Abstract
Background
The use of long-chain fatty acids (LCFAs) for energy is inhibited in inherited disorders of long-chain fatty acid oxidation (FAO). Increased energy demands during exercise can lead to cardiomyopathy and rhabdomyolysis. Medium-chain triglycerides (MCTs) bypass the block in long-chain FAO and may provide an alternative energy substrate to exercising muscle.
Objectives
To determine the influence of isocaloric MCT versus carbohydrate (CHO) supplementation prior to exercise on substrate oxidation and cardiac workload in participants with carnitine palmitoyltransferase 2 (CPT2), very long-chain acyl-CoA dehydrogenase (VLCAD) and long-chain 3-hydroxyacyl CoA dehydrogenase (LCHAD) deficiencies.
Design
Eleven subjects completed two 45-minute, moderate intensity, treadmill exercise studies in a randomized crossover design. An isocaloric oral dose of CHO or MCT-oil was administered prior to exercise; hemodynamic and metabolic indices were assessed during exertion.
Results
When exercise was pretreated with MCT, respiratory exchange ratio (RER), steady state heart rate and generation of glycolytic intermediates significantly decreased while circulating ketone bodies significantly increased.
Conclusions
MCT supplementation prior to exercise increases the oxidation of medium chain fats, decreases the oxidation of glucose and acutely lowers cardiac workload during exercise for the same amount of work performed when compared with CHO pre-supplementation. We propose that MCT may expand the usable energy supply, particularly in the form of ketone bodies, and improve the oxidative capacity of the heart in this population.
INTRODUCTION
Long-chain fatty acid oxidation (FAO) disorders are the result of recessively inherited deficiencies of enzymes in the mitochondrial fatty acid β-oxidation pathway. Subjects with long-chain FAO disorders often have recurrent episodes of rhabdomyolysis with elevated creatine kinase levels and chronic exercise intolerance1–3. Thus, patients are typically counseled on the potential risks of exercise and often adopt a relatively sedentary lifestyle.
Previous research has suggested that medium chain triglyceride (MCT) supplementation administered prior to exercise improves exercise tolerance in individuals with long-chain 3-hydroxyacyl CoA dehydrogenase (LCHAD) deficiency. A decrease in steady-state heart rate (HR) has also been observed in participants supplemented with MCT oil prior to exercise, suggesting a cardiac benefit 4. Changes in heart rate are noteworthy due to an increased incidence of cardiomyopathy among patients with long-chain fatty acid oxidation disorders 5–7. MCT bypasses the block in long-chain fatty acid (LCFA) oxidation and may provide a usable source of fatty acids for both the heart and exercising skeletal muscle. In a previous study, no significant difference in respiratory exchange ratio (RER) was observed when orange juice + MCT versus orange juice alone were administered prior to exercise 4, however these supplements were not isocaloric. Only subjects with LCHADD were included in this initial study; subjects with other long-chain FAO disorders that affect exercise tolerance may also benefit from a pre-exercise MCT supplement. The aims of the present study were to determine the effect of isocaloric MCT vs. CHO supplementation prior to exercise on substrate oxidation and cardiac function during submaximal exercise in subjects with a variety of long-chain FAO disorders. We hypothesized that subjects with different long-chain FAO disorders would respond to pre-exercise MCT supplementation in a similar manner.
METHODS
Recruitment
Subjects were recruited from previous studies and by physician referral. The first subject to enroll in this study began in 2007; in 2010, the last subject completed the trial. The inclusion criteria involved a confirmed diagnosis of a long-chain fatty acid oxidation disorder including very long-chain acyl-CoA dehydrogenase (VLCAD; OMIM # 201475), long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD; OMIM #609016), mitochondrial trifunctional protein (TFP; OMIM #609015) or carnitine palmitoyltransferase-2 (CPT2; OMIM #255110) deficiency, seven years of age or older and the ability to comply with the study protocol. The study was approved by the OHSU Institutional Review Board (OHSU eIRB 817, www.clinicaltrials.gov NCT00654004). Subjects over 18 years of age gave written informed consent to participate. Each participant under 18 years of age provided written assent in addition to written informed consent from a legal guardian.
Study design
Each subject completed the treadmill exercise test twice, at baseline and 4 months, in a randomized crossover design. Subjects were admitted to the inpatient research unit at OHSU for 3 nights per visit. A random sequence allocation was used to determine the order in which the pre-exercise supplement was administered.
Pre-Exercise Supplement & Dual-energy X-ray Absorptiometry (DEXA)
Lean body mass (LBM) in kilograms was determined by DEXA prior to treadmill testing. MCT, (8 kcals/gm), was administered prior to exercise testing relative to LBM at 0.5g MCT/kg LBM, and was then added to approximately 12 ounces of a calorie-free beverage. For the CHO (4 kcals/gm) supplemented exercise test, subjects consumed a full calorie juice or soda beverage at 1 gm CHO /kg LBM to achieve isocaloric supplementation.
Exercise Testing
Patients began treadmill ergometry 2 hours after a standardized low-fat lunch that provided approximately 25% of estimated total energy requirements. Randomized supplementation was administered 20 minutes prior to exercise. Four months later, subjects received the alternate beverage supplement and repeated treadmill ergometry at the same grade, speed and duration as the first test to keep work performed constant between trials.
Treadmill testing was performed on an SMC 2000 treadmill (Sensor Medics, Yorba Linda, CA) with continuous ECG monitoring using a Sensor Medics Cardiosoft digital system. Respiratory gases were collected using a mouthpiece or Hans-Rudolph mask and gas exchange was measured using a Sensor Medics VMAX 29 metabolic cart.
The exercise protocol was performed as follows: 3 minute warm up phase with a slow walk at 1.5 miles per hour at 0% grade followed by increases in rate and incline every 2 minutes until the subject’s heart rate achieved 60–70% of his/her predicted maximum heart rate. Subjects were asked to continue exercising at 60–70% of their predicted maximum heart rate for an additional 40 minutes after the warm up phase. Predicted maximum heart rate was calculated using the formula: 220 (beats per minute) – age in years. A 12-lead ECG was placed prior to exercise for the continual monitoring of the electrical activity and rate (bpm) of the heart during exercise. Using a blood pressure cuff, blood pressure (BP) data was also collected during exercise at least one time during the first 20 minutes and last 20 minutes of the test. Gas exchange was measured continuously. However, in some cases gas exchange was collected just during the first 15 minutes and the last 15 minutes of exercise, giving the subject a period of rest from wearing the collection apparatus. Heart rate, VO2 (mL/kg/min) & RER were recorded at one-minute intervals. Myocardial O2 consumption during exercise was estimated using a double product calculation, (systolic BP×HR), an indirect measure of ejection fraction (EF).
Blood Samples
An indwelling catheter was placed for repeated blood collection. Eight ml blood samples were drawn at pre-exercise (0 min), post-exercise (40 min), and recovery (60 min) time-points. Tetrahydrolipostatin was added to EDTA plasma to inhibit lipase hydrolysis of fatty acids ex-vivo. Free fatty acid levels were measured by a commercially available enzymatic kit that detects all medium and long-chain acyl-CoAs with 90–100% recovery (NEFA-2hr, Wako Chemicals USA, Inc, Richmond, VA). Acylcarnitines were measured in plasma by electrospray tandem mass spectrometry as previously described 8. Lactate, pyruvate, β-hydroxybutyrate and acetoacetic acid were measured in serum by gas chromatography-mass spectrometry 9.
Statistical Analysis
Blood analytes, and measures of exercise performance were collected for both exercise studies. Total area under the curve (TAUC) was calculated using the trapezoidal method for all parameters. The difference between the TAUC from the MCT and CHO supplemented tests were analyzed by paired t-tests with p < 0.05 being considered significant. The treatment effect and change over time during exercise testing was analyzed with a repeated measures (RM) ANOVA. Post-hoc paired t-tests were used to determine time point differences if the treatment effect was significant. Participants in whom accurate HR and/or RER data was not collected during a given time interval were excluded from the average and statistical analysis of that time point. Statistical analysis of blood analytes excluded participants in whom blood samples were not collected due to IV failure. All analysis was conducted using Prism Software (Version 5.0, Graphpad, La Jolla, CA).
RESULTS
Demographics
All subjects completed both arms of the trial and contributed to the results reported (see Table 1). Eight patients, (average age = 12 yrs), had a diagnosis (dx) of LCHADD. All LCHADD participants had alpha-subunit mutations. Three participants (average age = 25 yrs) with adult-onset CPT2 (n=2) and VLCAD (n=1) deficiencies also participated in this study.
Table 1.
Participant Characteristics
| Subject | Dx | Gene | Mutation | Residual Enzyme Activity in Cultured Fibroblasts |
Age (yrs) |
Gender | BMI |
|---|---|---|---|---|---|---|---|
| 1 | VLCAD | ACADVL #609575 |
Not identified | 0% of control VLCAD activity* |
20 | F | 31.3 |
| 2 | LCHAD | HADHA #600890 |
c. 1528G>C/ c.1528G>C |
17 | F | 27.8 | |
| 3 | LCHAD | HADHA #600890 |
c. 1528G>C/ c.1528G>C |
11.8** mol/min/mg protein |
14 | M | 19.3 |
| 4 | LCHAD | HADHA #600890 |
c.1528G>C/ c.1678C>T |
16 | M | 23.8 | |
| 5 | LCHAD | HADHA #600890 |
c. 1528G>C/ c.1528G>C |
7 | M | 16.6 | |
| 6 | CPT2 | CPT2 #600650 |
Not available | 20% of control CPT2 activity* | 37 | F | 29.9 |
| 7 | LCHAD | HADHA #600890 |
c. 1528G>C/ c.2102A>G |
7 | M | 19.2 | |
| 8 | LCHAD | HADHA #600890 |
c. 1528G>C/ c.1528C>T |
16 | F | 22.6 | |
| 9 | LCHAD | HADHA #600890 |
c.1528G>C/exon 3 splice A+3G |
9 | M | 27.4 | |
| 10 | CPT2 | CPT2 #600650 |
common mutations not detected*** |
0.060**** nmol/min/mg protein* |
17 | F | 22.7 |
| 11 | LCHAD | HADHA #600890 |
c.1528G> C/ c.1132C>T |
8 | M | 18.8 |
Dx = diagnosis; VLCAD = very long-chain acylCoA dehydrogenase deficiency; LCHAD = long-chain 3-hydroxyacylCoA dehydrogenase deficiency; CPT2= Carnitine Palmitoyltransferase 2 deficiency; Gene = specific gene with disease associated mutations & OMIM #; yrs = years; F = Female; M = male; BMI = body mass index (weight in kilograms/ (height in meters)2)
diagnosis confirmed by disease specific change in acylcarnitines during bouts of rhabdomyolysis
reference range for normal LCHAD activity = 43.6–89.9 nmol/min/mg protein
common mutations = p. 113S>L, p. 50P>H and p. 413 Q >fs; fs= frame shift
reference range for normal CPT 2 activity: 0.07 – 0.21 nmol/min/mg protein
Substrate Oxidation Data
Respiratory Exchange Ratio
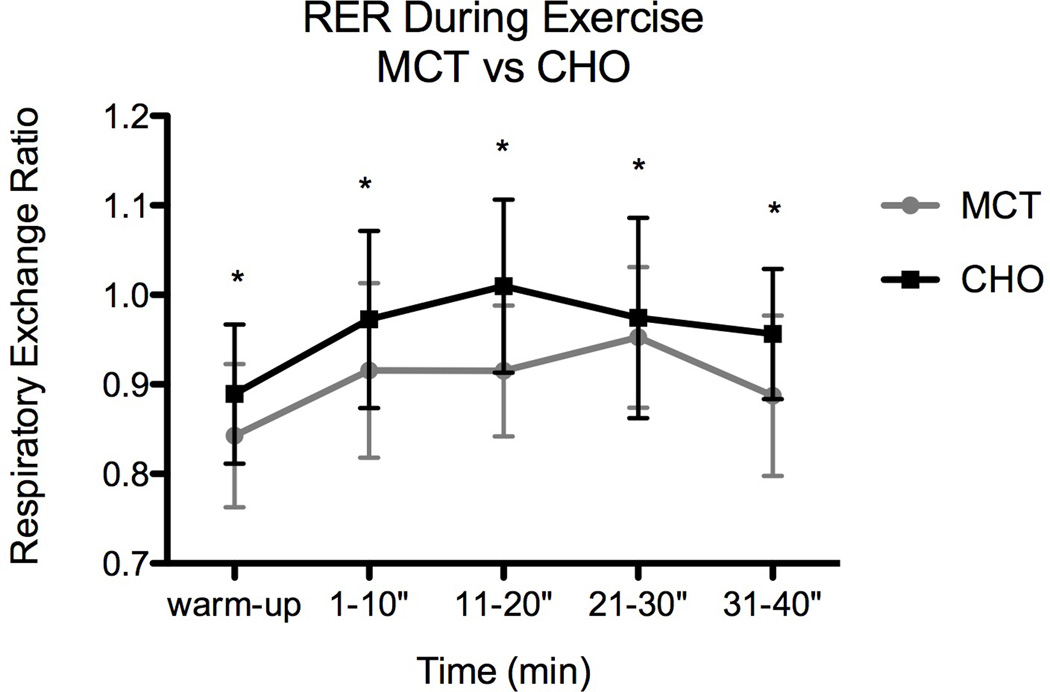
Total area under the curve (TAUC) for the MCT supplemented trial (3.648) was significantly lower than TAUC for the CHO supplemented trial (3.879; Figure 1). There was a significant MCT effect at baseline that increased over the course of the exercise trial [Fig 1 RM ANOVA: treatment (trt) p = 0.012, time p = 0.004]. The lower RER following MCT supplementation suggests that subjects oxidized more lipid during that bout of exercise than following CHO supplementation.
Fig 1.
Change in respiratory exchange ratio (RER) with and without MCT (*indicates significant difference). Standard deviation (SD) represented by error bars.
Oxygen Utilization
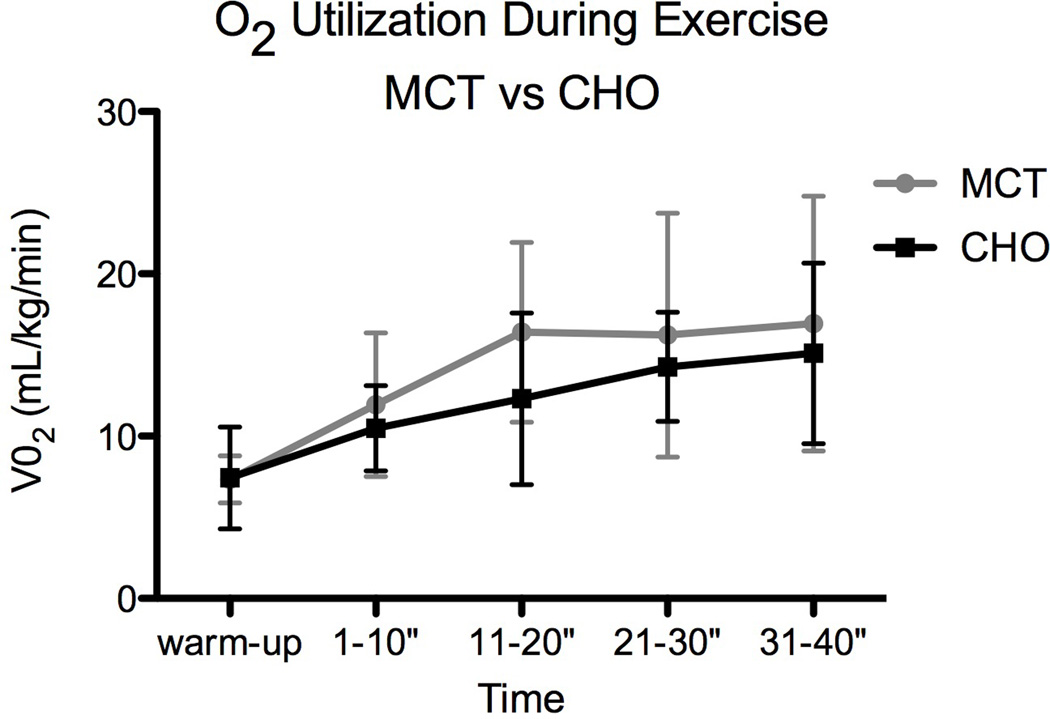
Because work performed was kept constant between trials, V02 in mL/kg/min did not significantly differ following MCT (VO2TAUC = 56.72) versus CHO (VO2TAUC = 48.34; Figure 2) supplementation. The level of exertion during both trials was approximately 4–4.5 metabolic equivalent tasks (METS). While treatment did not have a significant effect on V02, continuous exercise over 40 minutes did cause a significant increase in V02 over time (V02 RM ANOVA: trt p = 0.0805, time p < 0.0001).
Fig 2.
Change in oxygen utilization during exercise.
Ketones
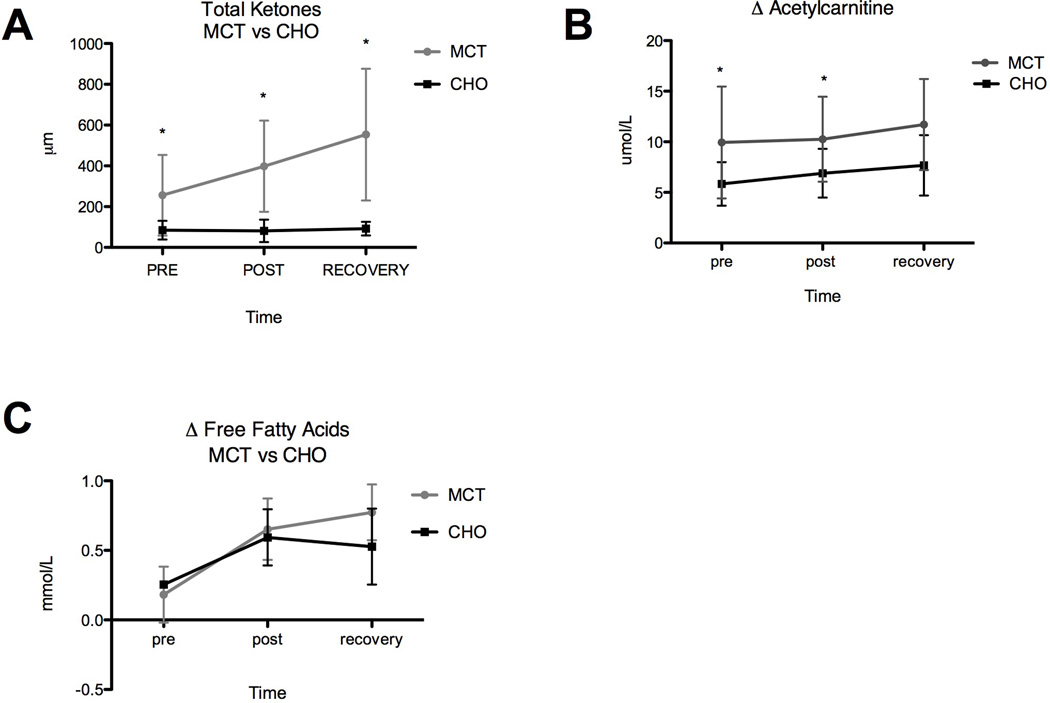
TAUC for serum ketone bodies (β-OH butyric acid and acetoacetic acid) was significantly higher when subjects (n=8) received MCT supplementation (TAUC = 802.8) prior to exercise versus pre-supplementation with CHO alone (TAUC = 169.8). The significant treatment effect was observed even prior to exercise (20 minutes after pre-exercise supplementation) but the increase with exercise over time was not significant (RM ANOVA: trt p < 0.0001; time p= 0.0723; see Figure 3a).
Fig 3.
(A) Change in total serum ketone bodies (B) acetylcarnitines and (C) free fatty acids, with and without MCT, measured immediately before, immediately after, and 20 minutes post exercise (*indicates significant difference).
Acetylcarnitine
TAUC for acetylcarnitine (C2) following pre-exercise supplementation with MCT (TAUC = 21.08) was significantly greater than TAUC following pre-supplementation with CHO alone (TAUC = 13.66; n=8). The effect of MCT was observed at both pre and post exercise time points but did not change over time (RM ANOVA: trt p = 0.001, time p = 0.412). The significant increase in circulating acetylcarnitine following pre-supplementation with MCT (see Fig 3b) mimics the simultaneous change in ketones and illustrates the use of MCT to generate both acetyl-CoA and ketones for energy.
Free Fatty Acids
Plasma FFAs TAUC did not differ significantly in response to MCT (TAUC = 1.130) versus CHO (TAUC = 0.9841) pre-supplementation, as illustrated by Figure 3c (n=4). Duration of exercise did, however, cause a significant rise in FFAs during both exercise trials (RM ANOVA: trt p = 0.3728, time p = 0.0009).
Lactate & Pyruvate
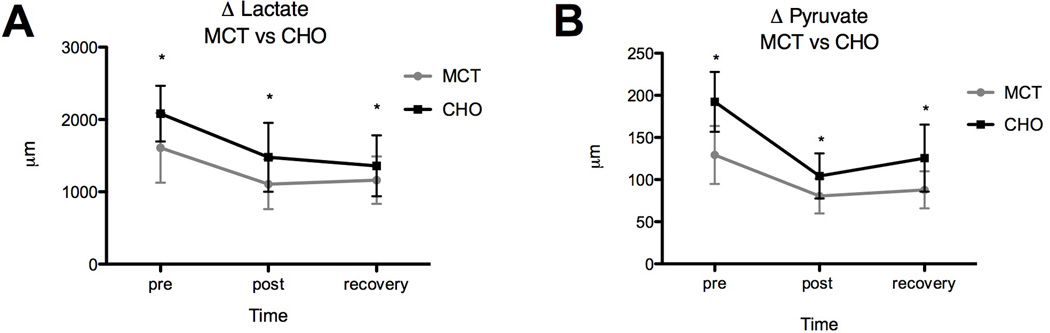
TAUC for serum lactate and pyruvate (see Figure 4a & 4b) was significantly lower when subjects (n=8) received MCT supplementation (lactate TAUC = 2491; pyruvate TAUC = 189.1) prior to exercise versus CHO alone (lactate TAUC = 3198; pyruvate TAUC = 263.3). The magnitude of the treatment effect decreased over time (lactate RM ANOVA: trt p = 0.0054, time p = 0.0002; pyruvate RM ANOVA: trt p < 0.0001, time p < 0.0001); plasma lactate and pyruvate dropped significantly during exercise, and plateaued or rose upon recovery.
Fig 4.
Difference in glycolytic intermediates during exercise with and without MCT; (A) change serum lactate, (B) change in serum pyruvate, measured immediately before, immediately after, and 20 minutes post exercise (*indicates significant difference).
Sum of Long-Chain Acylcarnitines
TAUC for the sum of long-chain acylcarnitines following pre-exercise supplementation with MCT (TAUC = 6.180) was not significantly different than TAUC following pre-supplementation with CHO alone (TAUC = 5.202). Neither treatment nor time significantly affected the sum of long-chain acylcarnitine species (RM ANOVA: trt p = 0.560, time p = 0.287). Despite significant changes in RER, the data suggests that in the presence of MCT, overall β-oxidation of LCFAs and the formation of long-chain acylcarnitines remains unchanged (Table 2).
Table 2.
Mean changes in long chain acylcarnitines during exercise
| Sum long-chain acylcarnitines*(nmol/L) | |||||
|---|---|---|---|---|---|
| MCT (n=8) | CHO (n=8) | MCT vs CHO | |||
| Mean | +/−SD | Mean | +/−SD | paired t test (p) | |
| Pre | 2.036 | 1.198 | 2.119 | 1.172 | 0.857 |
| Post | 3.592 | 3.303 | 2.685 | 1.510 | 0.349 |
| Recovery | 3.139 | 2.403 | 2.915 | 1.902 | 0.711 |
C12 + C12-0H + C12:1 + C12:1-0H + C14 + C14-0H + C14:1 + C14:1-0H + C14:2 + C16C + C16-0H + C16:1+ C16:1-0H+C18 + C18-0H+C18:1 + C18:1-0H + C18:2 + C18:2-0H
Creatine Kinase
All subjects fell within the normal range for creatine kinase (40–500 U/L) with the exception of 1 subject who exceeded this range before and after exercise pre-supplemented with CHO (see Table 3). Furthermore, there was not a significant difference in serum CK levels between interventions (TAUC MCT = 358.7, CHO = 530.6; RM ANOVA trt p = 0.3366, time p = 0.9785).
Table 3.
Change in creatine kinase levels during exercise with and without MCT
| MCT (n=8) | CHO (n=8) | Paired t-test | |||
|---|---|---|---|---|---|
| Mean | +/− SD | Mean | +/− SD | p | |
| Pre | 165 | 235 | 254 | 357 | 0.254 |
| Post | 182 | 243 | 265 | 370 | 0.287 |
| Recovery | 188 | 249 | 276 | 393 | 0.258 |
Heart Function Data
Heart Rate
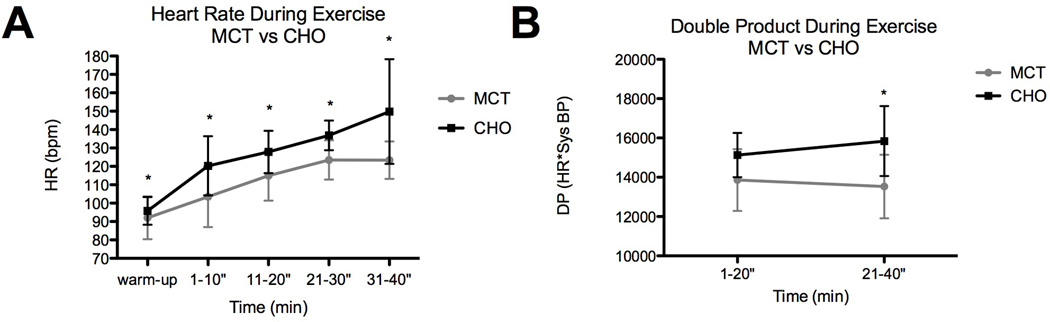
Heart rate was significantly lower during the exercise pretreated with MCT (TAUC = 449.7) when compared to CHO alone (TAUC = 507.9; RM ANOVA: trt p < 0.0001). A statistically significant difference was also observed between time-points (RM ANOVA: time p < 0.0001); the magnitude of the difference in HR increased with exercise (see Figure 5a).
Fig 5.
Difference in heart function during exercise with and without MCT; (A) change in heart rate, (B) change in double product.
Double Product
There was no difference in systolic BP between trials; therefore, as a result of the drop in HR, a significantly lower double product (DP) resulted when subjects were supplemented with MCT versus CHO alone (RM ANOVA trt p = 0.0134). Duration of exercise did not significantly affect DP (RM ANOVA time p = 0.7730). An average difference of 1265.67 in DP was observed during the first half of exercise; during the second half of exercise DP differed by 2311 (Figure 5b).
DISCUSSION
The current exercise study was designed to explore energy metabolism and cardiac performance during exercise in response to pre-exercise isocaloric supplementation with MCT versus CHO. The findings suggest that substrate utilization and cardiac workload were altered and may enhance the ability of individuals with defects in fatty acid oxidation to tolerate aerobic activity.
The significant decrease in RER following MCT supplementation indicates a change in substrate oxidation, with an increase in FAO and decrease in CHO oxidation. While the RER suggests a whole body increase in FAO, it does not differentiate between the oxidation of medium versus long-chain fatty acids. The measured difference in FAO was not related to a difference in FA availability because plasma FFA concentrations rose during both exercise tests; thus, the increase in FAO seems linked to the availability of the MCT as a substrate. While defects in long-chain β-oxidation diminish the use of long-chain fatty acids for energy, lipolysis and mobilization of fatty acids appear to occur normally in this population.
Following MCT supplementation, the significant increase in ketone production, in subjects who usually produce minimal ketones, suggests that the liver is able to oxidize MCT and produce ketones in the presence of MCT. The increase in acetylcarnitine with MCT supplementation suggests that acetyl-CoA is also generated as an energy source. The decrease in glycolytic intermediates suggests that MCT decreases the oxidation of CHO and may lower the risk of exercise-induced lactic acidosis in these patients.
With medium-chain fatty acids as an alternate lipid substrate, total oxidation of fat increased. However, unlike a previous study 4, the rate of partial LCFA β-oxidation and production of long-chain acylcarnitines were not altered. In a previous study, plasma OH-acylcarnitines were reduced, suggesting that total LCFA oxidation was suppressed following MCT supplementation 4. A difference between our current study and the previous research was that the participants’ metabolic control was better in the current study compared to those in the previous study. It is possible that because our subjects were already in excellent metabolic control, sums of hydroxyacylcarnitines could not be further improved upon. Both studies, however, demonstrate the ability of MCTs to change substrate oxidation during exercise by bypassing the defect in LCFA oxidation and increasing the oxidation of medium-chain fatty acids.
Work performed was kept constant between the two exercise tests by repeating the same treadmill grade and speed. However, a significant decrease in cardiac workload, identified by an approximate 20 bpm drop in HR, was observed following MCT pre-supplementation. This finding may be associated with the concurrent shift in cardiac substrate oxidation. The heart requires a constant supply of energy; in the presence of both carbohydrate and lipid, the heart will utilize fatty acid in preference to glucose 10,11. Furthermore, ketone bodies are oxidized in preference to fatty acids by cardiac muscle 12–14. In contrast to the oxidation of long-chain fatty acids and glucose, the oxidation of ketones is simple, requiring only a few steps and is not subject to any regulatory processes. In comparison with other tissues in the body, the enzymes required for the conversion of 3-hydroxybutyrate to acetyl CoA are highly active in the heart and facilitate the use of ketone bodies as an alternative energy source15,16.
Possible explanations of the change in cardiac workload observed in response to MCT pre-supplementation can be illustrated recognizing that cardiac output is a function of stroke volume and heart rate. Within subjects, cardiac output relates directly to oxygen uptake. As oxygen uptake was comparable between the two conditions, and because heart rate was lower, the stroke volume must have been greater with MCT supplementation.
Two hypotheses may explain that observation. First, MCT may have facilitated optimal contractile function and improved ejection fraction by increasing the preferred substrate of the myocardium, circulating ketone bodies.
Secondly, perhaps MCT itself temporarily provides a preferred substrate to generate ATP in the exercising cardiac and skeletal muscle. It is possible that in the absence of MCT and circulating ketone bodies, the heart compensates for the compromised ability to generate ATP by pumping harder, delivering more oxygen to the tissues in an effort to further ATP production and meet increased energy demands during a period of physical activity.
A similar change in HR has not been reported in healthy athletes given MCT prior to exercise 17,18. We suspect this finding is unique to subjects with a long-chain FAO disorder. We propose that MCT may expand the usable energy supply, such as ketone bodies, and improve the oxidative capacity of the heart, primarily during a period of increased workload.
In conclusion, MCT supplementation prior to exercise altered substrate oxidation and decreased heart rate during that bout of exercise. We believe this effect is unique to subjects with an inherited defect in long-chain FAO and would not be observed in otherwise healthy individuals18. Experimentally, it was necessary to separate MCT and CHO to understand the etiology of the differences we observed; however, in practice, MCT will be better tolerated when mixed with a carbohydrate-containing beverage, which can also be used for fuel. MCT supplementation of approximately 0.3–0.4 gm/kg total body weight mixed with a CHO containing beverage may allow subjects with a long-chain FAO disorder to safely exercise at a moderate intensity (60–70% max heart rate) for up to an hour. The definition of “moderate” is largely dependent on individual fitness; however, bicycling (<10 mph), briskly walking (3–3.4 mph) and light to moderate calisthenics have all been regarded as moderate intensity activities based on the standard MET 19. By altering substrate oxidation and decreasing heart rate in subjects with long-chain FAO disorders an active and healthy lifestyle can be facilitated.
Highlights.
Patients with inherited disorders of long-chain fatty acid oxidation can experience repeated episodes of rhabdomyolysis with exercise. Providing a pre-exercise supplement to fuel exercise induced energy demands such as carbohydrates or medium chain triglycerides (MCT) may improve exercise tolerance. Pre-exercise supplementation with MCT significantly increased fat oxidation and lowered exercise heart rate during a moderate intensity treadmill exercise compared to carbohydrate supplementation. MCT supplementation prior to exercise lowers cardiac workload in patients with long-chain fatty acid oxidation disorders.
Acknowledgements
The authors thank Gerard Vockley, MD, University of Pittsburgh, Georgirene D. Vladutiu, Ph.D. State University of New York, Buffalo NY, and Micheal Bennet, PhD, Children’s Hospital of Philidelphia for providing mutation and residual enzyme activity in cultured fibroblast results for our subjects.
Financial Disclosure:
Supported by the National Institutes of Health grant K01 NIDDK DK071869 (MBG) & Oregon Clinical & Translational Research Institute (OCTRI), grant ULI RR24140 from the National Center for Research Resources (NCRR)
Abbreviations
- AUC
area under the curve
- A-VO2
arteriovenous oxygen
- BMI
body mass index
- BP
blood pressure
- CHO
carbohydrate
- CK
creatine kinase
- CPT II
carnitine palmitoyl-transferase II
- CTRC
Clinical & Translational Research Center
- DEXA
dual energy x-ray absorpitometry
- DP
double product
- Dx
diagnosis
- ECG
electrocardiography
- EDTA
ethylenediaminetetraacetic acid
- EF
ejection fraction
- FAO
fatty acid oxidation
- FFA
free fatty acid
- FFM
fat free mass
- GC/MS
gas chromatography/mass spectrometry
- HR
heart rate
- IC
indirect calorimetry
- IRB
institutional review board
- IV
intravenous
- LBM
lean body mass
- LCFA
long-chain fatty acid
- LCHAD
long-chain 3-hydroxy acyl-CoA dehydrogenase deficiency
- MCT
medium chain triglyceride
- METS
metabolic equivalent tasks
- RER
respiratory exchange ratio
- RM
repeated measures
- RQ
respiratory quotient
- TAUC
total area under the curve
- TFP
trifunctional protein
- VCO2
rate of elimination of carbon dioxide
- VLCAD
very long-chain acyl-coenzyme A dehydrogenase
- VO2
oxygen uptake
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
The authors have no conflicts of interest relevant to this article to disclose.
References
- 1.den Boer ME, Wanders RJ, Morris AA, L IJ, Heymans HS, Wijburg FA. Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency: clinical presentation and follow-up of 50 patients. Pediatrics. 2002;109(1):99–104. doi: 10.1542/peds.109.1.99. [DOI] [PubMed] [Google Scholar]
- 2.Schaefer J, Jackson S, Dick DJ, Turnbull DM. Trifunctional enzyme deficiency: adult presentation of a usually fatal beta-oxidation defect. Ann Neurol. 1996 Oct;40(4):597–602. doi: 10.1002/ana.410400409. [DOI] [PubMed] [Google Scholar]
- 3.Vici CD, Burlina AB, Bertini E, et al. Progressive neuropathy and recurrent myoglobinuria in a child with long- chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency. J Pediatr. 1991;118(5):744–746. doi: 10.1016/s0022-3476(05)80039-3. [DOI] [PubMed] [Google Scholar]
- 4.Gillingham MB, Scott B, Elliott D, Harding CO. Metabolic control during exercise with and without medium-chain triglycerides (MCT) in children with long-chain 3-hydroxy acyl-CoA dehydrogenase (LCHAD) or trifunctional protein (TFP) deficiency. Mol Genet Metab. 2006 Sep–Oct;89(1–2):58–63. doi: 10.1016/j.ymgme.2006.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnet D, Martin D, Pascale De L, et al. Arrhythmias and conduction defects as presenting symptoms of fatty acid oxidation disorders in children. Circulation. 1999 Nov 30;100(22):2248–2253. doi: 10.1161/01.cir.100.22.2248. [DOI] [PubMed] [Google Scholar]
- 6.Moore R, Glasgow JF, Bingham MA, et al. Long-chain 3-hydroxyacylcoenzyme A dehydrogenase deficiency-- diagnosis, plasma carnitine fractions and management in a further patient. Eur J Pediatr. 1993;152(5):433–436. doi: 10.1007/BF01955905. [DOI] [PubMed] [Google Scholar]
- 7.Saudubray JM, Martin D, de Lonlay P, et al. Recognition and management of fatty acid oxidation defects: a series of 107 patients. J Inherit Metab Dis. 1999;22(4):488–502. doi: 10.1023/a:1005556207210. [DOI] [PubMed] [Google Scholar]
- 8.Smith EH, Matern D. Chapter 17 Acylcarnitine analysis by tandem mass spectrometry. Curr Protoc Hum Genet. 2010 Jan;(Unit 17 18):11–20. doi: 10.1002/0471142905.hg1708s64. [DOI] [PubMed] [Google Scholar]
- 9.Shoemaker JD, Elliott WH. Automated screening of urine samples for carbohydrates, organic and amino acids after treatment with urease. J Chromatogr. 1991 Jan 2;562(1–2):125–138. doi: 10.1016/0378-4347(91)80571-s. [DOI] [PubMed] [Google Scholar]
- 10.Neely JR, Morgan HE. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu Rev Physiol. 1974;36:413–459. doi: 10.1146/annurev.ph.36.030174.002213. [DOI] [PubMed] [Google Scholar]
- 11.Oey NA, den Boer ME, Wijburg FA, et al. Long-chain fatty acid oxidation during early human development. Pediatr Res. 2005 Jun;57(6):755–759. doi: 10.1203/01.PDR.0000161413.42874.74. [DOI] [PubMed] [Google Scholar]
- 12.Forsey RG, Reid K, Brosnan JT. Competition between fatty acids and carbohydrate or ketone bodies as metabolic fuels for the isolated perfused heart. Can J Physiol Pharmacol. 1987 Mar;65(3):401–406. doi: 10.1139/y87-067. [DOI] [PubMed] [Google Scholar]
- 13.Green A, Newsholme EA. Sensitivity of glucose uptake and lipolysis of white adipocytes of the rat to insulin and effects of some metabolites. Biochem J. 1979 May 15;180(2):365–370. doi: 10.1042/bj1800365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Little JR, Goto M, Spitzer JJ. Effect of ketones on metabolism of FFA by dog myocardium and skeletal muscle in vivo. Am J Physiol. 1970 Nov;219(5):1458–1463. doi: 10.1152/ajplegacy.1970.219.5.1458. [DOI] [PubMed] [Google Scholar]
- 15.Vanoverschelde JL, Wijns W, Kolanowski J, et al. Competition between palmitate and ketone bodies as fuels for the heart: study with positron emission tomography. Am J Physiol. 1993 Mar;264(3 Pt 2):H701–H707. doi: 10.1152/ajpheart.1993.264.3.H701. [DOI] [PubMed] [Google Scholar]
- 16.Williamson DH, Bates MW, Page MA, Krebs HA. Activities of enzymes involved in acetoacetate utilization in adult mammalian tissues. Biochem J. 1971 Jan;121(1):41–47. doi: 10.1042/bj1210041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeukendrup AE, Saris WH, Schrauwen P, Brouns F, Wagenmakers AJ. Metabolic availability of medium-chain triglycerides coingested with carbohydrates during prolonged exercise. J Appl Physiol. 1995;79(3):756–762. doi: 10.1152/jappl.1995.79.3.756. [DOI] [PubMed] [Google Scholar]
- 18.Jeukendrup AE, Thielen JJ, Wagenmakers AJ, Brouns F, Saris WH. Effect of medium-chain triacylglycerol and carbohydrate ingestion during exercise on substrate utilization and subsequent cycling performance. Am J Clin Nutr. 1998 Mar;67(3):397–404. doi: 10.1093/ajcn/67.3.397. [DOI] [PubMed] [Google Scholar]
- 19.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, Jacobs DR, Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000 Sep;32(9 Suppl):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]