Abstract
Purpose
To examine prescribing of biologic and nonbiologic disease-modifying anti-rheumatic drugs (nbDMARDs) in Rheumatoid Arthritis (RA) before and after publication of the American College of Rheumatology (ACR) treatment recommendations.
Methods
We identified biologic naïve RA patients cared for by US rheumatologists participating in the CORRONA registry with visits prior to and/or at least 6 months after publication of the ACR recommendations (time periods: 2/02 - 6/08 vs. 12/08 - 12/09). The population was divided into two mutually exclusive cohorts: 1) methotrexate (MTX) monotherapy users; and 2) multiple nbDMARD users. Initiation or dose escalation of biologic and nbDMARDs in response to active disease was assessed cross-sectionally and longitudinally in comparison to the ACR recommendations. The impact of the publication of the ACR recommendations on treatment practices was compared using logistic regression stratified by disease activity adjusting for clustering of physicians and geographic region.
Results
After one visit, 24 to 37% of MTX monotherapy users with moderate disease activity and poor prognosis or high disease activity received care consistent with the recommendations; it was 34 to 56% after 2 visits. In the multiple nbDMARD users, 30 to 47% of those with moderate or high disease activity received care consistent with the recommendation after one visit and 43 to 51% after 2 visits. Publication of the recommendations did not significantly change treatment patterns for active disease.
Conclusions
Substantial numbers of RA patients with active disease did not receive care consistent with the current ACR treatment recommendations. Innovative approaches to improve care are necessary.
Introduction
An estimated 1.3 million Americans are affected by rheumatoid arthritis (RA).1 The current management approach involves early and aggressive treatment with the goal of preventing the development of permanent joint damage and deformity as well as functional impairment.2 The majority of RA patients are first typically treated with traditional non-biologic disease modifying antirheumatic drugs (DMARDs). For patients with an inadequate response, non-biologic DMARDs can be used in combination or biologics can be used as add-on therapy to achieve better control.3 The American College of Rheumatology (ACR) published recommendations in 2008 for the use of non-biologic and biologic DMARDs with the goal of optimizing outcomes and providing the best evidence-based recommendations in relation to new therapies and new evidence for older therapies. The publication sought to provide guidance to clinicians on treatment strategies (Table 1).4 Specifically after a systematic literature review and a synthesis of the evidence, these recommendations were formulated based on clinical evidence and input from an expert panel using a formalized group process method including a modified Delphi panel and the RAND/UCLA appropriate method. Of note, these recommendations were developed specifically for specialists familiar with assessing RA disease activity and disease severity with the hope that they would be applied in conjunction with individualized assessment.
Table 1.
Summary of ACR recommendations based on disease activity and prior exposure to nonbiologic disease modifying anti-rheumatic drugs (DMARDs).
| Disease Activity | Methotrexate Monotherapy Users | Multiple nonbiologic DMARD Users |
|---|---|---|
| Low | Use of nonbiologic DMARDs* | Use of nonbiologic DMARD* |
| Moderate with good prognosis | Use of nonbiologic DMARDs* | Maximize nonbiologic DMARD therapy or initiate biologic** |
| Moderate with poor prognosis | Maximize nonbiologic DMARD therapy or initiate biologic** | Maximize nonbiologic DMARD therapy or initiate biologic** |
| High | Maximize nonbiologic DMARD therapy or initiate biologic** | Maximize nonbiologic DMARD therapy or initiate biologic** |
The recommendations do not specify dose escalation thus we only examined whether the patients were receiving nonbiologic DMARD therapy.
The recommendations suggest that if patients in these groups "fail" nonbiologic therapy, then biologic agents should be initiated. Therefore, for these groups we included escalation of nonbiologic DMARD therapy to be considered consistent with the recommendations.
Medication prescribing for rheumatoid arthritis is complex and is influenced by numerous factors including patients' clinical characteristics such as disease activity and disease severity, financial concerns, access to care, and both patient and provider preferences.5 Currently it is unknown whether providers use the published recommendations to guide treatment decision-making and whether treatment approaches vary based on disease activity. The aim of this study was to examine and compare the treatment of biologic naïve RA patients before and after publication of the ACR recommendations in a U.S. cohort of RA patients using data from the Consortium of Rheumatology Researchers of North America (CORRONA) registry. We sought to assess non-biologic and biologic DMARD use based on level of disease activity as well as to evaluate the impact of the ACR recommendations on medication patterns. In addition, we wanted to determine whether patients with moderate to high disease activity and features predicting poor prognosis received more aggressive treatment, hypothesizing that in the period of time following publication of the recommendations, a greater number of patients would receive care more consistent with the guidelines as compared to prior.
Methods
Data sources and data collection
CORRONA is a prospective US observational cohort of patients with arthritis who are enrolled by participating rheumatologists in both academic and private practice sites; the details have been previously described.6, 7 Data are collected from both patients and their treating rheumatologists, who gather information on disease duration, prognosis, disease severity and activity, medical comorbidities, use of medications including DMARDs, and adverse events.8 Follow-up assessments are requested at least as often as every 6 months and completed during routine clinical encounters. Approvals for participation in the CORRONA registry are obtained from respective Institutional Review Boards of participating academic sites and a central Institutional Review Board for private practice sites, and patients provide informed consent before enrollment.
Study population
There were 23,052 enrolled patients with rheumatoid arthritis and 137,444 visits entered into the CORRONA registry database for this population between October 1, 2001 to December 30, 2009. Over one hundred rheumatology practices enroll patients across 39 states with 310 participating rheumatologists. There are no disease activity requirements or comorbidity exclusion criteria. For the purposes of these analyses, RA patients were first divided into two cohorts based on their visit histories prior to and/or at least 6 months after the publication of the recommendations (published on June 15, 2008). Patients could be represented in one or both cohorts. Thus patients were identified from two different time periods: 2/1/02 - 6/15/08 and 12/1/08 -12/30/09. For each of the two cohorts, we selected those patients who were biologic naive, had an index visit and a follow-up visit within 6 months, a disease duration of >1 year, and were not in remission based on the Clinical Disease Activity Index (CDAI <2.8). In each time period, patients were further divided into two sub-cohorts based on their history of treatments and current medications at the baseline index visit: 1) “MTX monotherapy users” defined as those currently on methotrexate (MTX) or who had used MTX in the past without past or current use of other non-biologic DMARDs; and 2) “multiple non-biologic DMARD (nbDMARD) users” defined as those who were receiving or had received 2 or more non-biologic DMARDs. This strategy was employed to allow comparison with the ACR treatment recommendations which are based on prior medication usage.
Measures
To measure disease activity, we calculated the Clinical Disease Activity Index (CDAI) that includes a tender joint count for 28 joints, a swollen joint count for 28 joints, a patient global assessment, and an evaluator global assessment. Patients were stratified by CDAI score into low, moderate or high disease activity levels at the baseline visit.9 As the 2008 ACR treatment recommendations suggest different treatment strategies based on prognosis, we evaluated the prognosis in patients as defined. Specifically the prognosis was determined to be “Good” or “Poor” based on the absence or presence of a modified Health Assessment Questionnaire (mHAQ) of > 0.5 at the initial visit, rheumatoid factor positivity, presence of extra-articular disease (rheumatoid nodules or secondary Sjogren's), and erosive changes on x-ray. In order to allow comparisons with the ACR treatment recommendations, those with moderate disease activity in the MTX monotherapy cohort were stratified further based on prognosis (any features of poor prognosis present vs. all absent).
DMARD prescribing patterns
To evaluate DMARD prescribing patterns in the two study cohorts from before and after the ACR recommendations were published, we examined treatment regimens cross-sectionally and longitudinally within the time frame. First we assessed treatment practices based on the baseline visit (the first visit within the time period of interest). We categorized patients in one of 5 treatment regimens at the conclusion of the baseline visit as follows: 1) biologic DMARD initiated; 2) non-biologic DMARD initiated; 3) non-biologic DMARD dose increased; 4) nonbiologic DMARD therapy maintained without a dose increase; and 5) no DMARD used. These groups were mutually exclusive with patients being categorized based on the most aggressive treatment pattern. For example, if the patient was simultaneously started on a biologic and their MTX dose was increased, they would be considered as a biologic initiator. For the longitudinal analysis we identified patients with persistent disease activity at both the baseline visit and follow-up visit (a visit at least 3 months and up to 6 months after the baseline visit). For the MTX monotherapy users with moderate disease activity and poor prognosis or high disease activity at both the baseline and follow-up visits, we studied their treatment at the conclusion of their follow up visit using the same treatments regimens described above, including treatment changes initiated at the baseline visit, in between the baseline and follow-up visit, and at the conclusion of the follow-up visit. Similarly in the multiple nbDMARD users who had persistent moderate or high disease activity at both the initial and follow-up visit, we examined their treatment at the conclusion of the second visit. Finally, all patients care within each disease activity and prognosis category was grouped as either consistent with the ACR recommendations, or not.4
Statistical Analysis
Patient demographic and clinical characteristics were compared in the before and after ACR treatment recommendation populations. For continuous variables, means and standard deviations were estimated and t tests were used to test statistical differences between the groups. For dichotomous variables, percentages were estimated and chi square tests were used to test statistical differences between groups. In cross-sectional and longitudinal analyses, percentages of treatment strategies employed were calculated for each disease activity level. We compared the proportion of treatment regimens consistent with the ACR recommendations before and after publication using a mixed effect model logistic regression approach and considering clustering of physicians as a random effect. Models were adjusted for geographic region. Three sensitivity analyses were performed. In the first, we reran the analyses identifying the post-publication group based on at least 9 months following the publication of the recommendations rather than 6. For the second we excluded patients with heart disease, cancer and/or a demyelinating illness from both the pre and post publication groups as these conditions might influence treatment practices. For the third, we reran the primary analyses as well as the previous sensitivity analyses on those with RA disease duration of 5 years or less to assess whether this influenced treatment patterns. Statistical analyses were performed using STATA version 11.1.
Results
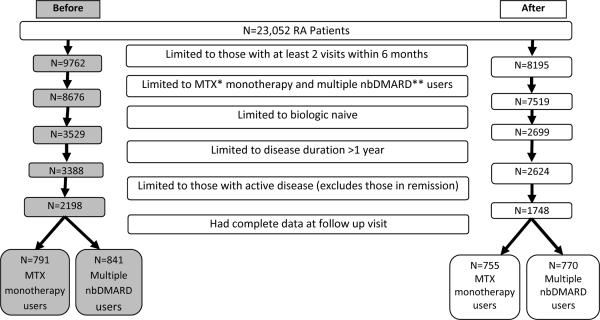
As shown in Figure 1, of the 23,052 RA patients, there were 1632 patients who met criteria for inclusion prior to the ACR recommendations (791 MTX monotherapy users and 841 mutliple nbDMARD users), and 1525 afterward (755 MTX monotherapy users and 770 multiple DMARD users). There were 204 treating rheumatologists, of whom 95 cared for patients in both the before and after cohorts. The baseline characteristics of these before and after cohorts are displayed in Table 2. In the cohort identified after publication of the ACR recommendations, patients were seen on average 11 months (SD 2.1) after the publication for the baseline visit and 15 months for the follow-up visit (SD 1.9). Among the MTX monotherapy users the mean age was 64 to 65, mean disease duration of 8.7 to 9.6 years and most had moderate disease activity (CDAI 11.5 to 13.4). There were significant, but minimal differences in the two sub-cohorts with respect to mean disease activity level. Among the multiple nbDMARD users, the mean age was 62 to 63, mean disease duration of 13.2 to 13.6 years and most had moderate disease activity (CDAI 12.2 to 12.7). There were borderline significant differences in the two sub-cohorts with respect to self-reported pain and Medicaid insurance status.
Figure 1. Identification of the study cohorts before and after publication of the ACR recommendations.
*MTX=Methotrexate **nbDMARD=nonbiologic disease modifying anti-rheumatic drug
Table 2.
The baseline characteristics of the two cohorts based on inclusion before or after publication of the ACR treatment recommendations.
| MTX Monotherapy Users | P value | Multiple Nonbiologic DMARD Users | P value | |||
|---|---|---|---|---|---|---|
| Before N=791 | After N=755 | Before N=841 | After N=770 | |||
| Demographics | ||||||
| Age (mean age, SD) | 64 (14) | 65(14) | 0.12 | 62 (13) | 63 (12) | 0.24 |
| Gender (% female) | 73 | 73 | 0.95 | 79 | 78 | 0.81 |
| Race (% white) | 83 | 86 | 0.09 | 86 | 85 | 0.78 |
| Insurance (%) | ||||||
| Private | 67 | 68 | 0.51 | 68 | 68 | 1.00 |
| Medicare | 53 | 48 | 0.11 | 48 | 50 | 0.42 |
| Medicaid | 6 | 5 | 0.27 | 7 | 5 | 0.06 |
| Clinical | ||||||
| RF Seropositivity (%) | 86 | 88 | 0.67 | 91 | 90 | 0.62 |
| Disease duration (mean years, SD) | 9.6 (9.9) | 8.7 (9.1) | 0.09 | 13.2 (10.4) | 13.6 (10.6) | 0.51 |
| Patient pain score (mean, SD) | 32.8 (23.9) | 32.7 (25.3) | 0.94 | 35.4 (24.5) | 37.6 (25.7) | 0.07 |
| mHAQ (mean, SD) | 0.37 (0.5) | 0.35 (0.4) | 0.35 | 0.42 (0.5) | 0.42 (0.5) | 0.82 |
| Use of prednisone ever (%) | 45 | 45 | 0.92 | 53 | 51 | 0.40 |
| CDAI (mean, SD) | 13.4 (10.5) | 11.5 (9.2) | <0.001 | 12.7 (10.3) | 12.2 (10.1) | 0.27 |
| Disease activity* (%) | <0.001 | 0.37 | ||||
| Low | 52 | 59 | 56 | 58 | ||
| Moderate | 29 | 30 | 29 | 29 | ||
| High | 19 | 12 | 16 | 13 | ||
Defined using the Clinical Disease Activity Index (CDAI)
Cross-sectional analyses
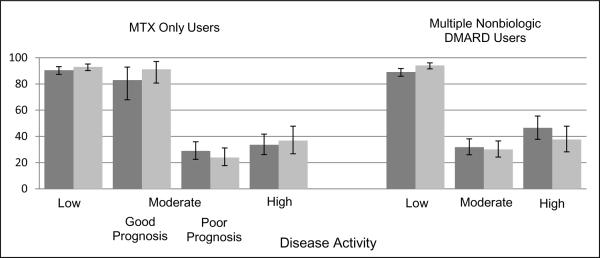
In the cross-sectional analysis examining treatment patterns both before and after publication of the ACR treatment recommendations, among the MTX monotherapy users with low disease activity, 91 to 93% received nonbiologic DMARDs while those medications were used in 83 to 91% of those with moderate disease activity and good prognosis. In those with moderate disease activity and a poor prognosis 17 to 19% had escalation of nonbiologic DMARD therapy, meaning either a dose increase or initiation of a nonbiologic DMARD, and 7–10% initiated a biologic at the conclusion of the initial visit. Approximately 60% were continued on MTX without a dose increase (median dose of MTX was 15.5 mg in the pre-recommendations group and 15.3 mg in the post-recommendations group) and 13% were not treated with any DMARDs. In those with high disease activity, 21% had escalation of nonbiologic DMARD therapy and 13 to 16% initiated a biologic. Maintenance of MTX without a dose increase occurred in 48 to 55% of patients (median dose of MTX was 15.3 mg in the pre-recommendations group and 16.2 mg in the post-recommendations group) while 8 to 18% did not receive any DMARDs.
In the cross-sectional analysis among the multiple nbDMARD cohort with low disease activity, prior to the publication of the recommendations 89% continued nonbiologic DMARDs while it was 94% afterwards, a significant increase (p=0.003). When examining treatment patterns both before and after publication of the ACR treatment recommendations, 21 to 23% of those with moderate disease activity had escalation of nonbiologic DMARD therapy, and 9% were initiated on a biologic. Nonbiologic DMARD therapy was maintained without a dose increase in 55 to 60% while 10 to 14% received no DMARD therapy. In those with high disease activity 22 to 32% had escalation of nonbiologic DMARD therapy and 15 to 16% initiated a biologic. Maintenance of nonbiologic DMARDs without a dose increase occurred in 41 to 51% of patient and 11 to 12% received no DMARD therapy.
Treatment categories were then combined based on whether the treatment was consistent with the treatment recommendations (Figure 2). The only significant increase in treatments consistent with the recommendations after the 2008 publication occurred in those with low disease activity in the multiple nbDMARD cohort. The other comparisons were not significant in both unadjusted and adjusted analyses controlling for clustering by physicians and geographic region (Table 3). The variability by physician was represented by median odds ratio, which ranged from 1.00–2.24.
Figure 2. Proportion of Patients Adherent to the ACR Recommendations Stratified by Disease Activity and Drug Use at the Conclusion of 1 Visit*.
*Dark grey columns = pre-publication treatment patterns, light grey columns = post-publication treatment patterns. Good prognosis means absence of a poor prognosis; Poor prognosis = mHAQ>0.5, presence of rheumatoid nodules, erosive changes on x-ray, rheumatoid factor positive and secondary Sjogren. Error bars represent 95% confidence intervals.
Table 3.
The unadjusted and adjusted likelihoods that publication of the recommendation influenced prescribing patterns relative to the ACR recommendations.*
| Disease activity | Unadjusted Odds Ratio (95% confidence interval) | Adjusted Odds Ratio (95% confidence interval) |
|---|---|---|
| MTX only users | ||
| Low | 1.56 (0.92,2.67) | 1.48 (0.87,2.52) |
| Moderate with good prognosis** | 2.14 (0.63,7.30) | 1.79 (0.50,6.38) |
| Moderate with poor prognosis** | 0.75 (0.45,1.24) | 0.77 (0.47,1.27) |
| High | 1.17 (0.66,2.09) | 1.15 (0.64,2.07) |
| Multiple nbDMARD users | ||
| Low | 2.23 (1.32,3.79) | 2.37 (1.39,4.02) |
| Moderate | 0.92 (0.62,1.37) | 0.89 (0.60,1.33) |
| High | 0.69 (0.41,1.18) | 0.70 0.41, 1.18) |
MTX= methotrexate; nbDMARDs=nonbiologic disease modifying anti-rheumatic drugs
Good prognosis means absence of a poor prognosis; Poor prognosis = mHAQ>0.5, presence of rheumatoid nodules, erosive changes on x-ray, rheumatoid factor positive and secondary Sjogren.
Longitudinal analyses
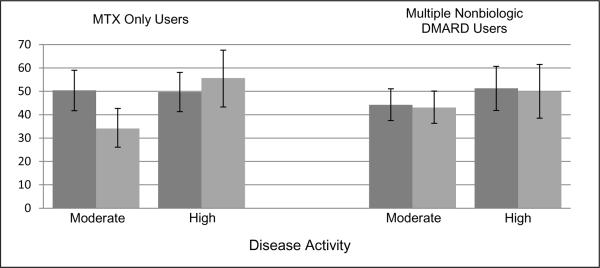
In the longitudinal analyses we also examined treatment patterns both before and after publication of the ACR treatment recommendations. First we examined those with persistent disease activity among the MTX monotherapy users. Of the patients with moderate disease activity and poor prognosis at both the baseline and follow-up appointment, 14 to 23% had their nonbiologic DMARD therapy escalated and 20 to 27% had a biologic initiated. Approximately 44 to 57% remained on a nonbiologic DMARD without any change and 6 to 9% received no DMARD therapy. In those with persistent high disease activity at both the baseline and follow-up visit, 19 to 23% had escalation of nonbiologic DMARD therapy and 31 to 33% initiated a biologic. There were 35 to 41% of patients who remained on nonbiologic DMARD therapy without a dose change and 3 to 16% who received no DMARD therapy. Treatment categories were then combined based on whether the treatment was consistent with the treatment recommendations and displayed in Figure 3. In adjusted analyses, prescribing practices were less consistent with the ACR recommendations after the publication in those with moderate disease activity and poor prognosis (OR 0.48, 95% CI 0.28 to 0.80), while it was not significantly different in those with high disease activity (OR 1.38, 95% CI 0.72 to 2.66).
Figure 3. Proportion of Patients Adherent to the ACR Recommendations after 2 Visits over 6 months with Persistent Active Disease.
*Dark grey columns = pre-publication treatment patterns, light grey columns = post-publication treatment patterns. Error bars represent 95% confidence intervals.
In the multiple nbDMARD users, we examined treatment patterns in those with persistent moderate or high disease activity at both the baseline and follow-up visit. Among those with moderate disease activity, escalation of nonbiologic agents occurred in 17 to 19%, biologic initiation in 25 to 26%, continuation of nonbiologic DMARD therapy without a dose increase in 45 to 50% with no DMARD use in 7 to 11%. In those with high disease activity, escalation of nonbiologic agents occurred in 17 to 26%, biologic initiation in 24 to 34%, continuation of nonbiologic DMARD therapy without dose increase in 34 to 41% with 9 to 15% receiving no DMARDs. When treatment categories were then combined based on whether the treatment was consistent with the treatment recommendations, publication of the ACR recommendations were not associated with any changes in prescribing patterns in those with moderate (OR 0.97, 95% CI 0.66 to 1.44) and high disease (OR 0.96, 95% CI 0.51 to 1.79) activity at 2 visits. The median odds ratios representing physician variability ranged from 1.00 to 2.28. The results from the cross-sectional and longitudinal analyses were not substantially changed when we excluded patients with heart disease, cancer and/or a demylinating illness, when we limited the post-publication group to those with the baseline visit at least 9 months after the publication and when we limited the population to those with 5 years or less RA disease duration.
Discussion
This manuscript examines treatment patterns among RA patients both before and after publication of the 2008 ACR treatment recommendations based on disease activity and prognosis. We found that adherence to the treatment recommendations was lower for patients with higher levels of disease activity, specifically because a majority of these higher risk patients were maintained on less aggressive therapies rather than being accelerated. The vast majority of MTX monotherapy users with low disease activity (90–93%) or moderate disease activity with a good prognosis (83–91%) received care consistent with the ACR treatment recommendations. However, only a quarter to a third of patients with moderate disease activity and poor prognosis (24 to 29%) or high disease activity (34 to 37%) had care consistent with recommendations. Similarly in the multiple nbDMARD users with moderate or high disease activity, only 31–47% had care consistent with the recommendations, meaning they had their non-biologic DMARD therapy optimized or were initiated on a biologic. In patients with persistent active disease (moderate disease activity with poor prognosis or severe disease activity) at both the initial and follow-up appointments, approximately 34 to 56% of patients had care consistent with the recommendations. In adjusted analyses, publication of the ACR treatment recommendations was not associated with a greater likelihood of prescribing treatments consistent with the recommendations in those with moderate or severe disease activity.
Deficits in the quality of care RA patients receive is not a new issue. MacLean and colleagues using data from the early 1990's examined the quality of care RA patients received for their arthritis based on the ACR recommendations at the time which included at least yearly appointments for RA, yearly monitoring of acute phase reactants and laboratory monitoring for DMARDs.10 While seeing a specialist was associated with greater quality, 25% of patients who saw rheumatologists still did not receive the recommended care. More recently Benhamou and colleagues in a French multi-center cohort study found that only 54% to 58% of early RA patients cared for by rheumatologists were treated according to 2 clinical practice guidelines which included initiating DMARDs.11
It is unlikely that our results reflect the care of a few select providers as there were 204 physicians caring for the patients in the cohorts. It is more likely that many factors contributed to the gaps we found between clinical practice and treatment recommendations. First, rheumatologists may not be aware of the disease activity at the time of the appointment. While it is possible for providers to calculate the CDAI, RAPID Score, or Global Arthritis Score at the time of the clinical encounter, in many instances these are only calculated after the fact, if at all.12–14 It is known that many rheumatologists rely instead on a variably determined qualitative Physician Global Assessment to inform their treatment decisions.13 It is also possible that rheumatologists are unaware of the ACR treatment recommendations, although these recommendations were published in a journal associated with the College, are available currently on the ACR website, and were highlighted in a plenary session at the annual meeting occurring immediately after their publication.4 Another possibility is that rheumatologists and patients consider the ACR recommendations to be too aggressive, even though several trials have demonstrated improved clinical outcomes among patients whose treatment regimens were accelerated in response to active disease activity.15–17 Alternatively they may think the recommendations are not generalizable to their patient population. Lastly, there may be safety concerns with the aggressive use of DMARDs.
Given that there was an increase in the proportion of patients receiving care consistent with the recommendation after two visits, it is possible that it may take time for providers and patients to respond to high levels of disease activity although the current recommendations specify aggressive medication use in those with persistent disease activity.18 As has been pointed out in the Institute of Medicine's report, lack of response to evidence-based treatment recommendations is typical of the care of all chronic diseases in the United States.19 The same has been documented for hypertension, hyperlipidemia, diabetes, and other diseases. In these cases, the failure to reliably accelerate treatments for patients with uncontrolled disease has been labeled “clinical inertia”, but calling attention to the problem has made little difference.20, 21 Moreover, a 17 year average time to adoption of new information and therapies into routine patient care has been reported.19
It is also critical to recognize that under-treatment in chronic disease care is in part related to the way care is typically delivered in the US and less likely due to knowledge deficits on the part of providers. For example, it requires a systems approach to capture disease activity status at every visit and relay that information to both the patient and provider. In many health care settings there is no infrastructure to allow that evaluation and feedback. In the case of the TICORA and BeST studies, there were dedicated staff to ensure serial measurement of disease activity, identification of the patients with persistent disease activity with notification to providers, acceleration of treatment for patients with active disease, and monitoring adherence and outcomes—steps critical to optimal disease management.15, 16
The strengths of this study were that we were able to identify a broad geographic distribution of US rheumatologists and RA patients with detailed clinical information on disease activity and medication use. However, the study does have limitations. We were unable to know whether patients were offered therapies but declined them. Rather we are only able to assess those medications that were prescribed. We also were unable to assess whether there were co-morbidities that precluded the use of some agents. However, we minimized this limitation by categorizing a wide range of medication strategies as being compliant with the ACR recommendations. Financial concerns may also have played a role in some of the treatment decisions, particularly when it comes to the biologics. However this was minimized by including dose escalation and initiation of non-biologic agents as being consistent with the treatment recommendations. While we examined treatment patterns for at least 6 months (and for at least 9 months in the sensitivity analysis) after publication of the recommendations, it may take more time for providers to incorporate these recommendations in clinical practice. Finally, many of the patients enrolled in the CORRONA registry did not meet the inclusion criteria for this study because of less frequent visit reports and/or prior or current biologic use. Further studies are planned to evaluate the disease activity status and treatments of the broader registry population.
In summary, this study is amongst the first to examine prescribing patterns before and after publication of the ACR treatment recommendations based on disease activity level. While the majority of patients with low disease activity received care consistent with the treatment recommendations, most with more active and aggressive disease did not. Even when we allowed 2 visits for providers to address ongoing active disease, approximately 50 to 60% of patients did not receive the recommended treatments. Additionally, publication of the ACR treatment recommendations had a minimal impact on prescribing practices. Improving RA quality of care will require addressing clinical inertia. Redesigning clinical care so that patients and providers have real-time information on disease activity as well as summaries of the risks and benefits of DMARD therapy based on disease activity is crucial and necessary to enable personalized treatment that is tailored to the specific needs of the patient and to improve long term disease outcomes.
Funding Acknowledgements
In the last two years, Abbott, Amgen, BMS, Centocor, Genentech, Lilly and Roche have supported CORRONA through contracted subscriptions to the database. The study design, data analysis and reporting of results in this manuscript were performed independent of all funding sources. Some of the investigators receive support from the National Institutes of Health (AR053856: LRH; AR053351: JRC; AR054412: JDG) and the Arthritis Foundation (JDG).
References
- 1.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I. Arthritis & Rheumatism. 2008;58(1):15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Aletaha D, Machold KP. Therapeutic strategies in early rheumatoid arthritis. Best Practice & Research Clinical Rheumatology. 2005;19(1):163–77. doi: 10.1016/j.berh.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 3.van Vollenhoven RF, Ernestam S, Geborek P, et al. Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1-year results of a randomised trial. Lancet. 2009;374(9688):459–66. doi: 10.1016/S0140-6736(09)60944-2. [DOI] [PubMed] [Google Scholar]
- 4.Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762–84. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 5.Curtis JR, Chen L, Harrold LR, Narongroeknawin P, Reed G, Solomon DH. Physician preference motivates the use of anti-tumor necrosis factor therapy independent of clinical disease activity. Arthritis Care & Research. 2010;62(1):101–7. doi: 10.1002/acr.20020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg JD, Bingham CO, 3rd, Abramson SB, Reed G, Sebaldt RJ, Kremer J. Effect of cardiovascular comorbidities and concomitant aspirin use on selection of cyclooxygenase inhibitor among rheumatologists. Arthritis Rheum. 2005;53(1):12–7. doi: 10.1002/art.20905. [DOI] [PubMed] [Google Scholar]
- 7.Kremer J. The CORRONA database. Ann Rheum Dis. 2005;64(Suppl 4):iv37–41. doi: 10.1136/ard.2005.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felson DT, Anderson JJ, Boers M, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum. 1993;36(6):729–40. doi: 10.1002/art.1780360601. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg JD, Harrold LR, Bentley MJ, Kremer J, Reed G, Strand V. Evaluation of composite measures of treatment response without acute-phase reactants in patients with rheumatoid arthritis. Rheumatology (Oxford) 2009;48(6):686–90. doi: 10.1093/rheumatology/kep054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacLean CH, Louie R, Leake B, et al. Quality of care for patients with rheumatoid arthritis. JAMA. 2000;284(8):984–92. doi: 10.1001/jama.284.8.984. [DOI] [PubMed] [Google Scholar]
- 11.Benhamou M, Rincheval N, Roy C, et al. The gap between practice and guidelines in the choice of first-line disease modifying antirheumatic drug in early rheumatoid arthritis: results from the ESPOIR cohort. J Rheumatol. 2009;36(5):934–42. doi: 10.3899/jrheum.080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aletaha D, Smolen JS. The Simplified Disease Activity Index (SDAI) and Clinical Disease Activity Index (CDAI) to monitor patients in standard clinical care. Best Practice & Research Clinical Rheumatology. 2007;21(4):663–75. doi: 10.1016/j.berh.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Harrington JT. The uses of disease activity scoring and the physician global assessment of disease activity for managing rheumatoid arthritis in rheumatology practice. J Rheumatol. 2009;36(5):925–9. doi: 10.3899/jrheum.081046. [DOI] [PubMed] [Google Scholar]
- 14.Pincus T, Yazici Y, Bergman MJ. RAPID3, an Index to Assess and Monitor Patients with Rheumatoid Arthritis, Without Formal Joint Counts: Similar Results to DAS28 and CDAI in Clinical Trials and Clinical Care. Rheumatic Disease Clinics of North America. 2009;35(4):773–8. doi: 10.1016/j.rdc.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet. 2004;364(9430):263–9. doi: 10.1016/S0140-6736(04)16676-2. [DOI] [PubMed] [Google Scholar]
- 16.Goekoop-Ruiterman YPM, de Vries-Bouwstra JK, Allaart CF, et al. Comparison of Treatment Strategies in Early Rheumatoid Arthritis. Annals of Internal Medicine. 2007;146(6):406–15. doi: 10.7326/0003-4819-146-6-200703200-00005. [DOI] [PubMed] [Google Scholar]
- 17.Möttönen T, Hannonen P, Leirisalo-Repo M, et al. Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis: a randomised trial. The Lancet. 1999;353(9164):1568–73. doi: 10.1016/s0140-6736(98)08513-4. [DOI] [PubMed] [Google Scholar]
- 18.Smolen JS, Aletaha D, Bijlsma JWJ, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 69:631–7. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medicine Io. Crossing the quality chasm: A new health system for the 21st century. National Academy Press; Washington, DC: 2001. [PubMed] [Google Scholar]
- 20.Phillips LS, Branch WT, Cook CB, et al. Clinical Inertia. Annals of Internal Medicine. 2001;135(9):825–34. doi: 10.7326/0003-4819-135-9-200111060-00012. [DOI] [PubMed] [Google Scholar]
- 21.Phillips LS, Twombly JG. It's Time to Overcome Clinical Inertia. Annals of Internal Medicine. 2008;148(10):783–5. doi: 10.7326/0003-4819-148-10-200805200-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]