin Oncol. 2008;38:589–595. doi: 10.1093/jjco/hyn078. [DOI] [PubMed] [Google Scholar]
- 13.Kim SH, Lee GW, Go SI, Cho SH, Kim HJ, Kim HG, et al. A phase II study of irinotecan, continuous 5-fluorouracil, and leucovorin (FOLFIRI) combination chemotherapy for patients with recurrent or metastatic gastric cancer previously treated with a fluoropyrimidine-based regimen. Am J Clin Oncol. 2010;33:572–576. doi: 10.1097/COC.0b013e3181bead7b. [DOI] [PubMed] [Google Scholar]
- 14.Takiuchi H, Goto M, Imamura H, Furukawa H, Imano M, Imamoto H, et al. Multi-center phase II study for combination therapy with paclitaxel/doxifluridine to treat advanced/recurrent gastric cancer showing resistance to S-1 (OGSG 0302) Jpn J Clin Oncol. 2008;38:176–181. doi: 10.1093/jjco/hyn003. [DOI] [PubMed] [Google Scholar]
- 15.Kim JA, Lee J, Han B, Park SH, Park JO, Park YS, et al. Docetaxel/cisplatin followed by FOLFIRI versus the reverse sequence in metastatic gastric cancer. Cancer Chemother Pharmacol. 2011;68:177–184. doi: 10.1007/s00280-010-1452-4. [DOI] [PubMed] [Google Scholar]
- 16.Shim HJ, Yun JY, Hwang JE, Bae WK, Cho SH, Chung IJ. Prognostic factor analysis of thirdline chemotherapy in patients with advanced gastric cancer. Gastric Cancer. 2011;14:249–256. doi: 10.1007/s10120-011-0032-6. [DOI] [PubMed] [Google Scholar]
- 17.Catalano V, Graziano F, Santini D, D'Emidio S, Baldelli AM, Rossi D, et al. Second-line chemotherapy for patients with advanced gastric cancer: who may benefit? Br J Cancer. 2008;99:1402–1407. doi: 10.1038/sj.bjc.6604732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piessen G, Messager M, Leteurtre E, Jean-Pierre T, Mariette C. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg. 2009;250:878–887. doi: 10.1097/SLA.0b013e3181b21c7b. [DOI] [PubMed] [Google Scholar]
- 19.Hyung WJ, Noh SH, Lee JH, Huh JJ, Lah KH, Choi SH, et al. Early gastric carcinoma with signet ring cell histology. Cancer. 2002;94:78–83. doi: 10.1002/cncr.10120. [DOI] [PubMed] [Google Scholar]
- 20.Zhang YH, Li Y, Chen C, Peng CW. Carcinoembryonic antigen level is related to tumor invasion into the serosa of the stomach: study on 166 cases and suggestion for new therapy. Hepatogastroenterology. 2009;56:1750–1754. [PubMed] [Google Scholar]
- 21.Park SH, Ku KB, Chung HY, Yu W. Prognostic significance of serum and tissue carcinoembryonic antigen in patients with gastric adenocarcinomas. Cancer Res Treat. 2008;40:16–21. doi: 10.4143/crt.2008.40.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah MA, Khanin R, Tang L, Janjigian YY, Klimstra DS, Gerdes H, et al. Molecular classification of gastric cancer: a new paradigm. Clin Cancer Res. 2011;17:2693–2701. doi: 10.1158/1078-0432.CCR-10-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueda K, Iwahashi M, Nakamori M, Nakamura M, Matsuura I, Yamaue H, et al. Carcinoembryonic antigen-specific suicide gene therapy of cytosine deaminase/5-fluorocytosine enhanced by the cre/loxP system in the orthotopic gastric carcinoma model. Cancer Res. 2001;61:6158–6162. [PubMed] [Google Scholar]
- 24.Toffoli G, Cecchin E. Clinical implications of genetic polymorphisms on stomach cancer drug therapy. Pharmacogenomics J. 2007;7:76–80. doi: 10.1038/sj.tpj.6500405. [DOI] [PubMed] [Google Scholar]
- 25.Golub TR, Slonim DK, Tamayo P, Huard C, Gaasenbeek M, Mesirov JP, et al. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science. 1999;286:531–537. doi: 10.1126/science.286.5439.531. [DOI] [PubMed] [Google Scholar]