Abstract
Background
Depression is a distressing side effect of cancer and its treatment. In the general population, exercise is an effective antidepressant.
Objective
We conducted a systematic review and meta-analysis to determine the antidepressant effect of exercise in cancer survivors.
Data Sources
In May, 2011, we searched MEDLINE, PsycInfo, EMBASE, CINAHL, CDSR, CENTRAL, AMED, Biosis Previews, and Sport Discus, and citations from relevant papers and reviews.
Study Eligibility Criteria
We included randomized controlled trials (RCTs) comparing exercise interventions to usual care in cancer survivors, utilizing a self-report inventory or clinician rating to assess depressive symptoms, and reporting symptoms pre- and post-intervention.
Study Appraisal
7,042 study titles were identified and screened, with 15 RCTs included.
Synthesis Methods
Effect sizes (ES) were reported as mean change scores. The Q test was conducted to evaluate heterogeneity of ES. Potential moderator variables were evaluated with examination of scatter plots and Wilcoxon rank-sum or Kruskal-Wallis tests.
Results
The overall ES, under a random effects model, was −0.22 (CI −0.43, −0.09, p = 0.04). Significant moderating variables (ps < .05) were exercise location, exercise supervision, and exercise duration.
Limitations
Only one study identified depression as the primary endpoint.
Conclusions
Exercise has modest positive effects on depressive symptoms with larger effects for programs that were supervised or partially supervised, not performed at home, and at least 30 minutes in duration.
Impact
Our results complement other studies showing that exercise is associated with reduced pain and fatigue and with improvements in quality of life among cancer survivors.
Keywords: physical activity, mental health, cancer survivorship
Depression is a distressing side-effect of cancer and cancer treatment and its prevalence varies by cancer site. Rates of depression in head and neck cancer tend to be the highest (25%–52%), while pancreatic, liver, colon, lung, brain, bladder/kidney, prostate, and Hodgkin lymphoma are all associated with lower rates ranging from 7%–9.7% (1–3). The prevalence of depression among breast cancer survivors ranges from 1.5%–46% (1, 4, 5).
Depression is characterized by feelings of sadness, hopelessness, changes in sleep and appetite, psychomotor retardation, and withdrawal from social contact. These cause reduced quality of life (QOL), impaired social and occupational functioning, and intermittent bouts of suffering. Depression is also associated with obesity, diabetes, and the development of cardiovascular disease (6–8). For some, clinical depression is also associated with non-compliance with cancer treatment and reduced survival (9). Thus, depression negatively affects the physical and psychological health of many survivors.
Several factors contribute to the development of depression in cancer survivors. Some have poor psychological adjustment to specific symptoms (e.g., sexual, bowel, fatigue), to the severity of symptoms (e.g., pain, fatigue), to the treatment itself (e.g., surgery and disfigurement in head, neck, and breast cancer), or to a poor cancer prognosis (10–13). Likewise, chemotherapy causes hair loss, nausea, weight gain, and effects on fertility and sexuality that may be perceived as distressing (14–16). For others, treatments cause hypothyroidism, electrolyte imbalances, or anemia that can increase the risk of a depressive episode (11). Agents such as steroids and interferon are associated with the development of depression and estrogen depleting interventions may alter serotonin, thus increasing depression risk (14). Finally, lifestyle factors such as tobacco and alcohol use can also contribute to depression risk, while a lack of emotional and social support can leave others feeling isolated and alone (11).
Current treatments for depression in cancer survivors include pharmacological interventions and psychotherapy. For many, these treatments are safe, effective and provide significant benefit. For others, they may have limited usefulness because of personal, behavioral, or biological factors. For example, those with head and neck cancer may have impairment in their ability to communicate, making psychotherapy more difficult (11). Similarly, pharmacological agents such as selective serotonin reuptake inhibitors (SSRIs) may be contraindicated for some survivors undergoing certain anti-hormonal therapies (17). Therefore, while existing treatments for depression benefit many cancer survivors, they do not benefit all and may have negative side effects.
Exercise has been identified as a treatment that may provide symptom relief for depression, as well as improve physical health outcomes in cancer survivors (18). In the general population, exercise is an effective antidepressant. Meta-analytic studies indicate that the effect size (ES) of exercise on depression is large, ranging from −0.72 to −1.4 (19–21). Individuals with moderate and more severe depression benefit similarly and exercise is equally effective for men and women across a wide range of ages. Exercise effects are comparable to psychotherapy and medication, particularly for those with mild to moderate depression (19, 20).
No previous meta-analysis has focused on the effects of exercise on depression, as a primary endpoint, in cancer survivors. Some meta-analyses have included depression but they have been limited by a broad definition of depression measures (e.g., emotional well-being, psychological distress, mood), were restricted to a single cancer site, included nonrandomized trials, or have not included more recent studies (22–26). The aim of this meta-analysis was to evaluate the current literature on the antidepressant effects of exercise in cancer survivors. Our review is more comprehensive in terms of the types of cancer included, as well as a more direct examination of the antidepressant effects of exercise. We hypothesized that exercise interventions would reduce depressive symptoms in cancer survivors relative to usual care. As a secondary exploratory aim, we examined potential moderating variables related to participant, cancer, and exercise characteristics.
Method
Search Strategy
We searched the following electronic databases to May 2011: MEDLINE, MEDLINE—In Process, PsycInfo, EMBASE, CINAHL, Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CENTRAL), Allied and Complementary Medicine (AMED), Biosis Previews, and Sport Discus. We used terms related to cancer (e.g., neoplasm, tumor, cancer), exercise (e.g., exercise, physical activity, yoga, strength training), and depression (e.g., cancer-related depression, quality of life, dysthymia). For example, we searched OVID MEDLINE with the following keywords: (cancer OR neoplasm) AND (exercise OR physical activity) AND (depression OR depressive disorder). We also hand-searched the reference lists of potentially relevant studies and of relevant review articles of exercise, cancer, and quality of life.
Selection Criteria
Studies were considered eligible for inclusion if 1) they were RCTs of adults diagnosed with cancer, 2) compared an exercise program with usual care, 3) the exercise program was chronic in nature (i.e., at least 4 weeks in duration), rather than studies examining acute bouts of exercise, 4) reported depressive symptoms pre- and post-intervention, 5) utilized a depression inventory or a clinician interview to quantify depressive symptoms, and 6) were published in English. Because “distress” is less well-defined and is often used to represent many conceptualizations of emotion and because an overall sense of “well-being” is often independent of depressed mood, we excluded studies utilizing QOL inventories or mood scales to assess depressive symptoms as these types of inventories measure constructs that are theoretically different (13, 27). Likewise, while we acknowledge that depressive symptoms often correlate highly with other constructs such as anxiety, negative affect, and stress, depression is thought to be distinct from these other constructs and, as a result, we limited inclusion to those studies that assessed depressive symptoms.
Data Extraction
Two reviewers (LLC & MHV) screened the titles and abstracts of papers that seemed potentially eligible and then reviewed those appearing relevant. There were no disagreements between reviewers regarding which papers were eligible for inclusion. Data extraction was conducted independently by both reviewers and disagreements were resolved by consensus.
Participant, cancer, intervention, and outcome assessment characteristics were extracted and coded. Participant data included: 1) age, 2) % female, 3) % Caucasian, 4) level of education (mean years of school or % high school or less), and 5) recruitment source. Cancer-related information included: 1) cancer site (breast, other), 2) cancer stage (nometastatic, other), and 3) treatment status at baseline (in primary treatment, other). Exercise intervention information included: 1) exercise format (group or individual), 2) type of delivery (in person, phone, web-based, other), 3) type of exercise program (aerobic, other), 4) mode of exercise (walking, other), 5) supervision of exercise (supervised, unsupervised, mixed supervision), 6) location of exercise (home, other), 7) exercise intervention length (≤ 12 weeks, > 12 weeks), 8) exercise session duration (≤ 30 min, > 30 min), and 9) exercise intensity. Finally, outcome assessment characteristics included: 1) primary outcome of interest reported in study, 2) depression inventory utilized, and 3) time point of follow-up assessment. No assumptions were made regarding these characteristics. Study quality was also coded using the PEDro Scale (28), which is widely used to rate the quality of RCTs. PEDro scores are summarized and high-quality studies are those with scores from 6 to 11, fair quality from 4 to 5, and poor quality less than 4.(29) Items 2–9 on the PEDro scale assess internal validity.(30) For studies not providing enough information to calculate an effect size, study authors were contacted for additional information.
Statistical Considerations
Effect sizes were obtained from the RCTs and were reported in terms of mean change scores (Cohen’s d). Some studies had available change scores (and standard deviations). For those that did not, we calculated change scores by subtracting the mean baseline score from the mean follow-up score and calculated the change score standard deviation by using:
Here, YBL and YFU are the mean baseline and follow-up scores. From this equation, we could first obtain Cov(YBL, YFU) from studies with an already calculated standard deviation or variance for the change scores, and then apply this value to compute the standard deviation for those studies that did not report change scores. Furthermore, separate Cov(YBL, YFU) values were obtained for exercise and control groups. Negative ES values indicated that intervention participants had improved depressive symptoms relative to the control group.
Meta-analysis was then conducted to examine the effect of exercise on depression. The Q test for heterogeneity was used to evaluate the assumption that ES were from the same population (31). Differences in ES among studies were analyzed both under a fixed-effects and a random-effects model, as recommended by Dersimonian and Laird (32). However, in order to acknowledge that there are likely person level and intervention level characteristics that may contribute to variation in the magnitude of the effect, the random-effects model ES is reported as our primary analysis (33). Publication bias was assessed via Egger’s test and graphical examination of a funnel plot of ES (34). All meta-analyses were conducted using Comprehensive Meta Analysis, Version 2.2.044.
To determine if any study variables moderated the effect of exercise on depression, differences in ES were evaluated with graphical examination of scatter plots and using the Wilcoxon rank-sum test for moderator variables with two levels and the Kruskal-Wallis test for moderator variables with three levels. Assessments involving these moderator variables were conducted in R 2.11.1.
Results
Preliminary abstract screening yielded 60 papers that appeared potentially appropriate (Figure 1). After review, 21 papers remained potentially relevant, with most being excluded because they utilized a mood inventory or QOL scale, were not RCTs, or reported secondary outcome data (e.g., adherence) rather than depression data. Of those 21, three studies of yoga were excluded because they employed relaxing, meditative forms of yoga rather than more physically active forms of yoga (35–37). Two studies were excluded because insufficient depression data was presented in the article and the authors no longer had access to the data (38, 39). One study was excluded because it did not utilize a usual care or no treatment control group, but utilized a group psychotherapy control group instead (40). No studies were excluded due to utilizing an exercise intervention of less than four weeks.
Figure 1.
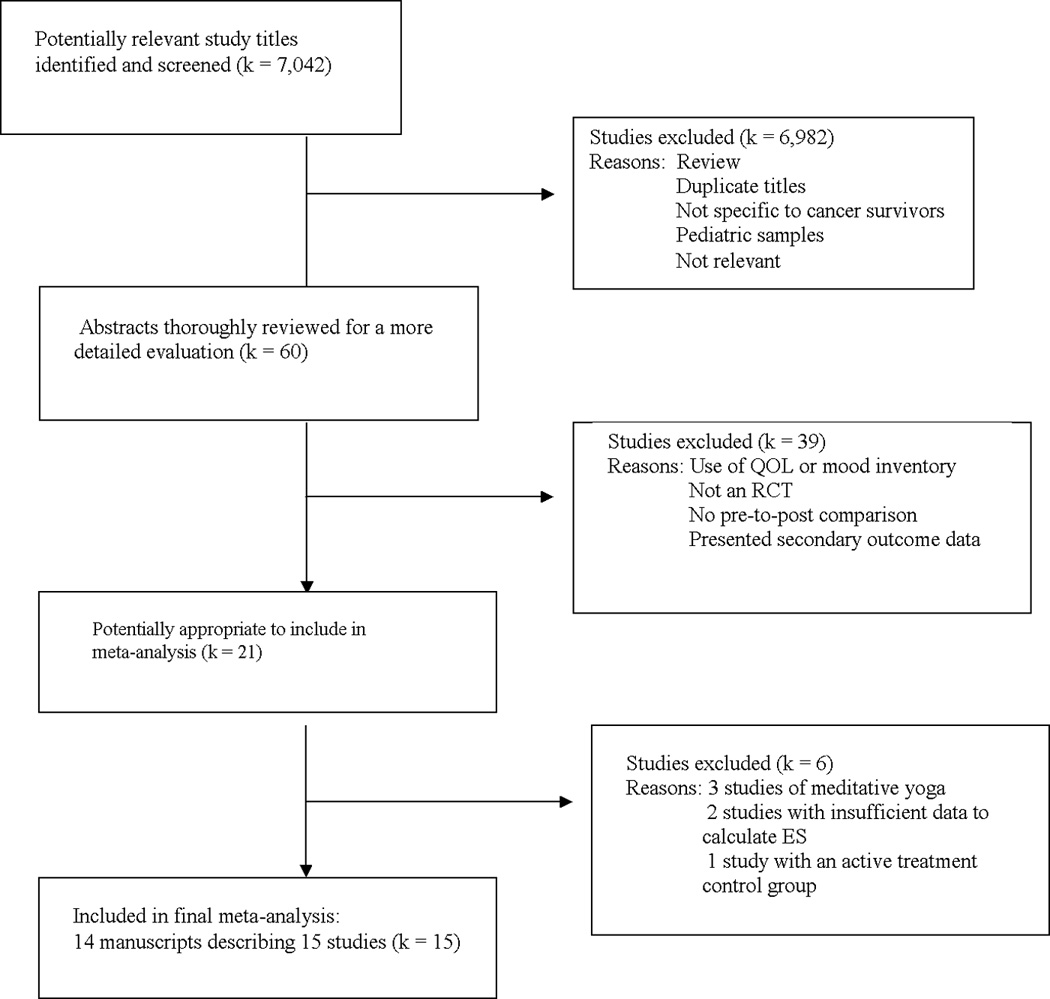
Flow diagram of study selection.
There were four studies in which multiple effect sizes were generated but only one comparison was included in our analyses. One study included a placebo exercise comparison group and a usual care group (41) and another study included a telephone counseling comparison group and a usual care group (42). Only the ES comparing exercise to usual care were included. One study compared an aerobic exercise program and a strength training program to usual care (43) and, thus, two ES were generated. Because there were no other studies that included strength training as a separate intervention arm, only the ES generated from the comparison of aerobic exercise to control was utilized. Further, one study (44) had three arms eligible for inclusion. This study utilized a control arm, an exercise intervention arm in which participants received the exercise intervention during their cancer treatment, and an exercise intervention arm in which participants received the exercise intervention following the completion of their treatment. Due to the dependency of the two ES (i.e., exercise during treatment compared to control and exercise following treatment compared to the same controls at a later time-point), we deemed it inappropriate to include both ES in the analyses or to average the ES. Consequently, we elected to include only the ES reflecting exercise post-treatment as there are multiple other factors during treatment that might influence depression. Therefore, 14 articles describing 15 RCTs (involving 1,371 participants) met the inclusion criteria and were included in this review. (41–54)
Characteristics of Studies
Nine studies (60%) utilized breast cancer survivors.(41, 42, 45–47, 49, 53, 54) The average age of participants was 51.6 yrs. Seven studies reported racial/ethnic information, with 76.9% of participants in those studies being Caucasian.(41, 42, 44, 45, 47, 50, 54) Of the 12 studies reporting information on cancer stage, nine (75%) utilized participants with non-metastatic cancer.(41, 42, 44–46, 49, 50, 54) (Table 1)
Table 1.
Sample and cancer characteristics of 15 RCTs examining the effects of exercise on depression in cancer survivors.
| Exercise Group (N) |
Control Group (N) |
Mea n Age |
% Female |
% Caucasian |
Recruitment Source |
Education | Cancer Site | Cancer Stage |
Treatment Status at Baseline |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Trial | ||||||||||
|
Badger et al (2007) |
Baseline: N = 23 Follow-up: N = 21 |
Baseline: N = 36 Follow-up: N = 33 |
54 |
100 |
85 |
Volunteers from cancer center, oncologists’ offices, support groups, etc. |
21% High School or less |
Breast |
I-III |
In primary treatment |
| Cadmus et al (2009) | Baseline: N = 25 Follow-up: N = 22 |
Baseline: N = 25 Follow-up: N = 23 |
54 | 100 | 94 | Letters to women identified through tumor registry and self-referral | 70% College degree | Breast | 0-IIIA | In primary treatment |
|
Cadmus et al (2009) |
Baseline: N = 37 Follow-up: N = 34 |
Baseline: N = 38 Follow-up: N = 33 |
56 | 100 | 84 | Letters to women identified through tumor registry and self-referral | 50% College degree | Breast | 0-IIIA | Completed primary treatment |
|
Courneya et al. (2003) |
Baseline: N = 69 Follow-up: N = 62 |
Baseline: N = 33 Follow-up: N = 31 |
60 | 42 | Not reported | Participants identified by oncologists at weekly Tumor Group at local cancer center | 39% College degree | Colorectal | Any stage | Unknown |
| Courneya et al (2007) |
Baseline: N = 78 Follow-up: N = 74 |
Baseline: N = 82 Follow-up: N = 73 |
49 |
100 |
Not reported |
Participants recruited from local cancer center, hospital, and regional cancer agency |
64% College degree |
Breast |
I-IIIA |
In primary treatment |
| Courneya et al. (2009) | Baseline: N = 60 Follow-up: N = 57 |
Baseline: N = 62 Follow-up: N = 60 |
53 | 41 | Not reported | Participants recruited from local cancer center | 52% College degree | Lymphoma | Any stage | Mix of in and completed treatment |
|
Culos-Reed et al. (2010) |
Baseline: N = 53 Follow-up: N = 42 |
Baseline: N = 47 Follow-up: N = 24 |
68 | 0 | Not reported | Not reported | 35% High school or less | Prostate | “Any stage” eligible | Mix of in and completed treatment |
|
Daley et al. (2007) |
Baseline: N = 34 Follow-up: N = 33 |
Baseline: N = 38 Follow-up: N = 33 |
51 | 100 | 98 | Letters to women identified through hospital records, as well as media advertisements and presentations to cancer support groups | 43.5% High school or less | Breast | “Non-metastatic cancers“ eligible | Completed primary treatment |
|
Dodd et al. (2010) |
Baseline: N = 36 Follow-up: N = 35 |
Baseline: N = 39 Follow-up: N = 38 |
51 | 100 | 75 | Participants recruited from six outpatient clinics | Mean of 16.2 years of school | Breast, Colorectal, & Ovarian | I-III | Completed primary treatment |
|
Kaltsatou et al. (2011) |
Baseline: N = 14 Follow-up: N = 14 |
Baseline: N = 13 Follow-up: N = 13 |
57 | 100 | Not reported | Participants recruited from three breast cancer survivor centers | Not reported | Breast | Stage not reported | Completed primary treatment |
|
Monga et al. (2007) |
Baseline: N = 13 Follow-up: N = 11 |
Baseline: N = 13 Follow-up: N = 10 |
68 | 0 | 33 | Volunteers were recruited from patients referred to radiotherapy service at the Veterans Affairs Medical Center | Mean of 12 years of school | Prostate | “Localized cancers“ eligible | In primary treatment |
|
Mutrie et al. (2007) |
Baseline: N = 107 Follow-up: N = 82 |
Baseline: N = 102 Follow-up: N = 92 |
52 | 100 | Not reported | Participants recruited during appointments at outpatient clinics for chemotherapy or radiotherapy at three oncology centers | Not reported | Breast | 0-III | In primary treatment |
|
Payne et al. (2008) |
Baseline: N = 10 Follow-up: N = 9 |
Baseline: N = 10 Follow-up: N = 9 |
65 | 100 | 90 | Participants recruited from breast cancer clinics at a university cancer center | 25% High school or less | Breast | Stage not reported | Completed primary treatment |
|
Perna et al (2010) |
Baseline: N = 27 Follow-up: N = 20 |
Baseline: N = 24 Follow-up: N = 19 |
51 | 100 | 56 | Participants identified by oncologists at weekly Tumor Group at local hospital | Not reported | Breast | I-IIIA | In primary treatment |
| Thorsen, et al. (2005) | Baseline: N = 69 Follow-up: N = 59 |
Baseline: N = 70 Follow-up: N = 52 |
39 | 68 | Not reported | Participants recruited from two university cancer clinics | Not reported | Lymphomas, Breast, Gynecologic, Testicular | Mix of stages | Completed primary treatment |
All studies included an aerobic exercise component (Table 2), with several also including a strength training component.(46, 51–54) Exercise programs were initiated either prior to or during adjuvant therapy in 47% of the studies.(42, 44–46, 49, 50) Exercise programs ranged from supervised, facility-based programs (41, 49, 50) to unsupervised home-based programs (42, 44, 45, 47, 48, 51), with a few studies having some of the exercise program supervised.(45, 46, 52, 54) The majority of the interventions ranged from four to 14 weeks with the longest being 52 weeks.(44) Table 3 presents depression assessment data.
Table 2.
Intervention characteristics of 15 RCTs examining the effects of exercise on depression in cancer survivors.
| Exercise Format & Delivery Mode |
Type of Exercise Program |
Mode of Aerobic Exercise |
Supervision of Exercise Program |
Exercise Bouts per Week |
Length of Exercise Bout |
Number of Weeks of Exercise |
Exercise Intensity |
Delivery Setting |
|
|---|---|---|---|---|---|---|---|---|---|
| Trial | |||||||||
| Badger et al (2007) | Individual | Aerobic | Walking | Unsupervised | 4 | Not reported | 6 | Not reported | Home |
| Phone Delivery | |||||||||
| Cadmus et al (2009) | Individual | Aerobic | Mix of types of aerobic | Unsupervised | 5 | 30 minutes | 24 | 60–80% Heart rate max | Home |
| Phone & Print Delivery | |||||||||
| Cadmus et al (2009) | Individual | Not reported | Mix of types of exercises | Mixed Supervision | 3/week supervised, 2/week unsupervised | 30 minutes | 24 | 60–80% Heart rate max | Community facility and home |
| In person Delivery | |||||||||
| Courneya et al. (2003) | Individual | Aerobic | Mix of types of aerobic | Unsupervised | 3–5 | 20–30 minutes | 16 | 65–75% Heart rate max | Home |
| In person Delivery | |||||||||
| Courneya et al. (2007) | Individual | Aerobic | Mix of types of aerobic | Supervised | 3 | 45 minutes | 17 | 60–80% VO2max | University facility |
| In person Delivery | |||||||||
| Courneya et al. (2009) | Individual | Aerobic | Cycle Ergometer | Supervised | 3 | 40–45 minutes | 12 | 60–75% VO2peak | University facility |
| In person Delivery | |||||||||
| Culos-Reed et al. (2010) | Both Individual & Group | Both Aerobic & Strength Training | Walking | Mixed Supervision | 3–5 | 60 minutes | 16 | “Moderate” | Mix of community facility & home |
| In person Delivery | |||||||||
| Daley et al. (2007) | Individual | Aerobic | Not reported | Supervised | 3 | 50 minutes | 8 | 65–85% Heart rate max | University facility |
| In person Delivery | |||||||||
| Dodd et al. (2010) | Individual | Aerobic | Mix of types of aerobic | Unsupervised | 3–5 | 20–30 minutes | 52 | 60–80% VO2peak | Home |
| Phone Delivery | |||||||||
| Kaltsatou et al. (2011) | Group | Both Aerobic & Strength Training | Dancing | Supervised | 3 | 60 minutes | 24 | 65–80% Heart rate max | Not reported |
| In person Delivery | |||||||||
| Monga et al. (2007) | Format not reported | Aerobic | Walking | Supervised | 3 | 30 minutes + 20 minutes of warm-up/cool-down | 8 | 65% Heart rate reserve | Medical facility |
| In person Delivery | |||||||||
| Mutrie et al. (2007) | Group | Both Aerobic & Strength Training | Mix of types of aerobic | Mixed Supervision | 3 | 45 minutes | 12 | 50–75% Heart rate max | Mix of community facility & home |
| In person Delivery | |||||||||
| Payne et al. (2008) | Individual | Aerobic | Walking | Unsupervised | 4 | 20 minutes | 14 | “Moderate” | Home |
| In person Delivery | |||||||||
| Perna et al (2010) | Individual | Mix of Aerobic & Strength Training | Walking | Mix of supervision | 3 | 30–40 minutes | 12 | 70–85% Heart rate max | Mix of medical facility & home |
| In person Delivery | |||||||||
| Thorsen, et al. (2005) | Individual | Mix of Aerobic & Strength Training | Mix of types of aerobic | Unsupervised | 2 | 30 minutes | 14 | 60–70% Heart rate max | Home |
| In person & print Delivery | |||||||||
Table 3.
Depression assessments of 15 RCTs examining the exercise effects on depressive symptoms in cancer survivors.
| Primary Outcome of Interest Assessed in Study |
Depression Inventory Utilized |
Baseline Depression Score M (SD) |
Follow-up Depression Score M (SD) |
|
|---|---|---|---|---|
| Trial | ||||
|
Badger et al (2007) |
Depression |
CES-D |
Intervention: 13.3 (2.4) |
6 weeks: Intervention: 11.32 (2.10) 14.6% reduction in symptoms |
| Control: 9.9 (1.8) | Control: 9.35 (1.57) 5.4% reduction in symptoms |
|||
| Groups did not significantly differ at follow-up | ||||
|
Cadmus et al (2009) |
Quality of Life |
CES-D |
Intervention: 10.7 (7.3) |
6 Months: Intervention: 7.9 (7.1) 26.2% reduction in symptoms |
| Control: 12.2 (6.5) | Control: 10.0 (7.6) 18.0% reduction in symptoms |
|||
| Groups did not significantly differ at follow-up | ||||
|
Cadmus et al (2009) |
Quality of Life |
CES-D |
Intervention: 9.3 (6.0) |
6 Months: Intervention: 9.6 (9.3) 3.2% increase in symptoms |
| Control: 9.2 (8.6) | Control: 10.8 (10.1) 17.4% increase in symptoms |
|||
| Groups did not significantly differ at follow-up | ||||
|
Courneya et al. (2003) |
Quality of Life |
CES-D |
Intervention: 9.6 (8.1) |
16 weeks: Intervention: 8.6 (8.7) 10.4% reduction in symptoms |
| Control: 10.1 (12.0) | Control: 9.6 (10.9) 5.0% reduction in symptoms |
|||
| Groups did not significantly differ at follow-up | ||||
|
Courneya et al. (2007) |
Quality of Life |
CES-D |
Intervention: 12.8 (9.8) |
9–24 (~20) weeks: Intervention: 9.7 (9.3) 24.2% reduction in symptoms |
| Control: 13.9 (9.7) | Control: 10.8 (9.4) 22.3% reduction in symptoms |
|||
| Groups did not significantly differ at follow-up | ||||
|
Courneya et al. (2009) |
Short Form CES-D |
Intervention: 7.7 (5.7) |
12 Weeks: Intervention: 5.4 (4.5) 29.9% reduction in symptoms |
|
| Quality of Life | Control: 6.0 (4.5) | Control: 6.1 (5.0) 1.7% increase in symptoms |
||
| Groups differed significantly (p < .05) at follow-up | ||||
|
Culos-Reed et al. (2010) |
Physical Activity Levels & Quality of Life |
CES-D |
Intervention: 8.6 (7.9) |
16 Weeks: Intervention: 8.2 (6.7) 4.7% reduction in symptoms |
| Control: 6.7 (6.4) | Control: 7.7 (8.6) 14.9% increase in symptoms |
|||
| Groups did not significantly differ at follow-up | ||||
|
Daley et al. (2007) |
Quality of Life |
BDI-II |
Intervention: 13.6 (9.1) |
8 week: Intervention: 6.0 (6.5) 55.9% reduction in depression |
| Control: 10.8 (7.7) |
Control: 10.3 (7.2) 4.6% reduction in depression |
|||
| Groups differed significantly (p < .05) at follow-up | ||||
|
Dodd et al. (2010) |
Fatigue |
CES-D |
Intervention: 13.1 (9.8) |
4–6 Months: Intervention: 13.0 (9.6) 0.8% reduction in symptoms |
| Control: 10.6 (6.5) | Control: 10.2 (8.6) 3.8% reduction in symptoms |
|||
| Groups did not significantly differ at follow-up | ||||
|
Kaltsatou et al. (2011) |
Physical Function |
BDI |
Intervention: 36.4 (7.2) |
24 Weeks: Intervention: 16.5 (1.7) 54.7% reduction in symptoms |
| Control: 33.4 (6.9) | Control: 22.3 (7.7) 33.2% reduction in symptoms |
|||
| Groups differed significantly (p < .05) at follow-up | ||||
|
Monga et al. (2007) |
Quality of Life/Fatigue |
BDI |
Intervention: 3.5 (5.4) |
8 Weeks: Intervention: 2.8 (1.8) 20% reduction in symptoms |
| Control: 3.6 (5.0) | Control: 4.2 (3.4) 16.7% increase in symptoms |
|||
| Groups did not significantly differ at follow-up | ||||
|
Mutrie et al. (2007) |
Quality of Life |
BDI |
Intervention: 11.8 (6.9) |
12 weeks: Intervention 8.6 (6.8) 27.1% reduction in symptoms |
| Control: 13.0 (7.4) | Control: 11.5 (8.6) 11.5% reduction in symptoms |
|||
| Groups did not significantly differ at follow-up | ||||
|
Payne et al. (2008) |
Fatigue/Biomarkers |
CES-D |
Intervention: 15.1 (7.9) |
14 week: Intervention: 12.7 (8.7) 5.9% reduction in symptoms |
| Control: 11.0 (6.7) | Control: 11.4 (7.9) 3.6% increase in symptoms |
|||
| Groups did not significantly differ at follow-up | ||||
|
Perna et al (2010) |
Fitness/Physical Activity Levels |
CES-D |
Intervention: 9.95 (8.0) |
3 Months: Intervention: 8.8 (8.4) 12.1% reduction in symptoms |
| Control: 8.6 (7.4) | Control: 12.4 (11.5) 44.2% increase in symptoms |
|||
| No direct comparison of depression scores between groups was conducted | ||||
|
Thorsen, et al. (2005) |
Quality of Life |
HADS |
Intervention: 2.9 (2.7) |
14 Weeks: Intervention: 2.2 (2.3) 24.1% reduction in symptoms |
|
Control: 3.1 (3.6) |
Control: 1.9 (2.5) 38.7% reduction in symptoms |
|||
| Groups did not significantly differ at follow-up |
CES-D = Center for Epidemiologic Studies Depression Scale
BDI = Beck Depression Inventory
HADS = Hospital Anxiety and Depression Scale
QOL = Quality of Life
Most studies utilized a “life as usual” approach to usual care.(41, 43, 45, 47–49, 51–53) Three studies added educational print material.(46, 50, 54) In one study (44), the usual care group received periodic phone calls to answer questions about cancer treatment and, in one study (42), usual care participants received both educational print material and periodic phone calls to answer cancer-related questions.
Study quality was very good overall. There was insufficient variability in the PEDro quality scores to examine this as a potential moderator, with all but three studies (44, 49, 54) earning a rating of “high” quality (see Table 4).
Table 4.
Study quality summary.
| Trials | Badger et al. (2007) | Cadmus et al. (2009) | Cadmus et al. (2009) | Courneya et al. (2003) | Courneya et al. (2007) | Courneya et al. (2009) | Culos-Reed et al. (2010) | Daley et al. (2007) | Dodd et al. (2010) | Kaltsatou et al. (2011) | Monga et al. (2007) | Mutrie et al. (2007) | Payne et al. (2008) | Perna et al. (2010) | Thorsen et al. (2005) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PEDro Criterion | |||||||||||||||
| 1. Eligibility criteria were specified. | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| 2. Subjects were randomly allocated to groups. | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| 3. Allocation was concealed. | X | X | X | X | X | X | -- | X | -- | -- | X | X | X | X | X |
| 4. The groups were similar at baseline regarding most important prognostic indicators. | X | X | X | X | X | X | X | X | X | X | X | X | -- | X | X |
| 5. There was blinding of all subjects. | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 6. There was blinding of all therapists who administered the therapy. | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 7. There was blinding of all assessors who measured at least one key outcome. | -- | X | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | X | -- | -- |
| 8. Measurements of at least one key outcome were obtained from more than 85% of the subjects initially allocated to groups. | X | X | X | X | X | X | -- | X | X | X | -- | X | X | -- | -- |
| 9. All subjects for whom outcome measurements were available received the treatment or control condition as allocated, or where this was not the case, data for at least one key outcome were analyzed by ‘intention to treat”. | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| 10. The results of between-group statistical comparisons are reported for at least one key outcome. | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| 11. The study provides both point measurements and measurements of variability for at least one key outcome. | X | X | X | X | X | X | X | X | -- | X | X | X | -- | -- | X |
| Total Criteria Met (item #1 is not included in summary score) | 7 | 8 | 7 | 7 | 7 | 7 | 5 | 7 | 5 | 6 | 7 | 7 | 6 | 5 | 7 |
Note: “X” = criterion is evidenced in article; “—” = criterion is not evidenced, not applicable, not coded, or could not be determined in article.
Criterion #5 is typically not applicable to behavioral interventions of exercise.
Note: Items 2–9 on the PEDro scale are utilized to assess internal validity.
Statistical Results
We found a small and significant overall mean ES of −0.22 (p = 0.04, CI −0.43, −0.009) under a random effects model, when comparing exercise interventions to control groups (Figure 2). The mean ES was slightly smaller, but still significant, under a fixed effects model (ES = −0.20, p =.001, CI −.32, −0.08). Neither the funnel plot nor Egger’s test, with a corresponding p-value = 0.62, showed evidence of publication bias. The test for heterogeneity was significant (p < .001), indicating that ES were not from the same population.
Figure 2.
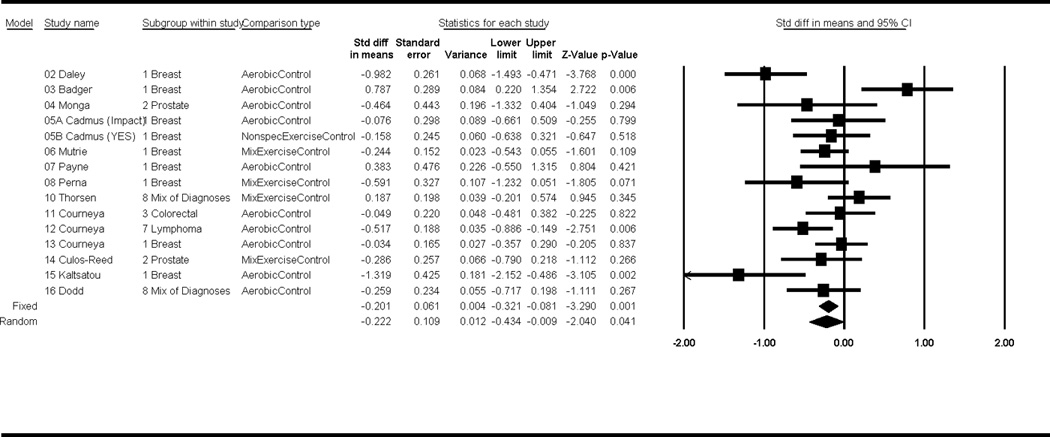
Forest plot of effect sizes.
Of the potential moderators examined (Figure 3), exercise location was significant (p = 0.04), with home-based exercise associated with increased depressive symptoms (ES = 0.16, CI −0.15, 0.47) compared to an improvement in depressive symptoms from exercise interventions in other locations such as community facilities, labs, and gyms (ES = −0.45, CI −0.77, −0.14). Supervised and partially supervised exercise produced reductions in depressive symptoms whereas non-supervised activity was associated with a small increase in depressive symptoms (Supervised: ES = −0.67, CI −1.11, −0.23; Mixed supervision: ES = −0.32, CI −0.50,−0.14; Unsupervised: ES = 0.25, CI −0.01, 0.50), p = 0.01. Further, exercise bout durations of >30 min had larger effects on depression than exercise bouts ≤ 30 min (>30 min bout: ES = −0.57, CI −0.91, −0.23; ≤ 30 min bout: ES = 0.01, CI −0.20, 0.22), p = 0.02.
Figure 3.
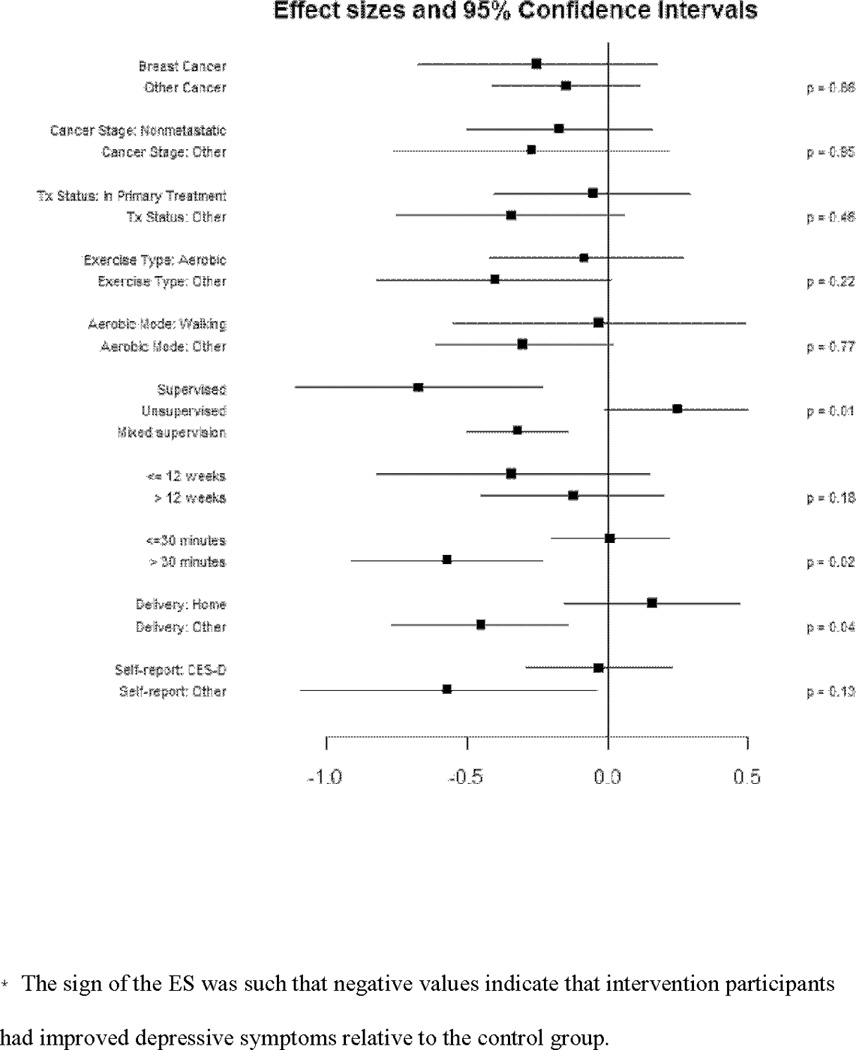
Forrest plot of ES from moderator variable analyses.
Discussion
In this study, exercise produced modest effects on depression in cancer survivors across cancer types (primarily breast), stages (predominantly early stage), treatment status at baseline, and baseline severity of depressive symptoms (most were not depressed). The major qualifier to this conclusion is that most studies did not target depression by selecting depressed cancer survivors, or subgroups of cancer survivors known to be at greater risk of depression, or by selecting exercise interventions known to have the greatest effects on depression in other populations. Thus, positive effects may be even larger for survivors actually experiencing significant levels of depressive symptoms and targeted with appropriate exercise interventions. Previous meta-analyses have reported inconsistent exercise effects. A meta-analyses of 82 RCTs (18 assessed depressive symptoms), involving 6,838 survivors and a mix of cancer types (most were breast cancer), reported no effect of exercise on depression (ES = 0.06, CI −0.26, 0.38) (26). Conversely, Duijts et al. (23) included 56 RCTs of breast cancer only (29 assessed depressive symptoms), representing 7,164 patients, and found that exercise interventions significantly reduced depressive symptoms (ES = −0.26, p < .016). A final meta-analysis of breast cancer only, included 9 controlled clinical trials (representing 452 patients) and concluded that too little information exists to determine the effect of exercise on mood disturbances (25).
Most participants in our meta-analysis scored within a “normal” range on depression inventories. Thus, a floor effect may have been observed. In the one study reporting depression outcomes separately for those who were and were not depressed at study entry, the authors found that both the depression rate and number of new episodes of depression were significantly lower in the exercise group as compared to the controls.(54) While our findings importantly suggest antidepressant benefits from exercise for cancer survivors, studies are needed that utilize depression as an entry criterion or target survivors at risk for depression.
Studies of depressed survivors are important if we are to understand the person, exercise, and cancer-related characteristics associated with the largest effects; consequently, several factors need consideration. For instance, while rates of depression may be similar across stages of cancer (including metastatic disease), the causes of distress may differ (55). Only three studies in this review examined survivors with metastatic cancer. Thus, we were likely insufficiently powered to examine cancer stage as a moderator. It is important that future researchers include those diagnosed with metastatic disease, as the antecedents to depression and their response to exercise may differ.
The specific cancer treatment a survivor receives may also affect depression and/or options for treatment. Women who have undergone chemotherapy, and, in particular taxane-based chemotherapies, may be at increased risk for emotional distress and depression (56, 57). Likewise, individuals receiving steroids, interferon, and estrogen depleting interventions may be at increased risk (14). Many of the studies reviewed herein lacked information regarding the specific types of medications survivors were receiving. In addition, traditional treatments for depression, such as SSRIs, may be contraindicated for those receiving certain types of cancer therapy. One study has shown that the SSRI, paroxetine, is associated with an increased risk of death from breast cancer in women undergoing treatment with tamoxifen (17). Therefore, researchers should carefully consider how medical treatments may play a role in the complex relationship between exercise and depression and enroll patients accordingly.
Third, exercise interventions in these studies were likely not designed to target depressive symptoms. Among depressed patients in the general population, several aspects of the exercise prescription have been identified as important. Although Craft and Landers (19) found that exercise bout duration was not a significant moderator of the effect of exercise on depression, Rethorst and colleagues (20) found that durations of 45–59 minute produced larger antidepressant benefits than shorter bouts of activity. In the current review, programs utilizing exercise bouts of >30 min produced the largest effects on depression; however, only six studies utilized exercise bouts of ≥ 45 min. Therefore, exercise bout duration may have been insufficient in some studies to effect depression. Similarly, an exercise frequency of five times per week was reported as being significantly more effective than two to four days of activity (20). In the current review, only three studies incorporated programs with an exercise frequency of ≥5 days/week. As a result, the exercise frequencies may have been inadequate to provide maximal affect on depressive symptoms. Lastly, in the general population, exercise programs of 10 – 16 weeks produced larger effects than programs < 9 weeks (19, 20). Conversely, although a non-significant difference, we found that effects sizes were larger for those studies utilizing programs of ≤12 weeks. It remains unclear whether these exercise programs characteristics, independently or in combination, contributed to the relatively small observed effect of exercise on depressive symptoms.
As research moves forward, studies can be improved in multiple ways. First, because depression was not the primary outcome of most trials included, no information was collected regarding the participant’s history of depression, ongoing medication or psychotherapeutic treatment for depression, length of current depressive symptoms, or additional psychological co-morbidities. Thus, the current literature is insufficient for understanding who might benefit most from exercise programs. Participant characteristics related to both depression and exercise (e.g., race, marital status, medical co-morbidities, SES) were lacking in most studies, making it impossible to examine these as potential moderators. Future studies should collect information pertaining to the onset, persistence, and treatment of depressive symptoms among participants, as well as pertinent demographic information that might be associated with both depression and exercise. As the risk for recurrence of a major depressive episode can be quite high (50–90%), those with a history of depression or psychological illness may be especially vulnerable to developing depression following a cancer diagnosis.
Race and socio-economic status (SES) are also important considerations. Research shows that low SES women with breast cancer have an increased risk of developing depression and that the symptom burden may differ by race and SES (58–60). Further, older African American cancer survivors who lost their job were three times more likely to develop depression than those who were employed (61). Similarly, among colorectal cancer survivors, race, but not employment status, was a determinant of depressive symptoms across time (62). The samples utilized herein were predominantly Caucasian and middle-class. Many studies did not provide information about race and even fewer alluded to the participant’s SES. Thus, we were not able to examine these as potential moderator variables. Future studies should include larger numbers of ethnic minority and low SES survivors as their risk for depression, cancer prognosis, and acceptance of exercise as a treatment option for depression may differ from Caucasian survivors of high SES.
Risk for the development of depression may also vary by cancer type.(1) Most studies in this meta-analysis were of breast cancer survivors. However, other cancers (e.g., head and neck, prostate, lung) are associated with higher rates of depression. We did not find significant differences in ES when comparing studies of breast cancer (N = 9, ES = −0.25, CI −0.68, 0.18) to “other cancers” (N = 6, ES = −0.15, CI 0.41, 0.12), which were two studies of “mixed diagnoses” (predominantly breast cancer), two studies of prostate cancer, and one study each of lymphoma and colorectal cancer. Thus, we were limited in our ability to compare exercise effects on various types of cancer.
Limited information can be gleaned from our review regarding exercise program characteristics that are most relevant for cancer survivors. Most of the studies reviewed utilized walking programs. Other types of aerobic activities and strength training programs should be examined. Clarification regarding mode of exercise may be important because exercise preference may predict exercise adherence and allowing survivors to choose enjoyable activities may be especially important when targeting depressive symptoms (63).
Among the studies we reviewed, all employed moderate-intensity activity. In the general population, even light-intensity exercise has been shown to be an effective antidepressant. Light-intensity activity may be preferred by some and may be easier to incorporate into one’s lifestyle, resulting in greater exercise frequency. Conversely, some survivors may enjoy and self-select more vigorous-intensity activities. Thus, it is essential that future researchers utilize and compare a variety of exercise intensities and varying lengths of exercise bouts so that evidence based recommendations can be made regarding the appropriate exercise prescription.
The timing of exercise interventions post-diagnosis must also be carefully considered. Half of the studies in this review examined exercise effects in participants undergoing active treatment. We found that treatment status at baseline was not a significant (p = 0.61) moderator. This suggests that exercise can lesson depressive symptoms among those who are and are not actively undergoing treatment during the exercise intervention. While this is very encouraging, only two studies examined exercise effects in survivors that were at least 12 months post-treatment(41, 45). For many, depression will resolve after diagnosis and treatment, but for some it persists or develops during post-treatment and lingers into long-term survivorship (1, 64, 65). Consequently, as the number of cancer survivors, and the average length of survivorship continues to increase,(66) it will be important to examine exercise effects across the various stages of survivorship.
Lastly, the location and supervision of the exercise are also important to consider. Craft and Landers (19) reported the largest antidepressant effects for those exercising in supervised laboratory settings. In the current review, we also found larger effects for those who participated in programs in which all or some part of the exercise was supervised and for those exercising in facilities as compared to home. Unfortunately, we actually found that home-based and unsupervised exercise were associated with increased depressive symptoms, suggesting caution in how exercise programs are implemented for survivors with distress. Conn (67) conducted a meta-analysis in healthy adults and also found that unsupervised exercise produced larger effects on depressive symptoms when the exercise was completed at a fitness center rather than at home. There may be therapeutic aspects to supervised exercise, such as working with an exercise instructor to learn new skills, collaboratively setting and achieving exercise goals, and receiving positive feedback and social interaction. Thus, more research is needed to determine the type of program, and program components, that lead to the largest antidepressant effect.
Conclusion
Elucidating effective treatments for depression in cancer survivors remains a primary challenge. Depression is associated with poor QOL, treatment non-adherence, and increased risk of relapse and mortality, independent of cancer stage or site.(9, 68–70) In this meta-analysis, exercise produced small improvements in depressive symptoms. Nevertheless, there was only one study that targeted depression as the primary endpoint. Most studies utilized samples that contained some depressed survivors but the majority were not experiencing depressive symptoms. We cannot be certain whether the modest improvements in depression caused by exercise participation in this study are sufficient to affect QOL, adherence to treatment, or mortality. However, our results complement the findings of RCTs and other meta-analyses showing that exercise is associated with reduced pain (71) and fatigue (29) and with improvements in QOL (24) among cancer survivors. As there are few negative side-effects of exercise participation, exercise should be recommended to cancer survivors who are experiencing depressive symptoms. Importantly, exercise represents a non-invasive, cost-effective, accessible treatment option, that if found effective, could be implemented into patient care for millions of survivors.
Acknowledgments
Support: The lead author, Dr. Craft, is supported by a career development award from the National Cancer Institute (#1K07CA134936-01A1). Kerry S. Courneya is supported by the Canada Research Chairs Program.
Footnotes
Financial Disclosure: There are no potential conflicts of interest to report.
References
- 1.Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004:57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- 2.Haisfield-Wolfe ME, McGuire DB, Soeken K, Geiger-Brown J, De Forge BR. Prevalence and correlates of depression among patients with head and neck cancer: a systematic review of implications for research. Oncol Nurs Forum. 2009;36:E107–E125. doi: 10.1188/09.ONF.E107-E125. [DOI] [PubMed] [Google Scholar]
- 3.Pirl WF. Evidence report on the occurrence, assessment, and treatment of depression in cancer patients. J Natl Cancer Inst Monogr. 2004:32–39. doi: 10.1093/jncimonographs/lgh026. [DOI] [PubMed] [Google Scholar]
- 4.Deschields T, Tibbs T, Fan MY, Taylor M. Differences in patterns of depression after treatment for breast cancer. Psychooncology. 2006;15:398–406. doi: 10.1002/pon.962. [DOI] [PubMed] [Google Scholar]
- 5.Honda K, Goodwin RD. Cancer and mental disorders in a national community sample: findings from the national comorbidity survey. Psychother Psychosom. 2004;73:235–242. doi: 10.1159/000077742. [DOI] [PubMed] [Google Scholar]
- 6.Arroyo C, Hu FB, Ryan LM, Kawachi I, Colditz GA, Speizer FE, et al. Depressive symptoms and risk of type 2 diabetes in women. Diabetes Care. 2004;27:129–133. doi: 10.2337/diacare.27.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carnethon MR, Kinder LS, Fair JM, Stafford RS, Fortmann SP. Symptoms of depression as a risk factor for incident diabetes: findings from the National Health and Nutrition Examination Epidemiologic Follow-up Study, 1971–1992. Am J Epidemiol. 2003;158:416–423. doi: 10.1093/aje/kwg172. [DOI] [PubMed] [Google Scholar]
- 8.Ferketich AK, Frid DJ. Depression and coronary heart disease: A review of the literature. Clin Geriatrics. 2001;9:1–8. [Google Scholar]
- 9.Somerset W, Stout SC, Miller AH, Musselman D. Breast cancer and depression. Oncology (Williston Park) 2004;18:1021–1034. discussion 35-6, 47–48. [PubMed] [Google Scholar]
- 10.Breen SJ, Baravelli CM, Schofield PE, Jefford M, Yates PM, Aranda SK. Is symptom burden a predictor of anxiety and depression in patients with cancer about to commence chemotherapy? Med J Aust. 2009;190:S99–s104. doi: 10.5694/j.1326-5377.2009.tb02480.x. [DOI] [PubMed] [Google Scholar]
- 11.Lydiatt WM, Moran J, Burke WJ. A review of depression in the head and neck cancer patient. Clin Adv Hematol Oncol. 2009;7:397–403. [PubMed] [Google Scholar]
- 12.Sharpley CF, Bitsika V, Christie DR. Understanding the causes of depression among prostate cancer patients: development of the Effects of Prostate Cancer on Lifestyle Questionnaire. Psychooncology. 2009;18:162–168. doi: 10.1002/pon.1382. [DOI] [PubMed] [Google Scholar]
- 13.Stommel M, Kurtz ME, Kurtz JC, Given CW, Given BA. A longitudinal analysis of the course of depressive symptomatology in geriatric patients with cancer of the breast, colon, lung, or prostate. Health Psychol. 2004;23:564–573. doi: 10.1037/0278-6133.23.6.564. [DOI] [PubMed] [Google Scholar]
- 14.Fann JR, Thomas-Rich AM, Katon WJ, Cowley D, Pepping M, McGregor BA, et al. Major depression after breast cancer: a review of epidemiology and treatment. Gen Hosp Psychiatry. 2008;30:112–126. doi: 10.1016/j.genhosppsych.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Lemieux J, Maunsell E, Provencher L. Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: a literature review. Psychooncology. 2008;17:317–328. doi: 10.1002/pon.1245. [DOI] [PubMed] [Google Scholar]
- 16.McInnes JA, Knobf MT. Weight gain and quality of life in women treated with adjuvant chemotherapy for early-stage breast cancer. Oncol Nurs Forum. 2001;28:675–684. [PubMed] [Google Scholar]
- 17.Kelly CM, Juurlink DN, Gomes T, Duong-Hua M, Pritchard KI, Austin PC, et al. Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: a population based cohort study. BMJ. 2010;340:c693. doi: 10.1136/bmj.c693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleishman SB. Treatment of symptom clusters: pain, depression, and fatigue. J Natl Cancer Inst Monogr. 2004:119–123. doi: 10.1093/jncimonographs/lgh028. [DOI] [PubMed] [Google Scholar]
- 19.Craft LL, Landers DM. The effect of exercise on clinical depression and depression resulting from mental illness: A meta-analysis. Journal of Sport & Exercise Psychology. 1998;20:339–357. [Google Scholar]
- 20.Rethorst CD, Wipfli BM, Landers DM. The Antidepressive Effects of Exercise A Meta-Analysis of Randomized Trials. Sports Medicine. 2009;39:491–511. doi: 10.2165/00007256-200939060-00004. [DOI] [PubMed] [Google Scholar]
- 21.Strathopoulou G, Powers MB, Berry AC, Smits AJ, Otto MW. Exercise interventions for mental health: A quantitative and qualitative review. Clinical Psychology Science and Practice. 2006;13:179–193. [Google Scholar]
- 22.Conn VS, Hafdahl AR, Porock DC, McDaniel R, Nielsen PJ. A meta-analysis of exercise interventions among people treated for cancer. Support Care Cancer. 2006;14:699–712. doi: 10.1007/s00520-005-0905-5. [DOI] [PubMed] [Google Scholar]
- 23.Duijts SF, Faber MM, Oldenburg HS, van Beurden M, Aaronson NK. Effectiveness of behavioral techniques and physical exercise on psychosocial functioning and health-related quality of life in breast cancer patients and survivors--a meta-analysis. Psychooncology. 2011;20:115–126. doi: 10.1002/pon.1728. [DOI] [PubMed] [Google Scholar]
- 24.Ferrer RA, Huedo-Medina TB, Johnson BT, Ryan S, Pescatello LS. Exercise interventions for cancer survivors: a meta-analysis of quality of life outcomes. Ann Behav Med. 2011;41:32–47. doi: 10.1007/s12160-010-9225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Markes M, Brockow T, Resch KL. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database of Systematic Reviews. 2006 doi: 10.1002/14651858.CD005001.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4:87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 27.Luckett T, Butow PN, King MT, Oguchi M, Heading G, Hackl NA, et al. A review and recommendations for optimal outcome measures of anxiety, depression and general distress in studies evaluating psychosocial interventions for English-speaking adults with heterogeneous cancer diagnoses. Supportive Care in Cancer. 2010;18:1241–1262. doi: 10.1007/s00520-010-0932-8. [DOI] [PubMed] [Google Scholar]
- 28.Verhagen AP, de Vet HCW, de Bie RA, Kessels AGH, Boers M, Bouter LM, et al. The delphi list: A criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. Journal of Clinical Epidemiology. 1998;51:1235–1241. doi: 10.1016/s0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 29.Brown JC, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferrer RA, Johnson BT. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20:123–133. doi: 10.1158/1055-9965.EPI-10-0988. [DOI] [PubMed] [Google Scholar]
- 30.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–721. [PubMed] [Google Scholar]
- 31.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 32.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 33.Hedges LV, Vevea JL. Fixed- and Random-Effects Models in Meta-Analysis. Psychological Methods. 1998;3:486–504. [Google Scholar]
- 34.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banerjee B, Vadiraj HS, Ram A, Rao R, Jayapal M, Gopinath KS, et al. Effects of an integrated yoga program in modulating psychological stress and radiation-induced genotoxic stress in breast cancer patients undergoing radiotherapy. Integr Cancer Ther. 2007;6:242–250. doi: 10.1177/1534735407306214. [DOI] [PubMed] [Google Scholar]
- 36.Danhauer SC, Mihalko SL, Russell GB, Campbell CR, Felder L, Daley K, et al. Restorative yoga for women with breast cancer: findings from a randomized pilot study. Psychooncology. 2009;18:360–368. doi: 10.1002/pon.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raghavendra RM, Vadiraja HS, Nagarathna R, Nagendra HR, Rekha M, Vanitha N, et al. Effects of a Yoga Program on Cortisol Rhythm and Mood States in Early Breast Cancer Patients Undergoing Adjuvant Radiotherapy: A Randomized Controlled Trial. Integrative Cancer Therapies. 2009;8:37–46. doi: 10.1177/1534735409331456. [DOI] [PubMed] [Google Scholar]
- 38.Ohira T, Schmitz KH, Ahmed RL, Yee D. Effects of weight training on quality of life in recent breast cancer survivors - The Weight Training for Breast Cancer Survivors (WTBS) Study. Cancer. 2006;106:2076–2083. doi: 10.1002/cncr.21829. [DOI] [PubMed] [Google Scholar]
- 39.Segar ML, Katch VL, Roth RS, Garcia AW, Portner TI, Glickman SG, et al. The effect of aerobic exercise on self-esteem and depressive and anxiety symptoms among breast cancer survivors. Oncol Nurs Forum. 1998;25:107–113. [PubMed] [Google Scholar]
- 40.Courneya KS, Friedenreich CM, Sela RA, Quinney HA, Rhodes RE, Handman M. The group psychotherapy and home-based physical exercise (group-hope) trial in cancer survivors: physical fitness and quality of life outcomes. Psychooncology. 2003;12:357–374. doi: 10.1002/pon.658. [DOI] [PubMed] [Google Scholar]
- 41.Daley AJ, Crank H, Saxton JM, Mutrie N, Coleman R, Roalfe A. Randomized trial of exercise therapy in women treated for breast cancer. J Clin Oncol. 2007;25:1713–1721. doi: 10.1200/JCO.2006.09.5083. [DOI] [PubMed] [Google Scholar]
- 42.Badger T, Segrin C, Dorros SM, Meek P, Lopez AM. Depression and anxiety in women with breast cancer and their partners. Nurs Res. 2007;56:44–53. doi: 10.1097/00006199-200701000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25:4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 44.Dodd MJ, Cho MH, Miaskowski C, Painter PL, Paul SM, Cooper BA, et al. A Randomized Controlled Trial of Home-Based Exercise for Cancer-Related Fatigue in Women During and After Chemotherapy With or Without Radiation Therapy. Cancer Nursing. 2010;33:245–257. doi: 10.1097/NCC.0b013e3181ddc58c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cadmus LA, Salovey P, Yu H, Chung G, Kasl S, Irwin ML. Exercise and quality of life during and after treatment for breast cancer: results of two randomized controlled trials. Psychooncology. 2009;18:343–352. doi: 10.1002/pon.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mutrie N, Campbell AM, Whyte F, McConnachie A, Emslie C, Lee L, et al. Benefits of supervised group exercise programme for women being treated for early stage breast cancer: pragmatic randomised controlled trial. BMJ. 2007;334:517. doi: 10.1136/bmj.39094.648553.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Payne JK, Held J, Thorpe J, Shaw H. Effect of exercise on biomarkers, fatigue, sleep disturbances, and depressive symptoms in older women with breast cancer receiving hormonal therapy. Oncol Nurs Forum. 2008;35:635–642. doi: 10.1188/08.ONF.635-642. [DOI] [PubMed] [Google Scholar]
- 48.Courneya KS, Friedenreich CM, Quinney HA, Fields AL, Jones LW, Fairey AS. A randomized trial of exercise and quality of life in colorectal cancer survivors. European Journal of Cancer Care. 2003;12:347–357. doi: 10.1046/j.1365-2354.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 49.Courneya KS, Sellar CM, Stevinson C, McNeely ML, Peddle CJ, Friedenreich CM, et al. Randomized Controlled Trial of the Effects of Aerobic Exercise on Physical Functioning and Quality of Life in Lymphoma Patients. Journal of Clinical Oncology. 2009;27:4605–4612. doi: 10.1200/JCO.2008.20.0634. [DOI] [PubMed] [Google Scholar]
- 50.Monga U, Garber SL, Thornby J, Vallbona C, Kerrigan AJ, Monga TN, et al. Exercise prevents fatigue and improves quality of life in prostate cancer patients undergoing radiotherapy. Archives of Physical Medicine and Rehabilitation. 2007;88:1416–1422. doi: 10.1016/j.apmr.2007.08.110. [DOI] [PubMed] [Google Scholar]
- 51.Thorsen L, Skovlund E, Stromme SB, Hornslien K, Dahl AA, Fossa SD. Effectiveness of physical activity on cardiorespiratory fitness and health-related quality of life in young and middle-aged cancer patients shortly after chemotherapy. J Clin Oncol. 2005;23:2378–2388. doi: 10.1200/JCO.2005.04.106. [DOI] [PubMed] [Google Scholar]
- 52.Culos-Reed SN, Robinson JW, Lau H, Stephenson L, Keats M, Norris S, et al. Physical activity for men receiving androgen deprivation therapy for prostate cancer: benefits from a 16-week intervention. Supportive Care in Cancer. 2010;18:591–599. doi: 10.1007/s00520-009-0694-3. [DOI] [PubMed] [Google Scholar]
- 53.Kaltsatou A, Mameletzi D, Douka S. Physical and psychological benefits of a 24-week traditional dance program in breast cancer survivors. J Bodyw Mov Ther. 2011;15:162–167. doi: 10.1016/j.jbmt.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Perna F, Craft L, Freund K, Skrinar G, Stone M, Kachnic L, et al. The effect of a cognitive behavioral exercise intervention on clinical depression in a multiethnic sample of women with breast cancer: A randomized controlled trial. International Journal of Sport and Exercise Psychology. 2010;8:36–47. [Google Scholar]
- 55.Kissane DW, Grabsch B, Love A, Clarke DM, Bloch S, Smith GC. Psychiatric disorder in women with early stage and advanced breast cancer: a comparative analysis. Aust N Z J Psychiatry. 2004;38:320–326. doi: 10.1080/j.1440-1614.2004.01358.x. [DOI] [PubMed] [Google Scholar]
- 56.Lee KC, Ray GT, Humkeler EM, Finley PR. Tamoxifen treatment and new- onset depression in breast cancer patients. Psychosomatics. 2008;48:205–210. doi: 10.1176/appi.psy.48.3.205. [DOI] [PubMed] [Google Scholar]
- 57.Thornton LM, Carson WE, 3rd, Shapiro CL, Farrar WB, Andersen BL. Delayed emotional recovery after taxane-based chemotherapy. Cancer. 2008;113:638–647. doi: 10.1002/cncr.23589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Post-treatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32:250–256. doi: 10.1188/05.ONF.250-256. [DOI] [PubMed] [Google Scholar]
- 59.Fu OS, Crew KD, Jacobson JS, Greenlee H, Yu G, Campbell J, et al. Ethnicity and persistent symptom burden in breast cancer survivors. J Cancer Surviv. 2009;3:241–250. doi: 10.1007/s11764-009-0100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hopwood P, Sumo G, Mills J, Haviland J, Bliss JM. The course of anxiety and depression over 5 years of follow-up and risk factors in women with early breast cancer: results from the UK Standardisation of Radiotherapy Trials (START) Breast. 2010;19:84–91. doi: 10.1016/j.breast.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 61.Agarwal M, Hamilton JB, Moore CE, Crandell JL. Predictors of depression among older African American cancer patients. Cancer Nurs. 2010;33:156–163. doi: 10.1097/NCC.0b013e3181bdef76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kurtz ME, Kurtz JC, Stommel M, Given CW, Given B. Predictors of depressive symptomatology of geriatric patients with colorectal cancer: a longitudinal view. Support Care Cancer. 2002;10:494–501. doi: 10.1007/s00520-001-0338-8. [DOI] [PubMed] [Google Scholar]
- 63.Brinthaupt TM, Kang M, Anshel MH. A delivery model for overcoming psycho-behavioral barriers to exercise. Psychology of Sport and Exercise. 2010;11:259–266. [Google Scholar]
- 64.Deschields T, Tibbs T, Fan MY, Taylor M. Differences in patterns of depression after treatment for breast cancer. Psychooncology. 2006;15:398–406. doi: 10.1002/pon.962. [DOI] [PubMed] [Google Scholar]
- 65.Patrick DL, Ferketich SL, Frame PS, Harris JJ, Hendricks CB, Levin B, et al. National Institutes of Health State-of-the-Science Conference Statement: Symptom Management in Cancer: Pain, Depression, and Fatigue, July 15–17, 2002. J Natl Cancer Inst. 2003;95:1110–1117. doi: 10.1093/jnci/djg014. [DOI] [PubMed] [Google Scholar]
- 66.National Cancer Institute. [cited 2010 July];Breast Cancer. 2010 Available from: www.cancer.gov/cancertopics/types/breast.
- 67.Conn VS. Depressive Symptom Outcomes of Physical Activity Interventions: Meta-analysis Findings. Annals of Behavioral Medicine. 2010;39:128–138. doi: 10.1007/s12160-010-9172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010;40:1797–1810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reich M. Depression and cancer: recent data on clinical issues, research challenges and treatment approaches. Curr Opin Oncol. 2008;20:353–339. doi: 10.1097/CCO.0b013e3282fc734b. [DOI] [PubMed] [Google Scholar]
- 70.Frick E, Tyroller M, Panzer M. Anxiety, depression and quality of life of cancer patients undergoing radiation therapy: a cross-sectional study in a community hospital outpatient centre. Eur J Cancer Care (Engl) 2007;16:130–136. doi: 10.1111/j.1365-2354.2006.00720.x. [DOI] [PubMed] [Google Scholar]
- 71.McNeely ML, Parliament MB, Seikaly H, Jha N, Magee DJ, Haykowsky MJ, et al. Effect of exercise on upper extremity pain and dysfunction in head and neck cancer survivors: a randomized controlled trial. Cancer. 2008;113:214–222. doi: 10.1002/cncr.23536. [DOI] [PubMed] [Google Scholar]


