Abstract
This introductory article to the special edition on glutamate neurotransmission in neuropsychiatric disorders provides an overview of glutamate neurotransmitter system physiology and pharmacology. Glutamate was only relatively recently recognized as the major excitatory neurotransmitter in the mammalian brain, in part due to its ubiquitious nature and diverse metabolic roles within the CNS. The extremely high concentration of glutamate in brain tissue paired with its excitotoxic potential, require tight physiological regulation of extracellular glutamate levels and receptor signaling in order to assure optimal excitatory neurotransmission but limit excitotoxic damage. In order to achieve this high level of control, the system has developed a complex physiology with multiple regulatory processes modulating glutamate metabolism, release, receptor signaling, and uptake. The basic physiology of the various regulatory components of the system including the rich receptor pharmacology is briefly reviewed. Potential contributions from each of the system’s components to the pathophysiology of neuropsychiatric illnesses are briefly discussed, as are the many new pharmacological targets for drug development provided by the system, especially as they pertain to the proceeding preclinical and clinical articles in this issue.
INTRODUCTION
The monoaminergic hypothesis of psychiatric disorders arose in the wake of the serendipitous discovery that tricyclic antidepressants and monoamine oxidase inhibitors had beneficial effects on mood, anxiety and psychosis via monoamine neurotransmitter (dopamine, serotonin and norepinephrine) reuptake, degradation and receptor dynamics. Monoamingergic research progressed apace, resulting in many important preclinical and clinical discoveries, enhancing our understanding of the pathophysiological mechanisms underlying many neuropsychiatric disorders and improving our ability to treat these devastating illnesses. However, several recent large clinical studies have made us increasingly aware of the limitations of our current armamentarium of psychotropic medications [STAR*D (Gaynes et al., 2009; Rush et al., 2006), STEP-BD (Perlis et al., 2006; Sachs et al., 2003), CATIE (Lieberman et al., 2005; Swartz et al., 2008)].
Presently, mounting evidence suggesting that the glutamatergic system also contributes to the pathophysiology of neuropsychiatric disorders is opening opportunities for the development of new models of pathogenesis, improved diagnostic tools and novel treatment strategies. A more complete understanding of glutamates roles in the pathogenesis and pathophysiology of neuropsychiatric disorders may allow for an increasingly rational approach to drug development for these common, disabling illnesses. The following review will briefly outline the extremely complex physiology and pharmacology of the glutamatergic neurotransmitter system, highlighting specific areas of interest to clinical neuroscience and drug discovery.
GLUTAMATE METABOLISM
Although glutamate was known to have central nervous system (CNS) effects for more than 75 years, it was not until 1984 that it was truly acknowledged as fulfilling the criteria of a neurotransmitter (Fonnum, 1984). Glutamate was originally speculated to serve a metabolic function in the CNS (Krebs, 1935), as it was found within numerous intracellular compartments including the cytosol and mitochondria of all CNS cell types. However, it is now known that despite its ubiquitous nature, levels of extracellular glutamate are indeed tightly regulated, thus allowing glutamate to function as the major excitatory neurotransmitter in the mammalian CNS. The tight control of glutamatergic neurotransmission is an energy-costly process, requiring multiple regulatory processes and high levels of glucose and oxygen consumption.
Like all amino acids, glutamate has a C-terminus and an N-terminus; the C-terminus and carbon backbone derive from glucose. Glucose crosses the blood-brain barrier via astrocytic end feet and, once intracellular, is broken down via glycolysis to pyruvic acid in the cytosol. Pyruvic acid enters the tricarboyxlic acid (TCA) cycle, which generates α-ketoglutarate and is later transaminated to receive an amino group from a branched chain amino acid donor, e.g. leucine, isoleucine and valine, and various amino group donors, e.g. aspartate, γ-aminobutyric acid (GABA) and alanine (Pellerin & Magistretti, 2004). It is important to note that in addition to it role as a neurotransmitter, glutamate also serves as a metabolic precursor to GABA and as a component of various amino acid-based derivatives, e.g. the antioxidant glutathione. Consistent with glutamate’s key role in multiple aspects of brain physiology, metabolic studies have determined that virtually all of the glucose that enters the CNS is eventually converted to glutamate (Shen et al., 1999).
GLUTAMATE RELEASE
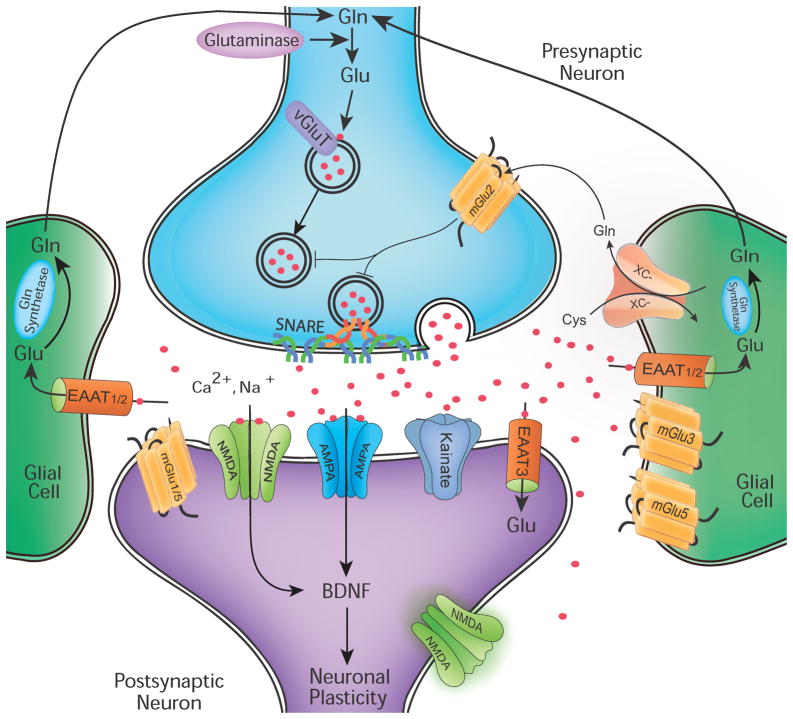
Cytosolic glutamate crosses the vesicular membrane via the activity of vesicular glutamate transporters (VGLUTs) (Takamori, 2006). VGLUTs are multimeric proton/glutamate antiporters. To date, three VGLUTs have been cloned. VGLUT1 and 2 are primarily expressed in glutamatergic neurons; whereas, VGLUT3 is somewhat unique in that it has been detected in GABAergic, cholinergic and monoaminergic neurons, although the function of VGLUT3 in these non-glutamatergic neuronal populations is unclear (Fremeau et al., 2004b). Interestingly, VGLUT1 and 2 are also expressed in glial cells and may play a role in the recently-identified release of glutamate from depolarized astrocytes (Bezzi et al., 2004; Montana et al., 2004). The loss of VGLUT expression via targeted knockout strategies results in the loss of glutamate packaging into synaptic vesicles and deleterious neuropsychiatric sequelae (Fremeau et al., 2004a; Gras et al., 2008; Moechars et al., 2006; Seal et al., 2008; Wallen-Mackenzie et al., 2006; Wallen-Mackenzie et al., 2010; Wojcik et al., 2004). In a Ca2+ and soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-dependent manner (Pang & Sudhof, 2010; Sudhof & Rothman, 2009), glutamate is released into the synaptic cleft to bind to and elicit its effects on postsynaptic receptors. Recent studies demonstrating that the packaging and release of vesicular glutamate are modulated by stress and psychotropic drugs (Musazzi et al., 2010), lead to speculation that this could be a critical site in relation to stress-related pathophysiology and possibly a target for drug development.
GLUTAMATE CLEARANCE AND CYCLING
Dysregulated excitatory neurotransmission, resulting in high concentrations of extracellular glutamate, and especially increased levels of extrasynaptic glutamate, leads to cellular damage (hence, the term “excitotoxicity”) (Hardingham et al., 2002; Ivanov et al., 2006; Leveille et al., 2008; Vanhoutte & Bading, 2003; Xu et al., 2009). Therefore, the rapid removal of extracellular glutamate must occur on a millisecond time scale to avoid cellular damage. Glutamate is actively removed from the synaptic cleft and transported into the cytosol against its concentration gradient via excitatory amino acid transporters (EAATs), primarily found on synaptically-associated astrocytic processes. Five such high-affinity transporters have been identified to date (O’Shea, 2002). EAAT1 is abundantly detected in the neocortex and cerebellum but appears to be restricted to astrocytes. EAAT2, the chief glutamate transporter in the forebrain, is expressed mostly in astrocytes but also, to a limited extent, in neurons. EAAT3 is neuron-specific and enriched in GABAergic presynaptic nerve endings. EAAT4 has only been detected in the dendrites of cerebellar Purkinje neurons. Finally, EAAT5 is retina-specific. In rodents, the homologues of EAAT1–3 are referred to as GLAST, GLT and EAAC1, respectively. The location of the EAATs relative to the geometry of synapse places them in a critical position to prevent glutamate spillover and activation of extrasynaptic glutamate transporters (Zheng et al., 2008). Considering individual astrocytes serve large numbers of synapses with minimal overlap in the synapses served by neighboring astrocytes, the failure of a single astrocyte could impair glutamate removal at thousands of synapses in some brain regions (Bushong et al., 2002). Interestingly, dysfunction of EAATs has specifically been implicated in the pathology of several neurodegenerative disorders (Beart & O’Shea, 2007), and has recently been related to learned helplessness behavior in rodent models (Zink et al., 2010). Other studies have identified reduced levels of EAATs in the brains of patients with mood and psychotic spectrum disorders (Bernard et al., 2010; Choudary et al., 2005; McCullumsmith & Meador-Woodruff, 2002; Sequeira et al., 2009).
Once in the cysotol, glutamine synthetase, an astrocyte and oligodendrocyte-specific enzyme, converts glutamate into glutamine in an ATP-requiring reaction with ammonia. Both astrocytes and neurons contain glutamine transporters that, under appropriate electrophysiological conditions, lead to the net exchange of glutamine from astrocytes-to-neurons. In neurons, the mitochondrial phosphate-specific enzyme, glutaminase, reconverts inert glutamine-to-glutamate for subsequent repackaging into synaptic vesicles. The cycling of glutamate/glutamine in astrocytes and neurons has been termed “the glutamine cycle” (see Figure for schematic). Thus, there are two pathways for the production of neuronal glutamate: (1.) the de novo production of glutamate from glucose and amino acid derivatives via energy metabolism and (2.) the recycling of glutamate from glutamine via glutamate reuptake, enzymatic activity of glutaminase and the activities of the glutamine transporters (Erecinska & Silver, 1990). Recent work has demonstrated a decreased rate of glutamate/glutamine cycling following chronic unpredictable stress exposure in rodents (Banasr et al., 2010), and suggests that glutamate clearance and cycling could be targets for future psychotropic drug development (Banasr et al., 2010; Mineur et al., 2007; Sattler & Rothstein, 2007).
Figure 1. Glutamatergic Neurotransmission.
Due to the risk of excitotoxic damage in the wake of excessive glutamatergic stimulation, precise physiological control of glutamate must be maintained in the mammalian CNS. Glutamine (Gln) is converted to glutamate (Glu) by glutaminase [though glutamate may also be derived from the TCA cycle (not shown)]. Glu is packaged into presynaptic vesicles by vesicular Glu transporter (VGLUT) proteins and synaptically released in an voltage-dependent manner through vesicular interactions with SNARE proteins. Synaptically-released Glu is recycled from the extracellular space by excitatory amino acid transporters (EAATs) expressed predominantly on astroglia. In astrocytes, Glu is converted to Gln by Gln synthetase and exported extracellularly to be taken up again by neurons. Additionally, system x-C is a cystine/glutamate antiporter expressed on glia that also contributes to Glu recycling. Glu receptors are present on presynaptic and postsynaptic neurons as well as on glial cells. These include both ionotropic receptors (NMDA, AMPA/KA) and metabotropic receptors (mGluRs). The effect of Glu is determined by the receptor subtype, localization (synaptic, perisynaptic and extrasynaptic), and interactions with various scaffolding and signaling proteins (not shown) in the postynaptic density. Glu receptor stimulation results not only in rapid ionotropic effects but also synaptic plasticity, e.g. long-term potentiation (LTP) and long-term depression (LTD), via cognate signal transduction cascades.
In addition to stimulated vesicular release of glutamate, some level of extracellular glutamate is maintained by a cystine-glutamate antiporter called system x-C. This antiporter exchanges extracellular cystine for intracellular glutamate in a 1:1 ratio. System x-C is highly expressed in the rodent and human brain, and most CNS cell types (neurons, astrocytes, microglia, vascular endothelial cells, ependymal cells of the choroid plexus and leptomeninges) express detectable levels of this antiporter. System x-C consists of a specific light chain, xCT, and a heavy chain, 4F2, linked by a disulfide bridge (Albrecht et al., 2010), and its activity is inhibited in the context of numerous neuropsychiatric insults including in vitro oxygen deprivation and in vivo chronic cocaine exposure (Baker et al., 2003; Fogal et al., 2007; Madayag et al., 2007). Interestingly, the activity of system x-C has been restored by numerous agents, e.g. interleukin 1-β (Jackman et al., 2010), N-acetylcysteine (Moussawi et al., 2009) and ceftriaxone (Knackstedt et al., 2010). In addition to it role in regulating levels of extracellular glutamate, system x-C is also the rate-limiting step in the formation of the potent antioxidant, glutathione (McBean, 2002).
GLUTAMATERGIC NEUROTRANSMISSION
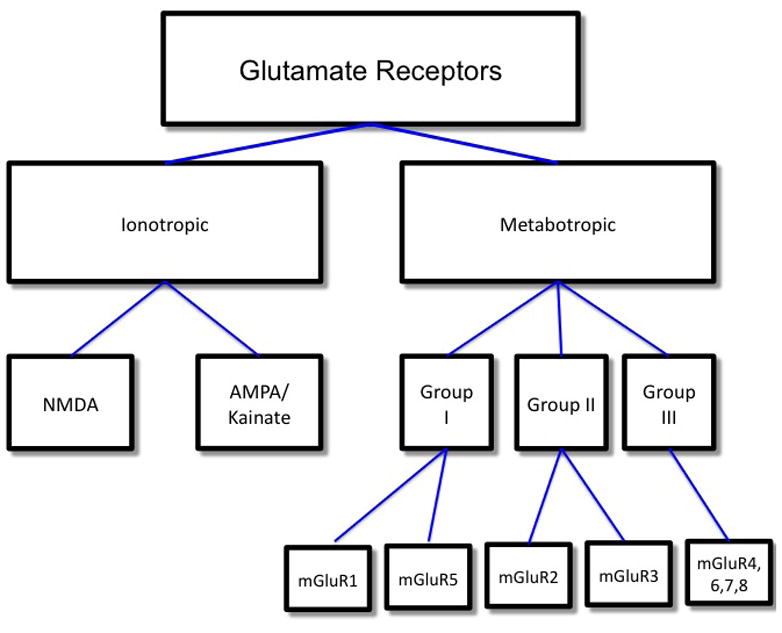
As described, glutamatergic synapses serve as excitatory relay stations between presynaptic nerve terminals and postsynaptic dendritic spines (axo-dendritic synapses) or adjacent nerve endings (axo-axonal synapses). Axo-dendritic glutamatergic synapses are easily recognizable via electron microscopy due to the thickened appearance of the postsynaptic membrane. These postsynaptic “densities” (PSDs) are ~50 nm thick conglomerations of membrane receptors, scaffolding proteins and second messenger effectors; some estimates suggest that each PSD may contain up to 100 proteins. Glutamate receptors may be divided into two broad categorizations: ionotropic and metabotropic receptors (see figure 2). Ionotropic glutamate receptors are ion channels that flux cations (Ca2+, Na+). Conformational changes “open” the channel in response to agonist binding. Metabotropic receptors, on the other hand, activate or inhibit second messenger systems via interactions with cognate G-proteins.
Figure 2.
Ionotropic Glutamate Receptors
Three classes of ionotropic glutamate receptors have been identified, which were named on the basis of agonist selectivity: N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazole proprionic acid (AMPA) and kainate (KA). Ionotropic glutamate receptors form tetrameric complexes of individual/heteromeric subunits. One of the most intriguing features of ionotropic glutamate receptors is their diversity of channel properties based on subunit composition and expression profile in the mammalian brain.
NMDA Receptors
NMDA receptors have the highest affinity for glutamate (EC50 1 μM). Three families of NMDA receptor subunits have been identified: (1.) NR1 (2.) NR2A-D (3.) NR3A-B. Via in situ hybridization studies, NR1 expression appears to be ubiquitous and obligatory in the brain; it is critical for neurodevelopment, as NR1 knockout mice die shortly after birth due to respiratory demise. Interestingly, hippocampal CA1-specific NR1-knockout mice display grossly normal development but impaired long-term potentiation (LTP), the molecular and electrophysiological correlate of learning and memory in CA1 hippocampal pyramidal neurons and impaired spatial memory in the Morris water maze (Tsien et al., 1996). NR2 mRNA displays differential expression and appears to be developmentally-regulated (Monyer et al., 1994). NR2A expression predominates in the neocortex and hippocampus while NR2B is primarily expressed in the forebrain. In contrast, NR2C and NR2D are intensely expressed in the cerebellum and diencephalon/lower brain stem (Nakanishi, 1992). NR3A is predominantly expressed in the neocortex and displays neurodevelopmental regulation; dysregulated NR3A development been proposed to contribute to the pathogenesis of schizophrenia (Das et al., 1998; Henson et al., 2008). Finally, NR3B mRNA expression is evident in the brainstem and alpha motor neurons of spinal cord (Chatterton et al., 2002; Matsuda et al., 2003; Matsuda et al., 2002; Nishi et al., 2001). More recently, NR3B has been detected in the cerebellum and hippocampus (Andersson et al., 2001; Bendel et al., 2005).
NMDA receptors are among the most tightly regulated in the mammalian brain and unique in requiring co-agonists for activation. At least six binding sites have been identified that regulate the probability of ion channel opening, viz., sites for two obligatory co-ligands (glutamate and glycine), polyamines and cations (Mg2+, Zn2+ and H+). NMDA receptor ligands are short-chain dicarboxlic amino acids (NMDA, glutamate, aspartate, etc.). Glutamate, the most potent neurochemical agonist identified in the CNS, and several competitive antagonists of the NMDA receptor including D-2-amino-5-phosphonopentanoic acid (D-AP5) and 3-(2-carboxypiperazin-4-yl)1-propeny-1-phosphonic acid (2R-CPPene) bind to the NR2 subunit of the tetrameric receptor complex. In contrast, glycine binds to a site on the NR1 subunit (Dingledine et al., 1999; Kleckner & Dingledine, 1988). The glycine-binding site on the NR1 subunit has gained clinical significance due to D-cycloserine’s binding at the same glycineB site. D-cycloserine is a partial agonist that has been proposed as a novel neuromodulatory agent to enhance the efficacy of evidence-based psychotherapies like exposure and response prevention in anxiety disorders (Danysz & Parsons, 1998; Krystal et al., 2009; Sheinin et al., 2001). Glycine transport requires the activity of specific glycine transporters (GlyT). Two such transporters have been identified to date, GlyT1 and GlyT2. Recent studies suggest GlyT inhibitors may provide an efficacious augmenting strategy in treatment-refractory schizophrenia (Lane et al., 2006; Lane et al., 2010).
Extracellular Mg2+ acts as an open-channel, voltage-dependent “pore blocker” to preclude cation flux (Nowak et al., 1984). Interestingly, Zn2+, while also a divalent cation, does not block the pore of the NMDA receptor. Instead, Zn2+ is an important allosteric modulator of some glutamate receptors and colocalizes to synaptic vesicles and is co-released with glutamate in select populations of synaptic vesicles, which possibly provides an additional mechanism to regulate glutamate receptor activation. Several additional NMDA receptor antagonists also exert their influence in an analogous voltage-dependent manner, e.g. phencyclidine (PCP), ketamine and MK-801. These noncompetitive antagonists have recently garnered significant attention both for their psychotomimetic (Balla et al., 2001; Javitt, 2007; Javitt et al., 2004; Krystal et al., 1994; Moghaddam & Adams, 1998; Patil et al., 2007; Umbricht et al., 2000) and rapidly-acting antidepressant-like properties (aan het Rot et al., 2010; Berman et al., 2000; Diazgranados et al., 2010; Mathew et al., 2009; Price et al., 2009; Valentine et al., 2011; Zarate et al., 2006).
Hydrogen ions (H+) are also critical endogenous allosteric modulators of glutamate receptors. At physiological pH, the presence of H+ decreases the frequency of channel opening due to H+ binding to NR2B. The polyamine regulatory sites of ionotropic glutamate receptors also play an important pH-dependent modulatory role. The binding of polyamines (spermine, spermidine) relieves the H+-mediated block and increases cation flux; however, the effect of polyamines reverses at higher concentrations (Traynelis et al., 1995). These pH-dependent effects may modulate NMDA receptor functioning in the context of increased metabolic demands and neurophysiological insults, e.g. excessive stimulation/activity, hypoxia and acidosis. Additionally, Ca2+ dependent calmodulin inactivation of NR1 subunits has also been proposed as a negative feedback mechanism leading to decreased channel open time/probability (Ehlers et al., 1996). Moreover, the Ca2+-calmodulin dependent phosphatase calcineurin inhibits the activity of ionotropic glutamate receptors via receptor dephosphorylation following the Ca2+-dependent activation of calmodulin (Tong et al., 1995).
Finally, glutamatergic neurotoxicity is increasingly thought to be mediated by the differential activation of extrasynaptic relative to synaptic NMDA receptors (Hardingham & Bading, 2010; Hardingham et al., 2002; Ivanov et al., 2006; Leveille et al., 2008; Vanhoutte & Bading, 2003; Xu et al., 2009). A recent series of studies suggests that excessive activation of extrasynaptic pathways specifically induces apopotic signal transduction cascades promoting neuronal cell death, while activation of synaptic NMDA receptors creates a “neuroprotective shield” via Ca2+-mediated signal transduction pathways promoting neuronal survival. The opposing neuroprotective and neurotoxic effects induced by activation of the synaptic and extrasynaptic NMDA receptors, respectively, are mediated by complex regulatory actions on several protective (anti-apoptotic, pro-survival and antioxidant) and pro-apoptotic genes (Hardingham et al., 2001a, 2001b; Papadia et al., 2008; Wu et al., 2001). Interestingly, memantine, a noncompetitive NMDA receptor antagonist, displays differential effects on synaptic and extrasynaptic NMDA receptors (Chen & Lipton, 2006). At low doses, memantine does not accumulate in the synaptic cleft to antagonize synaptic NMDA receptors; instead, it antagonizes extrasynaptic NMDA receptors which spares their exposure to high levels of extracellular glutamate in pathological states like ischemia and other neuropsychiatric processes (Chen et al., 1998; Xia et al., 2010).
AMPA/Kainate Receptors
AMPA receptors are also widely expressed in the mammalian CNS and mediate fast excitatory neurotransmission in response to glutamate binding (Palmer et al., 2005). AMPA receptor subunits are called GluR1–4; kainate receptor subunits are GluR5–7 and KA1–2. GluRs, in contrast to other amino acid and monoaminergic neurotransmitter receptors, contain an unusually large extracellular N-terminus. Upon forming a tetrameric complex of GluR1–4s, AMPA receptors mediate fast excitatory neurotransmission that can be blocked by specific quinoxalinediones including 6-nitro-7-sulphamobezo(f)quinoxaline-2,3-dione (NBQX), a potent and selective AMPA receptor antagonist. Kainate receptors are also tetrameric complexes of GluR5–7 and KA1–2 subunits. When expressed in heterologous systems, homomeric KA1 and/or KA2 containing-receptors are virtually inactive, and, therefore, appear to serve a modulatory function in contrast to the GluR5–7 subunits, which generate functional ligand-gated receptors (Alt et al., 2004; Herb et al., 1992; Howe, 1996).
The release of even small and brief (<1 millisecond) concentrations of glutamate into the synaptic cleft generates robust excitatory postsynaptic potentials (EPSPs). AMPA-mediated currents generate a fast upstroke and rapid current decay while NMDA-receptor activation provides a more prolonged phase of depolarization that can last several hundred milliseconds. EPSP generation is hypothesized to be controlled by AMPA receptor de/activation while the longer pharmacokinetics of NMDA receptor sensitization provides ample opportunity for spatial and temporal summation at numerous postsynaptic inputs. The higher affinity of glutamate for NMDA-to-AMPA receptors likely explains these pharmacokinetic differences, as prolonged receptor activation is often the result of slower dissociation of agonist and receptor.
AMPA receptor trafficking has been widely studied, especially its intracellular cycling and its potential physiological sequelae. Like all membrane receptors, AMPA receptors are synthesized in the soma and transported to the cell surface via the secretory pathway involving multiple membrane sorting steps and cytoskeleton transport proteins (Kapitein et al., 2010; Kennedy & Ehlers, 2006). Dendritic AMPA receptor localization to synapses is regulated via two mechanisms: (1) exocytic and endocytic trafficking and recycling, respectively, in the secretory pathway and (2) membrane diffusion from extrasynaptic-to-synaptic localizations (Groc & Choquet, 2006; Hoogenraad et al., 2010; Newpher & Ehlers, 2008; Wang et al., 2008).
A physiological role for AMPA receptor trafficking and surface diffusion has been hypothesized in learning and memory. An LTP-like strengthening of neocortical synapses occurs after sensory stimulation in vivo (Holtmaat & Svoboda, 2009; Kessels & Malinow, 2009), and this process appears dependent on AMPA receptor number, localization and facilitation at synapses (Takahashi et al., 2003). Learning in the hippocampus also appears to be regulated by AMPA receptor dynamics (Whitlock et al., 2006) as evidenced by the recruitment of AMPA receptors to mushroom-shaped dendritic spines in the CA1 region of the hippocampus 24 hours after fear conditioning (Matsuo et al., 2008). Stress hormones have recently been recognized to play a role in AMPA receptor trafficking (Groc et al., 2008; Krugers et al., 2010; Yuen et al., 2011), and may provide a mechanism for the dose-dependent (“inverted U”) facilitative and suppressive effects of corticosteroid hormones on synaptic plasticity and cognition (Martin et al., 2009). Further complexity in the regulation of ionotropic glutamatergic neurotransmission is provided by molecular variability at the transcriptional and post-transcriptional level. RNA editing of AMPA and kainate receptor subunits (Higuchi et al., 1993) and alternative splicing of mRNA transcripts (Sommer et al., 1990) modulate second messenger cascades critical for downstream intracellular effects.
METABOTROPIC GLUTAMATE RECEPTOTRS
Unlike ionotropic glutamate receptors that depend on cation flux, metabotropic glutamate receptors exert their effects via the recruitment and activation of intracellular trimeric G-proteins and downstream signal transduction pathways. Like all G-protein coupled receptors, metabotropic glutamate receptors are seven transmembrane domain-spanning receptors with an extracellular N-terminus and intracellular C-terminus, and, like AMPA receptors, they possess an especially large N-terminus. The metabotropic receptors (except mGluR8) localize primarily to perisynaptic and extrasynaptic locales on neurons and glial cells and modulate synaptic activity and plasticity. To date, eight metabotropic glutamate receptors have been identified (mGluR1–8), which have been further subdivided into three functional groups on the basis of amino acid homology, agonist binding and activated downstream signal transduction cascades (Kim et al., 2008). Group I metabotropic glutamate receptors consist of mGluR1 and mGluR5. They elicit their downstream effects by two mechanisms: (1.) phospholipase Cvia inositol-1,4,5-triphosphate (IP3) to release Ca2+ from intracellular stores and (2.) diacylgycerol (DAG) to stimulate protein kinase C. Group II metabotropic glutamate receptors (mGluR2 and mGluR3) and group III metabotropic glutamate receptors (mGluR4–8) are coupled to inhibitory G-proteins (Gi) that decrease intracellular cyclic adenosine monophosphate (cAMP) via inhibition of the adenylyl cyclase/protein kinase A pathway. Members of each class share approximately 70% sequence homology; across classes, there is approximately 45% sequence homology (Conn & Pin, 1997). Similar to ionotropic glutamate receptors, glutamate activates metabotropic glutamate receptors with varying degrees of affinity/avidity, and fairly selective agonists, antagonists and modulators have been identified and developed for the various receptor classes and subtypes.
Postsynaptic activation of metabotropic glutamate receptors has been demonstrated to modulate ion channel activity, and, as predicted, whether agonist binding to metabotropic glutamate receptors potentiates or inhibits channel activity depends on whether their cognate downstream signal transduction cascades. Tissue and cell type-specificity also exists in this regard (Kuzmiski & Bains, 2010). Metabotropic glutamate receptors localized to presynaptic membranes have been demonstrated to decrease both excitatory glutamatergic and inhibitory GABAergic neurotransmission (Pinheiro & Mulle, 2008). Although the precise mechanism(s) mediating presynaptic modulation has not been conclusively demonstrated, metabotropic glutamate receptors appear to elicit their diverse effects via the modulation of voltage-dependent presynaptic Ca2+ channels, thereby influencing quantal neurotransmitter release in a SNARE-dependent manner (Takahashi et al., 1996). There is presently intense effort to develop both positive and negative modulators of presynaptic group II and III metabotropic glutamate receptors in an effort to treat a plethora of neuropsychiatric illnesses (Nicoletti et al., 2010).
There is also great interest in developing strategies to modulate group I metabotropic glutamate receptor activity. Beyond its enhancing effects on ionotropic glutamate receptor activation, mGluR5 has been demonstrated to play a role in regulating local mRNA translation in dendritic spines (Weiler & Greenough, 1993; Weiler et al., 1997). Local protein synthesis at synapses is required for the long-lasting physiological and pathophysiological sequelae of group I metabotropic glutamate receptor activation including some receptors proposed in mediating metaplasticity. (Abraham, 2008; Aschrafi et al., 2005; Banko et al., 2006; Huber et al., 2000; Karachot et al., 2000; Merlin et al., 1998; Raymond et al., 2000; Vanderklish & Edelman, 2002). After initially discovering that group I metabotropic glutamate receptor signaling-dependent LTD is impaired in the hippocampus of FMR1 (Fragile X Mental Retardation gene 1) knock-out mice (Huber et al., 2002) and that the gene product of FMR1, FMRP (Fragile X Mental Retardation Protein), is a potent transcriptional repressor (Aschrafi et al., 2005; Bolduc et al., 2008; Dolen et al., 2007; Huber et al., 2002; Laggerbauer et al., 2001; Z. Li et al., 2001; Qin et al., 2005), Bear and colleagues proposed that the loss of FMRP in Fragile X Syndrome (FXS) leads to excessive local protein translation owing to the dysregulated mGluR5 stimulated-protein synthesis, and that antagonism of mGluR5 may abrogate the neurophysiatric sequalae of this disorder. There are now multiple studies confirming these hypotheses, especially mGluR5’s activation and downstream signal transduction hypersensitivity as critical pathogenic factors in FXS (Dolen & Bear, 2008; Osterweil et al., 2010). Other studies have demonstrated marked effects of mGluR5 modulators in a variety of animal models of neuropsychiatric and neurodegenerative disorders making this one of the most active areas in CNS drug discovery (Bird & Lawrence, 2009; Carroll, 2008; Cook, 2010; Gasparini et al., 2008; Krystal et al., 2010; Lindsley & Emmitte, 2009; Rodriguez & Williams, 2007; Simonyi et al., 2010)
INTRACELLULAR SIGNAL TRANSDUCTION FROM THE POSTSYNAPTIC DENSITY TO THE NUCLEUS
As mentioned, ionotropic and metabotropic glutamate receptors interact with postsynaptic proteins through their intracellular C-termini. Among the first discovered postsynaptic elements is the critically important “scaffolding” protein, postsynaptic density protein of 95 kDA (PSD-95). PSD-95 has been demonstrated to mechanically stabilize the synapse via the presynaptic-to-postsynaptic interaction of neuroligin and β-neurexin (Futai et al., 2007; Irie et al., 1997; Levinson et al., 2005; Nam & Chen, 2005; Schapitz et al., 2010; Song et al., 1999). PSD-95 also bridges glutamate receptors to the cytoskeleton. The C-terminus of the glutamate receptor subunit NR2 binds to PSD-95, and PSD-95 binds to α-actinin/F-actin, one of the major contributors to dendritic spine morphogenesis. PSD-95 also binds to postsynaptic signal transduction effectors via the activation of calmodulin/calmodulin-dependent kinase II (CaMKII). CAMKII mediates the phosphorylation of various protein kinases and, as discussed above, the translocation of AMPA receptors from more intracellular compartments to the PSD. A similar cycling process also occurs with KA receptors through PSD-95 and other scaffolding proteins with PDZ domains, e.g. glutamate receptor activating protein (GRIP) and SAP-97 (synapse-activating protein of 97 kDa). Metabotropic glutamate receptors, on the other hand, are found at more perisynaptic and extrasynaptic sites due to their interactions with similarly-localized “scaffolding” proteins (e.g. shank and homer). As such, presynaptic glutamate receptors are localized via these intracellular scaffolds, e.g. mGluR7 binds to the PDZ domain of protein interacting with C kinase-1 (PICK-1) (Bertaso et al., 2008; Boudin et al., 2000; Dev et al., 2000; El Far et al., 2000; Suh et al., 2008) and impaired mGluR7a-PICK1 interaction leads to absence-like seizures (Bertaso et al., 2008). Additionally, several recent studies have identified altered expression of postsynaptic proteins in rodent models and individuals suffering from a variety of neuropsychiatric diseases (Karolewicz et al., 2009; Kristiansen et al., 2010; Sifonios et al., 2009; Toro & Deakin, 2005), increasing their pathophysiological intrigue.
Scaffolding proteins directly or indirectly regulate small monomeric GTPases, and GTPases can either activate or silence transcription based on their specific downstream effectors. GTPases cycle between active GTP-bound and inactive-GDP bound forms, which are regulated by activating GEFs (guanyl exchange factors) and inhibiting GAPs (GTPase activating proteins). GEFs activate GTPases by promoting the exchange of bound GDP-for-GTP while GAPs inhibit GTPases by hydrolyzing bound GTP-to-GDP. As an example, a Ras-specific GEF associates with NR2B (Krapivinsky et al., 2003), and a Ras-specific GAP, synGAP, binds to PSD-95. If the Ras-specific GEF is activated via NR2B, GDP is exchanged for GTP, and this stimulates intracellular signal transduction cascades including the Raf-MEK-ERK and PI3K (phosphoinositide 3-OH kinase) pathways. Phosphorylated ERK translocates from the cytosol to the nucleus, where it activates the transcription factors CREB and Elk. The PI3K pathway activates protein kinase B (Akt/PKB), which stimulates nuclear translocation of the transcription factors, NFκB and CREB. The PI3K pathway also activates the MEKK-JNKK-JNK signal transduction cascade; via nuclear translocation of JNK, the transcription factors c-Jun, c-Fos and ATF2 are stimulated. Activation of these immediate-early genes mediates the transcription and translation of cytoskeletal proteins, enzymes of intermediary metabolism and neurotransmitter receptor subunits. In contrast, if Ras activity is inhibited via synGAP, the above signal transduction cascades are inhibited and transcription of the same target genes is reduced.
Cytoskeletal modulation is a critical mediator of glutamatergic receptor signaling because, as mentioned above, increased synaptic activity leads to morphological, biochemical and electrophysiological effects on the order of minutes. The Rho-family small GTPases Rac1 and Cdc42 promote dendritic spine morphogenesis via actin polymerization while Rho itself facilitates spine retraction via actin depolymerization (Krapivinsky et al., 2003). Numerous scaffolding proteins bind to RhoGEFs, e.g. PSD-95, and other scaffolds contain RhoGEF domains within their structure itself, e.g. kalirin (Alam et al., 1997) and trio (Debant et al., 1996). When overexpressed, GluR2 increases the size and density of dendritic spines while deletion of this subunit leads to spine retraction (Passafaro et al., 2003). Interestingly, acute ketamine exposure was recently shown to induce rapid dendritic spine morphogenesis via the activation of mammalian target of rapamycin (mTor), a serine/threonine protein kinase that signals though PI3K. Spine morphogenesis and the rapid antidepressant response of ketamine is speculated to occur via enhanced AMPA receptor activity, which is induced by the increased release of presynaptic glutamate in the context of acute NMDA receptor blockade (N. Li et al., 2010).
CONCLUSIONS
Glutamate is the main excitatory neurotransmitter in the mammalian CNS. Mostly due to the serendipitous discovery of antidepressants and antipsychotics that modulate monoaminergic neurontransmission and the relatively recent discovery of glutamate’s role as a true neurotransmitter, glutamate was initially understudied in neuropsychiatric disease. The discovery of ischemia-mediated glutamatergic excitotoxicity in stroke sparked initial interest in the glutamatergic system’s contribution to the pathophysiology of neuropsychiatric illnesses. Since this time, the number of studies implicating glutamatergic signaling in the diseased brain has swelled, and recent research has focused on glutamatergic neurotransmission as a rational therapeutic approach to disorders as diverse as schizophrenia, major depressive disorder, cocaine use disorders, FXS and amytrophic lateral sclerosis (ALS). The authors of the following articles will review the preclinical and clinical evidence for aberrant glutamatergic neurotransmission in neuropsychiatric disease and outline important future directions in diagnosis, prognosis and rational therapeutics.
Highlights.
This manuscript provides a general overview of Glutamate receptors and transporters
It provides a model of physiological regulation of glutamatergic neurotransmission
The manuscripts reviews how impaired physiological regulation of the glutamatergic receptors and transporter can be related to neuropsychiatric disorders
The manuscript describes specific viable drug targets within the glutamatergic system
Acknowledgments
We would like to thank Ms. Lisa Roach and Elizabeth Cooper for their assistance with the prepartation of the manuscript.
Support and Potential
This work was supported in part by NIMH R01 MH081211 (GS), NIMH T32 MH19961 (MN), and the Robert L. McNeil, Jr. Fellowship in Translational Research (MN) Dr. Sanacora has received consulting fees form Abbott, AstraZeneca, Bristol-Myers Squibb, Evotec, Eli Lilly & Co., Hoffman La-Roche and Johnson & Johnson, Novartis, and Novum Pharmaceuticals. He has also received additional grant support from AstraZeneca, Bristol-Myers Squibb, Hoffman La-Roche, Merck & Co., and Sepracor Inc. In addition Dr. Sanacora is a co-inventor on filed patent application by Yale University concerning the use of glutamate modulating drugs in the treatment of psychiatric disorders (PCTWO06108055A1).
Footnotes
Conflicts of Interest
Dr. Niciu and Mr. Kelmendi have no potential conflicts to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–45. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Alam MR, Johnson RC, Darlington DN, Hand TA, Mains RE, Eipper BA. Kalirin, a cytosolic protein with spectrin-like and GDP/GTP exchange factor-like domains that interacts with peptidylglycine alpha-amidating monooxygenase, an integral membrane peptide-processing enzyme. J Biol Chem. 1997;272:12667–75. doi: 10.1074/jbc.272.19.12667. [DOI] [PubMed] [Google Scholar]
- Albrecht P, Lewerenz J, Dittmer S, Noack R, Maher P, Methner A. Mechanisms of oxidative glutamate toxicity: the glutamate/cystine antiporter system xc- as a neuroprotective drug target. CNS Neurol Disord Drug Targets. 2010;9:373–82. doi: 10.2174/187152710791292567. [DOI] [PubMed] [Google Scholar]
- Alt A, Weiss B, Ogden AM, Knauss JL, Oler J, Ho K, Large TH, Bleakman D. Pharmacological characterization of glutamatergic agonists and antagonists at recombinant human homomeric and heteromeric kainate receptors in vitro. Neuropharmacology. 2004;46:793–806. doi: 10.1016/j.neuropharm.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Andersson O, Stenqvist A, Attersand A, von Euler G. Nucleotide sequence, genomic organization, and chromosomal localization of genes encoding the human NMDA receptor subunits NR3A and NR3B. Genomics. 2001;78:178–84. doi: 10.1006/geno.2001.6666. [DOI] [PubMed] [Google Scholar]
- Aschrafi A, Cunningham BA, Edelman GM, Vanderklish PW. The fragile X mental retardation protein and group I metabotropic glutamate receptors regulate levels of mRNA granules in brain. Proc Natl Acad Sci U S A. 2005;102:2180–5. doi: 10.1073/pnas.0409803102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–9. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Balla A, Koneru R, Smiley J, Sershen H, Javitt DC. Continuous phencyclidine treatment induces schizophrenia-like hyperreactivity of striatal dopamine release. Neuropsychopharmacology. 2001;25:157–64. doi: 10.1016/S0893-133X(01)00230-5. [DOI] [PubMed] [Google Scholar]
- Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry. 2010;15:501–11. doi: 10.1038/mp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banko JL, Hou L, Poulin F, Sonenberg N, Klann E. Regulation of eukaryotic initiation factor 4E by converging signaling pathways during metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2006;26:2167–73. doi: 10.1523/JNEUROSCI.5196-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beart PM, O’Shea RD. Transporters for L-glutamate: an update on their molecular pharmacology and pathological involvement. Br J Pharmacol. 2007;150:5–17. doi: 10.1038/sj.bjp.0706949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendel O, Meijer B, Hurd Y, von Euler G. Cloning and expression of the human NMDA receptor subunit NR3B in the adult human hippocampus. Neurosci Lett. 2005;377:31–6. doi: 10.1016/j.neulet.2004.11.064. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bernard R, Kerman IA, Thompson RC, Jones EG, Bunney WE, Barchas JD, Schatzberg AF, Myers RM, Akil H, Watson SJ. Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertaso F, Zhang C, Scheschonka A, de Bock F, Fontanaud P, Marin P, Huganir RL, Betz H, Bockaert J, Fagni L, Lerner-Natoli M. PICK1 uncoupling from mGluR7a causes absence-like seizures. Nat Neurosci. 2008;11:940–8. doi: 10.1038/nn.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, Volterra A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7:613–20. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- Bird MK, Lawrence AJ. The promiscuous mGlu5 receptor--a range of partners for therapeutic possibilities? Trends Pharmacol Sci. 2009;30:617–23. doi: 10.1016/j.tips.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Bolduc FV, Bell K, Cox H, Broadie KS, Tully T. Excess protein synthesis in Drosophila fragile X mutants impairs long-term memory. Nat Neurosci. 2008;11:1143–5. doi: 10.1038/nn.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudin H, Doan A, Xia J, Shigemoto R, Huganir RL, Worley P, Craig AM. Presynaptic clustering of mGluR7a requires the PICK1 PDZ domain binding site. Neuron. 2000;28:485–97. doi: 10.1016/s0896-6273(00)00127-6. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–92. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll FI. Antagonists at metabotropic glutamate receptor subtype 5: structure activity relationships and therapeutic potential for addiction. Ann N Y Acad Sci. 2008;1141:221–32. doi: 10.1196/annals.1441.015. [DOI] [PubMed] [Google Scholar]
- Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, Cui J, Tu S, Sevarino KA, Nakanishi N, Tong G, Lipton SA, Zhang D. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature. 2002;415:793–8. doi: 10.1038/nature715. [DOI] [PubMed] [Google Scholar]
- Chen HS, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem. 2006;97:1611–26. doi: 10.1111/j.1471-4159.2006.03991.x. [DOI] [PubMed] [Google Scholar]
- Chen HS, Wang YF, Rayudu PV, Edgecomb P, Neill JC, Segal MM, Lipton SA, Jensen FE. Neuroprotective concentrations of the N-methyl-D-aspartate open-channel blocker memantine are effective without cytoplasmic vacuolation following post-ischemic administration and do not block maze learning or long-term potentiation. Neuroscience. 1998;86:1121–32. doi: 10.1016/s0306-4522(98)00163-8. [DOI] [PubMed] [Google Scholar]
- Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, Myers RM, Bunney WE, Jr, Akil H, Watson SJ, Jones EG. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci U S A. 2005;102:15653–8. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–37. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Cook EH., Jr Reduction of increased repetitive self-grooming in ASD mouse model by metabotropic 5 glutamate receptor antagonism; randomized controlled trial of Early Start Denver Model. Autism Res. 2010;3:40–2. doi: 10.1002/aur.118. [DOI] [PubMed] [Google Scholar]
- Danysz W, Parsons CG. Glycine and N-methyl-D-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacol Rev. 1998;50:597–664. [PubMed] [Google Scholar]
- Das S, Sasaki YF, Rothe T, Premkumar LS, Takasu M, Crandall JE, Dikkes P, Conner DA, Rayudu PV, Cheung W, Chen HS, Lipton SA, Nakanishi N. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature. 1998;393:377–81. doi: 10.1038/30748. [DOI] [PubMed] [Google Scholar]
- Debant A, Serra-Pages C, Seipel K, O’Brien S, Tang M, Park SH, Streuli M. The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rho-specific guanine nucleotide exchange factor domains. Proc Natl Acad Sci U S A. 1996;93:5466–71. doi: 10.1073/pnas.93.11.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev KK, Nakajima Y, Kitano J, Braithwaite SP, Henley JM, Nakanishi S. PICK1 interacts with and regulates PKC phosphorylation of mGLUR7. J Neurosci. 2000;20:7252–7. doi: 10.1523/JNEUROSCI.20-19-07252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA., Jr A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Dolen G, Bear MF. Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol. 2008;586:1503–8. doi: 10.1113/jphysiol.2008.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–62. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD, Zhang S, Bernhadt JP, Huganir RL. Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit. Cell. 1996;84:745–55. doi: 10.1016/s0092-8674(00)81052-1. [DOI] [PubMed] [Google Scholar]
- El Far O, Airas J, Wischmeyer E, Nehring RB, Karschin A, Betz H. Interaction of the C-terminal tail region of the metabotropic glutamate receptor 7 with the protein kinase C substrate PICK1. Eur J Neurosci. 2000;12:4215–21. doi: 10.1046/j.1460-9568.2000.01309.x. [DOI] [PubMed] [Google Scholar]
- Erecinska M, Silver IA. Metabolism and role of glutamate in mammalian brain. Prog Neurobiol. 1990;35:245–96. doi: 10.1016/0301-0082(90)90013-7. [DOI] [PubMed] [Google Scholar]
- Fogal B, Li J, Lobner D, McCullough LD, Hewett SJ. System x(c)-activity and astrocytes are necessary for interleukin-1 beta-mediated hypoxic neuronal injury. J Neurosci. 2007;27:10094–105. doi: 10.1523/JNEUROSCI.2459-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F. Glutamate: a neurotransmitter in mammalian brain. J Neurochem. 1984;42:1–11. doi: 10.1111/j.1471-4159.1984.tb09689.x. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, Edwards RH. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science. 2004a;304:1815–9. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004b;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Futai K, Kim MJ, Hashikawa T, Scheiffele P, Sheng M, Hayashi Y. Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin. Nat Neurosci. 2007;10:186–95. doi: 10.1038/nn1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini F, Bilbe G, Gomez-Mancilla B, Spooren W. mGluR5 antagonists: discovery, characterization and drug development. Curr Opin Drug Discov Devel. 2008;11:655–65. [PubMed] [Google Scholar]
- Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60:1439–45. doi: 10.1176/ps.2009.60.11.1439. [DOI] [PubMed] [Google Scholar]
- Gras C, Amilhon B, Lepicard EM, Poirel O, Vinatier J, Herbin M, Dumas S, Tzavara ET, Wade MR, Nomikos GG, Hanoun N, Saurini F, Kemel ML, Gasnier B, Giros B, El Mestikawy S. The vesicular glutamate transporter VGLUT3 synergizes striatal acetylcholine tone. Nat Neurosci. 2008;11:292–300. doi: 10.1038/nn2052. [DOI] [PubMed] [Google Scholar]
- Groc L, Choquet D. AMPA and NMDA glutamate receptor trafficking: multiple roads for reaching and leaving the synapse. Cell Tissue Res. 2006;326:423–38. doi: 10.1007/s00441-006-0254-9. [DOI] [PubMed] [Google Scholar]
- Groc L, Choquet D, Chaouloff F. The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nat Neurosci. 2008;11:868–70. doi: 10.1038/nn.2150. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJ, Bading H. A calcium microdomain near NMDA receptors: on switch for ERK-dependent synapse-to-nucleus communication. Nat Neurosci. 2001a;4:565–6. doi: 10.1038/88380. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJ, Bading H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat Neurosci. 2001b;4:261–7. doi: 10.1038/85109. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11:682–96. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–14. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Henson MA, Roberts AC, Salimi K, Vadlamudi S, Hamer RM, Gilmore JH, Jarskog LF, Philpot BD. Developmental regulation of the NMDA receptor subunits, NR3A and NR1, in human prefrontal cortex. Cereb Cortex. 2008;18:2560–73. doi: 10.1093/cercor/bhn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herb A, Burnashev N, Werner P, Sakmann B, Wisden W, Seeburg PH. The KA-2 subunit of excitatory amino acid receptors shows widespread expression in brain and forms ion channels with distantly related subunits. Neuron. 1992;8:775–85. doi: 10.1016/0896-6273(92)90098-x. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Single FN, Kohler M, Sommer B, Sprengel R, Seeburg PH. RNA editing of AMPA receptor subunit GluR-B: a base-paired intron-exon structure determines position and efficiency. Cell. 1993;75:1361–70. doi: 10.1016/0092-8674(93)90622-w. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–58. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Hoogenraad CC, Popa I, Futai K, Martinez-Sanchez E, Wulf PS, van Vlijmen T, Dortland BR, Oorschot V, Govers R, Monti M, Heck AJ, Sheng M, Klumperman J, Rehmann H, Jaarsma D, Kapitein LC, van der Sluijs P. Neuron specific Rab4 effector GRASP-1 coordinates membrane specialization and maturation of recycling endosomes. PLoS Biol. 2010;8:e1000283. doi: 10.1371/journal.pbio.1000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe JR. Homomeric and heteromeric ion channels formed from the kainate-type subunits GluR6 and KA2 have very small, but different, unitary conductances. J Neurophysiol. 1996;76:510–9. doi: 10.1152/jn.1996.76.1.510. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–50. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–7. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl TW, Sudhof TC. Binding of neuroligins to PSD-95. Science. 1997;277:1511–5. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y, Medina I. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J Physiol. 2006;572:789–98. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman NA, Uliasz TF, Hewett JA, Hewett SJ. Regulation of system x(c)(-)activity and expression in astrocytes by interleukin-1beta: implications for hypoxic neuronal injury. Glia. 2010;58:1806–15. doi: 10.1002/glia.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Balla A, Burch S, Suckow R, Xie S, Sershen H. Reversal of phencyclidine-induced dopaminergic dysregulation by N-methyl-D-aspartate receptor/glycine-site agonists. Neuropsychopharmacology. 2004;29:300–7. doi: 10.1038/sj.npp.1300313. [DOI] [PubMed] [Google Scholar]
- Kapitein LC, Schlager MA, Kuijpers M, Wulf PS, van Spronsen M, MacKintosh FC, Hoogenraad CC. Mixed microtubules steer dynein-driven cargo transport into dendrites. Curr Biol. 2010;20:290–9. doi: 10.1016/j.cub.2009.12.052. [DOI] [PubMed] [Google Scholar]
- Karachot L, Shirai Y, Vigot R, Yamamori T, Ito M. Rapidly turned over protein maintains metabotropic synaptic transmission in Purkinje cells. Neuroreport. 2000;11:2903–6. doi: 10.1097/00001756-200009110-00015. [DOI] [PubMed] [Google Scholar]
- Karolewicz B, Szebeni K, Gilmore T, Maciag D, Stockmeier CA, Ordway GA. Elevated levels of NR2A and PSD-95 in the lateral amygdala in depression. Int J Neuropsychopharmacol. 2009;12:143–53. doi: 10.1017/S1461145708008985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MJ, Ehlers MD. Organelles and trafficking machinery for postsynaptic plasticity. Annu Rev Neurosci. 2006;29:325–62. doi: 10.1146/annurev.neuro.29.051605.112808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–50. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Lee J, Lee JY, Roche KW. Metabotropic glutamate receptors: phosphorylation and receptor signaling. J Neurosci Res. 2008;86:1–10. doi: 10.1002/jnr.21437. [DOI] [PubMed] [Google Scholar]
- Kleckner NW, Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988;241:835–7. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–4. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapivinsky G, Krapivinsky L, Manasian Y, Ivanov A, Tyzio R, Pellegrino C, Ben-Ari Y, Clapham DE, Medina I. The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron. 2003;40:775–84. doi: 10.1016/s0896-6273(03)00645-7. [DOI] [PubMed] [Google Scholar]
- Krebs HA. Metabolism of amino-acids: The synthesis of glutamine from glutamic acid and ammonia, and the enzymic hydrolysis of glutamine in animal tissues. Biochem J. 1935;29:1951–69. doi: 10.1042/bj0291951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen LV, Patel SA, Haroutunian V, Meador-Woodruff JH. Expression of the NR2B-NMDA receptor subunit and its Tbr-1/CINAP regulatory proteins in postmortem brain suggest altered receptor processing in schizophrenia. Synapse. 2010;64:495–502. doi: 10.1002/syn.20754. [DOI] [PubMed] [Google Scholar]
- Krugers HJ, Hoogenraad CC, Groc L. Stress hormones and AMPA receptor trafficking in synaptic plasticity and memory. Nat Rev Neurosci. 2010;11:675–81. doi: 10.1038/nrn2913. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Mathew SJ, D’Souza DC, Garakani A, Gunduz-Bruce H, Charney DS. Potential psychiatric applications of metabotropic glutamate receptor agonists and antagonists. CNS Drugs. 2010;24:669–93. doi: 10.2165/11533230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Tolin DF, Sanacora G, Castner SA, Williams GV, Aikins DE, Hoffman RE, D’Souza DC. Neuroplasticity as a target for the pharmacotherapy of anxiety disorders, mood disorders, and schizophrenia. Drug Discov Today. 2009;14:690–7. doi: 10.1016/j.drudis.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmiski JB, Bains JS. Metabotropic glutamate receptors: gatekeepers of homeostasis. J Neuroendocrinol. 2010;22:785–92. doi: 10.1111/j.1365-2826.2010.02020.x. [DOI] [PubMed] [Google Scholar]
- Laggerbauer B, Ostareck D, Keidel EM, Ostareck-Lederer A, Fischer U. Evidence that fragile X mental retardation protein is a negative regulator of translation. Hum Mol Genet. 2001;10:329–38. doi: 10.1093/hmg/10.4.329. [DOI] [PubMed] [Google Scholar]
- Lane HY, Huang CL, Wu PL, Liu YC, Chang YC, Lin PY, Chen PW, Tsai G. Glycine transporter I inhibitor, N-methylglycine (sarcosine), added to clozapine for the treatment of schizophrenia. Biol Psychiatry. 2006;60:645–9. doi: 10.1016/j.biopsych.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Lane HY, Lin CH, Huang YJ, Liao CH, Chang YC, Tsai GE. A randomized, double-blind, placebo-controlled comparison study of sarcosine (N-methylglycine) and D-serine add-on treatment for schizophrenia. Int J Neuropsychopharmacol. 2010;13:451–60. doi: 10.1017/S1461145709990939. [DOI] [PubMed] [Google Scholar]
- Leveille F, El Gaamouch F, Gouix E, Lecocq M, Lobner D, Nicole O, Buisson A. Neuronal viability is controlled by a functional relation between synaptic and extrasynaptic NMDA receptors. FASEB J. 2008;22:4258–71. doi: 10.1096/fj.08-107268. [DOI] [PubMed] [Google Scholar]
- Levinson JN, Chery N, Huang K, Wong TP, Gerrow K, Kang R, Prange O, Wang YT, El-Husseini A. Neuroligins mediate excitatory and inhibitory synapse formation:involvement of PSD-95 and neurexin-1beta in neuroligin-induced synaptic specificity. J Biol Chem. 2005;280:17312–9. doi: 10.1074/jbc.M413812200. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhang Y, Ku L, Wilkinson KD, Warren ST, Feng Y. The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucleic Acids Res. 2001;29:2276–83. doi: 10.1093/nar/29.11.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–23. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Lindsley CW, Emmitte KA. Recent progress in the discovery and development of negative allosteric modulators of mGluR5. Curr Opin Drug Discov Devel. 2009;12:446–57. [PubMed] [Google Scholar]
- Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, Grier MD, Baker DA. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci. 2007;27:13968–76. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Henley JM, Holman D, Zhou M, Wiegert O, van Spronsen M, Joels M, Hoogenraad CC, Krugers HJ. Corticosterone alters AMPAR mobility and facilitates bidirectional synaptic plasticity. PLoS One. 2009;4:e4714. doi: 10.1371/journal.pone.0004714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SJ, Murrough JW, Aan Het Rot M, Collins KA, Reich DL, Charney DS. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol. 2009:1–12. doi: 10.1017/S1461145709000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K, Fletcher M, Kamiya Y, Yuzaki M. Specific assembly with the NMDA receptor 3B subunit controls surface expression and calcium permeability of NMDA receptors. J Neurosci. 2003;23:10064–73. doi: 10.1523/JNEUROSCI.23-31-10064.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K, Kamiya Y, Matsuda S, Yuzaki M. Cloning and characterization of a novel NMDA receptor subunit NR3B: a dominant subunit that reduces calcium permeability. Brain Res Mol Brain Res. 2002;100:43–52. doi: 10.1016/s0169-328x(02)00173-0. [DOI] [PubMed] [Google Scholar]
- Matsuo N, Reijmers L, Mayford M. Spine-type-specific recruitment of newly synthesized AMPA receptors with learning. Science. 2008;319:1104–7. doi: 10.1126/science.1149967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBean GJ. Cerebral cystine uptake: a tale of two transporters. Trends Pharmacol Sci. 2002;23:299–302. doi: 10.1016/s0165-6147(02)02060-6. [DOI] [PubMed] [Google Scholar]
- McCullumsmith RE, Meador-Woodruff JH. Striatal excitatory amino acid transporter transcript expression in schizophrenia, bipolar disorder, and major depressive disorder. Neuropsychopharmacology. 2002;26:368–75. doi: 10.1016/S0893-133X(01)00370-0. [DOI] [PubMed] [Google Scholar]
- Merlin LR, Bergold PJ, Wong RK. Requirement of protein synthesis for group I mGluR-mediated induction of epileptiform discharges. J Neurophysiol. 1998;80:989–93. doi: 10.1152/jn.1998.80.2.989. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Picciotto MR, Sanacora G. Antidepressant-like effects of ceftriaxone in male C57BL/6J mice. Biol Psychiatry. 2007;61:250–2. doi: 10.1016/j.biopsych.2006.04.037. [DOI] [PubMed] [Google Scholar]
- Moechars D, Weston MC, Leo S, Callaerts-Vegh Z, Goris I, Daneels G, Buist A, Cik M, van der Spek P, Kass S, Meert T, D’Hooge R, Rosenmund C, Hampson RM. Vesicular glutamate transporter VGLUT2 expression levels control quantal size and neuropathic pain. J Neurosci. 2006;26:12055–66. doi: 10.1523/JNEUROSCI.2556-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–52. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- Montana V, Ni Y, Sunjara V, Hua X, Parpura V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J Neurosci. 2004;24:2633–42. doi: 10.1523/JNEUROSCI.3770-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–40. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–9. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musazzi L, Milanese M, Farisello P, Zappettini S, Tardito D, Barbiero VS, Bonifacino T, Mallei A, Baldelli P, Racagni G, Raiteri M, Benfenati F, Bonanno G, Popoli M. Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. PLoS One. 2010;5:e8566. doi: 10.1371/journal.pone.0008566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Nam CI, Chen L. Postsynaptic assembly induced by neurexin-neuroligin interaction and neurotransmitter. Proc Natl Acad Sci U S A. 2005;102:6137–42. doi: 10.1073/pnas.0502038102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–97. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD, Wroblewski JT, Pin JP. Metabotropic glutamate receptors: From the workbench to the bedside. Neuropharmacology. 2010 doi: 10.1016/j.neuropharm.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi M, Hinds H, Lu HP, Kawata M, Hayashi Y. Motoneuron-specific expression of NR3B, a novel NMDA-type glutamate receptor subunit that works in a dominant-negative manner. J Neurosci. 2001;21:RC185. doi: 10.1523/JNEUROSCI.21-23-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–5. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- O’Shea RD. Roles and regulation of glutamate transporters in the central nervous system. Clin Exp Pharmacol Physiol. 2002;29:1018–23. doi: 10.1046/j.1440-1681.2002.03770.x. [DOI] [PubMed] [Google Scholar]
- Osterweil EK, Krueger DD, Reinhold K, Bear MF. Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. J Neurosci. 2010;30:15616–27. doi: 10.1523/JNEUROSCI.3888-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CL, Cotton L, Henley JM. The molecular pharmacology and cell biology of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Pharmacol Rev. 2005;57:253–77. doi: 10.1124/pr.57.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Sudhof TC. Cell biology of Ca2+-triggered exocytosis. Curr Opin Cell Biol. 2010;22:496–505. doi: 10.1016/j.ceb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadia S, Soriano FX, Leveille F, Martel MA, Dakin KA, Hansen HH, Kaindl A, Sifringer M, Fowler J, Stefovska V, McKenzie G, Craigon M, Corriveau R, Ghazal P, Horsburgh K, Yankner BA, Wyllie DJ, Ikonomidou C, Hardingham GE. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008;11:476–87. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passafaro M, Nakagawa T, Sala C, Sheng M. Induction of dendritic spines by an extracellular domain of AMPA receptor subunit GluR2. Nature. 2003;424:677–81. doi: 10.1038/nature01781. [DOI] [PubMed] [Google Scholar]
- Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13:1102–7. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Neuroenergetics: calling upon astrocytes to satisfy hungry neurons. Neuroscientist. 2004;10:53–62. doi: 10.1177/1073858403260159. [DOI] [PubMed] [Google Scholar]
- Perlis RH, Ostacher MJ, Patel JK, Marangell LB, Zhang H, Wisniewski SR, Ketter TA, Miklowitz DJ, Otto MW, Gyulai L, Reilly-Harrington NA, Nierenberg AA, Sachs GS, Thase ME. Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Am J Psychiatry. 2006;163:217–24. doi: 10.1176/appi.ajp.163.2.217. [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, Mulle C. Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nat Rev Neurosci. 2008;9:423–36. doi: 10.1038/nrn2379. [DOI] [PubMed] [Google Scholar]
- Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66:522–6. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M, Kang J, Burlin TV, Jiang C, Smith CB. Postadolescent changes in regional cerebral protein synthesis: an in vivo study in the FMR1 null mouse. J Neurosci. 2005;25:5087–95. doi: 10.1523/JNEUROSCI.0093-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CR, Thompson VL, Tate WP, Abraham WC. Metabotropic glutamate receptors trigger homosynaptic protein synthesis to prolong long-term potentiation. J Neurosci. 2000;20:969–76. doi: 10.1523/JNEUROSCI.20-03-00969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AL, Williams R. Recent progress in the development of allosteric modulators of mGluR5. Curr Opin Drug Discov Devel. 2007;10:715–22. [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Sachs GS, Thase ME, Otto MW, Bauer M, Miklowitz D, Wisniewski SR, Lavori P, Lebowitz B, Rudorfer M, Frank E, Nierenberg AA, Fava M, Bowden C, Ketter T, Marangell L, Calabrese J, Kupfer D, Rosenbaum JF. Rationale, design, and methods of the systematic treatment enhancement program for bipolar disorder (STEP-BD) Biol Psychiatry. 2003;53:1028–42. doi: 10.1016/s0006-3223(03)00165-3. [DOI] [PubMed] [Google Scholar]
- Sattler R, Rothstein JD. Targeting an old mechanism in a new disease-protection of glutamatergic dysfunction in depression. Biol Psychiatry. 2007;61:137–8. doi: 10.1016/j.biopsych.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Schapitz IU, Behrend B, Pechmann Y, Lappe-Siefke C, Kneussel SJ, Wallace KE, Stempel AV, Buck F, Grant SG, Schweizer M, Schmitz D, Schwarz JR, Holzbaur EL, Kneussel M. Neuroligin 1 is dynamically exchanged at postsynaptic sites. J Neurosci. 2010;30:12733–44. doi: 10.1523/JNEUROSCI.0896-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Akil O, Yi E, Weber CM, Grant L, Yoo J, Clause A, Kandler K, Noebels JL, Glowatzki E, Lustig LR, Edwards RH. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron. 2008;57:263–75. doi: 10.1016/j.neuron.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V, Rehal S, Klempan T, Gratton A, Benkelfat C, Rouleau GA, Mechawar N, Turecki G. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS One. 2009;4:e6585. doi: 10.1371/journal.pone.0006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinin A, Shavit S, Benveniste M. Subunit specificity and mechanism of action of NMDA partial agonist D-cycloserine. Neuropharmacology. 2001;41:151–8. doi: 10.1016/s0028-3908(01)00073-9. [DOI] [PubMed] [Google Scholar]
- Shen J, Petersen KF, Behar KL, Brown P, Nixon TW, Mason GF, Petroff OA, Shulman GI, Shulman RG, Rothman DL. Determination of the rate of the glutamate/glutamine cycle in the human brain by in vivo 13C NMR. Proc Natl Acad Sci U S A. 1999;96:8235–40. doi: 10.1073/pnas.96.14.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifonios L, Trinchero M, Cereseto M, Ferrero A, Cladouchos ML, Macedo GF, Reines A, Wikinski S. An enriched environment restores normal behavior while providing cytoskeletal restoration and synaptic changes in the hippocampus of rats exposed to an experimental model of depression. Neuroscience. 2009;164:929–40. doi: 10.1016/j.neuroscience.2009.08.059. [DOI] [PubMed] [Google Scholar]
- Simonyi A, Schachtman TR, Christoffersen GR. Metabotropic glutamate receptor subtype 5 antagonism in learning and memory. Eur J Pharmacol. 2010;639:17–25. doi: 10.1016/j.ejphar.2009.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer B, Keinanen K, Verdoorn TA, Wisden W, Burnashev N, Herb A, Kohler M, Takagi T, Sakmann B, Seeburg PH. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science. 1990;249:1580–5. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- Song JY, Ichtchenko K, Sudhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci U S A. 1999;96:1100–5. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–7. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh YH, Pelkey KA, Lavezzari G, Roche PA, Huganir RL, McBain CJ, Roche KW. Corequirement of PICK1 binding and PKC phosphorylation for stable surface expression of the metabotropic glutamate receptor mGluR7. Neuron. 2008;58:736–48. doi: 10.1016/j.neuron.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz MS, Stroup TS, McEvoy JP, Davis SM, Rosenheck RA, Keefe RS, Hsiao JK, Lieberman JA. What CATIE found: results from the schizophrenia trial. Psychiatr Serv. 2008;59:500–6. doi: 10.1176/ps.2008.59.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Forsythe ID, Tsujimoto T, Barnes-Davies M, Onodera K. Presynaptic calcium current modulation by a metabotropic glutamate receptor. Science. 1996;274:594–7. doi: 10.1126/science.274.5287.594. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Svoboda K, Malinow R. Experience strengthening transmission by driving AMPA receptors into synapses. Science. 2003;299:1585–8. doi: 10.1126/science.1079886. [DOI] [PubMed] [Google Scholar]
- Takamori S. VGLUTs: ‘exciting’ times for glutamatergic research? Neurosci Res. 2006;55:343–51. doi: 10.1016/j.neures.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Tong G, Shepherd D, Jahr CE. Synaptic desensitization of NMDA receptors by calcineurin. Science. 1995;267:1510–2. doi: 10.1126/science.7878472. [DOI] [PubMed] [Google Scholar]
- Toro C, Deakin JF. NMDA receptor subunit NRI and postsynaptic protein PSD-95 in hippocampus and orbitofrontal cortex in schizophrenia and mood disorder. Schizophr Res. 2005;80:323–30. doi: 10.1016/j.schres.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Hartley M, Heinemann SF. Control of proton sensitivity of the NMDA receptor by RNA splicing and polyamines. Science. 1995;268:873–6. doi: 10.1126/science.7754371. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–38. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry. 2000;57:1139–47. doi: 10.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, Krystal JH, Sanacora G. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Res. 2011;191:122–7. doi: 10.1016/j.pscychresns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderklish PW, Edelman GM. Dendritic spines elongate after stimulation of group 1 metabotropic glutamate receptors in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 2002;99:1639–44. doi: 10.1073/pnas.032681099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte P, Bading H. Opposing roles of synaptic and extrasynaptic NMDA receptors in neuronal calcium signalling and BDNF gene regulation. Curr Opin Neurobiol. 2003;13:366–71. doi: 10.1016/s0959-4388(03)00073-4. [DOI] [PubMed] [Google Scholar]
- Wallen-Mackenzie A, Gezelius H, Thoby-Brisson M, Nygard A, Enjin A, Fujiyama F, Fortin G, Kullander K. Vesicular glutamate transporter 2 is required for central respiratory rhythm generation but not for locomotor central pattern generation. J Neurosci. 2006;26:12294–307. doi: 10.1523/JNEUROSCI.3855-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen-Mackenzie A, Wootz H, Englund H. Genetic inactivation of the vesicular glutamate transporter 2 (VGLUT2) in the mouse: what have we learnt about functional glutamatergic neurotransmission? Ups J Med Sci. 2010;115:11–20. doi: 10.3109/03009730903572073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Edwards JG, Riley N, Provance DW, Jr, Karcher R, Li XD, Davison IG, Ikebe M, Mercer JA, Kauer JA, Ehlers MD. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell. 2008;135:535–48. doi: 10.1016/j.cell.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, Greenough WT. Metabotropic glutamate receptors trigger postsynaptic protein synthesis. Proc Natl Acad Sci U S A. 1993;90:7168–71. doi: 10.1073/pnas.90.15.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, Irwin SA, Klintsova AY, Spencer CM, Brazelton AD, Miyashiro K, Comery TA, Patel B, Eberwine J, Greenough WT. Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc Natl Acad Sci U S A. 1997;94:5395–400. doi: 10.1073/pnas.94.10.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–7. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, Brose N, Rosenmund C. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci U S A. 2004;101:7158–63. doi: 10.1073/pnas.0401764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GY, Deisseroth K, Tsien RW. Activity-dependent CREB phosphorylation: convergence of a fast, sensitive calmodulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway. Proc Natl Acad Sci U S A. 2001;98:2808–13. doi: 10.1073/pnas.051634198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia P, Chen HS, Zhang D, Lipton SA. Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J Neurosci. 2010;30:11246–50. doi: 10.1523/JNEUROSCI.2488-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kurup P, Zhang Y, Goebel-Goody SM, Wu PH, Hawasli AH, Baum ML, Bibb JA, Lombroso PJ. Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J Neurosci. 2009;29:9330–43. doi: 10.1523/JNEUROSCI.2212-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, Yan Z. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry. 2011;16:156–70. doi: 10.1038/mp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zheng K, Scimemi A, Rusakov DA. Receptor actions of synaptically released glutamate: the role of transporters on the scale from nanometers to microns. Biophys J. 2008;95:4584–96. doi: 10.1529/biophysj.108.129874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink M, Vollmayr B, Gebicke-Haerter PJ, Henn FA. Reduced expression of glutamate transporters vGluT1, EAAT2 and EAAT4 in learned helpless rats, an animal model of depression. Neuropharmacology. 2010;58:465–73. doi: 10.1016/j.neuropharm.2009.09.005. [DOI] [PubMed] [Google Scholar]