Summary
The association of type 2 diabetes with elevated plasma triglyceride (TG) and very low-density lipoproteins (VLDL), and intrahepatic lipid accumulation represents a pathophysiological enigma and an unmet therapeutic challenge. Here we uncover a link between insulin action through FoxO1, bile acid (BA) composition, and altered lipid homeostasis that brings new insight to this longstanding conundrum. FoxO1 ablation brings about two signature lipid abnormalities of diabetes and the metabolic syndrome, elevated liver and plasma TG. These changes are associated with deficiency of 12α-hydroxylated BAs and their synthetic enzyme, Cyp8b1, that hinders the TG-lowering effects of the BA receptor, Fxr. Accordingly, pharmacological activation of Fxr with GW4064 overcomes the BA imbalance, restoring hepatic and plasma TG levels of FoxO1-deficient mice to normal levels. We propose that generation of 12α-hydroxylated products of BA metabolism represents a signaling mechanism linking hepatic lipid abnormalities with type 2 diabetes, and a treatment target for this condition.
Introduction
The metabolic syndrome and diabetes are linked to abnormalities in lipid metabolism that can lead to cardiovascular disease and hepatic steatosis (DeFronzo, 2010). This dyslipidemia poses a double therapeutic challenge: on one hand, its non-LDL based mechanism renders it poorly responsive to statins and cholesterol absorption inhibitors; on the other, macrovascular complications arising from it are singularly unresponsive to tight glycemia control and remain the leading cause of death of diabetic patients (National Institute of Diabetes and Digestive and Kidney Diseases, 2005).
In addition to its role in causing hyperglycemia through excessive glucose production (HGP) (Lin and Accili, 2011), the liver is also the source of increased triglyceride (TG) levels in diabetes (Choi and Ginsberg, 2011). Interestingly, the latter antedate the onset of hyperglycemia, seemingly indicating that alterations of hepatic TG metabolism are an intrinsic component of the metabolic syndrome (Sorensen et al., 2011).
FoxO1 promotes HGP (Haeusler et al., 2010b; Matsumoto et al., 2007) and regulates different aspects of liver sensitivity to insulin. Genetic ablation studies show that FoxO1 is dispensable for insulin regulation of hepatic lipid metabolism (Dong et al., 2008; Wan et al., 2011). This finding led to a model in which insulin signaling to glucose and lipid production diverges at Akt, with FoxO1 regulating HGP, and mTOR or other serine kinases–such as atypical Pkc–regulating lipid synthesis and secretion (Biddinger et al., 2008b; Li et al., 2010). However, it has proven difficult to obtain in vivo evidence for selective insulin resistance, e.g., for a condition in which FoxO1 signaling is suppressed, but mTOR or Pkc signaling is preserved (Brown and Goldstein, 2008; Haeusler and Accili, 2008).
The hypothesis of this work was that FoxO1 mediates aspects of insulin-dependent hepatic lipid metabolism that have not been identified in previous studies because animals were not challenged appropriately. Using genetic (crosses with low-density lipoprotein receptor knockout mice, Ldlr−/−) or dietary approaches (cholesterol-rich, western-type diet–WTD) in mice lacking hepatic FoxO1 (L-FoxO1), we discovered that FoxO1 is required to suppress hepatic and plasma TG levels through qualitative, rather than quantitative regulation of BA synthesis.
BAs are potent regulators of hepatic cholesterol and TG metabolism, and affect insulin sensitivity and glucose disposal. Bile acid sequestrants reduce glucose and cholesterol levels in type 2 diabetics (Staels and Kuipers, 2007). In addition to their established role in cholesterol and fat absorption as well as cholesterol detoxification, BAs activate the nuclear receptor Fxr, and the transmembrane receptor Tgr5 (Thomas et al., 2008). In this work, we show that FoxO1 is required for expression of a subset of BA synthetic genes, and that alterations of this gene set lead to an unusual BA profile that impairs the ability of Fxr to reduce TG levels, and can be reversed by pharmacological Fxr activation. The demonstration that insulin regulates hepatic lipid metabolism through FoxO1-dependent modulation of BA profiles, points to alterations of BA composition as an exploitable opportunity in diabetes therapeutics.
Results
Western diet exacerbates lipid abnormalities in mice lacking liver FoxO1
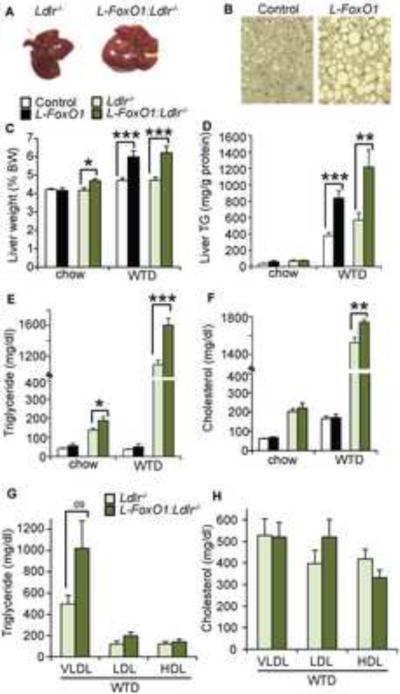
We examined liver and plasma lipids in L-FoxO1 mice and L-FoxO1:Ldlr−/− mice either on a regular chow diet or on WTD. After WTD feeding, weight and adiposity increased similarly in all groups (data not shown). Livers of L-FoxO1 and L-FoxO1:Ldlr−/− mice were paler, larger, and laden with larger lipid droplets compared to controls (Figure 1A–C), due to a large increase in liver TG content (Figure 1D). In addition, L-FoxO1:Ldlr−/− mice showed significantly higher serum TG compared to Ldlr−/− on both chow and WTD (Figure 1E). The increase in TG was associated with a large, though not statistically significant, increase of VLDL-TGs, as assessed by density ultracentrifugation (Figure 1G). We did not detect a difference in TG secretion rate (Figure S1A). Total serum cholesterol was also elevated in fasted L-FoxO1:Ldlr−/− mice on WTD (Figure 1F), with trends toward higher LDL-and lower HDL-cholesterol (Figure 1H).
Figure 1. Lipid parameters after WTD feeding.
(A) Livers and (B) Hematoxylin and eosin staining of liver sections after WTD feeding. (C) Liver weights as a percentage of body weight (n=7–14). (D) Liver TG (n=7–8). (E) Total serum TG and (F) cholesterol in chow and WTD-fed L-FoxO1 and L-FoxO1:Ldlr−/− and respective controls, after a 5 hour fast (n=7–12). (G) Ultracentrifuge-fractionated TG and (H) cholesterol in WTD-fed L-FoxO1:Ldlr−/− and controls (n=5). *P<0.05, **P < 0.01, ***P < 0.001, by Student's t-tests. Data are presented as mean ± S.E.M. See also Figures S1 and S2.
We also assessed aortic atherosclerotic plaque area in WTD-fed L-FoxO1:Ldlr−/− and controls, but found no differences between the two groups (Figure S1B–C). We reckoned that this unexpected finding of normal plaque size despite elevated TG and cholesterol might be due to the concurrent increase in insulin sensitivity of the FoxO1 mutants, as seen previously in chow-fed mice (Matsumoto et al., 2007). To test this hypothesis, we measured glucose and insulin levels in WTD-fed mice. We found that WTD tended to raise both glucose and insulin, but L-FoxO1 and L-FoxO1:Ldlr−/− mice showed lower fasting glucose and insulin (Figure S2A–D), and better glucose tolerance (Figure S2E–H) than littermate controls. There were no changes to the response to insulin injection (Figure S2I,J). These observations reveal that hepatic FoxO1 ablation preserves insulin sensitivity of glucose metabolism during WTD, possibly contributing to the lower-than-expected plaque size (Tabas et al., 2010).
The elevated serum TG of L-FoxO1:Ldlr−/− mice, as well as the increased liver TG in WTD-fed L-FoxO1 and L-FoxO1:Ldlr−/− mice, resemble key aspects of the lipid profile of the metabolic syndrome (Isomaa et al., 2001), despite the improvements in glucose metabolism and insulin sensitivity due to FoxO1 ablation. The findings could be construed to support a model in which lipid abnormalities in the metabolic syndrome arise as a consequence of excessive (or preserved) sensitivity to the lipogenic actions of insulin (Brown and Goldstein, 2008; Haeusler and Accili, 2008).
Altered enterohepatic bile acid composition in L-FoxO1 mice
To understand the mechanism underlying the alterations in lipid levels, we performed comprehensive, unbiased liver metabolite analyses. As the liver abnormalities were identical in the Ldlr-competent and Ldlr-deficient background, we limited subsequent analyses to the former. Notable metabolite changes in L-FoxO1 mice that were consistent with the hypertriglyceridemia included increases of glycerolipids (data not shown) and very long chain fatty acids (FAs) (≥20 carbons), with decreases of some shorter FAs (Figure S3A). But the most striking changes were among BA species (Figure 2A). Because BAs are known to reduce TGs (Lefebvre et al., 2009; Thomas et al., 2008), we hypothesized that alterations of bile acid metabolism link FoxO1 deficiency to increased TG levels.
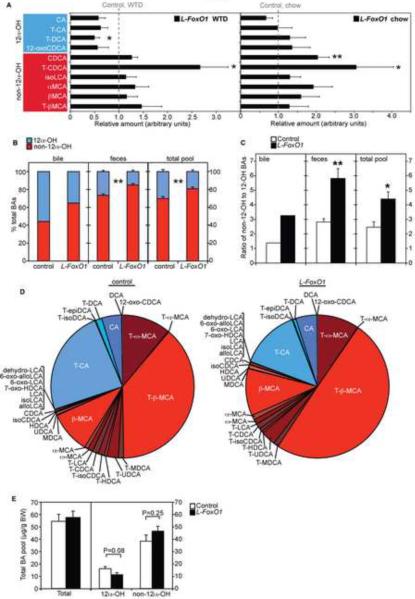
Figure 2. Metabolomics and bile acid composition.
(A) Hepatic BAs in L-FoxO1 mice, relative to controls, on WTD, and chow, separated by origination from 12α-hydroxylation (n=8). Note that 12-oxo-CDCA does not have a hydroxyl group at the 12 position, but it is derived by oxidation of CA by bacteria (Ridlon et al., 2006). A complete list of BA abbreviations is available in Supplemental Table 4. (B) 12α-OH (blue) and non-12α-OH (red) BAs, as a percentage of total in gallbladder bile (pooled from 5 mice per genotype), feces (n=3–4), and total BA pool (n=5–6), in WTD-fed L-FoxO1 mice and controls. (C) Ratio of non-12α-OH BAs to 12α-OH BAs in bile, feces, and total pool. (D) Composition of total BA pool in WTD-fed L-FoxO1 mice and controls (mean values of n=5–6). (E) Quantitation of total BAs, 12α-OH BAs and non-12α-OH BAs in the total BA pool from WTD-fed mice (n=5–6). *P < 0.05, **P < 0.01, by Welch's two-sample t-test (A) or Student's t-tests (B,C,E). Data are presented as mean ± S.E.M. See also Figure S3.
BAs are synthesized in liver from cholesterol in two multi-step pathways, leading to the formation of primary BAs cholic acid (CA), chenodeoxycholic acid (CDCA) and, in mice, muricholic acids (MCAs) (Russell, 2009). After secretion into the duodenum, these are converted to secondary BAs by microbiota, and largely recycled to the liver (Ridlon et al., 2006). We identified ten hepatic BA species with a distinct distribution: In WTD-fed L-FoxO1 mice, products that had undergone hydroxylation at the carbon 12 position (12α–OH) all had relative values less than one, indicating a decrease compared to controls, a trend that showed P < 0.05 for T-DCA (Figure 2A, left panel). Conversely, all products that had never undergone 12α-hydroxylation had values greater than one, indicating an increase compared to controls, and for T-CDCA this trend achieved P < 0.05 (Figure 2A, left panel). In chow-fed L-FoxO1 mice, we found that the major 12α–OH BA, CA, showed a trend towards decrease (P = NS) while two non-12α-OH BAs, CDCA and T-CDCA, showed significant increases (P = 0.001 and 0.03, respectively) (Figure 2A, right panel). Moreover, WTD itself increased two non-12α-OH BAs (Figure S3B), suggesting that the BA composition defects of L-FoxO1 mice are exacerbated by WTD.
We quantitated BAs in gallbladder bile and feces, as well as the total BA pool in WTD-fed mice, by LC-MS/MS (Alnouti et al., 2008). In every case, L-FoxO1 mice showed relative deficiency of 12α–OH BAs, leading to a marked increase in the ratios of non-12α-OH:12α-OH BAs, or MCAs:CA (Figure 2B–D, Figure S3C,D). However, total BA pool size was unchanged, as the deficiency was compensated for by an increase in non-12α-OH BAs (Figure 2E). We also found no difference in conjugated versus unconjugated BAs (Figure S3E). Quantitation of all detected BAs is available in Table S1.
FoxO1 ablation affects expression of bile acid, fatty acid, and cholesterol synthesis genes
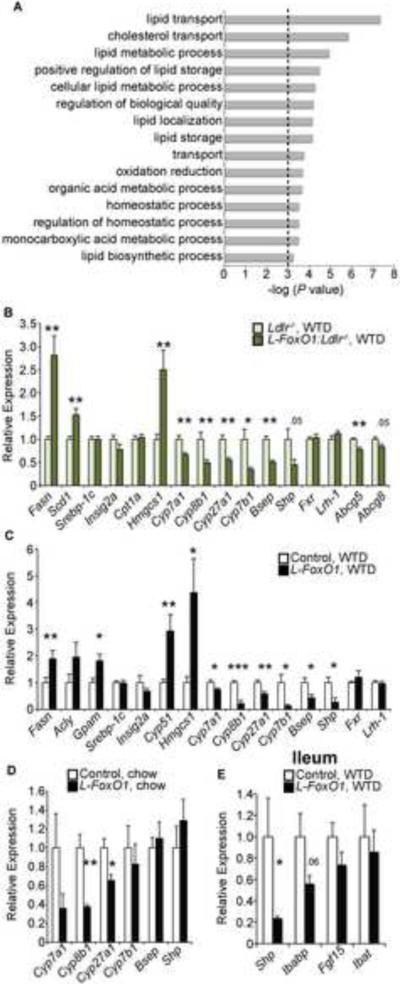
We hypothesized that alterations of the BA pool in FoxO1-deficient livers reflected altered FoxO1-dependent transcription. Accordingly, we surveyed gene expression changes by an unbiased exon microarray approach to identify possible effectors (Table S2). Gene ontology enrichment analyses demonstrated highly significant changes in lipid metabolism, transport, storage, and biosynthesis in response to FoxO1 ablation after WTD feeding (Figure 3A) (Eden et al., 2009).
Figure 3. Gene expression changes due to hepatic FoxO1 ablation.
(A) Gene ontology enrichment analysis, based on microarrays of liver tissue from WTD-fed L-FoxO1 and L-FoxO1:Ldlr−/− versus littermate controls. Only non-redundant pathways, where P< 0.001 for the pathway, are shown. (B–D) Relative hepatic gene expression by qPCR in (B) WTD-fed L-FoxO:Ldlr−/−, (C) WTD-fed L-FoxO1, and (D) chow-fed L-FoxO1 mice. (E) Relative gene expression by qPCR in ileum from WTD-fed L-FoxO1 mice. (n=6–7 for all) *P <0.05, **P < 0.01, ***P < 0.001, by Student's t-tests. Data are presented as mean ± S.E.M.
We saw a striking dysregulation of BA synthesis genes in L-FoxO1 mice. Consistent with the changes in BA composition, Cyp8b1, encoding the 12α-hydroxylase enzyme, was sharply downregulated in all tested conditions (Figure 3B–D), as were enzymes involved in other steps of BA metabolism, including Cyp7a1, Cyp27a1, Cyp7b1 (Figure 3B–D, Table S2). Canalicular cholesterol transporters Abcg5 and Abcg8, two FoxO1 targets (Biddinger et al., 2008a), were also downregulated, as were the canalicular bile salt export pump (Bsep), and some sinusoidal BA uptake transporters (Figure 3B,C, Table S2).
Consistent with their high liver TGs, very long chain FAs, and glycerolipids, WTD-fed L-FoxO1 (and L-FoxO1:Ldlr−/−) mice showed increased expression of genes involved in FA synthesis, elongation, glycerolipid formation, and lipid storage, including Fasn, Acly, and Gpam (Figure 3B–C, Table S2). However, these changes occurred in the absence of changes to known regulators of lipid synthesis and oxidation, including sterol response element binding protein (Srebp)-1c and its post-translational activator Insig-2a (Goldstein et al., 2006), liver × receptor–α (Lxrα, encoded by Nr1h3), Pparα and its target genes (Figure 3B,C). Carbohydrate response element binding protein (Chrebp) was reduced, but its target Pklr was unchanged (Table S2). Genes involved in lipoprotein assembly and turnover Lpl, Angptl3, Angptl4, Apoc3, and Mttp showed minor, or no changes (Table S2).
Cholesterol biosynthesis genes, such as Hmgcs1, Hmgcr, and Cyp51, were increased in WTD-fed L-FoxO1 and L-FoxO1:Ldlr−/− mice (Figure 3B,C, Table S2). Srebp-2, which promotes cholesterol biosynthesis genes as well as its own expression, also tended to be elevated. These findings might be related to the elevated serum cholesterol levels in L-FoxO1:Ldlr−/− mice. Their significance in L-FoxO1 mice will be discussed below.
Changes in known BA regulators cannot explain the defects of L-FoxO1 mice
The observed changes in BAs could potentially explain the alterations of TG and cholesterol homeostasis seen in L-FoxO1 mice. Such changes could arise either as a direct effect of impaired FoxO1-dependent transcription of BA synthetic enzymes, or as an indirect effect of the FoxO1 knockout on known regulators of BA synthetic genes. To examine the latter possibility, we measured expression of nuclear receptors required to regulate BA synthesis. Hepatic Fxr (encoded by Nr1h4) represses Cyp7a1 and Cyp8b1 by inducing the nuclear co-repressor Shp (encoded by Nr0b2), which inhibits transcription factor Lrh-1 (encoded by Nr5a2) (Goodwin et al., 2000; Lu et al., 2000). However, Fxr and Lrh-1 were expressed normally, and Shp was reduced, as were other Fxr target genes, including Bsep, Scarb1, and Fetub (Figure 3B,C, Table S2). Fxr activation in the small intestine also represses hepatic Cyp7a1 through Fgf15 and its receptor, Fgfr4 (Chiang, 2009). However, hepatic Fgfr4 and its co-factor β-Klotho (encoded by Klb) were expressed normally. Fgf15 was not expected to be affected directly by loss of liver FoxO1, as it is made in ileum. Consistent with this, we found that Fgf15 mRNA tended to be slightly reduced, although this didn't reach statistical significance. Two intestinal Fxr targets, Ibabp and Shp also tended to be reduced (Figure 3E, Table S2). The apparent reduction of Fxr activity would be expected to result in higher expression of Cyp7a1 and Cyp8b1. Since we saw the opposite result (Figure 3B–D), we conclude that Fxr activity cannot be directly responsible for the defects in BA synthesis genes.
We examined other transcriptional regulators of Cyp7a1, including Lxrα, Hnf4α (hepatocyte nuclear factor 4α), Pparα, Pgc1α (PPARγ coactivator 1α and Rev-erb (encoded by Nr1d2) (Chiang, 2009), but neither they nor their target genes were changed (Table S2). Thus, these data support the alternative hypothesis that reduction of BA synthetic genes is a primary effect of FoxO1 ablation in L-FoxO1 mice.
Regulation of bile acid synthesis genes by FoxO1
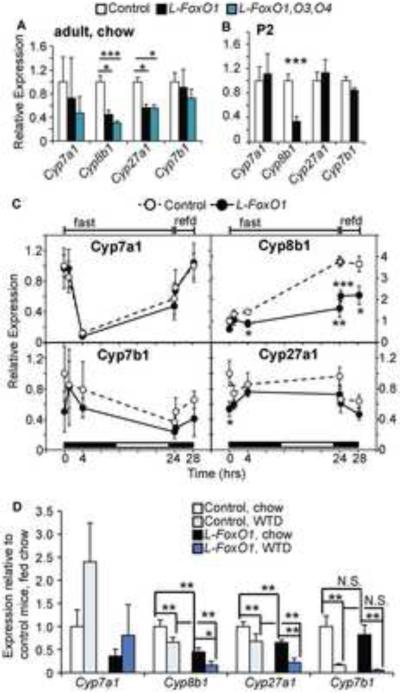
We next interrogated whether the observed changes in BA synthetic genes can be imputed to FoxO1. Based on the dearth of 12α-OH BAs in L-FoxO1 mice, we expected a primary requirement for FoxO1 to promote Cyp8b1. FoxO1 is also reported to promote Cyp7a1 in rodents (Li et al., 2006; Shin and Osborne, 2009). We compared single hepatic FoxO1 knockouts with compound hepatic knockouts of three FoxO isoforms (L-FoxO1,O3,O4) (Haeusler et al., 2010b). Loss of FoxOs reduced the expression of Cyp8b1, Cyp7a1, and Cyp27a1 compared to control littermates, and the reduction in Cyp8b1 tended to be exacerbated by triple knockout, compared to single FoxO1 knockout (Figure 4A). The changes in Cyp8b1 were identified as early as post-natal day 2 in neonates (Figure 4B), and are thus likely to represent primary effects of the FoxO ablation, rather than acquired or compensatory changes (Haeusler et al., 2010b; Matsumoto et al., 2007). In contrast, Cyp7a1, Cyp27a1, and Cyp7b1 were expressed normally at this age, suggesting that changes occur later in life (Figure 4B). Although this developmental snapshot might not adequately capture the complex developmental regulation of BA synthetic genes, the decrease of Cyp8b1 emerges as a key feature of this model. In fact, when we examined the nutritional regulation of these genes, we found only Cyp8b1 to be induced by fasting, in a fashion consistent with its regulation by FoxO1 (Gafvels et al., 1999). Moreover, this regulation was lost in L-FoxO1 mice, indicating that FoxO1 is required for Cyp8b1 induction in vivo (Figure 4C).
Figure 4. Regulation of BA synthetic genes.
(A) Hepatic gene expression in chow-fed L-FoxO1 and L-FoxO1,O3,O4 mice. Values are shown relative to same-strain littermate controls (n=6–8). (B) Hepatic gene expression from mice sacrificed on postnatal day 2 (P2) (n=6). (C) Hepatic gene expression during a fasting-refeeding time course (n=5–6). Mice were fasted up to 24 hours, starting at 8pm, or fasted 24 hours then refed chow for 0.25 or 4.25 hours. Black and white boxes at the bottom of each graph indicate the light/dark cycle. (D) Comparison of gene expression on chow and WTD (n=6–7). *P<0.05, **P < 0.01, ***P < 0.001, by Student's t-tests (A–C) or two-way analysis of variance (D). Data are presented as mean ± S.E.M.
Finally, we assessed the effect of WTD on these genes. Cyp7a1 tended to be induced to the same extent in L-FoxO1 and control mice, consistent with an Lxr-mediated effect, in response to cholesterol contained in the WTD (Kalaany and Mangelsdorf, 2006) (Figure 4D). Cyp8b1, Cyp27a1 and Cyp7b1 were repressed by WTD (Figure 4D). Thus, it appears that the physiologic response to WTD entails repression of Cyp8b1 with simultaneous induction of Cyp7a1, the rate-limiting enzyme of BA synthesis. This combined effect explains the limited rise of some non-12α-OH BAs in normal mice. In the absence of FoxO1, the basal deficiency of Cyp8b1 is compounded by the failure to fully induce Cyp7a1, resulting in the characteristic BA profile of L-FoxO1 mice.
Lipid defects in L-FoxO1 mice are secondary to impaired BA signaling, not to altered glucose homeostasis
We hypothesized that lack of 12α-OH BAs renders the BA pool of L-FoxO1 mice abnormally hydrophilic (Heuman, 1989), and affects TG metabolism and cholesterol absorption (Lefebvre et al., 2009). First, we examined TG metabolism. Hydrophobic BAs are known to reduce TGs (Angelin et al., 1978; Bilz et al., 2006; Miller and Nestel, 1974; Watanabe et al., 2004). This is possibly through the actions of Fxr, as Fxr−/− mice display hypertriglyceridemia, whereas Fxr activation reduces TGs (Evans et al., 2009; Kong et al., 2009; Maloney et al., 2000; Sinal et al., 2000; Watanabe et al., 2004; Zhang et al., 2006). However, hydrophilic BAs are unable to activate Fxr (Makishima et al., 1999; Parks et al., 1999), thus the hydrophilic BA pool of L-FoxO1 mice might explain the reduced Fxr activity.
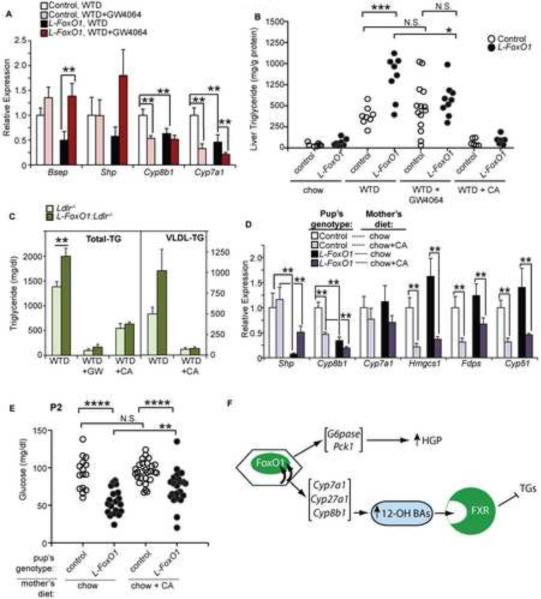
We hypothesized that the hypertriglyceridemia of L-FoxO1 mice was due to low Fxr activity and tested the hypothesis by treating WTD-fed control and L-FoxO1 mice with low doses of the Fxr agonist GW4064 (Maloney et al., 2000). This treatment did not affect body weight (Figure S4A), but was sufficient to activate Fxr, as shown by the induction of Bsep and the repression of Cyp7a1 and Cyp8b1 (Figure 5A). In control mice, this dose of GW4064 had no effect on liver TG; however in L-FoxO1 mice, liver TG decreased to control levels (Figure 5B). Additionally, we treated mice with cholic acid (CA), which is known to reduce TGs (Lefebvre et al., 2009; Watanabe et al., 2004). Consistent with our hypothesis, CA reduced liver TGs in both control and L-FoxO1 mice, and eliminated differences between genotypes (Figure 5B). GW4064 and CA had similar effects in L-FoxO1:Ldlr−/− mice (Figure S4B).
Figure 5. Defects in BA composition, lipid, and glucose metabolism in L-FoxO1 mice.
(A) Hepatic gene expression showing FXR activation due to GW4064 treatment (n=6–7). (B) Liver TGs from L-FoxO1 and control mice fed WTD supplemented with either GW4064 (0.035%) or CA (0.5%) for 3 weeks. (C) Serum total and VLDL-TGs in L-FoxO1:Ldlr−/− and Ldlr−/− controls, after GW4064 (4 daily treatments orally) or supplementation of WTD with CA (1% for 1 week). For comparison in B and C, we also show lipid levels from mice fed chow and WTD (without treatment); these are the same mice whose mean values are shown in Figure 1. (D) Hepatic gene expression and (E) blood glucose levels from P2 neonates, where nursing mothers ate either chow or chow supplemented with CA. Expression of Cyp8b1 and Cyp7a1 from untreated mice are the same measurements presented in Figure 4B. (F) Model of metabolic functions of FoxO1 in liver. Data are presented as mean ± S.E.M. *P<0.05, **P < 0.01, ***P < 0.001, by Student's t-tests (B,C,E) or two-way analysis of variance (A,D). N.S.: not Significant; HGP: Hepatic Glucose Production. See also Figure S4.
In L-FoxO1:Ldlr−/− and Ldlr−/− mice, GW4064 or CA treatment also reduced serum TGs, and eliminated differences between genotypes (Figure 5C). The decrease was primarily, though not exclusively, in VLDL-TGs (Figure 5C, Figure S4C). Together, these data support the hypothesis that after WTD feeding, L-FoxO1 mice develop hypertriglyceridemia due to a dearth of endogenous Fxr ligands (i.e. 12α-OH BAs).
Second, we examined cholesterol homeostasis. Absorption of dietary cholesterol is promoted by hydrophobic, micelle-forming, BAs (Murphy et al., 2005; Wang et al., 2003). L-FoxO1 mice are thus expected to be ineffective at absorbing cholesterol, thereby activating Srebp-2 (Heuman et al., 1989; Murphy et al., 2005). Furthermore, the effect of WTD to repress cholesterol synthetic genes was blunted in L-FoxO1 mice (Table S3), consistent with inefficient Srebp-2 inhibition, providing an explanation for the inappropriate activation of this pathway (Figure 3B,C, Table S2). As these effects of BA are Fxr-independent, we expected that CA, but not GW4064, would increase liver cholesterol and normalize cholesterol synthetic genes in L-FoxO1 mice. Indeed CA, but not GW4064, increased liver cholesterol and normalized Hmgcs1 in L-FoxO1 mice (Figure S4D–F), although the increase in liver cholesterol after CA treatment may also cause cellular stress.
Finally, we examined glucose metabolism. We expected that the improved glucose phenotype of L-FoxO1 mice was independent of the BA phenotype, because FoxO1 directly regulates gluconeogenic genes (Lin and Accili, 2011), and because the BA composition of L-FoxO1 mice predicts worse, not improved, glycemia (Staels and Kuipers, 2007). To test this, we measured blood glucose in L-FoxO1 neonates, while the nursing mothers (Forsyth et al., 1983) were fed either chow alone or chow containing 0.5% CA. This dose was effective, as treated neonates showed induction of Shp and repression of Cyp8b1 and Cyp7a1 (Figure 5D).
As we have previously observed (Haeusler et al., 2010b; Matsumoto et al., 2007), L-FoxO1 neonates from chow-fed mothers showed hypoglycemia (Figure 5E). After supplementing mothers with CA, L-FoxO1 neonates still showed hypoglycemia compared to controls (Figure 5E). Intriguingly, CA treatment slightly elevated the glycemia of L-FoxO1 neonates, without affecting that of controls. We predicted that this may reflect a slight improvement in absorption of milk, which contains a high percentage of lipid, including cholesterol (Meier et al., 1965). Consistent with this, cholesterol synthetic genes were repressed by CA treatment (Figure 5D). Overall, these data suggest that the regulation of glucose metabolism by FoxO1 is independent of its effect on BA composition.
Discussion
The constellation of metabolic dysfunctions known as the metabolic syndrome includes abnormalities of glucose and lipid metabolism that are purportedly caused by defective insulin signaling. The liver is a key site of development of mixed abnormalities of insulin signaling that affect glucose and lipid metabolism (Haeusler and Accili, 2008). However, while the mechanism by which insulin regulates HGP has been well characterized (Lin and Accili, 2011), its control over TG synthesis, secretion, and clearance is less defined (Choi and Ginsberg, 2011). In this work we show that insulin signaling through FoxO1 controls production of 12α-OH BAs, and we propose that these molecules act as regulatory mediators, relaying a signal from insulin to TG synthesis.
This discovery can be integrated into a new model for the role of the insulin-Akt-FoxO1 pathway in regulating multiple aspects of hepatic metabolism (Figure 5F). In normal physiology, FoxO1 maintains the production of 12α-OH BAs by regulating Cyp8b1, and potentially other BA synthetic genes. In turn, these BAs maintain normal Fxr activity, curbing TG levels. In overt diabetes, FoxO1 becomes trapped inside the nucleus, due to impaired ability of insulin to cause its nuclear export, compounded by hyperglycemia (Haeusler et al., 2010a; Kitamura et al., 2005). In this condition, BAs would become skewed towards excess 12α-OH BAs. And, indeed, diabetic models such as alloxan-treated rodents, NOD mice, and mice lacking hepatic insulin receptors show disproportionately high 12α-OH BAs (Akiyoshi et al., 1986; Biddinger et al., 2008a; Uchida et al., 1985; Uchida et al., 1996).
Compellingly, the pattern of increased 12α-OH BAs has also been identified in human patients with diabetes (Brufau et al., 2010). However in humans, contrary to rodents, the most abundant non-12α-OH BA, CDCA, is hydrophobic and is the most potent FXR ligand (Makishima et al., 1999; Parks et al., 1999). Thus FOXO1 induction of CYP8B1, promoting CA formation, at the expense of CDCA, would cause a relative reduction in FXR activation, as CA and its conjugated form T-CA are less potent FXR ligands (Makishima et al., 1999; Parks et al., 1999). The constitutive activation of the FOXO1-CYP8B1-12α-OH BA pathway during diabetes would be expected to result in reduced FXR activity, raising TG.
It is of interest that L-FoxO1 mice express inadequate Cyp8b1 and have a distinct functional defect in 12α-hydroxylation; however, they do not phenocopy Cyp8b1−/− mice (Li-Hawkins et al., 2002; Murphy et al., 2005). The latter presumably have normal Fxr activity, as they express normal Shp and Bsep, and reportedly have no steatosis (Li-Hawkins et al., 2002; Murphy et al., 2005). This is likely due to their compensatory upregulation of Cyp7a1, associated with an expanded BA pool, including a substantial increase in CDCA (Li-Hawkins et al., 2002). In contrast, L-FoxO1 mice show low Cyp7a1 and Cyp27a1 under several different conditions and low Cyp7b1 after WTD feeding, suggesting that FoxO1 is required for full expression of multiple of these genes. Mice lacking Lrh-1 specifically in liver show several similarities to L-FoxO1 mice, including reduced expression of Cyp8b1 and deficiency of 12-OH BAs (Lee et al., 2008; Mataki et al., 2007). However the two models are not identical: Lrh-1 knockouts express normal levels of Cyp7a1, and they have not been reported to develop steatosis. The latter may be attributed to the fact that (1) they have not been challenged with WTD feeding, and (2) Lrh-1 is reportedly required for full induction of Fasn, the rate limiting enzyme of FA synthesis (Matsukuma et al., 2007). This pattern makes L-FoxO1 mice a unique model of concerted impairment of BA synthesis, creating an environment that is permissive for the development of hyperlipidemia, and suggests that the present phenotype is unlikely to be due to a single FoxO1 target gene, but is rather due to the multiple abnormalities of BA production seen in this model.
Prior observations that gain of FoxO1 function in liver results in hyperlipidemia (Kamagate et al., 2008; Matsumoto et al., 2006) seem to be at odds with our current findings, but there are key differences. Transgenic mice overexpressing FoxO1 become hyperglycemic, introducing a major confounder (Kamagate et al., 2008; Nakae et al., 2002). And acute overexpression of FoxO1 by adenovirus causes a paradoxical increase in Akt activity that is hardly seen in diabetes (Matsumoto et al., 2006). Therefore, both models subsume independent downstream signaling events (hyperglycemia, Akt activation) that independently contribute to excess lipid production. In contrast, L-FoxO1 mice have improved glycemia and insulin sensitivity, without ancillary Akt activation.
In addition to their role as FXR agonists, BAs also signal through TGR5. We have not yet explored the consequences of this model on TGR5 signaling, but there may be effects on other aspects of the L-FoxO1 phenotype, such as energy expenditure and metabolic efficiency (Thomas et al., 2008). The present data raise the possibility that altered BA composition is part of the underlying pathogenesis of diabetes, and that redressing BA composition may therapeutically benefit a wide population of patients.
Experimental Procedures
Mice and diets
Only male mice were studied, with the exception of the experiment in P2 mice, for which we did not separate male and female neonates. L-FoxO1 and L-FoxO1,O3,O4 mice have been described (Haeusler et al., 2010b; Matsumoto et al., 2007). L-FoxO1 mice were backcrossed more than 9 generations to C57BL/6J and crossed with Ldlr−/− mice (both from The Jackson Laboratories), except mice used in Figure 4A–C, which were on a mixed genetic background (C57BL/6J × 129 × FVB). Mice were fed either standard chow diet (Purina), or WTD, containing 42% kcal from fat and 0.2% cholesterol (TD 88137, Harlan Teklad), starting at 4–7 weeks of age. Mice were kept on the diet for 10–13 weeks before sacrifice. For GW4064 or CA feeding, mice were fed WTD for 9–10 weeks then switched to the same WTD, crushed to powder and mixed with either 0.035% GW4064 (Tocris), or sodium cholate (Sigma): 0.5% for 3 weeks or 1 day (Watanabe et al., 2004) (for liver lipids and gene expression, respectively in L-FoxO1), or 1% for 1 week (L-FoxO1:Ldlr−/−). GW4064 treatment in L-FoxO1:Ldlr−/− mice was delivered by oral gavage, dissolved in corn oil, at a dose of 18 mg/kg/day for 3 days and a final dose of 9 mg/kg, 5 hrs before sacrifice. Mice are housed in a 12-hour light/dark cycle, with the dark cycle between 7pm–7am. For the timecourse experiment shown in Figure 4C, mice were first “synchronized” by removing food for 4 hours (12–4pm) then replacing food for 4 hours (4–8pm). The 24-hour fast began at 8pm, and mice were sacrificed before the fast (“0”) and periodically throughout the fast. Refed mice were refed chow at 8pm, following the full 24-hour fast. The light/dark cycle was maintained during this experiment.
Glucose metabolic tests
Ad libitum fed measurements were taken between 8–10 am and 5h- fasted measurements taken between 1–3 pm, using OneTouch glucose monitor and strips (Lifescan) and insulin ELISA (Millipore). Intraperitoneal glucose and insulin tolerance tests were performed as described (Haeusler et al., 2010b; Nakae et al., 2002). For phosphorylated Akt analysis, mice were fasted 5h, anesthetized, and 5 mU of insulin (NovoLog, Novo Nordisk) were injected into the inferior vena cava. Livers were collected 5 minutes after injection. Antibodies against total and phospho-Ser473 Akt were from Cell Signaling.
Lipid metabolic tests
To measure hepatic lipids, livers were extracted as described (Folch et al., 1957). Liver and serum lipids were measured by colorimetric assay: TG (Infinity, Thermo Scientific), cholesterol E and free cholesterol (Wako). Lipoprotein fractions were separated by density ultracentrifugation as described (Haeusler et al., 2010a).
Bile acid measurements and metabolomics
A complete list of BA abbreviations is available in Supplemental Table 4. The metabolomics study was performed after 10 weeks on chow or WTD. Livers were collected after a 5-hour fast and snap-frozen. Hepatic BAs (relative values) were measured by LC-MS/MS (-ESI), with the exception of α-MCA, which was measured by GC/MS (Metabolon). The relative increase of T-CDCA in L-FoxO1 mice is underestimated in both WTD and chow-fed mice, as it was detectable in only 4 out of 16 control mice, but 12 out of 16 L-FoxO1 mice; samples below the limit of detection were considered to be at the lowest detectable level. Gallbladder bile was collected by syringe from mice fed the WTD for 10 weeks and pooled (5 per genotype). Fecal samples were collected for two consecutive days, after 8 weeks WTD feeding. For total BA pool, gallbladder and small intestine were collected together with whole liver, minus a small piece used to confirm genotype. For bile, feces, and total BA pool, BAs were extracted and measured by LC-MS/MS as described (Alnouti et al., 2008). Detailed methods are provided in the supplement.
Gene expression
RNA was isolated using TRIzol (Invitrogen). GeneChip Mouse Exon 1.0 ST Arrays were from Affymetrix. Expression analysis was performed using Partek Genomics Suite (Partek). For qPCR, cDNA was synthesized using qScript (QantaBioSciences), and qPCR was performed using SyBr Green (New England Biolabs). Genes were normalized to 18 S. Primer sequences are available upon request.
Statistical analyses
Data are presented as mean ± S.E.M. Data were analyzed by two-tailed Student's t-tests, with the following exceptions: metabolomics data were analyzed by Welch's two-sample t-tests; microarray data was analyzed by analysis of variance (ANOVA), using Partek Genomics Suite; and data comparing two variables (Figures 4D, 5A, 5D, and Figure S4E,F) were analyzed by two-way ANOVA. P < 0.05 was considered significant.
Supplementary Material
Highlights.
-
-
Hypertriglyceridemia in mice lacking liver FoxO1 (L-FoxO1)
-
-
Bile acid synthetic genes are suppressed in L-FoxO1 mice
-
-
Bile acid composition is deficient in 12α-hydroxylated species
-
-
FXR activation rescues the hypertriglyceridemia of L-FoxO1 mice
Acknowledgements
Supported by NIH grants F32HL103103 to RAH, HL87123 and DK58282 to DA, and DK63608 (Columbia University Diabetes & Endocrinology Research Center). We thank A. Tall, I. Tabas, D. Mangelsdorf, J. Horton, and U. Pajvani for valuable comments and discussions, Ana Flete for technical assistance, and members of the Accili laboratory for discussion of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions R.A.H. and D.A. designed and analyzed experiments and wrote the paper. R.A.H. performed experiments. M.P-H and C.D.K. performed bile acid measurements by LC-MS/MS. C.L.W. performed aortic root lesion analysis. All authors edited the manuscript.
References
- Akiyoshi T, Uchida K, Takase H, Nomura Y, Takeuchi N. Cholesterol gallstones in alloxan-diabetic mice. J Lipid Res. 1986;27:915–924. [PubMed] [Google Scholar]
- Alnouti Y, Csanaky IL, Klaassen CD. Quantitative-profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;873:209–217. doi: 10.1016/j.jchromb.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelin B, Einarsson K, Hellstrom K, Leijd B. Effects of cholestyramine and chenodeoxycholic acid on the metabolism of endogenous triglyceride in hyperlipoproteinemia. J Lipid Res. 1978;19:1017–1024. [PubMed] [Google Scholar]
- Biddinger SB, Haas JT, Yu BB, Bezy O, Jing E, Zhang W, Unterman TG, Carey MC, Kahn CR. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat Med. 2008a;14:778–782. doi: 10.1038/nm1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Aleman JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008b;7:125–134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilz S, Samuel V, Morino K, Savage D, Choi CS, Shulman GI. Activation of the farnesoid × receptor improves lipid metabolism in combined hyperlipidemic hamsters. Am J Physiol Endocrinol Metab. 2006;290:E716–722. doi: 10.1152/ajpendo.00355.2005. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Brufau G, Stellaard F, Prado K, Bloks VW, Jonkers E, Boverhof R, Kuipers F, Murphy EJ. Improved glycemic control with colesevelam treatment in patients with type 2 diabetes is not directly associated with changes in bile acid metabolism. Hepatology. 2010 doi: 10.1002/hep.23831. [DOI] [PubMed] [Google Scholar]
- Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends in endocrinology and metabolism: TEM. 2011;22:353–363. doi: 10.1016/j.tem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53:1270–1287. doi: 10.1007/s00125-010-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XC, Copps KD, Guo S, Li Y, Kollipara R, DePinho RA, White MF. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008;8:65–76. doi: 10.1016/j.cmet.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden E, Navon R, Steinfeld I, Lipson D, Yakhini Z. GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics. 2009;10:48. doi: 10.1186/1471-2105-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Mahaney PE, Borges-Marcucci L, Lai K, Wang S, Krueger JA, Gardell SJ, Huard C, Martinez R, Vlasuk GP, et al. A synthetic farnesoid × receptor (FXR) agonist promotes cholesterol lowering in models of dyslipidemia. Am J Physiol Gastrointest Liver Physiol. 2009;296:G543–552. doi: 10.1152/ajpgi.90585.2008. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Forsyth JS, Ross PE, Bouchier IA. Bile salts in breast milk. Eur J Pediatr. 1983;140:126–127. doi: 10.1007/BF00441660. [DOI] [PubMed] [Google Scholar]
- Gafvels M, Olin M, Chowdhary BP, Raudsepp T, Andersson U, Persson B, Jansson M, Bjorkhem I, Eggertsen G. Structure and chromosomal assignment of the sterol 12alpha-hydroxylase gene (CYP8B1) in human and mouse: eukaryotic cytochrome P-450 gene devoid of introns. Genomics. 1999;56:184–196. doi: 10.1006/geno.1998.5606. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- Haeusler RA, Accili D. The double life of Irs. Cell Metab. 2008;8:7–9. doi: 10.1016/j.cmet.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler RA, Han S, Accili D. Hepatic FoxO1 ablation exacerbates lipid abnormalities during hyperglycemia. J Biol Chem. 2010a;285:26861–26868. doi: 10.1074/jbc.M110.134023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler RA, Kaestner KH, Accili D. FoxOs function synergistically to promote glucose production. J Biol Chem. 2010b;285:35245–35248. doi: 10.1074/jbc.C110.175851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuman DM. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J Lipid Res. 1989;30:719–730. [PubMed] [Google Scholar]
- Heuman DM, Hylemon PB, Vlahcevic ZR. Regulation of bile acid synthesis. III. Correlation between biliary bile salt hydrophobicity index and the activities of enzymes regulating cholesterol and bile acid synthesis in the rat. J Lipid Res. 1989;30:1161–1171. [PubMed] [Google Scholar]
- Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- Kalaany NY, Mangelsdorf DJ. LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annu Rev Physiol. 2006;68:159–191. doi: 10.1146/annurev.physiol.68.033104.152158. [DOI] [PubMed] [Google Scholar]
- Kamagate A, Qu S, Perdomo G, Su D, Kim DH, Slusher S, Meseck M, Dong HH. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J Clin Invest. 2008;118:2347–2364. doi: 10.1172/JCI32914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura YI, Kitamura T, Kruse JP, Raum JC, Stein R, Gu W, Accili D. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–163. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Kong B, Luyendyk JP, Tawfik O, Guo GL. Farnesoid × receptor deficiency induces nonalcoholic steatohepatitis in low-density lipoprotein receptor-knockout mice fed a high-fat diet. J Pharmacol Exp Ther. 2009;328:116–122. doi: 10.1124/jpet.108.144600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Schmidt DR, Cummins CL, Choi M, Peng L, Zhang Y, Goodwin B, Hammer RE, Mangelsdorf DJ, Kliewer SA. Liver receptor homolog-1 regulates bile acid homeostasis but is not essential for feedback regulation of bile acid synthesis. Mol Endocrinol. 2008;22:1345–1356. doi: 10.1210/me.2007-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci U S A. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Kong X, Owsley E, Ellis E, Strom S, Chiang JY. Insulin regulation of cholesterol 7alpha-hydroxylase expression in human hepatocytes: roles of forkhead box O1 and sterol regulatory element-binding protein 1c. J Biol Chem. 2006;281:28745–28754. doi: 10.1074/jbc.M605815200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Hawkins J, Gafvels M, Olin M, Lund EG, Andersson U, Schuster G, Bjorkhem I, Russell DW, Eggertsen G. Cholic acid mediates negative feedback regulation of bile acid synthesis in mice. J Clin Invest. 2002;110:1191–1200. doi: 10.1172/JCI16309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell metabolism. 2011;14:9–19. doi: 10.1016/j.cmet.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6:507–515. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- Maloney PR, Parks DJ, Haffner CD, Fivush AM, Chandra G, Plunket KD, Creech KL, Moore LB, Wilson JG, Lewis MC, et al. Identification of a chemical tool for the orphan nuclear receptor FXR. J Med Chem. 2000;43:2971–2974. doi: 10.1021/jm0002127. [DOI] [PubMed] [Google Scholar]
- Mataki C, Magnier BC, Houten SM, Annicotte JS, Argmann C, Thomas C, Overmars H, Kulik W, Metzger D, Auwerx J, et al. Compromised intestinal lipid absorption in mice with a liver-specific deficiency of liver receptor homolog 1. Mol Cell Biol. 2007;27:8330–8339. doi: 10.1128/MCB.00852-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukuma KE, Wang L, Bennett MK, Osborne TF. A key role for orphan nuclear receptor liver receptor homologue-1 in activation of fatty acid synthase promoter by liver × receptor. J Biol Chem. 2007;282:20164–20171. doi: 10.1074/jbc.M702895200. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Han S, Kitamura T, Accili D. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest. 2006;116:2464–2472. doi: 10.1172/JCI27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 2007;6:208–216. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Meier H, Hoag WG, McBurney JJ. Chemical characterization of inbred-strain mouse milk. I. Gross composition and amino acid analysis. J Nutr. 1965;85:305–308. doi: 10.1093/jn/85.3.305. [DOI] [PubMed] [Google Scholar]
- Miller NE, Nestel PJ. Triglyceride-lowering effect of chenodeoxycholic acid in patients with endogenous hypertriglyceridaemia. Lancet. 1974;2:929–931. doi: 10.1016/s0140-6736(74)91134-9. [DOI] [PubMed] [Google Scholar]
- Murphy C, Parini P, Wang J, Bjorkhem I, Eggertsen G, Gafvels M. Cholic acid as key regulator of cholesterol synthesis, intestinal absorption and hepatic storage in mice. Biochim Biophys Acta. 2005;1735:167–175. doi: 10.1016/j.bbalip.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Nakae J, Biggs WH, 3rd, Kitamura T, Cavenee WK, Wright CV, Arden KC, Accili D. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat Genet. 2002;32:245–253. doi: 10.1038/ng890. [DOI] [PubMed] [Google Scholar]
- National Institute of Diabetes and Digestive and Kidney Diseases . National Diabetes Statistics fact sheet: general information and national estimates on diabetes in the United States. U.S. Department of Health and Human Services, National Institute of Health; Bethesda, MD: 2005. [Google Scholar]
- Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- Russell DW. Fifty years of advances in bile acid synthesis and metabolism. J Lipid Res. 2009;50(Suppl):S120–125. doi: 10.1194/jlr.R800026-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DJ, Osborne TF. FGF15/FGFR4 integrates growth factor signaling with hepatic bile acid metabolism and insulin action. J Biol Chem. 2009;284:11110–11120. doi: 10.1074/jbc.M808747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Sorensen LP, Sondergaard E, Nellemann B, Christiansen JS, Gormsen LC, Nielsen S. Increased VLDL-Triglyceride Secretion Precedes Impaired Control of Endogenous Glucose Production in Obese, Normoglycemic Men. Diabetes. 2011;60:2257–2264. doi: 10.2337/db11-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staels B, Kuipers F. Bile acid sequestrants and the treatment of type 2 diabetes mellitus. Drugs. 2007;67:1383–1392. doi: 10.2165/00003495-200767100-00001. [DOI] [PubMed] [Google Scholar]
- Tabas I, Tall A, Accili D. The impact of macrophage insulin resistance on advanced atherosclerotic plaque progression. Circ Res. 2010;106:58–67. doi: 10.1161/CIRCRESAHA.109.208488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- Uchida K, Makino S, Akiyoshi T. Altered bile acid metabolism in nonobese, spontaneously diabetic (NOD) mice. Diabetes. 1985;34:79–83. doi: 10.2337/diab.34.1.79. [DOI] [PubMed] [Google Scholar]
- Uchida K, Satoh T, Takase H, Nomura Y, Takasu N, Kurihara H, Takeuchi N. Altered bile acid metabolism related to atherosclerosis in alloxan diabetic rats. J Atheroscler Thromb. 1996;3:52–58. doi: 10.5551/jat1994.3.52. [DOI] [PubMed] [Google Scholar]
- Wan M, Leavens KF, Saleh D, Easton RM, Guertin DA, Peterson TR, Kaestner KH, Sabatini DM, Birnbaum MJ. Postprandial Hepatic Lipid Metabolism Requires Signaling through Akt2 Independent of the Transcription Factors FoxA2, FoxO1, and SREBP1c. Cell Metabolism. 2011;14:516–527. doi: 10.1016/j.cmet.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DQ, Tazuma S, Cohen DE, Carey MC. Feeding natural hydrophilic bile acids inhibits intestinal cholesterol absorption: studies in the gallstone-susceptible mouse. Am J Physiol Gastrointest Liver Physiol. 2003;285:G494–502. doi: 10.1152/ajpgi.00156.2003. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, Moore DD, Auwerx J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, Willson TM, Edwards PA. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A. 2006;103:1006–1011. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.