Abstract
Exposure to tobacco smoke during pregnancy is associated with a range of adverse Outcomes in offspring, including cognitive deficits and increased incidence of attention deficit-hyperactivity disorder (ADHD), but there is considerable controversy concerning the causal role of tobacco smoke in these outcomes. To determine whether developmental exposure to the primary psychoactive ingredient in tobacco smoke, nicotine, may cause long-lasting behavioral alterations analogous to those in ADHD, male Sprague-Dawley rats underwent a chronic neonatal nicotine administration regimen which models third-trimester human exposure. Male rat pups were administered nicotine (6 mg/kg/day) by oral gastric intubation on postnatal days 1–7. In adulthood, rats were tested in two decision-making tasks (risky decision making and delay discounting) as well as in free-operant responding for food reward and the elevated plus maze (EPM). Chronic neonatal nicotine attenuated weight gain during nicotine exposure, but there were no effects on performance in either decision-making task, and only a modest decrease in arm entries in the EPM in one sub-group of rats. These data are consistent with previous findings that developmental nicotine exposure has no effect on delay-discounting, and they extend these findings to risky decision making as well. They further suggest that at least some neurocognitive alterations associated with prenatal tobacco smoke exposure in humans may be due to genetic or other environmental factors, including non-nicotine components of tobacco smoke.
Introduction
A large proportion (15%) of women are reported to smoke during pregnancy, despite ample available information about possible adverse outcomes for their children (Substance Abuse and Mental Health Services Administration 2010). Smoking during pregnancy is associated with reduced birth weight, impairments in cognitive function, and increased incidence of attention deficit-hyperactivity disorder (ADHD) in offspring (Winzer-Serhan 2008). Despite these strong associations, however, it has been difficult to establish causal relations between prenatal tobacco smoke exposure and adverse Outcomes (particularly cognitive/behavioral outcomes), as smoking during pregnancy often occurs in the presence of a variety of other risk factors for such adverse outcomes, including young maternal age, poor education, and low socioeconomic status.
To circumvent these confounding factors, animal models have been used to test directly for causal roles of smoking in adverse outcomes in offspring. The majority of studies in animal models have examined the effects of nicotine (the major psychoactive ingredient in tobacco smoke), although a handful have examined effects of tobacco smoke itself (Gaworski et al. 2004; Golub et al. 2007; Slotkin 2008; Winzer-Serhan 2008). These studies typically involve administration of nicotine to either pregnant females or early neonates, as the early neonatal period in rats and mice corresponds roughly to the third trimester of human development (Dobbing and Sands 1979; Winzer-Serhan 2008). Despite the strong associations between cognitive and behavioral deficits in humans exposed to prenatal smoking, the outcomes of these animal studies have been mixed. The effects of prenatal nicotine on tests of learning and memory (which typically engage the hippocampus) are generally mild, and in some cases may be due to effects on non-mnemonic factors such as anxiety or motor function (Levin et al. 1993; Eppolito and Smith 2006; Huang et al. 2007; Winzer-Serhan 2008). Effects on locomotor activity in rodent models have also been variable, with different studies reporting hyperactivity, hypoactivity, or no change (Romero and Chen 2004; LeSage et al. 2006; Huang et al. 2007; Schneider et al. 2011).
Recently, there has been interest in modeling cognitive aspects of ADHD in rodent models of prenatal nicotine exposure. ADHD is associated with suboptimal decision making, including elevations in both impulsive and risky decision making (Barkley et al. 2001; DeVito et al. 2008; Paloyelis et al. 2009). However, a recent study of the effects of prenatal nicotine exposure in rats found no effects on performance in a delay-discounting task, which assesses impulsive decision making (Schneider et al. 2011). This lack of effect could suggest that prenatal nicotine exposure may play only a minimal causal role in cognitive features of ADHD; however, the same nicotine exposure regimen had lasting effects on performance of the 5 choice serial reaction time task (5CSRTT), including deficits in attention and increased motoric impulsivity, which are Thought to model some of the core features of ADHD (Robbins 2002; Winstanley et al. 2006; Schneider et al. 2011). Hence, it appears that prenatal nicotine exposure has the potential to play a causal role in at least some features of ADHD.
Individuals with ADHD show elevated risk taking (preference for larger rewards accompanied by greater probabilities of adverse outcomes, relative to smaller, safer rewards (Toplak et al. 2005; Garon et al. 2006; Malloy-Diniz et al. 2007; DeVito et al. 2008; Masunami et al. 2009; Drechsler et al. 2010)), but it is not known whether this type of decision making can be influenced by early nicotine exposure. The purpose of the present studies was to determine how developmental nicotine exposure in rats affects performance in a test of risky decision making (involving choices between small safe rewards and large rewards accompanied by risk of punishment, (Simon et al. 2009; Mitchell et al. in 2012). For comparison, rats were also tested on a delay-discounting task (Evenden and Ryan 1996; Simon et al. 2007; Schneider et al. 2011). Finally, additional measures of locomotor activity, reward motivation, and anxiety were collected, as these factors can be affected in both rodent nicotine exposure models and ADHD, and have the potential to influence performance on decision-making tasks (Huang et al. 2007; Franke et al. 2008; Luman et al. 2010: for review see Sagvolden et al. 2005). We used a rat neonatal nicotine exposure model to address the effects of nicotine during the brain growth spurt period, which takes place during the third trimester in humans but during early postnatal development in rats (Dobbing and Sands 1979; Winzer-Serhan 2008). During this early neonatal period in rodents, several developmental processes occur in cortical structures, including neuronal maturation, synaptogenesis, and circuit formation, all of which take place in utero in humans (Bayer et al. 1993; de Graaf-Peters et al. 2006). Administration of nicotine during postnatal days (P)1–7 produces robust effects on measures of anxiety, but few if any effects on learning and memory (Huang et al. 2006; Huang et al. 2007). Here we evaluated the consequences of neonatal nicotine exposure on behavioral alterations analogous to those in ADHD.
Methods
Subjects
Five timed virgin-mated pregnant Sprague-Dawley rats (Harlan, Houston, TX) arrived between gestational day 14 and 16, and were housed in accordance with the rules of the Texas A&M University Laboratory Animal Care Committee and NIH guidelines. Litters were born to all five female rats; the day pups were born was termed P0, and litters were culled to 8 pups per litter on P1. At P21, rats were weaned and pair-housed under standard care conditions until adulthood.
At approximately 10 weeks of age, male rats from these litters (n = 12 control and 12 nicotine exposed) were transferred to individual housing and kept on a 12h light/dark cycle (lights on at 0800 hours) with free access to food and water except as noted. During behavioral testing, rats were maintained at 85% of their free-feeding weight, with allowances for growth (except during testing in the elevated plus maze (EPM), when rats were given free access to food). All behavioral procedures were conducted during the light cycle (0900 – 1500) and were approved by the Texas A&M University Laboratory Animal Care Committee and followed NIH guidelines.
Nicotine exposure
These experiments employed a neonatal gastric intubation model, which allows delivery of controlled amounts of nicotine to rat pups with minimal stress, maternal separation, or risk of injury (Huang et al. 2006). In addition, because pups are individually treated, nicotine doses can be adjusted to body weight, and littermates can be assigned to milk-control and nicotine+milk treatment groups. Nicotine is highly membrane-permeable and brain nicotine levels comparable to those in blood are achieved within seconds. However, due to slower absorption of nicotine from the acidic milieu of the stomach, peak nicotine blood levels of 150 to 200 ng/ml serum are achieved within 30 min of oral nicotine administration (unpublished results), which are comparable to levels found in heavy smokers. The pattern of administration in the chronic neonatal nicotine model also mimics that found in Smokers, in whom blood nicotine levels tend to decrease at night. Pups were treated from P1 to P7 as previously described (Huang et al., 2006). Briefly, pups were given milk formula (Enfamil with iron; Mead Johnson & Company, Evansville, IN) with or without nicotine, in a volume of 1/36 their total body weight three times a day (0900, 1300, 1700) using the oral gastric intubation method. For the nicotine exposed group, rat pups were given 2 mg/kg/dose nicotine free base (calculated as the weight of the free base, Sigma Chemical, St. Louis, MO). The control group received milk formula only. Within each litter, half of the pups were nicotine exposed, and half were controls.
Apparatus
Testing in the decision-making and fixed ratio (FR) responding tasks was conducted in standard behavioral test chambers (Coulbourn Instruments, Whitehall, PA) housed within sound-attenuating isolation cubicles. Each chamber was equipped with a recessed food-pellet-delivery trough fitted with a photobeam to detect head entries and a 1.12 W lamp to illuminate the food trough, which was located 2 cm above the floor in the center of the front wall. Forty-five mg grain-based food pellets (PJAI, Test Diet, Richmond, IN) could be delivered into the food trough. Two retractable levers were located to the left and right of the food trough, 11 cm above the floor. A 1.12 W house light was mounted on the rear wall of the isolation cubicle. The floor of the test chamber was composed of steel rods connected to a shock generator that delivered scrambled footshocks. Locomotor activity was assessed throughout each session with an infrared activity monitor mounted on the ceiling of the test chamber. This monitor consisted of an array of infrared (body heat) detectors focused over the entire test chamber. Movement in the test chamber (in x, y, or z planes) was defined as a relative change in the infrared energy falling on the different detectors in the array. Test chambers were interfaced with a computer running Graphic State software (Coulbourn Instruments), which controlled programmed events and data collection.
Behavioral Procedures
Rats were tested in the behavioral procedures in the order in which they are described below.
Shaping
Rats were approximately 12 weeks old at commencement of shaping. Shaping procedures for the decision-making tasks followed those used previously (Cardinal et al. 2000; Simon et al. 2007; Simon et al. 2009). Following training to respond at the food trough upon food delivery, rats were trained to press a single lever (either the left or right, counterbalanced across groups; the other lever was retracted during this phase of training) to receive a single food pellet. After reaching a criterion of 50 lever presses in 30 min, rats were then trained on the opposite lever under the same criterion. This was followed by further shaping sessions in which both levers were retracted and rats were trained to nose poke into the food trough during simultaneous illumination of the trough and house lights. When a nose poke occurred, a single lever was extended (left or right, pseudorandomly determined, such that each lever was presented once in every two-trial block), and a lever press resulted in immediate delivery of a single food pellet. Immediately following the lever press, the trough light was extinguished and the lever was retracted. Rats were trained to a criterion of 30 presses on each lever within 60 min.
Risky decision-making task
The risky decision-making task assesses the degree of preference for small safe rewards vs. large risky rewards that are accompanied by different probabilities of punishment. Testing procedures followed Simon et al. (2009). Sessions were 60 min in duration and consisted of 5 blocks of 18 trials each. Each 40-s trial began with a 10-s illumination of the food trough and house lights. A nose poke into the food trough extinguished the trough light and triggered extension of either a single lever (forced-choice trials) or of both levers simultaneously (free-choice trials). If rats failed to nose poke within the 10 s time window, the lights were extinguished and the trial was scored as an omission. A press on one lever (either left or right, balanced across rats) resulted in immediate delivery of a single food pellet (the small safe reward).
A press on the other lever resulted in immediate delivery of three food pellets (the large risky reward). However, selection of this lever was also accompanied by a possible 1 s footshock (0.3 mA) which occurred immediately following food delivery, contingent on a preset probability specific to each trial block. The large reward was delivered following every choice of the large-reward lever, regardless of whether or not the footshock occurred. The probability of footshock accompanying the large reward was set at 0% during the first 18-trial block. In subsequent 18-trial blocks, the probability of footshock increased to 25, 50, 75, and 100%. Each 18-trial block began with 8 forced-choice trials in which only a single lever was extended and which were used to establish the punishment contingencies in effect for that block (4 for each lever), followed by 10 free-choice trials (Cardinal and Howes 2005; Simon et al. 2007; Simon et al. 2009; St Onge and Floresco 2009). Once either lever was pressed, both levers were immediately retracted. Failure to press either lever within 10 s of their extension resulted in the levers being retracted and lights extinguished, and the trial was scored as an omission.
Food delivery was accompanied by re-illumination of both the food trough and house lights, which were extinguished upon entry to the food trough to collect the food or after 10 s, whichever occurred sooner. On the forced-choice trials (in which only one lever was present) the probability of shock following a press on the large-reward lever was dependent across the four trials in each block. For example, in the 25% risk block, one and only one of the four forced-choice trials (randomly selected) always resulted in shock, and in the 75% risk block, three and only three of the four forced-choice trials always resulted in shock. In contrast, the probability of shock on each of the free-choice trials (in which both levers were present) was entirely independent, such that the probability of shock on each trial was the same, irrespective of shock delivery on previous trials in that block.
Delay-discounting task
Testing in the delay-discounting task took place in a separate set of behavioral test chambers which were identical in design to those used for the risky decision-making task. However, no additional shaping was needed prior to testing in the delay-discounting task, as it was similar in design to the risky decision-making task. A detailed description of this task is provided in Mendez et al. (2010). Each 60-min session consisted of 5 blocks of 12 trials each. Each 60-s trial began with a 10-s illumination of the food trough and house lights. A nose-poke into the food trough during this time extinguished the food trough light and triggered extension of either a single lever (forced-choice trials) or of both levers simultaneously (free-choice trials). Trials on which rats failed to nose-poke during this 10-s window were scored as omissions. Each block consisted of 2 forced-choice trials followed by 10 free-choice trials. A press on one lever (either left or right, counterbalanced across subjects) resulted in one food pellet (the small reward) delivered immediately. A press on the other lever resulted in three food pellets (the large reward) delivered after a variable delay. Failure to press either lever within 10 s of their extension resulted in the levers being retracted and lights extinguished, and the trial was scored as an omission. Once either lever was pressed, both levers were retracted for the remainder of the trial. The delay duration increased between each block of trials (0 s, 4 s, 8 s, 16 s, 32 s), but remained constant within each block (Evenden and Ryan 1996; Cardinal et al. 2000; Winstanley et al. 2003; Simon et al. 2007).
Fixed ratio responding
Testing took place in the same apparatus as that used in the delay-discounting task, except that only the response lever that previously produced the small reward was used, and it remained extended throughout the duration of the session (hence no additional shaping was needed). Sessions were 30 min in duration, during which rats were free to press the lever to receive a single food pellet under different FR schedules (FR1, 3, 10, 20, 40; (Mendez et al. 2009). Each FR schedule was presented only once (one schedule/day), with the schedules presented in ascending order. Following completion of this testing, rats were returned to free feeding.
Elevated plus maze
The EPM consisted of two opposing closed arms and two opposing open arms (42.7 cm length × 15.2 cm width/arm; arm enclosure height: 22.9 cm) attached to a central platform, all at a height of 73 cm from the floor. The 10-min test sessions began with the rat facing the left open arm, with behavior recorded using a camera suspended overhead. Variables of interest were the time spent in the closed and open arms, and the number of entries into the closed and open arms (Schulteis et al. 1998; Wingard and Packard 2008).
Data Analysis
Raw data files were exported from Graphic State software and compiled using a custom macro written for Microsoft Excel (Dr. Jonathan Lifshitz, University of Kentucky). Statistical analyses were conducted in SPSS 18.0. To assess stability of performance in the decision-making tasks, repeated-measures ANOVAs were conducted on group data across 5 consecutive sessions (both for the nicotine and control groups separately and for both groups together). Stable behavior was defined by the absence of a main effect of session, the absence of an interaction between session and trial block, and the presence of a main effect of trial block (Winstanley et al. 2003; Mar and Robbins 2007; Simon et al. 2009; Simon et al. 2010). The effects of nicotine exposure in the decision-making tasks were assessed using two-way ANOVA, with drug condition as a between-subjects variable and trial block (i.e. – level of risk or duration of delay) as a repeated-measures variable. Baseline locomotor activity in these tasks was measured by averaging activity across all inter-trial interval (ITI) segments (in which no lights or levers were present). Shock reactivity in the risky decision-making task was defined as activity (movement units) during the 1 s shock periods, averaged across the test session. Performance on the FR schedules (number of lever presses and rewards earned) and the EPM was assessed using unpaired t-tests for each measure. In all cases, p values less than .05 were considered significant, and errors are presented as SEMs.
Results
Body weight
There was no sipgnificant difference in body weight between control and nicotine exposed rats on P1 (control = 6.9 ± 0.1 (SEM) g, nicotine = 6.9 ± 0.1 g, t(22) = 0.33, NS). On P8 (the day after the last nicotine administration), nicotine-exposed rats weighed significantly less than controls (control = 20.0 ± 0.5 g, nicotine = 18.5 ± 0.5 g, t(22) = 2.24, p < 0.05), indicating that nicotine attenuated the normal developmental increase in body weight during this time period. However, these body weight differences were transient, as they were not evident at the start of behavioral testing (control = 368.8 ± 3.6 g, nicotine = 363.6 ± 6.2 g t(22) = 0.10, NS).
Risky decision-making task
There were no differences between control and nicotine exposed rats in the number of days required for shaping (control = 4.8 ± 0.4 days, nicotine = 5.0 ± 0.4 days, t(22) = 0.48, NS). Once lever pressing was shaped, rats were trained on the risky decision-making task for a total of 49 days, at which point stable performance (see Methods) was observed. Figure 1a shows mean performance across the final 5 test sessions of the risky decision-making task for control and neonatal nicotine exposed rats. Analysis of the mean percent choice of the large, risky reward averaged across the last 5 sessions of testing (Simon et al. 2009; Mendez et al. 2010) using a two-factor ANOVA (drug condition X trial block) revealed a significant main effect of trial block (F(4, 88) = 41.55, p <0.05), such that all rats decreased their choice of the large reward as the risk of punishment increased across the course of the test session; however, there was neither a significant main effect (F(1, 22) = 0.21, NS) nor interaction (F(4, 88) = 0.35, NS) involving drug condition. Additional analyses of ITI locomotor activity, shock reactivity, and trial omissions revealed no differences between groups (ts < 1.34, NS). Similar analyses of omissions of the large risky forced-choice trials also revealed no significant difference between groups (mean number of omissions/session, control = 4.0 ± 1.6 trials, nicotine = 3.7 ± 1.5 trials, t(22) = 0.18, NS).
Figure 1.
Effects of chronic neonatal nicotine exposure on risky decision making and delay-discounting. a). There were no effects of nicotine exposure on choice performance in the risky decision-making task. Graph shows mean (±SEM) percent choice of the large risky reward (vertical axis) under varying degrees of risk of punishment (horizontal axis). Data from control rats are shown as open symbols and nicotine exposed rats as closed symbols. b). There were no effects of nicotine exposure on choice performance in the delay-discounting task. Graph shows mean (±SEM) percent choice of the large delayed reward (y axis) under varying delays to large reward delivery (x axis). Other conventions as in 1a.
Delay-discounting task
Rats were trained in the delay-discounting task for 37 days, at which point stable performance was observed. Figure 1b shows mean performance across the final 5 test sessions of the delay-discounting task for control and neonatal nicotine exposed rats. Similar to the risky decision-making task, analysis of the mean percent choice of the large, delayed reward (averaged across the last 5 sessions of testing) revealed a significant main effect of trial block (F(4, 88) = 82.74, p <0.05), such that all rats decreased their choice of the large reward as the delay to its delivery increased across the course of the test session, but neither a significant main effect (F(1,22) = 0.85, NS) nor interaction (F(4, 88) = 0.59, NS) involving drug condition were detected. Additional analyses of ITI locomotor activity and trial omissions revealed no significant differences between groups (ts < 0.53, NS).
Fixed ratio responding
Figure 2 shows the number of lever presses emitted by control and neonatal nicotine exposed rats under different FR schedules. One rat in the nicotine group died prior to commencing the FR responding tests. In the remaining rats, unpaired t-tests comparing performance of control and nicotine exposed rats on each ratio revealed no significant differences between groups in either lever presses or food pellets earned (ts(21) < 1.10, NS).
Figure 2.
Effects of chronic neonatal nicotine exposure on responding under different FR schedules. Nicotine exposure did not alter the number of lever presses or number of rewards earned (not shown) on any of the schedules. Graph shows mean (+SEM) lever presses (vertical axis) under each FR schedule (horizontal axis), with data from control rats shown in open bars and nicotine exposed rats in closed bars.
Elevated plus maze
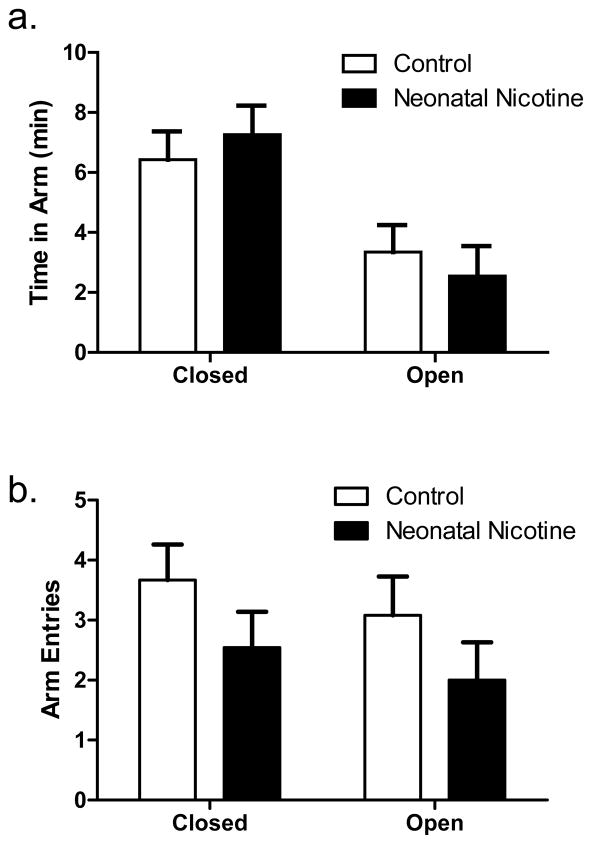
Figure 3 shows both the total time and number of entries in closed and open arms in the EPM for control and neonatal nicotine exposed rats. Comparisons of control and nicotine exposed rats’ performance on the EPM revealed no significant differences between groups in either time spent in the open or closed arms or the number of open or closed arm entries (unpaired t-tests, ts(21) < 1.33, NS).
Figure 3.
Effects of chronic neonatal nicotine exposure on performance in the elevated plus maze. Nicotine exposure had no effects on either time spent in the open and closed arms or the numbers of entries into the open and closed arms. Graph a) shows mean (+SEM) time spent in open and closed arms (vertical axis), and b) shows mean (+SEM) entries into open and closed arms (vertical axis), with data from control rats shown in open bars and nicotine exposed rats in closed bars.
Relations between nicotine-induced attenuation of weight gain and behavioral measures
The neonatal nicotine exposure regimen used here retards the normal gain in body weight during the period of exposure (Huang et al. 2006, 2007), but in unpublished experiments we have observed some variability across litters in the degree to which nicotine exposure produces this effect. In the current study, within-litter comparisons of percent weight gain from P1 to P8 between control and nicotine exposed rats revealed significant or near-significant effects in three of the five litters used (ns ranged from 2–4 rats/condition/litter, mean percent weight gains for these three litters: controls = 184.6 ± 1.7, 186.4 ± 5.1, 227.6 ± 4.0%; nicotine = 167.0 ± 3.9, 165.5 ±2.1, 191.5 ± 11.7%; unpaired t-tests, ps 0.05: nicotine appeared to attenuate weight gain in the other two litters as well, but it did not approach statistical significance). To determine whether the magnitude of nicotine-induced growth attenuation within each litter was related to subsequent behavioral performance, we conducted separate analyses of behavioral data in rats from the three litters that showed the most substantial attenuation of weight gain (n = 6 nicotine exposed and 6 control).
Similar to the results with all litters included, there were no effects of neonatal nicotine exposure on any measures of performance in the decision-making or FR responding tasks. In the EPM, however, there was a main effect of neonatal nicotine on total arm entries (control = 9.3 ± 1.2 entries, nicotine = 4.6 ± 1.5 entries t(9) = 2.49, p < 0.05), with nicotine-exposed rats making fewer overall arm entries than controls. When arm entries were broken down by arm type (closed vs. open), nicotine-exposed rats made significantly fewer entries than controls into closed arms (control = 5.0 ± 0.6 entries, nicotine = 2.6 ± 0.7 entries t(9) = 2.71, p < 0.05), but this effect did not reach significance for entries into open arms (control = 4.3 ± 0.7 entries, nicotine = 2.0 ± 0.8 entries t(9) = 2.21, p = 0.05). There were no significant effects of nicotine exposure on the percentage of time spent in either the open or closed arms (ts(9) < 0.67, NS).
To further examine relations between performance on the EPM and other behavioral tasks, Pearson’s correlations were conducted between the various measures of task performance (among all rats in the experiment). There were no significant correlations between EPM measures and either mean percent choice of the large reward in the decision-making tasks or lever presses or rewards earned in the FR responding task (ps > 0.06). In addition, consistent with previous findings (Simon et al. 2009), there were no significant correlations (either bivariate or partial correlations controlling for nicotine exposure condition) between performance in the risky decision making and delay-discounting tasks (rs < 0.35, NS).
Discussion
Exposure to cigarette smoke during fetal development is associated with adverse outcomes, including increased incidence of ADHD, but the extent to which such exposure plays a causal role in these outcomes has not been clear (D’Onofrio et al. 2008; Pauly and Slotkin 2008; Thapar et al. 2009). The present study sought to determine whether exposure to nicotine in a rat model of human third trimester development (chronic neonatal nicotine, Huang et al. 2006) causes lasting alterations in performance of behavioral tasks that model aspects of decision making impaired in ADHD. There were no effects of neonatal nicotine on either risky or impulsive decision making (delay discounting) in adulthood, suggesting that the presence of these behaviors in individuals exposed to cigarette smoke during pregnancy may be due to factors other than nicotine exposure (Khalil et al. 2000; Clemens et al. 2009; Thapar et al. 2009; Mitchell et al., 2012).
The risky decision-making task assesses preference for a small safe reward over a large risky reward that is accompanied by different probabilities of punishment. The task demands, which pit motivation to obtain rewards against motivation to avoid adverse outcomes, are similar to those of human decision-making tasks in which individuals with ADHD show elevated risk-taking, such as the Iowa Gambling Task and the Cambridge Gamble Task (although in human subjects, the adverse outcomes are loss of money or points, rather than physical punishment: Toplak et al. 2005; Garon et al. 2006; Malloy-Diniz et al. 2007; DeVito et al. 2008; Masunami et al. 2009; Simon et al. 2009; Drechsler et al. 2010; Mitchell et al., 2012). To our knowledge, risk-taking behavior has not been studied specifically in individuals exposed to tobacco smoke prenatally; however, the present findings suggest that any associations observed in this population might be due to genetic or other environmental factors.
A recent report by Schneider et al. (2011) showed that nicotine exposure in a different rat model (maternal exposure to nicotine in drinking water during pregnancy) resulted in impaired performance on the 5CSRTT in adulthood, including decreased accuracy and increased premature responses, which are thought to reflect impaired attentional processing and greater impulsive action, respectively (Robbins 2005). These findings suggest that some behaviors characteristic of ADHD can be caused by prenatal nicotine exposure (at least in that model). Consistent with the present results, Schneider et al. (2011) also found in their model that prenatal nicotine had no effect on performance on a delay-discounting task. These data support the validity of the negative effects observed in the delay-discounting task using the neonatal nicotine model, and are consistent with the idea that different forms of impulsivity (“motor” impulsivity, as assessed by the 5CSRTT, and “cognitive” or “choice” impulsivity, as assessed by the delay-discounting task) are supported by dissociable neural mechanisms (Evenden 1999; Winstanley et al. 2006).
One of the most reliable outcomes of developmental nicotine exposure is low birth weight and attenuated weight gain during early development (Winzer-Serhan 2008). In the present study, nicotine exposed rats gained less weight than controls during the period of nicotine administration. This finding is consistent with previous work in this model (Huang et al. 2006; Winzer-Serhan 2008), and validates the effectiveness of the nicotine exposure regimen. However, in unpublished data we have observed variability between litters in the degree to which neonatal nicotine attenuates weight gain, with the difference between nicotine exposed and control rats being greater in some litters than others. To determine whether this variability was related to performance in the behavioral tasks, data from the subset of litters (3 of the 5) in which there was the greatest effect of nicotine on body weight were analyzed separately. In this subset of rats, there were still no effects of nicotine exposure on any of the measures in the decision-making tasks; however, in the EPM, nicotine exposed rats had significantly fewer arm entries than controls. These data are consistent with previous work in the chronic neonatal nicotine and other models, in which nicotine exposure caused a similar decrease in open arm entries (Vaglenova et al. 2004; Huang et al. 2007), and provide further evidence for the efficacy of the nicotine-exposure regimen. Unlike previous work in the chronic neonatal nicotine model, however, there were no effects of nicotine exposure in the present study on time spent in the open arms in the EPM, possibly related to the difference in ages at which the rats were tested (10 mo at time of EPM testing in the present study vs. 2.5 mo in our previous work, (Huang et al. 2007) or to the rats’ extensive prior handling and exposure to other behavioral tasks. The decrease in arm entries may also reflect decreased locomotor activity, which has been observed in this and other developmental nicotine-exposure models, although such a decrease was not evident as assessed in the behavioral test chambers during the decision-making tasks (Eppolito and Smith 2006; Huang et al. 2007; Lesage et al. 2006)
Prenatal exposure to tobacco smoke in humans is associated with higher incidence of substance use, and developmental nicotine exposure in animal models leads to increased sensitivity to drugs of abuse (Hellstrom-Lindahl and Nordberg 2002; Levin et al. 2006; Franke et al. 2007; McQuown et al. 2007). There is also some evidence for prenatal nicotine-induced alterations in behavior guided by natural (non-drug) rewards, in that prenatal nicotine exposure reduced responding for food on several FR schedules (FR1, 2, and 5) in adolescent rats (Franke et al. 2008). No such effects were observed in the present study, suggesting differential effects of pre- vs. neonatal nicotine-exposure regimens and/or that effects of developmental nicotine on reward motivation in adolescence are attenuated by adulthood.
Although the neonatal nicotine exposure regimen employed here did not affect performance in either decision-making task, performance in these tasks is sensitive to nicotine in adult rats. Acute nicotine administration can decrease preference for the large risky reward in the risky decision-making task, and can either increase or decrease impulsive decision making in delay-discounting tasks (Dallery and Locey 2005; Anderson and Diller 2010; Kolokotroni et al. 2011; Mitchell et al., 2012). Two months of chronic daily nicotine administration causes increases in impulsive decision making that persist for one month (but no longer) following nicotine cessation (Dallery and Locey 2005). This could suggest that either the neonatal nicotine exposure regimen in the present study was too brief to cause lasting alterations in impulsive decision making, or that the effects of nicotine on impulsive decision making dissipated by the time rats in the present study were tested. However, given prior evidence that neither prenatal nicotine exposure (through maternal drinking water) nor adolescent nicotine administration (3 × 0.4 mg/kg, s.c., daily from P34–43) alters impulsive decision making in adulthood (Counotte et al. 2009; Schneider et al. 2011), it seems more likely that developmental nicotine exposure simply does not affect this type of impulsivity (although a more extensive examination of different doses, routes and times of administration, and times of testing will be necessary to fully support this conclusion). This absence of effects of developmental nicotine on impulsive decision making suggests the possibility that such behaviors in individuals exposed to tobacco smoke prenatally may be due to genetic or other environmental factors, including non-nicotine components of tobacco smoke (Thapar et al. 2009). These other components of tobacco smoke are of particular interest, as compounds present in tobacco smoke (e.g. – monoamine oxidase inhibitors) can cause significant biochemical effects which would be expected to alter neurodevelopment (Khalil et al. 2000; Clemens et al. 2009). It will be important in future work to determine whether the outcomes of studies employing developmental exposure to tobacco smoke are comparable to those using nicotine.
Acknowledgments
We thank Dr. Mark Packard for the use of the EPM apparatus, and Rebecca Simmons, Amy Blankenship, and Alixe Christie for technical assistance. Supported by R01DA024671 (BS), R01DA016487 (UHWS) and T32MH065728 (IAM & JCD).
References
- Anderson KG, Diller JW. Effects of acute and repeated nicotine administration on delay discounting in Lewis and Fischer 344 rats. Behav Pharmacol. 2010 doi: 10.1097/FBP.0b013e328340a050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA, Edwards G, Laneri M, Fletcher K, Metevia L. Executive functioning, temporal discounting, and sense of time in adolescents with attention deficit hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD) J Abnorm Child Psychol. 2001;29:541–556. doi: 10.1023/a:1012233310098. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14(1):83–144. [PubMed] [Google Scholar]
- Cardinal RN, Howes NJ. Effects of lesions of the nucleus accumbens core on choice between small certain rewards and large uncertain rewards in rats. BMC Neurosci. 2005;6:37. doi: 10.1186/1471-2202-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology (Berl) 2000;152(4):362–375. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Clemens KJ, Caille S, Stinus L, Cador M. The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats. Int J Neuropsychopharmacol. 2009;12(10):1355–1366. doi: 10.1017/S1461145709000273. [DOI] [PubMed] [Google Scholar]
- Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer AN, De Vries TJ, Smit AB, Pattij T. Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology. 2009;34(2):299–306. doi: 10.1038/npp.2008.96. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Van Hulle CA, Waldman ID, Rodgers JL, Harden KP, Rathouz PJ, Lahey BB. Smoking during pregnancy and offspring externalizing problems: an exploration of genetic and environmental confounds. Dev Psychopathol. 2008;20(1):139–164. doi: 10.1017/S0954579408000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Locey ML. Effects of acute and chronic nicotine on impulsive choice in rats. Behav Pharmacol. 2005;16(1):15–23. doi: 10.1097/00008877-200502000-00002. [DOI] [PubMed] [Google Scholar]
- de Graaf-Peters VB, De Groot-Hornstra AH, Dirks T, Hadders-Algra M. Specific postural support promotes variation in motor behaviour of infants with minor neurological dysfunction. Dev Med Child Neurol. 2006;48(12):966–972. doi: 10.1017/S001216220600212X. [DOI] [PubMed] [Google Scholar]
- DeVito EE, Blackwell AD, Kent L, Ersche KD, Clark L, Salmond CH, Dezsery AM, Sahakian BJ. The effects of methylphenidate on decision making in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;64(7):636–639. doi: 10.1016/j.biopsych.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3(1):79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Drechsler R, Rizzo P, Steinhausen HC. Decision making with uncertain reinforcement in children with attention deficit/hyperactivity disorder (ADHD) Child Neuropsychol. 2010;16(2):145–161. doi: 10.1080/09297040903190774. [DOI] [PubMed] [Google Scholar]
- Eppolito AK, Smith RF. Long-term behavioral and developmental consequences of pre- and perinatal nicotine. Pharmacol Biochem Behav. 2006;85(4):835–841. doi: 10.1016/j.pbb.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146(4):348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1996;128(2):161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Franke RM, Belluzzi JD, Leslie FM. Gestational exposure to nicotine and monoamine oxidase inhibitors influences cocaine-induced locomotion in adolescent rats. Psychopharmacology (Berl) 2007;195(1):117–124. doi: 10.1007/s00213-007-0876-y. [DOI] [PubMed] [Google Scholar]
- Franke RM, Park M, Belluzzi JD, Leslie FM. Prenatal nicotine exposure changes natural and drug-induced reinforcement in adolescent male rats. Eur J Neurosci. 2008;27(11):2952–2961. doi: 10.1111/j.1460-9568.2008.06253.x. [DOI] [PubMed] [Google Scholar]
- Garon N, Moore C, Waschbusch DA. Decision making in children with ADHD only, ADHD-anxious/depressed, and control children using a child version of the Iowa Gambling Task. J Atten Disord. 2006;9(4):607–619. doi: 10.1177/1087054705284501. [DOI] [PubMed] [Google Scholar]
- Gaworski CL, Carmines EL, Faqi AS, Rajendran N. In utero and lactation exposure of rats to 1R4F reference cigarette mainstream smoke: effect on prenatal and postnatal development. Toxicol Sci. 2004;79(1):157–169. doi: 10.1093/toxsci/kfh083. [DOI] [PubMed] [Google Scholar]
- Golub MS, Slotkin TA, Tarantal AF, Pinkerton KE. Visual recognition memory and auditory brainstem response in infant rhesus monkeys exposed perinatally to environmental tobacco smoke. Brain Res. 2007;1151:102–106. doi: 10.1016/j.brainres.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Hellstrom-Lindahl E, Nordberg A. Smoking during pregnancy: a way to transfer the addiction to the next generation? Respiration. 2002;69(4):289–293. doi: 10.1159/000063261. [DOI] [PubMed] [Google Scholar]
- Huang LZ, Hsiao SH, Trzeciakowski J, Frye GD, Winzer-Serhan UH. Chronic nicotine induces growth retardation in neonatal rat pups. Life Sci. 2006;78(13):1483–1493. doi: 10.1016/j.lfs.2005.07.047. [DOI] [PubMed] [Google Scholar]
- Huang LZ, Liu X, Griffith WH, Winzer-Serhan UH. Chronic neonatal nicotine increases anxiety but does not impair cognition in adult rats. Behav Neurosci. 2007;121(6):1342–1352. doi: 10.1037/0735-7044.121.6.1342. [DOI] [PubMed] [Google Scholar]
- Khalil AA, Steyn S, Castagnoli N., Jr Isolation and characterization of a monoamine oxidase inhibitor from tobacco leaves. Chem Res Toxicol. 2000;13(1):31–35. doi: 10.1021/tx990146f. [DOI] [PubMed] [Google Scholar]
- Kolokotroni KZ, Rodgers RJ, Harrison AA. Acute nicotine increases both impulsive choice and behavioural disinhibition in rats. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2296-2. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Gustaf E, Dufek MB, Pentel PR. Effects of maternal intravenous nicotine administration on locomotor behavior in pre-weanling rats. Pharmacol Biochem Behav. 2006;85(3):575–583. doi: 10.1016/j.pbb.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Briggs SJ, Christopher NC, Rose JE. Prenatal nicotine exposure and cognitive performance in rats. Neurotoxicol Teratol. 1993;15(4):251–260. doi: 10.1016/0892-0362(93)90006-a. [DOI] [PubMed] [Google Scholar]
- Levin ED, Lawrence S, Petro A, Horton K, Seidler FJ, Slotkin TA. Increased nicotine self-administration following prenatal exposure in female rats. Pharmacol Biochem Behav. 2006;85(3):669–674. doi: 10.1016/j.pbb.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luman M, Tripp G, Scheres A. Identifying the neurobiology of altered reinforcement sensitivity in ADHD: a review and research agenda. Neurosci Biobehav Rev. 2010;34(5):744–754. doi: 10.1016/j.neubiorev.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Malloy-Diniz L, Fuentes D, Leite WB, Correa H, Bechara A. Impulsive behavior in adults with attention deficit/ hyperactivity disorder: characterization of attentional, motor and cognitive impulsiveness. J Int Neuropsychol Soc. 2007;13(4):693–698. doi: 10.1017/S1355617707070889. [DOI] [PubMed] [Google Scholar]
- Mar AC, Robbins TW. Curr Protoc Neurosci. Unit 8. Chapter 8. 2007. Delay discounting and impulsive choice in the rat; p. 22. [DOI] [PubMed] [Google Scholar]
- Masunami T, Okazaki S, Maekawa H. Decision-making patterns and sensitivity to reward and punishment in children with attention-deficit hyperactivity disorder. Int J Psychophysiol. 2009;72(3):283–288. doi: 10.1016/j.ijpsycho.2009.01.007. [DOI] [PubMed] [Google Scholar]
- McQuown SC, Belluzzi JD, Leslie FM. Low dose nicotine treatment during early adolescence increases subsequent cocaine reward. Neurotoxicol Teratol. 2007;29(1):66–73. doi: 10.1016/j.ntt.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez IA, Williams MT, Bhavsar A, Lu AP, Bizon JL, Setlow B. Long-lasting sensitization of reward-directed behavior by amphetamine. Behav Brain Res. 2009;201(1):74–79. doi: 10.1016/j.bbr.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez IA, Simon NW, Hart N, Mitchell MR, Nation JR, Wellman PJ, Setlow B. Self-administered cocaine causes long-lasting increases in impulsive choice in a delay discounting task. Behav Neurosci. 2010;124(4):470–477. doi: 10.1037/a0020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MR, Vokes CV, Blankenship AL, Simon NW, Setlow B. Effects of acute administration of drugs of abuse on risky decision-making in rats. 2012 doi: 10.1007/s00213-011-2363-8. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paloyelis Y, Asherson P, Kuntsi J. Are ADHD symptoms associated with delay aversion or choice impulsivity? A general population study. J Am Acad Child Adolesc Psychiatry. 2009;48(8):837–846. doi: 10.1097/CHI.0b013e3181ab8c97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly JR, Slotkin TA. Maternal tobacco smoking, nicotine replacement and neurobehavioural development. Acta Paediatr. 2008;97(10):1331–1337. doi: 10.1111/j.1651-2227.2008.00852.x. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163(3–4):362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Chemistry of the mind: neurochemical modulation of prefrontal cortical function. J Comp Neurol. 2005;493(1):140–146. doi: 10.1002/cne.20717. [DOI] [PubMed] [Google Scholar]
- Romero RD, Chen WJ. Gender-related response in open-field activity following developmental nicotine exposure in rats. Pharmacol Biochem Behav. 2004;78(4):675–681. doi: 10.1016/j.pbb.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav Brain Sci. 2005;28(3):397–419. doi: 10.1017/S0140525X05000075. discussion 419–468. [DOI] [PubMed] [Google Scholar]
- Schneider T, Ilott N, Brolese G, Bizarro L, Asherson PJ, Stolerman IP. Prenatal exposure to nicotine impairs performance of the 5-choice serial reaction time task in adult rats. Neuropsychopharmacology. 2011;36(5):1114–1125. doi: 10.1038/npp.2010.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Yackey M, Risbrough V, Koob GF. Anxiogenic-like effects of spontaneous and naloxone-precipitated opiate withdrawal in the elevated plus-maze. Pharmacol Biochem Behav. 1998;60(3):727–731. doi: 10.1016/s0091-3057(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Simon NW, I, Mendez A, Setlow B. Cocaine exposure causes long-term increases in impulsive choice. Behav Neurosci. 2007;121(3):543–549. doi: 10.1037/0735-7044.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Gilbert RJ, Mayse JD, Bizon JL, Setlow B. Balancing risk and reward: a rat model of risky decision making. Neuropsychopharmacology. 2009;34(10):2208–2217. doi: 10.1038/npp.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, LaSarge CL, Montgomery KS, Williams MT, Mendez IA, Setlow B, Bizon JL. Good things come to those who wait: attenuated discounting of delayed rewards in aged Fischer 344 rats. Neurobiol Aging. 2010;31(5):853–862. doi: 10.1016/j.neurobiolaging.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA. If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol Teratol. 2008;30(1):1–19. doi: 10.1016/j.ntt.2007.09.002. [DOI] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB. Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology. 2009;34(3):681–697. doi: 10.1038/npp.2008.121. [DOI] [PubMed] [Google Scholar]
- SubstanceAbuseandMentalHealthServicesAdministration. Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings N. S. H.-A. Rockville, MD: 2010. Office of Applied Studies, HHS Publication No. SMA 10-4856Findings. [Google Scholar]
- Thapar A, Rice F, Hay D, Boivin J, Langley K, van den Bree M, Rutter M, Harold G. Prenatal smoking might not cause attention-deficit/hyperactivity disorder: evidence from a novel design. Biol Psychiatry. 2009;66(8):722–727. doi: 10.1016/j.biopsych.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplak ME, Jain U, Tannock R. Executive and motivational processes in adolescents with attention-deficit-hyperactivity disorder (ADHD) Behav Brain Funct. 2005;1(1):8. doi: 10.1186/1744-9081-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaglenova J, Birru S, Pandiella NM, Breese CR. An assessment of the long-term developmental and behavioral teratogenicity of prenatal nicotine exposure. Behav Brain Res. 2004;150(1–2):159–170. doi: 10.1016/j.bbr.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Wingard JC, Packard MG. The amygdala and emotional modulation of competition between cognitive and habit memory. Behav Brain Res. 2008;193(1):126–131. doi: 10.1016/j.bbr.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on a delay-discounting task in rats. Psychopharmacology (Berl) 2003;170(3):320–331. doi: 10.1007/s00213-003-1546-3. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26(4):379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzer-Serhan UH. Long-term consequences of maternal smoking and developmental chronic nicotine exposure. Front Biosci. 2008;13:636–649. doi: 10.2741/2708. [DOI] [PubMed] [Google Scholar]