Abstract
Rationale
Analyses of circulating cell membrane-derived microvesicles (MV) have come under scrutiny as potential diagnostic and prognostic biomarkers of disease. However, methods to isolate, label and quantify MV have been neither systematized nor validated.
Objective
To determine how pre-analytical, analytical and post-analytical factors affect plasma MV counts, markers for cell of origin and expression of procoagulant surface phosphatidylserine.
Methods and Results
Peripheral venous blood samples were collected from healthy volunteers and patients with cardiovascular disease and/or diabetes. Effects of blood sample collection, anticoagulant and sample processing to platelet free plasma (PFP), and MV isolation, staining and storage (freeze-thaw) and cytometer design were evaluated with replicate samples from these populations. The key finding is that use of citrate or EDTA anticoagulants decreases or eliminates microvesicles from plasma by inducing adhesion of the microvesicles to platelets or other formed elements. Protease inhibitor anticoagulants, including heparin, preserve MV counts. A centrifugation protocol was developed in which recovery of isolated MV was high with resolution down to the equivalent light scatter of 0.2 micron latex beads. Each procedure was systematically evaluated for its impact on the MV counts and characteristics.
Conclusion
This study provides a systematic methodology for MV isolation, identification and quantification, essential for development of MV as diagnostic and prognostic biomarkers of disease.
Keywords: anticoagulants, flow cytometry, microparticle, phosphatidylserine, pre-analytical and analytical variables
1. INTRODUCTION
During cell activation, apoptosis or intercellular interactions, sealed unilamellar plasma membrane vesicles are shed into circulation (Lynch and Ludlam, 2007; Piccin et al., 2007; Cocucci et al., 2009). The terms ‘microvesicles’ and ‘microparticles’ have been interchanged, but ‘microvesicles’ (MV) distinguishes membrane-derived vesicles from other microparticles including lipoproteins, protein aggregates, non-membranous debris, and exosomes. The concentration and composition of MV in the circulation depend upon their cells of origin and the stimuli that trigger their production. Thus, populations of circulating MV bear pathophysiological information, and so have potential for use as sensitive and specific biomarkers for presymptomatic disease. MV have been investigated for prognosis in coronary artery syndrome, aneurysm, thrombosis, pulmonary embolism, thrombotic thrombocytopenic purpura, paroxysmal nocturnal hemoglobinuria, heparin induced thrombocytopenia, sickle cell disease, sepsis, rheumatoid disease, multiple sclerosis, preeclampsia, myeloproliferative disorder and some types of cancer (Zwaal and Schroit, 1997; Berckmans et al., 2001; VanWijk et al., 2003; Morel et al., 2006; Zwicker et al., 2007; Toth et al., 2008a; Toth et al., 2008b). We reported that concentrations of platelet and endothelium-derived MV were elevated in plasma samples from recently-menopausal women who were at low risk for cardiovascular disease by Framingham scores but who had unexpected coronary calcification (Jayachandran et al., 2008).
Methods for isolation, identification, characterization and, especially, enumeration of circulating MV have not been validated completely. Several reviews of the topic have emphasized the need for validation of pre-analytical procedures, including anticoagulants and isolation methods, and for analytical procedures, including reagent compositions, instrument settings and calibration (Kim et al., 2002; Horstman et al., 2004; Jy et al., 2004; Michelsen et al., 2006; Enjeti et al., 2007; Lynch and Ludlam, 2007; Shet, 2008; Dey-Hazra et al., 2010; van Ierssel et al., 2010; Ayers et al., 2011; Yuana et al., 2011). The present study was undertaken to define pre-analytical, analytical and post-analytical factors in MV analysis and to refine, standardize and validate methods for isolation, identification, quantification and characterization of MV in peripheral blood samples.
2. MATERIALS AND METHODS
2.1. Antibodies and other reagents
Annexin-V and mouse anti-human CD42a, CD61 and 62E conjugated with fluorescein isothiocynate (FITC) or R-phycoerythrin (PE) and TruCOUNT™ (4.2μm) beads were purchased from BD Biosciences, San Jose, CA. Fluorescent latex beads (1 μm and 2μm) were purchased from Sigma-Aldrich, Saint Louis, Missouri. Fluoresbrite® Microparticles (0.2 μm, 0.5 μm, 1 μm and 2 μm) were purchased from Polysciences, Inc., Warrington, PA. Soybean trypsin inhibitor was purchased from Sigma, St. Louis, MO, hirudin from CIBA GEIGY Ltd, Basle, Switzerland, and paraformaldehyde (16% solution, EM grade) from Electron Microscopy Sciences, Hatfield, PA. Blood collection tubes were purchased from Becton, Dickson and Company, Franklin Lakes, NJ.
2.2. Samples
All studies were approved by the Mayo Clinic Institutional Review Board. Blood samples were collected from 120 male and female participants (19 - 85 years of age) who were either apparently healthy or diagnosed with type II diabetes, coronary artery disease (CAD) with and without diabetes, or prior stroke or venous thromboembolism. These participants were selected to provide a wide range of MV counts and properties. Samples were collected through a 19 gauge butterfly needle by slow-fill syringe, and after discard of the initial 2-3 mL, dispensed into tubes containing either 15% ethylenediaminetetraacetic acid (EDTA), 3.2% NaCitrate (citrate; 0.11 M), Acid Citrate Dextrose (ACD, Solution B), sodium heparin (68 USP Units) or a mix of 1μM hirudin plus a factor Xa inhibitor (10 μM Soybean Trypsin Inhibitor or 10 μM Tick Anticoagulant Peptide; H&S). The use of the trypsin inhibitor, which on its own is a weak anticoagulant, has supplanted that of the tick anticoagulant, no longer available. We have not established that addition of either Xa inhibitor is essential, but we have determined (unpublished observation) that factor X can become activated in plasma anticoagulated only with hirudin. Platelet P-selectin, PAC-1 binding and phosphatidylserine were determined as described (Jayachandran et al., 2008).
2.3. Isolation of blood MV
The method is published in part (Jayachandran et al., 2008). Essentially platelet free plasma (PFP) was prepared from anticoagulated blood by double centrifugation at 3000 × g for 15 min. The PFP (0.5 - 1mL) was centrifuged at 20,000 × g for 30 minutes in an angle-head rotor. The supernatant plasma was subjected to a second centrifugation at 60,000 × g for 30 min; this supernatant was then stored at −80° C for subsequent analysis. The MV pellet obtained from each centrifugation was reconstituted by vortex mixing (1 – 2 min) with 0.5 – 1 mL of Hanks’/HEPES (130 mM NaCl, 5.4mM KCl 1.3mM CaCl2, 0.8mM MgSO4, 0.44mM Na2HPO4, 20mM HEPES, pH 7.4). All solutions were filtered twice through 0.2 μm membrane (Millipore) filters. Each washed suspension containing MV was then centrifuged again at 20,000 × g or 60,000 × g for 30 min and the resulting pellet reconstituted with 0.5 or 1 mL of fresh buffer.
2.4. Flow cytometry
Unless otherwise indicated, all analyses used a FACSCanto II cytometer (BD Biosciences, San Jose, CA). A sample of isolated MV (50μL) was incubated with 4μL of annexin-V-FITC and PE-conjugated mouse anti-human CD42a or CD61) for 25-30 min. These times and concentrations had been optimized by titration of each reagent. Where indicated, stained MV were fixed by dilution with 400 μL of 1% paraformaldehyde for 15 min. For calculation of counts, TruCOUNT™ beads (50μL) were added immediately prior to analysis by flow cytometry. Gain settings were adjusted to place the TruCOUNT™ beads in the upper log for scatter. Unfiltered Isoton® II diluent from Beckman Coulter, Fullerton, CA, was used in cytometers. Compensation for channel spill was calculated using the auto-compensation feature from recorded values of separate and combined unstained and single-stained MV. Auto-calculated compensation parameters were verified monthly. All antibodies were filtered twice through 0.2 μm membrane filters. Unfiltered buffers and antibodies contain interfering numbers of chemical microparticles (data not shown). MV are defined in this study as events <1 μm in diameter and positive for annexin-V and cell-specific markers. The thresholds were set with isotype control fluorescent antibodies. MV prepared and stained in phosphate buffered saline or HEPES buffered saline (HBS; pH 7.4) without calcium served as negative controls for annexin-V. The absolute count of MV either in the absence or presence of single or dual staining was calculated with the relation:
where GMV is the number of events in the MV gate, GTC is the number of events in the TruCOUNT™ bead gate, and TC is the number of TruCOUNT™ beads added to the sample of volume V (Shet et al., 2003; Jayachandran et al., 2008). Except for comparison of instruments, the FACSCanto™ flow cytometer was used for all other measurements. Unless otherwise indicated data are shown as mean ± SD.
2.5. Direct analysis of plasma
PFP (5μL) was diluted 1:20 with Hanks’/HEPES (pH7.4), and then 4 μL of fluorochrome-conjugated annexin-V and cell-specific antibodies were added. These mixtures were briefly vortexed and incubated in the dark for 25-30 min at room temperature. The mixture was diluted with 800μL of Hanks’/HEPES or buffered saline solution (HBS; 20mM HEPES, 150mM NaCl, 2.5mM calcium) and 100μL of TruCOUNT™ beads.
3. RESULTS
3.1. Data acquisition
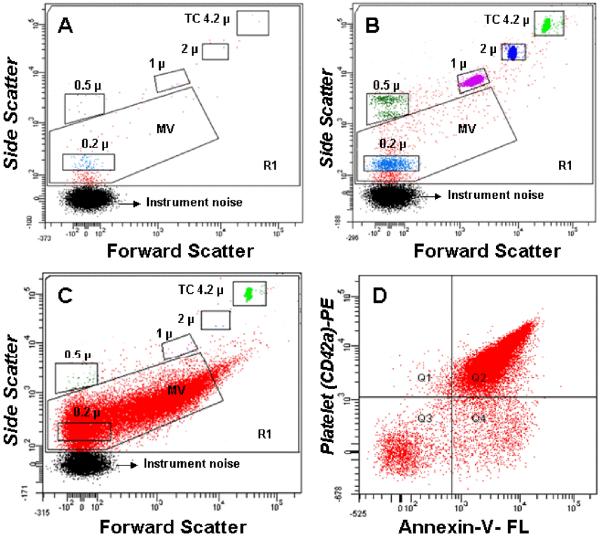
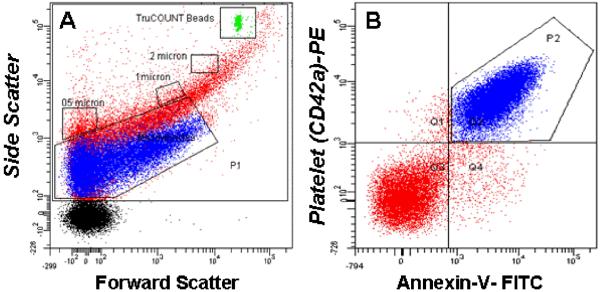
Side scatter events from size calibration beads of 0.2 μm, 0.5 μm, 1μm and 2μm were resolved from instrument noise with the 18-bit FACSCanto (105-channel) flow cytometer (Figure 1). Inspection of the scatter plot (Figure 1B) indicates that 0.2 μm is the lower limit for beads, which have a higher index of refraction, and therefore lower size threshold, than membrane vesicles (Koch et al., 1966; Foladori et al., 2008; Lacroix et al., 2010; Yuana et al., 2011). More than 90% of MV isolated from plasma showed scatter intensities lower than that of 1μm beads (Figure 1C). Fluorescence events from anti-CD42a and annexin V from within the MV scatter gate accounted for more than 99% of events (Figure 1C). For the sample shown in Figure 1D, all but a small fraction (Q4) of counts were positive for both ligands, a finding typical for platelet MV (Jayachandran et al., 2008) MV counts were calculated from the nominal number of beads added per volume of sample, with a minimum of 1000 TruCOUNT™ bead events (typically 2500) per analysis. The coefficient of variation of ten aliquots of 0.5, 1 and 2 μm beads was 7.2%, 2.6% and 2.4%, and MV counts calculated with the TruCOUNT™ internal standard were not significantly affected by flow rate.
Figure 1.
Representative dot plots from the FACSCanto flow cytometer. R1 is the gate for all events, MV is the microvesicle gate, and the remainders are gates for the calibration beads, including TruCOUNT™ (TC). A. Reagent blank: buffer plus fluorochrome-conjugated annexin V and anti-CD 42a; B. Reagents plus calibration beads; C. Forward and side scatter of isolated microvesicles stained with Fluorescein (FL)-annexin-V and Phycoerythrin (PE)-anti-CD42a (GPIX) and the TruCOUNT™ internal calibrator; D. The quadrant lines dividing positive from negative events are drawn to include the maximal number of positive events above 95% the events seen with the isotype controls.
3.2. Preanalytical issues
3.2.1. Anticoagulants
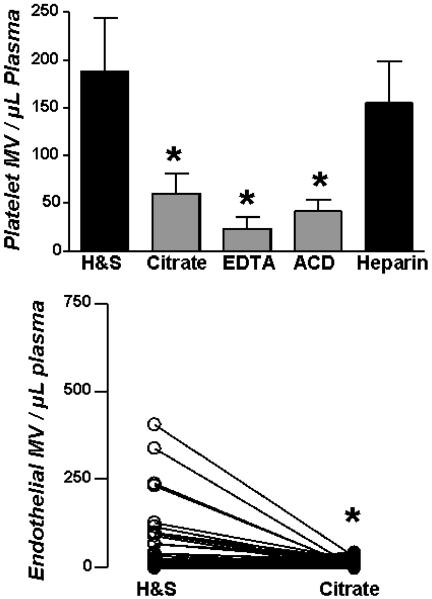
The choice of anticoagulant had a substantial impact on both platelet and endothelial MV counts (Figure 2). Both platelet and endothelial MV were fewer in preparations from blood collected in calcium chelating anticoagulants versus protease inhibitors. When counts were above the 90th percentile, endothelial (CD62-E positive) MV were effectively eliminated (P<0.003) in preparations from blood collected in sodium citrate compared to H&S. When counts were in the range typical of healthy donors, the counts for platelet MV in citrate plasma averaged 30 – 40% of the counts in hirudin & soy bean trypsin inhibitor (H&S;endothelial MV were not detected in plasmas from the normal donor pool). However, the ratio of annexin-V positive to negative MV was not sensitive to anticoagulant (r2=0.08). MV recovery was the same from blood collected in Vacutainer or non-Vacutainer tubes containing the same concentration of calcium chelating and protease inhibitor anticoagulants (not shown).
Figure 2.
Impact of anticoagulants. Upper panel, platelet (CD42a) MV obtained in blood from the same individuals collected in different anticoagulants. Data are shown as mean ± SD; n=12. Lower panel, endothelial (CD62E) MV. Lines connect paired data from 63 individuals. Asterisks denote p<0.05 relative to H&S.
Does calcium chelation suppress MV recovery or do protease inhibitors stimulate shedding? The results with endothelial MV suggest suppression. We had observed that there was a window of as long as 10 minutes between phlebotomy and mixing of the blood with anticoagulant during which the MV count was stable. Accordingly, blood (1 mL aliquots) without anticoagulant was centrifuged immediately for two minutes at 8000g or for 10 minutes at 3000g, and then anticoagulants were added to these platelet poor plasmas (PPP). Addition of any anticoagulant to PPP thus prepared from non-anticoagulated blood yielded the same number of annexin-V positive MV as blood collected in H&S anticoagulant (Figure 3).
Figure 3.
Anticoagulants in cell-free plasma. Anticoagulants as indicated were added to plasma obtained by immediate brief centrifugation of blood collected without anticoagulant. Data are shown as mean ± SD; n=12. The last bar is the MV count from whole blood (WB) collected in H&S anticoagulant from the same individuals.
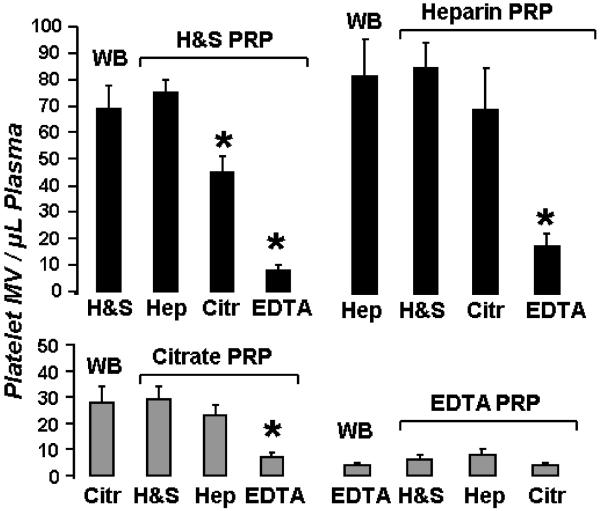
The basis for the loss of MV with removal of calcium was addressed by shifting the point of addition of anticoagulants. Adding either calcium chelating or protease inhibitor anticoagulants to isolated MV did not alter MV counts (data not shown). When calcium chelators were added to the platelet rich plasma (PRP) prepared from the first 800 × g spin of blood collected in H&S or heparin (Figure 4, top), platelet MV counts decreased to an extent similar to that seen in whole blood with citrate or EDTA anticoagulants (Figure 4, bottom). In contrast, addition of H&S or heparin to PRP prepared from blood collected in calcium chelating anticoagulants did not further affect numbers of MV (Figure 4, bottom).
Figure 4.
Point of loss of MV with chelating anticoagulants. Top: Whole blood (WB) was collected in H&S (left) or heparin (Hep; right), centrifuged to obtain PRP and then the indicated anticoagulants were added. Bottom: Whole blood was collected with citrate (left) or EDTA (right) anticoagulant, centrifuged to obtain PRP, and then the indicated anticoagulants were added. Then, PFP was prepared from each sample and analyzed for platelet MV counts. Data are shown as mean ± SD; n=10. Asterisks denote P<0.05 against WB.
3.2.2. Time and temperature
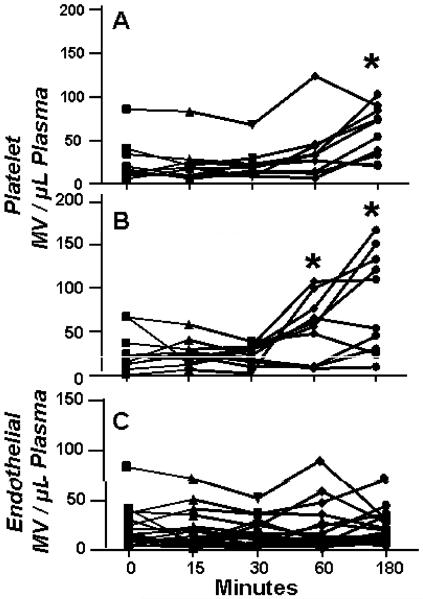
Whole blood collected in either citrate or H&S was distributed into 1.5mL tubes and maintained at either room temperature (ca. 22°C) or 33°C for up to 3 hours, during which MV counts were obtained at intervals. For whole blood collected in citrate, counts of annexin-V positive and platelet MV decreased within 15 minutes and were significantly lower after one hour at either temperature (data not shown). In contrast, counts of annexin-V and platelet MV did not change significantly within the first hour at either temperature in blood collected in H&S but increased significantly thereafter (Figure 5). The increase in counts of stained MV was greater at room temperature than at 33°C. However, the percentage of platelets expr essing surface P-selectin, activated glycoprotein αIIbβ3, phosphatidylserine remained <5% in all samples. Counts of endothelial MV did not change during the three hours at either temperature.
Figure 5.
Stability of MV counts in blood in vitro. Blood was collected in H&S and maintained at either (A) 33°C or (B) room temperature. At the indicated times samples (n=10) were centrifuged and analyzed for platelet MV counts. Endothelial MV (C) were analyzed in samples from blood at both temperatures. Each line represents MV recovered from a single individual. Asterisks denote P<0.05 relative to zero.
3.2.3. Recovery after centrifugation
Centrifugation of PFP at 20,000 × g recovered on average 80% of the MV measured by direct staining of PFP (r2=0.8). More than 90% of platelet MV were recovered after a wash with Hanks’/HEPES of MV pelleted by the 20,000 × g centrifugation (n=66). Less than 10% additional platelet MV were isolated when plasma supernatants from the 20,000 × g centrifugation were centrifuged a second time at 60,000 × g for 30 min. Typical of isolation procedures, the recovery increased from a low of 50% at the lowest MV counts up to 80% at the highest counts. Scatter signals from MV isolated by ultracentrifugation (Figure 1B) were better resolved than those obtained from samples analyzed by direct staining of PFP or unwashed MV (Figure 6), which showed substantial populations of microparticles negative for all stains (Figure 6, red dots).
Figure 6.
Direct staining of MV in dilute plasma. A. Dot plot of MV contaminated with plasma, color-coded according to staining for CD42a and annexin-V (blue); B. Quadrant plot of the MV gate in A.
3.2.4. Storage and freeze-thaw
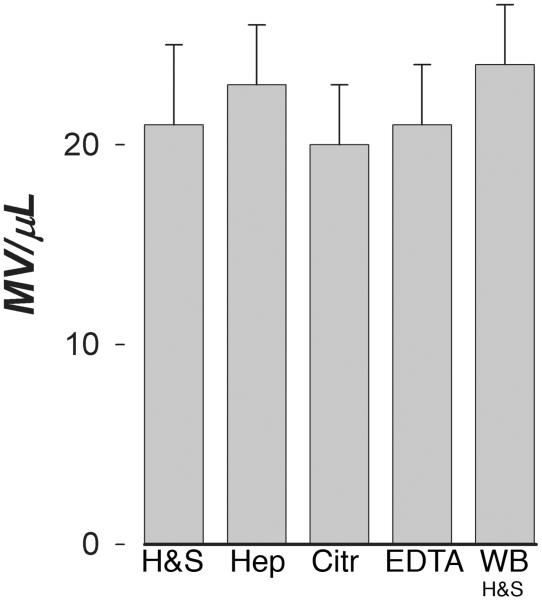
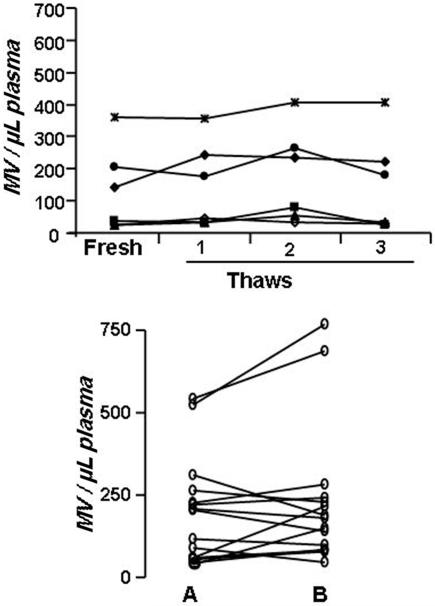
Counts of MV were the same when isolated from either PFP or PPP stored at either −40°C or −80°C for more than a year. Up to three f reeze thaw cycles of PFP had no effect on MV counts, irrespective of initial counts (Figure 7). Once isolated, counts of isolated MV were stable during storage at room temperature for 3-4 days. However, a single freeze and thaw of isolated MV at either −20°C, −40°C or −80°C lowered the count by 10 - 15%. .
Figure 7.
Stability of MV counts in plasma after freezing. Plasma frozen by placing the samples (1 mL) in a −70°C freezer were then subjected to repeated freeze-thaw cycles (top panel) or left undisturbed for a minimum of one year and then (lower panel) thawed and assayed (B) for comparison to the values obtained for the original pre-frozen sample (A).
3.3. Analytical issues
3.3.1. Count calibration
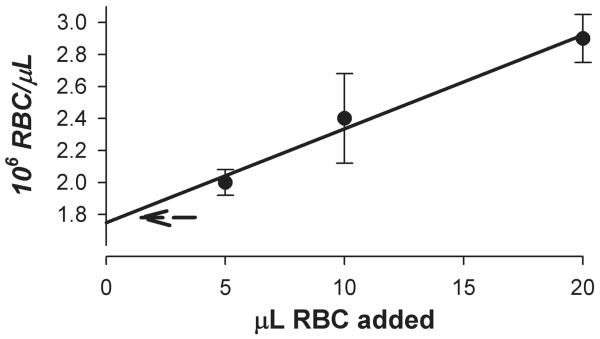
The assumption that the nominal TruCOUNT™ bead count is valid was verified by a cross-check with erythrocyte counts and a validated Coulter counter (Figure 8). As the erythrocyte count in each sample increased above the order of the (constant) TruCOUNT™ bead concentration, the red blood cells (RBC) event rate increased in linear proportion to the RBC count while the TruCOUNT™ bead event rate declined. Because the TruCOUNT™ calibration is in the denominator (Methods), the calculated erythrocyte count showed a systematic increase (solid line/symbols) above that obtained with the Coulter counter (dashed line). Extrapolation of the linear increase to the erythrocyte count of zero intersected the count axis within 5% of the Coulter counter value, and showed a systematic error of +10% when the count rate was 1000 times that of the TruCOUNT™ rate. Because analysis with other bead calibrators has been published (Robert et al., 2008), we analyzed mixtures of BD TruCOUNT™ beads (4.2 μm) with Beckman-Coulter Flow-Check (10 μm) beads for counts obtained by scatter and by fluorescence. In all cases, scatter and fluorescence data were congruent. Two lots of the Flow-Check beads yielded counts of 50% of nominal or less when the TruCOUNT™ count rates were of the order of 20 - 30/sec. At lower bead dilutions (higher count rates), the BD beads yielded proportional counts whereas the Flow-Check beads were disproportionately undercounted. We did not investigate this disparity further.
Figure 8.
Validation of TruCOUNT™ bead calibration. A suspension of washed fixed erythrocytes (1.8 × 106/μL) were counted with a calibrated particle counter (Beckman Coulter ACT diff 2) and then diluted as indicated into 0.5 mL of Hanks’/HEPES solution containing 5000 TruCOUNT™ beads (10/μL). Erythrocyte counts (solid circles) were calculated from FACSCanto data by reference to the internal standard TruCOUNT™ beads as described in Methods. The dashed arrow is the initial erythrocyte count determined with the Coulter counter.
4. DISCUSSION
Distinct populations of circulating MV have been observed in a variety of disease conditions, often related to inflammatory processes (Zwaal and Schroit, 1997; Berckmans et al., 2001; VanWijk et al., 2003; Morel et al., 2006; Jayachandran et al., 2008; Jayachandran et al., 2009). However, the potential for MV as biomarkers has been limited by inadequate validation and standardization of sample preparation, reagents and instrument parameters (Jy et al., 2004; Lynch and Ludlam, 2007). The importance of sample acquisition and processing to obtain platelet poor plasma has been addressed (Jy et al., 2004; Shah et al., 2008; Shet, 2008; Dignat-George et al., 2009) but without a systematic analysis of individual parameters. The present investigation was undertaken to fill this gap in the literature by evaluating factors that affect MV analysis in terms of venous sample collection, anticoagulants, isolation techniques, staining methods and storage and cytometer settings. An important feature of the present approach was to assess all of these parameters on blood from diverse groups of healthy and diseased individuals so that findings may be generalized.
4.1. Anticoagulant
A paramount finding of this study is the impact of anticoagulants on MV recovery. Counts of platelet and endothelial MV were substantially lower in blood collected in citrate or EDTA than in blood collected in protease inhibitors, either H&S or heparin. This effect of anticoagulants was interpreted by Shah et al. as arising from microvesiculation in vitro with protease inhibitor anticoagulants (Shah et al., 2008). However, results of the present study provide an alternative conclusion, first because endothelial MV, which cannot be generated in blood in vitro, were effectively removed with chelation of whole blood. Therefore, the difference in MV counts obtained in calcium chelating anticoagulants compared to protease inhibiting anticoagulants reflects loss with chelation rather than gain with protease inhibitors. This conclusion is verified by the finding that adding any anticoagulant to platelet-free plasma prepared without an anticoagulant had no effect on MV counts, which were congruent with those obtained from whole blood anticoagulated by protease inhibition. Chelation-induced association of the MV with platelets is adequate to account for this phenomenon, as it can be recapitulated with PRP prepared from blood collected in protease inhibiting anticoagulants. Because the degree of loss with chelation is unpredictable, with relative proportions of annexin-V positive and negative platelet MV not falling in predictable register, all prior work on MV from blood anticoagulated by citrate, ACD and EDTA (Jy et al., 2004) may need reevaluation. That said, our MV counts from citrated plasma lie within the lower group of the wide range among published studies (Yuana et al., 2011). Platelet MV counts remained constant when either H&S or heparin anticoagulated blood was maintained for up to 60 minutes at 33°C, and for 30 minutes at room temperature, but thereafter increased. The temperature effect is commensurate with the sensitivity of platelet shape change as blood cools (Tablin et al., 2000). Counts of endothelial MV did not change over time at either temperature, to indicate that the increase reflected release of MV from the platelets.
4.2. Bead calibration
There is growing and compelling evidence that flow cytometry resolves only the largest membrane vesicles, which comprise a near-negligible portion of the total (Koch et al., 1966; Foladori et al., 2008; Zwicker et al., 2009; Lacroix et al., 2010; Yuana et al., 2010; Chandler et al., 2011; Yuana et al., 2011). Visualization of plasma or thrombin-stimulated platelet microvesicles by atomic force microscopy (Yuana et al., 2010) indicates a median diameter of 60 nm. Counts obtained from the AFM images averaged 1000 times those obtained by flow cytometry using an isolation and staining protocol similar to ours, and which yielded similar counts. Direct measurement of placental and plasma MV by refractive index-independent particle tracking with simultaneous extraction of translational diffusion coefficients likewise detected the order of 107 cellular MV/μL of plasma, more than four orders of magnitude times that detected by flow cytometry (Dragovic et al., 2011). Using synthetic microvesicles of defined size, Chandler et al (Chandler et al., 2011) verified that the most sensitive flow cytometers cannot detect single microvesicles smaller than about 400 nm. And finally measurements of microvesicle procoagulant activity directly in plasma (Mallat et al., 2000; Owen et al., 2011) yield activities at least 3 – 4 orders of magnitude higher than can be accounted for by annexin-V positive microvesicle counts obtained by flow cytometry. However, microvesicle analysis by flow cytometry has yielded correlations to inflammatory and vascular pathophysiology, and continues to dominate the MV literature, so continuing standardization and validation of reagents and sample preparation remains essential in the face of the high variability among laboratories (Yuana et al., 2011).
The basis for the disparity between BD TruCOUNT™ and Beckman-Coulter calibrators is not clear. Undercounting of a calibrator might account for exceptionally high MV counts (Shah et al., 2008). We validated the TruCOUNT™ calibration against a washed erythrocyte suspension counted with a Coulter counter. The TruCOUNT™ beads are provided in single use tubes, whereas the Flow-Check are provided in a single bottle for repetitive sampling and thus might be prone to sampling error secondary to incomplete mixing. However, we found a fresh bottle of Flow-Check beads to yield under-counts comparable to those of a nearly exhausted bottle. We did not evaluate the disparity with a cytometer other than the FACSCanto, but no theoretical basis for a cytometer-specific disparity is obvious.
4.3. Isolation and staining
Isolation of MV with 20,000 × g centrifugation of platelet free plasma resulted in the loss of as much as 20% of the counts obtained with direct staining, but the fidelity of the signal was higher. This enhanced resolution may reflect the removal of microparticulate lipids and proteins aggregates. Although it adds a significant pre-analytical step, isolation rather than direct staining is essential for analyzing batches of samples, as plasma clogs the flow tubes and carries over. At best, a 1:200 dilution of the plasma is required for optimal staining and analysis and so decreases the sensitivity for low abundant MV signals.
Unfixed samples yielded variably fewer MV, which could arise from MV aggregation or adsorption to the sample tubes and instrument plumbing. We have not evaluated other possible inhibitors of MV loss, but there may be alternatives to paraformaldehyde, which provide acceptable inter-assay variability of <10%.
4.4. Storage
Up to three freeze-thaw cycles of PFP did not affect MV recovery. Storage of PPP (prepared by single centrifugation of PFP at 3,000 × g for 15 minutes) for more than a year minimally and randomly affected MV recovery.
5. CONCLUSIONS
Among pre-analytical procedures that affect yield and reproducibility of microvesicle analysis, choice of anticoagulant and centrifugation protocols for preparing platelet free plasma and isolated microvesicles have major influences. Other significant influences arise from time and temperature between phlebotomy and initial centrifugation, freezing of the isolated microvesicles and internal calibration. Freezing of plasma, essential for large scale studies, has no effect on the microvesicle counts.
Highlights.
Protease inhibitors as anticoagulates preserve microvesicle counts found in whole blood.
Washed preparations of microvesicles provide the best fidelity for signal.
Microvesicle counts are stable for at least one year in frozen platelet poor plasma.
Microvesicle counts are stable up to three freeze thaw cycles of frozen platelet poor plasma.
Acknowledgments
Robert D. Litwiller, Teresa Kimlinger and Benjamin J. Sticha provided technical assistance. Drs. Robert Frey, Sreekumaran Nair, Srinivasan Manivannan and Arshad Jahangir provided blood samples from their study participants.
Sources of funding: This work was supported by Novel Methodology Award from the Mayo Foundation for Medical Education and Research, CTSA grant UL1 RR024150 from the National Center for Research Resources (NCRR), a grant from the Aurora Foundation to the Kronos Longevity Research Institute; NHLBI grants HL78638, HL83141 and HL83797 and HL090639; and the American Heart Association-Scientist Development Grant AHA30503Z. .
Abbreviations
- MV
microvesicles
- PRP
platelet rich plasma
- PFP
platelet free plasma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ayers L, et al. Measurement of circulating cell-derived microparticles by flow cytometry: sources of variability within the assay. Thromb. Res. 2011;127:370–7. doi: 10.1016/j.thromres.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Berckmans RJ, et al. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb. Haemost. 2001;85:639–46. [PubMed] [Google Scholar]
- Chandler WL, et al. A new microparticle size calibration standard for use in measuring smaller microparticles using a new flow cytometer. J Thromb Haemost. 2011;9:1216–24. doi: 10.1111/j.1538-7836.2011.04283.x. [DOI] [PubMed] [Google Scholar]
- Cocucci E, et al. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Dey-Hazra E, et al. Detection of circulating microparticles by flow cytometry: influence of centrifugation, filtration of buffer, and freezing. Vasc Health Risk Manag. 2010;6:1125–33. doi: 10.2147/VHRM.S13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignat-George F, et al. Centrifugation is a crucial step impacting microparticle measurement. Platelets. 2009;20:225–226. doi: 10.1080/09537100902795500. [DOI] [PubMed] [Google Scholar]
- Dragovic RA, et al. Sizing and phenotyping of cellulars vesicles using Nanoparticle Tracking Analysis. Nanomedicine. 2011 doi: 10.1016/j.nano.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjeti A, et al. Detection and measurement of microparticles: an evolving research tool for vascular biology. Semin. Thromb. Hemost. 2007;33:771–779. doi: 10.1055/s-2007-1000369. [DOI] [PubMed] [Google Scholar]
- Foladori P, et al. Use of silica microspheres having refractive index similar to bacteria for conversion of flow cytometric forward light scatter into biovolume. Water Res. 2008;42:3757–66. doi: 10.1016/j.watres.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Horstman LL, et al. New horizons in the analysis of circulating cell-derived microparticles. Keio J. Med. 2004;53:210–30. doi: 10.2302/kjm.53.210. [DOI] [PubMed] [Google Scholar]
- Jayachandran M, et al. Characterization of blood borne microparticles as markers of premature coronary calcification in newly menopausal women. Am J Physiol Heart Circ Physiol. 2008;295:931–938. doi: 10.1152/ajpheart.00193.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayachandran M, et al. Circulating microparticles and endogenous estrogen in newly menopausal women. Climacteric. 2009;12:177–84. doi: 10.1080/13697130802488607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jy W, et al. Measuring circulating cell-derived microparticles. J Thromb Haemost. 2004;2:1842–50. doi: 10.1111/j.1538-7836.2004.00936.x. [DOI] [PubMed] [Google Scholar]
- Kim HK, et al. Optimized flow cytometric assay for the measurement of platelet microparticles in plasma: pre-analytic and analytic considerations. Blood Coagul. Fibrinolysis. 2002;13:393–397. doi: 10.1097/00001721-200207000-00003. [DOI] [PubMed] [Google Scholar]
- Koch AL, et al. Deduction of the cell volume and mass from forward scatter intensity of bacteria analyzed by flow cytometry. J Microbiol Met. 1966;27:49–61. [Google Scholar]
- Lacroix R, et al. Overcoming limitations of microparticle measurement by flow cytometry. Semin. Thromb. Hemost. 2010;36:807–18. doi: 10.1055/s-0030-1267034. [DOI] [PubMed] [Google Scholar]
- Lynch SF, Ludlam CA. Plasma microparticles and vascular disorders. Br. J. Haematol. 2007;137:36–48. doi: 10.1111/j.1365-2141.2007.06514.x. [DOI] [PubMed] [Google Scholar]
- Mallat Z, et al. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation. 2000;101:841–843. doi: 10.1161/01.cir.101.8.841. [DOI] [PubMed] [Google Scholar]
- Michelsen A, et al. Development of a time-resolved immunoflurometric assay for quantifying platelet-derived microparticles in human plasma. Thromb. Res. 2006;117:705–711. doi: 10.1016/j.thromres.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Morel O, et al. Procoagulant Microparticles: Disrupting the Vascular Homeostasis Equation? Arterioscler. Thromb. Vasc. Biol. 2006;26:2594–2604. doi: 10.1161/01.ATV.0000246775.14471.26. [DOI] [PubMed] [Google Scholar]
- Owen BA, et al. Procoagulant activity, but not number, of microparticles increases with age and in individuals after a single venous thromboembolism. Thromb. Res. 2011;127:39–46. doi: 10.1016/j.thromres.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccin A, et al. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 2007;21:157–171. doi: 10.1016/j.blre.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Robert S, et al. Standardization of platelet-derived microparticle counting using calibrated beads and a Cytomics FC500 routine flow cytometer: a first step towards multicenter studies? J Thromb Haemost. 2008;7:190–97. doi: 10.1111/j.1538-7836.2008.03200.x. [DOI] [PubMed] [Google Scholar]
- Shah MD, et al. Flow cytometric measurement of microparticles: Pitfalls and protocol modifications. Platelets. 2008;19:365–372. doi: 10.1080/09537100802054107. [DOI] [PubMed] [Google Scholar]
- Shet AS. Characterizing blood microparticles: Technical aspects and challenges. Vasc Health Risk Manag. 2008;4:769–774. doi: 10.2147/vhrm.s955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shet AS, et al. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102:2678–83. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- Tablin F, et al. Animal models for studies on cold-induced platelet activation in human beings. J. Lab. Clin. Med. 2000;135:339–346. doi: 10.1067/mlc.2000.105619. [DOI] [PubMed] [Google Scholar]
- Toth B, et al. Platelet-derived microparticles and coagulation activation in breast cancer patients. Thromb. Haemost. 2008a;100:663–9. [PubMed] [Google Scholar]
- Toth B, et al. Circulating microparticles in breast cancer patients: a comparative analysis with established biomarkers. Anticancer Res. 2008b;28:1107–12. [PubMed] [Google Scholar]
- van Ierssel SH, et al. Flow cytometric detection of endothelial microparticles (EMP): effects of centrifugation and storage alter with the phenotype studied. Thromb. Res. 2010;125:332–9. doi: 10.1016/j.thromres.2009.12.019. [DOI] [PubMed] [Google Scholar]
- VanWijk MJ, et al. Microparticles in cardiovascular diseases. Cardiovasc. Res. 2003;59:277–87. doi: 10.1016/s0008-6363(03)00367-5. [DOI] [PubMed] [Google Scholar]
- Yuana Y, et al. Pre-analytical and analytical issues in the analysis of blood microparticles. Thromb. Haemost. 2011;105:396–408. doi: 10.1160/TH10-09-0595. [DOI] [PubMed] [Google Scholar]
- Yuana Y, et al. Atomic force microscopy: a novel approach to the detection of nanosized blood microparticles. J Thromb Haemost. 2010;8:315–23. doi: 10.1111/j.1538-7836.2009.03654.x. [DOI] [PubMed] [Google Scholar]
- Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–1132. [PubMed] [Google Scholar]
- Zwicker JI, et al. Cancer-associated thrombosis. Crit. Rev. Oncol. Hematol. 2007;62:126–36. doi: 10.1016/j.critrevonc.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Zwicker JI, et al. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin. Cancer Res. 2009;15:6830–40. doi: 10.1158/1078-0432.CCR-09-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]