Abstract
While grammatical aspects of language are preserved, executive deficits are prominent in Lewy body spectrum disorder (LBSD), including Parkinson’s disease (PD), Parkinson’s dementia (PDD) and dementia with Lewy bodies (DLB). We examined executive control during sentence processing in LBSD by assessing temporary structural ambiguities. Using an on-line word detection procedure, patients heard sentences with a syntactic structure that has high-compatibility or low-compatibility with the main verb’s statistically preferred syntactic structure, and half of the sentences were lengthened strategically between the onset of the ambiguity and its resolution. We found selectively slowed processing of lengthened ambiguous sentences in the PDD/DLB subgroup. This correlated with impairments on measures of executive control. Regression analyses related the working memory deficit during ambiguous sentence processing to significant cortical thinning in frontal and parietal regions. These findings emphasize the role of prefrontal disease in the executive limitations that interfere with processing ambiguous sentences in LBSD.
Keywords: Parkinson’s, Lewy body, syntactic ambiguity, working memory, frontal
INTRODUCTION
Parkinson’s disease (PD) is thought to be primarily a motor disorder, although non-motor aspects of functioning also can be compromised (Rodriguez-Oroz et al., 2009). Perhaps the most commonly cited cognitive impairment is a limitation in executive control (Brown & Marsden, 1990; Brown, Soliveri, & Jahanshahi, 2000; Dalrymple-Alford, Kalders, Jones, & Watson, 1994; Marie et al., 1999; A. Price & Shin, 2009). By comparison, language difficulties are not thought to be directly affected in PD (Bayles, 1990). In this study, we assessed a challenging aspect of language that appears to depend in part on executive control – the resolution of a syntactic ambiguity in a sentence.
Cognitive impairments in PD may be subtle at onset. A progressive reduction in cognitive functioning can reach the status of dementia (PDD) in 11% to 36% of PD patients (Giladi et al., 2000; Girotti et al., 1988; Lees, 1985; Parashos, Johnson, Erickson-Davis, & Wielinski, 2009), and may be seen in up to 80% of patients over time (Dag Aarsland, Andersen, Larsen, & Lolk, 2003; Buter et al., 2008; Hely, Reid, Adena, Halliday, & Morris, 2008). Dementia in PD is associated with a proliferation of Lewy bodies in the cerebral cortex. This histopathologic picture is identical to that seen in dementia with Lewy bodies (DLB), a condition that is said to differ clinically from PDD due to the relatively later onset of a motor disorder in DLB (McKeith et al., 2005). We refer to this family of conditions as Lewy body spectrum disorder (LBSD). We acknowledge that this view of PD, PDD, and DLB as a spectrum of cognitive and extrapyramidal motor disorders is not universally accepted (Aarsland et al., 2003; Downes et al., 1998). Nevertheless, the shared histopathologic feature of Lewy body proliferation is the basis for our regarding these conditions as a spectrum of disorders, where differences in the degree of cognitive and motor impairment are related to the relative amount of disease burden in specific brain regions mediating cognitive and motor functioning (Double et al., 1996; Harrington et al., 1994).
Cognitive deficits in LBSD may extend to language (Bastiaanse & Leenders, 2009; Chenery, Angwin, & Copland, 2008; Colman et al., 2009; Grossman, 1999; Hochstadt, 2009; Pereira et al., 2009). Some prior work has demonstrated difficulties processing grammatical relationships during sentence processing in non-demented patients with PD (Grossman, 1999; Grossman, Lee, Morris, Stern, & Hurtig, 2002; Grossman, Zurif, et al., 2002; Lee, Grossman, Morris, Stern, & Hurtig, 2003). This deficit has been attributed to a limitation in executive resources such as working memory that contribute to interpreting complex grammatical relationships in a sentence. Rare studies have examined language processing in the demented spectrum of patients with LBSD (Parashos et al., 2009; Piatt, Fields, Paolo, Koller, & Troster, 1999). A detailed analysis of a semi-structured speech sample recently revealed an impairment in speech production in patients with DLB and PDD (Ash et al., 2011). These patients had simplified grammatical and narrative expression, and this correlated with executive difficulties on non-linguistic measures such as category naming fluency. Patients with DLB were more impaired quantitatively than patients with PDD, and both PDD and DLB patients were significantly more impaired than non-demented patients with PD.
An important challenge in everyday conversation is that the meaning of over one-third of our utterances is ambiguous (Elsness, 1984; Thompson & Mulac, 1991). This is often due to a syntactic ambiguity. A sentence with a temporary syntactic ambiguity may begin with a noun phrase and verb phrase suggesting one syntactic parsing, but continues with sentence constituents suggesting a different parse. Consider a sentence like “Citizens heard the election result on the radio.” The principle of minimal attachment (Ferreira & Henderson, 1991; Frazier & Rayner, 1982) leads one to interpret the phrase “the election result” as the direct object of “heard” (i.e. citizens listened to a report of an election on the radio). Analyses of language corpora show that some verbs are statistically more likely to appear in some syntactic contexts than others (Garnsey, Pearlmutter, Myers, & Lotocky, 1997). From this perspective, this interpretation of the sentence is reasonable because the verb “hear” is most frequently associated with a direct object. When the structure of a sentence matches the most frequent structure associated with its verb, we refer to this as “high compatibility.”
Next consider the sentence “Citizens heard the election result had been fixed.” Here, the verb “heard” is embedded in a sentence with a sentential complement rather than a direct object. If the listener makes the high probability assumption that the phrase following “heard” is its direct object, a structural ambiguity emerges when “had been fixed” is encountered. This must be revised to reflect the correct, lower probability interpretation of the sentence. We refer to this type of sentence as having “low compatibility” because the structure of the sentence does not match the most likely structure in which the verb can be embedded. Although the sentence is perfectly acceptable grammatically, the coherence of the sentence requires re-parsing in order to capture the less common structure associated with “heard”, where “the election result” is the subject of “was fixed” in a sentence complement (cf., Ferreira & Henderson, 1991; Gibson & Perlmutter, 2000; Just & Carpenter, 1992; MacDonald et al., 1994; Mason et al., 2003; Trueswell et al., 1993). Listeners may need to retain portions of a sentence in working memory during the course of processing before its full meaning can be derived, and may rely on subconscious decision-making based on subtle probabilistic biases to arrive at the most likely meaning of an ambiguous sentence.
Some work has assessed the resolution of lexical semantic ambiguities in PD (Chenery et al., 2008; Copland, 2003; Copland, Chenery, & Murdoch, 2000, 2001; Copland, Sefe, Ashley, Hudson, & Chenery, 2009; Gadsby, Arnott, & Copland, 2008). This has been related to deficits in executive control. fMRI studies of healthy adults implicate prefrontal regions during resolution of a lexical semantic ambiguity (Mason & Just, 2007). These findings emphasize the important role of working memory in processing lexical semantic ambiguities, and the likely support of frontal neocortex in this process.
Although we are not aware of studies examining syntactic ambiguities in LBSD, prior fMRI work examining the neural basis for resolving a temporary structural ambiguity has emphasized the important contribution of different regions within the frontal lobe (Novais-Santos et al., 2007). The results revealed two patterns of activation. On the one hand, both high-compatibility and low-compatibility sentences recruited inferior frontal regions, and the contrast of lengthened and unlengthened sentences additionally demonstrated inferior parietal activation. Other work has emphasized significant activation in this frontal-parietal distribution when evaluating sentences lengthened strategically to stress grammatical processing (Cooke et al., 2001). This resembles the activation pattern seen in non-linguistic studies of verbal working memory (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Ramnani & Owen, 2004; E. E. Smith, Marshuetz, Geva, & Grafman, 2002; Wager & Smith, 2003). On the other hand, a direct comparison of high-compatibility and low-compatibility sentences in the Novais-Santos study showed activation of dorsolateral prefrontal cortex (dlPFC). Other work involving resolution of an ambiguous sentence also has shown activation of dlPFC (G. Kuperberg, Lakshmanan, Caplan, & Holcomb, 2006; G. R. Kuperberg, Holcomb, Sitnikova, & Greve, 2003; Mason & Just, 2007; Mason, Just, Keller, & Carpenter, 2003; Rodd, Davis, & Johnsrude, 2005). This may be related in part to an on-line decision-making process that assesses probabilistic relationships and biases subjects towards a more likely interpretation (Badre & D’Esposito, 2007; Casey et al., 2001; Huettel, Song, & McCarthy, 2005; Rowe & Passingham, 2001; C. D. Smith et al., 2001).
In this context, we examined the neuroanatomic basis for resolution of a temporary structural ambiguity in LBSD by assessing the regional distribution of cortical atrophy with volumetric MRI. LBSD patients appear to have prefrontal cortical atrophy (Burton et al., 2009; Burton, McKeith, Burn, Williams, & O’Brien, 2004; Sauer, ffytche, Ballard, Brown, & Howard, 2006; Tam, Burton, McKeith, Burn, & O’Brien, 2005; Whitwell et al., 2007). Other work using functional imaging has emphasized the role of parietal disease (Samuel et al., 1997). In the present study, we examined regional gray matter atrophy in comparison to a group of healthy, age-matched seniors using voxel-based morphometry (VBM). This quantitative technique establishes the regional anatomic distribution of statistically significant atrophy. Moreover, because this technique is quantitative, it allows us to relate behavioral performance directly to gray matter atrophy.
In sum, we assessed the resolution of a temporary syntactic ambiguity in LBSD by assessing performance with high- versus low-compatibility verb-sentence combinations, and with strategically lengthened versus unlengthened sentences. Given the apparent contribution of limited executive control to the language deficits in LBSD, an important methodological feature of the present study is the use of an on-line technique to minimize the risk that an observed deficit can be attributed to task-related demands (Grossman, Lee, et al., 2002; Lee et al., 2003; C. Price & Grossman, 2005; Tyler, 1985). We hypothesized that LBSD patients would have greater difficulty than controls at resolving a temporary structural ambiguity in a sentence, and that this would be related to neuropsychological performance on measures of executive control. Moreover, we hypothesized that these patients would have cortical atrophy involving at least portions of the frontal lobe, and that regression analyses would relate difficulty processing ambiguous sentences to specific regions within the frontal lobe.
METHODS
Subjects
We studied 43 patients with LBSD. This included 26 non-demented patients with PD and 17 patients with DLB/PDD. We also examined 19 healthy seniors. As shown in Table 1, these patient groups were matched for education, but PDD/DLB patients were older than PD patients and controls. Correlation studies failed to reveal any correlation of sentence processing performance with age in any of the patient groups, and statistical analyses reported below co-varied for age. Thus, we do not feel that this difference in age is playing a prominent role in our observations. PD and PDD/DLB patients differed from controls in their performance on the MMSE, as expected, although all patients had mild cognitive deficits overall. Exclusionary criteria included evidence for a stroke, hydrocephalus or other neurological disorder, a primary psychiatric disorder such as schizophrenia or major depression, or a medical condition such as hypothyroidism or meningoencephalitis that could impair cognitive functioning. Patients were on a stable dosage of medication during the course of their participation in the study, and no patients were taking large doses of sedating medications such as benzodiazepines or anti-cholinergics that could interfere with performance. All subjects participated in an informed consent process approved by the Institutional Review Board of the University of Pennsylvania.
TABLE 1.
MEAN (+STANDARD DEVIATION) DEMOGRAPHIC AND CLINICAL FEATURES IN CONTROLS AND LEWY BODY SPECTRUM DISORDER PATIENTS
| PARKINSON’S DEMENTIA/ LEWY BODY DISEASE |
PARKINSON’S DEMENTIA |
LEWY BODY DISEASE |
PARKINSON’S DISEASE |
CONTROL | |
|---|---|---|---|---|---|
| AGE | 73.8 (6.5)1 | 74.8 (7.3) | 72.9 (5.9) | 65.6 (8.2) | 63.9 (9.5) |
| EDUCATION | 16.1 (2.9) | 16.3 (3.3) | 16.0 (2.6) | 15.8 (3.2) | 15.2 (2.3) |
| MMSE | 25.1 (3.2)1,2 | 26.5 (2.4) | 23.8 (3.4) | 28.3 (1.8)1 | 29.4 (0.7) |
| MOTOR | |||||
| UPDRS | 25.21 (11.67) | 31.9 (13.5) | 18.6 (3.0) | 25.00 (8.38) | - |
| Hoehn & Yahr score | 2.5 (0.67) | 2.3 (0.8) | 2.7 (0.4) | 2.12 (0.65) | - |
| NEUROPSYCHOLOGY | |||||
| Trails B | −7.32 (17.1) | −2.9 (5.6) | −11.2 (22.8) | −1.23 (3.7) | - |
| Stroop | −4.7 (9.4) | 0.2 (0.7) | −9.0 (11.5) | 0.11 (0.93) | - |
| Sentence grammatical comprehension | −4.04 (3.42) | −2.5 (3.5) | −5.6 (3.1) | −0.29 (1.76) | - |
| Sentence-picture match | −3.47 (5.26) | −1.8 (2.1) | −5.6 (7.6) | −0.24 (2.12) | - |
| Pyramid and palm tree | −5.26 (5.69) | −2.4 (2.1) | −8.1 (6.8) | −1.31 (1.4) | - |
| Semantic categorization | −2.21 (3.33) | −2.9 (3.9) | −1.2 (2.3) | −0.07 (1.01) | - |
NOTE Differs from controls at p<0.01, according to a t-test
Differs from non-demented PD patients at p<0.01, according to a t-test
Neuropsychology values are z-scores relative to age- and education-matched controls, where z<−1.96 differs from controls at a p-value of 0.05.
We examined neuropsychological performance on patients using measures of executive control, grammatical comprehension, and semantic processing. Performance is summarized in Table 1 as z-scores relative to 25 age- and education-matched controls:
Executive control
Trails B (written alternating number and letter percent correct in 180 sec); Stroop (naming font of color word percent correct in 180 sec);
Grammatical comprehension
Sentence-picture matching (two-choice picture matching for conjoined, subject-relative and object-relative sentences with cleft or center-embedded structure); Sentence grammatical comprehension (agent probe of aural, center-embedded sentences);
Lexical comprehension
Pyramid and Palm Tree (two-choice word-word and picture-picture associativity matching); Semantic categorization (semantic category membership judgment of printed words and color photos).
Materials
Subjects heard 160 experimental sentences, including 80 sentences with a temporary structural ambiguity having high verb compatibility (e.g., “The tired passenger claimed the luggage was unidentified at the airport”) and 80 with low verb compatibility (e.g., “The tired passenger claimed the luggage eagerly at the airport”), using biased verbs based on Garnsey et al. (1997). All sentences had the same initial format (NP-V-NP) with identical words, and were disambiguated at the second-to-last phrase (a terminal phrase was included to minimize any confounds due to wrap-up effects). In half of the experimental sentences, the post-verbal NP was a direct object (DO) followed by an adverbial phrase; in the remainder, the post-verbal NP was the subject of a sentence complement (SC). In half of each type of sentence, the verb occurred statistically more often in a DO structure, and the verb in the remainder occurred more often in a SC structure. These verb preferences were matched across conditions, according to ratings derived from Garnsey et al (1997), and all sentences were grammatically correct and semantically coherent. Thus, verbs preferring a DO structure that were embedded in a DO sentence structure, and verbs preferring a SC structure that were embedded in a SC sentence structure, were considered “high compatible.” By comparison, verbs preferring a DO structure that were embedded in a SC sentence structure, and verbs preferring a SC structure that were embedded in a DO sentence structure, were considered “low compatible.” In half of each type of sentence, moreover, a four-word prepositional phrase was inserted between the onset of ambiguity and the point at which the ambiguity is resolved (e.g., “The tired passenger claimed the luggage from the rotating carousel at the airport”). Subjects also heard 80 filler sentences with another ambiguity that was not of theoretical interest. The sentences were produced aurally with a natural rate and prosodic contour, digitized with Pratt, and presented by computer to patients in a pseudo-random order.
Procedure
The task used an on-line word detection paradigm (Davis et al., 2002; Grossman et al., 2002; Lee et al, 2003; Price et al, 2005; Tyler et al, 2010). At 500 msec following a warning signal, a target content word was presented, and 500 msec later subjects heard the sentence containing the target word. Subjects were instructed to press a response key (the space bar of the computer) as soon as the target word was heard. The target was placed immediately following the resolution of the ambiguity (i.e. the experimental condition, where target word detection is occurring during the processing window for ambiguity resolution) or preceding the verb by two words (i.e. the control condition, where the target word is occurring before the ambiguity is encountered). Each sentence was presented in two different, counter-balanced blocks, where the target word immediately followed the point of ambiguity resolution in half of the sentences in each block and preceding the point of ambiguity in the remaining sentences. The types of sentences were counter-balanced across blocks. To insure that patients were listening to sentence content, we probed 20% of sentences at their completion for a simple content question.
Statistical analyses
We analyzed the data for the experimental condition and the control condition using mixed analysis of covariance (MANCOVA), where the group was a between-subject effect, and the working memory factor and the compatibility factor were within-subject effects. Age was included as a covariate because of the difference in age for the PDD/DLB subgroup. Post-hoc tests were performed within this analysis. We used Pearson correlations to relate latencies on the measure of ambiguity resolution to neuropsychological performance.
Imaging analysis
Fourteen LBSD patients, including 8 patients with PD and 6 patients with PDD/DLB, had a volumetric T1-weighted brain MRI scan within one year of the task. These patients did not differ statistically from the larger set of LBSD patients on any demographic or language measures. Thirteen patients had MRI scans acquired using a GE 1.5T scanner with 1.2-mm slice thickness and a 144 × 256 matrix. For 1 patient and for 64 age-matched controls (mean age = 71.5±7.1 years; gender = 36 females; handedness = all dextrals; MMSE ≥28 for all), images were collected using a SIEMENS Trio 3.0T scanner with 1-mm slice thickness and a 195 × 256 matrix. Images from both scanners were deformed into a standard local template space with a 1-mm3 resolution using PipeDream (https://sourceforge.net/projects/neuropipedream/) and Advanced Normalization Tools (ANTS, http://www.picsl.upenn.edu/ANTS/). These tools have been validated as stable and reliable for performing multivariate normalization (Avants, Epstein, Grossman, & Gee, 2008; Klein et al., 2009). Both PipeDream and ANTS mapped T1 structural MRI images to an optimal template space, using diffeomorphic and symmetric registration methods (Avants & Gee, 2004; Avants et al., 2010). The registered images were segmented into gray matter thickness maps using template-based priors and then registered to MNI-template space for statistical comparisons. Gray matter thickness images were smoothed in SPM5 (http://www.fil.ion.ucl.ac.uk/spm/sortware/spm5) using a 4-mm full-width half-maximum Gaussian kernel to minimize individual gyral variations.
In SPM5, a two-sample t-test contrasted gray matter thickness between patients with LBSD and healthy controls to identify regions of significant cortical thinning, covarying for scanner. For this atrophy analysis, an explicit mask was defined by generating a mean gray matter image from the healthy controls in order to limit the analysis to voxel-wise comparisons within gray matter. We used a p<0.01 height threshold, 100-voxel extent, and accepted clusters with a peak voxel z-score > 3.09 (p<0.001).
The regression module in SPM5 was used to relate gray matter thinning to a measure of ambiguous sentence processing, in particular, the processing latency in experimental sentences lengthened at the critical locus of ambiguity resolution. We performed a whole-brain analysis, and then used an explicit mask so that we could examine the relationship between narrative performance and gray matter thinning in brain areas known to be significantly atrophied from the prior analysis of whole brain gray matter thinning. We interpreted only regions where narrative performance was related to atrophied gray matter areas because we knew that these implicated regions are diseased and because it would be difficult to explain with confidence significant associations between non-atrophied regions and patients’ performance. For the regression analysis, we used a statistical height threshold of p<0.05 and accepted clusters containing a peak with z-score > 3.09 (p<0.001) and an extent greater than 50 voxels. Coordinates for all accepted clusters were converted to Talairach space (Talairach & Tournaux, 1988).
RESULTS
Behavioral results
The latencies for performance with the experimental stimuli are summarized in Figure 1. A MANCOVA analyzed these latencies with a group (3: control, PD, PDD/DLB) X working memory (2: lengthened, unlengthened) X compatibility (2: high-compatible, low-compatible) design, co-varying for age. This revealed a main effect for group that approached significance [F(2,58)=2.96; p<0.06]. PDD/DLB patients were significantly more impaired than PD patients and controls [p<0.05]. We also observed a significant interaction effect for group X working memory [F(2,58)=3.29; p<0.05], but we found no effect for the compatibility factor. PDD/DLB patients were significantly slower than controls in their processing of lengthened sentences [p<0.05]; this was true for both high compatibility sentences [p<0.05] and low compatibility sentences [p<0.05]. A paired-sample t-test also showed that PDD/DLB patients are significantly slower in their performance with lengthened sentences compared to the own performance with unlengthened sentences [t(16)=5.04; p<0.001]. This pattern was due in large part to the DLB subgroup (mean ±S.D. latency = 1488.45 ±422 msec), and these patients were significantly slower than the PDD subgroup (mean ±S.D. latency = 970.48 ±340 msec) for lengthened sentences [p<0.001]. By comparison, non-demented PD patients did not differ from controls in their processing of the lengthened sentences, nor in their processing of lengthened sentences compared to their own performance with unlengthened sentences.
FIGURE 1.
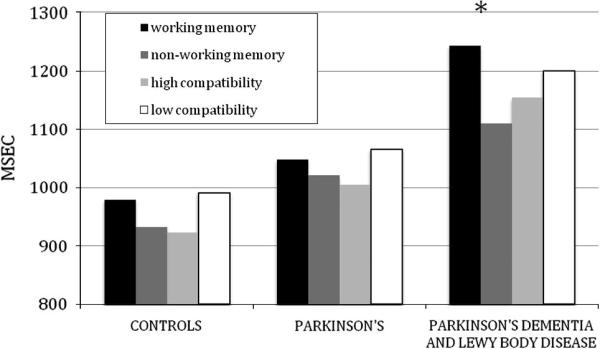
LATENCY PERFORMANCE FOR WORKING MEMORY AND COMPATIBILITY PERFORMANCE IN LEWY BODY SPECTRUM DISORDER PATIENTS AND CONTROLS
For the control stimuli, by comparison, a MANCOVA using the same design revealed significant main effect for group [F(2,58)=3.92; p<0.05] and for working memory [F(1,58)=7.52;p<0.01], but there was no interaction effect, emphasizing that no group suffered from a selective deficit for working memory burden when the probe was located in a position unrelated to a processing demand at two words prior to the critical processing window. In particular, PDD/DLB patients did not differ in their own performance for lengthened and unlengthened sentences.
We performed correlations to relate performance on the lengthened sentences to measures of executive control, grammatical comprehension and semantic processing. As shown in Table 2, latency to respond to the target word in experimental stimuli with lengthened sentences in PDD/DLB correlated with executive measures. We did not find significant correlations between lengthened sentence performance and other neuropsychological measures of grammatical comprehension and semantic processing in PDD/DLB. Nor did we find significant correlations between measures of executive control and performance with lengthened sentences in non-demented patients with PD. Although the small sample size warrants great interpretive caution, we also note that the DLB subgroup of patients had correlations that approach significance for measures of executive control [Trails B: r(6)=−0.66; p=0.07; Stroop: r(6)=−0.58; p=0.13] but other neuropsychological measures did not correlate with lengthened sentences. This pattern of performance was seen to a lesser extent in PDD [Trails B: r(5)=−0.33; p=0.47; Stroop: r(5)=0.62; p=0.13].
TABLE 2.
CORRELATIONS OF NEUROPSYCHOLOGICAL PERFORMANCE WITH STRATEGICALLY LENGTHENED SENTENCES IN LEWY BODY SPECTRUM DISORDER1
| PARKINSON’S DEMENTIA/ LEWY BODY DISEASE |
PARKINSON’S DEMENTIA |
LEWY BODY DISEASE |
PARKINSON’S DISEASE |
|
|---|---|---|---|---|
| EXECUTIVE | ||||
| Trails B | −0.58 (15)* | −0.33 (7) | −0.67 (8) | 0.19 (26) |
| Stroop | −0.62 (15)* | 0.63 (7) | −0.58 (8) | −0.14 (26) |
| GRAMMAR COMP | ||||
| Sent comp | −0.42 (6) | −0.15 (3) | −0.16 (3) | −0.34 (14) |
| Sent-pic match | −0.37 (9) | −0.33 (5) | −0.21 (4) | −0.62 (11)* |
| SEMANTIC | ||||
| Pyramid and Palm | −0.35 (16) | 0.05 (8) | −0.13 (8) | −0.10 (15) |
| Semantic category | 0.38 (10) | 0.04 (6) | 0.94 (4) | −0.07(22) |
NOTE* indicates significant correlation at p<0.05. Numbers in parentheses indicate the numbers of patients who performed the task. Not all patients performed all tasks because of scheduling difficulties, intercurrent unrelated medical issues, or technical difficulties.
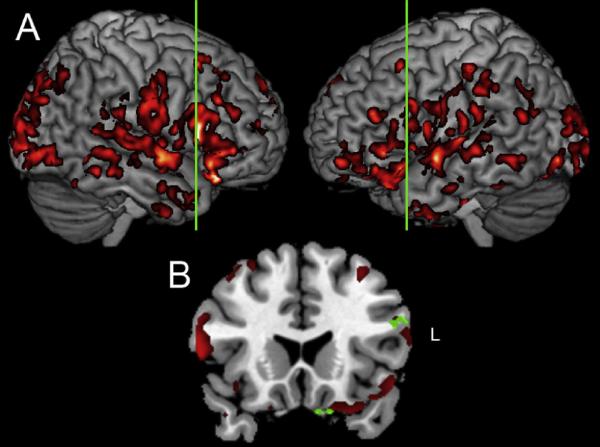
Imaging results
The imaging results are summarized in Figure 2, and the peaks of significantly thinned cortical areas are found in Table 3. Significant gray matter atrophy in LBSD compared to controls can be seen in frontal, temporal, parietal, and occipital regions bilaterally (Figure 2, Panel A). A regression analysis related lengthened working memory stimuli to left dorsolateral prefrontal and left ventral medial frontal regions (Figure 2, Panel B) as well as right inferior parietal cortex (not shown).
FIGURE 2.
PANEL A: GRAY MATTER THINNING (RED-YELLOW) IN LEWY BODY SPECTRUM DISORDER, AND
PANEL B: REGRESSION ANALYSIS (GREEN) RELATING RESPONSE LATENCY FOR EXPERIMENTAL SENTENCES LENGTHENED AT THE CRITICAL LOCUS OF AMBIGUITY RESOLUTION TO GRAY MATTER ATROPHY1
NOTE
1. Vertical green line in Panel A indicates location of coronal slice used to illustrate regression analysis in Panel B (y=56).
TABLE 3.
PEAK COORDINATES OF SIGNIFICANT AREAS OF CORTICAL THINNING IN LEWY BODY SPECTRUM DISORDER, AND SIGNIFICANT REGRESSIONS RELATING CORTICAL THINNING TO SENTENCE PROCESSING PERFORMANCE
| Anatomic Locus (Brodmann Area) | Coordinates | z-score | Cluster Size (voxels) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| LEWY BODY SPECTRUM DISORDER ATROPHY | |||||
| L inferior frontal (47) | −63 | 2 | −4 | 4.19 | 3477 |
| L inferior frontal (47/11/38) | −52 | 22 | −12 | 4.07 | 4331 |
| L inferior frontal (47/45) | −56 | 33 | −2 | 3.87 | 1234 |
| L inferior frontal (47) | −49 | 14 | −1 | 3.47 | 197 |
| L inferior frontal (11) | −35 | 40 | −18 | 3.09 | 310 |
| L inferior frontal (10/11) | −32 | 55 | −10 | 3.65 | 599 |
| L dorsolateral prefrontal (45/10) | −46 | 40 | 19 | 3.78 | 451 |
| L dorsolateral prefrontal (9/46) | −51 | 16 | 27 | 3.49 | 890 |
| L dorsolateral prefrontal (8) | −32 | 21 | 46 | 3.56 | 247 |
| L medial frontal (10) | −6 | 46 | 10 | 3.42 | 290 |
| L medial frontal (11/10) | −4 | 48 | −14 | 3.78 | 1104 |
| L anterior cingulate (24/32) | −7 | 3 | 37 | 3.24 | 504 |
| L insula (13) | −45 | −15 | 15 | 3.13 | 638 |
| R inferior frontal (45/44/47) | 56 | 19 | 5 | 4.73 | 11918 |
| R dorsolateral prefrontal (9/46) | 45 | 31 | 26 | 3.69 | 1171 |
| R dorsolateral prefrontal (10) | 34 | 56 | 11 | 3.11 | 444 |
| R dorsal frontal (6) | 33 | −2 | 45 | 3.19 | 241 |
| R medial frontal (10/32) | 10 | 50 | −1 | 3.18 | 681 |
| R medial frontal (9/10) | 1 | 58 | 30 | 3.12 | 1147 |
| L posterolateral temporal (37/27) | −23 | −36 | −8 | 4.05 | 3790 |
| L posterolateral temporal (20/21) | −42 | −1 | −39 | 3.76 | 1539 |
| L posterolateral temporal (22/40) | −60 | −51 | 22 | 3.46 | 719 |
| L posterolateral temporal (21) | −63 | −45 | −3 | 3.42 | 743 |
| L posterolateral temporal (21/20) | −67 | −37 | −18 | 3.31 | 202 |
| L posterolateral temporal (21/20) | −57 | −10 | −19 | 3.13 | 277 |
| L anterior temporal (38/13) | −30 | 5 | −12 | 3.34 | 1562 |
| L medial temporal (36/28) | −24 | −8 | −31 | 3.61 | 659 |
| L temporal-occipital (37/19) | −44 | −58 | −10 | 3.29 | 978 |
| R posterolateral temporal (21/22) | −50 | −77 | −3 | 3.96 | 1233 |
| R posterolateral temporal (37/20) | 46 | −55 | −16 | 3.51 | 2142 |
| R posterolateral temporal (21/22) | 67 | −35 | 1 | 3.45 | 1309 |
| R posterolateral temporal (21/20) | 65 | −43 | −10 | 3.45 | 339 |
| R posterolateral temporal (40/42) | 63 | −27 | 23 | 3.26 | 852 |
| R anterior temporal (21/22) | 58 | −3 | −5 | 4.02 | 14484 |
| R middle temporal (20) | 40 | −12 | −31 | 3.18 | 946 |
| R temporal-occipital (21/37) | −20 | −99 | 11 | 3.43 | 3040 |
| L inferior parietal (43/40) | −67 | −15 | 18 | 3.58 | 693 |
| L inferior parietal (40/2/1) | −59 | −22 | 24 | 3.49 | 967 |
| L superior parietal (2/40) | −63 | −25 | 36 | 3.34 | 730 |
| L anterior parietal (2/1/3) | −54 | −19 | 48 | 3.71 | 2018 |
| L lateral parietal-occipital (19) | 40 | −20 | 48 | 3.91 | 378 |
| R parietal-occipital (19/18/37) | 30 | −71 | 39 | 3.88 | 6768 |
| L lateral occipital (18) | −40 | −91 | −4 | 3.37 | 683 |
| L medial occipital (18) | −7 | −72 | −3 | 3.80 | 182 |
| L medial occipital (17) | −22 | −96 | −11 | 3.18 | 124 |
| R medial occipital (18) | 6 | −76 | −1 | 3.89 | 392 |
| R medial occipital (18/17) | 12 | −97 | 9 | 3.36 | 1040 |
| R medial occipital (19) | 19 | −89 | 32 | 3.20 | 1357 |
| R medial occipital (19) | 7 | −83 | 36 | 3.18 | 651 |
| R medial occipital (18/17) | 8 | −94 | −7 | 3.13 | 1411 |
| R posterior cingulate (31) | 3 | −59 | 25 | 3.14 | 1426 |
| REGRESSION ANALYSES | |||||
| EXPERIMENTAL SENTENCES LENGTHENED AT THE CRITICAL LOCUS OF AMBIGUITY RESOLUTION | |||||
| L dorsolateral prefrontal (45) | −51 | 20 | 20 | 3.16 | 57 |
| L medial frontal (11) | −8 | 17 | −24 | 3.21 | 156 |
| R frontal (4) | 50 | −8 | 40 | 3.26 | 71 |
| R inferior parietal (40) | 53 | −29 | 22 | 3.16 | 63 |
DISCUSSION
The present study found that processing sentences with a temporary syntactic ambiguity is significantly impaired in the demented spectrum of patients with LBSD. They were particularly impaired with the ambiguous sentences requiring additional working memory support. This was related in part to disease in left frontal and parietal regions.
In the current study, we examined the ability of patients to establish intra-sentential relationships between words in sentences with relatively simple syntactic structures. Unlike previous work, the syntactic structure of the sentence stimuli in the present study was ambiguous. We found that LBSD patients are slowed in their processing of ambiguous stimuli. This effect was most evident in PDD/DLB patients, and particularly patients with DLB. This slowing appeared to be due in part to disproportionate difficulty with the subset of experimental stimuli where a prepositional phrase was inserted between the onset of the syntactic ambiguity and its resolution, implicating a working memory limitation in the patients’ deficit. Responses to the target word in these stimuli were significantly slowed, presumably because of the concurrent working memory demands that were occurring at the time that the target word occurred. Patients with PDD and DLB have working memory difficulty on neuropsychological measures, and correlation analyses associated difficulty on these experimental stimuli with their performance on measures of executive control. All sentence materials were fully coherent and we did not ask patients to make off-line, resource-demanding coherence judgments of the sentence’s grammatical or semantic properties. These findings are consistent with previous work showing that knowledge of the grammatical rules integrating sentence constituents is largely preserved in LBSD, but that supporting resources such as working memory may be difficult for LBSD patients to implement during complex grammatical processing (Grossman, Lee, et al., 2002; Grossman, Zurif, et al., 2002; Lee et al., 2003). Our observations are consistent with a model of sentence comprehension that includes linguistic components such as the syntactic rules that govern long-distance relationships between words in a sentence, and the recruitment of executive resources such as working memory as needed to help support sentence processing (Wingfield and Grossman, 2006).
Several studies have demonstrated difficulty processing homophones in LBSD (Chenery et al., 2008; Copland, 2003; Copland et al., 2000, 2001; Copland et al., 2009; Gadsby et al., 2008). These words are ambiguous because multiple meanings are associated with a single phonological representation. A priming procedure was used with varying inter-stimulus intervals to examine the time course for activating words with multiple meanings. The investigators reported that all meanings of a word become activated in PD when there is a very brief interval between the prime and the target, as seen in control subjects. While control subjects are able to identify the specific meaning of a homophone that is appropriate to its context during a longer inter-stimulus interval, PD patients had difficulty priming over this prolonged time course that is thought to reflect a more controlled form of processing (Copland et al., 2000, 2001). This has been attributed to a variety of executive control limitations. Competing accounts have included difficulty inhibiting competing representations and limited attention-mediated ability to sustain an appropriate representation in the face of decay over time (Copland, 2003; Gurd & Oliveira, 1996). More recently, Copland and his co-workers have shown difficulty with suppression of an incorrect meaning during a subsequent presentation of a priming pair (Copland et al., 2009). As in the present work, this implicates working memory limitations in the context of a sentence, where it is helpful to maintain a relevant meaning over a sustained period of time (Copland et al., 2000, 2001; Gadsby et al., 2008).
Other possible explanations of the patients’ impairment seem less likely. We do not think that this deficit can be attributed to degraded representations of grammatical structures since we focused on sentences with relatively simple active declarative structures, we contrasted these with sentences containing a simple sentence complement where the complementizer was removed, and we did not find a difference between these two types of sentence stimuli. Moreover, correlation analyses showed that performance in PDD/DLB was not related to neuropsychological measures of grammatical comprehension. We also evaluated the processing speed of patients when encountering a verb in a statistically less preferred or “low compatibility” sentential context compared to a statistically more common or “high compatibility” context. We did not find that executive resource limitations make the low compatibility sentences differentially more difficult in LBSD than the high compatibility sentences. This suggests that potential executive resource limitations that may be needed to switch between high- and low-compatibility sentences are not differentially impaired. LBSD patients are generally slowed in their information processing, but we do not think that a slowed motor response alone can explain the patients’ deficit. While analyses of performance with control stimuli showed a main effect for group as well as a main effect for working memory, there was no interaction effect, emphasizing that responses to stimuli with greater working memory demands were not disproportionately affected in LBSD patients when the target was located in a position that is not associated with processing working memory demands.
In the present study, non-demented patients with PD were carefully selected to rule out any evidence of a cognitive deficit. However, we did find a difference within the demented spectrum of LBSD patients: The DLB subgroup of LBSD patients was particularly impaired in their performance on this experimental measure. The source of the disproportionate deficit in DLB is not clear. On the basis of clinical observations, there has been a sustained attempt to distinguish between PDD and DLB. Patients with PDD typically present with a motor disorder, and cognitive difficulties may eventually emerge over a matter of years. By comparison, patients with DLB typically present with cognitive difficulties but only minimal motor impairment (McKeith, et al., 2005). Nevertheless, histopathologic examinations of the brains of patients with PDD and DLB reveal identical abnormalities, suggesting that these conditions are one and the same. Unfortunately, the numbers of patients with DLB or PDD were too small in the present study to pursue these issues empirically. Regardless of the specific basis for the discrepancy between PDD and DLB, our work suggests an intermediate position, where cognitive difficulties are statistically more pronounced in DLB than PDD, but the cognitive impairments in DLB and PDD are qualitatively similar. Observations such as these have led us to believe that patients with synuclein-immunoreactive inclusions may have a spectrum of clinical presentations, ranging from a purely motor disorder seen in non-demented patients with PD to an overwhelmingly cognitive syndrome seen in DLB. Additional work is needed to test this hypothesis by examining cognitive difficulties in PDD and DLB comparatively and in a longitudinal manner.
Few quantitative studies of MRI atrophy in LBSD have been published. We observed statistically significant gray matter thinning in frontal, temporal, parietal and occipital regions. We performed a regression analysis to relate performance on the present study to a specific distribution of cortical volume. This analysis revealed that left frontal and right parietal regions play a significant role in the deficits processing syntactically ambiguous sentence in LBSD. This is consistent with previous work showing that sentence expression deficits in LBSD are due in part to executive control limitations mediated by disease in frontal and parietal brain regions (Ash et al., 2011). Other studies of language have associated frontal brain regions with executive resources during language processing. For example, in an fMRI study of healthy young adults, we used the same stimuli as the present report to demonstrate frontal and parietal activation during processing of ambiguous sentences (Novais-Santos et al., 2007). Other fMRI studies examining working memory during sentence processing also have shown activation in this frontal region (Cooke et al., 2001, 2005). Regarding the relationship between working memory processing in ambiguous sentences and right inferior parietal cortical thinning, fMRI studies of healthy seniors have shown activation of this parietal area during the processing of non-ambiguous sentences with lengthened segments that stressed working memory (Grossman et al., 2002).
The basis for the association of ambiguous sentence processing difficulty with left ventral medial frontal cortex is less clear. While this area is not typically activated in studies of language processing, a recent fMRI study examining anaphora has demonstrated activation of this area during “risky” associations of a pronoun with an antecedent noun (McMillan et al., in press). From a neuroeconomic perspective, risk of miscommunication emerged in this study of anaphora due to the necessary association of a gender-marked pronoun with an antecedent, gender-neutral noun, and additional work is needed to examine this hypothesis. Finally, we observed an association of difficulty processing ambiguous sentences with a region of cortical thinning that has its peak in right motor cortex. The basis for this association is unclear. Additional work is needed to investigate the basis for these findings.
Several caveats should be kept in mind while interpreting the results of our study. We examined only a small number of patients, and follow-up work is needed to assess the group differences we described with larger numbers of patients. While we used an on-line technique to obtain response latencies reflecting the time course of sentence processing, we were able to relate these behavioral observations to a neural substrate only indirectly, and it would be valuable to obtain direct measures of the time course involved in processing these materials using techniques such as magnetoencephalography that are sensitive to fine-grained temporal and spatial resolution.
With these caveats in mind, we conclude that patients with LBSD are compromised in their processing of sentences with a temporary syntactic ambiguity. While several cognitive and linguistic components contribute to resolving an ambiguous sentence, it appears that an executive resource limitation involving working memory plays a prominent role in the difficulty interpreting ambiguous sentences in the demented spectrum of LBSD patients. Finally, regression analysis underlined the importance of frontal and parietal disease in their deficit.
Key Points.
Executive deficits contribute to ambiguous sentence processing
Executive deficits impair sentence processing in Lewy body spectrum disorders
This is related to frontal cortical thinning
Acknowledgments
This work was supported in part by NIH (NS53488, AG17586, NS44266, AG15116, and AG32953)
LITERATURE CITED
- Aarsland D, Litvan I, Salmon D, Galasko D, Wentzel-Larsen T, Larsen JP. Performance on the dementia rating scale in Parkinson’s disease with dementia and dementia with Lewy bodies: Comparison with progressive supranuclear palsy and Alzheimer’s disease. Journal of Neurology, Neurosurgery and Psychiatry. 2003;74:1215–1220. doi: 10.1136/jnnp.74.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash S, Gross RG, McMillan C, Boller A, Morgan B, Dreyfuss M, et al. Impairments of speech fluency in Lewy body spectrum disorder. Brain and Language. doi: 10.1016/j.bandl.2011.09.004. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash S, McMillan C, Gross RG, Cook P, Morgan B, Boller A, et al. The organization of narrative discourse in Lewy body spectrum disorder. Brain and Language. 2011 doi: 10.1016/j.bandl.2011.05.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B, Gee JC. Geodesic estimation for large deformation anatomical shape and intensity averaging. Neuroimage. 2004;23:S139–S150. doi: 10.1016/j.neuroimage.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Avants B, Yushkevich P, Pluta J, Minkoff D, Korczykowski M, Detre J, et al. The optimal template effect in hippocampus studies of diseased populations. Neuroimage. 2010;49(3):2457–2466. doi: 10.1016/j.neuroimage.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, D’Esposito M. Functional Magnetic Resonance Imaging Evidence for a Hierarchical Organization of the Prefrontal Cortex. [Article] Journal of Cognitive Neuroscience. 2007;19(12):2082–2099. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- Bastiaanse R, Leenders KL. Language and Parkinson’s disease. Cortex. 2009;45(8):912–914. doi: 10.1016/j.cortex.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Bayles KA. Language and Parkinson disease. Alzheimer Dis Assoc Disord. 1990;4(3):171–180. doi: 10.1097/00002093-199040300-00005. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Braun AR, Guillemin A, Hosey L, Varga M. The neural organization of discourse: An H215O-PET study of narrative production in English and American sign language. Brain. 2001;124:2028–2044. doi: 10.1093/brain/124.10.2028. [DOI] [PubMed] [Google Scholar]
- Brown RG, Marsden CD. Cognitive function in Parkinson’s disease: from description to theory. Trends Neurosci. 1990;13(1):21–29. doi: 10.1016/0166-2236(90)90058-i. [DOI] [PubMed] [Google Scholar]
- Brown RG, Soliveri P, Jahanshahi M. Executive processes in Parkinson’s disease -- random number generation and response suppression. Neuropsychologia. 2000;36:1355–1362. doi: 10.1016/s0028-3932(98)00015-3. [DOI] [PubMed] [Google Scholar]
- Burton EJ, Barber R, Mukaetova-Ladinska EB, Robson J, Perry RH, Jaros E, et al. Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain. 2009;132(Pt 1):195–203. doi: 10.1093/brain/awn298. [DOI] [PubMed] [Google Scholar]
- Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT. Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain. 2004;127(Pt 4):791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Forman SD, Franzen P, Berkowitz A, Braver TS, Nystrom LE, et al. Sensitivity of prefrontal cortex to changes in target probability: a functional MRI study. Human Brain Mapping. 2001;13:26–33. doi: 10.1002/hbm.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenery HJ, Angwin AJ, Copland DA. The basal ganglia circuits, dopamine, and ambiguous word processing: A neurobiological account of priming studies in Parkinson’s disease. Journal of the International Neuropsychological Society. 2008;14(03):351–364. doi: 10.1017/S1355617708080491. [DOI] [PubMed] [Google Scholar]
- Colman KS, Koerts J, van Beilen M, Leenders KL, Post WJ, Bastiaanse R. The impact of executive functions on verb production in patients with Parkinson’s disease. Cortex. 2009;45(8):930–942. doi: 10.1016/j.cortex.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Copland DA. The basal ganglia and semantic engagement: Potential insights from semantic priming in individuals with subcortical vascular lesions, Parkinson’s disease, and cortical lesions. Journal of the International Neuropsychological Society. 2003;9(07):1041–1052. doi: 10.1017/S1355617703970081. [DOI] [PubMed] [Google Scholar]
- Copland DA, Chenery HJ, Murdoch BE. Understanding Ambiguous Words in Biased Sentences: Evidence of Transient Contextual Effects in Individuals with Nonthalamic Subcortical Lesions and Parkinson’s Disease. Cortex. 2000;36(5):601–622. doi: 10.1016/s0010-9452(08)70541-0. [DOI] [PubMed] [Google Scholar]
- Copland DA, Chenery HJ, Murdoch BE. Discourse Priming of Homophones in Individuals With Dominant Nonthalamic Subcortical Lesions, Cortical Lesions and Parkinson’s Disease. [Article] Journal of Clinical & Experimental Neuropsychology. 2001;23(4):538. doi: 10.1076/jcen.23.4.538.1233. [DOI] [PubMed] [Google Scholar]
- Copland DA, Sefe G, Ashley J, Hudson C, Chenery HJ. Impaired semantic inhibition during lexical ambiguity repetition in Parkinson’s disease. Cortex. 2009;45(8):943–949. doi: 10.1016/j.cortex.2009.02.023. [DOI] [PubMed] [Google Scholar]
- Dalrymple-Alford JC, Kalders AS, Jones RD, Watson RW. A central executive deficit in patients with Parkinson’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 1994;57:360–367. doi: 10.1136/jnnp.57.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Double KL, Halliday GM, McRitchie DA, Reid WG, Hely MA, Morris JG. Regional brain atrophy in idiopathic parkinson’s disease and diffuse Lewy body disease. Dementia. 1996;7(6):304–313. doi: 10.1159/000106896. [DOI] [PubMed] [Google Scholar]
- Downes JJ, Priestley NM, Doran M, Ferran J, Ghadiali E, Cooper P. Intellectual, mnemonic, and frontal functions in dementia with Lewy bodies: A comparison with early and advanced Parkinson’s disease. Behav Neurol. 1998;11(3):173–183. [PubMed] [Google Scholar]
- Farag C, Troiani V, Bonner M, Powers C, Avants B, Gee JC, et al. Hierarchical organization of scripts: Converging evidence from fMRI and frontotemporal dementia. Cerebral Cortex. 2010;20:2453–2463. doi: 10.1093/cercor/bhp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby N, Arnott WL, Copland DA. An Investigation of Working Memory Influences on Lexical Ambiguity Resolution. Neuropsychology. 2008;22(2):209–216. doi: 10.1037/0894-4105.22.2.209. [DOI] [PubMed] [Google Scholar]
- Garnsey SM, Pearlmutter NJ, Myers E, Lotocky MA. The contributions of verb bias and plausibility to the comprehension of temporarily ambiguous sentences. Journal of Memory and Language. 1997;37:58–93. [Google Scholar]
- Gibson E, Pearlmutter NJ. Distinguishing serial and parallel processing. Journal of Psycholinguistic Research. 2000;29:231–240. doi: 10.1023/a:1005153330168. [DOI] [PubMed] [Google Scholar]
- Giladi N, Treves TA, Paleacu D, Shabtai H, Orlov Y, Kandinov B, et al. Risk factors for dementia, depression and psychosis in long-standing Parkinson’s disease. J Neural Transm. 2000;107(1):59–71. doi: 10.1007/s007020050005. [DOI] [PubMed] [Google Scholar]
- Girotti F, Soliveri P, Carella F, Piccolo I, Caffarra P, Musicco M, et al. Dementia and cognitive impairment in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51(12):1498–1502. doi: 10.1136/jnnp.51.12.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross RG, Camp E, Gunawardena D, McMillan C, Cook P, Morgan B, et al. Impairments of narrative comprehension in Lewy body spectrum disorders. 2011. submitted.
- Grossman M. Sentence processing in Parkinson’s disease. Brain and Cognition. 1999;40:387–413. doi: 10.1006/brcg.1999.1087. [DOI] [PubMed] [Google Scholar]
- Grossman M, Lee C, Morris J, Stern MB, Hurtig HI. Assessing resource demands during sentence processing in Parkinson’s disease. Brain and Language. 2002;80:603–616. doi: 10.1006/brln.2001.2630. [DOI] [PubMed] [Google Scholar]
- Grossman M, Zurif EB, Lee C, Prather P, Kalmanson J, Stern MB, et al. Information processing speed and sentence comprehension in Parkinson’s disease. Neuropsychology. 2002;16:174–181. doi: 10.1037//0894-4105.16.2.174. [DOI] [PubMed] [Google Scholar]
- Gurd JM, Oliveira RM. Competitive inhibition models of lexical-semantic processing: Experimental evidence. Brain and Language. 1996;54:414–433. doi: 10.1006/brln.1996.0083. [DOI] [PubMed] [Google Scholar]
- Harrington CR, Perry RH, Perry EK, Hurt J, McKeith IG, Roth M, et al. Senile dementia of Lewy body type and Alzheimer type are biochemically distinct in terms of paired helical filaments and hyperphosphorylated tau protein. Dementia. 1994;5(5):215–228. doi: 10.1159/000106727. [DOI] [PubMed] [Google Scholar]
- Hochstadt J. Set-shifting and the on-line processing of relative clauses in Parkinson’s disease: results from a novel eye-tracking method. Cortex. 2009;45(8):991–1011. doi: 10.1016/j.cortex.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Amunts K, Bhattacharyya R, Patkin D, Jeffries K, Zilles K, et al. Activation of Broca’s area during the production of spoken and signed language: a combined cytoarchitectonic mapping and PET analysis. Neuropsychologia. 2003;41:1868–1876. doi: 10.1016/s0028-3932(03)00125-8. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCarthy G. Decisions under Uncertainty: Probabilistic Context Influences Activation of Prefrontal and Parietal Cortices. J. Neurosci. 2005;25(13):3304–3311. doi: 10.1523/JNEUROSCI.5070-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46(3):786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg G, Lakshmanan BM, Caplan D, Holcomb PJ. Making sense of discourse: An fMRI study of causal inferencing across sentences. Neuroimage. 2006;33:343–361. doi: 10.1016/j.neuroimage.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Holcomb PJ, Sitnikova T, Greve D. Distinct patterns of neural modulation during the processing of conceptual and syntactic anomalies. J. Cogn. Neurosci. 2003;15:272–293. doi: 10.1162/089892903321208204. [DOI] [PubMed] [Google Scholar]
- Lee C, Grossman M, Morris J, Stern MB, Hurtig HI. Attentional resource and processing speed limitations during sentence processing in Parkinson’s disease. Brain and Language. 2003;85:347–356. doi: 10.1016/s0093-934x(03)00063-4. [DOI] [PubMed] [Google Scholar]
- Lees AJ. Parkinson’s disease and dementia. Lancet. 1985;1(8419):43–44. doi: 10.1016/s0140-6736(85)90986-9. [DOI] [PubMed] [Google Scholar]
- Marie RM, Barre L, Dupuy B, Viader F, Defer G, Baron JC. Relationships between striatal dopamine denervation and frontal executive tests in Parkinson’s disease. Neuroscience Letters. 1999;260:77–80. doi: 10.1016/s0304-3940(98)00928-8. [DOI] [PubMed] [Google Scholar]
- Mason RA, Just MA. Lexical ambiguity in sentence comprehension. Brain Research. 2007;1146:115–127. doi: 10.1016/j.brainres.2007.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RA, Just MA, Keller TA, Carpenter PA. Ambiguity in the brain: What brain imaging reveals about the processing of syntactically ambiguous sentences. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29:1319–1338. doi: 10.1037/0278-7393.29.6.1319. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- Novais-Santos S, Gee J, Shah M, Troiani V, Work M, Grossman M. Resolving sentence ambiguity with planning and working memory resources: Evidence from fMRI. NeuroImage. 2007;37(1):361–378. doi: 10.1016/j.neuroimage.2007.03.077. [DOI] [PubMed] [Google Scholar]
- Parashos SA, Johnson ML, Erickson-Davis C, Wielinski CL. Assessing cognition in Parkinson disease: use of the cognitive linguistic quick test. J Geriatr Psychiatry Neurol. 2009;22(4):228–234. doi: 10.1177/0891988709342721. [DOI] [PubMed] [Google Scholar]
- Pereira JB, Junque C, Marti MJ, Ramirez-Ruiz B, Bartres-Faz D, Tolosa E. Structural brain correlates of verbal fluency in Parkinson’s disease. Neuroreport. 2009;20(8):741–744. doi: 10.1097/WNR.0b013e328329370b. [DOI] [PubMed] [Google Scholar]
- Piatt AL, Fields JA, Paolo AM, Koller WC, Troster AI. Lexical, semantic, and action verbal fluency in Parkinson’s disease with and without dementia. J Clin Exp Neuropsychol. 1999;21(4):435–443. doi: 10.1076/jcen.21.4.435.885. [DOI] [PubMed] [Google Scholar]
- Price A, Shin JC. The impact of Parkinson’s disease on sequence learning: Perceptual pattern learning and executive function. Brain and Cognition. 2009;69(2):252–261. doi: 10.1016/j.bandc.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Price C, Grossman M. Verb agreements during on-line sentence processing in Alzheimer’s disease and frontotemporal dementia. Brain and Language. 2005;94:217–232. doi: 10.1016/j.bandl.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: Insights into function from anatomy and neuroimaging. Nature Reviews: Neuroscience. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Rodd JM, Davis MH, Johnsrude IS. The neural mechanisms of speech comprehension: fMRI studies of semantic ambiguity. Cereb. Cortex. 2005;15:1261–1269. doi: 10.1093/cercor/bhi009. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Oroz M, Jahanshahi M, Krack P, Litvan I, Macias R, Bezard E, et al. Initial clinical manifestations of Parkinson’s disease: Features and pathophysioogical mechanisms. Lancet Neurology. 2009;8:1128–1139. doi: 10.1016/S1474-4422(09)70293-5. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Passingham RE. Working memory for location and time: Activity in prefrontal area 46 relates to selection rather than maintenance in memory. Neuroimage. 2001;14:77–86. doi: 10.1006/nimg.2001.0784. [DOI] [PubMed] [Google Scholar]
- Samuel M, Ceballos-Baumann AO, Blin J, Uema T, Boecker H, Passingham R, et al. Evidence for lateral premotor and parietal overactivity in Parkinson’s disease during sequential and bimanual movements: A PET study. Brain. 1997;120:963–976. doi: 10.1093/brain/120.6.963. [DOI] [PubMed] [Google Scholar]
- Sauer J, ffytche DH, Ballard C, Brown RG, Howard R. Differences between Alzheimer’s disease and dementia with Lewy bodies: an fMRI study of task-related brain activity. Brain. 2006;129:1780–1788. doi: 10.1093/brain/awl102. [DOI] [PubMed] [Google Scholar]
- Smith CD, Anderson AH, Kryscio RJ, Schmitt FA, Kindy MS, Blonder LX, et al. Differences in functional magnetic resonance imaging activation by category in a visual confrontation naming task. Journal of Neuroimaging. 2001;11:165–170. doi: 10.1111/j.1552-6569.2001.tb00028.x. [DOI] [PubMed] [Google Scholar]
- Smith EE, Marshuetz C, Geva A, Grafman J. Handbook of Neuropsychology. Volume 7. Elsevier Science; New York: 2002. Working memory: Findings from neuroimaging and patient studies; pp. 55–72. [Google Scholar]
- Talairach J, Tournaux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme; New York: 1988. [Google Scholar]
- Tam CWC, Burton EJ, McKeith IG, Burn DJ, O’Brien JT. Temporal lobe atrophy on MRI in Parkinson disease with dementia: A comparison with Alzheimer disease and dementia with Lewy bodies. Neurology. 2005;64(5):861–865. doi: 10.1212/01.WNL.0000153070.82309.D4. [DOI] [PubMed] [Google Scholar]
- Troiani V, Fernandez-Seara MA, Wang Z, Detre JA, Ash S, Grossman M. Narrative speech production: An fMRI study using continuous arterial spin labeling. NeuroImage. 2008;40(2):932–939. doi: 10.1016/j.neuroimage.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK. Real-time comprehension processes in agrammatism: a case study. Brain and Language. 1985;26:259–275. doi: 10.1016/0093-934x(85)90042-2. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cognitive, Affective and Behavioral Neuroscience. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Weigand SD, Shiung MM, Boeve BF, Ferman TJ, Smith GE, et al. Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer’s disease. Brain. 2007;130:708–719. doi: 10.1093/brain/awl388. [DOI] [PMC free article] [PubMed] [Google Scholar]