Abstract
(±)-3,4-Methylenedioxymethamphetamine (MDMA) is an illicit drug that evokes transporter-mediated release of serotonin (5-HT) in the brain. 5-HT transporter (SERT) proteins are also expressed in non-neural tissues (e.g., blood), and evidence suggests that MDMA targets platelet SERT to increase plasma 5-HT. Here we tested two hypotheses related to the effects of MDMA on circulating 5-HT. First, to determine if MDMA metabolites might contribute to actions of the drug in vivo, we used in vitro microdialysis in rat blood specimens to examine the effects of MDMA and its metabolites on plasma 5-HT. Second, to determine whether effects of MDMA on plasma 5-HT might be used as an index of central SERT activity, we carried out in vivo microdialysis in blood and brain after intravenous MDMA administration. The in vitro results show that test drugs evoke dose-related increases in plasma 5-HT ranging from two- to sevenfold above baseline, with MDMA and its metabolite, (±)-3,4-methylenedioxyamphetamine (MDA), producing the largest effects. The ability of MDMA and related analogs to elevate plasma 5-HT is correlated with their potency as SERT substrates in rat brain synaptosomes. The in vivo results reveal that MDMA causes concurrent increases in extracellular 5-HT in blood and brain, but there are substantial individual differences in responsiveness to the drug. Collectively, our findings indicate that MDMA and its metabolites increase plasma 5-HT by a SERT-dependent mechanism, and suggest the possibility that measures of evoked 5-HT release in blood may reflect central SERT activity.
Keywords: Blood, Brain, MDMA, Microdialysis, Platelet, Serotonin (5-HT), Serotonin transporter (SERT)
1. Introduction
(±)-3,4-Methylenedioxymethamphetamine (MDMA or Ecstasy) is a widely abused illicit drug. The popularity of MDMA is most likely related to its unique profile of psychotropic actions, which includes amphetamine-like stimulant effects coupled with feelings of increased emotional sensitivity and closeness to the others (Liechti and Vollenweider, 2001; Parrot et al., 2001). Ecstasy tablets ingested by humans contain a racemic mixture of (+) and (-) isomers of MDMA, and both enantiomers display biological activity (Baumann et al., 2007; Fantegrossi et al., 2009). Upon systemic administration, MDMA is extensively metabolized by hepatic mechanisms in humans and laboratory animals (de la Torre et al., 2004; Baumann et al., 2009). N-demethylation of MDMA yields the amphetamine analog MDA. O-demethylenation of MDMA and MDA gives rise to catechol metabolites 3,4-dihydroxymethamphetamine and 3,4-dihydroxyamphetamine, which are further O-methylated to form 4-hydroxy-3-methoxymethamphetamine (HMMA) and 4-hydroxy-3-methoxyamphetamine (HMA), respectively (de la Torre et al. 2004).
MDMA and MDA are known to cause the release of 5-HT, dopamine and norepinephrine from neurons by acting as substrates for monoamine transporter proteins (Crespi et al., 1997; Rothman et al., 2001). Like other transporter substrates, MDMA and MDA bind to plasma membrane transporters and are translocated into the cytoplasm where they promote nonexocytotic transmitter release (Rudnick and Wall, 1992; Verrico et al., 2007). The precise mechanism underlying transporter-mediated release is not completely understood but probably involves drug-induced phosphorylation of cytoplasmic domains on the transporter, which triggers reversal of normal transporter flux (i.e., reverse transport) (Robertson et al., 2009; Sitte and Freissmuth, 2010). It is noteworthy that few studies have examined the molecular mechanism of the various hydroxylated metabolites of MDMA (see Forsling et al., 2002; Escobedo et al., 2005).
Historical evidence has implicated central 5-HT neurons in the regulation of mood, and the acute release of neuronal 5-HT mediates most of the subjective effects produced by MDMA in humans (Liechti and Vollenweider, 2001; Parrot, 2001). In vivo microdialysis studies in rat brain demonstrate that MDMA evokes large dose-dependent increases in extracellular 5-HT, and to a lesser extent dopamine, consistent with the molecular mechanism of the drug (Gudelsky and Nash, 1996; Baumann et al., 2008). MDMA-induced release of 5-HT is blocked by the uptake inhibitor fluoxetine, confirming the critical role of 5-HT transporter (SERT) proteins in this effect. Because SERT sites are widely expressed in non-neuronal tissues such as blood and lung (Ramamoorthy et al., 1993; Ni and Watts, 2006), it seems logical to assume that MDMA has direct actions in the periphery. In the bloodstream, plasma 5-HT levels are kept very low (i.e., ~1 nM) by SERT-mediated uptake of 5-HT into platelets and by metabolism to 5-hydroxyindolacetic acid (5-HIAA) (Mercado et al., 2010). Recent studies in rats demonstrate that administration of MDMA and other amphetamine analogs causes dose-dependent elevations in plasma 5-HT. The ability of amphetamine-like drugs to increase plasma 5-HT is related to their potency as SERT substrates, suggesting that platelet SERT proteins are involved in this process (Zolkowska et al., 2006). Here we use the term “transporter substrate” to describe those drugs which cause transporter-mediated transmitter release via a reverse transport process.
The experiments in the present study were designed to address two hypotheses related to effects of MDMA on circulating 5-HT in rats. First, to determine if metabolites of MDMA might contribute to the actions of the drug in vivo, we used in vitro microdialysis to compare the ability of MDMA and its metabolites (i.e., MDA, HMMA and HMA) to release 5-HT in blood specimens obtained from drug-naïve catheterized rats. Second, to determine whether MDMA-induced changes in plasma 5-HT might reflect central SERT activity, we carried out simultaneous microdialysis in blood and brain after intravenous MDMA administration. Our findings demonstrate that MDMA and MDA evoke dose-related elevations in plasma 5-HT, whereas HMMA and HMA are less effective. The ability of MDMA and its metabolites to increase plasma 5-HT correlates with SERT-mediated release of 5-HT from synaptosomes, suggesting a role for platelet SERT proteins. Finally, the blood and brain microdialysis data reveal a positive relationship between MDMA-induced 5-HT release in both compartments, but large individual differences in responsiveness to the drug were noted.
2. Materials and Methods
2.1. Animals
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 250-300 g were double-housed (lights on: 0600-1800 h) in conditions of controlled temperature (22 ± 2°C) and humidity (45 ± 5%), with free access to food and water. Experiments were carried out in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) as revised in 1996. Vivarium facilities were fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and study procedures were approved by the NIDA, Intramural Research Program (IRP), Animal Care and Use Committee.
2.2. Drugs and reagents
(±)-3,4-Methylenedioxymethamphetamine HCl (MDMA, FW 229.7), (±)-3,4-methylenedioxyamphetamine HCl (MDA, FW 217.7), (+)-methamphetamine HCl (methamphetamine, FW 185.7), (+)-amphetamine sulfate (amphetamine, FW 368.5) and pentobarbital sodium were obtained from the National Institute on Drug Abuse, IRP Pharmacy. (±)-4-Hydroxy-3-methoxymethamphetamine HCl (HMMA, FW 232.7) and (±)-4-hydroxy-3-methoxyamphetamine HCl (HMA, FW 217.7) were synthesized by Dr. Bruce E. Blough. Monochloroacetic acid was obtained from Mallinckrodt Baker Inc. (Phillipsburg, NJ, USA), while all other reagents required for analytical assays were obtained from Sigma Chemical Co. (St Louis, MO, USA). For in vitro studies, drug solutions were prepared fresh daily by diluting powders in Ringer's solution which contained 150 mM NaCl, 3 mM KCl, 1.4 mM CaCl2, and 0.8 mM MgCl2. For in vivo studies, MDMA was diluted in sterile saline immediately prior to i.v. administration.
2.3. Surgical procedures
After two weeks of acclimation to the vivarium, rats were transported to the surgery room and anesthetized with sodium pentobarbital (60 mg/kg, i.p.). All rats used for blood specimen withdrawal received an indwelling intravenous (i.v.) catheter. Catheters made of Silastic tubing (Dow Corning, Midland, MI, USA) were filled with sterile saline and surgically implanted into the jugular vein as previously described (Baumann et al., 2009). Briefly, the proximal end of the catheter was inserted into the r. jugular vein and advanced to the atrium, whereas the distal end was exteriorized on the nape and plugged with a metal stylet. In rats used for brain microdialysis, intracranial guide cannulae were implanted immediately after the catheter surgery. Stereotaxic surgery was carried out as previously described (Baumann et al., 2008). Rats were placed into a stereotaxic apparatus, and guide cannulae (CMA 12, CMA Microdialysis, North Chelmsford, MA, USA) were implanted according to the coordinates: 1.6 mm anterior and 1.6 mm lateral to bregma, 6.0 mm ventral to the surface of dura. At the end of the procedure, the proximal cannula tip resided just above the n. accumbens, while the distal tip was exteriorized on top of the head and plugged with a metal stylet. Rats were single-housed postoperatively and allowed at least one week to recover.
2.4. In vitro microdialysis in blood
Between 8-9 AM, rats bearing indwelling jugular catheters were moved into the testing room and allowed to acclimate to the surroundings for 1 h. Feeding trays were removed and wire lids were placed atop the cages. Polyethylene extension tubes (30 cm) were filled with sterile saline, connected to i.v. catheters, and threaded outside the cages. Catheters were flushed with 0.3 ml of 48 IU/ml heparin saline to facilitate blood withdrawal. Blood specimens (0.3 ml) were withdrawn from drug-naïve donor rats; up to four specimens were drawn from each rat during an experiment, and each rat was used only twice for blood withdrawal, allowing at least one week between experimental sessions. Specimens were gently transferred to 300 μl polypropylene tubes containing 20 μl of 1000 IU/ml heparin and kept at room temperature. An equal volume of sterile saline was infused into the catheters after each blood withdrawal to maintain volume and osmotic homeostasis. Microdialysis probes (4 mm exchange surface, MAB 6, SciPro, Inc., Sanborn, NY, USA) were immediately placed into blood specimens and perfused with Ringer's solution pumped at a flow rate of 1.0 μl/ min. Dialysate efflux was collected at 15 min intervals and assayed for 5-HT and 5-HIAA using high performance liquid chromatography with electrochemical detection (HPLC-ECD). Each blood specimen was dialyzed for 60 min, to generate four dialysate samples. Two baseline dialysate samples were collected before addition of test drugs, and two dialysate samples were collected thereafter. Solutions of test drug were prepared in Ringers’ solution, transferred to perfusion syringes, and delivered into blood via reverse dialysis. Concentrations of 1, 3 or 10 μM were administered, with only a single dose examined in each blood specimen. In vitro probe recoveries were performed before and after microdialysis using a 10 pg/5 μl standard of 5-HT in Ringers’ solution.
2.5. In vitro release assays in synaptosomes
The ability of MDMA and its analogs to evoke the release of radiolabeled substrates was evaluated in rat brain synaptosomes as previously described (Rothman et al., 2001) with minor modifications (Rothman et al., 2003). Briefly, in vitro release assays were conducted using [3H]5-HT as the radiolabeled substrate for SERT or [3H]MPP+ as the radiolabeled substrate for norepinephrine transporters (NET) and dopamine transporters (DAT). Rats were exposed to 2 minutes of carbon dioxide narcosis, followed by decapitation and brain removal. Whole brain minus cerebellum (for SERT and NET assays) or striatum (for DAT assays) was homogenized in ice-cold 10% sucrose containing 1 μM reserpine. For SERT release assays, 100 nM nomifensine and GBR12935 were added to the sucrose solution to block uptake of [3H]5-HT into norepinephrine and dopamine terminals. For the NET release assays, 100 nM GBR12935 and citalopram were added to block [3H]MPP+ uptake into dopamine and 5-HT terminals. For DAT release assays, 100 nM desipramine and citalopram were added to block [3H]MPP+ uptake into norepinephrine and 5-HT terminals. After 12 strokes with a Potter-Elvehjem homogenizer, homogenates were centrifuged at 1000 × g for 10 min at 4 oC, and the supernatants (i.e., synaptosomes) were retained on ice. Synaptosomes were incubated to steady state (60 min) with 5 nM [3H]5-HT or [3H]MPP+ in Krebs-phosphate buffer (without BSA; pH 7.4) which contained 154.4 mM NaCl, 2.9 mM KCl, 1.1 mM CaCl2, 0.83 mM MgCl2, 5 mM glucose, 1 mg/mL ascorbic acid, 50 μM pargyline plus 1 μM reserpine in a polypropylene beaker with stirring at 25°C. To commence the assay, 850 μl of preloaded synaptosomes were added to 12 x 75 mm polystyrene test tubes that contained 150 μl test drug in assay buffer plus 1 mg/ml BSA. After 5 min ([3H]5-HT assays) or 30 min ([3H]MPP+ assays) the release reaction was terminated by dilution with 4 ml wash buffer (10 mM Tris-HCl pH 7.4 containing 0.9% NaCl at 25 °C) followed by rapid vacuum filtration over Whatman GF/B filters using a Brandel Harvester. Filters were rinsed twice with 4 ml wash buffer using the Brandel Harvester, and the retained tritium was counted by a Taurus liquid scintillation counter at 40% efficiency after an overnight extraction in 3 ml scintillation cocktail.
2.6. In vivo microdialysis experiments
On the evening prior to brain microdialysis, extension tubes were connected to catheters, and microdialysis probes (2 mm exchange surface, CMA/12, CMA/Microdialysis) were inserted into the n. accumbens via the guide cannulae. Dialysis probes were perfused in situ overnight with Ringer's solution pumped at a flow rate of 1.0 μl/ min. The next morning, catheters were flushed with 0.3 ml of 48 IU/ml heparin saline to facilitate blood withdrawal. Brain dialysate samples were collected at 20-min intervals and blood specimens were drawn on the same schedule. Brain dialysates were immediately assayed for endogenous 5-HT using HPLC-ECD as described elsewhere (Baumann et al., 2008), whereas blood specimens were transferred to 300 μl tubes and dialyzed for 20 min. Blood dialysates were assayed for 5-HT and 5-HIAA using HPLC-ECD, as described for the in vitro studies. Once three baseline dialysates were collected from brain and blood, rats received 0.3 mg/kg i.v. MDMA at time 0 followed by 1 mg/kg i.v. MDMA 60 min later. MDMA was dissolved in sterile saline immediately before use and doses are expressed as the salt. Three dialysate samples from brain and blood were collected and assayed after each drug condition.
2.7. HPLC-ECD analysis of 5-HT and 5-HIAA
Aliquots of the dialysate (5 μl) were injected onto a microbore HPLC column (Unijet, 100 × 1 mm, 5 μM ODS, Bioanalytical Systems, Inc, West Lafayette, IN, USA) that was coupled to an amperometric detector (Model LC-4C, BAS, Inc.). A glassy carbon electrode was set at a potential of +650 mV relative to Ag/AgCl reference. Mobile phase consisted of 180 μM Na2EDTA, 150 mM monochloroacetic acid, 125 mM NaOH, and 318 μM sodium octanesulfonic acid, with 4.5 % MeOH and 4.5% CH3CN per liter of water (final pH= 3.15). Mobile phase was pumped through the column at 60 μl/min (Model 260D, Teledyne ISCO, Lincoln, NE, USA). Chromatographic data were acquired on-line and exported to a Millennium software system (Waters Associates, Milford, MA, USA). The concentration of 5-HT in dialysate samples was compared to known standards, and the lower limit of detection was 0.05 pg/5 μL (0.047 nM).
2.8. Statistical analyses
Statistical analyses were carried out using Prism 5 (GraphPad Software, Inc., San Diego, CA, USA) unless otherwise noted. Raw 5-HT values are mean ± S.E.M. expressed as pg/5 μl sample. For in vitro microdialysis studies, the first 2 dialysate samples collected before any treatment were considered baseline. In vitro time-course data were evaluated by two-way ANOVA (dose x time) to determine main effects and interactions for each test drug. Mean drug effects were then calculated at each dose by dividing the mean 5-HT value (pg/5 μl) for two post-drug samples by the mean 5-HT value for two pre-drug samples, and this ratio was multiplied by 100 (Matthews et al., 1990). Mean effects were analyzed by one-way ANOVA followed by Tukey's post hoc test to determine differences across dose for each drug, and differences between drugs at each dose. EC50 values were determined from release data using the nonlinear least squares curve fitting program MLAB-PC (Civilized Software, Bethesda, MD, USA). The mean drug effects on plasma 5-HT at the 10 μM dose were plotted against the corresponding pEC50 values for transporter-mediated release in synaptosomes, then these data were analyzed using Pearson's correlation coefficient. For in vivo studies, the first 3 dialysate samples from blood and brain before any treatment were considered baseline. In vivo data were evaluated by one-way ANOVA followed by Tukey's post hoc test. The relationship between dialysate 5-HT from blood and brain at individual time points was evaluated using Pearson's correlation coefficient. Mean group data, and data from individual rats, were subjected to correlation analyses. A value of P < 0.05 was considered the minimum criterion for statistical significance.
3. Results
3.1. Baseline 5-HT levels in plasma
For all animals used in the microdialysis experiments (N = 40 rats), the mean basal concentration of dialysate 5-HT in blood was 0.54 ± 0.04 pg/5 μL (i.e., 0.51 ± 0.04 nM). Baseline plasma 5-HT levels did not differ significantly among the various treatment groups for the in vitro and in vivo experiments. It should be noted that microdialysis probes had in vitro recovery rates of approximately 25% and this value did not change before, during or after experiments where probes were immersed in sequential blood specimens. Preliminary studies have shown that in vitro probe recoveries determined in artificial salt solution do not necessarily reflect probe recovery characteristics in complex biological matrices. Thus, we did not “correct” 5-HT values for probe recovery.
3.2. In vitro microdialysis experiments
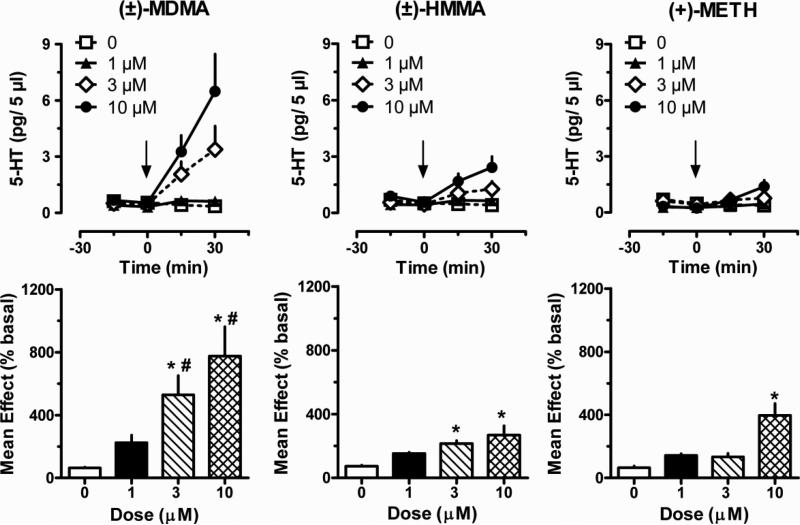
Figure 1 depicts the effects of MDMA, HMMA and methamphetamine on plasma 5-HT, when drugs were administered directly into blood specimens via reverse dialysis. The raw time-course data for MDMA showed significant main effects of dose [F(3,64) = 10.72; P < 0.0001] and time [F(3,64) = 10.32; P < 0.0001], with an interaction between these factors [F(9,64) = 4.168; P < 0.001]. The mean effect data demonstrated that MDMA increased plasma 5-HT to 2-, 5- and 7.5-fold above control values after 1, 3 and 10 μM, respectively. Effects of MDMA on 5-HT were significant with respect to 0 dose control at 3 and 10 μM doses [F(3,16) = 7.65; P < 0.01]. HMMA significantly increased plasma 5-HT levels, and there were main effects of dose [F(3,64) = 10.51; P < 0.0001], time [F(3,64) = 6.42; P < 0.001], and an interaction between factors [F(9,64) = 2.57; P < 0.01]. Mean effect data showed that HMMA had less robust actions when compared to MDMA, nevertheless HMMA significantly increased plasma 5-HT above control at 3 and 10 μM doses [F(3,16) = 7.21; P < 0.01]. The methamphetamine data revealed significant effects of dose [F(3,64) = 4.87; P < 0.01] and time [F(3,64)=6.51; p<0.001], with an interaction between these factors [F(9,64)=4.24; p<0.001]. Mean effect data showed that methamphetamine significantly increased plasma 5-HT above control only at the 10 μM dose [F(3,16) = 13.24; P < 0.0001)]. A comparison of mean effects across all three drugs demonstrated that MDMA elicited significantly greater elevations in plasma 5-HT than HMMA or methamphetamine at the 3 μM [F(2,12) = 8.15; P < 0.01] and 10 μM doses [F(2,12) = 4.76; P < 0.05]. Plasma 5-HIAA levels were not affected by MDMA, HMMA or methamphetamine at any dose (data not shown).
Figure 1.
Effects of MDMA, HMMA and methamphetamine on dialysate 5-HT concentrations measured in blood specimens obtained from drug-naïve catheterized rats. Blood specimens were withdrawn and dialyzed in vitro as described under Materials and Methods. Test drugs were dissolved in Ringer's solution to yield the appropriate concentration and added directly to blood specimens via reverse dialysis at 0 min. Top panels show time-course 5-HT data (pg/5 μl) whereas bottom panels depict mean drug effects at each dose (% basal). Data are mean ± S.E.M. for N=5 rats/group. * P < 0.05 compared to 0 dose group for a given drug; # P < 0.05 compared to HMMA and methamphetamine at a given dose.
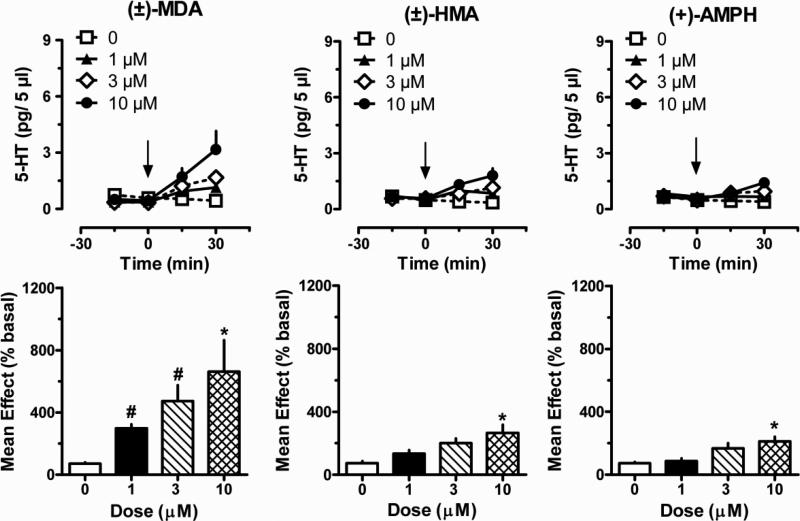
Figure 2 depicts the effects of MDA, HMA and amphetamine on plasma 5-HT. Time-course data for MDA showed significant main effects of dose [F(3,64) = 6.43; P < 0.001] and time [F(3,64) = 13.00; P < 0.0001], with an interaction between factors [F(9,64) = 3.60; P < 0.001]. The mean effect data demonstrated that MDA increased plasma 5-HT to 3-, 4.5- and 6.5-fold above control after 1, 3 and 10 μM. Effects of MDA on 5-HT were significant with respect to 0 dose control at the 10 μM dose [F(3,16) = 4.19; P < 0.01]. HMA significantly increased plasma 5-HT levels, with main effects of dose [F(3,64) = 10.08; P < 0.0001], time [F(3,64) = 8.57; P < 0.0001], and an interaction between factors [F(9,64) = 3.65; P < 0.001] . Mean effect data showed that HMA caused smaller effects when compared to MDA, but HMA significantly increased plasma 5-HT above control at 10 μM [F(3,16) = 6.20; P < 0.01]. Amphetamine had significant effects on dose [F(3,64) = 4.17; P < 0.01] and time [F(3,64) = 2.77; P < 0.05], with an interaction between factors [F(9,64) = 2.05; P < 0.05]. Mean effect data revealed that amphetamine significantly increased plasma 5-HT above control only at the 10 μM dose [F(3,16) =7.62; P < 0.01]. A comparison of mean effects across all three drugs demonstrated that MDA elicited significantly greater elevations in plasma 5-HT than HMA or amphetamine at the 1 μM [F(2,12) = 26.95; P < 0.001] and 3 μM doses [F(2,12) = 6.94; P < 0.01]. Plasma 5-HIAA levels were not affected by MDA, HMA or methamphetamine at any dose (data not shown).
Figure 2.
Effects of MDA, HMA and amphetamine on dialysate 5-HT concentrations measured in blood specimens obtained from drug-naïve catheterized rats. Blood specimens were withdrawn and dialyzed in vitro as described under Materials and Methods. Drugs were dissolved in Ringer's solution to yield the appropriate concentration and added directly to blood specimens via reverse dialysis at 0 min. Top panels show time-course 5-HT data (pg/5 μl) whereas bottom panels depict mean drug effects at each dose (% basal). Data are mean ± S.E.M. for N=5 rats/group. * P < 0.05 compared to 0 dose group for a given drug; # P < 0.05 compared to HMA and amphetamine at a given dose.
3.3. In vitro release of tritiated transporter substrates
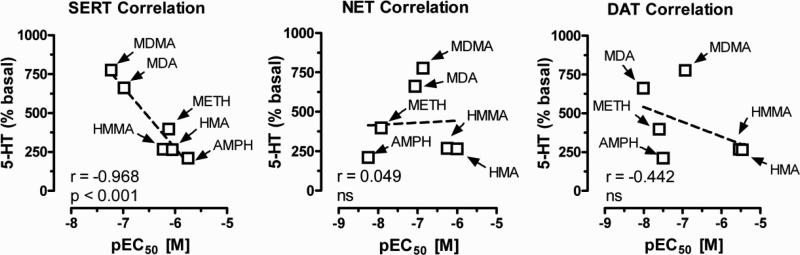
Previous studies from our laboratory have reported structure-activity relationships for amphetamine-type drugs as monoamine releasers in rat brain synaptosomes (Baumann et al., 2007; Rothman et al., 2001; Rothman et al., 2003). Such studies reveal that the EC50 value for inducing efflux of radiolabeled substrate (i.e., [3H]5-HT) is an accurate measure of a drug's releasing potency. The data in Table 1 summarize the effects of MDMA and other test drugs on the release of radiolabeled substrate under conditions that were optimized for SERT, NET and DAT. All of the test drugs examined were fully efficacious (i.e., Emax of ~100%) in their ability to release radiolabeled substrate from synaptosomes. MDMA and MDA displayed the highest potency as SERT-mediated releasers of [3H]5-HT, with EC50 values of 58 ± 7 and 104 ± 14 nM, respectively. However, both drugs were relatively non-selective in this regard, and had comparable potencies across all three transporters. HMMA and HMA were about 10-fold less active as releasers at SERT and NET when compared to MDMA and MDA, while these metabolites had even lower potency at DAT. Methamphetamine and amphetamine had the weakest effects on SERT-mediated release of [3H]5-HT, but both drugs were powerful releasers of [3H]MPP+ via NET and DAT. For example, methamphetamine exhibited 60- and 30-fold greater potency at NET and DAT as compared to effects at SERT. The data in Figure 3 demonstrate that the ability of test drugs to increase plasma 5-HT at the 10 μM dose is negatively correlated with the pEC50 values for SERT-mediated release of [3H]5-HT from synaptosomes (r=-0.968; P < 0.001). Importantly, because lower pEC50 values indicate higher potency, the data in Figure 3 actually reveal a positive relationship between SERT potency and ability to increase plasma 5-HT. The extent of 5-HT release in blood is not related to drug effects on NET- or DAT-mediated release of [3H]MPP+, ruling out a role for these transporters. Taken together, the correlation data suggest that MDMA and related analogs increase plasma 5-HT in whole blood by a process involving SERT proteins, most likely those found on platelets.
Table 1.
Effects of MDMA and related analogs on the release of tritiated transporter substrates in synaptosomes.
| Test drug | SERT-mediated release of [3H]-5-HT EC50 (nM) | NET-mediated release of [3H]-MPP+ EC50 (nM) | DAT-mediated release of [3H]-MPP+ EC50 (nM) |
|---|---|---|---|
| (±)-MDMA | 58 ± 7 | 138 ± 14 | 118 ± 8 |
| (±)-HMMA | 589 ± 76 | 625 ± 66 | 2884 ± 364 |
| (+)-Methamphetaminea | 735 ± 45 | 12 ± 1 | 24 ± 2 |
| (±)-MDA | 104 ± 14 | 88 ± 8 | 98 ± 7 |
| (±)-HMA | 897 ± 74 | 694 ± 64 | 3423 ± 244 |
| (+)-Amphetaminea | 1765 ± 94 | 7 ± 1 | 25 ± 4 |
Transporter-mediated release assays were carried out in rat brain synaptosomes as described in Materials and Methods, with data expressed as mean ± S.D. for three separate experiments.
Data for (+)-methamphetamine and (+)-amphetamine are from Baumann et al., 2007.
Figure 3.
Relationship between drug-induced increases in plasma 5-HT and pEC50 values for release of tritiated substrates in rat brain synaptosomes. Mean effects of drugs on plasma 5-HT (i.e., % basal values) were obtained from Figures 2 and 3, whereas pEC50 values were calculated from the molar concentration for each test drug given in Table 1. The mean increase in plasma 5-HT at the 10 μM dose was plotted against the pEC50 value for that drug to release [3H]-5-HT under SERT conditions, and [3H]-MPP+ under NET or DAT conditions. Pearson's correlation coefficient “r” and P value for significance are given.
3.4. In vivo microdialysis experiments
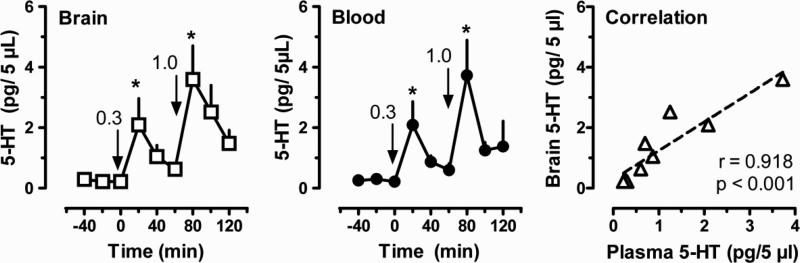
Figure 4 depicts the effects of i.v. MDMA administration on dialysate concentrations of 5-HT in brain and blood from conscious rats undergoing in vivo microdialysis in n. accumbens. For each individual rat, dialysate samples from brain and blood were collected and analyzed concurrently. MDMA significantly increased dialysate 5-HT in brain after 0.3 mg/kg [F(5,30) = 3.39; P < 0.05] and 1.0 mg/kg doses [F(5,30) = 5.44; P < 0.01]. This stimulatory effect was dose-dependent, with 0.3 and 1.0 mg/kg doses elevating 5-HT to 6- and 12-fold above pre-injection baseline, respectively. MDMA also significantly increased plasma 5-HT levels after 0.3 mg/kg [F(5,30) = 4.416; P < 0.01] and 1.0 mg/kg doses [F(5,30) = 5.106; P < 0.05]. This stimulatory effect was dose-dependent, with 0.3 and 1.0 mg/kg doses elevating 5-HT 6- and 10-fold, respectively. The 5-HT concentrations in the first sample after each drug dose were significantly elevated compared to preinjection control in brain and blood, and effects of MDMA were markedly similar in both compartments. Figure 4 also shows the correlation between mean dialysate 5-HT levels measured in brain and blood at each time point. The data reveal a highly significant positive correlation between blood and brain dialysate 5-HT concentrations (P < 0.001), suggesting MDMA-induced 5-HT release in blood may reflect 5-HT release in the brain.
Figure 4.
In vivo effects of MDMA administration on dialysate 5-HT concentrations measured in brain and blood from conscious rats. Dialysate 5-HT in brain is shown in the left panel; dialysate 5-HT in blood is shown in the middle panel; correlation between mean brain and blood measures at each time point is shown in the right panel. MDMA was dissolved in sterile saline and administered by the i.v. route; 0.3 mg/kg was given at 0 min followed by 1.0 mg/kg at 60 min. Dialysates from brain were obtained at 20 min intervals, while blood specimens were withdrawn on the same schedule and immediately dialyzed. 5-HT was assayed by HPLC-ECD as described in Materials and Methods. Data are mean ± S.E.M. for N=6 rats/group, expressed as pg/5 μL samples. * P < 0.05 with respect to preinjection baseline. Pearson's correlation coefficient “r” and P value for significance are given in the right panel.
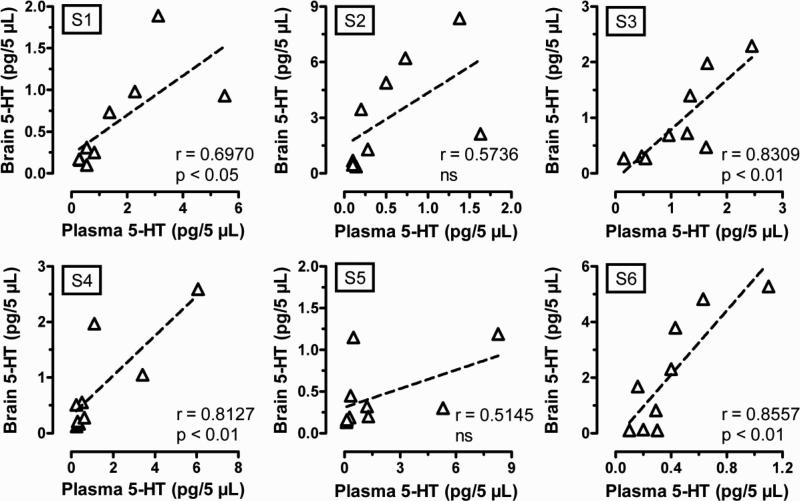
The findings in Figures 4 indicate that there is a strong positive relationship between extracellular 5-HT levels in brain and blood, but inspection of the raw data revealed that there was a highly variable response to MDMA among the individual rats. While all rats displayed dose-dependent increases in 5-HT release, the pg values of 5-HT and magnitude of responses were different between animals. In order to further study these inter-individual differences in responsiveness, we analyzed correlations between dialysate levels of 5-HT in brain and blood for each of the 6 individual rats used for this experiment. The data in Figure 5 show that there is a significant correlation between brain and blood 5-HT values in most of the cases, but two of six rats tested in this study did not show a significant correlation between the 5-HT levels in both compartments.
Figure 5.
Correlation between brain and blood 5-HT levels obtained after MDMA administration for each individual rat tested. MDMA was dissolved in sterile saline, and 0.3 mg/kg i.v. was given at 0 min followed by 1.0 mg/kg i.v. at 60 min. Serial samples from blood and brain were withdrawn at 20-min intervals and were immediately dialyzed as described under Materials and Methods. The data show effects determined before and after injections, and are expressed as pg/5 μL values. Pearson's correlation coefficient “r” and P value are given for the data from each individual rat.
4. Discussion
It is well established that amphetamine, methamphetamine and MDMA promote transporter-mediated release of 5-HT in nervous tissue (Crespi et al., 1997; Rothman et al., 2001; Sitte and Freissmuth, 2010). These drugs also increase plasma concentrations of 5-HT when administered in vivo (Zolkowska et al., 2006), and this effect is related to the ability of a given drug to release [3H]5-HT from synaptosomes. Because platelets and neurons express the same SERT protein (Ramamoorthy et al., 1993; Ni and Watts, 2006), the potency of drugs to release [3H]5-HT should be similar in both cell types. Indeed, data reported by Schuldiner et al. (1993) show that the EC50 value for fenfluramine to increase efflux of [3H]5-HT from platelets is comparable to the corresponding value determined in rat brain synaptosomes (Rothman et al., 2001). Such observations support the notion that amphetamine analogs act as substrates for platelet SERT proteins, thereby explaining their ability to increase plasma 5-HT (see Rudnick and Wall, 1992).
Here we wished to examine whether metabolites of MDMA might contribute to the effects of the drug on plasma 5-HT in rats. Pharmacokinetic studies in humans and rodents have shown that MDMA is metabolized to form HMMA, MDA and HMA after the administration of behaviorally-relevant doses of the drug (de la Torre, 2004; Goni-Allo et al., 2008; Kolbrich et al., 2008; Baumann et al., 2009). In particular, HMMA is a prominent metabolite of MDMA in both species, and could therefore exert pharmacological effects in the periphery. Kolbrich et al. (2008) demonstrated that human subjects given 1.6 mg/kg MDMA (p.o. route) exhibit peak plasma concentrations (Cmax) of 291 ng/ml (1.36 μM) for MDMA and 173 ng/ml (0.81μM) for HMMA. The same investigators showed that MDA and HMA are minor MDMA metabolites, with plasma Cmax values at least ten-fold lower than that of MDMA. A similar profile of MDMA metabolites is found in rats given low-dose MDMA treatments. Baumann et al. (2009) administered 2 mg/kg MDMA (i.p. route) to male Sprague-Dawley rats and reported plasma Cmax of 210 ng/ml (0.99 μM) for MDMA, 186 ng/ml (0.87 μM) for HMMA, 21 ng/ml (0.11 μM) for MDA and 13 ng/ml ( 0.07 μM) for HMA. Collectively, the pharmacokinetic data from humans and rats indicate that circulating concentrations of MDMA and HMMA can reach peak levels of ~1 μM whereas concentrations of MDA and HMA are about ten-fold lower.
In the present study, we assessed the effects of MDMA and its metabolites on plasma 5-HT by administering these drugs directly into whole blood specimens via reverse dialysis. Consistent with previously published data (Zolkowska et al., 2006), we found that MDMA caused potent dose-related increases in plasma 5-HT. It is also shown for the first time that MDA produces marked elevations in plasma 5-HT, whereas HMMA and HMA are less effective. MDMA and MDA exerted robust effects on plasma 5-HT when perfused through the microdialysis probe at doses of 3 and 10 μM. Given that in vitro probe recovery for 5-HT is about 25%, one can assume that the number of drug molecules penetrating the dialysis membrane during reverse dialysis is a small fraction of the concentration present in the perfusion fluid. When viewed in the context of the pharmacokinetic data described above, our in vitro microdialysis data suggest that HMMA, and perhaps MDA, could make minor contributions to the effects of systemically administered MDMA. Importantly, we confirm that the molecular machinery for increasing plasma 5-HT is found within the blood itself, and does not require other tissues (e.g., lung or gut), since 5-HT release was evoked from whole blood specimens incubated in vitro. The ability of drugs to increase plasma 5-HT correlated with their pEC50 values for SERT-mediated release of [3H]5-HT from synaptosomes, but not their pEC50 values for release of [3H]MMP+ via NET or DAT. To the best of our knowledge, the EC50 values for HMMA and HMA reported here (see Table 1) represent the first assessment of the releasing capabilities for these metabolites. Taken together, our in vitro results support the hypothesis that effects of MDMA and its metabolites on plasma 5-HT most likely involve interaction of the drugs at SERT proteins expressed on blood platelets. Future studies should be carried out to examine this hypothesis directly.
Measuring plasma 5-HT is technically challenging. Given that 99% of blood 5-HT is stored in platelets, and platelets are fragile, even minor disturbance or damage to platelets during specimen handling will cause platelet activation or lysis, generating artificial increases in plasma 5-HT. The method that we developed to measure dialysate 5-HT in whole blood specimens minimizes trauma to platelets (e.g., the method does not require centrifugation) and permits an accurate determination of plasma 5-HT. The mean baseline level of dialysate 5-HT in blood collected in these studies (N = 40) was 0.54 ± 0.04 pg/5 μl (i.e., 0.51 ± 0.04 nM). Because in vitro probe recoveries averaged approximately 25%, the “actual” corrected baseline level of plasma 5-HT in our experiments is likely in the range of 2 nM. This level is comparable with plasma 5-HT levels in humans, which are reportedly in the low nanomolar range (Herve et al., 1995).
As noted above, it is generally accepted that SERT expressed in platelets is identical to the one found in nervous tissue (Ni and Watts, 2006). The same single-copy gene encodes for SERT in platelets and neurons (Ramamoorthy et al., 1993), and polymorphisms affecting SERT expression have been shown to influence SERT function in human platelets and brain in a similar manner (Nobile et al. 1999; Heinz et al., 2000). However, despite the apparent similarities between neuronal and platelet SERT, there is no consensus on whether platelet SERT can be used as a valid model of the neuronal protein (Rausch et al., 2005; Uebelhack et al., 2006). At the present time, it is technically difficult to measure SERT binding and function in living human brain. Imaging methods are limited by a number of factors, especially a lack of selective SERT ligands (Elkving et al., 2007; Huang et al., 2010). It would be advantageous to have a reliable peripheral index of SERT-mediated effects of drugs in the brain. To this end, we wanted to see if SERT-mediated release of 5-HT in rat blood specimens could be related to comparable release in the brain. Our working hypothesis was that the activity of SERT in blood and brain would be similar, so drug-induced 5-HT release in blood could be used to provide information about drug actions in the brain. By simultaneously measuring the effects of MDMA injections on dialysate 5-HT in blood and brain, we found parallel dose-related increases in extracellular 5-HT in both compartments. Interestingly, a striking and significant positive correlation was noted between 5-HT release in blood and brain when considering the mean group data for analysis. However, when examining the data from individual rats, we found that there is not always a significant correlation between 5-HT levels in blood and brain.
While there is no simple explanation for the lack of consistent correlations between dialysate 5-HT in blood and brain, there are many possible sources of variability in 5-HT measures. First, there appears to be substantial individual differences in responsiveness to MDMA among the rats tested. For example, some rats exhibited robust behaviors (i.e., 5-HT syndrome) after MDMA administration while others did not (data not shown). The molecular underpinnings of individual differences in drug responsiveness are not known but could be related to differential expression of SERT across subjects. Another factor giving rise to individual differences in plasma 5-HT could be the extent of non-specific 5-HT release from platelets across blood specimens. In particular, behavioral activation can interfere with the collection of blood specimens, via twisting or tangling of the extension catheter; difficulty with blood removal or slight physical impacts to the sampling syringe can activate platelets, causing 5-HT release events (see Viisoreanu and Gear, 2007). Collectively, our in vivo data suggest that MDMA administration increases extracellular 5-HT in blood and brain in a similar manner, but the relationship between peripheral and central effects of the drug may not always be significant. More investigation is needed to determine the complex relationships between platelet and neuronal SERT function in vivo.
There are potential clinical implications of our findings. First, it seems that our method developed in rats could be refined and applied to clinical studies where repeated blood specimens are available for HPLC analysis. Clinical studies could measure the in vivo effects of therapeutic or abused drugs on plasma 5-HT to gauge the effects of those drugs on platelet SERT, and possibly relate this to brain SERT function. In humans, interpreting this type of data would be complicated by SERT polymorphisms, life-time drug exposures, and other factors. Despite such limitations, the concept of identifying peripheral biomarkers for central SERT activity is of great interest. Second, the peripheral SERT substrate activity of MDMA may have relevance to public health. It is well established that (±)-fenfluramine and (+)-fenfluramine are potent SERT substrates that increase the risk for primary pulmonary hypertension in humans (Rothman et al., 1999). The mechanisms underlying the effects of fenfluramines on lung function are not completely understood, but pulmonary SERT proteins and extracellular 5-HT have been implicated in lung pathology (Dempsie and MacLean, 2008). Recently, methamphetamine abuse has been identified as a risk factor for pulmonary hypertension, an effect that could be related to drug interactions at SERT (Chin et al., 2006; Rothman and Baumann, 2007). It is tempting to speculate that chronic abuse of Ecstasy might increase the risk for pulmonary complications in human users due to peripheral SERT substrate activity of MDMA and MDA. Further clinical investigations are warranted to examine this question.
In summary, our in vitro findings support the hypothesis that platelet SERT is involved with the elevation of plasma 5-HT produced by amphetamine-type drugs. It was demonstrated that MDMA and its metabolites produce significant dose-dependent increases in plasma 5-HT when administered directly into blood specimens via reverse dialysis. The structure-activity data show that compounds with potent SERT substrate activity, such MDMA and MDA, are considerably more effective in elevating plasma 5-HT when compared with HMMA and HMA. By simultaneously measuring the effects of MDMA administration on dialysate 5-HT in blood and brain, we demonstrated that there is a positive relationship between SERT activity in brain and blood when analyzing the data of the whole group. Nevertheless, there is substantial variability between subjects in responsiveness to the drug, suggesting caution should be exercised when attempting to glean information about central SERT function from assessment of transporter-mediated release of 5-HT in the bloodstream.
Acknowledgements
This research was generously supported by intramural funding of the Neurosciences Research Program, IMIM-Hospital del Mar, Barcelona, Spain, DIUE de la Generalitat de Catalunya (2009 SGR 718) and by grants from the National Institute on Drug Abuse, 5R01BA017987-01 and Intramural Research Program [Grant 1R01 DA017987].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baumann MH, Wang X, Rothman RB. 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacology. 2007;189:407–424. doi: 10.1007/s00213-006-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Franken FH, Rutter JJ, Rothman RB. Tolerance to 3,4-methylenedioxymethamphetamine (MDMA) in rats exposed to single high-dose binges. Neuroscience. 2008;152:773–784. doi: 10.1016/j.neuroscience.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Zolkowska D, Kim I, Scheidweiler KB, Rothman RB, Huestis MA. Effects of dose and route of administration on pharmacokinetics of (+/-)-3,4-methylenedioxymethamphetamine in the rat. Drug Metab. Dispos. 2009;37:2163–2170. doi: 10.1124/dmd.109.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin KM, Channick RN, Rubin LJ. Is methamphetamine use associated with idiopathic pulmonary arterial hypertension? Chest. 2006;130:1657–1663. doi: 10.1378/chest.130.6.1657. [DOI] [PubMed] [Google Scholar]
- Crespi D, Mennini T, Gobbi M. Carrier-dependent and Ca(2+)-dependent 5-HT and dopamine release induced by (+)-amphetamine, 3,4-methylendioxymethamphetamine, pchloroamphetamine and (+)-fenfluramine. Br. J. Pharmacol. 1997;121:1735–1743. doi: 10.1038/sj.bjp.0701325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre R, Farre M, Roset PN, Pizarro N, Abanades S, Segura M, Segura J, Cami J. Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition. Ther. Drug Monit. 2004;26:137–144. doi: 10.1097/00007691-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Dempsie Y, MacLean MR. Pulmonary hypertension: therapeutic targets within the serotonin system. Br. J. Pharmacol. 2008;155:455–462. doi: 10.1038/bjp.2008.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkving B, Madsen J, Knudsen GM. Neuroimaging of the serotonin reuptake site requires high-affinity ligands. Synapse. 2007;61:882–888. doi: 10.1002/syn.20443. [DOI] [PubMed] [Google Scholar]
- Escobedo I, O'Shea E, Orio L, Sanchez V, Segura M, de la Torre R, Farre M, Green AR, Colado MI. A comparative study on the acute and long-term effects of MDMA and 3,4-dihydroxymethamphetamine on brain monoamine levels after i.p. or striatal adminstration in mice. Br. J. Pharmacol. 2005;144:231–241. doi: 10.1038/sj.bjp.0706071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Murai N, Mathúna BO, Pizarro N, de la Torre R. Discriminative stimulus effects of 3,4-methylenedioxymethamphetamine and its enantiomers in mice: pharmacokinetic considerations. J. Pharmacol. Exp. Ther. 2009;329:1006–1015. doi: 10.1124/jpet.109.150573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsling ML, Fallon JK, Shah D, Tilbrook GS, Cowan DA, Kicman AT, Hutt AJ. The effect of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) and its metabolites on neurohypophysial hormone release from isolated rat hypothalamus. Br. J. Pharmacol. 2002;135:649–658. doi: 10.1038/sj.bjp.0704502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goni-Allo B, Mathuna BO, Segura M, Puerta E, Lasheras B, de la Torre R, Aguirre N. The relationship between core body temperature and 3,4-methylenedioxymethamphetamine metabolism in rats: implications for neurotoxicity. Psychopharmacology. 2008;197:263–278. doi: 10.1007/s00213-007-1027-1. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Nash JF. Carrier-mediated release of serotonin by 3,4-methylenedioxymethamphetamine: implications for serotonin–dopamine interactions. J. Neurochem. 1996;66:243–249. doi: 10.1046/j.1471-4159.1996.66010243.x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Jones DW, Mazzanti C, Goldman D, Ragan P, Hommer D, Linnoila M, Weinberger DR. A relationship between serotonin transporter genotype, in vivo protein expression and alcohol neurotoxicity. Biol. Psychiatry. 2000;47:643–649. doi: 10.1016/s0006-3223(99)00171-7. [DOI] [PubMed] [Google Scholar]
- Herve P, Launay JM, Scrobohaci ML, Brenot F, Simonneau G, Petitpretz P, Poubeau P, Cerrina J, Duroux P, Drouet L. Increased plasma serotonin in primary pulmonary hypertension. Am. J. Med. 1995;99:249–254. doi: 10.1016/s0002-9343(99)80156-9. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zheng MQ, Gerdes JM. Development of effective PET and SPECT imaging agents for the serotonin transporter: has a twenty-year journey reached its destination? Curr. Top. Med. Chem. 2010;10:1499–1526. doi: 10.2174/156802610793176792. [DOI] [PubMed] [Google Scholar]
- Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA. Plasma pharmacokinetics of 3,4-methylenedioxymethamphetamine after controlled oral administration to young adults. Ther. Drug Monit. 2008;30:320–332. doi: 10.1097/FTD.0b013e3181684fa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Vollenweider FX. Which neuroreceptors mediate the subjective effects of MDMA in humans? A summary of mechanistic studies. Hum. Psychopharmacol. 2001;16:589–598. doi: 10.1002/hup.348. [DOI] [PubMed] [Google Scholar]
- Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. Br. Med. J. 1990;333:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado CP, Kilic F. Molecular mechanisms of SERT in platelets: regulation of plasma serotonin levels. Mol. Interv. 2010;10:231–241. doi: 10.1124/mi.10.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W, Watt SW. 5-Hydroxytryptamine in the cardiovascular system: focus on the serotonin transporter (SERT). Clin. Exp. Pharmacol. Physiol. 2006;33:575–583. doi: 10.1111/j.1440-1681.2006.04410.x. [DOI] [PubMed] [Google Scholar]
- Nobile M, Begni B, Giorda R, Frigerio A, Marino C, Molteni M, Ferrarese C, Battaglia M. Effects of serotonin transporter promoter genotype on platelet serotonin transporter functionality in depressed children and adolescents. J. Am. Acad. Child Adolesc. 1999;38:1396–1402. doi: 10.1097/00004583-199911000-00014. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Human psychopharmacology of ecstasy (MDMA): a review of 15 years of empirical research. Hum. Psychopharmacol. 2001;16:557–77. doi: 10.1002/hup.351. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, Ganapathy V, Blakely RD. Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc. Natl. Acad. Sci. 1993;90:2542–2546. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch JL, Johnson ME, Li J, Hutcheson J, Carr BM, Corley KM, Gowans AB, Smith J. Serotonin transport kinetics correlated between human platelets and brain synaptosomes. Psychopharmacology. 2005;180:391–398. doi: 10.1007/s00213-005-2178-6. [DOI] [PubMed] [Google Scholar]
- Robertson SD, Matthies HJG, Galli A. A closer look at amphetamine-induced reverse transport and trafficking of the dopamine and norepinephrine transporters. Mol. Neurobiol. 2009;39:73–80. doi: 10.1007/s12035-009-8053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Methamphetamine and idiopathic pulmonary arterial hypertension: role of the serotonin transporter. Chest. 2007;132:1412–1413. doi: 10.1378/chest.07-0235. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Ayestas MA, Dersch CM, Baumann MH. Aminorex, fenfluramine and chlorphentermine are serotonin transporter substrates. Implications for pulmonary hypertension. Circulation. 1999;100:869–875. doi: 10.1161/01.cir.100.8.869. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Vu N, Partilla JS, Roth BL, Hufeisen SJ, Compton-Toth BA, Birkes J, Young R, Glennon RA. In vitro characterization of ephedrine stereoisomers at biogenic amine transporters and the receptorome reveals selective actions as norepinephrine transporter substrates. J. Pharmacol. Exp. Ther. 2003;307:138–145. doi: 10.1124/jpet.103.053975. [DOI] [PubMed] [Google Scholar]
- Rudnick G, Wall SC. The molecular mechanism of “ecstasy” [3,4-methylenedioxymethamphetamine (MDMA)]: serotonin transporters are targets for MDMA-induced serotonin release. Proc. Natl. Acad. Sci. 1992;89:1817–1821. doi: 10.1073/pnas.89.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner S, Steiner-Mordoch S, Yelin R, Wall SC, Rudnick G. Amphetamine derivatives interact with both plasma membrane and secretory vesicle biogenic amine transporters. Mol. Pharmacol. 1993;44:1227–1231. [PubMed] [Google Scholar]
- Sitte HH, Freissmuth M. The reverse operation of Na+/Cl- coupled neurotransmitter transporters: Why amphetamines take two to tango. J. Neurochem. 2010;112:340–355. doi: 10.1111/j.1471-4159.2009.06474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebelhack R, Franke L, Herold N, Plotkin M, Amthauer H, Felix R. Brain and platelet serotonin transporter in humans: correlation between [123I]-ADAM SPECT and serotonergic measurements in platelets. Neurosci. Lett. 2006;406:153–158. doi: 10.1016/j.neulet.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Viisoreanu D, Gear A. Effect of physiologic shear stresses and calcium on agonist-induced platelet aggregation, secretion and thromboxane A2 formation. Thromb. Res. 2007;120:885–892. doi: 10.1016/j.thromres.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Verrico CD, Miller GM, Madras BK. MDMA (Ecstasy) and human dopamine, norepinephrine and serotonin transporters: implications for MDMA-induced neurotoxicity and treatment. Psychopharmacology. 2007;189:489–503. doi: 10.1007/s00213-005-0174-5. [DOI] [PubMed] [Google Scholar]
- Zolkowska D, Rothman RB, Baumann MH. Amphetamine analogs increase plasma serotonin: Implications for cardiac and pulmonary disease. J. Pharmacol. Exp. Ther. 2006;318:604–610. doi: 10.1124/jpet.106.101618. [DOI] [PubMed] [Google Scholar]