Abstract
Cigarette smoking is prevalent in cocaine/methamphetamine-dependent patients and associated with significant morbidity and mortality, yet, the provision of smoking cessation treatment in conjunction with substance use disorder (SUD) treatment is not standard practice. This is due, in part, to clinician concern that combining smoking cessation treatment with SUD treatment could lead to poorer SUD outcomes. The NIDA Clinical Trials Network is conducting a 10-week, two-group, randomized trial to evaluate the impact of providing smoking cessation treatment (SCT) with SUD treatment as usual (TAU), compared to TAU alone, in smokers who are in outpatient treatment for cocaine or methamphetamine dependence. Approximately 528 participants, recruited from 12 community treatment programs, will be randomized into the trial. The present paper describes key design decisions made during protocol development. The trial is designed to evaluate the relationship between cigarette smoking and stimulant use, which prior research suggests is linked, and should contribute to our understanding of how best to address the co-occurring problems of nicotine dependence and cocaine/ methamphetamine-dependence. Unique aspects of the trial include the primary question of interest, which concerns the impact of providing SCT on SUD outcomes rather than on smoking outcomes, and the intensity of the SCT chosen, which includes bupropion, nicotine replacement, and two psychosocial interventions.
Keywords: cocaine, methamphetamine, smoking cessation, bupropion, contingency management
1. Introduction
Each year, cigarette smoking accounts for an estimated 443,000 deaths and $96.8 billion in productivity losses in the United States [1]. The prevalence of smoking in illicit drug abusers is 49 – 98%, a rate substantially higher than the 19.8% smoking prevalence in the general population [2]. A link between cigarette smoking and non-nicotine stimulant abuse has been established in both clinical and laboratory studies. The results from clinical studies suggest that the rate of smoking in cocaine abusers is 75-80% [3,4,5] and that smoking cigarettes is associated with more severe addiction, including more frequent cocaine use, a greater likelihood of injecting or smoking cocaine, and more severe employment and legal difficulties [6]. An outpatient study in methamphetamine-dependent individuals reported that 65% of the participants were current cigarette smokers [7]. Human laboratory studies have found that cocaine administration increases the rate of cigarette smoking [8,9] and that mecamylamine, a nicotine antagonist, reduces cue-induced cocaine craving [10]. Despite the pervasiveness of smoking in stimulant abusers and the deadly consequences of smoking in addicted individuals [11], smoking-cessation treatment is typically not provided in community substance use disorder (SUD) treatment programs. Failure to provide smoking-cessation treatment concurrently with SUD treatment stems in part from concern that smoking cessation might impact negatively on non-nicotine substance use outcomes [12]. Prochaska et al. [13] completed a meta-analysis of nine studies in which the impact of smoking-cessation treatment on non-nicotine drug/alcohol abstinence was assessed; the findings suggested that smoking-cessation treatment might actually improve substance use outcomes. However, it is important to note that the nine studies analyzed included mainly alcohol-dependent, and, to a lesser extent, methadone-maintained participants, and did not include outpatient cocaine/methamphetamine abusers. A trial evaluating the impact of smoking-cessation treatment during outpatient SUD treatment to stimulant-dependent individuals has not been conducted. To address this gap, the National Institute on Drug Abuse (NIDA) Clinical Trials Network (CTN) has developed the trial: “Smoking-Cessation and stimulant treatment (S-CAST): Evaluation of the impact of concurrent outpatient smoking-cessation and stimulant treatment on stimulant-dependence outcome.” S-CAST is somewhat unique in that it is being conducted at community treatment programs as opposed to traditional research settings and its primary focus is on the impact of smoking cessation treatment on stimulant-dependence outcomes as opposed to the effectiveness of the smoking cessation treatment on smoking abstinence. The present paper describes the key design considerations associated with this unique trial.
2. Research design and study organization
2.1 Research questions
The primary research question addressed by the S-CAST study is whether adding smoking cessation treatment (SCT) including bupropion, nicotine replacement, and two psychosocial interventions to substance abuse treatment as usual (TAU) improves, worsens, or has no effect on stimulant-use outcomes. The target population is smokers enrolled in outpatient treatment for cocaine or methamphetamine dependence. Secondary research questions addressed include: 1. whether TAU+SCT, relative to TAU, impacts non-stimulant drug-abuse outcomes; 2. whether TAU+SCT, relative to TAU, improves smoking cessation outcomes; and 3. whether there is an association between smoking cessation and stimulant use outcomes.
2.2 Research design
S-CSAT is a 10-week, intent-to-treat, 2-group randomized controlled trial with follow-up visits at 3 and 6 months post-smoking quit date. Eligible participants are randomized to the TAU+SCT or TAU arms. Participants who are randomized to the TAU+SCT arm have a target quit smoking day at the end of study week 3. The 10-week treatment phase is considered optimal in that it provides the length of dosing required for bupropion treatment to have an effect [14] and provides the time-frame required to complete the assessments needed to answer the research questions while conserving resources that would be required for a longer treatment phase. The follow-up visits were included since follow-ups are standard in smoking cessation research, with the effectiveness of smoking cessation treatment largely judged on long-term abstinence rates. The study schema is provided in Figure 1.
Figure 1. Study Schema.
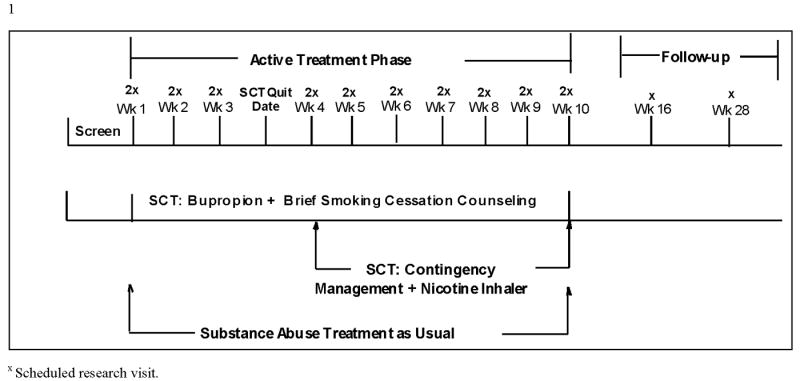
The S-CAST protocol development team considered three possible research designs:
TAU +SCT versus TAU + attention/placebo control versus TAU;
TAU + SCT versus TAU + attention/placebo control
TAU+SCT versus TAU
The first and second designs include a condition that controls for attention and placebo effects of the active SCT intervention. Although elegant, these designs were eliminated due to lack of resources required to complete a three-arm trial (design 1) and the complexity of the control intervention that would be needed to match the active SCT intervention (designs 1 and 2). The third design (TAU versus TAU+SCT) was ultimately selected since this design uniquely addresses the primary research question being asked about the impact that adding smoking cessation treatment to TAU has on stimulant use outcomes.
Failing to include attention and placebo controls for the SCT intervention does, of course, leave open the possibility that any differential treatment effect is the result of the additional attention or other non-treatment-specific factors. However, the development team noted that the intensity of SCT, designed to promote maximal impact on smoking cessation, might not necessarily have a positive impact on stimulant use outcomes. For example, asking stimulant-dependent patients to address smoking concurrently with stimulant dependence might be overwhelming to the degree that patients drop out of treatment, which may be associated with worse outcomes. In addition, a distinction must be made between the impact of smoking cessation treatment intervention and smoking cessation per se. If all individuals exposed to treatment had better outcomes irrespective of their smoking status, then we might conclude that the treatment itself was an active factor and that the absence of an attention/placebo control condition was a limitation of the study. Our hypothesis, however, is that the positive impact of TAU+SCT on stimulant use outcomes will occur due to reduced stimulant priming from exposure to nicotine and related cues. In this case, a positive effect on stimulant use outcomes would be found only in the TAU+SCT participants who achieve smoking abstinence. In this case, the absence of an attention/placebo control would be irrelevant.
2.3 Study setting
The S-CAST study is being conducted by NIDA's CTN. The CTN was established in 1999 in response to an Institute of Medicine report [15] delineating the relative lack of information exchange between substance abuse researchers and substance abuse community treatment providers. In the CTN, substance abuse researchers work with treatment providers to design clinically meaningful trials and to implement them in “real world” settings utilizing existing staff as interventionists and clinic patients as participants. The CTN links substance abuse researchers and treatment providers through nodes, with each node consisting of a Regional Research and Training Center (RRTC) which is affiliated with at least one university, and multiple Community Treatment Programs (CTPs) that work with the RRTC.
2.4 Site selection process
To be eligible for study participation, a CTP needed to treat a sufficient number of stimulant-dependent individuals to randomize 44 within an 18-month time-frame, and to have medical staffing with enough time available to safely and effectively conduct a medication trial. In addition, since the design of the S-CAST trial requires a treatment as usual group in which smoking-cessation treatment is not provided, sites that provided smoking-cessation treatment in outpatient programming were not eligible for participation. This latter criterion ruled out one site in Seattle, Washington as well as several sites in New York State, where the provision of smoking cessation treatment in SUD treatment is mandated. Other criteria upon which sites were evaluated included performance in prior trials and diversity of the potential study sample. Site selection was completed using the following process: 1) A call for site nominations was sent to all RRTC PIs, 2) Twenty-six sites completed a survey describing their program and the patients that apply for treatment, 3) the S-CAST Executive Committee reviewed the surveys and selected 16 sites for teleconference interviews, and 4) Final site selections were made by the Executive Committee. Based on the information obtained during the interviews, four sites were not eligible for study participation either by failing to meet site inclusion criteria or other factors significantly impacting feasibility of implementation. The selection process resulted in 12 regionally diverse sites being selected for participation: Adapt (Roseburg, OR), Addiction Medicine Services (Pittsburgh, PA), Behavioral Health Services of Pickens County (Pickens, SC), Dorchester Alcohol and Drug Commission (Summerville, SC), Gateway Community Services (Jacksonville, FL), Gibson Recovery Center (Cape Girardeau, MO), La Frontera Center (Tucson, AZ), Lexington Richland Alcohol and Drug Abuse Council (Columbia, SC), Maryhaven (Columbus, OH), Matrix Institute on Addictions (Rancho Cucamonga, CA), Nexus Recovery Center (Dallas, TX), and Tarzana Treatment Centers (Tarzana, CA).
2.5 Study population
The study population includes adults (≥18) who smoke at least 7 cigarettes per day, wish to stop smoking, meet DSM-IV-TR criteria for cocaine and/or methamphetamine dependence, are enrolled in outpatient SUD treatment, and are scheduled to attend treatment for at least 10 weeks post-randomization. In addition to meeting criteria for current methamphetamine or cocaine dependence, it was suggested that self-report of stimulant use in the past 28 days be required. However, this criterion was not included because prior studies have shown that a significant proportion of patients who apply for outpatient psychosocial counseling treatment report no substance use in the prior 28 days. Thus, requiring self-reported stimulant use would rule out a significant proportion of CTP patients, limiting the generalizability of findings. Second, qualitative research suggests that some crack cocaine dependent patients smoke cigarettes to slow the rate at which they smoke crack (i.e., they use cigarettes between “rocks” so they do not use up their crack supply too quickly; [16]). Requiring recent self-reported use might result in enrolling a substantial number of participants who use cigarettes as a “break” from crack use. Of relevance to this issue is a small, post-hoc analysis of cocaine-dependent outpatients (cigarette smokers and non-smokers), which found that nicotine dependence was associated with worse treatment outcomes in patients who had a cocaine-negative urine screen at admission [17]. For patients with a cocaine-positive urine screen, nicotine dependence was not related to outcome. These findings suggest that it might be important to address nicotine dependence in cocaine-negative patients.
2.6 Inclusion and exclusion criteria
The eligibility criteria were developed to include a representative sample while ensuring the safety of study participants. Most exclusion criteria reflect safety issues associated with bupropion (e.g., conditions/medication that increase the risk of seizures, etc; criteria in Table 1).
Table 1. Eligibility criteria for the S-CAST study.
| Inclusion Criteria |
| Potential participants must: |
|
| Exclusion Criteria |
| Potential participants must not: |
|
2.7 Randomization
Consistent with the above rationale for inclusion of both currently active and currently abstinent stimulant users, eligible participants are stratified within sites, according to whether a stimulant-positive (amphetamines or cocaine) UDS result is obtained during screening, and randomized 1:1 at a centralized site to TAU+SCT or TAU.
3. Study treatments
Past research suggests that smoking cessation rates in substance abusing populations are poor [18,19] but that cessation rates can be improved by combining smoking cessation interventions [20]. Consequently, the S-CAST protocol team, in conjunction with recommendations from the expert review panel (Protocol Review Board) attempted to select an optimal combination of evidence-based smoking cessation interventions, as described below. This strategy was designed to maximize smoking quit rates thereby allowing an assessment of smoking abstinence on stimulant use patterns.
3.1 Bupropion Extended-Release (XL)
The S-CAST trial is being conducted with cocaine/methamphetamine-dependent smokers at sites that are relatively new to research. Consequently, the non-nicotine medication needed to be one whose efficacy for smoking-cessation was established and which had a well-known safety profile. Bupropion SR met both criteria. The FDA approved it for this indication [14] and it has been studied in hundreds of clinical trials, and used in psychiatric and general medical practice since 1996. It has also been evaluated for treating adult ADHD [21], as well as cocaine [22,23,24] and methamphetamine dependence [25,7]. The bioequivalence of extended-release (XL) and SR bupropion has been established, with medication compliance greater for the XL formulation [26]. Also considered was Varenicline, a partial agonist for the α4β2 nicotinic acetylcholine receptor, which has had FDA approval for smoking cessation treatment since 2006. However, the lack of safety data in stimulant-dependent populations, coupled with study sites that are relatively new to research and have relatively limited medical services made it a less desirable candidate for the S-CAST trial from a safety perspective. Thus, the medication condition selected for the TAU+SCT arm was bupropion XL. The dosage regimen is consistent with recommended practice: 150 mg/day for study days 1-3, then 300 mg/day of bupropion XL for study days 4 through week 10. At the final week 10 visit, TAU+SCT participants are given a 3-day bupropion XL dose taper, in which 150 mg is taken once per day.
3.2 Nicotine Inhaler
Five forms of nicotine replacement therapy (NRT; gum, patch, nasal spray, inhaler, and lozenge) are FDA approved as smoking-cessation aids and data suggest that NRT used in combination with bupropion might improve smoking cessation outcomes [14]. The nicotine inhaler was of interest in that a small trial revealed a higher smoking cessation rate in the group receiving buproprion and the nicotine inhaler relative to groups receiving either medication alone [27]. The results from this trial coupled with the good side effect and tolerability profile of NRT [14], led to the inclusion of the nicotine inhaler in the S-CAST trial. It is recognized, however, that this decision was somewhat arbitrary and that other forms of NRT may be equally useful in combination with bupropion. In S-CAST, the TAU+SCT participants are prescribed 6-16 nicotine cartridges per day ad libitum starting with the smoking quit date through week 10. Following week 10, participants are provided with a decreasing number of cartridges per week during a 3-week taper.
3.3 Counseling support: Smoke Free and Living It©
The USPHS Clinical Practice Guideline recommends that both effective pharmacologic and psychosocial approaches be used in smoking-cessation treatment [14]. A prior CTN trial conducted with individuals in outpatient SUD treatment found that group smoking-cessation counseling was associated with poor attendance and low recruitment [19], thus, the protocol development team turned to a program that could be provided individually. “Smoke Free and Living It, ©” is a brief, individualized smoking-cessation counseling program designed to provide education, problem-solving skills, and social support that was developed by the Mayo Clinic Nicotine Research Program. A prior CTN smoking-cessation trial that used this program had a high level of treatment adherence by counselors and a high rate of attendance by participants [28]. Its brevity, consisting of weekly 10-minute sessions, also appealed to CTP providers from a sustainability standpoint. In S-CAST, “Smoke Free and Living It©” counseling is provided weekly during study weeks 1-10 by a trained interventionist.
3.4 Contingency Management
Contingency management (CM) is an intervention in which patients receive rewards contingent on a target behavior, such as providing drug-free urines, attending treatment, or taking medication. When used to reinforce abstinence, it has been shown to reduce substance use during the time it is applied [29]. In a meta-analytic evaluation of the effectiveness of CM for tobacco use, 11 studies were included with an estimated effect size of d= 0.31 [30]. One of these studies evaluated the effectiveness of CM for smoking cessation in methadone-maintained participants and found that contingency management nearly doubled the smoking abstinence rate during the active treatment phase, although this advantage was not maintained at the 6- and 12-month follow-up visits [18]. A small study (N=20) evaluating the efficacy of CM in reducing carbon monoxide (CO) levels to ≤8 ppm in cocaine-abusing smokers in outpatient treatment found a significantly greater CO reduction in the contingent, relative to the non-contingent, groups [31]. In the S-CAST trial, CM, which should serve to significantly increase smoking abstinence during the active treatment phase, is being used in conjunction with bupropion XL, nicotine inhaler, and smoking-cessation counseling, which are effective in increasing long-term smoking abstinence.
The CM procedure utilized in early research typically followed the reinforcement schedule developed by Higgins et al. [32] in which participants received vouchers for goods/services of escalating value based on maintaining a target behavior (e.g., drug-free urines, treatment attendance, etc.). While highly effective, this procedure has been criticized as being more costly than most CTPs can afford, which led to the development of an intermittent reinforcement approach in which participants earn chances to draw for prizes based on maintaining a target behavior [33]. This prize-based approach has been effective in a number of studies, including a CTN trial where it was found to increase treatment retention and negative urine drug screens in stimulant abusers [34].
In S-CAST, prize-based CM is used to reinforce negative CO (i.e., CO < 4 ppm) results during the post-quit phase. The CO cut-off of < 4 ppm is consistent with findings that the use of 2-3 ppm produces the most accurate identification of smoking abstinence whereas 8-10 ppm, which has been traditionally used to verify abstinence, can result in smokers being falsely classified as abstinent [35,36]. In order to encourage continuous abstinence, the number of draws earned escalates with each consecutive week of abstinence and resets if evidence of smoking is obtained. A unique feature of the S-CAST CM protocol, however, is that participants who delay their quit attempt or quit and subsequently relapse do not start at the week 4 earning level when they initiate or resume abstinence. Rather, they begin to earn abstinence incentives at the level specified in the escalating schedule for that week. This ability to earn larger amounts later in the program is meant to encourage late quitters or those who initially fail to continue trying to quit throughout the active treatment phase. Table 2 outlines the escalating draw schedule. Participants who quit immediately and maintain continuous abstinence earn approximately $380 in prizes during the 10-week study. This is consistent with amounts that have previously been efficacious for improving treatment outcomes when the target was stimulant negative urines [34].
Table 2. Number of prize drawings per week as a function of negative CO levels.
| Study Week | Number draws for negative CO | Bonus for negative CO since week 4 | Max Draw/Sample |
|---|---|---|---|
| 4 | 4 | 0 | 4 |
| 4 | 4 | 0 | 4 |
| 5 | 5 | 1 | 6 |
| 5 | 5 | 1 | 6 |
| 6 | 6 | 1 | 7 |
| 6 | 6 | 1 | 7 |
| 7 | 7 | 1 | 8 |
| 7 | 7 | 1 | 8 |
| 8 | 8 | 1 | 9 |
| 8 | 8 | 1 | 9 |
| 9 | 9 | 1 | 10 |
| 9 | 9 | 1 | 10 |
| 10 | 10 | 1 | 11 |
| 10 | 10 | 1 | 11 |
| Total | 110 |
4. Assessments
4.1 Primary outcome measure
The primary outcome is whether a participant is stimulant-free during each week of the active treatment phase as assessed by stimulant-negative urine drug screens (UDS) and self-report of no stimulant use during weeks 1-10. This outcome was selected as primary since it provides an objective measure of recent stimulant use. At the group level, this outcome translates into the percentage of participants in each study arm who are stimulant-free during each week of the active treatment phase. During the active treatment phase, participants are scheduled to provide two urine samples per week on nonconsecutive days. To avoid falsification, urine samples are collected using temperature monitoring and the validity of urine samples is checked with the use of a commercially available adulterant test. In cases where the temperature reading/adulterant test indicates a non-valid sample, an attempt is made to obtain a second urine sample. A rapid UDS system that screens for drugs of abuse including cocaine, methamphetamine, amphetamine, opioids, benzodiazepines, and marijuana is used to analyze the urine samples (Branan Medical Corporation).
4.2 Secondary outcome measures
Secondary drug-abuse outcomes include days of stimulant-use and days with any substance-use (i.e., alcohol, tobacco and/or other substance use), treatment utilization, and craving. The Timeline Follow-back (TLFB) [37,38] is a widely employed and well validated method used at each study visit to capture self-report drug use information for each day elapsed since the previous study visit. Treatment utilization is defined as the percent of scheduled treatment hours attended as obtained from clinic records. Craving is assessed on a weekly basis with the Brief Substance Craving Scale [39].
Smoking outcomes are a secondary focus of the study. Point-prevalence abstinence, defined as self-report of not smoking in the previous seven days, confirmed by a carbon monoxide (CO) level <8 ppm [40], is assessed weekly. A CO cut-off of 8-10 ppm is the standard used by the majority of smoking cessation trials and, thus, the protocol development team chose an 8 ppm cut-off to ensure comparability with other smoking cessation trials. In addition, a combination of daily self-reported smoking data and weekly CO levels will be used to determine continuous abstinence during post-quit days 15 – 42. This time frame was selected based on the recommendation that a two-week post-quit grace period be included in smoking-cessation studies during which time smokers can smoke without it being counted as a treatment failure [41]. The use of a 4-week abstinence period is consistent with FDA standards for approving smoking-cessation medications [41]. To be scored as achieving continuous abstinence, the participant must report no smoking on any of the days during this four-week period and deliver no positive CO samples (>8 ppm) during that period with at least one CO assessment per week.
5. Data and Safety Monitoring
Safety measures assessed weekly include vital signs, adverse events and the Hospital Anxiety and Depression Scale [42], a brief, validated instrument that screens for both depression and anxiety [43]. An independent CTN Data Safety and Monitoring Board (DSMB) examines accumulating data to assure protection of participants' safety while the study's scientific goals are being met. The DSMB recommends to the sponsor (NIDA) whether there is support for continuation of the trial, or evidence that study procedures should be changed, or if the trial should be halted, for reasons relating to the safety of the study participants, the efficacy of the treatment under study, or inadequate trial performance (e.g., poor recruitment).
6. Sample size, power, and effect size
The probability of stimulant-free urines in the TAU group is one key assumption in determining sample size. A review of the stimulant UDS results for cocaine-dependent participants from three prior CTN studies produced probabilities ranging from 0.55 to 0.75, with the lower probabilities (i.e., 0.55) requiring larger sample sizes. Based on these prior studies, we assumed a probability of 0.6 for stimulant-free urines in the TAU group. A second key consideration in calculating sample size is the size of the group difference to be detected. S-CAST is powered to detect the lower limit of a clinically meaningful effect. Specifically, S-CAST is powered to detect a group difference if the probability of stimulant-free urines in the TAU+SCT group is at least 0.7 (vs .6 in the TAU group).
The minimum sample size required to achieve 80% power using an alpha level of 0.05 (two-sided) and a 1:1 randomization ratio was computed based on a repeated measures study design that takes into account that the data are binary and can be correlated across repeated measurements [44]. We assumed a compound symmetry covariance structure to be conservative (correlation between 2 successive time points does not change over time), as well as a maximum correlation between any 2 time points of 0.65. The higher the assumed correlation between time points, referred to as phi, the less information that we gain from successive measurements, thus requiring a larger sample size. The assumption of 0.65 for S-CAST was based on the phi calculated for the stimulant UDS results from the subgroup of cocaine dependent participants in a prior CTN study that evaluated the impact of concurrently providing smoking-cessation treatment with SUD treatment. The sample size yielded from the assumptions outlined above is 528, with 264 in each treatment group. If in fact the covariance structure is not compound symmetric and the correlation is close to the assumed 0.65 at time points close together and decreases with distance, a smaller sample size would be allowed. The sample size assumptions were also assessed during a planned interim analysis and the sample size was found to be adequate.
7. Analytic Plan
7.1 Primary Outcome Analysis
The primary hypothesis is that a significantly greater percentage of TAU+SCT, relative to TAU, participants will be stimulant-free during the weeks of the active treatment phase. For each participant, a stimulant-free week is defined as a week in which both urine samples test negative for stimulants and the participant self-reports no illicit stimulant use. A stimulant-positive week is defined as a week in which at least one urine sample tests positive for a stimulant or during which the participant self-reports illicit stimulant use.
However, in the analysis of data from any clinical trial, strategies must be employed for handling missing data. In this trial, data for a week in which both urine samples are missing and the participant self-reports no illicit stimulant use will be treated as missing. For a week in which one stimulant-free UDS is produced, the second urine sample is missing, and the participant self-reports no illicit stimulant use or the self-report data are missing will be treated as negative (Table 3).
Table 3. Stimulant-use assignment for study week as a function of time line follow back and stimulant urine drug screen results.
| Urine Drug Screen -Stimulant result | Time Line Follow Back (TLFB) | ||||
|---|---|---|---|---|---|
| Result | Result | At least one positive day | All days negative | Some days negative, some missing | All days missing |
| - | - | + | - | - | - |
| + | - | + | + | + | + |
| + | + | + | + | + | + |
| + | 0 | + | + | + | + |
| 0 | 0 | + | Missing | Missing | Missing |
| - | 0 | + | - | - | - |
Note: '0′=missing, '+'=positive and '-'=negative
Data will be analyzed using a generalized linear mixed model (GLMM) for repeated-measures analysis [45]. Suppose Y represents the vector of observed response data, X and Z the fixed and random effects design matrices, and α and β be the vectors of fixed and random effects, respectively. Then, the GLMM model formulation assumes that
where E(Y|β) is the expected value of Y conditional on the random effects vector, g-1 is the inverse link function, and Xα + Zβ the linear predictor. The response variable Y will be modeled using the logit link function, i.e., g(μ) = log(μ / (1 - μ)). Fixed effects will include treatment group and week, while random effects will include site. While several statistical packages, such as R, have procedures for analyzing GLMM models, we will fit the model using SAS Proc GLIMMIX as follows:
proc glimmix;
class site treatment;
model Y = treatment week treatment*week / dist=binary link=logit covb solution;
random intercept / subject=site type=cs;
The model formulation implies the same treatment effect in each site. The effect of the intervention on change in response from baseline will be addressed by the treatment by week interaction parameter. Due to the possibility that CTPs may differ in their success at helping participants become/remain stimulant-free, each regression model will adjust for site effects. Site will be included in the GLMM model as a random effect based on the participation of 12 CTPs. Assumptions of linearity will be examined by adding a restricted cubic spline to represent nonlinearity [46]. Assumptions for the covariance structure that provides the best fit to the data will be analyzed using the COVTEST statement under SAS Proc GLIMMIX. Since GLMM inference is only valid if data are missing at random (MAR), logistic regression analyses will be conducted to identify patterns of attrition and to determine if there is differential attrition by treatment condition. A binary indicator variable for missing data will be regressed on treatment assignment and other covariates. Variables that are associated with attrition at or below the α=0.10 level of significance will be included in subsequent analyses where the assumption of data missing at random (conditional on covariates) is required [47].
If the random effects model unexpectedly fails to converge, then a general estimating equation (GEE) approach will be employed treating site as a fixed effect. If this model is utilized, then missing data (see Table 3) will be imputed using standard multiple imputation procedures, since GEE inference is only valid if data are missing completely at random (MCAR). SAS Proc MI will be used to create five complete data sets using multiple imputation. Each data set subsequently be analyzed using SAS Proc GENMOD (the GEE procedure), and the results combined using SAS Proc MIANALYZE.
7.2 Secondary Outcome Analyses
For all repeated measures mixed model and generalized linear mixed model (GLMM) analyses, the response variable will be modeled using the appropriate link function. The effect of the intervention on change in response from baseline will be addressed by the treatment by week interaction parameter. Each model will adjust for possible site differences by including site as a random effect. The SAS procedure MIXED will be used for continuous variables, while GLIMMIX will be used for binary data. Before modeling is commenced, assumptions of linearity and covariance structure will be examined.
8. Current status of the trial
As of the writing of this paper, all 12 study sites were actively randomizing participants, with data collection expected to be complete in July 2012.
9. Summary
The S-CAST study addresses an important gap in the literature by evaluating the impact of providing smoking cessation treatment to smokers who are in outpatient treatment for cocaine/methamphetamine dependence. Several aspects of S-CAST make it unique including the research question, which is not focused on the effectiveness of the smoking cessation treatment per se but, rather, seeks to address the concern of some treatment providers that the provision of concurrent smoking-cessation treatment could result in poorer cocaine/methamphetamine use outcomes. To this end, the S-CAST study includes two conditions: substance abuse treatment as usual (TAU) and TAU with smoking cessation treatment (SCT).
Another unique aspect of S-CAST is a design that focuses on providing an optimally effective smoking-cessation intervention that could be provided safely by community treatment programs. The protocol design team placed heavy emphasis on this aspect of the trial given the poor smoking abstinence rates obtained in prior smoking cessation trials with substance abusing populations [18,19]. The focus of S-CAST on providing an optimally effective smoking intervention is clear in both the number of smoking cessation treatments provided, which includes two medication and two psychosocial interventions, and in the form of the individual treatment interventions. For example, smoking cessation trials traditionally have used the SR formulation of bupropion, which is the formulation approved by the FDA for smoking cessation. However, the SR formulation requires twice a day dosing while the XL formulation requires once a day dosing and the bioequivalence of the two formulations has been established. Since it has been demonstrated that compliance with the XL formulation is significantly better than compliance with the SR formulation [26], bupropion XL was selected for use in S-CAST. The contingency management intervention was also designed to maximize the likelihood of quitting and remaining abstinent from cigarettes and is specifically designed to encourage individuals who have difficulty quitting at the target quit date to continue trying to quit throughout the trial. This mix of smoking-cessation interventions should help a significant proportion of the participants assigned to the TAU+SCT arm to quit smoking. This in turn should enable an evaluation of the impact that smoking cessation has on stimulant use outcomes. Based on laboratory studies finding a link between cocaine use/craving and nicotine, we hypothesize that stimulant outcomes will be better in those who quit smoking than in those who do not. S-CAST is thus designed to answer questions of interest to clinicians as well as more basic researchers and should contribute significantly to our understanding of how best to address the co-occurring problems of nicotine dependence and cocaine/methamphetamine-dependence. Addressing this problem has public health implications since smoking is so prevalent among cocaine/methamphetamine-dependent individuals and is associated in this, as in other populations, with long-term deadly consequences.
Acknowledgments
Support: This work was supported by NIDA grant: U10-DA013732 to the University of Cincinnati (Dr. Somoza).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention. Smoking-attributable mortality, years of potential life lost and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226–1228. [PubMed] [Google Scholar]
- 2.Schroeder SA. A 51-Year-Old woman with bipolar disorder who wants to quit smoking. JAMA. 2009;301(5):522–231. doi: 10.1001/jama.2008.982. [DOI] [PubMed] [Google Scholar]
- 3.Budney AJ, Higgins ST, Hughes JR, Bickel WK. Nicotine and caffeine use in cocaine-dependent individuals. J Subst Abuse. 1993;5:117–130. doi: 10.1016/0899-3289(93)90056-h. [DOI] [PubMed] [Google Scholar]
- 4.Sees KL, Clark HW. When to begin smoking cessation in substance abusers. J Subst Abuse Treat. 1993;10:189–195. doi: 10.1016/0740-5472(93)90044-3. [DOI] [PubMed] [Google Scholar]
- 5.Gorelick DA, Simmons MS, Carriero N, Tashkin DP. Characteristics of smoked drug use among cocaine smokers. Am J Addiction. 1997;6:237–245. [PubMed] [Google Scholar]
- 6.Roll JM, Higgins ST, Budney AJ, Bickel WK, Badger GJ. A comparison of cocaine-dependent cigarette smokers and non-smokers on demographic, drug use and other characteristics. Drug Alcohol Depen. 1996;(40):195–201. doi: 10.1016/0376-8716(96)01219-7. [DOI] [PubMed] [Google Scholar]
- 7.Shoptaw S, Henzerling KG, Rotherman-Fuller E, Steward T, Wang J, Swanson AN, De La Garza R, Newton T, Ling W. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depen. 2008b;96:222–232. doi: 10.1016/j.drugalcdep.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemeth-Coslett R, Henningfield JE, Katz J, Goldberg S. Effect of cocaine on rate of cigarette smoking. Pharmacol Biochem Behav. 1986;25:303. [Google Scholar]
- 9.Roll JM, Higgins ST, Tidey J. Cocaine use can increase cigarette smoking: evidence from laboratory and naturalistic settings. Exp Clin Psychopharm. 1997;5(3):263–268. doi: 10.1037//1064-1297.5.3.263. [DOI] [PubMed] [Google Scholar]
- 10.Reid M, Mickalian J, Delucchi K, Berger SP. A nicotine antagonist, mecamylamine, reduces cue-induced craving in cocaine-dependent subjects. Neuropsychopharmacol. 1999;20(3):297–307. doi: 10.1016/S0893-133X(98)00076-1. [DOI] [PubMed] [Google Scholar]
- 11.Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, Melton J. Mortality following inpatient addictions treatment. JAMA. 1996;275:1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- 12.Ziedonis DM, Guydish J, Williams J, Steinberg M, Foulds J. Barriers and solutions to addressing tobacco dependence in addiction treatment programs. Alcohol Res Health. 2006;29(3):228–235. [PMC free article] [PubMed] [Google Scholar]
- 13.Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation with individuals in substance abuse treatment or recovery. J Consult Clin Psych. 2004;72(6):1144–1156. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- 14.Fiore MC, Jaén CR, Baker TB, et al. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2008. Treating Tobacco Use and Dependence: 2008 Update. [Google Scholar]
- 15.Lamb S, Greenlick MR, McCarty D, editors. Bridging the gap between practice and research, forging partnerships with community-based drug and alcohol treatment. Washington, DC: National Academy Press; 1998. [PubMed] [Google Scholar]
- 16.Wiseman EJ, McMillan DE. Rationale for cigarette smoking and for mentholation cocaine and nicotine-dependent outpatients. Comprehensive Psychiatry. 1998;39:358–363. doi: 10.1016/s0010-440x(98)90048-7. [DOI] [PubMed] [Google Scholar]
- 17.Patkar AA, Vergare MJ, Thornton CC, Weinstein SP, Murray HW, Leone FT. Nicotine dependence and treatment outcome among African American cocaine-dependent patients. Nicotine Tob Res. 2003;5(3):411–418. doi: 10.1080/1462220031000094178. [DOI] [PubMed] [Google Scholar]
- 18.Shoptaw S, Rotheram-Fuller E, Yang X, Frosch D, Nahom D, Jarvik ME, Rawson RA, Ling W. Smoking cessation in methadone maintenance. Addiction. 2002;97:1317–1328. doi: 10.1046/j.1360-0443.2002.00221.x. [DOI] [PubMed] [Google Scholar]
- 19.Reid MS, Fallon B, Sonne S, Flammino F, Nunes EV, Jiang H, Kourniotis E, Lima J, Brady R, Burgess C, Arfken C, Pihlgren E, Giordano L, Starosta A, Robinson J, Rotrosen J. Smoking cessation treatment in community-based substance abuse rehabilitation programs. J Subst Abuse Treat. 2008;35:68–77. doi: 10.1016/j.jsat.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Ebbert JO, Croghan IT, Sood A, Schroeder DR, Hays JT, Hurt RD. Varenicline and bupropion sustained-release combination therapy for smoking cessation. Nicotine Tob Res. 2009;11:234–239. doi: 10.1093/ntr/ntn031. [DOI] [PubMed] [Google Scholar]
- 21.Reimherr FW, Hedges DW, Strong RE, Marchant BK, Williams ED. Bupropion SR in adults with ADHD: a short-term, placebo-controlled trial. Neuropsychiatr Dis Treat. 2005;1(3):245–251. [PMC free article] [PubMed] [Google Scholar]
- 22.Margolin A, Kosten TR, Avants SK, Wilkins J, Ling W, Beckson M, Arndt IO, Cornfish J, Ascher JA, Li SH, Bridge P. A multicenter trial of bupropion for cocaine dependence in methadone-maintained patients. Drug Alcohol Depen. 1995;40:125–131. doi: 10.1016/0376-8716(95)01198-6. [DOI] [PubMed] [Google Scholar]
- 23.Poling J, Oliveto A, Petry N, Sofuoglu M, Gonsai K, Gonzalez G, Martell B, Kosten TR. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Arch Gen Psychiatry. 2006;63:219–228. doi: 10.1001/archpsyc.63.2.219. [DOI] [PubMed] [Google Scholar]
- 24.Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Hao UH, Wang PC, Bholat MA, Ling W. Bupropion hydrochloride versus placebo, in combination with cognitive behavioral therapy, for the treatment of cocaine abuse/dependence. J Addict Dis. 2008a;27(1):13–23. doi: 10.1300/J069v27n01_02. [DOI] [PubMed] [Google Scholar]
- 25.Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, Chiang N, Kahn R, Vocci F, Ling W, Pearce VJ, McCann M, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Weis D. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacol. 2008;33:1162–1170. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- 26.Stang P, Young S, Hogue S. Better patient persistence with once-daily bupropion compared with twice-daily bupropion. Am J Ther. 2007;14:20–24. doi: 10.1097/MJT.0b013e31802b5954. [DOI] [PubMed] [Google Scholar]
- 27.Croghan IV, Hurt RD, Dakhil SR, Croghan GA, Sloan JA, Novotny PJ, Rowland KM, Bernath A, Loots ML, Le-Lindqwister NA, Tschetter LK, Garneau SC, Flynn KA, Ebbert LP, Wender DB, Loprinzi CL. Randomized comparison of a nicotine inhaler and bupropion for smoking cessation and relapse prevention. Mayo Clin Proc. 2007;82(2):186–195. doi: 10.4065/82.2.186. [DOI] [PubMed] [Google Scholar]
- 28.Winhusen TM, Somoza EC, Brigham GS, Liu DS, Green CA, Covey LS, Croghan IT, Adler LA, Weiss R, Leimberger JD, Lewis DF, Dorer EM. Impact of attention-deficit/hyperactivity disorder (ADHD) treatment on smoking cessation intervention in ADHD smokers: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010;71(12):1680–1688. doi: 10.4088/JCP.09m05089gry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- 30.Prendergast ML, Hall EA, Roll J, Warda U. Use of vouchers to reinforce abstinence and positive behaviors among clients in a drug court treatment program. J Subst Abuse Treat. 2008;35:125–136. doi: 10.1016/j.jsat.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiseman EJ, Williams DK, McMillan DE. Effectiveness of payment for reduced carbon monoxide levels and noncontingent payments on smoking behaviors in cocaine-abusing outpatients wearing nicotine or placebo patches. Exp Clin Psychopharm. 2005;13(2):102–110. doi: 10.1037/1064-1297.13.2.102. [DOI] [PubMed] [Google Scholar]
- 32.Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives improve outcome in outpatient treatment of cocaine dependence. Arch Gen Psychiatry. 1994;51:568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- 33.Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes, and they will come: contingency management for treatment of alcohol dependence. J Consult Clin Psychol. 2000;68:250–257. doi: 10.1037//0022-006x.68.2.250. [DOI] [PubMed] [Google Scholar]
- 34.Petry NM, Peirce JM, Stitzer ML, Blaine J, Roll JM, Cohen A, Obert J, Killeen T, Saladin ME, Cowell M, Kirby KC, Sterling R, Royer-Malvestuto C, Hamilton J, Booth RE, Macdonald M, Liebert M, Rader L, Burns R, DiMaria J, Copersino M, Stabile PQ, Kolodner K, Li R. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs. Arch Gen Psychiatry. 2005;62:1148–1156. doi: 10.1001/archpsyc.62.10.1148. [DOI] [PubMed] [Google Scholar]
- 35.Javors MA, Hatch JP, Lamb RJ. Cut-off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction. 2005;100(2):159–67. doi: 10.1111/j.1360-0443.2004.00957.x. [DOI] [PubMed] [Google Scholar]
- 36.Cropsey KL, Eldridge GD, Weaver MF, Villalobos GC, Stitzer ML. Expired carbon monoxide levels in self-reported smokers and nonsmokers in prison. Nicotine Tob Res. 2006;8(5):653–9. doi: 10.1080/14622200600789684. [DOI] [PubMed] [Google Scholar]
- 37.Sobell LC, Sobell MB. Timeline follow back: A technique for assessing self-reported ethanol consumption. In: Allen J, Lit-ten R, editors. Techniques to Assess Alcohol Consumption. New Jersey: Humana Press, Inc; 1992. pp. 19–28. [Google Scholar]
- 38.Fals-Stewart W, O'Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol. 2000;68:134–44. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- 39.Mezinskis J, Dyrenforth S, Goldsmith J, Cohen M, Somoza E. Craving and withdrawal symptoms for various drugs of abuse. Psychiatric Annals. 1998;28:577–583. [Google Scholar]
- 40.Hurt RD, Krook JE, Croghan IT, Loprinzi CL, Sloan JA, Novotny PJ, Kardinal CG, Knost JA, Tirona MT, Addo F, Morton RF, Michalak JC, Schaefer PL, Porter PA, Stella PJ. Nicotine patch therapy based on smoking rate followed by bupropion for prevention of relapse to smoking. J Clin Oncol. 2003;21(5):914–20. doi: 10.1200/JCO.2003.08.160. [DOI] [PubMed] [Google Scholar]
- 41.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5:603. [PubMed] [Google Scholar]
- 42.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 43.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale: an updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 44.Rochon J. Application of GEE procedures for sample size calculations in repeated measures experiments. Statistics in Medicine. 1998;17:1643–1658. doi: 10.1002/(sici)1097-0258(19980730)17:14<1643::aid-sim869>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 45.Brown H, Prescott P. Applied Mixed Models in Medicine. John Wiley & Sons Ltd.; 1999. [Google Scholar]
- 46.Harrell F. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer; 2001. [Google Scholar]
- 47.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. Springer; 2000. [Google Scholar]


