Summary
Plants monitor changes in day length to coordinate flowering with favorable seasons to increase their fitness. The day-length specific induction of FLOWERING LOCUS T (FT) regulated by CONSTANS (CO) is the crucial aspect of photoperiodic flowering in Arabidopsis thaliana. Recent studies elucidated some mechanisms of CO-dependent FT induction. Here, we demonstrate another mechanism of CO-dependent FT regulation. Our results indicate that CO protein regulates FT transcription partially by forming a complex with ASYMMETRIC LEAVES 1 (AS1) protein, which regulates leaf development partly by controlling gibberellin (GA) levels. We identified AS1 as a CO-interacting protein in yeast and verified their interaction in vitro and in planta. We also showed that the AS1 temporal and spatial expression pattern overlapped with that of CO. In addition, as1 mutants showed GA-independent delayed flowering under different light/dark conditions. FT expression levels in the as1 mutants and the SUC2:CO-HA/as1 line under long-day and 12-h light/12-h dark conditions were reduced compared to wild-type plants and the SUC2:HA-CO line, respectively. Moreover, AS1 directly bound to the specific regions of the FT promoter in vivo. These results indicate that CO forms a functional complex with AS1 to regulate FT expression and that AS1 plays different roles in two regulatory pathways, both of which concomitantly regulate a precise timing of flowering.
Keywords: Arabidopsis, ASYMMETRIC LEAVES1 (AS1), CONSTANS (CO), FLOWERING LOCUS T (FT), Flowering time, Photoperiodic pathway
Introduction
Plants primarily transition from a vegetative phase to a reproductive phase on a seasonal basis. This transition is precisely controlled by various environmental signals, such as light, temperature, and the availability of water and nutrients, and by endogenous factors, such as differences in developmental stages. In the model plant Arabidopsis thaliana, various signaling pathways regulate the flowering response (Baurle and Dean, 2006; Amasino, 2010). Plants sense changes in day length (=photoperiod) to regulate the timing of flowering (Kobayashi and Weigel, 2007; Turck et al., 2008; Song et al., 2010). For Arabidopsis thaliana, a longer day accelerates the transition to flowering through the photoperiodic pathway (Kobayashi and Weigel, 2007; Turck et al., 2008). The difference in day length is perceived in the leaf, at a site for the induction of the transmissible floral inductive signals termed florigen (Corbesier et al., 2007; Tamaki et al., 2007). Day-length measurement requires the integration of temporal information provided by the circadian system and light signals perceived by various photoreceptors (Suarez-Lopez et al., 2001; Yanovsky and Kay, 2002; Valverde et al., 2004; Sawa et al., 2007). The prolonged cold exposure known as vernalization, as well as more subtle ambient temperature changes, also influence the timing of flowering (Kim et al., 2009; Amasino, 2010; McClung and Davis, 2010).
Flowering, especially under short-day conditions, is regulated through the action of the phytohormone gibberellins (Eriksson et al., 2006). The autonomous pathway in which mutations affect the timing of flowering under various conditions shares some components with the vernalization pathway (Mouradov et al., 2002; Simpson, 2004; Feng et al., 2011). These pathways converge on regulating the expression of common target genes referred to as the floral integrators, such as FLOWERING LOCUS T (FT), SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1), and LEAFY (LFY) genes (Simpson and Dean, 2002; Hayama and Coupland, 2003; Sung et al., 2003; Amasino, 2005; Parcy, 2005). Having interconnected regulatory networks among these pathways enables plants to precisely coordinate the timing of flowering with the optimal environments. (Mouradov et al., 2002; Vandenbussche and Van Der Straeten, 2004; Baurle and Dean, 2006).
In Arabidopsis, photoperiodic flowering is mainly regulated through the function of CONSTANS (CO) and FT proteins (Suarez-Lopez et al., 2001; Valverde et al., 2004; Abe et al., 2005; Wigge et al., 2005). CO, which is a B-box zinc-finger-type transcription factor, accelerates flowering through the induction of FT expression in the leaf vasculature (Putterill et al., 1995; Robson et al., 2001; Takata and Goto, 2003; An et al., 2004). Mutations in CO and FT genes result in delayed flowering under long day conditions, whereas overexpression of these genes accelerates flowering regardless of photoperiods (Samach et al., 2000). The circadian-clock regulated timing of CO gene expression and light-dependent stability regulation of CO protein are crucial processes to control photoperiodic induction of FT (Suarez-Lopez et al., 2001; Yanovsky and Kay, 2002; Valverde et al., 2004; Sawa et al., 2007; Imaizumi, 2010). FT protein is synthesized in the leaf and translocated to the shoot apex, where it induces the expression of floral identity genes that trigger transcriptional cascades of floral development (Abe et al., 2005; Wigge et al., 2005; Corbesier et al., 2007; Jaeger and Wigge, 2007; Mathieu et al., 2007). FT protein is thought to play a major role as a florigen. FT orthologs are also identified as having similar characteristics as a florigen in other plant species such as rice and cucumber (Lin et al., 2007; Tamaki et al., 2007).
Recent advances in Arabidopsis research have provided some insights into the molecular mechanisms of CO-dependent FT regulation. Through the C-terminal CCT (CONSTANS, CO-LIKE and TOC1) domain, CO protein physically interacts with HEME ACTIVATOR PROTEIN (HAP) components (also known as NUCLEAR FACTOR-Y), HAP3 and HAP5 proteins, that form the trimeric CCAAT-binding transcription factor complex (Wenkel et al., 2006). The loss of several HAP3 and HAP5 genes caused late flowering, which resembles the flowering phenotype of the co mutants (Laubinger et al., 2006 Kuminoto et al., 2008). In addition, CO potentially interacts with other transcription factors in vivo, as it has been shown that CO may bind to TGAGC MOTIF-BINDING FACTOR 4 (TGA4) (Song et al., 2008). Recent results have indicated that CO by itself might also directly bind to the specific cis-elements in the FT promoter through its CCT domain (Tiwari et al., 2010). These results suggest that CO protein may induce FT transcription through various mechanisms. CO also binds to CONSTITUTIVE PHOTOMORPHOGENIC 1 (COP1) and SUPPRESSOR OF PHYA-105 (SPA) proteins, which together form the E3 ubiquitin ligase complex that degrades CO protein in the dark (Laubinger et al., 2006; Jang et al., 2008). This dark-dependent degradation of CO ensures that FT is not induced in the dark.
To further understand the molecular mechanisms of CO dependent FT induction, we aimed to find additional factors that may participate in FT regulation together with CO. We isolated ASYMMETRIC LEAVES 1 (AS1) Myb-type transcription factor as a CO interacting protein. AS1 controls leaf patterning by regulating cell differentiation in part through direct repression of class-1 KNOTTED-like homeobox (KNOX) gene expression at leaf primordia (Byrne et al., 2000; Hay et al., 2002; Guo et al., 2008; Hay and Tsiantis, 2010). KNOX proteins repress the expression of the GA biosynthesis gene, AtGA20ox1; thus AS1 indirectly regulates GA biosynthesis (Hay et al., 2002; Hay and Tsiantis, 2010; Ikezaki et al., 2010). The GA signaling pathway is detrimental to proper leaf development (Hay et al., 2002; Hay and Tsiantis, 2010). Reduced GA biosynthesis or GA signaling enhances the phenotype of the as1 mutants, indicating the possible role of AS1 in the regulation of GA signaling (Hay et al., 2002). AtGA20ox1 overexpression induces GA overproduction phenotypes including early flowering in SD (Huang et al., 1998; Coles et al., 1999). Conversely, the ga20ox1 mutation, together with the ga20ox2 mutation, causes the reduction of GA levels and also late flowering phenotype of the mutants in both LD and SD (Coles et al., 1999; Rieu et al., 2008). These results indicate that AS1 may be involved in the control of flowering time by regulating active GA levels (Ikezaki et al., 2010). In addition to the possible role of AS1 in GA-mediated flowering regulation, our data indicated that AS1 functions as a floral regulator involved in regulation of FT mediated by direct interaction with CO in the photoperiodic pathway.
Results
Isolation of AS1 as a CO interacting protein
CO contains two functional domains: two tandem repeats of B-box domains at the N-terminus and the CCT domain at the C-terminus (Khanna et al., 2009). Currently published data demonstrated that the CCT domain is an important domain in terms of regulating CO flowering function. Through the CCT domain, CO interacts with the HAP trimetric transcription factor complex, which regulates FT expression (Wenkel et al., 2006), as well as the COP1-SPAs E3 ubiquitin ligase complex, which controls the CO protein stability (Laubinger et al., 2006; Jang et al., 2008; Liu et al., 2008). The B-box domain is also predicted to be involved in the protein-protein interaction (Robson et al., 2001; Torok et al, 2001). In addition, there are five co mutant alleles that have mutations in the B-box domains, indicating that the B-box domain may also be important for the CO function (Robson et al., 2001). We previously found that TGA4 transcription factor interacts with the CO B-box domain in vitro and in yeast, although the purpose of this interaction remains elusive (Song et al., 2008). Therefore, the role of the CO B-box domain still hasn’t been well defined in the regulation of flowering time.
To understand the mechanisms by which CO regulates FT transcription, especially through the B-box domains, yeast two-hybrid screening was performed. A truncated CO protein that possesses two B-box domains was used as bait to isolate potential unidentified CO interacting transcription factors. We screened an Arabidopsis transcription factor library of roughly 1100 transcription factors (Song et al., 2008) and isolated a clone containing ASYMMETRIC LEAVES 1 (AS1) cDNA as a potential interactor of the partial CO peptides (Figure 1a). When AS1 was used as bait, we reciprocally detected the interaction of AS1 with CO in yeast (Figure 1a). To further define the interacting domain of CO with AS1, we tested AS1 interaction with various truncated CO proteins (Figure 1b). As expected, AS1 interacted with the truncated CO proteins that contain two B-box domains (Figure 1c). The truncated CO protein that possesses only the first B-box domain (designated B1) was not sufficient to bind to AS1 (Figure 1c). These results indicate that either the second B-box domain or both B-box domains may be involved in the interaction with AS1.
Figure 1. CO binds to AS1 in vitro and in planta.
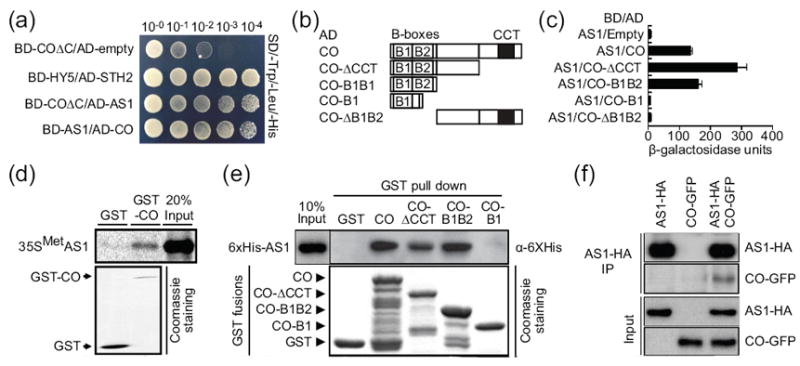
(a) Yeast two-hybrid assay for the CO-AS1 interaction. Serial dilutions of yeast cells harboring different combination of bait and prey constructs were grown on the selection media (SD-Trp, Leu, and His) for the protein-protein interaction assay. For the positive protein-protein interaction control, the HY5 and STH2 interaction (Datta et al., 2007) was used as a reference. (b) Schematic representation of the truncated CO proteins. The constructs containing the truncated CO genes were used in two-hybrid assay shown in (c). (c) Yeast two-hybrid assay to map the AS1 interaction site of CO. Different combinations of truncated CO proteins and the full length of AS1 were expressed in yeast. β-galactosidase (LacZ) activities that indicate the strength of the interaction were quantified. Similar mean values were observed in three independent assays. (d) GST pull-down assay for verifying the CO-AS1 interaction. Radioisotope labeled AS1 protein was co-purified with GST-CO protein in vitro. (e) In vitro binding assay to confirm the interaction domain of CO to AS1 observed in (c). (f) Coimmunoprecipitation assay for the CO-AS1 interaction in planta. AS1-HA and/or CO-GFP proteins were transiently expressed in tobacco leaves. AS1-HA proteins were precipitated against anti-AS1 antibody, and the presence of CO-GFP was analyzed. The AS1-HA and CO-GFP proteins were detected by anti-HA and anti-CO antibodies, respectively.
To verify the interaction observed in yeast, in vitro binding assay was performed using a recombinant glutathione S-transferase (GST)-fused full length of CO protein and a full length of AS1 protein. More AS1 protein was precipitated with GST-CO than with GST alone (Figure 1d). We also performed another GST pull down assay to confirm the result shown in Figure 1c, and found that the truncated CO protein that contains only two B-box domains was sufficient for the interaction in vitro (Figure 1e). We also tested whether four known co mutations, which cause missense mutations in the B-box domains (Robson et al., 2001), affect the interaction with AS1. All four CO variants attenuated the binding to AS1 in vitro (Figure S1), further supporting the notion that the B-box domains are important for the interaction with AS1.
We further confirmed the interaction of CO and AS1 by coimmunoprecipitation analysis using the Nicotiana benthamiana transient expression system. AS1-HA specifically coimmunoprecipitated with CO-GFP when both AS1-HA and CO-GFP were expressed in tobacco leaves (Figure 1f). In addition, when AS1-GFP and CO-RFP were co-expressed in Arabidopsis protoplasts, both GFP and RFP fluorescence co-localized in the nucleus (Figure 2a), indicating that both CO and AS1 exist in the same domain in the nucleus. Together with the results of yeast two-hybrid analysis, these results imply that CO may physically associate with AS1 in the nucleus in vivo.
Figure 2. Vascular and diurnal expression of AS1.
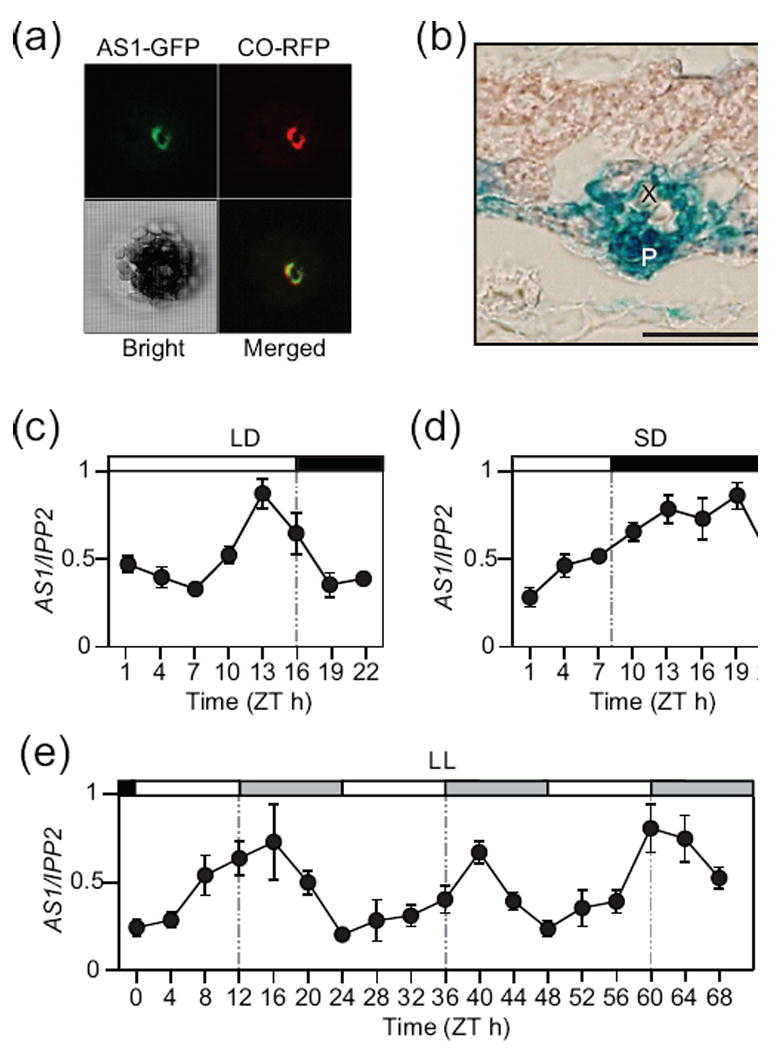
(a) Nuclear localization of AS1-GFP and CO-RFP proteins in Arabidopsis leaf protoplast. Scale bar is 10 μm. (b) Transverse section of a rosette leaf of the gAS:GUS plant. P, phloem; X, xylem. A scale bar is 50 μm. (c-e) Temporal expression profiles of AS1 in wild-type plants grown in LD (c), SD (d), or continuous light conditions (e). AS1 expression levels were quantified by real-time PCR. All Data points represent an average of three biological replicates and include error bars (SEM). Open, filled, and grey bars represent light, dark, and subjective dark periods, respectively.
Spatial and temporal expression patterns of AS1
CO expression is restricted in specific tissues and at specific times of the day. CO is mainly expressed in leaf phloem tissues (Takada and Goto, 2003; An et al., 2004). The peak expression of CO occurs in the late afternoon and at night (Suarez-Lopez et al., 2001), while CO protein stabilized only in the afternoon of long days (Valverde et al., 2004). If CO interacts with AS1 in vivo, we predicted that the spatial and temporal expression patterns of AS1 might overlap with those of CO. To study the spatial expression pattern of AS1, we analyzed the GUS expression pattern derived from the AS1 genomic fragment fused to the β-glucuronidase gene (AS1:AS1-GUS). GUS activity was predominantly found in the phloem tissues of leaves, cotyledons, hypocotyls, and roots (Figures 2b and S2) (Iwakawa et al., 2007). The expression patterns were similar within plants grown under various growth conditions (Figure S2). This result indicates that CO and AS1 expression overlaps in leaf vascular tissues.
We then analyzed the temporal expression patterns of AS1 under long-day (LD) and short-day (SD) conditions. Under these conditions, AS1 mRNA levels changed throughout the day with an afternoon peak in LD and with a broad night peak in SD (Figure 2c,d). In LD, the AS1 peak coincides with the daytime peak of CO (Suarez-Lopez et al., 2001). The results of the diurnal expression patterns of AS1 also prompted us to examine whether AS1 expression is controlled by the circadian clock. Therefore, we analyzed AS1 expression under continuous light (LL) conditions. The AS1 mRNA level oscillated diurnally under LL conditions (Figure 2e), indicating that AS1 expression is regulated by the circadian clock. Together with the AS1 spatial expression pattern, our data indicated that AS1 is largely expressed in the same spatiotemporal domain of CO. In addition, these data are consistent with the possibility that CO and AS1 work together.
AS1 is a positive regulator of flowering
Since CO regulates flowering time, we investigated the effect of as 1 mutation on flowering time in response to changes in photoperiods. We used as1-1, as1-2, and a newly identified T-DNA insertion as1 allele (SAIL 915_A12, designated as as 1-21, Figure S3) for the analysis. The as1 mutations had little effect on the timing of flowering in LD (Figure 3a). In SD, all the as1 mutants examined showed slightly late flowering (Figure 3b). It has been shown that some flowering time mutants and wild-type accessions respond differently to various day-length conditions, and exhibit stronger flowering phenotypes under certain day-length conditions (Giakountis et al., 2010). Therefore, we also tested the as1 flowering phenotype under various day-length conditions. Under 12-hour light/12-hour dark conditions, all three as1 alleles exhibited distinct late flowering (Figure 3c). In addition, we found that as1 showed a late flowering phenotype when the day is shorter than 14 hours (Figure S3g).
Figure 3. AS1 regulates flowering time under various day-length conditions.
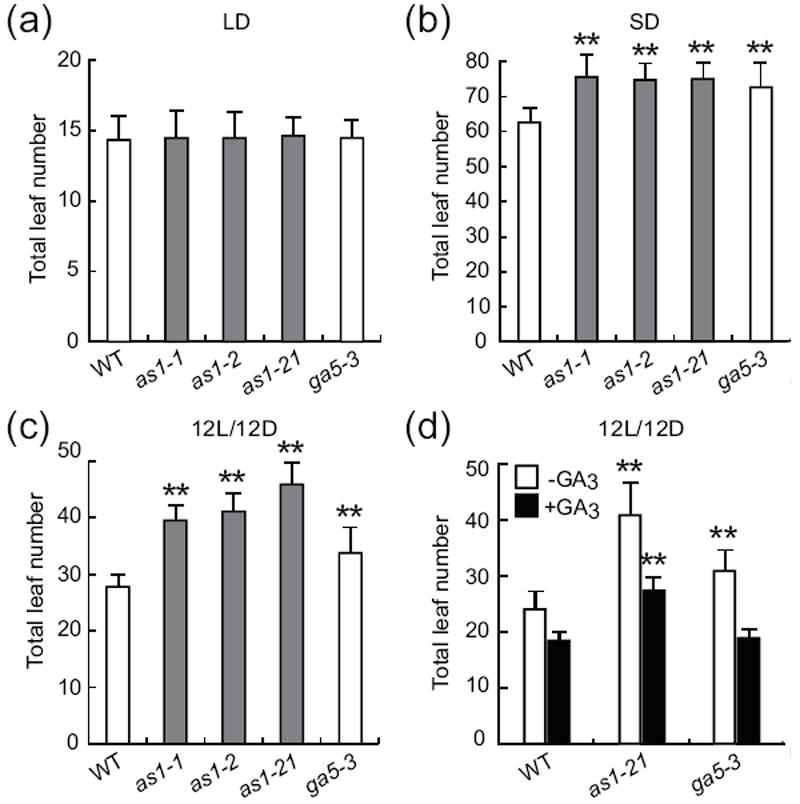
(a-c) Flowering time of as1 alleles under different day-length conditions. Flowering time of as1 alleles, wild type plants, and ga5-3 mutant (recently renamed Atga20ox1) were measured under LD (a), SD (b), and 12L/12D (c) conditions. At least 16 plants per each line were analyzed, and the means ±SD are shown in all panels. ** indicates p≤ 0.01 calculated using Student’s t test. (d) Effects of the GA3 application for flowering time. GA3 (100 μM) was sprayed twice a week to the plants grown in 12L/12D conditions until they started to flower. Error bars show SD; n = 12-18 plants.
AS1 is also thought to be a positive regulator in the GA signaling pathway. The as1 phenotype is enhanced by the inhibition of GA signaling and/or biosynthesis (Hay et al., 2002). AS1 directly suppresses the expression of KNOX genes (Figure S4a) (Guo et al., 2008), and the KNOX transcription factors repress the expression of GA biosynthesis gene, GA-20 oxidase 1 (AtGA20ox1) (Hay et al., 2002; Hay et al., 2010; Ikezaki et al, 2010). Therefore, the loss of as1 function indirectly reduces AtGA20ox1 expression and subsequently decreases the rate of active GA biosynthesis (Figure S4b) (Ikezaki et al, 2010).
Because the severe GA-biosynthesis deficient mutant ga1-3 failed to flower specifically in SD, GA plays a crucial role in floral induction especially under SD conditions (Wilson et al., 1992). At least, GA signals regulate the expression of LEAFY (LFY) (Eriksson et al., 2006). The mutation in AtGA20ox1 also delays the timing of the floral transition in SD, and the late flowering phenotype was rescued by application of exogenous GA (Coles et al., 1999; Rieu et al, 2008). Therefore, we postulated that the as1 mutant flowering phenotype described above could be partly caused by changes in the GA signaling levels due to the misexpression of AtGA20ox1. To test this possibility, we compared the flowering time of the as1 alleles with the AtGA20ox1 mutant, ga5-3. The flowering phenotype of the as1 alleles was similar to that of the ga5-3 mutant under both LD and SD conditions (Figure 3a,b). Under the 12L/12D conditions, the ga5-3 displayed a slight late-flowering phenotype, while the as1 mutants flowered even later than the ga5-3 plants (Figure 3c). In addition, the application of exogenous GA3 to the as1 mutant could not completely rescue the delayed flowering phenotype. On the contrary, the GA3 application to the ga5-3 mutant complemented the flowering phenotype observed in the 12L/12D conditions (Figure 3d). This result indicates that, at least under the 12L/12D conditions, AS1 regulates flowering by both GA-dependent and GA-independent mechanisms.
An as1 mutation enhances the late flowering phenotype of co mutant
Since our biochemical results indicate the presence of the CO-AS1 complex, we reasoned that if the GA-independent AS1 flowering function requires functional CO, this AS1 function would not be observed when the co mutation is present. To test this possibility we generated an as1 co double mutant using the possible co null mutant (co-101; Takada and Goto, 2003), which we found that it has a T-DNA insertion in the same position to the null co-10 allele (data not shown) (Laubinger et al., 2006). When we analyzed flowering phenotype under five different photoperiods (from SD to LD), we found that the co as1 double mutant always demonstrated a later flowering than the co mutant (Figure 4a). When the co as1 double mutant was grown under the 12L/12D conditions with exogenous GA3 application, the flowering phenotype of the double mutant was indistinguishable with that of the co mutant (Figure 4b). This result shows that the later flowering phenotype of the co as1 mutant caused by the addition of as1 mutation was rescued by the treatment of GA application and that the GA-independent as1 flowering phenotype did not exist when the co is mutated. This result implies that the GA-independent AS1 flowering function seems to be dependent on the CO function.
Figure 4. Effect of double mutations in AS1 and CO genes in flowering time.
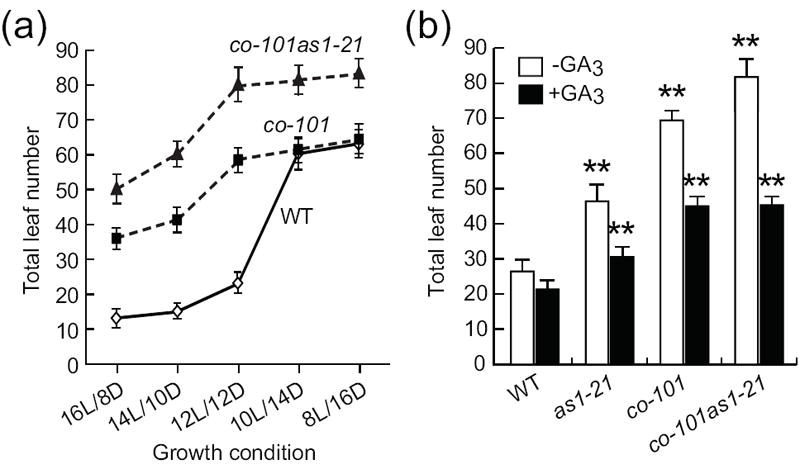
(a) Flowering times of co-101 as1-21 double mutants under different day-length conditions. At least 18 plants per each line were analyzed. Error bars represent SD (n = 10-14). (b) Application of exogenous GA3 to 12L/12D-grown plants. Error bars show SD; n = 14-18 plants.
Our flowering experiments demonstrated that AS1 is involved in the GA-dependent flowering time regulation, and that under certain photoperiods AS1 is also involved in the GA-independent flowering time regulation, possibly through the function of CO.
AS1 is involved in the CO dependent regulation of FT expression
Given that AS1 likely interacts with CO in vivo (Figure 1) and that AS1 may partially regulate flowering through CO function (Figure 4), we hypothesized that the as1 mutation might also affect the expression level of FT, a direct target of CO activity. FT transcript levels in the as1 mutant were slightly lower than those in the wild type plants in LD and 12L/12D (Figure 5b,c), although the CO expression in LD between the as1 mutant and the wild type plants looked identical (Figure 5a). This suggests that AS1 may facilitate CO inducing FT. Since CO protein is likely expressed at very low levels, and because CO may be recruited to form various protein complexes (Valverde et al., 2004; Wenkel et al., 2006; Song et al., 2008), we postulated that the endogenous abundance of CO protein could become a limiting factor to analyze the specific role of a potential CO-AS1 complex. To overcome this potential limitation, we utilized transgenic plants in which CO expression levels were elevated specifically in phloem tissues where AS1 is also expressed. To further test whether the as1 mutation attenuates the ability of CO to induce FT, we examined FT expression in the SUC2:CO-HA transgenic plants with or without the as1 mutation. For this experiment, the as1 mutation was introduced into SUC2:CO-HA plants by genetic cross, and we confirmed that CO expression levels in the transgenic plants with or without the as1 mutation were similar (Figure 5d). In the SUC2:CO-HA plants, the FT expression level was drastically higher than that in wild type plants in LD and 12L/12D (Figure 5e,f). In the SUC:CO-HA/as1-21 line, the FT expression level was still higher than that in the wild type; however, it was markedly lower than that in the SUC2:CO-HA plants (Figure 5e,f). This result suggests that the elevated CO levels (=sensitized conditions) facilitate the depiction of the AS1 contribution to CO dependent FT expression. These results indicate that AS1 may assist CO in inducing FT under these conditions and this could be a GA-independent role of AS1 in flowering time regulation. We also analyzed whether the difference in FT expression between the SUC2:CO-HA and SUC:CO-HA/as1-21 lines caused the difference in flowering time and found that the flowering time of both lines was very similar in LD and 12L/12D conditions (Figure S5), indicating that the FT expression levels in both lines are still high enough to induce a similar early flowering.
Figure 5. The as1 mutation reduces the expression of FT.
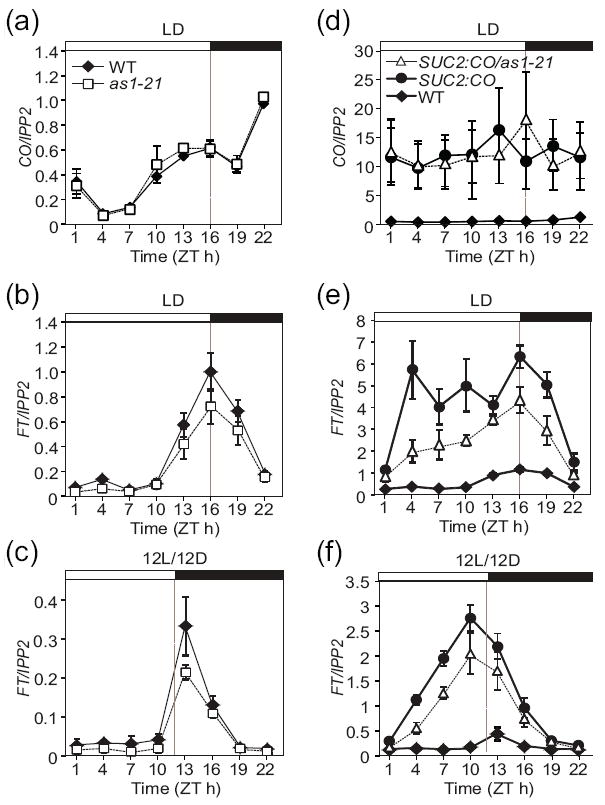
Plants were grown in LD (a,b,d,e) and 12L/12D (c,f) conditions. (a-c) Daily CO (a) and FT (b,c) mRNA expression patterns were examined in WT and as1 plants. (d-f) Daily CO (d) and FT (e,f) mRNA expression patterns were examined in WT, SUC2:CO-HA, and SUC2:CO-HA/as1-21 plants. CO and FT levels were normalized to IPP2 expression levels. The means ± SEM of normalized values of three or four independent experiments are shown.
AS1 binds to FT promoter in vivo
We have shown that AS1 may form a complex with CO (Figure 1), and that AS1 may participate in CO-dependent FT induction (Figure 5). Since CO likely binds to the FT promoter directly to regulate FT transcription (Tiwari et al., 2010), we examined whether AS1 also binds to the FT promoter. We performed chromatin immunoprecipitation (ChIP) analysis using plants expressing functional Hemagglutinin (HA)-tagged AS1 (Figures S3e and S6; previously described by Theodoris et al., 2003) and examined whether AS1-HA protein was coimmunoprecipitated with the FT chromatin (Figure 6a). The FT promoter sequences were most highly enriched around amplicons 8 and 11 in the 35S:AS1-HA line relative to the as1 mutant (Figure 6a). These contain the regions that CO proteins may associate with the FT promoter (Adrian et al., 2010; Tiwari et al., 2010). Our ChIP results indicate a presence of AS1 to the regions adjacent to a transcriptional start site of FT.
Figure 6. AS1 associates with FT promoter.
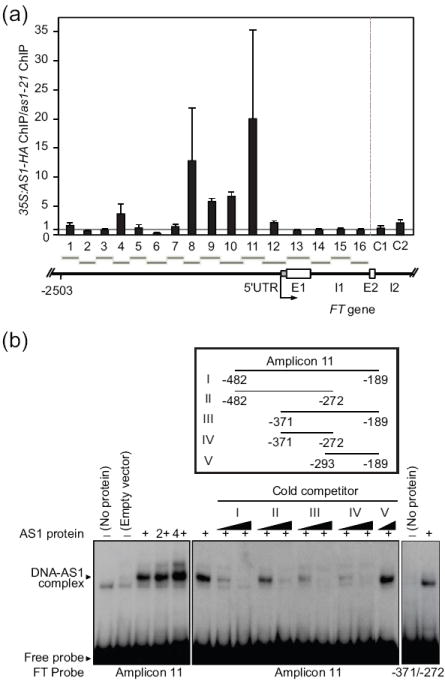
(a) Chromatin immunoprecipitation (ChIP) analysis of AS1-HA on FT promoter. The 10-day-old 35S:AS1-HA plants and the as1-21 mutant were harvested at 12 hours after the light onset in the 12L/12D conditions. The ratios between the specific enrichment values in the 35S:AS1-HA samples and that in the as1 sample on each region were calculated from three or four independent ChIP assays, respectively. C1 (ACT2) and C2 (UBQ10), which were described previously (Sawa et al., 2007), were used as controls. The dotted line in the upper panel indicates no enrichment. Schematic drawing of the FT gene and the locations of amplified regions are shown below the graph. Grey and white boxes represent 5’-untranslated (UTR) region and exons, respectively. E and I denote exon and intron. (b) Electrophoretic mobility shift assay (EMSA) of AS1 protein. The amount of AS1-DNA complex increases related to the increment of the AS1 protein added to the reaction (left panel). The right panel shows a competition experiment. The same reaction was incubated with two concentrations (1:10 and 1:100 molar ratios) of various non-labeled competitors, except the -293/-189 fragment (only 1:100). Locations of competitor DNA fragments are depicted above the gel image. Negative number 1 (-1) represents the first nucleotide on the 5’ side of the transcription start site.
Since AS1 is a Myb transcription factor, we next analyzed a possible direct binding of AS1 to the FT promoter sequences in vitro. We detected specific binding of AS1 to the DNA fragment that corresponds to the amplicon 11 region in vitro (Figures 6b and S7a). Although we could not successfully identify specific cis-elements for AS1 binding, our competition experiments further narrowed down the AS1 binding region to the sequence positions of -371/-272 (Figure 6b). This suggests that the region likely contains an AS1 binding site. Based on the Arabidopsis transient expression analysis, this region also participated in the CO-dependent induction of the FT:GUS reporter expression, although this region does not contain either the CCAAT-binding sites or the CORE (CO responsive element) sequences (Tiwari et al., 2010). Under our conditions, the presence of CO protein in the reaction did not affect the binding affinity of AS1 to the amplicon 11 sequences (Figure S7b), suggesting that CO may not be required for AS1 to bind the FT promoter. Together with our ChIP results, our data indicate that AS1 binds to the region around the transcriptional start site of FT in vivo.
Discussion
In this manuscript we reported the identification of another CO-interacting protein, AS1. We have shown the evidence that CO directly binds to AS1 (Figure 1). AS1 diurnal expression is regulated by the circadian clock and its spatiotemporal expression pattern largely overlaps with that of CO (Figure 2). The as1 mutants showed the late flowering phenotype under the 12L/12D and SD conditions, and at least part of the late flowering phenotype is not caused by as1-induced alteration of GA biosynthesis (Figure 3). In addition, the as1 mutation reduced the expression of FT without changing CO expression (Figure 5). Furthermore, we have demonstrated that AS1 binds to the FT promoter in vivo (Figure 6). These results indicate that AS1 is involved in the regulation of FT transcription by possibly interacting with CO on the FT promoter. Since AS1 was originally isolated as an interacting protein to the CO B-box domains, our results also indicate the potential role of the CO B-box domains in the flowering regulation.
The CO B-box domain is involved in the floral regulation
Five out of eight co alleles, all of which showed different degrees of the late flowering phenotype, have mutations in the B-box domains (four missense mutations and one deletion) (Robson et al., 2001). This indicates the functional importance of the CO B-box domains for the regulation of photoperiodic flowering. In contrast, most of the known CO interacting proteins that regulate flowering time bind to the CCT domain but not to the B-box domains (Laubinger et al., 2006; Jang et al., 2008; Liu et al., 2008). Thus, the molecular function of the B-box domains still remained elusive. Our results imply that the transcriptional factor AS1 binds to the CO B-box domains and regulates flowering in part through the function of CO. Based on the flowering phenotype of the as1 mutants, it is improbable that the attenuated binding to AS1 is a sole cause of the co mutant phenotypes. We have previously shown that CO may bind to a bZIP transcription factor TGA4 (Song et al., 2008). These results allow us to infer that CO may recruit several different types of transcription factors, other than HAP proteins, that regulate flowering through direct binding to the B-box domains. It would be intriguing to find out what other transcription factors bind to CO B-box domains and when and how the interaction occurs.
AS1 has GA-dependent and -independent roles in the flowering time regulation
Both the photoperiod pathway and the GA pathway contribute to the control of floral induction even under LD conditions (Reeves and Coupland, 2001). Because of the presence of the photoperiod pathway, a defect in the GApathway by itself does not have a detrimental effect on flowering in LD, but it is necessary for the regulation of flowering when the CO/FT module is also absent (Blazquez and Weigel, 2000; Reeves and Coupland, 2001; Eriksson et al., 2006). Thus there is interdependence between these pathways.
We demonstrated that the as1 mutants showed the late flowering phenotype when the day was shorter than 14 hours. Under the 12-hour day length, the application of bioactive GA could not completely rescue the as1 flowering phenotype. In addition, when the co as1 double mutant was treated with the same amount of GA, the flowering phenotype of the double mutant was indistinguishable with that of the co mutant. Moreover, the co as1 double mutant also showed distinct later flowering than the co single mutant. Furthermore, the results of our biochemical analysis indicated that AS1 forms a complex with CO protein. Together with published data regarding the role of AS1 in the GA-dependent pathway (Ikezaki et al., 2010), our results support the notion that AS1 likely has dual roles (GA-dependent and GA-independent) in the regulation of flowering time. One role is regulating the GA levels that affect flowering, and the other is to function as an accessory protein of CO that participates in FT induction under various day-length conditions. FT expression levels in the as1 mutant are lower than that in wild type plants at least in LD and 12L/12D conditions, although as1 flowered at the same time as the wild-type plants in LD. This indicates that the FT expression level in the as1 mutant in LD was still high enough to induce early flowering, which is similar to the wild type plants. Thus, in LD as long as CO is fully active, the contribution of AS1 in photoperiodic flowering seems to be negligible. However, under day-length conditions of 14 hours and shorter, AS1 contributes more to flowering time regulation. Even under SD conditions, the GA-dependent mechanism maintains the basal level of floral induction and AS1 contributes to this mechanism by indirectly regulating the expression of the AtGA20ox1 gene (Ikezaki et al., 2010). When the day is getting longer, in addition to the AS1-depednent GA-level regulation mechanism, AS1 seemed to facilitate CO to activate FT transcription by potentially direct binding to CO itself. This AS1-dependent CO activation mechanism may enhance the effect of day-length changes on CO activation and subsequently on floral transition especially when CO activity is still relatively low (such as under 12L/12D conditions). Thus AS1 plays a dual role to regulate flowering time under a broad range of photoperiods, and the contribution of AS1 in each pathway changes depending on the day-length conditions. This is a unique example of one protein that is shared between two different pathways that together regulate the same developmental transition to flowering. Our data also indicated that the late flowering phenotype of co was partially rescued by the application of GA3. It still remains to be revealed whether the AS1-CO complex is involved in GA biosynthesis or GA signaling.
In nature, the changes in day length occur gradually throughout the year. Together with other published results (Reeves and Coupland, 2001; Giakountis et al, 2010), our data suggest that as the days lengthen from winter to spring, Arabidopsis plants likely utilize both the GA pathway and the photoperiodic pathway to regulate the timing of flowering. Under these conditions, AS1 seems to have an active role in the regulation of flowering in these two different pathways. Having multiple pathways connected to each other to regulate flowering time enables Arabidopsis plants to more easily adapt to broader seasonal conditions.
Experimental Procedures
Plant materials and growth conditions
All Arabidopsis thaliana plants, WT (Col-0), as1-1, as1-2, as1-21, ga5-3, and co-101, are in the Col background (Theodoris et al., 2003; Takata and Goto, 2003; Hisamatsu et al., 2005). as1-1, as1-2, ga5-3 and co-101 seeds were gifts from Drs. M. Freeling (University of California, Berkeley), G.T. Kim (Dong-A University), M. Koshioka (National Institute of Floricultural Science), and K. Goto (Research Institute for Biological Sciences), respectively. We also used a new as1 allele (designated as as1-21, ABRC stock number CS841207), which is identified using LB3 (Sessions et al., 2002) and AS1 gene specific primers (Table S1). For flowering time experiments, plants were grown on soil in 16-h light/8-h dark (16L/8D, LD), 14-h light/10-h dark (14L/10D), 12-h light/12-h dark (12L/12D), 10-h light/14-h dark (10L/14D), and 8-h light/16-h dark (8L/16D, SD) conditions under cool white fluorescent lights (70-90 μmol·m-1·s-1). Flowering time was measured by counting total leaf number when plants were bolted.
Generation of transgenic plants
For AS1:AS1-GUS (gAS1:GUS), AS1:AS1-HA (gAS1:HA), SUC2:AS1-HA, and SUC2:CO-HA (SUC2:CO) constructs, Cauliflower Mosaic Virus 35S promoter in pCAMBIA1303 vector was replaced by either the AS1 genomic fragment (3805 bp) or the SUC2 promoter (An et al., 2004). The AS1 genomic fragment consisting of 2701 bp promoter and AS1 ORF (1104 bp) was amplified from the BAC clone F13M22 and cloned into the GUS coding sequence in the pCAMBIA1303 vector to generate AS1:AS1-GUS. The SUC2 promoter and full-lengths of AS1 and CO cDNA were sequentially inserted in front of the GUS coding sequence, generating SUC2:AS1-GUS and SUC2:CO-GUS. The HA sequences containing a stop codon were inserted in front of the GUS gene to generate non-translational fusion with GUS for SUC2:AS1-HA, SUC2:CO-HA, and AS1:AS1-HA constructs. For 35S:AS1-HA, the full-length of AS1 was introduced into pCAMBIA1303 vector, and then the HA sequences were cloned in front of GUS.
Yeast two-hybrid analysis
The experimental procedures of the GATEWAY based yeast two-hybrid assay were based on the manufacturer’s protocol (Invitrogen). For the yeast two-hybrid screening, the CO cDNA encoding the CO N-terminus (designated as COΔC, amino acid positions: 1-130) was introduced in pDEST32 vector to generate the bait construct BD-COΔC. First, the BD-COΔC plasmid was transformed into the yeast strain pJ69-A (James et al., 1996). Next, Arabidopsis transcription factor (TF) library, which contains about 1100 TF cDNAs in pDEST22 AD vector, was transformed into the yeast cells that possess the BD-COΔC plasmid. The transformants were selected on SD agar medium lacking Tryptophan (Trp), Leucine (Leu), Histidine (His) for 6 days. Positive colonies were isolated and grown in SD liquid medium lacking Trp, Leu, and His for 2 days, and plasmids were isolated from the yeast cells. The insert DNA fragment was amplified using GAD-F and attB2 adapter primers (Table S1). To confirm the results of the screening, we performed the filter lift β-galactosidase assay.
The full-length of AS1 cDNA and the CO deletion clones were amplified using primers described in Table S1. The PCR products of AS1 and CO fragments were inserted into the pDEST32 bait and pDEST22 prey vectors, respectively. Both bait and prey constructs were co-transformed in pJ69-4A, and the transformants were selected on SD agar medium lacking Trp, Leu, His, and Adenine (Ade). Four to five independent colonies per each combination were transferred into SD liquid medium lacking Trp and Leu. After 2-day incubation, the β-galactosidase activity was quantified using o-nitrophenyl-β-D-galatopyranoside (ONPG).
GST pull down assay
The full-length of CO cDNA was cloned into pGEX-5X-1 (GE) vector. pGEX-5X-1 and pGEX-5X-1-CO plasmids were transformed in E. coli BL21(DE3) strain. GST and GST-CO proteins were purified using glutathione-sepharose beads (Sigma). The full-length of AS1 cDNA was inserted into pDEST17 vector (Invitrogen) to generate 6xHis-AS1. 35S-labled AS1 proteins were synthesized using pDEST17-AS1 in the T′n′T in vitro transcription-translation system (Promega) according to the manufacturer’s instructions. Equal amounts (5 μl) of 35S-labled translation products and the GST-beads were added to 50 μl of binding buffer [50 mM Tris, pH 7.5, 150 mM NaCl, 0.5% Nonidet P-40, 10 mM EDTA, 2 mM EGTA, and protease inhibitors (complete, Roche)], and incubated at 4°C for 2 h with gentle mixing. After washing the beads with the binding buffer and the proteins bound to the beads were extracted by heating in Laemmli buffer. The extracted proteins and 0.5 μl of 35S-labled AS1 (input) were fractionated on the SDS-PAGE gel and subjected to autoradiography.
The full-length and the truncated versions of CO were cloned into the GST fusion vector pDEST15 (Invitrogen). All GST- and 6xHis-fused proteins were expressed in E. coli BL21 (DE3). GST and GST-fusion proteins were immobilized to the glutathione beads. For in vitro GST pull-down assay, 5 μg of total E. coli extracts containing 6xHis-AS1 protein were mixed with GST or GST-CO proteins attached to the glutathione-beads in the binding buffer [150 mM NaCl (for Figure 1d,e) or 250 mM NaCl (Figure S1)]. After 2 h incubation at 4°C, the beads were washed with the binding buffer. The proteins attached to the beads were extracted in Laemmli buffer. The AS1 proteins co-purified with GST-CO were detected by western blot using anti-6xHis antibody (PerkinElmer).
Gene expression analysis
To analyze CO and FT mRNA abundance, plants were grown for 8 or 10 days (LD or 12L/12D, respectively) on the agar media containing half-strength of Linsmaier and Skoog (LS) salts and 2% sucrose and harvested every 3 hours on day 8 or 10. Two μg of total RNA isolated by illustra RNAspin Mini (GE Healthcare) was used for cDNA synthesis using iScript cDNA Synthesis kit (Bio-Rad). Transcripts of CO, FT, and IPP2 were measured by quantitative-PCR (Sawa et al., 2007). IPP2 was used as a control to normalize the amount of cDNA. Sequences of the Q-PCR primers are listed in Table S1.
Intracellular distribution of CO and AS1 in Arabidopsis protoplasts
GFP- and RFP-fusion vectors and the methods for isolating protoplasts and transformation were described previously (Choi et al., 2005). Full-length AS1 and CO cDNAs were fused into GFP and RFP coding sequences in p326-GFP and p326-RFP vectors to generate 35S:AS1-GFP and 35S:CO-RFP constructs, respectively.
Coimmunoprecipitation and western blot
Tobacco (Nicotiana benthamiana) transient system was used for the coimmunoprecipitation assay as described in (Baudry et al., 2010). The full-length of CO cDNA was cloned into pMDC83 vector (Curtis and Grossniklaus, 2003) by LR recombination reaction (Invitrogen) to generate CO-GFP construct. pCAMBIA1303-35S:AS1-HA and pMDC83-CO plasmids were transformed into the agrobacteria GV3101. Overnight cultured agrobacterium carrying 35S:AS1-HA or 35S:CO-GFP were individually infiltrated or co-infiltrated into 3-week-old tobacco leaves. After 4 days, tissues were harvested. For coimmunoprecipitation assay, anti-AS1 antibody were pre-incubated with Protein A agarose (Invitrogen) in binding buffer [100 mM NaCl, 50 mM Tris pH 7.5, 0.2% triton X-100, 1 mM DTT, 50 μM MG132, protease inhibitors, and phosphatase inhibitors (sodium fluoride and sodium orthovanadate)] at 4°C. Approximately 2 mg of protein extracts were added and further incubated for 2 hours with gentle agitation. Immune complexes were extracted in Laemmli buffer by heating at 90°C for 2 min and subjected to SDS-PAGE.
To detect AS1-HA and CO-GFP proteins, anti-AS1 and anti-CO antibodies were used. The full-length of CO cDNA was cloned to pDEST17 vector (Invitrogen) to generate 6xHis-CO. pDEST17-AS1 and pDEST17-CO were introduced into E. coli BL21-AI cells to induce 6xHis-AS1 and 6xHis-CO proteins, respectively. His-fusion proteins were purified using Ni-NTA agarose (Qiagen) under denatured conditions according to the manufacturer’s manual and used for antibody generation in Rabbit. To purify antibodies, each 6xHis-fusion protein was immobilized on a PVDF membrane and then incubated with Rabbit serum containing anti-AS1 or anti-CO antibody at room temperature for 2 hours. After the membrane was washed five times with 1X TBS at 4°C for 10 min, the antibodies were eluted in 200 mM Glycine pH 2.5 at 4°C for 10 min and then were quickly neutralized by 2 M Tirs-HCl pH 8.8. Specificities of anti-AS1 and anti-CO were tested using epitope-tagged overexpression plants (Figure S8).
For western blot, proteins were separated on 10% SDS-PAGE, transferred to a PVDF membrane (Hybond-P, Amersham Biosciences), and probed with anti-GFP (Abcam), anti-HA (Abcam), anti-AS1, or anti-CO antibodies. Immunoreactive proteins were visualized by ECL kits (GE).
Chromatin immunoprecipitation (ChIP) assay
Procedures for the chromatin isolation and immunoprecipitation of protein-chromatin DNA were described previously (Bowler et al., 2004). Briefly, 12-day-old seedlings were vacuum infiltrated with 1% formaldehyde and subsequently 0.125 M glycine and were ground to a find powder. Arabidopsis chromatin pellet was isolated and sonicated in nuclei lysis buffer [50 mM Tris-HCl pH8.0, 10 mM EDTA, 1% SDS, 1 mM PMSF, 50 μM MG132, protease inhibitors, and phosphatase inhibitors (Roche)] to shear DNA to approximately 0.5 - 1 kb DNA fragments. The chromatin solution was diluted with ChIP dilution buffer (16.7 mM Tris-HCl pH 8.0, 1.1% Triton X-100, 1.2 mM EDTA, 167 mM NaCl, 1 mM PMSF, 50 μM MG132, protease inhibitors, and phosphatase inhibitors). Protein A agarose beads coated by sheared Salmon sperm DNA (Upstate) were pretreated with anti-AS1 antibody or rabbit serum, which is used as a negative control, and incubated with chromatin solution for 2-3 hours at 4°C. Precipitated DNA was analyzed by quantitative PCR. See Table S1 for the primer sequences used for the amplification of FT genomic regions designated as amplicons 1 to 16.
Electrophoretic mobility shift assay (EMSA)
6xHis-AS1 fusion protein used in the GST pull-down assay was used for the EMSA. The crude proteins containing 6xHis-AS1 were extracted in buffer consisting 20 mM HEPES-KOH pH 7.9, 25 mM KCl, 0.1 mM EDTA, 0.5 mM DTT, 10% glycerol and protease inhibitors and used without further purification. A method for the EMSA was described previously (Song et al., 2008).
Supplementary Material
Interactions between AS1 and CO proteins that contains B-box mutations.
AS1 is mainly expressed in the vascular tissues.
Location of the T-DNA insertion in the as1-21 mutant.
Expression levels of KNAT1 and AtGA20ox1 in as1 mutants.
Flowering time of SUC2:CO as1 plants under various photoperiods.
Effects of AS1 overexpression on flowering time and leaf shape regulation.
AS1 specifically binds to FT promoter sequences in vitro.
Anti-AS1 and anti-CO antibodies can detect AS1 and CO expressed in plants.
Primer sequences used in this study.
Acknowledgments
We thank S Ito and N Iranon for critical reading of manuscript, M Freeling, GT Kim, M Koshioka and K Goto for mutant seeds, and HJ Kim, SY Shin, SA Shim, SJ Jeon (Gyeongsang National University), and S Kim (Seoul National University) for technical assistance. This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST) (No. 2010-001280), Korea Research Foundation Grant (KRF-2008-314-C00362 and KRF-2007-0053802) to J.C.H, by a grant from the Next-Generation BioGreen 21 Program (SSAC, grant#: PJ008109), Rural Development Administration, Republic of Korea to S.Y.L, and by National Institutes of Health Grant GM079712 to T.I.
Footnotes
SUPPORTING INFORMATION The following materials are available in the online version of this article.
References
- Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science. 2005;309:1052–1056. doi: 10.1126/science.1115983. [DOI] [PubMed] [Google Scholar]
- Adrian J, Farrona S, Reimer JJ, Albani MC, Coupland G, Turck F. cis-Regulatory elements and chromatin state coordinately control temporal and spatial expression of FLOWERING LOCUS T in Arabidopsis. Plant Cell. 2010;22:1425–1440. doi: 10.1105/tpc.110.074682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amasino R. Vernalization and flowering time. Curr Opin Biotechnol. 2005;16:154–158. doi: 10.1016/j.copbio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Amasino R. Seasonal and developmental timing of flowering. Plant J. 2010;61:1001–1013. doi: 10.1111/j.1365-313X.2010.04148.x. [DOI] [PubMed] [Google Scholar]
- An H, Roussot C, Suarez-Lopez P, Corbesier L, Vincent C, Pineiro M, Hepworth S, Mouradov A, Justin S, Turnbull C, Coupland G. CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development. 2004;131:3615–3626. doi: 10.1242/dev.01231. [DOI] [PubMed] [Google Scholar]
- Baudry A, Ito S, Song YH, Strait AA, Kiba T, Lu S, Henriques R, Pruneda-Paz JL, Chua NH, Tobin EM, Kay SA, Imaizumi T. F-box proteins FKF1 and LKP2 act in concert with ZEITLUPE to control Arabidopsis clock progression. Plant Cell. 2010;22:606–622. doi: 10.1105/tpc.109.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baurle I, Dean C. The timing of developmental transitions in plants. Cell. 2006;125:655–664. doi: 10.1016/j.cell.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Blazquez MA, Weigel D. Integration of floral inductive signals in Arabidopsis. Nature. 2000;404:889–892. doi: 10.1038/35009125. [DOI] [PubMed] [Google Scholar]
- Bowler C, Benvenuto G, Laflamme P, Molino D, Probst AV, Tariq M, Paszkowski J. Chromatin techniques for plant cells. Plant J. 2004;39:776–789. doi: 10.1111/j.1365-313X.2004.02169.x. [DOI] [PubMed] [Google Scholar]
- Byrne ME, Barley R, Curtis M, Arroyo JM, Dunham M, Hudson A, Martienssen RA. Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature. 2000;408:967–971. doi: 10.1038/35050091. [DOI] [PubMed] [Google Scholar]
- Choi K, Kim S, Kim SY, Kim M, Hyun Y, Lee H, Choe S, Kim SG, Michaels S, Lee I. SUPPRESSOR OF FRIGIDA3 encodes a nuclear ACTIN-RELATED PROTEIN6 required for floral repression in Arabidopsis. Plant Cell. 2005;17:2647–2660. doi: 10.1105/tpc.105.035485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles JP, Phillips AL, Croker SJ, Garcia-Lepe R, Lewis MJ, Hedden P. Modification of gibberellin production and plant development in Arabidopsis by sense and antisense expression of gibberellin 20-oxidase genes. Plant J. 1999;17:547–556. doi: 10.1046/j.1365-313x.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, Coupland G. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science. 2007;316:1030–1033. doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 2003;133:462–469. doi: 10.1104/pp.103.027979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Hettiarachchi C, Johansson H, Holm M. SALT TOLERANCE HOMOLOG2, a B-box protein in Arabidopsis that activates transcription and positively regulates light-mediated development. Plant Cell. 2007;19:3242–3255. doi: 10.1105/tpc.107.054791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson S, Bohlenius H, Moritz T, Nilsson O. GA4 is the active gibberellin in the regulation of LEAFY transcription and Arabidopsis floral initiation. Plant Cell. 2006;18:2172–2181. doi: 10.1105/tpc.106.042317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Jacob Y, Veley KM, Ding L, Yu X, Choe G, Micheals SD. Hypomorphic alleles reveal FCA-independent roles for FY in the regulation of FLOWERING LOCUS C. Plant Physiol. 2011;155:1425–1434. doi: 10.1104/pp.110.167817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giakountis A, Cremer F, Sim S, Reymond M, Schmitt J, Coupland G. Distinct patterns of genetic variation alter flowering responses of Arabidopsis accessions to different daylengths. Plant Physiol. 2010;152:177–191. doi: 10.1104/pp.109.140772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Thomas J, Collins G, Timmermans MC. Direct repression of KNOX loci by the ASYMMETRIC LEAVES1 complex of Arabidopsis. Plant Cell. 2008;20:48–58. doi: 10.1105/tpc.107.056127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A, Kaur H, Phillips A, Hedden P, Hake S, Tsiantis M. The gibberellin pathway mediates KNOTTED1-type homeobox function in plants with different body plans. Curr Biol. 2002;12:1557–1565. doi: 10.1016/s0960-9822(02)01125-9. [DOI] [PubMed] [Google Scholar]
- Hay A, Tsiantis M. KNOX genes: versatile regulators of plant development and diversity. Development. 2010;137:3153–3165. doi: 10.1242/dev.030049. [DOI] [PubMed] [Google Scholar]
- Hayama R, Coupland G. Shedding light on the circadian clock and the photoperiodic control of flowering. Curr Opin Plant Biol. 2003;6:13–19. doi: 10.1016/s1369-5266(02)00011-0. [DOI] [PubMed] [Google Scholar]
- Hisamatsu T, King RW, Helliwell CA, Koshioka M. The involvement of gibberellin 20-oxidase genes in phytochrome-regulated petiole elongation of Arabidopsis. Plant Physiol. 2005;138:1106–1116. doi: 10.1104/pp.104.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Raman AS, Ream JE, Fujiwara H, Cerny RE, Brown SM. Overexpression of 20-oxidase confers a gibberellin-overproduction phenotype in Arabidopsis. Plant Physiol. 1998;118:773–781. doi: 10.1104/pp.118.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezaki M, Kojima M, Sakakibara H, Kojima S, Ueno Y, Machida C, Machida Y. Genetic networks regulated by ASYMMETRIC LEAVES1 (AS1) and AS2 in leaf development in Arabidopsis thaliana: KNOX genes control five morphological events. Plant J. 2010;61:70–82. doi: 10.1111/j.1365-313X.2009.04033.x. [DOI] [PubMed] [Google Scholar]
- Imaizumi T. Arabidopsis circadian clock and photoperiodism: time to think about location. Curr Opin Plant Biol. 2010;13:83–89. doi: 10.1016/j.pbi.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakawa H, Iwasaki M, Kojima S, Ueno Y, Soma T, Tanaka H, Semiarti E, Machida Y, Machida C. Expression of ASYMMETRIC LEAVES2 gene in the adaxial domain of Arabidopsis leaves represses cell proliferation in this domain and is critical for the development of properly expanded leveas. Plant J. 2007;51:173–184. doi: 10.1111/j.1365-313X.2007.03132.x. [DOI] [PubMed] [Google Scholar]
- Jaeger KE, Wigge PA. FT protein acts as a long-range signal in Arabidopsis. Curr Biol. 2007;17:1050–1054. doi: 10.1016/j.cub.2007.05.008. [DOI] [PubMed] [Google Scholar]
- James P, Halladay J, Craig EA. Genomic libraries and a host strain degsigned for high efficient two-hybrid selection in yeast. Genetics. 1996;144:1425–1436. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Marchal V, Panigrahi KC, Wenkel S, Soppe W, Deng XW, Valverde F, Coupland G. Arabidopsis COP1 shapes the temporal pattern of CO accumulation conferring a photoperiodic flowering response. Embo J. 2008;27:1277–1288. doi: 10.1038/emboj.2008.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R, Kronmiller B, Maszle DR, Coupland G, Holm M, Mizuno T, Wu SH. The Arabidopsis B-box zinc finger family. Plant Cell. 2009;21:3416–3420. doi: 10.1105/tpc.109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Doyle MR, Sung S, Amasino RM. Vernalization: winter and the timing of flowering in plants. Annu Rev Cell Dev Biol. 2009;25:277–299. doi: 10.1146/annurev.cellbio.042308.113411. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Weigel D. Move on up, it’s time for change -mobile signals controlling photoperiod-dependent flowering. Genes Dev. 2007;21:2371–2384. doi: 10.1101/gad.1589007. [DOI] [PubMed] [Google Scholar]
- Kumimoto RW, Adam L, Hymus GJ, Repetti PP, Reuber TL, Marion CM, Hempel FD, Ratcliffe OJ. The Nuclear Factor Y subunits NF-YB2 and NF-YB3 play additive roles in the promotion of flowering by inductive long-day photoperiods in Arabidopsis. Planta. 2008;228:709–723. doi: 10.1007/s00425-008-0773-6. [DOI] [PubMed] [Google Scholar]
- Laubinger S, Marchal V, Le Gourrierec J, Wenkel S, Adrian J, Jang S, Kulajta C, Braun H, Coupland G, Hoecker U. Arabidopsis SPA proteins regulate photoperiodic flowering and interact with the floral inducer CONSTANS to regulate its stability. Development. 2006;133:3213–3222. doi: 10.1242/dev.02481. [DOI] [PubMed] [Google Scholar]
- Lin MK, Belanger H, Lee YJ, Varkonyi-Gasic E, Taoka KI, Miura E, Xoconostle-Cazares B, Gendler K, Jorgensene RA, Phinney B, Lough TJ, Lucas WJ. FLOWERING LOCUS T protein may act as the long-distance florigenic signal in the cucurbits. Plant Cell. 2007;19:1488–1506. doi: 10.1105/tpc.107.051920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LJ, Zhang YC, Li QH, Sang Y, Mao J, Lian HL, Wang L, Yang HQ. COP1-mediated ubiquitination of CONSTANS is implicated in cryptochrome regulation of flowering in Arabidopsis. Plant Cell. 2008;20:292–306. doi: 10.1105/tpc.107.057281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu J, Warthmann N, Kuttner F, Schmid M. Export of FT protein from phloem companion cells is sufficient for floral induction in Arabidopsis. Curr Biol. 2007;17:1055–1060. doi: 10.1016/j.cub.2007.05.009. [DOI] [PubMed] [Google Scholar]
- McClung CR, Davis SJ. Ambient thermometers in plants: from physiological outputs towards mechanisms of thermal sensing. Curr Biol. 2010;20:R1086–1092. doi: 10.1016/j.cub.2010.10.035. [DOI] [PubMed] [Google Scholar]
- Mouradov A, Cremer F, Coupland G. Control of flowering time: interacting pathways as a basis for diversity. Plant Cell. 2002;14(Suppl):S111–130. doi: 10.1105/tpc.001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcy F. Flowering: a time for integration. Int J Dev Biol. 2005;49:585–593. doi: 10.1387/ijdb.041930fp. [DOI] [PubMed] [Google Scholar]
- Putterill J, Robson F, Lee K, Simon R, Coupland G. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell. 1995;80:847–857. doi: 10.1016/0092-8674(95)90288-0. [DOI] [PubMed] [Google Scholar]
- Reeves PH, Coupland G. Analysis of flowering time control in Arabidopsis by comparison of double and triple mutants. Plant Physiol. 2001;126:1085–1091. doi: 10.1104/pp.126.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieu I, Ruiz-Rivero O, Fernandez-Garcia N, Griffiths J, Powers SJ, Gong F, Linhartova T, Eriksson S, Nilsson O, Thomas SG, Philips AL, Hedden P. The gibberellin biosynthetic genes AtGA20ox1 and AtGA20ox2 act, partially redundantly, to promote growth and development throughout the Arabidopsis life cycle. Plant J. 2008;53:488–504. doi: 10.1111/j.1365-313X.2007.03356.x. [DOI] [PubMed] [Google Scholar]
- Robson F, Costa MM, Hepworth SR, Vizir I, Pineiro M, Reeves PH, Putterill J, Coupland G. Functional importance of conserved domains in the flowering-time gene CONSTANS demonstrated by analysis of mutant alleles and transgenic plants. Plant J. 2001;28:619–631. doi: 10.1046/j.1365-313x.2001.01163.x. [DOI] [PubMed] [Google Scholar]
- Samach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer Z, Yanofsky MF, Coupland G. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science. 2000;288:1613–1616. doi: 10.1126/science.288.5471.1613. [DOI] [PubMed] [Google Scholar]
- Sawa M, Nusinow DA, Kay SA, Imaizumi T. FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science. 2007;318:261–265. doi: 10.1126/science.1146994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions A, Burke E, Presting G, Aux G, McElver J, Patton D, Dietrich B, Ho P, Bacwaden J, Ko C, Clarke JD, Cotton D, Bullis D, Snell J, Miguel T, Hutchison D, Kimmerly B, Mitzel T, Katagiri F, Glazebrook J, Law M, Goff SA. A high-throughput Arabidopsis reverse genetics system. Plant Cell. 2002;14:2985–2994. doi: 10.1105/tpc.004630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GG, Dean C. Arabidopsis, the Rosetta stone of flowering time? Science. 2002;296:285–289. doi: 10.1126/science.296.5566.285. [DOI] [PubMed] [Google Scholar]
- Simpson GG. The autonomous pathway: epigenetic and post-transcriptional gene regulation in the control of Arabidopsis flowering time. Curr Opin Plant Biol. 2004;7:570–574. doi: 10.1016/j.pbi.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Song YH, Song NY, Shin SY, Kim HJ, Yun DJ, Lim CO, Lee SY, Kang KY, Hong JC. Isolation of CONSTANS as a TGA4/OBF4 interacting protein. Mol Cells. 2008;25:559–565. [PubMed] [Google Scholar]
- Song YH, Ito S, Imaizumi T. Similarities in the circadian clock and photoperiodism in plants. Curr Opin Plant Biol. 2010;13:594–603. doi: 10.1016/j.pbi.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Lopez P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature. 2001;410:1116–1120. doi: 10.1038/35074138. [DOI] [PubMed] [Google Scholar]
- Sung ZR, Chen L, Moon YH, Lertpiriyapong K. Mechanisms of floral repression in Arabidopsis. Curr Opin Plant Biol. 2003;6:29–35. doi: 10.1016/s1369-5266(02)00014-6. [DOI] [PubMed] [Google Scholar]
- Takada S, Goto K. TERMINAL FLOWER2, an Arabidopsis homolog of HETEROCHROMATIN PROTEIN1, counteracts the activation of FLOWERING LOCUS T by CONSTANS in the vascular tissues of leaves to regulate flowering time. Plant Cell. 2003;15:2856–2865. doi: 10.1105/tpc.016345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K. Hd3a protein is a mobile flowering signal in rice. Science. 2007;316:1033–1036. doi: 10.1126/science.1141753. [DOI] [PubMed] [Google Scholar]
- Theodoris G, Inada N, Freeling M. Conservation and molecular dissection of ROUGH SHEATH2 and ASYMMETRIC LEAVES1 function in leaf development. Proc Natl Acad Sci USA. 2003;100:6837–6842. doi: 10.1073/pnas.1132113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari SB, Shen Y, Chang HC, Hou Y, Harris A, Ma SF, McPartland M, Hymus GJ, Adam L, Marion C, Belachew A, Repetti PP, Reuber TL, Ratcliffe OJ. The flowering time regulator CONSTANS is recruited to the FLOWERING LOCUS T promoter via a unique cis-element. New Phytol. 2010;187:57–66. doi: 10.1111/j.1469-8137.2010.03251.x. [DOI] [PubMed] [Google Scholar]
- Torok M, Etkin LD. Two B or not two B? Overview of the rapidly expanding B-box family of proteins. Differentiation. 2001;67:63–71. doi: 10.1046/j.1432-0436.2001.067003063.x. [DOI] [PubMed] [Google Scholar]
- Turck F, Fornara F, Coupland G. Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu Rev Plant Biol. 2008;59:573–594. doi: 10.1146/annurev.arplant.59.032607.092755. [DOI] [PubMed] [Google Scholar]
- Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science. 2004;303:1003–1006. doi: 10.1126/science.1091761. [DOI] [PubMed] [Google Scholar]
- Vandenbussche F, Van Der Straeten D. Shaping the shoot: a circuitry that integrates multiple signals. Trends Plant Sci. 2004;9:499–506. doi: 10.1016/j.tplants.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Wenkel S, Turck F, Singer K, Gissot L, Le Gourrierec J, Samach A, Coupland G. CONSTANS and the CCAAT box binding complex share a functionally important domain and interact to regulate flowering of Arabidopsis. Plant Cell. 2006;18:2971–2984. doi: 10.1105/tpc.106.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D. Integration of spatial and temporal information during floral induction in Arabidopsis. Science. 2005;309:1056–1059. doi: 10.1126/science.1114358. [DOI] [PubMed] [Google Scholar]
- Wilson RN, Heckman JW, Somerville CR. Gibberellin Is Required for Flowering in Arabidopsis thaliana under Short Days. Plant Physiol. 1992;100:403–408. doi: 10.1104/pp.100.1.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky MJ, Kay SA. Molecular basis of seasonal time measurement in Arabidopsis. Nature. 2002;419:308–312. doi: 10.1038/nature00996. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Interactions between AS1 and CO proteins that contains B-box mutations.
AS1 is mainly expressed in the vascular tissues.
Location of the T-DNA insertion in the as1-21 mutant.
Expression levels of KNAT1 and AtGA20ox1 in as1 mutants.
Flowering time of SUC2:CO as1 plants under various photoperiods.
Effects of AS1 overexpression on flowering time and leaf shape regulation.
AS1 specifically binds to FT promoter sequences in vitro.
Anti-AS1 and anti-CO antibodies can detect AS1 and CO expressed in plants.
Primer sequences used in this study.


