Abstract
Memory B cells (MBCs) are a key component of long term humoral immunity to many human infectious diseases. Despite their importance, we know little about the generation or maintenance of antigen- (Ag)-specific MBCs in humans in response to infection. A frequently employed method for quantifying Ag-specific MBCs in human peripheral blood (Crotty et al., 2004) relies on the ability of MBCs but not naïve B cells to differentiate into antibody secreting cells (ASCs) in response to polyclonal activators and Toll-like receptor agonists in vitro and the measurement of Ag-specific ASCs by ELISPOT assays. Here we report on studies to optimize the efficiency of this ELISPOT-based assay and to apply this assay to the detection of Plasmodium falciparum (Pf)-specific MBCs in adults living in a malaria endemic area where immunity to Pf is acquired through natural infection. We show that the addition of IL-10 to in vitro cultures of human peripheral blood mononuclear cells increased the efficiency of the assay from 10% to over 90% without increasing the ASC burst size and without any substantial increase in background from naïve B cells or plasma cells (PCs). Using this assay we were able to quantify the frequency of Pf-specific MBCs in peripheral blood of adults living in a malaria endemic area. Thus, this highly efficient assay appears to be well suited to field studies of the generation and maintenance of MBCs where the volumes of blood obtainable are often limiting.
Keywords: Memory B cells, B cell ELISPOT, Plasmodium falciparum, malaria
1. Introduction
A hallmark of adaptive immunity is Ag-specific immunological memory, the ability to respond more rapidly and robustly to re-exposure to an Ag. Indeed, all current vaccines are predicated on the phenomenon of immunological memory (Sallusto F, 2010). However, despite its importance we still have an incomplete understanding of the cellular and molecular mechanisms that underlie the generation, maintenance and activation of immunological memory. For many infectious diseases neutralizing antibodies (Abs) play a critical role in protective immune responses (Sallusto F, 2010), and thus the mechanisms that underlie the generation and maintenance of B cell memory are of considerable interest. Of particular interest is an understanding of the acquisition of B cell memory in infectious diseases for which we currently have no vaccines, including malaria.
Long-term humoral memory is encoded in both memory B cells (MBCs) and long-lived plasma cells (LLPCs) that are generated during a primary immune response to vaccination or infection. LLPCs reside in the bone marrow and are responsible for maintaining serum antibody (Ab) levels in the absence of Ag re-exposure (Radbruch A, 2006; Wrammert J, 2009). Because of their inaccessibility to routine sampling in humans, LLPCs are rarely directly assayed. However, the presence of Ag-specific Ab in serum in the absence of re-exposure to an Ag is likely an accurate reflection of the presence of Ag-specific LLPCs. MBCs are responsible for the rapid, high titer, high affinity secondary Ab responses elicited upon re-exposure to a pathogen or booster vaccination (Radbruch A, 2006; Wrammert J, 2009). MBCs reside both in the lymphoid tissues and in the peripheral circulation. The results of a recent study in humans receiving a smallpox vaccine provided evidence that although the majority of vaccine-specific MBCs were in the spleen, the frequency of Ag-specific MBCs in the spleen paralleled the frequency observed in peripheral blood throughout the course of the response (Blink EJ, 2005). Furthermore, a recent study using a mouse model of malaria indicated MBCs and PCs in peripheral blood mononuclear cells (PBMCs) reflect Plasmodium-specific B cell responses in the spleen and bone marrow (Nduati EW, 2010). Based on these observations, the frequencies of MBCs in peripheral blood appear to be a good indication of the total number of MBCs in an individual.
Here we focus on the development of B cell memory to Pf malaria because it kills nearly one million people annually and there is no vaccine. Immunity to clinical malaria is slow to develop requiring years of repeated infections (Langhorne J, 2008; Crompton PD, 2010; Weiss GE, 2010). Abs are known to play a central role in protection from malaria (Cohen S, 1961) but the B cell biology that underlies the slow acquisition of immunity is just beginning to be investigated. Therefore it is important to develop highly efficient assays that provide the tools for assessing MBCs in the PBMCs of individuals acquiring malaria immunity through natural infection. Evidence is accumulating that the immune systems of individuals living in malaria endemic areas may differ from those in non-malaria endemic areas. For example, in one study we determined that individuals in Mali, in an area of high malaria transmission, have greatly expanded populations of atypical or ‘exhausted’ MBCs (Weiss GE, 2009). In another, we found that Malian adults were relatively refractory to the potentiating effects of CpG-containing vaccines as compared to individuals in the U.S. in that while CpG-containing vaccines resulted in a significant increase in the number of MBCs and the Ab titer in the U.S., there was no difference in MBC number or antibody titer when CpG-containing or non-CpG-containing vaccines were administered to Malian adults (Crompton PD, 2009). Thus, it is essential that assays to detect Pf-specific MBCs are validated in field samples from individuals living in malaria endemic areas.
An assay frequently used to detect Ag-specific human MBCs, described by Crotty et al. 2004 (Crotty S et al, 2004), relies on the selective ability of MBCs to proliferate and differentiate into Ab secreting cells (ASCs) in vitro in response to a combination of pokeweed mitogen (PWM), fixed S. aureus, Cowan strain (SAC) and the TLR9 agonist CpG oligonucleotide (ODN-2006) over a five to six day culture period. Ag-specific and total ASCs were quantified in ELISPOT assays using plates coated with either Ag or human Ig-specific antibodies to capture Ag-specific and total IgG, respectively. Crotty et al. showed that peripheral blood MBCs (defined as CD19+CD20+CD27+) from individuals immunized with anthrax vaccine differentiated into anthrax protective antigen (PA)-specific ASCs in this assay, but naïve B cells (defined as CD19+CD20+CD27−) did not. PA-specific MBCs represented up to 2% of circulating IgG+ B cells in immune individuals and were essentially undetectable in non-immune individuals. Thus, this assay provided a means of identifying Ag-specific MBCs in human peripheral blood.
Using peripheral blood samples from vaccinated U.S. individuals we determined the efficiency of this assay by enumerating the actual number of tetanus toxoid (TT)-specific MBCs in a given sample using a sensitive and specific flow cytometry technique (Amanna I.J., 2006) and comparing this number to the number of MBCs which respond to the stimulation cocktail used by Crotty et al. We report that only ~10% of TT-specific MBCs enumerated by flow cytometry respond in a limiting dilution version of the in vitro assay described by Crotty et al. and that the efficiency of this assay could be significantly improved, to nearly 100%, by the addition of IL-10 to the five day culture. This increase in efficiency in a method that can be done with whole PBMCs and does not require isolation of B cells as do other highly efficient assays (Amanna I.J., 2006) makes this protocol valuable in field studies in malaria endemic areas, where isolation of B cells is impractical due to both sample number and sample size. We provide evidence that this modified assay can be successfully used to quantify Pf-specific MBCs in semi-immune adults in Kenya. The increased efficiency of this modified assay allows for the measurement of MBCs from smaller blood volumes than was possible with the original assay that should facilitate field studies, particularly those involving children.
2. Materials and methods
2.1 Samples and sample preparation
Peripheral blood was obtained from healthy, anonymous, adults at the National Institutes of Health blood bank and peripheral blood mononuclear cells (PBMCs) were isolated from whole blood or elutriated mononuclear cell buffy coats obtained by lymphapheresis within six hours of blood collection, usingby density gradient centrifugation. Samples were diluted in an equal volume of PBS, layered over a 20% volume of Ficoll-Hypaque (Amersham Biosciences) and centrifuged for 20 min at room temperature at 1800 × g with the brake off. This study was approved by the Ethics Committee of the Faculty of Medicine, Pharmacy, and Odonto-Stomatology, and the IRB at the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) protocol # 06-I-N147. Written, informed consent was obtained from all study participants. Samples from malaria-exposed individuals were collected at Kilifi District Hospital situated 60 km north of Mombasa on the Kenyan coast. This study was approved by the Kenyan Medical Research Institute, Ethical Review Committee under the Scientific Steering Committee protocol number 1131. Written, informed consent was obtained from all study participants. PBMCs were washed twice with sterile phosphate buffered saline (PBS) (KD Medical), and were either used fresh or cryopreserved in fetal bovine serum (FBS) (Gibco) containing 7.5% dimethyl sulfoxide (DMSO) (Sigma-Aldrich), kept at −80°C for 24 h, and then stored at −196°C in liquid nitrogen. Frozen PBMCs were rapidly thawed in a 37°C water bath and then added to warm complete media; RPMI 1640 plus L-glutamine supplemented with 10% heat-inactivated FBS, 10,000 IU/ml each of penicillin and streptomycin, and 50 µM β-Mercaptoethanol (all media reagents from Invitrogen). Cells were washed, resuspended in complete media and counted using trypan blue (BioWhittaker) dye exclusion to detect viable cells.
2.2 Detection of TT-specific memory B cells by flow cytometry
Tetanus toxoid (TT) –specific cells were detected by flow cytometry according to Amanna and Slifka 2006 (Amanna I.J., 2006). In this technique Ag-specific B cells are labeled using Ag conjugated to two different flourochromes, then Ag-specific B cells which stain positive for both flourochromes are counted, yielding much greater specificity and lower background than is achieved using either flourochrome alone. TT (Biologic Laboratories) and human serum albumin (HSA) were biotinylated using the EZ-Link™Sulfo-NHS-LCBiotin kit (Pierce), according to the manufacturer’s protocol. In brief, 450 µg of TT protein or 300 µg of HSA protein were incubated with biotin at a molar ratio of 1 mole of protein per 10 moles of biotin and dialyzed against PBS/0.01% NaN3 to remove excess biotin. TT was conjugated to Alexa Fluor® 488 (Molecular Probes) according to the manufacturer’s direction. 10 × 106 PBMCs were incubated in PBS with 3% FBS and 0.03% Sodium Azide in 50 µl volumes 1 h at 4°C with 0.1ug of either TT-biotin or HSA-biotin and 0.3ug of TT- Alexa Fluor® 488 in addition to B cell markers CD20-PECy5.5 (clone HI47) (Invitrogen) and IgD-PE (clone IA6-2) (BD Pharmingen). Biotinylated samples were subsequently incubated with streptavidin-conjugated APC (Molecular Probes) diluted 1:500 for 30 min at 4°C. Labeled cells were analyzed on a FACSCalibur flow cytometer (BD Biosciences) and data analyzed using FlowJo software (Tree Star).
2.3 Phenotypic analysis by flow cytometry and cell sorting
All phenotypic analyses were performed using mouse mAbs specific for human B cell markers conjugated to fluorophores as follows: CD19-PE-Cy5.5 (clone SJ25C1), CD27-PE (clone CLB-27/1), CD20-APC-Alexa Fluor 750 (clone HI47) (Invitrogen), CD38-APC (clone HIT2), and IgD-FITC (clone IA6-2) (BD Pharmingen). For phenotypic analysis 1×106 PBMCs were incubated with fluorophore-conjugated antibodies in PBS with 3% FBS and 0.03% Sodium Azide for 20 min at 4°C. Labeling strategies were as follows: 1) CD19-PE-Cy5.5, CD27-PE, CD38-APC; 2) CD19-PE-Cy5.5, CD27-PE, CD20-APC-Alexa Fluor® 750, IgD-FITC. All antibodies were titrated per lot of antibody used. No significant differences were observed in the proportions of B-cell populations when defined by these two labeling methods. Labeled cells were fixed with 2% paraformaldehyde at room temperature for 10 min (paraformaldehyde was not washed off), analyzed on a FACSCalibur flow cytometer (BD Biosciences) and data analyzed using FlowJo software (Tree Star). For sorting, PBMC were washed in PBS and platelets were removed by low-speed centrifugation through FBS (Gibco, Grand Island, NY). Cells were labeled with strategy 1) described above, and sorted on a FACSAria cell-sorting system (BD Biosciences) as follows; non B cells (CD19 ), naïve B cells (CD19+ CD27 ), MBCs (CD19+CD27+) and PCs (CD19+CD27++ CD38+++).
2.4 Memory B cell ELISPOT assay
Whole PBMCs or sorted populations of MBCs, naïve B cells or PCs were cultured at 1x106cells/ml in complete media in 24 well plates alone or with stimulants optimized by titrations for optimal MBC activation, specifically 2.5 µg/ml CpG oligodeoxynucleotide ODN-2006 (Operon Technologies); 1/10,000 dilution Protein A from Staphylococcus aureus Cowan (SAC), 1/100,000 dilution pokeweed mitogen (Sigma-Aldrich). CpG ODN-2006 is classified as a CpG-B and is known to activate B cells. When indicated the wells of 24 well culture plates were incubated overnight at 4°C with 0.5 µg/ml CD40-specific Ab and washed. In addition, where indicated, 10 ng/ml IL-2, 15 ng/ml IL-4, 150 ng/ml IL-5, 150 ng/ml IL-6, 10-150 ng/ml IL-10, or 100 ng/ml IL-21 (Cell Sciences) or 50 ng/ml BAFF (R&D Systems) were added to cultures. Cells were incubated for 1-10 days (5 or 6 days unless otherwise specified), washed in complete media warmed to 37°C, counted and plated on prepared ELISPOT plates. Filterbottom 96-well ELISPOT plates (Millipore Multiscreen MSIP HTS IP Sterile plate 0.45um, hydrophobic, high-protein binding) were prepared by pre-treating with ethanol (ethanol exposure on plate filters should not exceed 1 minute) washing 3 times with PBS and incubating plates overnight at 4°C with either: 10 µg/ml polyclonal goat antibodies specific for human IgG (Caltag) to detect all IgG-secreting cells; 1% bovine serum albumin (BSA) as a non-specific protein control or 5 µg/ml of either TT, Pf-apical membrane antigen 1 (AMA1) or Pf-merozoite surface protein 1 (MSP1) in PBS. Plates were blocked by incubation with a solution of 1% BSA in RPMI for 2 h at 37°C. Serial dilution of cells from 5 to 6 day cultures were plated in ELISPOT plates in the culture media described above in duplicate or triplicate at concentrations of 4×104–3×102 PBMC/well to detect total IgG+ ASCs and 5×105–4×103 PBMC/well to detect Ag-specific ASCs, incubated at 37°C in a 5% CO2 incubator for 5 h, then washed 4 times each with PBS and PBS-0.05% Tween 20. Goat Abs specific for human IgG Fc conjugated to alkaline phosphatase (Jackson ImmunoResearch Laboratories) diluted 1:1000 in PBS-0.05% Tween 20 with 1% FBS were added to wells and incubated overnight at 4°C. Plates were washed four times each in PBST, PBS, and ddH2O, developed using 100 µl/well BCIP/NBT (Calbiochem) for 10 min, washed thoroughly with ddH2O and dried in the dark. ELISPOTS were quantified using Cellular Technologies LTD plate-reader and results analyzed using Cellspot software. For paired comparisons of fresh vs frozen samples, the Wilcoxon signed rank test was used. GraphPad Prism was used to perform statistical analyses.
2.5 Measurement of memory B-cell frequencies by limiting dilution analysis
PBMCs were cultured at 37 °C in 200 µl volumes in 96 well plates at concentrations of 5×103–5×102 PBMC/well to detect total IgG+ MBCs, 2×105–1×104 PBMC/well to detect TT-specific MBCs, and 1×105–1×104 PBMC/well to detect AMA1- and MSP1-specific MBCs. For IgG and TT a minimum of 48 wells per dilution per condition were plated. For AMA1 and MSP1 a minimum of 18 wells per dilution per condition were plated. By definition, only dilutions where ≤33.3% of wells were positive for ASCs, and ≥ 66.6% of wells were clear negatives were used in analysis, with each positive well taken as a surrogate for a single responding MBC in order to determine frequency of responding MBCs in total PBMCs. When MBCs were detected by ELISPOT, PBMCs were incubated for 5-6 days, then transferred from 96 well culture plates to prepared 96 well ELISPOT plates. In ELISPOT analysis wells were considered positive if there were >3 ASCs per well. When MBCs were detected by ELISA, PBMCs were incubated for 10 days, then supernatants were collected and frozen. For ELISA analysis wells were considered positive if the optical density (OD) was ≥ the average OD + 3SD of the average OD of wells with supernatants from unstimulated cells. Wells cross-reactive with KLH were excluded from analysis.
2.6 ELISA
For Ag-specific ELISAs, 96-well flat-bottom ELISA plates were incubated overnight at 4°C with TT, AMA1, or MSP1 at 10 µg/ml in PBS, blocked 1 h at room temperature with 200 µl/well of PBS/10% FCS (Sigma-Aldrich), then incubated overnight at 4 °C with 30 µl of cell culture supernatant. Plates were then washed and incubated with 100 µl/well of alkaline phosphatase-conjugated goat anti-human IgG diluted 1/2000 in PBS with 1% FCS and 0.05% Tween (Sigma-Aldrich), followed by 100 µl/well p-nitrophenyl phosphate (pNPP) in alkaline phosphatase buffer (Sigma-Aldrich) for 30 min, and the reaction stopped with 50 µl/well 2 M NaOH. The optical density (OD) of each plate was read at 405 nm.
3. Results and Discussion
3.1 Quantifying antigen-specific MBCs by flow cytometry
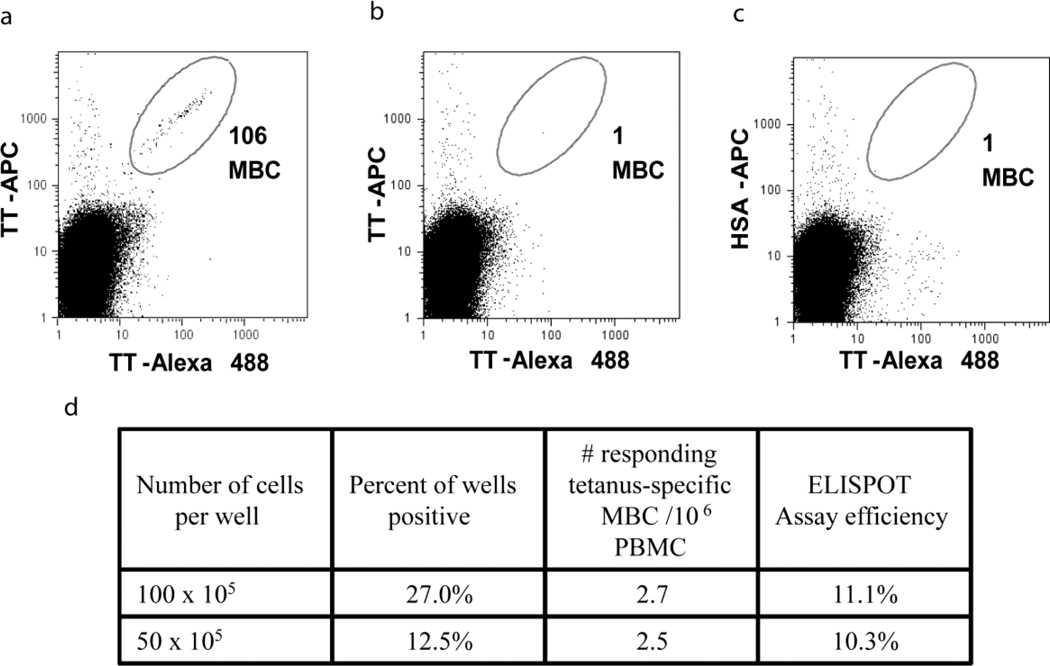
To quantify Ag-specific MBCs in vaccinated U.S. individuals we used a sensitive flow cytometry-based assay described for detecting TT-specific MBCs by Amanna and Slifka (Amanna I.J., 2006). We chose to measure TT for the assessment of the MBC ELISPOT assay because our attempts to detect Pf-specific MBCs using the Ags apical membrane antigen 1 (AMA1) and merozoite surface protein 1 (MSP1), were unsuccessful, because of the instability and non-specific binding of these proteins. PBMCs were incubated with biotinylated TT (TT-b) and TT conjugated to Alexa Fluor® 488 (TT-Alexa 488), followed by incubation with streptavidin-conjugated APC (SA-APC). As a control Ag, biotinylated human serum albumin (HSA-b) and SA-APC were used. Gating on CD20+, IgD− MBCs, we detected 106 TT-specific MBCs per 3.5×106 PBMCs or approximately 30 per 106 PBMCs that bound to both TT-b/SA-APC and to TT-Alexa 488 (Fig.1a). The specificity of the staining was confirmed using a cold competition control where PBMCs were incubated with unlabelled TT prior to incubation with TT-Alexa Fluor® 488 and TT-b/SA-APC (Fig. 1b). A mis-matched Ag control, was also used in which case PBMCs were stained with TT-Alexa Fluor® 488 and HSA-b/SA-APC (Fig.1c). Essentially no double labeled cells were detected in either case. The frequency of approximately 30 TT-specific MBCs per 106 PBMCs, detected in our PBMC samples is on the order of that reported by Amanna and Slifka (Amanna I.J., 2006) who observed ~19 TT-specific MBCs per 106 PBMCs, assuming that CD20+ B cells were 15%-20% of total PBMCs in their assay.
Figure 1.
The frequency of TT-binding MBCs in vaccinated U.S. individuals by FACS and ELISPOT using PSC stimulation. (a) TT-specific MBCs are identified by gating on MBCs that stain positive for TT-Alexa Fluor® 488 and TT-b/SA-APC. (b) Cold competition control, where cells are incubated with unlabeled TT before staining with TT-Alexa Fluor® 488 and TTb/ SA-APC. (c) Mis-matched Ag control, where cells are stained with TT-Alexa Fluor® 488 and b-HSA/SA-APC. (d) Tabular results of LD ELISPOTs of the same PBMC samples assayed by FACS at two dilutions using PSC as stimulation. Assay efficiency was determined by dividing the number of TT-specific MBCs which differentiated into ASCs (as determined by LD) by the total number of TT-specific MBCs present (as determined by FACS).
3.2 Quantifying TT-specific MBCs by limiting dilution ELISPOT
To determine the frequency of TT-specific MBCs in the in vitro culture assay described by Crotty et al. we modified the assay, culturing PBMC in complete media containing PWM, SAC and CpG (PSC) at limiting dilution (LD) in 96 well plates rather than in bulk culture 24 well plates. We determined that LD, when greater than 37% of the wells were negative for TT-specific ASCs, was reached when cells were cultured at 50,000 to 100,000 PBMC per well in 96 well plates for TT-specific MBCs. Wells were scored positive when there were ≥ 3 ASCs per well, and a minimum of 48 wells were used per dilution per condition. We detected on average 2.5-2.7 TT-specific MBCs per 106 PBMCs (Fig.1d). Thus, only ~1 in 10 TT-specific MBCs detected by flow cytometry responded by differentiating into ASCs in the LD assay in vitro.
3.3 Increasing the efficiency of the MBC ELISPOT assay
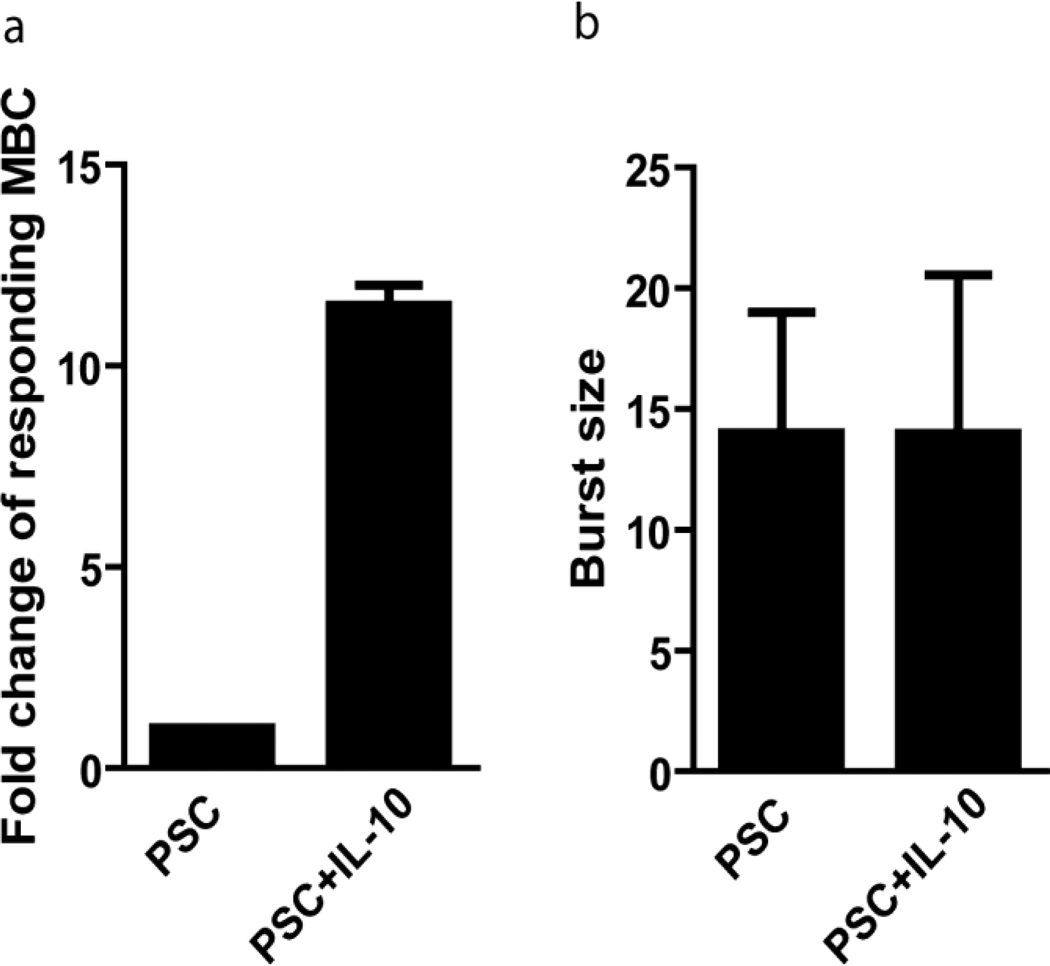
To optimize the efficiency of the ELISPOT assay, several cytokines and stimulatory factors known to have a role in the differentiation of MBCs and ASCs, including anti-CD40, IL-2, IL-21, IL-6, IL-4, IL-10 and BAFF, were added at physiologic concentrations in various combinations with PSC to cultures in 24 well plates. Combinations included: PSC and either IL-4, IL-5, IL-6, IL-10, BAFF or anti-CD40; PSC+IL-4+BAFF; PC+IL-10; PSC+IL-4+BAFF; IL-4+BAFF, anti-CD40+IL-4; anti-CD40+IL-10, and BAFF+IL-21. The total number of ASCs detected on anti-Ig coated ELISPOT plates from the 24 well plate cultures was only consistently significantly increased by a combination of PSC and IL-10. IL-10 is known to induce MBCs to differentiate into PCs (Arpin C,, 1995; Yoon SO, 2009) and the addition of IL-10 has been shown to result in a 17 fold increase in ASCs derived from MBCs as compared to cultures without IL-10 (Choe J, 1998). It has also been shown that IL-10 preferentially induces MBCs to differentiate into ASCs, while having little effect on the differentiation of naïve B-cells (Arpin C, 1997). LD analyses showed that as compared to PBMCs cultured in PSC alone, the addition of IL-10 to PSC increased the number of responding IgG+ MBCs by approximately 10 fold (p=0.002) (Fig. 2a). We also determined the burst size, which is the number of ASCs that result from a single MBC, by enumerating the IgG+ ASCs in each positive well at limiting dilution and found no difference in the average burst size in PSC- versus PSC+IL-10-containing cultures (Fig. 2b). This indicates that while PSC+IL-10 increases the number of MBCs responding to stimulation to differentiate into ASCs, PSC+IL-10 does not increase the number of ASCs that result from a single MBC over that achieved by PSC alone.
Figure 2.
The addition of IL-10 to PSC increased the efficiency of MBC differentiation to ASCs by 10 fold. Comparison of two stimulation cocktails at LD; PSC, or PSC+IL-10. (a) Average fold change in the number of IgG+ MBCs differentiating into ASCs in PSC or PSC+ IL-10 p=0.002 (b) Average number of daughter ASCs arising from each responding IgG+ MBC in PSC or PSC+IL-10.
3.4 Establishing the selectivity of the modified assay for MBCs
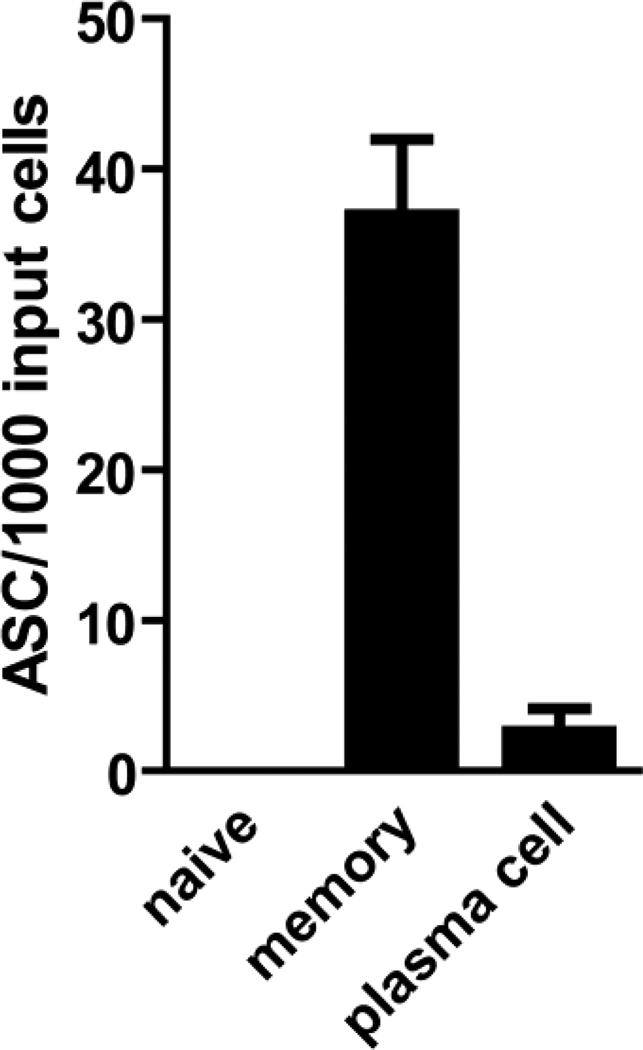
In order to confirm that only MBCs were responsive to stimulation with PSC+IL-10, platelet-depleted PBMCs were sorted into non B cells (CD19−), MBCs (CD19+, CD27+ gating out CD27++, CD38+++ PCs), naïve B cells (CD19+, CD27−) and PCs (CD19+, CD27++, CD38+++) as discussed in the methods portion. These populations were >98% pure as estimated by flow cytometry. For these experiments non-B-cells (CD19−) were added back to sorted populations of B cells to simulate the environment present in whole PBMC cultures and cultured in bulk-culture format. The combination of PSC+IL-10 stimulated MBCs to differentiate into ASCs and had no measurable effect on the differentiation of naïve B cells (Fig. 3). Approximately four ASCs were detected per 1000 input cells in the sorted PC population that could be due to a low frequency of PCs surviving in the five-day cultures. If so, in fresh whole PBMCs in which PCs represent approximately 0.5-2.0% of B cells these PCs would be estimated to compose 0.012% of the IgG producing ASCs which should be inconsequential to the assay. In addition, no PCs were detected by ELISPOT within 24 h of thawing PBMCs, suggesting that PCs either do not survive freezing or are unable to produce Ab after freezing and thawing and thus do not raise the background in assays using frozen cells.
Figure 3.
Determination of the selectivity of the PSC+IL-10 stimulation cocktail for MBCs. To determine the specificity of the PSC+IL-10 stimulation cocktail, naïve B cells (CD19+, CD27−), MBCs (CD19+, CD27+), and PCs (CD19+, CD27++,CD38+++) were sorted and cultured with CD19+ B cell-depleted PBMCs in PSC+IL-10. Shown are the number of IgG+ ASCs per 1000 sorted input B-cells.
3.5 Comparison of fresh versus frozen samples and determination of optimal assay culture time
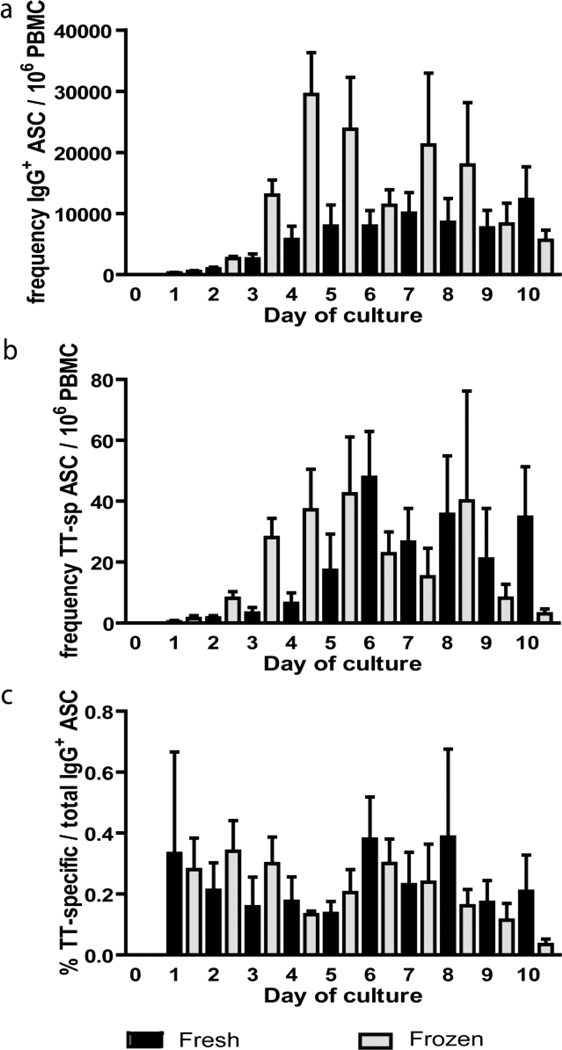
In longitudinal studies of the immune response to malaria in endemic areas it is not always possible to perform cellular assays on fresh PBMCs collected in field studies and consequently these samples are often frozen. In addition freezing samples allows PBMCs collected at different time points to be assayed simultaneously. To determine if ELISPOT results differed significantly between fresh and frozen samples and to determine the optimal culture time for detecting MBCs, we cultured PBMCs with PSC+IL-10 and performed ELISPOTs every 24 h for 10 days on fresh and frozen-thawed PBMCs from the same peripheral blood sample. Frozen samples were stored in liquid nitrogen for > 2 months. The frequency of total IgG+ and TT-specific ASCs increased for the first five to six days of culture for both fresh and frozen samples, and there were no significant increases in the frequencies of either total IgG+ (Fig. 4a) or TT-specific (Fig. 4b) ASCs from day five through ten of culture (p>0.05 for all comparisons). From day one of culture through day five of culture frozen samples had a greater frequency of total IgG+ ASCs per total PBMCs relative to the fresh samples in paired analysis (p ≤ 0.0313 for each comparison), possibly due to the reduced ability of some populations in PBMCs that limit the development of ASCs to survive the freeze-thaw. There were no significant differences between fresh and frozen samples in paired analysis from day six through day ten of culture. As expected, in comparing the percentage of IgG+ TT-specific ASCs per total IgG+ ASCs there were no significant differences between fresh and frozen samples at any time during culture (Fig. 4c). Based on this cumulative data, we would recommend that ELISPOTs be done on day five through eight of culture and if fresh and frozen samples are to be compared, that ELISPOTs be done on day six through eight of culture.
Figure 4.
ELISPOTS done in fresh and frozen aliquots of the same sample are comparable. Comparison of ELISPOTs using PSC+IL-10 stimulation done on fresh PBMCs (black) or the same samples frozen then thawed and assayed (gray). (a) The frequency of IgG+ ASC per 106 PBMCs as determined by ELISPOTs done every 24 hours for ten days. (b) The frequency of TT-specific ASC per 106 PBMCs as determined by ELISPOTs done every 24 hours for ten days. (c) The percentage of TT-specific ASCs per total IgG+ ASCs as determined by ELISPOTs is consistent throughout the ten day culture period.
3.6 Detecting Pf-specific MBCs in individuals living in malaria endemic areas
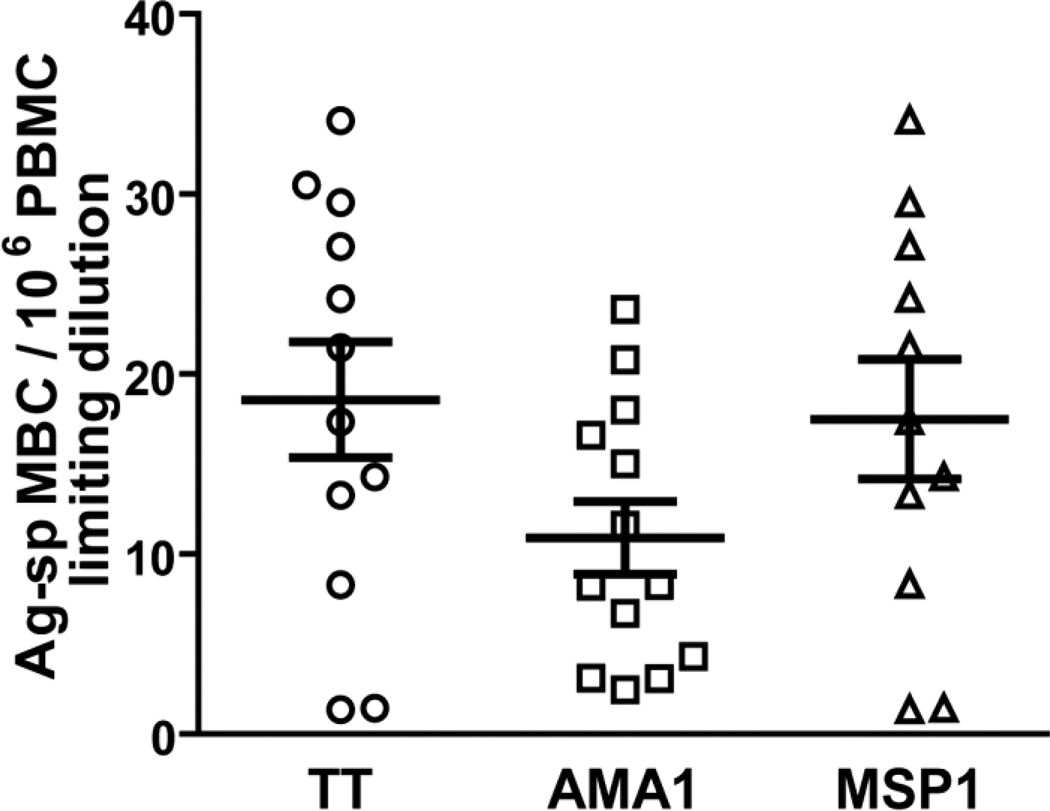
The frequency of MBCs specific for TT and for two blood stage Pf Ags, AMA1 and MSP1, were determined in the PBMCs of adults living in a malaria endemic area of Kenya. PBMCs were cultured at LD in PSC+IL-10 for ten days and the culture supernatants assayed by ELISA for TT-, AMA1-, and MSP1-specific Ab as a surrogate for ASCs. The results showed an average frequency of 19 TT-specific MBCs per 106 PBMC in 12 adults (Fig. 5). The average frequencies of MBCs specific for AMA1 and MSP1, were 11 and 18 per 106 PBMCs measured in 13 and 11 individuals respectively (Fig. 5). The frequencies we observed for Pf-specific MBCs were comparable to Ag-specific MBC frequencies reported in other studies (Dorfman J.R. et al., 2005; Amanna I.J., 2006; Amanna I.J., 2007; Harris AM, 2009; Weiss GE, 2010).
Figure 5.
The frequency of Ag-specific MBCs by LD in PBMCs of adults exposed to malaria. Total PBMC were cultured in PSC+IL-10 for 10 days. The 10-day culture supernatants were analyzed by ELISA for IgG antibodies that bound to TT, AMA1 and MSP1 or, as a negative control antigen, KLH. Cultures showing binding to KLH were excluded from the analysis because of potential cross-reactivity or non-specific binding. Frequencies were calculated from standard single hit kinetic curves. Shown are the mean frequencies of Ag-specific MBCs per 106 PBMCs. Error bars indicate SEM for n = 12, 13 and 11 individuals for TT, AMA1 and MSP1, respectively.
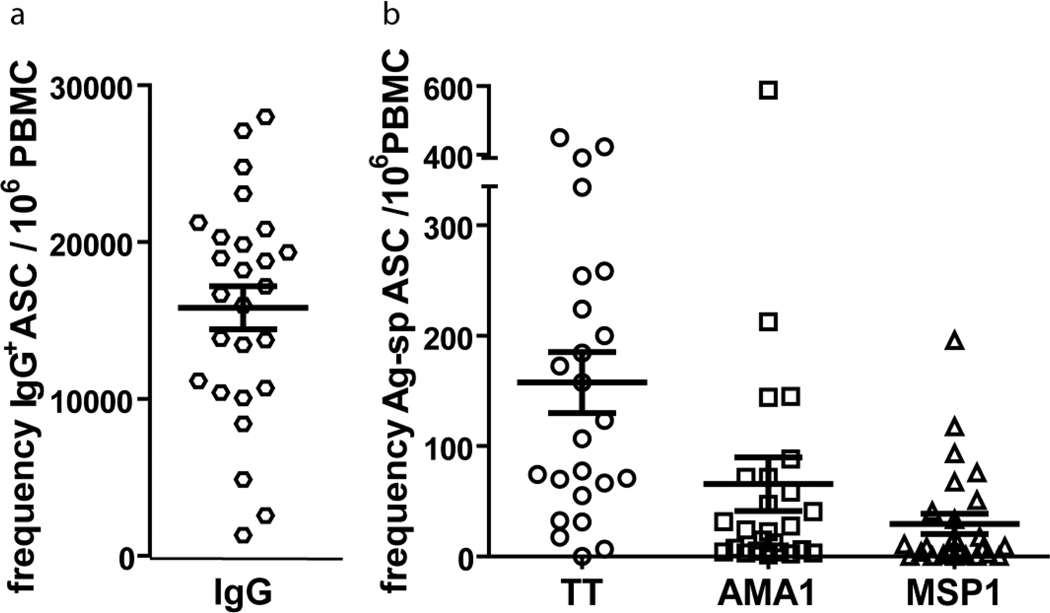
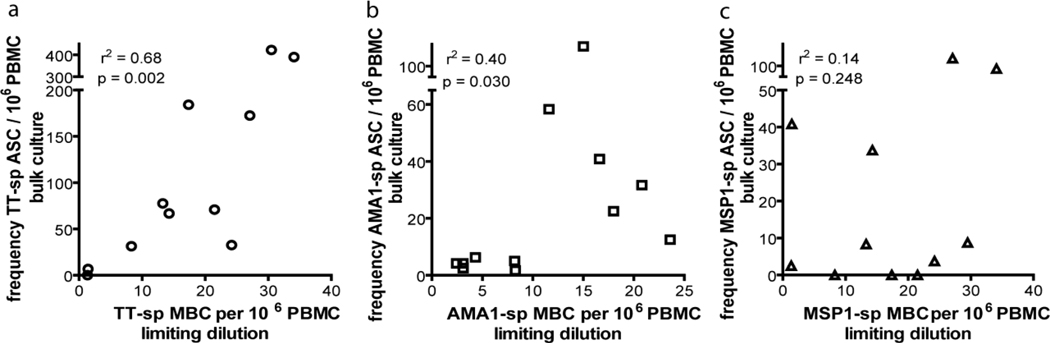
LD analyses are more expensive and time consuming than the bulk cultures for reading out ASCs. We therefore determined if there is a correlation between the frequency of MBCs determined in LD by ELISA and the number of ASCs differentiating from MBCs in bulk cultures (Fig. 6). Shown is the frequency of IgG+-ASCs (Fig. 6a), and TT-, MSP1- and AMA1-specific ASCs per 106 PBMCs (Fig. 6b). We observed a correlation between the number of MBCs detected in LD and the number of ASCs in five day bulk culture for TT, AMA1, and MSP1, although the correlation was stronger for TT than for either AMA1 or MSP1 (Fig. 7).
Figure 6.
Numbers of total IgG-secreting and Ag-specific ASCs in bulk assay cultures of PBMCs from malaria exposed adults. PBMCs were cultured for 5 days in PSC+IL-10. The total number of total IgG+, TT-, AMA1, and MSP1-specific ASCs were quantified in ELISPOT assays as described in the methods section. Shown are the mean numbers of total IgG+ (a) or Ag-specific (b) ASCs per 106 PBMC. Error bars indicate SEM for n = 26, 24, 25 and 26 for total IgG, TT, AMA1 and MSP1, respectively.
Figure 7.
Ag-specific MBC frequencies correlate with Ag-specific ASC numbers. The correlations between the frequencies of Ag-specific MBCs determined by LD and the numbers of Ag-specific ASCs by bulk assay cultures in PBMC of adults exposed to malaria for (a) TT, (b) AMA1 and (c) MSP1. Spearman rank correlations are shown.
Collectively, the results presented here demonstrate that the efficiency of the in vitro assay described by Crotty et al. to detect Ag-specific MBCs can be increased significantly by the addition of IL-10 to the cultures. The addition of IL-10 increased the frequency of the MBCs activated in culture but did not influence the ASC burst size nor did it alter the selectivity of the assay for MBCs. In addition the assay proved efficacious in detecting MBCs in field samples from adults living in a malaria endemic area of Africa. The increased efficiency of this modified assay allows for the measurement of MBC frequencies from smaller blood volumes than was possible with the original assay, which is important in field studies, particularly those involving children in which blood volumes are limiting. The further application of this assay to the study of the acquisition and maintenance of B cell memory through natural infection may ultimately aid in the design and development of a highly effective malaria vaccine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amanna IJ, Slifka MK. Quantitation of rare memory B cell populations by two independent and complementary approaches. J Immunol Methods. 2006;317:175–185. doi: 10.1016/j.jim.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- Arpin C, Banchereau J, Liu YJ. Memory B cells are biased towards terminal differentiation: a strategy that may prevent repertoire freezing. J Exp Med. 1997;186:931–940. doi: 10.1084/jem.186.6.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpin C, Dechanet J, van Kooten C, Merville P, Grouard G, Briere F, Banchereau J, Liu YJ. Generation of memory B cells and plasma cells in vitro. Science. 1995;268:720–722. doi: 10.1126/science.7537388. [DOI] [PubMed] [Google Scholar]
- Blink EJ, Light A, Kallies A, Nutt SL, Hodgkin PD, Tarlinton DM. Early appearance of germinal center-derived memory B cells and plasma cells in blood after primary immunization. J Exp Med. 2005:545–554. doi: 10.1084/jem.20042060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe J, Choi YS. IL-10 interrupts memory B cell expansion in the germinal center by inducing differentiation into plasma cells. Eur J Immunol. 1998;28:508–515. doi: 10.1002/(SICI)1521-4141(199802)28:02<508::AID-IMMU508>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Cohen S, McGregor IA, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- Crompton P, Kayala MA, Traore B, Kayentao K, Ongoiba A, Weiss GE, Molina DM, Burk CR, Waisberg M, Jasinskas A, Tan X, Doumbo S, Doumtabe D, Kone Y, Narum DL, Liang X, Doumbo OK, Miller LH, Doolan DL, Baldi P, Felgner PL, Pierce SK. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. PNAS. 2010;107:6958–6963. doi: 10.1073/pnas.1001323107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton PD, Mircetic M, Weiss G, Baughman A, Huang CY, Topham DJ, Treanor JJ, Sanz I, Lee FE, Durbin AP, Miura K, Narum DL, Ellis RD, Malkin E, Mullen GE, Miller LH, Martin LB, Pierce SK. The TLR9 ligand CpG promotes the acquisition of Plasmodium falciparum-specific memory B cells in malaria-naive individuals. J Immunol. 2009;182:3318–3326. doi: 10.4049/jimmunol.0803596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J. Immunol. Methods. 2004;286:111–122. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Dorfman JR, Bejon P, Ndungu FM, Langhorne J, Kortok MM, Lowe BS, Mwangi TW, Williams TN, K M. B cell memory to 3 Plasmodium falciparum blood-stage antigens in a malaria-endemic area. J Infect Dis. 2005;10:1623–1630. doi: 10.1086/429671. [DOI] [PubMed] [Google Scholar]
- Harris AM, Bhuiyan MS, Chowdhury F, Khan AI, Hossain A, Kendall EA, Rahman A, LaRocque RC, Wrammert J, Ryan ET, Qadri F, Calderwood SB, Harris JB. Antigen-specific memory B-cell responses to Vibrio cholerae O1 infection in Bangladesh. Infect Immun. 2009;77:3850–3856. doi: 10.1128/IAI.00369-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9:725–732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- Nduati EW, Ng DH, Ndungu FM, Gardner P, Urban BC, Langhorne J. Distinct kinetics of memory B-cell and plasma-cell responses in peripheral blood following a blood-stage Plasmodium chabaudi infection in mice. PLoS One. 2010;5:e15007. doi: 10.1371/journal.pone.0015007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dörner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G, Traore B, Kayentao K, Ongoiba A, Doumbo S, Doumtabe D, Kone Y, Dia S, Guindo A, Traore A, Huang CY, Miura K, Mircetic M, Li S, Baughman A, Narum DL, Miller LH, Doumbo OK, Pierce SK, Crompton PD. The Plasmodium falciparum-specific human memory B cell compartment expands gradually with repeated malaria infections. PLoS Path. 2010;6:e1000912. doi: 10.1371/journal.ppat.1000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss GE, Crompton PD, Li S, Walsh LA, Moir S, Traore B, Kayentao K, Ongoiba A, Doumbo OK, Pierce SK. Atypical Memory B Cells Are Greatly Expanded in Individuals Living in a Malaria-Endemic Area. J. Immunol. 2009;183:2176–2182. doi: 10.4049/jimmunol.0901297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrammert J, Miller J, Akondy R, Ahmed R. Human immune memory to yellow fever and smallpox vaccination. J Clin Immunol. 2009;29:151–157. doi: 10.1007/s10875-008-9267-3. [DOI] [PubMed] [Google Scholar]
- Yoon SO, Zhang X, Berner P, Choi YS. IL-21 and IL-10 have redundant roles but differential capacities at different stages of Plasma Cell generation from human Germinal Center B cells. J. Leukoc Biol. 2009;86:1311–1318. doi: 10.1189/jlb.0409268. [DOI] [PubMed] [Google Scholar]