Abstract
The repair of DNA double-stranded breaks (DSBs) is essential for cell viability and genome stability. Aberrant repair of DSBs has been linked with cancer predisposition and aging. During the repair of DSBs by non-homologous end joining (NHEJ), DNA ends are brought together, processed and then joined. In eukaryotes, this repair pathway is initiated by the binding of the ring-shaped Ku heterodimer and completed by DNA ligase IV. The DNA ligase IV complex, DNA ligase IV/XRRC4 in humans and Dnl4/Lif1 in yeast, is recruited to DNA ends in vitro and in vivo by an interaction with Ku and, in yeast, Dnl4/Lif1 stabilizes the binding of yKu to in vivo DSBs. Here we have analyzed the interactions of these functionally conserved eukaryotic NHEJ factors with DNA by electron microscopy. As expected, the ring-shaped Ku complex bound stably and specifically to DNA ends at physiological salt concentrations. At a ratio of 1 Ku molecule per DNA end, the majority of DNA ends were occupied by a single Ku complex with no significant formation of linear DNA multimers or circular loops. Both Dnl4/Lif1 and DNA ligase IV/XRCC4 formed complexes with Ku-bound DNA ends, resulting in intra- and intermolecular DNA end bridging even with non-ligatable DNA ends. Together these studies, which provide the first visualization of the conserved complex formed by Ku and DNA ligase IV at juxtaposed DNA ends by electron microscopy, suggest that the DNA ligase IV complex mediates end-bridging by engaging two Ku-bound DNA ends.
Keywords: non-homologous end joining, DNA ligase, Ku, end-bridging
1. Introduction
DNA double-stranded breaks (DSBs) arise from a variety of sources, including exposure to endogenous and exogenous DNA damaging agents, and in a programmed manner during meiosis and immmunoglubulin gene rerrangement. There are two main pathways that repair DSBs: in one pathway, the DNA ends are re- joined in a reaction involving end resection followed by strand invasion into a homologous duplex (homologous recombination, HR) whereas in the non-homologous end joining (NHEJ) pathway, broken ends are simply spliced together [1, 2]. In mammals, defects in DSB repair have been linked with an increased incidence of chromosomal rearrangements, cancer, and premature aging [3–6].
The fundamental mechanism of NHEJ is conserved among eukaryotes and also in some prokaryotes [2, 7, 8]. This repair pathway is initiated by the binding of a dimeric ring-shaped Ku complex, which is a homodimer in prokaryotes and a heterodimer in eukaryotes, to the DNA end. The binding of Ku protects the DNA end from degradation and also recruits other NHEJ proteins to the DNA end. Assembly of yeast NHEJ factors at in vivo DSBs has been examined by chromatin immunoprecipitation (ChIP). These studies revealed that, while yeast Ku (yKu) is necessary for the subsequent recruitment of the other factors [9–11], Dnl4/Lif1 is required for the stable association of yKu at in vivo DSBs [11]. This stable association of yKu and Dnl4/Lif1 at DNA ends, which is dependent upon a direct interaction between these complexes, inhibits end resection, thereby preventing the repair of the DSB by HR [11]. In human cells, the recruitment of DNA ligase IV/XRCC4 to in vivo DSBs is also dependent upon Ku. However, the catalytic subunit of the DNA-dependent protein kinase (DNA PKcs), which is independently recruited by Ku to DNA ends to form the DNA dependent protein kinase, appears to play the major role in stabilizing Ku binding at the DSB [2, 8, 12].
Although biochemical studies with purified human and yeast proteins have shown that the binding of Ku to DNA ends enhances subsequent DNA binding by the DNA ligase complex, there are contradictory reports as to whether the presence of Ku enhances or inhibits DNA joining [11, 13–17]. Nonetheless, the biochemical, molecular genetic and cell biology studies in both mammals and yeast provide compelling evidence that the conserved complex formed between Ku (yKu) and DNA ligase IV/XRCC4 (Dnl4/Lif1) at DNA ends plays a critical role in the repair of DSBs by NHEJ in eukaryotes. Here we have combined biochemical approaches with electron microscopy (EM), to provide novel insights into the molecular architecture of DNA-protein complexes formed by Ku (yKu) and DNA ligase IV/XRCC4 (Dnl4/Lif1).
2. Results
2.1. Formation of DNA-protein complexes by yKu and Dnl4/Lif1 on oligonucleotide substrates
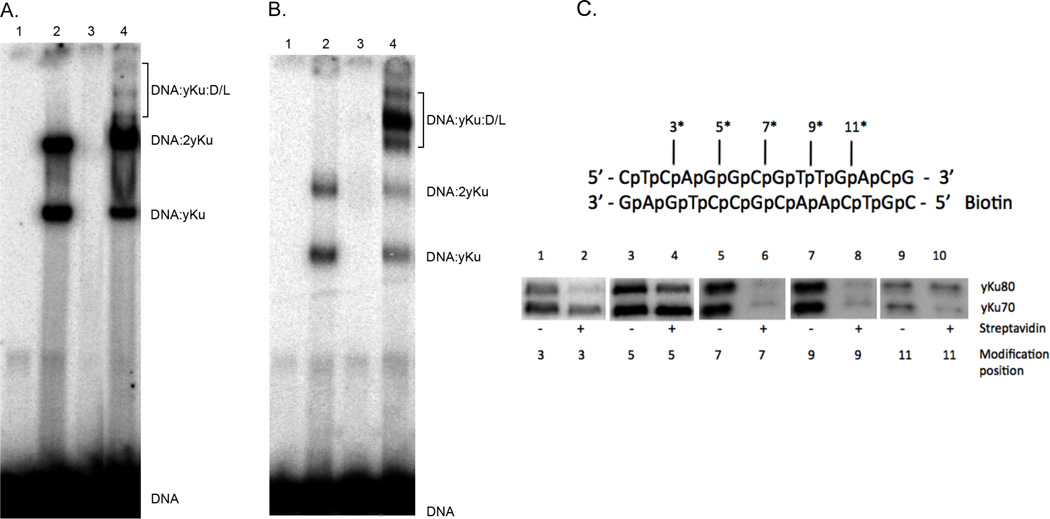
Previously, Dynan and colleagues used photocrosslinking to determine the relative positions of Ku and DNA PKcs in DNA-proteins complexes formed on a DNA end [18, 19]. Notably, these studies revealed that Ku was displaced inward along the DNA duplex as a consequence of DNA PKcs binding. In initial studies, we examined the formation of DNA protein complexes by purified yKu and Dnl4/Lif1 on a 45 bp oligonucleotide duplex in electrophoretic mobility shift assays. Two DNA-protein complexes were formed by yKu without (Fig. 1A) or with glutaraldehyde cross-linking (Fig. 1B). Since these assays contained 1 yKu per 5 DNA ends, we suggest that the two DNA-protein complexes correspond to DNA molecules with a yKu complex bound at either one or both ends. This is consistent with the structure of the yKu-DNA protein complexes visualized by electron microscopy (Figs. 2 and 3). In contrast, no DNA-complexes were detected in similar assays with Dnl4/Lif1 (Fig. 1A and B). When yKu and Dnl4/Lif1 were co-incubated, slower migrating DNA-protein complexes were detected at low levels without cross-linking (Fig. 1A) but at much higher levels after cross-linking (Fig. 1B). Since glutaraldehyde cross-linking did not significantly increase the amount of DNA-protein complex formed by yKu and Dnl4/Lif1 on a 500 bp linear DNA substrate [11], it appears that the length of the DNA substrate to which yKu is bound impacts the efficiency and/or stability of yKu-dependent DNA binding by Dnl4/Lif1. This was confirmed in surface plasmon resonance assays comparing the binding of Dnl4/Lif1 to chip surfaces with either 45 bp or 500 bp by DNA duplexes immobilized and pre-loaded with yKu (data not shown).
Figure 1. DNA-protein complex by yKu and Dnl4/Lif1 in EMSAs; Photocrosslinking of yKu to aryl azide-modified linear duplex DNA.
Radiolabeled 45 bp linear duplex DNA (25 nM) was incubated for 15 minutes at room temperature with; lane 1, no addition; lane 2, yKu (10 nM); lane 3, Dnl4/Lif1 (D/L, 20 nM) and lane 4, yKu (10 nM) and Dnl4/Lif1 (D/L, 20 nM). Incubation was continued for an additional 10 minutes at room temperature without (Panel A) or with 0.2% glutaraldehyde (Panel B). After separation by polyacrylamide (5%) gel electrophoresis, labeled DNA species in were visualized by phosphorImager analysis (Molecular Dynamics). The positions of the DNA substrate (DNA) and DNA-protein complexes formed with yKu (yKu) and Dnl4/Lif1 (D/L) are indicated. (C) Upper panel, schematic of the 14 bp DNA substrate used for photocrosslinking. The positions of phosphorothioate modifications within the top strand (asterisk) are indicated. Bottom panel, radiolabeled DNA substrates (7 nM) containing a single activated aryl azide modification within the top strand at the indicated distance from the end; lanes 1 and 2, 3 nucleotides; lanes 3 and 4, 5 nucleotides; lanes 5 and 6, 7 nucleotides; lanes 7 and 8, 9 nucleotides and lanes 9 and 10, 11 nucleotides. DNA (7 nM) and yKu (40 nM) were incubated with yKu (40 nM) in the presence or absence of streptavidin as indicated for 30 minutes at 25°C. Reactions were irradiated with 254 nM UV light for 2 minutes to trap DNA-protein complexes. After separation by 7.5% SDSPAGE electrophoresis, covalently-linked labeled DNA-protein complexes visualized by phosphorImager analysis.
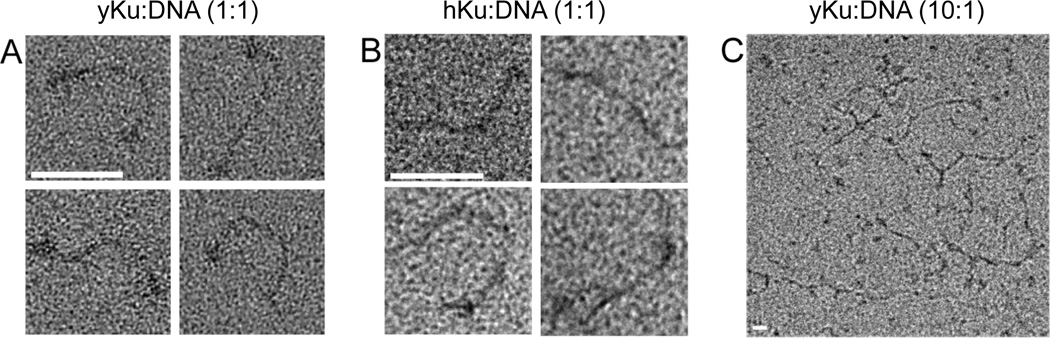
Figure 2. Positive stain images of yKu (Ku) interacting with a linear duplex DNA.
Samples from reactions containing; (A) equimolar amounts of yKu and DNA ends; (B) equimolar amounts of human Ku and DNA ends; (C) 10-fold excess of yKu relative to DNA ends. Scale bars represent 50nm.
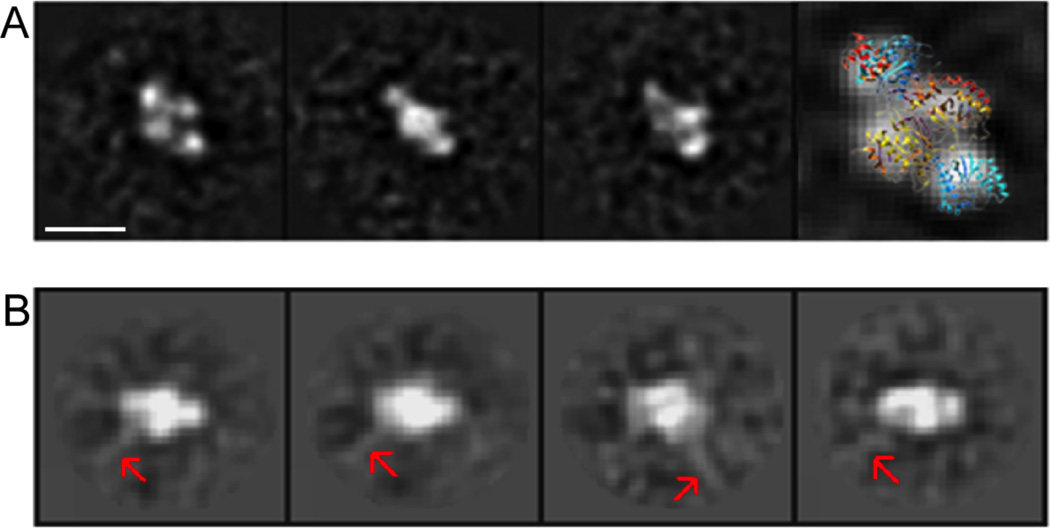
Figure 3. Negative stain images yKu and yKu-DNA complexes.
(A) A gallery of class averages of negatively stained yKu70 particles in the absence of DNA, after 2D single particle data processing as described in Materials and Methods. The last panel on the right shows a spatial representation of 3D atomic structure of human Ku docked over a 2D class average. (B) Selected class averages of yKu particles in the presence of 580 bp DNA (1:1 molar ration Ku to DNA ends). Red arrows indicate DNA fragments protruding out from the yKu particle. Scale bars represent 10 nm.
It has been shown that XRCC4 preferentially binds to DNA duplexes of greater than 200 bp [20], and so it is possible that the Lif1 subunit of the Dnl4-Lif1 has similar DNA binding properties that account for the enhanced formation of DNA-protein complexes with yKu on longer DNA duplexes. Since we were able to trap DNA-protein complexes containing yKu and Dnl4/Lif1 on an oligonucleotide substrate under cross-linking conditions, we performed photocross-linking studies with DNA oligonucleotide substrates which contain a single photoactivatable residue. Using a biotinylated 14 bp duplex with a photoactivatable residue at different positions, we trapped yKu-DNA complexes (Fig. 1C). In the absence of streptavidin, yKu70 and yKu80 were cross-linked in approximately equal amounts along the oligonucleotide. When streptavidin was present, the pattern was different with preferential binding of Ku70 at the unblocked end (Fig. 1 C, compare lanes 1 and 2, and 3 and 4), very little binding in the central region of the oligoncleotide (Fig. 1 C, compare lanes 5 and 6, and 7 and 8), and preferential binding of Ku80 adjacent to the blocked end (Fig. 1 C, compare lanes 9 and 10). This binding pattern is very similar to that with human Ku [19] and is consistent with the crystal structure of a Ku bound to a DNA end [21]. In assays with a 27 bp oligonucleotide substrates, the addition of Dnl4/Lif1 did not alter the pattern of Ku binding and we were unable to detect the cross-linking of either Dnl4 or Lif1 to the DNA substrate (data not shown), suggesting that either formation of DNA-protein complexes containing Dnl4/Lif1 was too inefficient for detection or Dnl4/Lif1 exclusively contacts the yKu ring bound to the DNA end without changing its position.
2.2. Analysis of yKu(Ku) and yKu(Ku)-DNA complexes by electron microscopy using positive staining
Since DNA-protein complexes containing human Ku and DNA ligase IV/XRCC4 have been visualized by atomic force microscopy [15], we utilized electron microscopy to gain higher resolution information about these complexes. In initial studies, DNA-protein complexes formed when yKu was incubated with 580 bp linear duplex DNA with 4 nucleotide cohesive 3’ overhangs at a 1:1 ratio of yKu to DNA ends were visualized by positive staining. Our images revealed that most DNA duplexes had increased density at both ends that presumably corresponds to yKu binding (Fig, 2A). No DNA looping or end-to-end linking of linear DNA monomers was observed under these conditions. Similar results were obtained with human Ku although the binding of human Ku to DNA ends was less efficient than that of yKu (Fig. 2B). Previous studies have shown Ku binding to internal regions of the linear DNA molecules and have ascribed this to Ku translocating along the DNA duplex [22]. Under our experimental conditions (100 mM NaCl and a 1:1 ratio of protein complex to DNA ends), yKu was only seen at DNA ends but, at lower NaCl concentrations, yKu was detected along the length of the DNA duplex (Fig. S1).
Although it has been reported that Ku has end-bridging activity [17, 22], we failed to observe any DNA end bridging at a 1:1 ratio of yKu to DNA ends. This prompted us to examine DNA-protein complex formation at higher concentrations of yKu. At a 10:1 ratio of yKu to DNA ends in 100 mM NaCl, we observed DNA multimers and loops with, 36% of the DNA molecules involved in intermolecular contacts and 22% in intramolecular contacts (Fig. 2C). The presence of bulkier density regions on the linked DNA molecules compared with the density observed at the ends of the linear DNA molecules suggests that the inter- and intramolecular linking of DNA molecules occurs via protein-protein interactions between yKu molecules. In addition, Ku binding occurs along the length of the DNA molecule when it is in 10-fold excess compared with DNA ends (Fig 2C).
2.3. Visualization of yKu(Ku) and yKu(Ku)-DNA complexes by negative staining
To determine the stoichiometry of the complexes formed by yKu at DNA ends, we carried out single particle EM studies of negatively stained samples. In initial studies with yKu alone, 2D classification and alignment of individual particle images gave rise to class averages showing a clear four-lobed structure (Fig. 3A) that matches very well with the shape and size of human Ku determined by x-ray crystallography [21] (PDB code: 1JEY) (Fig. 3A, rightmost panel). The four-lobed yKu structure was also evident in negatively stained samples of yKu that had been pre-incubated with linear duplex DNA at a 1:1 ratio of yKu to DNA ends with no evidence of self association (Fig. 3B). After image processing with multi-reference alignment and classification, we were able to visualize class-averages which resembled those obtained with yKu alone (Fig. 3A), Notably, the class averages obtained in the presence of DNA have a faint line protruding outward at an angle from the yKu density that we attribute to the DNA duplex exiting the yKu ring before it becomes too flexible for visualization after averaging (Fig. 3B). As expected, the putative DNA density only protrudes from side of the Ku density, indicating that Ku binds and caps the DNA end. Furthermore, the orientation of the DNA duplex relative to the yKu ring is consistent with the position of the DNA duplex in the crystal structure of the human Ku-DNA complex [21] (PDB 1JEY).
2.4. DNA-protein complexes formed by yKu(Ku) and Dnl4/Lif1(DNA ligase IV/XRCC4) visualized by positive staining
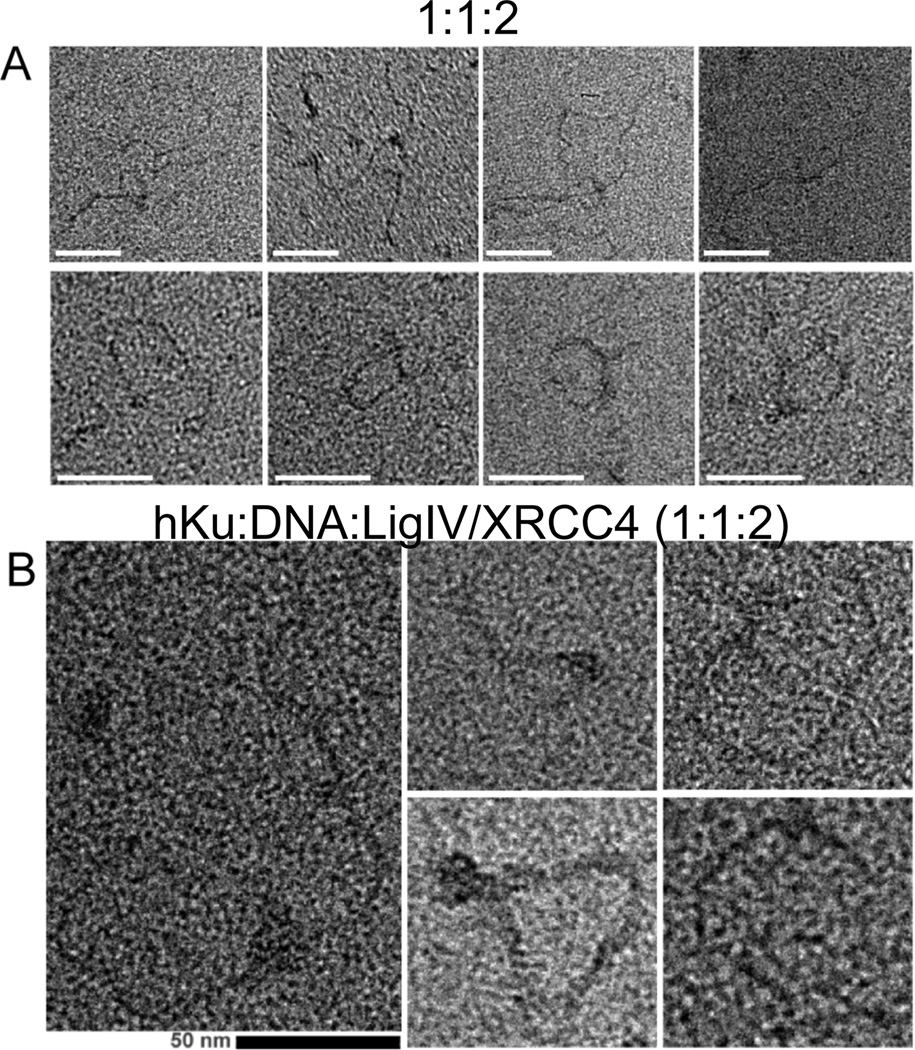
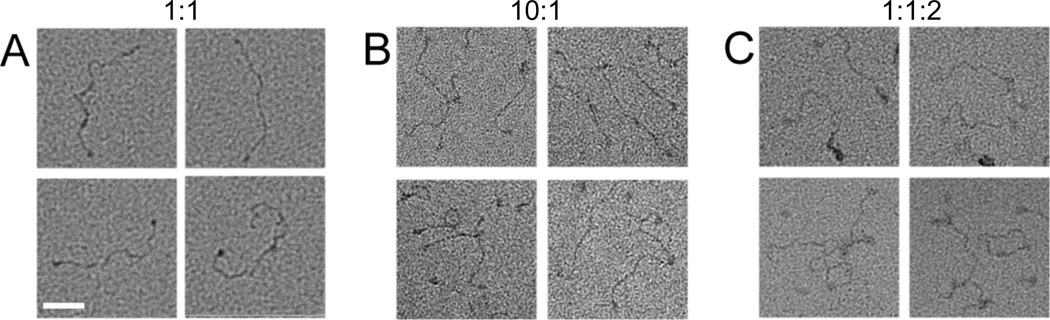
Next we examined the effect of Dnl4/Lif1 on the DNA-protein complexes formed by yKu at a 1:1 ratio of yKu to ligatable DNA ends by positive staining. Preincubation of yKu and Dnl4/Lif1 with linear duplex DNA resulted in linear DNA multimers generated by end-to-end linking of monomers (Fig. 4A, top row) and circular loops, generated by intramolecular linking of the linear DNA duplexes (Fig. 4A, bottom row). Under these conditions, 14% of the DNA molecules were linear monomers, 14% were circular loops and 72% were linear multimers. Similar experiments with human Ku and DNA ligase IV/XRCC4 also resulted in the formation of linear DNA multimers and circular loops albeit to a lesser extent than was observed with the yeast proteins (Fig. 4B). In contrast, no DNA–protein complexes were formed by either Dnl4/Lif1 or DNA ligase IV/XRCC4 alone (data not shown). To eliminate the possibility that the DNA multimers and loops are generated by ligation, similar assays were carried out with the non-ligatable, phosphatase-treated linear DNA duplex. As expected, removal of the terminal 5’ phosphate groups had no effect on the types of DNA-protein complexes formed by yKu at a 1:1 (Fig. 5A) and a 10:1 ratio to DNA ends (Fig. 5B). Notably, the inclusion of Dnl4/Lif1 in reactions containing yKu and DNA ends at a 1:1 ratio resulted in the conversion of the linear monomers capped by yKu into linear DNA multimers and circular loops (Fig. 5C). Similar results were obtained with human Ku and DNA ligase IV/XRCC4 (data not shown). Thus, the Dnl4/Lif1(DNA ligase IV/XRCC4)-dependent end-to–end linking of DNA molecules capped by yKu(Ku) is due to end-bridging rather than ligation.
Figure 4. Positive stain EM of DNA-protein complexes formed by yKu and Dnl4/Lif1 and by human Ku and DNA ligase IV/XRCC4.
A selection of positive stain images showing end-to-end linked linear oligomers (top row) and DNA loops (bottom rows) formed by co-incubation of; (A) yKu and Dnl4/Lif1 with 580 bp linear duplex DNA (1:2:1 molar ratio); (B) Human Ku and DNA ligase IV/XRCC4 with 580 bp linear duplex DNA (1:2:1 molar ratio). Protein complexes appear as bulky density regions at contacts between DNA strands. Scale bars represent 50 nm.
Figure 5. Positive stain images of the complexes formed by yKu and Dnl4/Lif1 with non-ligatable DNA.
Samples from reactions containing; (A) equimolar amounts of yKu and non-ligatable DNA ends; (B) 10-fold excess of yKu relative to non-ligatable DNA ends; (C) yKu and Dnl4/Lif1 with non-ligatable DNA ends (1:2:1 molar ratio). Protein complexes appear as bulky density regions Scale bars represent 50 nm.
2.5. DNA-protein complexes formed by yKu(Ku) and Dnl4/Lif1(DNA ligase IV/XRCC4) visualized by negative staining
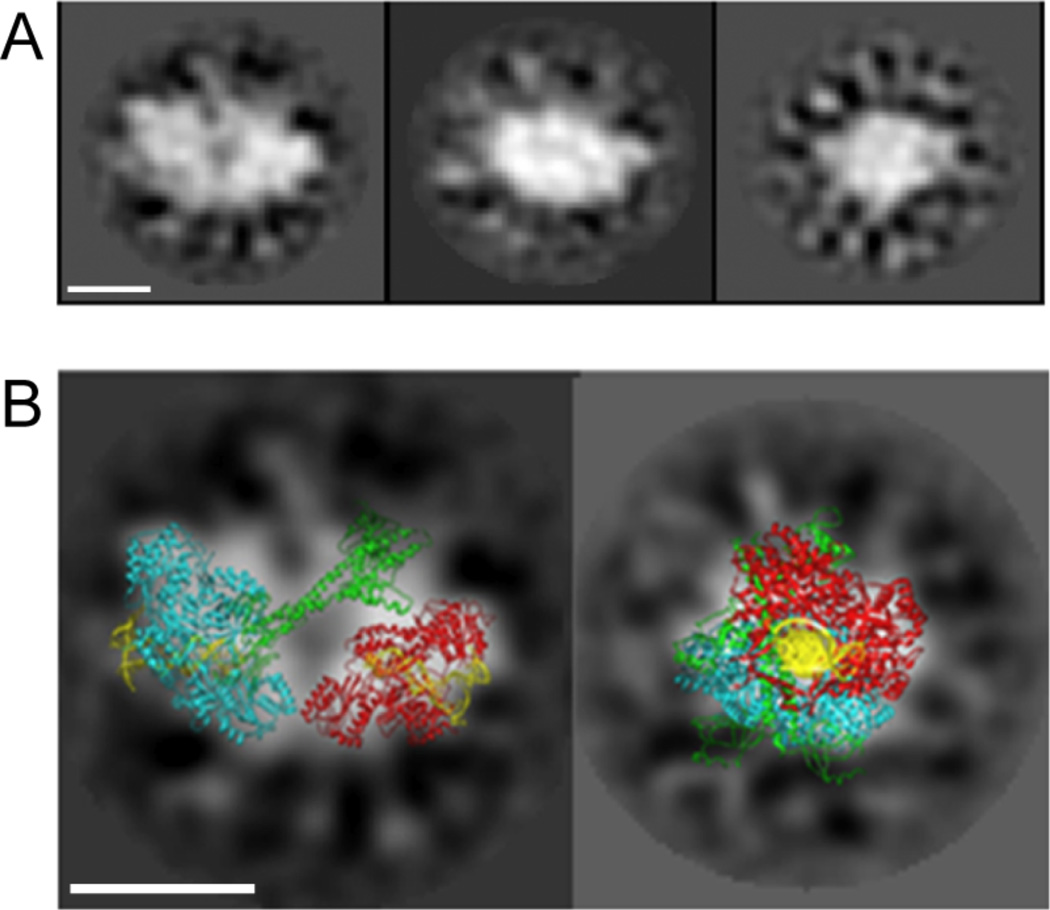
The DNA-protein complexes formed by co-incubation of yKu and Dnl4/Lif1 and DNA were analyzed by single-particle image processing after negative staining (Fig. 6). The class averages obtained identify particles that are much larger than those observed with yKu in the presence and absence of DNA. Under these conditions, approximately 32% of the particles analyzed were significantly larger than those observed with either yKu or Dnl4/Lif1 alone. In Figure 6A, three examples of class averages corresponding to different views of the larger complex are shown. The apparent mass is compatible with two molecules of yKu and at least one Dnl4/Lif1 complex. To gain insights into the architecture of this larger DNA-protein complex, the crystal structure of human Ku with DNA [21] and Dnl4/Lif1 [23] were superimposed onto the best-defined class averages (Fig. 6B). The image on the right is interpreted as a “side” view of the complex, with two yKu70 heterodimers and the ligase complex overlapping one another. The image on the left shows a complex in which two yKu heterodimers with their characteristic 4-lobed densities are linked by additional density that presumably corresponds to one or possibly two (not shown) Dnl4/Lif1 complexes in what we define as a “front” view. This overlay also shows the putative orientation of the two linear DNA duplexes in the NHEJ complex positioned close to each other in an end-to-end fashion within the two yKu rings that are linked via protein-protein interactions with Dnl4/Lif1. It was, however, not possible to determine the exact number of Dnl4/Lif1 complexes contributing to the EM densities by 2D docking, for several reasons; (i) the small number of preferential views that were found; (ii) overlap of densities from the different complexes in 2D, and (iii) only parts of the Dnl4/Lif1 structure is known [23].
Figure 6. Negative stain EM of DNA-protein complexes formed by yKu and Dnl4/Lif1.
(A) Gallery of 2D class averages of the DNA-protein complex formed by yKu and Dnl4/Lif1. (B) Docking of 3D atomic stuctures of human Ku-DNA and Dnl4/Lif1 complexes over 2D class averages from a “front” and a “side” view. Ku proteins are shown as ribbon diagrams in cyan and red, Dnl4/Lif1 in green and DNA fragments are shown in yellow (PDB 1JEQ). The extra density between Ku dimers is mostly attributed to one or possibly two Dnl4/Lif1 complexes.
3. Discussion
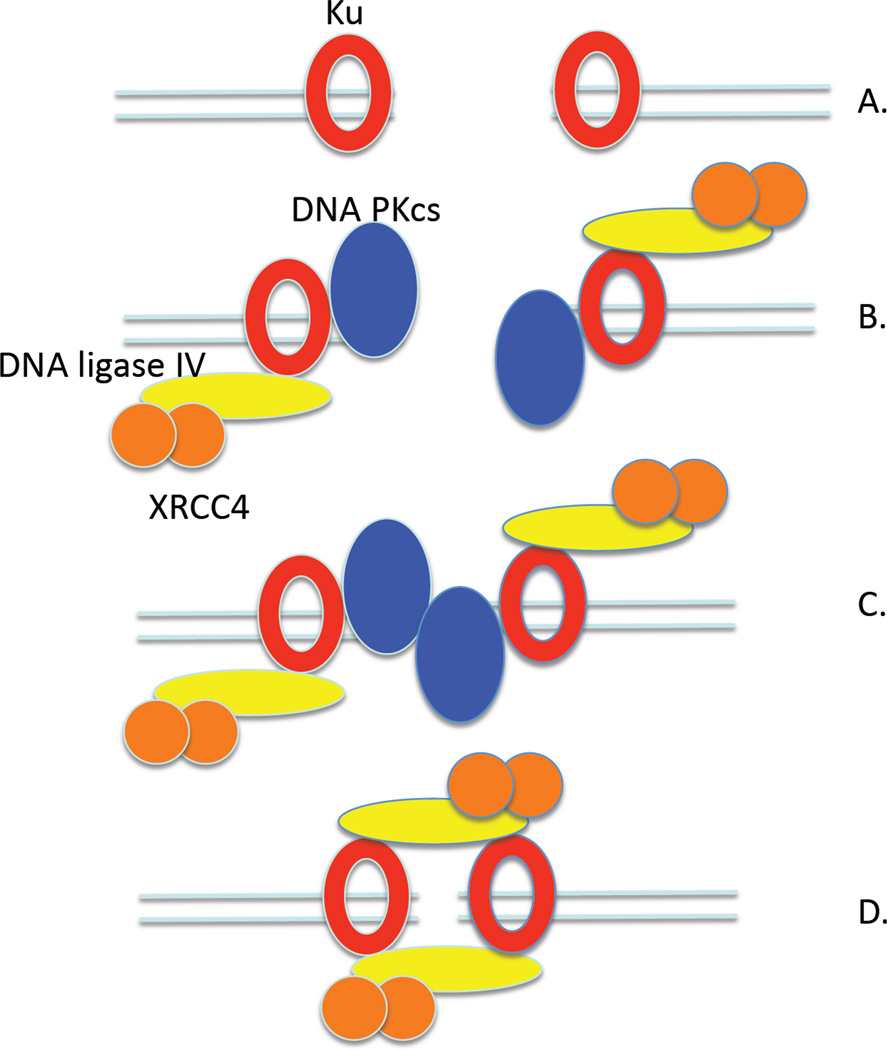
The conserved ring-shaped Ku dimer is not only a key component of the eukaryotic NHEJ DNA repair pathway [2, 7, 8] but also plays a major role in determining repair pathway choice [24]. Genetic inactivation of Ku results in increased repair of DSBs by homologous recombination [11, 25, 26] and an error-prone alternative NHEJ pathway [24]. Ku binds to DNA ends (Fig. 7A), concomitantly inhibiting resection and recruiting other NHEJ factors [9–12, 27]. Surprisingly, it has been shown that Dnl4/Lif1 stabilizes the binding of yKu to in vivo DSBs [11], indicating that the DNA ligase acts throughout the NHEJ reaction from end-binding to ligation (Fig. 7).
Figure 7. Model for repair of a DSB by NHEJ.
(A) Induction of a DSB results in recruitment of Ku (yKu) heterodimers to bind to the DNA ends. (B) The Ku bound at the DNA ends recruits other NHEJ factors, including DNA ligase IV/XRCC4(Dnl4/Lif1) and DNA PKcs in humans. The binding of DNA PKcs results in the inward displacement of the Ku ring along the DNA. (C) End-bridging occurs via interactions between the DNA PKcs molecules (In yeast, end-bridging is mediated by the MRX complex), resulting in activation of the kinase activity of DNA PK. Phosphorylation in trans of the DNA PKcs molecules results in large conformational change in the DNA PKcs molecules and their release from the end-joining complex. (D) The DNA ends are held together by the combined DNA-protein complex in which one or two DNA ligase IV/XRCC4(Dnl4/Lif1) molecules provide a link between the two Ku-bound DNA ends.
Here we have used a combination of biochemical and biophysical approaches to characterize the DNA-protein complexes formed by yKu and Dnl4/Lif1 and by human Ku and DNA ligase IV/XRCC4. In accord with studies on human Ku [19, 21], yKu binds to DNA ends, covering a region of about 12 bp with the yKu70 subunit contacting the DNA closest to the end. As expected [19, 21], visualization of yKu and human Ku by electron microscopy using positive and negative staining showed that, at physiological salt concentrations, yKu and human Ku was predominantly located at DNA ends (Fig. 7A). Free and DNA-bound yKu particles visualized by negative staining were indistinguishable indicating that DNA binding does not induce major conformational changes. A novel feature of our EM reconstruction of the yKu-DNA complex, which was a good fit with the atomic resolution x-ray crystal structure of the human Ku-DNA complex [21] and the EM reconstruction of human Ku [28], was that the DNA strand was visible as it exited the yKu particle.
The end-to-end alignment of DNA molecules mediated by protein-protein interactions is the key step in the repair of DSBs by NHEJ. Although Ku has been reported to have end-bridging activity [17, 22], DNA PKcs and Mre11/Rad50/Xrs2 appear to be the major end-bridging factors in human and yeast NHEJ, respectively [14, 15, 29]. When we incubated yKu with DNA ends at a 1:1 ratio, DNA end bridging was not observed even though the majority of DNA ends were bound by yKu. We did, however, observe intra- and inter-molecular linking of linear DNA molecules at higher concentrations of yKu and also human Ku, suggesting that yKu(Ku) has weak end-bridging activity. When DNA PKcs binds to a DNA end bound by Ku, the Ku ring is translocated inward along the duplex (Fig. 7B), leaving the DNA PKcs subunit at the end [18, 28]. Thus, the DNA PKcs subunit is appropriately positioned to mediate end-bridging (Fig. 7C). We envision a similar scenario in yeast NHEJ, in which the DNA ends are juxtaposed via interactions with Mre11 dimer within the Mre11/Rad50/Xrs2 complex [14, 30]. Since Dnl4/Lif1 is required for the stable binding of yKu to in vivo DSBs [11] and the recruitment of DNA ligase IV/XRCC4 to DSBs is Ku-dependent in human cells [12], we envision that the ligase complex is recruited to the protein complexes at both DNA ends prior to end-bridging (Fig. 7B).
The presence of large protein complexes at the bridged DNA ends presents a potential problem for the subsequent end-processing and ligation reactions. In the case of DNA PKcs, it appears likely that phosphorylation in trans induces a large conformational change in DNA PKcs that results either in the release of DNA PKcs from the ends or the exposure of the DNA ends [31]. We suggest that the weak end bridging activity of Ku and other NHEJ factors contributes to holding the bridged DNA ends together during the latter stages of NHEJ after the departure of the major end-bridging factors (Fig. 7D). Since DNA ligase IV/XRCC4 (Dnl4/Lif1) has intermolecular DNA joining activity [14, 15, 32, 33], this complex must be capable of simultaneously engaging two DNA ends. The linking of linear DNA molecules with cohesive but non-ligatable ends by DNA ligase IV/XRCC4 has been observed by atomic force microscopy [14]. Notably, under conditions where neither yKu or Dnl4/Lif1 alone mediated end bridging, the combination of these factors resulted in the intra- and intermolecular linking of both ligatable and non-ligatable linear DNA molecules. In these reactions, we observed larger protein complexes residing on the DNA that contained two yKu(Ku) molecules linked by additional density that is presumably contributed by Dnl4/Lif1 (DNA ligase IV/XRCC4). Our studies did not definitively determine the number of DNA ligase complexes present in these larger complexes. Furthermore, although yKu and Ku enhance the binding of Dnl4/Lif1 and DNA ligase IV/XRCC4, respectively to DNA ends [11, 13], we were unable to detect a complex of yKu and Dnl4/Lif1 at the DNA end that was significantly different than that formed by yKu alone using DNA footprinting. It is conceivable that Dnl4/Lif1 interacts with the C-terminus of the yKu80 subunit of yKu that protrudes from the yKu ring [9, 21] and does not engage the DNA end until it is aligned for ligation. In the model shown in Figure 7D, the DNA ligase complex is not interacting with the DNA end but instead is positioned to interact with another yKu-bound DNA end with bridging occurring between two DNA ends, each of which is capped by the Ku and DNA ligase complexes. An attractive feature of this arrangement is that there are two DNA ligase molecules to catalyze the two ligation events required to complete the repair of a DSB. Alternatively, it is possible that end bridging occurs as a result of one Dnl4/Lif1 complex engaging two yKu-bound DNA ends.
Our studies have provided important new insights into the conserved complex formed between Ku and the DNA ligase that completes NHEJ at DNA ends. Another NHEJ factor, Nej1 in yeast and XLF in humans, that is structurally related to XRCC4(Lif1) interacts with and stimulates the DNA ligase complex and is recruited to DNA ends in a Ku-dependent manner [34–41]. Notably, Nej1(XLF) stimulates ligations of mismatched DNA ends and the turnover of the DNA ligase complex [33, 42–44]. Further studies are needed to characterize the DNA-protein complex formed by yKu(Ku), Nej1(XLF), and Dnl4/Lif1(DNA ligase IV/XRCC4).
4. Materials and Methods
4.1. Protein Purification
Yeast Ku and Dnl4/Lif1 and human Ku and DNA ligase IV/XRCC4 were purified as described previously [14, 15, 45, 46].
4.2. Electrophoretic Mobility Shift Assay
A 45-mer oligonucleotide was radiolabeled, and after purification using a BioRad P30 spin column, annealed to a complementary 45-mer oligonucleotide. The labeled 45 bp DNA substrate (25 nM) was incubated for 15 min at room temperature with Ku and Dnl4/Lif1, either alone or in combination, in reaction mixtures (20 µl) containing 10 mM HEPES pH 7.5, 100 mM NaCl, 2 mM dithiothreitol, and 5% glycerol. Where indicated, DNA-protein complexes were cross-linked by incubation with glutaraldehyde at a final concentration of 0.2% for 10 minutes at room temperature. After separation by polyacrylamide gel electrophoresis (5% acrylamide v/v), labeled DNA species were visualized in the dried gel by PhosphorImager analysis (Molecular Dynamics).
4.3. Aryl Azide Photocross-linking
To prepare top strand DNA probes (Fig. 1C), oligonucleotides with a single phosphorothioate modification were synthesized and annealed to unmodified, complementary, bottom strand DNA with a biotin group at the 5' end. After gel purification, DNA duplexes were dissolved in 10 mM Tris-HCl, pH 7.9, 1 mM EDTA, and 100 mM NaCl and then radiolabeled at the 5' end of the top strand using [γ-32P] ATP and T4 polynucleotide kinase. Radiolabeled DNAs were incubated for 3 h in the dark at room temperature in a reaction containing 150 nM DNA, 40 mM MOPS, pH 7.0, 60% MeOH, and 12 mM 4-azidophenacyl bromide (Sigma). Unreacted reagents were removed using Biospin P30 columns (BioRad).
Radiolabeled DNA duplexes were incubated with yKu in the presence or absence of streptavidin prior to irradiation with 365 nm ultraviolet light (Stratalinker UV Crosslinker 2400, Stratagene) for 120 s. Reactions were terminated by the addition of SDS-PAGE sample buffer (62.5 mM Tris-HCl, pH 6.8, 5% glycerol, 2% SDS, 5% 2-mercaptoethanol, 1% bromphenol blue) and heating for 4 min at 95°C. Labeled proteins were resolved by 7.5% SDS-PAGE and detected by PhosphorImager analysis (Molecular Dynamics).
4.5. EM sample preparation
Plasmid DNA was digested with Pst1 to generate a 580 bp fragment that was purified using the Qiagen Qiaquick kit after separation by agarose gel electrophoresis. To prevent ligation, the 580 bp fragment was incubated calf intestine alkaline phosphatase and then purified as described above.
Purified human and yKu (2 pmoles or 20 pmoles) were incubated with the 580 bp linear DNA fragment (2 pmoles DNA ends) in 60 µl of 10 mM HEPES pH 7.5, 100 mM NaCl, 3 mM EDTA and 0.05% Tween 20 for 15 minutes at 30°C. yKu/DNA/Dnl4/Lif1 complexes were formed in a stepwise manner. First, 2 pmoles of yKu and 1 pmol of 580 bp linear DNA (2 pmoles of DNA ends) and incubated for 15 minutes at 30°C in the buffer conditions described above. After the addition of 4 pmoles of Dnl4/Lif1, incubation was continued for 15 minutes at 30 °C. The final concentrations for EM were 33 nM yKu, 33 nM DNA ends and, when present, 66 nM Dnl4/Lif1. Reactions with the homologous human proteins were carried under the same conditions.
Samples for electron microscopy observation were prepared over holey carbon grids coated with a thin layer of continuous carbon and following glow-discharge treatment. For negative staining, 4 µl of yKu-DNA or yKu-DNA-Dnl4/Lif1 sample was deposited on the grid for 30 seconds to 1 minute, then stained in four consecutive 75 µl drops of 3% uranyl acetate stain prior to blotting completely dry.
Positive stain techniques were adapted from Dubochet and colleagues [47]. yKu-DNA or yKu-DNA-Dnl4/Lif1 samples (4 µl) were incubated on the grid for 30 seconds, then stained with 4µl of 1% uranyl acetate for 30s. Excess stain was blotted off before washing the sample grid with five 75 µl successive drops of water on Parafilm. Finally, the grid was blotted dry.
4.6. Microscopy and Image Processing
Samples were observed in a Tecnai 12 transmission electron microscope (FEI) operating at 120kV. Micrographs were recorded at a magnification of 49,000× using low dose search and focusing conditions. Images were digitized using a Nikon SuperCool Scan 8000 scanner to a final 2.54 Å/pixel at the specimen scale. For the single particle analysis of negatively stained samples, approximately 4,000 images of complexes were picked using the boxer software (EMAN package [48]) for the yKu-DNA-Dnl4/Lif1 data set and approximately 2,000 images of complexes were boxed for the yKu-DNA data set. Initial 2D analysis of the data was carried out using a combination of automatic classification utilizing an in-house implementation of the self-organizing network algorithm [48] (V.H. Ramey, http://cryoem.berkeley.edu), and a classical multi-reference alignment scheme in IMAGIC [49] (Image Science Software GmbH, Berlin, Germany). Briefly, classes were chosen from the data through a genetic algorithm where the most viable crossing survives. The resulting class-averages then served as references to align the particle data in IMAGIC, using cross correlation as scoring function to find the best matching reference, angle and shifts in the x and y directions. The maximum of cross-correlation defined the new orientation that was then adopted for each particle before undergoing the next cycle or classification. Repeating this process for several cycles, particles were iteratively aligned with respect to each other and classified into a smaller and smaller number of classes. Particles were initially classified into 500 classes, and iteratively processed to obtain a final number of 10 classes after 10 cycles, which represented the most distinct views of the complexes. In the case of the yKu-DNA-Dnl4/Lif1 data set, a first cycle of alignment and classification was run to separate classes that were attributed to Ku dimers or Dnl4/Lif1 complexes alone. This could be determined by comparison with control data sets (see Fig. 3, data not shown for Dnl4/Lif1) and a published EM structure for DNA ligase IV/XRCC4 [50]. Larger classes, containing NHEJ complexes and representing 32% of the data, were subsequently selected and analyzed again separately.
The atomic model for the human Ku-DNA complex and Dnl4/Lif1, (PDB codes: 1JEY and 1Z56 respectively) were manually positioned using Chimera [51] (supported by NIH grant P41 RR-01081) to match the views corresponding to our class averages from 2D images. The image of the best “2D docking” was then saved with a transparent background and superimposed with the corresponding class-average in Photoshop (Adobe).
Highlights.
To resolve DSBs DNA ends are brought together by protein complexes
Ku heterodimers recognize specifically DNA ends as shown by EM
Ku alone is not sufficient for DNA end bridging but requires Dnl4/Lif1 (Ligase IV/XRCC4)
Ligase IV mediates end-bridging by bringing two Ku-bound DNA ends.
Supplementary Material
Figure S1. “Chains” of yKu binding along dsDNA in lower salt concentration buffer conditions (50mM NaCl). The length is consistent with a 580bp DNA strand.
Figure S2. At higher concentration of yKu (10:1:2 molar ratio), the same types of DNA multiplexes are formed, but protein complexes also bind along the DNA.
Acknowledgements
This work was supported by National Institutes of Health Grants GM47251 (to A.E.T.), and CA92584 (to A.E.T. and E.N.) E.N. is a Howard Hughes Medical Institute Investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare there are no conflicts of interest.
References
- 1.San Filippo J, Sung P, Klein H. Mechanism of eukaryotic homologous recombination. Annu Rev Biochem. 2008;77:229–257. doi: 10.1146/annurev.biochem.77.061306.125255. [DOI] [PubMed] [Google Scholar]
- 2.Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem. 79:181–211. doi: 10.1146/annurev.biochem.052308.093131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171–182. doi: 10.1016/s0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson DO, Sekiguchi JM, Chang S, Frank KM, Gao Y, DePinho RA, Alt FW. The nonhomologous end-joining pathway of DNA repair is required for genomic stability and the suppression of translocations. Proc Natl Acad Sci U S A. 2000;97:6630–6633. doi: 10.1073/pnas.110152897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharpless NE, Ferguson DO, O'Hagan RC, Castrillon DH, Lee C, Farazi PA, Alson S, Fleming J, Morton CC, Frank K, Chin L, Alt FW, DePinho RA. Impaired nonhomologous end-joining provokes soft tissue sarcomas harboring chromosomal translocations, amplifications, and deletions. Mol Cell. 2001;8:1187–1196. doi: 10.1016/s1097-2765(01)00425-7. [DOI] [PubMed] [Google Scholar]
- 6.Vogel H, Lim DS, Karsenty G, Finegold M, Hasty P. Deletion of Ku86 causes early onset of senescence in mice. Proc Natl Acad Sci U S A. 1999;96:10770–10775. doi: 10.1073/pnas.96.19.10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hefferin ML, Tomkinson AE. Mechanism of DNA double-strand break repair by non-homologous end joining. DNA Repair (Amst) 2005;4:639–648. doi: 10.1016/j.dnarep.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Weterings E, Chen DJ. The endless tale of non-homologous end-joining. Cell Res. 2008;18:114–124. doi: 10.1038/cr.2008.3. [DOI] [PubMed] [Google Scholar]
- 9.Palmbos PL, Daley JM, Wilson TE. Mutations of the Yku80 C terminus and Xrs2 FHA domain specifically block yeast nonhomologous end joining. Mol Cell Biol. 2005;25:10782–10790. doi: 10.1128/MCB.25.24.10782-10790.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmbos PL, Wu D, Daley JM, Wilson TE. Recruitment of Saccharomyces cerevisiae Dnl4-Lif1 complex to a double-strand break requires interactions with Yku80 and the Xrs2 FHA domain. Genetics. 2008;180:1809–1819. doi: 10.1534/genetics.108.095539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Hefferin ML, Chen L, Shim EY, Tseng HM, Kwon Y, Sung P, Lee SE, Tomkinson AE. Role of Dnl4-Lif1 in nonhomologous end-joining repair complex assembly and suppression of homologous recombination. Nat Struct Mol Biol. 2007;14:639–646. doi: 10.1038/nsmb1261. [DOI] [PubMed] [Google Scholar]
- 12.Mari PO, Florea BI, Persengiev SP, Verkaik NS, Bruggenwirth HT, Modesti M, Giglia-Mari G, Bezstarosti K, Demmers JA, Luider TM, Houtsmuller AB, van Gent DC. Dynamic assembly of end-joining complexes requires interaction between Ku70/80 and XRCC4. Proc Natl Acad Sci U S A. 2006;103:18597–18602. doi: 10.1073/pnas.0609061103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nick McElhinny SA, Snowden CM, McCarville J, Ramsden DA. Ku recruits the XRCC4-ligase IV complex to DNA ends. Mol Cell Biol. 2000;20:2996–3003. doi: 10.1128/mcb.20.9.2996-3003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Trujillo K, Ramos W, Sung P, Tomkinson AE. Promotion of Dnl4-catalyzed DNA end-joining by the Rad50/Mre11/Xrs2 and Hdf1/Hdf2 complexes. Mol Cell. 2001;8:1105–1115. doi: 10.1016/s1097-2765(01)00388-4. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Trujillo K, Sung P, Tomkinson AE. Interactions of the DNA ligase IV-XRCC4 complex with DNA ends and the DNA-dependent protein kinase. J Biol Chem. 2000;275:26196–26205. doi: 10.1074/jbc.M000491200. [DOI] [PubMed] [Google Scholar]
- 16.Kysela B, Doherty AJ, Chovanec M, Stiff T, Ameer-Beg SM, Vojnovic B, Girard PM, Jeggo PA. Ku stimulation of DNA ligase IV-dependent ligation requires inward movement along the DNA molecule. J Biol Chem. 2003;278:22466–22474. doi: 10.1074/jbc.M303273200. [DOI] [PubMed] [Google Scholar]
- 17.Ramsden DA, Gellert M. Ku protein stimulates DNA end joining by mammalian DNA ligases: a direct role for Ku in repair of DNA double-strand breaks. Embo J. 1998;17:609–614. doi: 10.1093/emboj/17.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoo S, Dynan WS. Geometry of a complex formed by double strand break repair proteins at a single DNA end: recruitment of DNA-PKcs induces inward translocation of Ku protein. Nucleic Acids Res. 1999;27:4679–4686. doi: 10.1093/nar/27.24.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoo S, Kimzey A, Dynan WS. Photocross-linking of an oriented DNA repair complex. Ku bound at a single DNA end. J Biol Chem. 1999;274:20034–20039. doi: 10.1074/jbc.274.28.20034. [DOI] [PubMed] [Google Scholar]
- 20.Modesti M, Hesse JE, Gellert M. DNA binding of Xrcc4 protein is associated with V(D)J recombination but not with stimulation of DNA ligase IV activity. EMBO J. 1999;18:2008–2018. doi: 10.1093/emboj/18.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker JR, Corpina RA, Goldberg J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature. 2001:607–614. doi: 10.1038/35088000. [DOI] [PubMed] [Google Scholar]
- 22.Cary RB, Peterson SR, Wang J, Bear DG, Bradbury EM, Chen DJ. DNA looping by Ku and the DNA-dependent protein kinase. Proc Natl Acad Sci U S A. 1997;94:4267–4272. doi: 10.1073/pnas.94.9.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dore AS, Furnham N, Davies OR, Sibanda BL, Chirgadze DY, Jackson SP, Pellegrini L, Blundell TL. Structure of an Xrcc4-DNA ligase IV yeast ortholog complex reveals a novel BRCT interaction mode. DNA Repair (Amst) 2006;5:362–368. doi: 10.1016/j.dnarep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Fattah F, Lee EH, Weisensel N, Wang Y, Lichter N, Hendrickson EA. Ku regulates the non-homologous end joining pathway choice of DNA double-strand break repair in human somatic cells. PLoS Genet. 6:e1000855. doi: 10.1371/journal.pgen.1000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clikeman JA, Khalsa GJ, Barton SL, Nickoloff JA. Homologous recombinational repair of double-strand breaks in yeast is enhanced by MAT heterozygosity through yKU-dependent and -independent mechanisms. Genetics. 2001:579–589. doi: 10.1093/genetics/157.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pierce AJ, Hu P, Han M, Ellis N, Jasin M. Ku DNA end-binding protein modulates homologous repair of double-strand breaks in mammalian cells. Genes Dev. 2001;15:3237–3242. doi: 10.1101/gad.946401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yano K, Morotomi-Yano K, Wang SY, Uematsu N, Lee KJ, Asaithamby A, Weterings E, Chen DJ. Ku recruits XLF to DNA double-strand breaks. EMBO Rep. 2008;9:91–96. doi: 10.1038/sj.embor.7401137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivera-Calzada A, Spagnolo L, Pearl LH, Llorca O. Structural model of full-length human Ku70-Ku80 heterodimer and its recognition of DNA and DNA-PKcs. EMBO Rep. 2007;8:56–62. doi: 10.1038/sj.embor.7400847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeFazio LG, Stansel RM, Griffith JD, Chu G. Synapsis of DNA ends by DNA-dependent protein kinase. Embo J. 2002;21:3192–3200. doi: 10.1093/emboj/cdf299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams RS, Moncalian G, Williams JS, Yamada Y, Limbo O, Shin DS, Groocock LM, Cahill D, Hitomi C, Guenther G, Moiani D, Carney JP, Russell P, Tainer JA. Mre11 dimers coordinate DNA end bridging and nuclease processing in double-strand-break repair. Cell. 2008;135:97–109. doi: 10.1016/j.cell.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammel M, Yu Y, Mahaney BL, Cai B, Ye R, Phipps BM, Rambo RP, Hura GL, Pelikan M, So S, Abolfath RM, Chen DJ, Lees-Miller SP, Tainer JA. Ku and DNA-dependent protein kinase dynamic conformations and assembly regulate DNA binding and the initial non-homologous end joining complex. J Biol Chem. 285:1414–1423. doi: 10.1074/jbc.M109.065615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X, Ballin D, Della-Maria J, Tsai M-S, White EJ, Tomkinson AE, Wilson GM. Distinct kinetcis of humna DNA ligases I, IIIa, IIIb and IV reveal direct DNA sensing ability and differential physiological functions in DNA repair. DNA Repair (Amst) 2009;8:961–968. doi: 10.1016/j.dnarep.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Tomkinson AE. Yeast Nej1 is a key participant in the initial end binding and final ligation steps of nonhomologous end joining. J Biol Chem. 286:4931–4940. doi: 10.1074/jbc.M110.195024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahnesorg P, Smith P, Jackson SP. XLF interacts with the XRCC4-DNA ligase IV complex to promote DNA nonhomologous end-joining. Cell. 2006;124:301–313. doi: 10.1016/j.cell.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 35.Andres SN, Modesti M, Tsai CJ, Chu G, Junop MS. Crystal structure of human XLF: a twist in nonhomologous DNA end-joining. Mol Cell. 2007;28:1093–1101. doi: 10.1016/j.molcel.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 36.Buck D, Malivert L, de Chasseval R, Barraud A, Fondaneche MC, Sanal O, Plebani A, Stephan JL, Hufnagel M, le Deist F, Fischer A, Durandy A, de Villartay JP, Revy P. Cernunnos, a novel nonhomologous end-joining factor, is mutated in human immunodeficiency with microcephaly. Cell. 2006;124:287–299. doi: 10.1016/j.cell.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 37.Deshpande RA, Wilson TE. Modes of interaction among yeast Nej1, Lif1 and Dnl4 proteins and comparison to human XLF, XRCC4 and Lig4. DNA Repair (Amst) 2007;6:1507–1516. doi: 10.1016/j.dnarep.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kegel A, Sjostrand JO, Astrom SU. Nej1p, a cell type-specific regulator of nonhomologous end joining in yeast. Curr Biol. 2001;11:1611–1617. doi: 10.1016/s0960-9822(01)00488-2. [DOI] [PubMed] [Google Scholar]
- 39.Ooi SL, Shoemaker DD, Boeke JD. A DNA microarray-based genetic screen for nonhomologous end-joining mutants in Saccharomyces cerevisiae. Science. 2001;294:2552–2556. doi: 10.1126/science.1065672. [DOI] [PubMed] [Google Scholar]
- 40.Valencia M, Bentele M, Vaze MB, Herrmann G, Kraus E, Lee SE, Schar P, Haber JE. NEJ1 controls non-homologous end joining in Saccharomyces cerevisiae. Nature. 2001;414:666–669. doi: 10.1038/414666a. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Chirgadze DY, Bolanos-Garcia VM, Sibanda BL, Davies OR, Ahnesorg P, Jackson SP, Blundell TL. Crystal structure of human XLF/Cernunnos reveals unexpected differences from XRCC4 with implications for NHEJ. Embo J. 2008;27:290–300. doi: 10.1038/sj.emboj.7601942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu H, Pannicke U, Schwarz K, Lieber MR. Length-dependent binding of human XLF to DNA and stimulation of XRCC4.DNA ligase IV activity. J Biol Chem. 2007;282:11155–11162. doi: 10.1074/jbc.M609904200. [DOI] [PubMed] [Google Scholar]
- 43.Riballo E, Woodbine L, Stiff T, Walker SA, Goodarzi AA, Jeggo PA. XLF-Cernunnos promotes DNA ligase IV-XRCC4 re-adenylation following ligation. Nucleic Acids Res. 2009;37:482–492. doi: 10.1093/nar/gkn957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai CJ, Kim SA, Chu G. Cernunnos/XLF promotes the ligation of mismatched and noncohesive DNA ends. Proc Natl Acad Sci U S A. 2007;104:7851–7856. doi: 10.1073/pnas.0702620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen X, Pascal J, Vijayakumar S, Wilson GM, Ellenberger T, Tomkinson AE. Human DNA ligases I, III, and IV-purification and new specific assays for these enzymes. Methods Enzymol. 2006;409:39–52. doi: 10.1016/S0076-6879(05)09003-8. [DOI] [PubMed] [Google Scholar]
- 46.Tseng HM, Tomkinson AE. A physical and functional interaction between yeast Pol4 and Dnl4-Lif1 links DNA synthesis and ligation in nonhomologous end joining. J Biol Chem. 2002;277:45630–45637. doi: 10.1074/jbc.M206861200. [DOI] [PubMed] [Google Scholar]
- 47.Dubochet J, Ducommun M, Zollinger M, Kellenberger E. A new preparation method for dark-field electron microscopy of biomacromolecules. J Ultrastruct Res. 1971;35:147–167. doi: 10.1016/s0022-5320(71)80148-x. [DOI] [PubMed] [Google Scholar]
- 48.Ludtke SJ, Baldwin PR, Chiu W. EMAN: semiautomated software for high-resolution single-particle reconstructions. J. Struct. Biol. 1999;128:82–97. doi: 10.1006/jsbi.1999.4174. [DOI] [PubMed] [Google Scholar]
- 49.van Heel M, Harauz G, Orlova EV, Schmidt R, Schatz M. A new generation of the IMAGIC image processing system. J Struct Biol. 1996;116:17–24. doi: 10.1006/jsbi.1996.0004. [DOI] [PubMed] [Google Scholar]
- 50.Recuero-Checa MA, Dore AS, Arias-Palomo E, Rivera-Calzada A, Scheres SH, Maman JD, Pearl LH, Llorca O. Electron microscopy of Xrcc4 and the DNA ligase IV-Xrcc4 DNA repair complex. DNA Repair (Amst) 2009;8:1380–1389. doi: 10.1016/j.dnarep.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 51.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. “Chains” of yKu binding along dsDNA in lower salt concentration buffer conditions (50mM NaCl). The length is consistent with a 580bp DNA strand.
Figure S2. At higher concentration of yKu (10:1:2 molar ratio), the same types of DNA multiplexes are formed, but protein complexes also bind along the DNA.