Abstract
Since a combination of flt3 ligand plasmid (pFL) and CpG-oligodeoxynucleotides (ODN)3 as a dendritic cell (DC)-targeting double mucosal adjuvant elicited ovalbumin-specific secretory IgA (S-IgA) antibody (Ab) responses, we examined whether this double adjuvant could induce influenza-specific protective immunity in aged mice. A double adjuvant plus A/Puerto Rico/8/34 (PR8)-hemagglutinin (HA) induced increased numbers of CD11b+ CD11c+DCs and both CD4+ Th1- and Th2-type responses in the nasopharyngeal-associated lymphoreticular tissue, nasal passages and cervical lymph nodes. Further, increased levels of PR8-HA-specific S-IgA Ab responses were detected in the upper respiratory tact (URT) of aged and young adult mice given nasal PR8-HA with this double adjuvant. Thus, when mice were challenged with PR8 virus via the nasal route, both aged and young adult mice given nasal vaccine exhibited complete protection. Further, IgA-deficient mice nasally immunized with a double adjuvant influenza vaccine failed to provide protection against PR8 challenge. These results indicate that a nasal double adjuvant successfully induces PR8-HA-specific IgA Ab responses in both young adult and aged mice, which are essential for the prevention of influenza infection in the murine URT.
Keywords: Influenza, Mucosal vaccine, DC, Aged mice
Introduction
Influenza virus infection remains a serious respiratory disease since the virus often escapes from pre-existing host immunity by altering the antigenic properties of its surface hemaggultinin (HA). In addition, new types of viruses including highly pathogenic avian influenza (HPAI; H5N1) as well as swine influenza viruses (H1N1) cause disease in humans [1-3]. In this regard, effective vaccine development is essential for the prevention of seasonal and pandemic influenza. However, it is difficult to achieve effective protection by using currently available influenza vaccines in all age groups. In fact, the severity and mortality caused by the infectious pathogens invading mucosal surfaces such as the influenza virus and the bacterial pathogen Streptococcus pneumoniae (the pneumococcus) are sharply increased in the elderly [4-6]. Influenza virus infection caused an annual mean of 36,000 deaths during 1990-1999 and annual average of 226,000 hospitalizations during 1979-2001 in the United States [7, 8]. Over 90 % of these deaths and over 50 % of hospitalizations occur among persons older than 65 years of age. Thus, persons over 85 years of age showed substantially higher rates of death than any other age group [8-10]. In addition, an impaired response to influenza infection and vaccination in the elderly may be the clinically most relevant fact associated with infectious diseases in aging [11-13]. Even when the antigenic match between influenza vaccine and circulating virus is close, vaccination provides only 30–40 % protection in subjects aged > 65 years, whereas 70–90 % protection was seen in those < 65 years of age [14]. The currently available trivalent inactivated influenza vaccines are particularly ineffective in preventing deaths among the elderly with associated chronic conditions [9, 14-16]. In this regard, a molecular and cellular understanding of the impaired immune response to pathogens and the development of novel vaccines for the elderly would be clinically relevant and important.
Since the toll-like receptor (TLR)9 ligand CpG-oligodeoxynucleotide (ODN) induced protection when used as a mucosal adjuvant [17], nasal delivery of CpG-ODN plus formalin-inactivated influenza virus or hepatitis B virus surface Ag successfully induced Ag-specific Ab responses in both external secretions and plasma of mice [18, 19]. Additional work showed that mice given nasal recombinant protective antigen (PA) of anthrax toxin plus CpG-ODN exhibited high levels of PA-specific neutralizing IgG2a and S-IgA Ab responses [20]. These results suggest that although CpG-ODN has been shown to specifically stimulate plasmacytoid DCs (pDCs) in systemic lymphoid tissues [21], mucosal administration of CpG-ODN targets mucosal pDCs to enhance both innate and acquired immune responses.
Flt3 ligand (FL) has also been used as a DC-targeting mucosal adjuvant. Thus, mice which received nasal ovalbumin (OVA) plus a naked cDNA plasmid expressing FL cDNA (pFL) or adenovirus expressing FL (Ad-FL) elicited DC-mediated Ag-specific S-IgA and IgG Ab responses in both mucosal and systemic lymphoid tissues [22, 23]. Importantly, when both CpG-ODN and pFL were employed as a combined nasal adjuvant, prolonged Ag-specific mucosal IgA Ab responses were induced along with a balanced Th1- and Th2-type cytokine response [24]. Further, this combined double adjuvant successfully induced Ag-specific S-IgA Ab responses in external secretions of aged mice [24].
Based upon these findings, this study was designed to determine whether a nasal influenza vaccine together with pFL and CpG-ODN would enhance influenza-specific immunity for the prevention of influenza infection in both young adult and aged mice.
Materials and Methods
Mice
Young adult (8- to 10-week-old) female BALB/c and C57BL/6 mice were purchased from the Frederick Cancer Research Facility (National Cancer Institute, Frederick, MD). Aged (18-20 month-old) BALB/c mice were obtained from the National Institute of Aging. IgA gene-deficient (IgA-/-) mice were kindly provided by the Baylor College of Medicine [25, 26]. Mice were housed in microisolators, maintained in horizontal laminar flow cabinets, and provided sterile food and water in a specific pathogen-free facility at the University of Alabama at Birmingham. All mice used in these experiments were free of bacterial and viral pathogens.
HA vaccine
Vaccine Ag (split product virus vaccine) was prepared from influenza virus A/Puerto Rico/8/34 (PR8, H1N1) by the methods of Davenport et al. [27]. The plasmid pORF9-mFLt3L (pFL), comprising the pORF9-mcs vector (pORF) and the full-length mouse FL cDNA gene (InvivoGen, San Diego, CA), was prepared as described previously [22]. Plasmid DNA (< 0.1 endotoxin) was purified using QIAGEN Plasmid Giga Kits (QIAGEN, Valencia, CA). A synthetic oligodeoxynucleotide (ODN) containing CpG motif 1826 (CpG-ODN) was also used as a nasal adjuvant [20].
Nasal immunization and challenge
Both young adult and aged BALB/c mice were anesthetized with 0.2 (young mice) or 0.3 (aged mice) ml of ketamine (10 mg/ml) and xylazine (1.0 mg/ml) diluted in 0.9 % NaCl2 (saline) and immunized by the addition dropwise of 4 μl of PBS containing the required dose of PR8-HA (0.2 or 2.0 μg) together with pFL (50 μg) plus CpG-ODN (10 μg) into each nostril. In some experiments, native cholera toxin (nCT, 1.0 μg) was employed as nasal adjuvant. Mice immunized nasally with PR8-HA alone or non-immunized mice were used as negative controls. Mice were nasally immunized with PR8-HA plus pFL and CpG-ODN or nCT three times at weekly intervals. This immunization procedure limits the spread of vaccine in the nasal cavity. One week after the last immunization, the mice were challenged nasally with a small (2 μl) or a large (20 μl) volume of 40 LD50 dose of A/PR/8/34 (PR8; H1N1) virus [28-30].
Sample collection
Nasal washes (NWs), lung washes (LWs) and plasma were collected one week after the last immunization as described previously [31, 32]. Both samples were centrifuged to remove cellular debris and subjected to a HA-specific ELISA [33]. In some experiments, NWs, LWs and plasma samples were collected three days after the challenge with PR8 virus, and the virus titers were determined using a plaque assay with Madin-Darby canine kidney cells, as described previously [8].
HA-specific ELISA
The isotype of PR8-HA-specific Abs in NWs, LWs and plasma were determined by ELISA as previously described [28, 29, 31]. Briefly, 96-well Falcon microtest assay plates (BD Biosciences, San Jose, CA) were coated with purified PR8-HA (1.0 μg/ml). After blocking (1 % BSA in PBS), 2-fold serial dilutions of samples were added and incubated overnight at 4° C. HRP-labeled goat anti-mouse μ, γ or α heavy chain-specific Abs (Southern Biotechnology Associates, Birmingham, AL) were added and the color reaction was developed for 15 min at room temperature with 100 μl of 1.1 mM 2, 2’-azino bis (3-ethylbenz-thiazoline-6-sulfonic acid) (EMD Biosciences, La Jolla, CA). Endpoint titers were expressed as the reciprocal log2 of the last dilution that gave an optical density at 415 nm of 0.1 greater than background.
HA-specific ELISPOT assay
Mononuclear cells from the posterior cervical lymph nodes (CLNs) were isolated aseptically by a mechanical dissociation method using gentle teasing through stainless steel screens as described previously [34, 35]. For isolation of mononuclear cells from nasal passages (NPs) and nasopharyngeal-associated lymphoreticular tissue (NALT), a modified dissociation method was used based upon a previously described protocol [36, 37]. Isolated cells were subjected to a PR8-HA-specific ELISPOT assay in order to enumerate influenza-virus-specific Ab-forming cells (AFCs), as described previously [32, 38]. Briefly, a 96-well plate with a nitrocellulose base (Millititer HA; Millipore, Billerica, MA) was coated with the PR8 HA (1.0 μg/ml), and the numbers of PR8-HA-specific AFCs were quantified using a CTL-ImmunoSpot® analyzer (Cellular Technology Limited, Shaker Heights, OH).
Flow cytometric analysis
Intracellular cytokine analysis was performed as described previously [39-41]. Mononuclear cells (2 × 106 /ml) isolated from NALT, CLNs and NPs were harvested one week after the last immunization and restimulated with PMA (50 ng/ml) and ionomycin (500 ng/ml) in the presence of GolgiStop™ (1 μM) for 3 h at 37° C. Cells were then incubated with allophycocyanin (APC)-conjugated anti-mouse CD4 (Becton Dickinson, San Diego, CA) and FITC-conjugated anti-mouse CD3ε (BD PharMingen) monoclonal Abs (mAbs) followed by intracellular staining with PE-labeled anti-IFN-γ or -IL-4 mAb. Aliquots of mononuclear cells isolated from various tissues were stained with FITC-conjugated anti-mouse CD11b, PE-labeled anti-mouse CD11c, APC-labeled anti-mouse B220 and biotinylated anti-mouse CD8α mAbs followed by streptavidin-PerCP-Cy5.5 (BD PharMingen). All samples were subjected to flow cytometric analysis by FACSCalibur® (Becton Dickinson, San Jose, CA).
Statistics
The data are expressed as the mean ± one standard error of mean (SEM). The mouse groups were compared with non-immunized mice using an unpaired Mann-Whitney U test with Statview software (Abacus Concepts, Cary, NC) designed for Macintosh computers. Values of p < 0.05 were considered significant.
Results
A combined nasal adjuvant enhances influenza-specific Ab responses
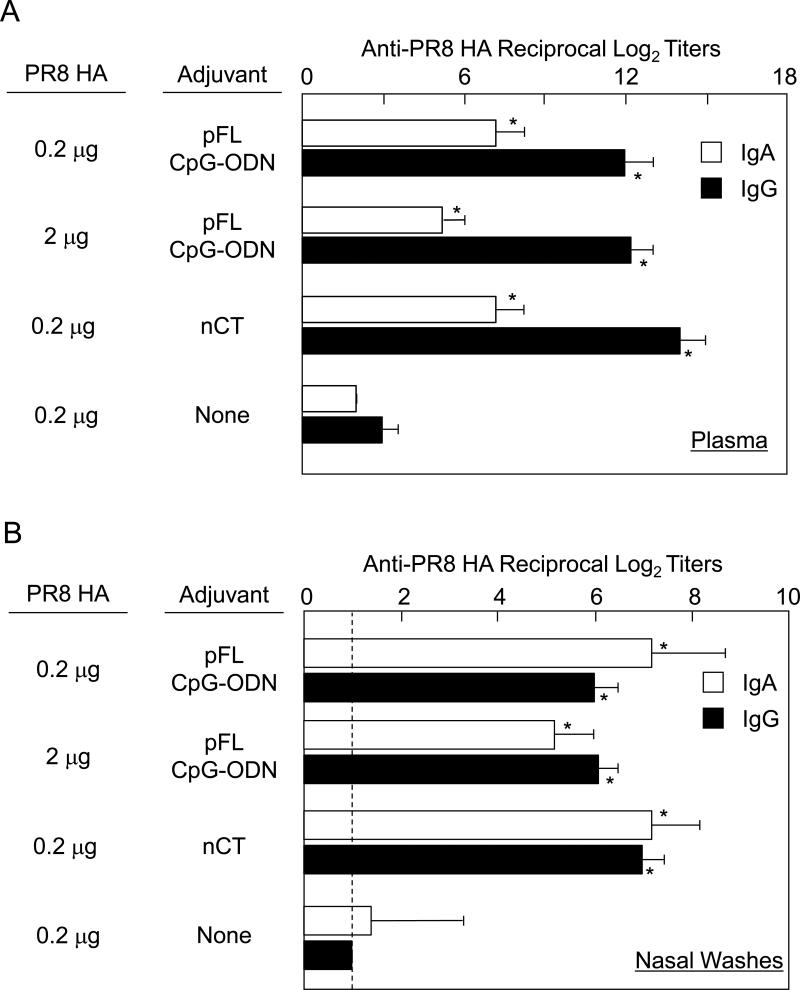
We initially examined whether nasal administration of pFL and CpG-ODN as adjuvant would enhance PR8-HA-specific immune responses. Mice were nasally immunized with a small dose (0.2 μg/mouse) or a larger dose (2.0 μg/mouse) of PR8-HA plus combined adjuvant or PR8-HA alone three times at weekly intervals. As positive controls, mice were given nasal PR8-HA (0.2 μg/mouse) plus 1 μg of nCT on the same schedule. One week after the last immunization, increased levels of IgA anti-PR8 Ab responses were seen in plasma of mice given nasal PR8-HA plus double adjuvant regardless of the dose of PR8-HA given (Figure 1A). Thus, nasal combined adjuvant induced significantly increased levels of PR8-HA-specific IgA Abs than those seen in mice nasally immunized with PR8-HA alone. Further, NWs of mice given the double adjuvant exhibited elevated levels of PR8-HA-specific secretory IgA (S-IgA) Abs (Figure 1B). PR8-HA-specific IgA Ab responses in NWs and plasma were comparable to those seen in mice given nCT as mucosal adjuvant. Increased levels of anti-PR8-HA IgG Abs were induced in plasma of mice nasally immunized with PR8-HA plus double adjuvant although the levels were lower than those in mice given nCT as mucosal adjuvant. Importantly, increased levels of PR8-HA-specific IgG Abs, which were comparable to those seen in mice given nasal PR8-HA plus nCT, were induced in NWs of mice given nasal PR8-HA with the combined adjuvant (Figure 1B). These results show that pFL and CpG-ODN markedly enhance PR8-HA-specific IgA and IgG Ab responses in NWs and plasma when employed as nasal adjuvant with influenza HA Ag.
Figure 1.
PR8 HA-specific Ab responses in nasal washes and plasma of young adult (8-10 weeks old) BALB/c mice. Mice were nasally immunized with PR8 HA (0.2 or 2.0 μg / dose) plus pFL (50 μg) and CpG-ODN (10 μg) or nCT (1.0 μg) three times at weekly intervals. Naïve mice and mice given nasal PR8 HA alone (0.2 μg / dose) were used as controls. One week after the last nasal immunization, plasma (A) and nasal washes (B) were collected and subjected to PR8 HA-specific ELISA. The values shown are the mean ± SEM of 10 mice in each experimental group. *p < 0.05 when compared with the control groups.
Balanced Th1- and Th2-type cytokine responses
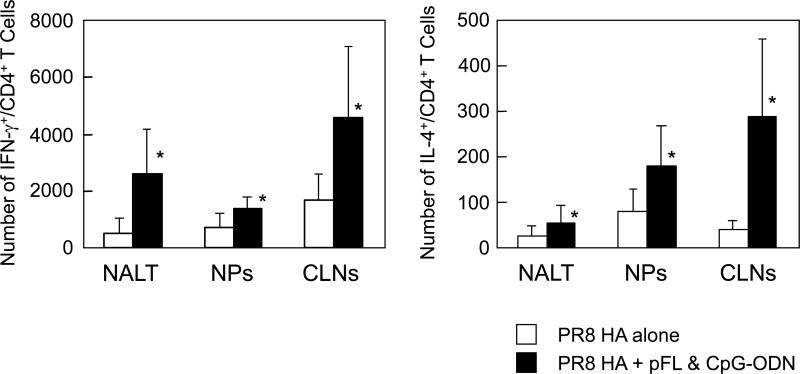
Since our previous study showed that pFL and CpG-ODN as nasal adjuvant induced a balanced Th1- and Th2-type cytokine response, we next examined intracellular IFN-γ and IL-4 production by CD4+ T cells of mice given nasal PR8-HA plus pFL and CpG-ODN (Figure 2). NALT of mice given nasal combined adjuvant contained approximately five fold higher numbers of IFN-γ+/CD4+ and two times higher numbers of IL-4+/CD4+ T cells than those seen in controls. Further, two to three-fold higher levels of IFN-γ+/CD4+ and seven-fold higher levels of IL-4+/CD4+ T cells were induced in CLNs. The numbers of IFN-γ+/CD4+ and IL-4+/CD4+ T cells in NPs were doubled when mice were nasally immunized with PR8-HA plus pFL and CpG-ODN. These results suggest that the combination of pFL and CpG-ODN as nasal adjuvant induce both Th1- and Th2-type cytokine responses in mucosal inductive and effector tissues in the URT.
Figure 2.
Numbers of IFN-γ and IL-4 producing CD4+ T cells in NALT, NPs and CLNs. Mononuclear cells were isolated from young adult mice given nasal PR8 HA plus pFL and CpG-ODN or PR8 HA alone one week after the last immunization. Cells were stained with FITC conjugated anti-CD4 mAb followed by additional intracellular staining with PE-tagged anti-IFN-γ or -IL-4 mAbs. Samples were subjected to flow cytometric analysis by FACSCalibur®. The numbers of IFN-γ and IL-4 producing CD4+ T cells were determined by subtracting those numbers present in naïve mice. The values shown are the mean ± SEM of 10 mice in each experimental group. *p < 0.05 when compared with the control groups.
Increased numbers of DCs co-expressing CD11b or B220
We next investigated the frequency and phenotypes of CD11c+ DC subsets in NALT, NPs and CLNs (Table 1). Our results showed that nasal vaccination with PR8-HA plus pFL and CpG-ODN increased the numbers of CD11c+ DC subsets which co-expressed CD11b+ or B220 molecules in the nasal cavity and draining lymph nodes. In addition, the CD11c+ CD11b- CD8α- B220- DC subset was also significantly elevated in NALT and NPs. Further, increased numbers of CD11c+ CD11b- CD8α+ B220+ DCs were noted in CLNs of mice given nasal double adjuvant than were seen in non-immunized mice. These results show that two major subsets of DCs co-expressing CD11b or B220 are induced by the combined adjuvant when given nasally with PR8-HA.
TABLE 1.
Actual numbers of DCs in nasal mucosa of mice given nasal PR8 plus pFL and CpG-ODN
| CD11c+ DCs (× 102 cells/mouse) |
||||||
|---|---|---|---|---|---|---|
| CD8+ | CD8- | CD8- | CD8+ | CD8- | ||
| CD11b- | CD11b+ | CD11b- | CD11b- | CD11b- | ||
| Tissue | Nasal Immunization | B220- | B220-- | B220+ | B220+ | B220- |
| NALT | None | 15 ± 2 | 36 ± 5 | 76 ± 9 | 11 ± 9 | 33 ± 3 |
| PR8 | 22 ± 4 | 41 ± 15 | 68 ± 6 | 17 ± 5 | 30 ± 5 | |
| PR8 + pFL/CpG-ODN | 25 ± 1 | 108 ± 1* | 167 ± 11* | 65 ± 18* | 63 ± 6* | |
| NPs | None | 16 ± 4 | 122 ± 38 | 18 ± 11 | < 10 | 35 ± 19 |
| PR8 | 24 ± 2 | 96 ± 11 | 10 ± 2 | < 10 | 16 ± 1 | |
| PR8 + pFL/CpG-ODN | 35 ± 1* | 456 ± 5* | 205 ± 31* | 44 ± 5* | 333 ± 1* | |
| CLNs | None | 59 ± 10 | 64 ± 15 | 113 ± 13 | 64 ± 15 | 48 ± 9 |
| PR8 | 66 ± 8 | 12 ± 1 | 129 ± 4 | 32 ± 3 | 75 ± 7 | |
| PR8 + pFL/CpG-ODN | 63 ± 12 | 163 ± 13* | 203 ± 20* | 204 ± 42* | 55 ± 14 | |
a) Young adult (8- to 10-weeks-old) BALB/c mice were nasally immunized with inactivated influenza PR8 Ag (0.2 μg) with or without adjuvant three times at weekly intervals.
b) Values represent means ± SEM of DCs induction of 5-10 mice in each group.
c) Asterisk (*) represent statistically differences in result between immunized and control groups.
Induction of PR8-HA-specific S-IgA Ab responses in aged mice
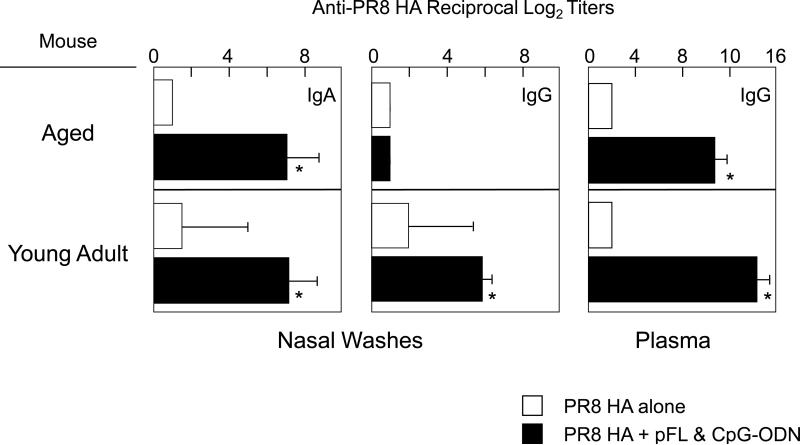
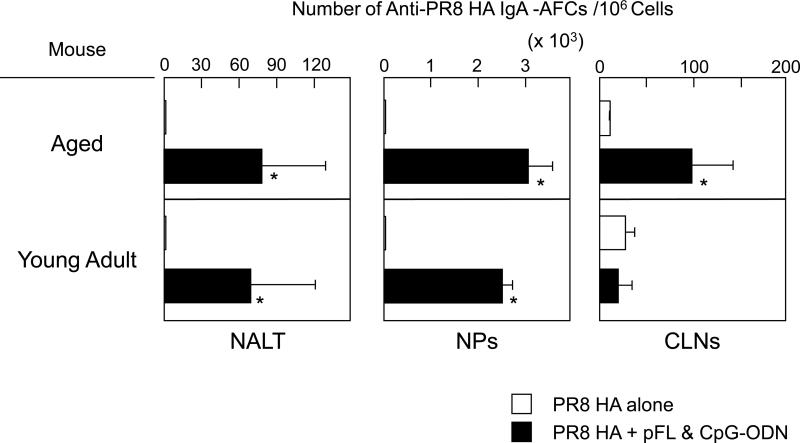
We next examined whether a combined nasal adjuvant could effectively induce PR8-HA-specific Ab responses in aged mice. When aged mice were nasally immunized with small doses of PR8-HA (0.2 μg/mouse) with the combined adjuvant, significantly increased levels of anti-PR8-HA IgA Abs were seen in NWs when compared with those detected in aged mice given nasal PR8-HA alone (Figure 3). Importantly, these PR8-HA-specific S-IgA Ab responses in NWs were comparable to those seen in young adult mice (Figure 3). In contrast, HA-specific IgG Ab responses in NWs of aged mice were significantly lower than those responses in young adult mice (Figure 3). The levels of PR8-HA-specific IgG Abs in plasma of aged mice given a combined adjuvant were higher than were seen in aged mice immunized with PR8-HA alone; however, these responses were lower than those seen in young adult mice (Figure 3). The numbers of IgA AFCs in NALT and NPs of aged mice given pFL plus CpG-ODN as a nasal adjuvant were significantly increased when compared with aged mice given nasal PR8-HA alone (Figure 4). These numbers were essentially the same as those of young adult mice nasally immunized with PR8-HA and combined adjuvant (Figure 4). Interestingly, CLNs of aged mice given nasal PR8-HA and combined adjuvant showed higher numbers of IgA AFCs when compared with young adult mice (Figure 4).
Figure 3.
PR8 HA-specific Ab responses in nasal washes and plasma of aged (18-20 month-old) BALB/c mice. Both aged and young adult mice were nasally immunized with PR8 HA (0.2 μg) plus pFL (50 μg) and CpG-ODN (10 μg) three times at weekly intervals. One week after the last nasal immunization, nasal washes and plasma were isolated and subjected to PR8 HA-specific ELISA. Mice nasally immunized with PR8 HA (0.2) without adjuvant were used as controls. The values shown are the mean ± SEM of 10 mice in each experimental group. *p < 0.05 when compared with the control group.
Figure 4.
Numbers of PR8 HA-specific IgA Ab forming cells (AFCs) in aged and young adult mice immunized nasally with PR8 HA (0.2 μg / dose) plus pFL and CpG-ODN or PR8 alone. One week after the last nasal immunization, mononuclear cells were obtained from NALT, NPs and CLNs and subjected to PR8 HA-specific ELISPOT assay. The values shown are the mean ± SEM of 10 mice in each experimental group. *p < 0.05 when compared with the control group.
A Role for HA-specific S-IgA Abs in protection from influenza infection
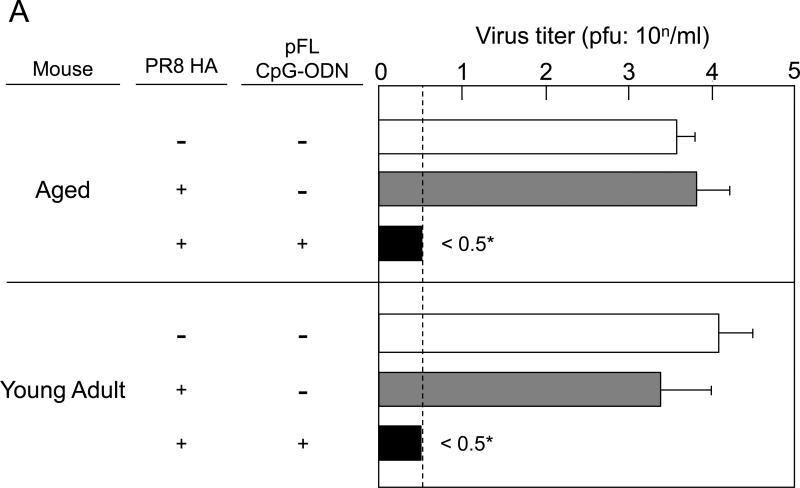
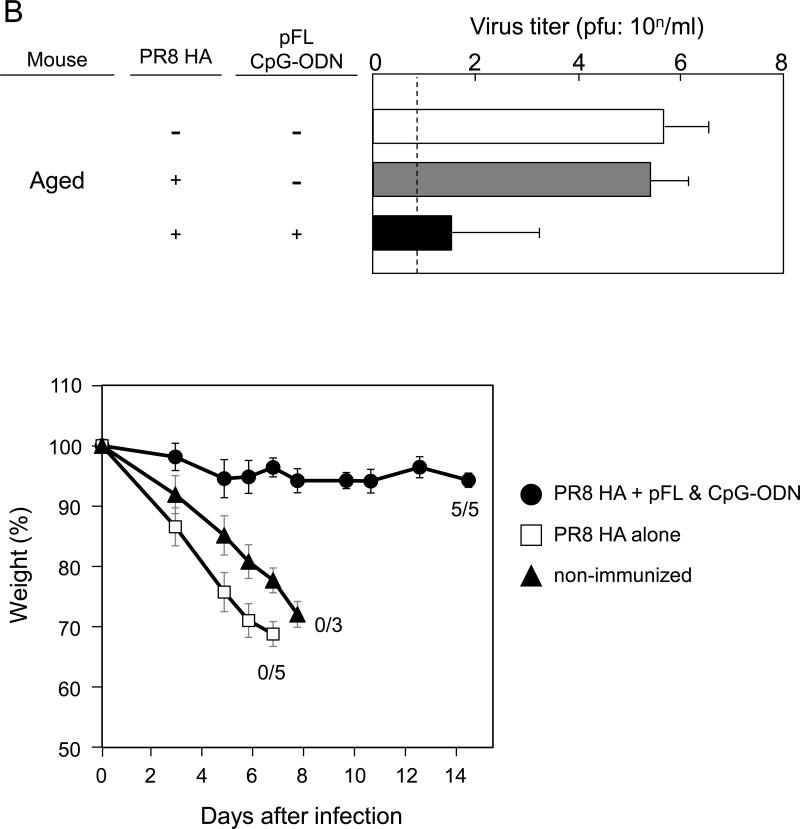
To determine functional properties of PR8-HA-specific S-IgA Abs, vaccinated aged mice were challenged with a 40 LD50 dose of live PR8 influenza virus one week after the last immunization (Figure 5A). This infectious procedure limits initial viral replication predominantly to the URT and mice recover from the influenza infection over the next 2 weeks (2, 28, 42). NWs and plasma from aged and young adult mice given pFL and CpG-ODN as a combined nasal adjuvant resulted in essentially no detectable virus (Figure 5A). On the other hand, both age groups given nasal PR8-HA alone showed high virus titers in both NWs and plasma (Figure 5A). These results suggest that PR8-HA-specific S-IgA Abs in the URT play a central role in the prevention of influenza virus infection. Thus, even aged mice that possess lower PR8-HA-specific IgG Abs in plasma were able to protect the upper respiratory tract from influenza infection. Of importance, aged mice given a combined adjuvant showed significantly lower virus titers and essentially no weight loss after challenged with a lethal dose (20 μl of 40 LD50) of PR8. On the other hand, non-immunized mice and mice given PR8 alone failed to provide immunity and died within 10 days after the challenge (Figure 5B). These findings showed that a combination of pFL and CpG ODN is an effective nasal adjuvant to provide protection in the upper and lower respiratory tracts of aged mice against influenza infection.
Figure 5.
Protection against PR8 influenza challenge in aged mice. Both aged and young adult mice were nasally immunized with PR8 HA (0.2 μg / dose) in the presence or absence of pFL and CpG-ODN three times at weekly intervals. (A) One week after the last nasal immunization, mice were nasally challenged with 2 μl of PR8 influenza virus (40 LD50). Nasal washes were collected and virus titers were determined three days after the challenge. (B) In some experiments, mice were challenged with 20 μl of PR8 influenza virus (40 LD50). The virus titers in nasal washes were determined three days after the challenge. The body weight of individual mice were determined daily for two weeks. The values shown are the mean ± SEM of 6 or 10 mice in each experimental group. *p < 0.05 when compared with the control group.
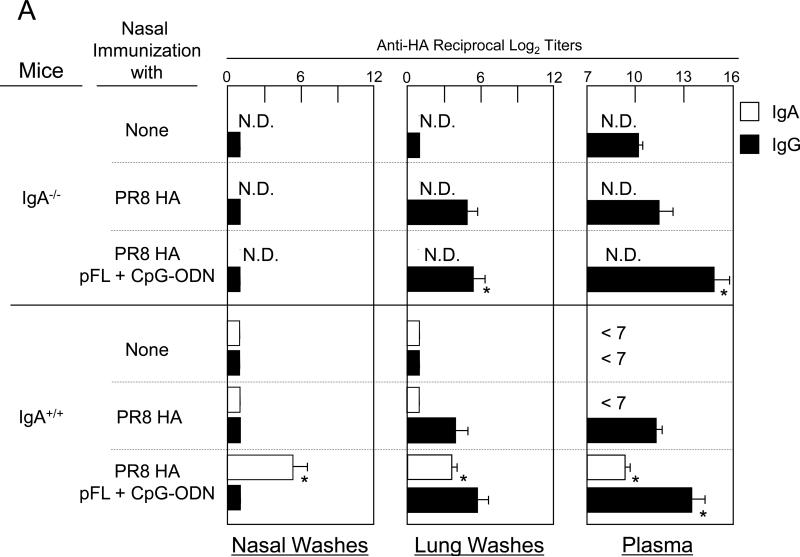
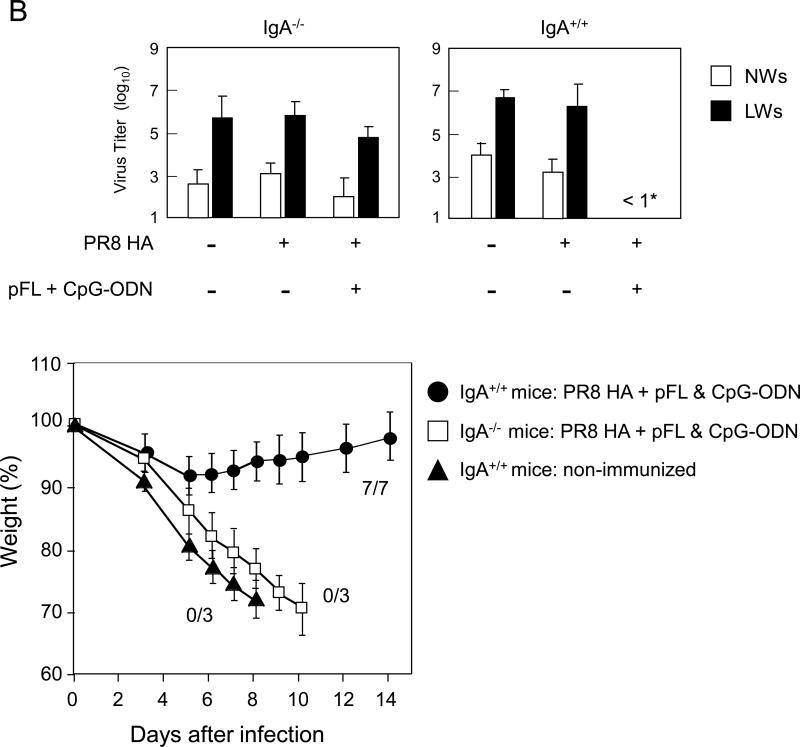
In order to determine possible roles for influenza-specific S-IgA Ab responses in the upper respiratory tract, young adult IgA deficient (IgA-/-) and their genetic background control mice were nasally immunized with PR8-HA plus pFL and CpG-ODN, and were compared with mice given nasal PR8-HA alone (Figure 6A). Significantly increased levels of PR8-HA-specific IgG Ab responses were induced in LWs and plasma of young adult IgA-/- mice given nasal combined adjuvant. In contrast, C57BL/6 wild-type mice nasally immunized with PR8-HA plus pFL and CpG-ODN showed increased levels of mucosal IgA and plasma IgG Abs in NWs, LWs and plasma than those in wild-type mice given nasal PR8-HA alone. Thus, when all groups of mice were nasally challenged with live PR8 influenza virus, only the wild-type mouse group that received the combined adjuvant influenza vaccine showed significantly lower virus titers in NWs, LWs and plasma. On the other hand, young adult IgA-/- mice given nasal PR8-HA plus combined adjuvant failed to prevent virus infection in the URT and plasma. Furthermore, when mice were challenged with a larger volume of PR8 virus (lung challenge), IgA-/- mice mice nasally immunized with PR8 plus pFL and CpG ODN showed high virus titers in both NWs and LWs. Thus these mice resulted in significant weight loss and subsequent died within 10 days after the challenge (Figure 6). These results clearly show that influenza-specific S-IgA Ab responses are key players for prevention from influenza infection in the respiratory tract.
Figure 6.
A lack of PR8 HA-specific IgA Abs is associated with a lack of mucosal protection against PR8 HA influenza virus. Both IgA-/- mice (8- to 10-week-old) and genetic background normal C57BL/6 mice (8- to 10-week-old) were nasally immunized with PR8 HA (2.0 μg /dose) in the presence or absence of pFL and CpG-ODN three times at weekly intervals. One week after the last nasal immunization, mice nasally challenged with 2 μl of PR8 influenza virus (40 LD50). Nasal washes, lung washes and plasma were collected three days after the challenge and subjected to : (A) PR8 HA-specific ELISA and a plaque assay with Madin-Darby canine kidney (MDCK) cells in order to determine virus titers. (B) In some experiments, both IgA-/- and C57BL/6 mice given nasal vaccine were challenged with 20 μl of PR8 influenza virus (40 LD50). The virus titers in nasal washes and lung washes were determined three days after the challenge. The weight loss was determined daily and the survival rates were observed for 2 weeks after this challenge. The values shown are the mean ± SEM of 5-6 mice in each experimental group. *p < 0.05 when compared with the control group.
Discussion
The present study examined whether a combined nasal adjuvant, pFL and CpG-ODN induces influenza virus-specific functional S-IgA Abs in URT mucosa of aged mice. Our results are the first to show that a combined double adjuvant successfully induced influenza-specific S-IgA Ab responses in aged mice. Further, S-IgA Abs effectively prevented influenza infection in the URT. It is well accepted that pathogen-specific S-IgA Abs play a key role as the first defense line against infectious diseases [43]. Although current influenza vaccines which are licensed for parenteral administration in humans induce neutralizing Abs against one surface glycoprotein HA and are highly effective against homologous virus infection, these vaccines do not elicit S-IgA Abs and are less effective against heterologous drift viral infection [44, 45]. In contrast, influenza-specific IgA Abs can bind not only the vaccine strain, but also some drift influenza virus strains for cross-protection [43]. Further, other studies have shown that the effectiveness of the vaccine diminished in older persons when compared with younger healthy persons [46]. Thus, a more serious infection is seen in elderly persons than in young adults [45]. These reports clearly emphasize the importance of induction of influenza-specific S-IgA Ab responses in the elderly. Although it has been shown that mucosal adjuvants or delivery systems successfully induced Ag-specific mucosal S-IgA Abs [47], many attempts have failed to elicit protective mucosal immunity in the elderly. Indeed, the potent mucosal adjuvant nCT was unsuccessful in enhancing Ag-specific S-IgA Ab responses in aged mice [24, 48]. In this regard, our recent study showed that nasal immunization with OVA plus a combination of pFL and CpG-ODN elicited long-lasting Ag-specific mucosal immunity [24]. Of importance, enhanced OVA-specific mucosal S-IgA Ab responses were also achieved in aged mice [24]. Thus, our current findings further extend the concept of pFL and CpG-ODN as an effective combined nasal adjuvant for the induction of protective mucosal immunity against influenza virus infection.
It has been shown that specific Abs play a major role in the protection against influenza infection. Although it has been shown that effective protection can be provided by influenza-specific systemic IgG without mucosal IgA Ab responses [25], it is still possible that influenza-specific S-IgA Ab responses are a necessary component for providing effective immunity against these respiratory pathogens in the elderly. Indeed, our current study showed that influenza virus colonization was totally prevented in the presence of virus-specific S-IgA Ab responses in aged mice, despite the fact that reduced levels of PR8-HA specific IgG Ab responses were seen. Thus, IgA-/- mice given PR8-HA plus combined adjuvant showed high virus titers in the LWs and plasma despite increased titers of HA-specific IgG Abs in plasma. Furthermore, IgA-/- mice failed to provide protective immunity against a lethal dose of influenza virus. The different results obtained in the present study from the previous findings [49, 50] may be due to the functional properties of influenza-specific IgG Abs. Indeed, others reported that influenza-specific IgG1 but not IgG2a Ab responses were induced in IgA-/- mice [50]. Thus, significantly low levels of IFN-γ with high levels of IL-4 production were induced by influenza vaccination [50]. On the other hand, our present study showed a more balanced induction of IFN-γ and IL-4. Thus, both PR8 HA-specific IgG1 and IgG2a Ab responses were induced in IgA-/- mice given nasal PR8 HA plus pFL and CpG ODN (data not shown). Thus, our current results clearly show that influenza-specific S-IgA Abs at the surface of the nasal mucosa block invasion of and/or reduce growth of influenza viruses in the URT. To support this view, it was reported that influenza HA-specific S-IgA Ab responses play a key role in protection against influenza in the URT and provide cross-protection against infection with a variant virus within the same subtype [51]. Further, passive transfer of influenza-specific purified IgA Abs from NWs of immunized mice provided protection against lethal infection in naïve mice [52]. Thus, induction of influenza-specific S-IgA Abs would result in the inhibition of disease development, less severe clinical involvement and early recovery even when the pathogens invade the host.
Although induction of pathogen-specific S-IgA Ab responses by mucosal vaccination is essential for protecting the host from infection, these responses often depend upon exaggerated Th1- or Th2-type responses, which elicit undesired inflammation or allergy depending upon the type of adjuvant used. In order to develop effective and safe vaccines, it is essential to use a mucosal adjuvant which induces a more balanced Th1- and Th2-type response for support of Ag-specific S-IgA Abs without inflammatory or allergic side effects. In this regard, our previous study showed that nasal pFL and CpG-ODN elicited balanced Th1- and Th2-type cytokine responses for support of Ag-specific S-IgA Abs when co-administered with OVA. Our present results further show that this nasal adjuvant elicits PR8-HA-specific S-IgA Ab responses with balanced Th1- and Th2-type cytokine responses when mice were nasally immunized with PR8 HA plus combined adjuvant (Figure 2). These findings show that a balanced Th1 and Th2 cytokine response is induced even when viral Ag is employed.
It has been shown that TLR9-expressing plasmacytoid DCs (pDCs) induce Ag-specific Th1 and CTL responses when stimulated with CpG-ODN [53-55]. Since our results showed that increased frequencies of pDCs in NALT, NPs and CLNs of mice given nasal PR8-HA plus double adjuvant (Table 1), it is most likely that nasal CpG-ODN targets pDCs in the nasal cavity for the induction of Th1-type cytokine responses. Previous studies showed that nasal pFL expanded CD8 α+ DCs for the induction of IL-4 producing CD4+ T cells [22]. Our present study also showed increased levels of IL-4 production by CD4+ T cells; however, the numbers of CD11b- but not CD8-expressing DCs were significantly elevated in NALT and CLNs. The expansion of CD11b-expressing DCs may be due to the nature of Ag in the vaccine preparation. Since the PR8-HA preparation contains not only HA glycoprotein but also viral-related cell membranes, these components could alter the targeting subset of DCs. To support this prediction, our previous study showed that adenovirus expressing FL cDNA as nasal adjuvant resulted in expansion of CD11b-expressing DCs for the induction of Th1- and Th2-type cytokine responses [23].
In summary, our present study clearly shows that a combination of pFL and CpG-ODN as nasal adjuvant targets mucosal DCs to mediate a more balanced Th1- and Th2-type of cytokine response for induction of PR8 HA-specific Ab responses in the nasal mucosa and systemic immune compartments. Importantly, this combined nasal adjuvant successfully induces PR8-HA-specific S-IgA Ab responses in aged mice for protection against influenza virus infection in the upper respiratory tract. This is an important finding which demonstrates the important functional properties of influenza-specific S-IgA Abs for protection in aged mice, which is achieved by nasal administration of pFL and CpG-ODN as nasal adjuvant. Therefore, this combined adjuvant regimen possesses the potential for use with mucosal vaccines in order to induce protective immunity from infectious diseases in the elderly.
Highlights.
Nasal pFL and CpG ODN induce influenza-specific mucosal immunity in aged mice. A combination of pFL and CpG ODN elicits a balanced Th1 and Th2 response.
Asanuma et.al.
Acknowledgements
We would like to thank Dr. Jerry R. McGhee for his discussion and editorial help and Ms. Sheila D. Turner for the final preparation of the manuscript. This work was supported by U.S. Public Health Service grants AG 025873 and DE 012242 from the National Institutes of Health as well as the Japan Society of the Promotion of Science (JSPS) program entitled “Young Researcher Overseas Visits Program for Vitalizing Brain Circulation”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beigel J, Bray M. Current and future antiviral therapy of severe seasonal and avian influenza. Antiviral Res. 2008;78:91–102. doi: 10.1016/j.antiviral.2008.01.003. [PubMed: 18328578] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dawood FS, Jain S, Finelli L, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15. doi: 10.1056/NEJMoa0903810. [PubMed: 19423869] [DOI] [PubMed] [Google Scholar]
- 3.Wright PF, Neumann G, Kawaoka Y. Orthomyxoviruses: Influenza in humans – past pandemic and the H5N1 epidemic. In: Knipe DM, Howley PM, Griffin DE, et al., editors. Fields Virology. 5th ed. Lippincott-Raven Publishers PA; 2007. pp. 1697–701. [PubMed: 6852914] [Google Scholar]
- 4.Bernstein JM. Treatment of community-acquired pneumonia--IDSA guidelines. Infectious Diseases Society of America. Chest. 1999;115:9S–13S. doi: 10.1378/chest.115.suppl_1.9s. [PubMed: 10084453] [DOI] [PubMed] [Google Scholar]
- 5.Mufson MA. Pneumococcal Pneumonia. Curr Infect Dis Rep. 1999;1:57–64. doi: 10.1007/s11908-999-0011-9. [PubMed: 11095768] [DOI] [PubMed] [Google Scholar]
- 6.Webster RG. Immunity to influenza in the elderly. Vaccine. 2000;18:1686–9. doi: 10.1016/s0264-410x(99)00507-1. [PubMed: 10689149] [DOI] [PubMed] [Google Scholar]
- 7.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [PubMed: 12517228] [DOI] [PubMed] [Google Scholar]
- 8.Tobita K, Sugiura A, Enomote C, Furuyama M. Plaque assay and primary isolation of influenza A viruses in an established line of canine kidney cells (MDCK) in the presence of trypsin. Med Microbiol Immunol. 1975;162:9–14. doi: 10.1007/BF02123572. [PubMed: 1214709] [DOI] [PubMed] [Google Scholar]
- 9.Pawelec G. Immunity and ageing in man. Exp Gerontol. 2006;41:1239–42. doi: 10.1016/j.exger.2006.09.005. [PubMed: 17118601] [DOI] [PubMed] [Google Scholar]
- 10.Pawelec G, Koch S, Franceschi C, Wikby A. Human immunosenescence: does it have an infectious component? Ann N Y Acad Sci. 2006;1067:56–65. doi: 10.1196/annals.1354.009. [PubMed: 16803971] [DOI] [PubMed] [Google Scholar]
- 11.Castle SC. Clinical relevance of age-related immune dysfunction. Clin Infect Dis. 2000;31:578–85. doi: 10.1086/313947. [PubMed: 10987724] [DOI] [PubMed] [Google Scholar]
- 12.Targonski PV, Jacobson RM, Poland GA. Immunosenescence: role and measurement in influenza vaccine response among the elderly. Vaccine. 2007;25:3066–9. doi: 10.1016/j.vaccine.2007.01.025. [PubMed: 17275144] [DOI] [PubMed] [Google Scholar]
- 13.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–40. doi: 10.1001/jama.292.11.1333. [PubMed: 15367555] [DOI] [PubMed] [Google Scholar]
- 14.Fukuda F, Bridges CB, Brammer TL. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1999;48:1–28. [PubMed: 10366138] [PubMed] [Google Scholar]
- 15.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [PubMed: 16213065] [DOI] [PubMed] [Google Scholar]
- 16.Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann Intern Med. 1995;123:518–27. doi: 10.7326/0003-4819-123-7-199510010-00008. [PubMed: 7661497] [DOI] [PubMed] [Google Scholar]
- 17.Krieg AM, Efler SM, Wittpoth M, Al Adhami MJ, Davis HL. Induction of systemic TH1-like innate immunity in normal volunteers following subcutaneous but not intravenous administration of CPG 7909, a synthetic B-class CpG oligodeoxynucleotide TLR9 agonist. J Immunother. 2004;27:460–71. doi: 10.1097/00002371-200411000-00006. [PubMed: 15534490] [DOI] [PubMed] [Google Scholar]
- 18.McCluskie MJ, Davis HL. CpG DNA is a potent enhancer of systemic and mucosal immune responses against hepatitis B surface antigen with intranasal administration to mice. J Immunol. 1998;161:4463–6. [PubMed: 9794366] [PubMed] [Google Scholar]
- 19.Moldoveanu Z, Love-Homan L, Huang WQ, Krieg AM. CpG DNA, a novel immune enhancer for systemic and mucosal immunization with influenza virus. Vaccine. 1998;16:1216–24. doi: 10.1016/s0264-410x(98)80122-9. [PubMed: 9682382] [DOI] [PubMed] [Google Scholar]
- 20.Boyaka PN, Tafaro A, Fischer R, Leppla SH, Fujihashi K, McGhee JR. Effective mucosal immunity to anthrax: neutralizing antibodies and Th cell responses following nasal immunization with protective antigen. J Immunol. 2003;170:5636–43. doi: 10.4049/jimmunol.170.11.5636. [PubMed: 12759444] [DOI] [PubMed] [Google Scholar]
- 21.Krug A, Rothenfusser S, Hornung V, et al. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol. 2001;31:2154–63. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [PubMed: 11449369] [DOI] [PubMed] [Google Scholar]
- 22.Kataoka K, McGhee JR, Kobayashi R, Fujihashi K, Shizukuishi S. Nasal Flt3 ligand cDNA elicits CD11c+CD8+ dendritic cells for enhanced mucosal immunity. J Immunol. 2004;172:3612–619. doi: 10.4049/jimmunol.172.6.3612. [PubMed: 15004163] [DOI] [PubMed] [Google Scholar]
- 23.Sekine S, Kataoka K, Fukuyama Y, et al. A novel adenovirus expressing Flt3 ligand enhances mucosal immunity by inducing mature nasopharyngeal-associated lymphoreticular tissue dendritic cell migration. J Immunol. 2008;180:8126–34. doi: 10.4049/jimmunol.180.12.8126. [PubMed: 18523277] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukuiwa T, Sekine S, Kobayashi R, et al. A combination of Flt3 ligand cDNA and CpG ODN as nasal adjuvant elicits NALT dendritic cells for prolonged mucosal immunity. Vaccine. 2008;26:4849–59. doi: 10.1016/j.vaccine.2008.06.091. [PubMed: 18625280] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harriman GR, Bogue M, Rogers P, et al. Targeted deletion of the IgA constant region in mice leads to IgA deficiency with alterations in expression of other Ig isotypes. J Immunol. 1999;162:2521–9. [PubMed: 10072491] [PubMed] [Google Scholar]
- 26.Harriman GR, Bradley A, Das S, Rogers-Fani P, Davis AC. IgA class switch in I alpha exon-deficient mice. Role of germline transcription in class switch recombination. J Clin Invest. 1996;97:477–85. doi: 10.1172/JCI118438. [PubMed: 8567970] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davenport FM, Hennessy AV, Brandon FM, Webster RG, Barrett CD, Jr., Lease GO. Comparisons of serologic and febrile responses in humans to vaccination with influenza a viruses or their hemagglutinins. J Lab Clin Med. 1964;63:5–13. [PubMed: 14102904] [PubMed] [Google Scholar]
- 28.Kadowaki S, Chen Z, Asanuma H, Aizawa C, Kurata T, Tamura S. Protection against influenza virus infection in mice immunized by administration of hemagglutinin-expressing DNAs with electroporation. Vaccine. 2000;18:2779–88. doi: 10.1016/s0264-410x(00)00087-6. [PubMed: 10812219] [DOI] [PubMed] [Google Scholar]
- 29.Tamura SI, Asanuma H, Ito Y, et al. Superior cross-protective effect of nasal vaccination to subcutaneous inoculation with influenza hemagglutinin vaccine. Eur J Immunol. 1992;22:477–81. doi: 10.1002/eji.1830220228. [PubMed: 1537382] [DOI] [PubMed] [Google Scholar]
- 30.Yetter RA, Lehrer S, Ramphal R, Small PA., Jr. Outcome of influenza infection: effect of site of initial infection and heterotypic immunity. Infect Immun. 1980;29:654–62. doi: 10.1128/iai.29.2.654-662.1980. [PubMed: 7216433] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamura S, Ito Y, Asanuma H, et al. Cross-protection against influenza virus infection afforded by trivalent inactivated vaccines inoculated intranasally with cholera toxin B subunit. J Immunol. 1992;149:981–8. [PubMed: 1634780] [PubMed] [Google Scholar]
- 32.Tamura S, Iwasaki T, Thompson AH, et al. Antibody-forming cells in the nasal-associated lymphoid tissue during primary influenza virus infection. J Gen Virol. 1998;79(Pt 2):291–9. doi: 10.1099/0022-1317-79-2-291. [PubMed: 9472613] [DOI] [PubMed] [Google Scholar]
- 33.Asanuma H, Hirokawa K, Uchiyama M, et al. Immune responses and protection in different strains of aged mice immunized intranasally with an adjuvant-combined influenza vaccine. Vaccine. 2001;19:3981–9. doi: 10.1016/s0264-410x(01)00129-3. [PubMed: 11427274] [DOI] [PubMed] [Google Scholar]
- 34.Kimoto H, Nagaoka H, Adachi Y, et al. Accumulation of somatic hypermutation and antigen-driven selection in rapidly cycling surface Ig+ germinal center (GC) B cells which occupy GC at a high frequency during the primary anti-hapten response in mice. Eur J Immunol. 1997;27:268–79. doi: 10.1002/eji.1830270140. [PubMed: 9022029] [DOI] [PubMed] [Google Scholar]
- 35.Wu HY, Nikolova EB, Beagley KW, Eldridge JH, Russell MW. Development of antibody-secreting cells and antigen-specific T cells in cervical lymph nodes after intranasal immunization. Infect Immun. 1997;65:227–35. doi: 10.1128/iai.65.1.227-235.1997. [PubMed: 8975916] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asanuma H, Inaba Y, Aizawa C, Kurata T, Tamura S. Characterization of mouse nasal lymphocytes isolated by enzymatic extraction with collagenase. J Immunol Metho. 1995;187:41–51. doi: 10.1016/0022-1759(95)00165-7. [PubMed: 7490457] [DOI] [PubMed] [Google Scholar]
- 37.Hiroi T, Iwatani K, Iijima H, Kodama S, Yanagita M, Kiyono H. Nasal immune system: distinctive Th0 and Th1/Th2 type environments in murine nasal-associated lymphoid tissues and nasal passage, respectively. Eur J Immunol. 1998;28:3346–53. doi: 10.1002/(SICI)1521-4141(199810)28:10<3346::AID-IMMU3346>3.0.CO;2-P. [PubMed: 9808204] [DOI] [PubMed] [Google Scholar]
- 38.Asanuma H, Thompson AH, Iwasaki T, et al. Isolation and characterization of mouse nasal-associated lymphoid tissue. J Immunol Metho. 1997;202:123–31. doi: 10.1016/s0022-1759(96)00243-8. [PubMed: 9107301] [DOI] [PubMed] [Google Scholar]
- 39.Harris DP, Haynes L, Sayles PC, et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1:475–82. doi: 10.1038/82717. [PubMed: 11101868] [DOI] [PubMed] [Google Scholar]
- 40.Mauri C, Mars LT, Londei M. Therapeutic activity of agonistic monoclonal antibodies against CD40 in a chronic autoimmune inflammatory process. Nat Med. 2000;6:673–9. doi: 10.1038/76251. [PubMed: 10835684] [DOI] [PubMed] [Google Scholar]
- 41.Hussell T, Spender LC, Georgiou A, O'Garra A, Openshaw PJ. Th1 and Th2 cytokine induction in pulmonary T cells during infection with respiratory syncytial virus. J Gen Virol. 1996;77(Pt 10):2447–55. doi: 10.1099/0022-1317-77-10-2447. [PubMed: 8887477] [DOI] [PubMed] [Google Scholar]
- 42.Tamura S, Miyata K, Matsuo K, et al. Acceleration of influenza virus clearance by Th1 cells in the nasal site of mice immunized intranasally with adjuvant-combined recombinant nucleoprotein. J Immunol. 1996;156:3892–900. [PubMed: 8621928] [PubMed] [Google Scholar]
- 43.Tamura S, Kurata T. Defense mechanisms against influenza virus infection in the respiratory tract mucosa. Jpn J Infect Dis. 2004;57:236–47. [PubMed: 15623947] [PubMed] [Google Scholar]
- 44.Couch RB, Kasel JA. Immunity to influenza in man. Annu Rev Microbiol. 1983;37:529–49. doi: 10.1146/annurev.mi.37.100183.002525. [PubMed: 6357060] [DOI] [PubMed] [Google Scholar]
- 45.Wright PF, Neumann G, Kawaoka Y. Orthomyxoviruses: Vaccine. In: Knipe DM, Howley PM, Griffin DE, et al., editors. Fields Virology. 5th ed. Lippincott-Raven Publishers PA; 2007. pp. 1727–31. [Google Scholar]
- 46.Bridges CB, Harper SA, Fukuda K, Uyeki TM, Cox NJ, Singleton JA. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2003;52:1–34. quiz CE31-4. [PubMed: 12755288] [PubMed] [Google Scholar]
- 47.Wright PF, Murphy BR, Kervina M, Lawrence EM, Phelan MA, Karzon DT. Secretory immunological response after intranasal inactivated influenza A virus vaccinations: evidence for immunoglobulin A memory. Infect Immun. 1983;40:1092–5. doi: 10.1128/iai.40.3.1092-1095.1983. [PubMed: 6852914] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hagiwara Y, McGhee JR, Fujihashi K, et al. Protective mucosal immunity in aging is associated with functional CD4+ T cells in nasopharyngeal-associated lymphoreticular tissue. J Immunol. 2003;170:1754–62. doi: 10.4049/jimmunol.170.4.1754. [PubMed: 12574339] [DOI] [PubMed] [Google Scholar]
- 49.Mbawuike IN, Pacheco S, Acuna CL, Switzer KC, Zhang Y, Harriman GR. Mucosal immunity to influenza without IgA: an IgA knockout mouse model. J Immunol. 1999;162:2530–7. [PMID: 10072492] [PubMed] [Google Scholar]
- 50.Zhang Y, Pacheco S, Acuna CL, Switzer KC, Wang Y, Gilmore X, Harriman GR, Mbawuike IN. Immunoglobulin A-deficient mice exhibit altered T helper 1-type immune responses but retain mucosal immunity to influenza virus. Immunology. 2002;105:286–94. doi: 10.1046/j.0019-2805.2001.01368.x. [PMID: 11918690] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liew FY, Russell SM, Appleyard G, Brand CM, Beale J. Cross-protection in mice infected with influenza A virus by the respiratory route is correlated with local IgA antibody rather than serum antibody or cytotoxic T cell reactivity. Eur J Immunol. 1984;14:350–6. doi: 10.1002/eji.1830140414. [PubMed: 6609824] [DOI] [PubMed] [Google Scholar]
- 52.Tamura S, Funato H, Hirabayashi Y, et al. Cross-protection against influenza A virus infection by passively transferred respiratory tract IgA antibodies to different hemagglutinin molecules. Eur J Immunol. 1991;21:1337–44. doi: 10.1002/eji.1830210602. [PubMed: 1646112] [DOI] [PubMed] [Google Scholar]
- 53.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [PubMed: 11130078] [DOI] [PubMed] [Google Scholar]
- 54.Klinman DM, Currie D, Gursel I, Verthelyi D. Use of CpG oligodeoxynucleotides as immune adjuvants. Immunol Rev. 2004;199:201–6. doi: 10.1111/j.0105-2896.2004.00148.x. [PubMed: 15233736] [DOI] [PubMed] [Google Scholar]
- 55.Wagner H. Bacterial CpG DNA activates immune cells to signal infectious danger. Adv Immunol. 1999;73:329–68. doi: 10.1016/s0065-2776(08)60790-7. [PubMed: 10399010] [DOI] [PubMed] [Google Scholar]