Abstract
Although the selection of appropriate clinical sites has a significant impact on the successful conduct of clinical trials, no generally accepted model is available for site selection. Use of an appropriate site selection process is even more pertinent when conducting large scale, practical clinical trials in practice settings.
This report provides a rationale for selecting sites by identifying both a set of basic site selection criteria important to most trials as well as criteria specific to the features of a particular study’s design. In this two-tier system, although all these criteria must be met, some criteria are firm and viewed as essential for a site to conduct the trial. Other criteria, such as those that support study recruitment or participant retention, are flexible. These flexible criteria may be addressed through several alternative solutions that meet the original intent of the criterion.
We illustrate how the study specific features and requirements of Stimulant Reduction Intervention using Dosed Exercise (STRIDE), a multisite clinical trial evaluating the efficacy of exercise or health education, added to treatment as usual for stimulant abuse are linked to firm and flexible site selection criteria. We also present an iterative, multi-step approach to site selection including building awareness about the study and screening and evaluating sites using these criteria.
This simple model could maximize the chance that selected sites will implement a study successfully and achieve trial aims. It may be helpful to researchers who are developing criteria and methods for site selection for specific clinical trials.
Keywords: Site selection, clinical trials, effectiveness, efficacy, substance use, exercise
1. Introduction
Site selection has a significant impact on the successful conduct of clinical trials. Often clinical trials do not achieve recruitment targets, have substantial missing data due to attrition or missed contacts, or have other data quality concerns. Any of these can compromise achievement of trial aims or the generalizability of findings. In efficacy trials it is not uncommon for a third of sites to recruit no patients, and a third to recruit 70–80% of the targeted enrollment [1], raising questions about generalizability. Although there is substantial attention paid in the literature to selecting study participants, there is no generally accepted model or tools available for use in site selection. Matching the criteria for selection of study sites to the requirements and specific features of a trial increases the chances that the trial will be conducted efficiently and that sufficient data of good quality will be available to achieve the study aims.
Site selection criteria for efficacy trials focus on qualifications, experience and reputation of the investigator; availability of local patients, labs, data about the patient population, and presence and quality of the local Institutional Review Board (IRB) [1]; prevalence and incidence of the disorder to be studied [2]; and adequacy of staff and facilities, time, commitment, competing demands, security and storage [3]. These characteristics are necessary but not sufficient to support good study performance.
Clinical research networks [4], such as those for cancer or cardiovascular diseases [5,6], psychiatric disorders [7–9] and substance abuse [10] allow for the conduct of trials in real-world populations that are more generalizable than the typical efficacy samples recruited in research settings [11]. Descriptions of network trial infrastructures can be found in the literature [12,13]. However, we were unable to locate any guidance about how to select real-world sites that will perform well in a hybrid efficacy/effectiveness model for a practical clinical trial.
This report aims to provide a step in the development of a model and tools for use by researchers in selecting trial sites. It describes the rationale for determining site selection criteria and illustrates site selection methods for the Stimulant Reduction Intervention using Dosed Exercise (STRIDE) study. STRIDE is a multisite clinical trial evaluating a novel intervention for adults with stimulant abuse, treated in community-based addiction treatment programs in the National Institute on Drug Abuse’s (NIDA) Clinical Trials Network (CTN). The model for identifying selection criteria and selecting sites is applicable to trials at any point on the efficacy/effectiveness continuum.
2. METHODS
2.1. Description of the NIDA Funded Clinical Trials Network (CTN)
The CTN develops and implements clinical trials that generate and validate treatments that address the practical needs of community-based addiction treatment providers [10]. The CTN infrastructure includes geographically diverse nodes. Each node includes a university-based regional research training center, led by a Principal Investigator (PI), a researcher, and partnerships with public and private community-based addiction treatment providers and other providers that treat patients with addictions (Community Treatment Programs [CTPs]), referred to here as sites). The regional research training center provides research and administrative oversight of studies conducted in its affiliated sites with the assistance of NIDA’s Center for the Clinical Trials Network and central data and clinical coordinating centers. Lead Investigators of CTN studies select sites for trials from among the large group of community-based treatment programs affiliated with or interested in affiliation with the CTN.
2.2 Description of the Study
STRIDE, which is in the process of implementation at the time of submission of this manuscript, aims to compare the efficacy of high intensity exercise or health education added to substance abuse treatment as usual in 330 stimulant-abusing adults in nine addiction treatment sites. Description of the study rationale and design will be reported elsewhere. Participants are randomized to receive supervised exercise or health education three times a week during a 12-week acute phase intervention. This is followed by combined home based and weekly supervised exercise or weekly health education for 24 additional weeks in a continuation phase, a total of 60 scheduled visits. This is a marathon compared with most substance abuse treatment trials.
STRIDE is a hybrid efficacy effectiveness trial. Study interventions are added to treatment as usual in community-based treatment sites for patients with minimal exclusion criteria, enhancing external validity and generalizability. However, exercise is a novel intervention for the treatment of stimulant abuse. It is therefore also important to enhance internal validity to help determine the efficacy of this new intervention. Designed to meet both requirements, study entry occurs while each participant is in a restrictive residential treatment setting to increase the chance that participants will be present to receive the initial dose of study interventions. Interventions continue as the participant is discharged to community-based outpatient treatment, aftercare, and/or 12 step groups over the course of 36 weeks of drug treatment as usual. The primary outcome, percent of days abstinent, is evaluated during the acute phase of the study. The study is being conducted by a study Lead Investigator and study team (MHT, DW, TG, KR), located at the University of Texas Southwestern Medical Center. Research teams with a site Principal Investigator, project manager, two research coordinators, and exercise and health education facilitators are located at each participating site. It is being conducted in accordance with the principles of the Declaration of Helsinki. Sites were selected for two waves with wave 1 beginning enrollment about 7 months before wave 2 to allow for any implementation issues to be identified and resolved in a smaller group of sites.
2.3. Rationale for Determining Site Selection
To develop a set of criteria to use for site selection for STRIDE, we saw the need for basic standard site selection criteria as well as those driven by the study design. Table 1 summarizes both.
Table 1.
Site Selection Criteria for a Novel Intervention
| Criteria | Firm vs. Flexible | If Flexible, Alternate Solution |
|---|---|---|
| Basic Criteria Common to Most Trials | ||
| Access to study population | Firm | |
| Availability of space for research staff, equipment, and storage | Firm | |
| Ability to identify a principal investigator and research team from within or outside the site | Firm | |
| Access to an IRB | Firm | |
| Lack of competing demands that would interfere with conduct of the study | Firm | |
| Criteria Based on Study Features | ||
| Use of a novel intervention | ||
| Flexibility to allow specific study interventions and frequent study visits to be added to usual treatment | Firm | |
| Maintaining the integrity of the intervention | ||
| No formal exercise program greater than one hour per week or willing to restrict access to any existing exercise program during residential treatment | Firm | |
| Interventions continue as participant transitions among treatments | ||
| Offers continued treatment at various levels of intensity over extended periods of time, as close to 36 weeks as feasible | Flexible | Transition to community-based treatment close enough geographically to return to study visits or other solution that supports high likelihood of retention |
| Willing to allow patients to come back to study visits for 36 weeks, regardless of where they are receiving treatment and regardless of their relapse or treatment continuation status | Firm | |
| Maximizing initial dose of intervention/minimizing restrictiveness of setting after initial dose | ||
| Residential length of stay of about 3 to 4 weeks | Flexible | Willing to provide alternatives for 24 hour living arrangements or additional days of residential treatment if payor authorized days insufficient or has alternate means of maximizing the return of participants after discharge from residential treatment |
| Residential length of stay that does not exceed about a month while participant is randomized. Patients transition to community-based treatment with minimal restrictions on access to the community after the initial dosing period of 3 to 4 weeks. | Firm | |
| Maximizing recruitment, retention, and study visit attendance | ||
| Patient flow of 10–12 new stimulant abusing patients admitted to residential treatment a month | Flexible | Any that has a high probability of entering 2–3 participants per month |
| Strong retention in each phase of a site’s treatment program and in transitions between levels of care | Flexible | Any that has a high probability of retaining participants |
| Residential and outpatient programs are at the same location or in close physical proximity | Flexible | Any with a high probability of maximizing study attendance |
| Residential and outpatient programs are part of the same organization | Flexible | Any that will be effective in coordinating study visits between treatment and study and engaging treatment staff as appropriate across the continuum of care in retention efforts |
| Access to medical evaluation and maximal exercise testing | ||
| Access, within 24–48 hours, to a medical facility for evaluation | Firm | |
| Internet access | ||
| Internet access for web-based adherence program | Firm | |
| Administrative support | ||
| Administrative support for the specific study, understanding its burden on the site | Firm | |
| Strong minority representation in the study | ||
| Enough sites with good access to large minority populations | Flexible | Specific sites need not have good access to minority populations as long as sufficient selected sites do have this access |
2.3.1. Basic Site Selection Criteria
There are basic requirements for sites common to most trials. These characteristics, such as access to the study population; availability of space for research staff, equipment and storage; ability to identify a site Principal Investigator and research team; access to an IRB; and lack of competing demands, such as multiple other studies, that would interfere with the conduct of the study, were necessary for participation in STRIDE or any other study. All these requirements were firm.
2.3.2. Site Selection Criteria Associated with STRIDE Design Features
However, the STRIDE study design also has a number of novel features, developed to meet its requirements for internal and external validity, each of which suggested specific site characteristics that could increase the site’s chance of being successful in the study. Some criteria were firm requirements while others were more flexible indicators that signaled how a site might perform in key areas such as recruitment and retention. The flexible criteria had to be met but they could be met with an alternate solution that met the original intent of the criterion.
2.3.2.1. Use of a novel intervention – exercise or health education
Using exercise as an augmentation intervention with stimulant abusers is novel and study visits are integrated into a participant’s treatment day. A participating site therefore had to have the flexibility to allow this novel intervention to be added to usual treatment as well as to allow for frequent exercise or heath education study visits during the treatment programs.
2.3.2.2. Maintaining the integrity of the intervention
As well, since STRIDE evaluates the efficacy of exercise or health education, the dose of exercise received is best tracked and managed in both groups. Although step counters are worn by participants to measure activity levels, participants’ access to exercise outside study intervention visits had to be limited while in the residential setting. If the dose of exercise received by participants is dissimilar to that required by the study, it would be more difficult to assess whether our intervention is efficacious. As well, if the participants receiving health education increase their frequency of exercise, group differences could be attenuated. Sites could not have a formal exercise program greater than one hour per week or had to be willing to restrict access to exercise for participants in both treatment groups. This was a firm criterion applicable to the residential stay.
2.3.2.3. Interventions that continue as participants transition among settings and intensities of treatment, starting with residential treatment
The study was uniquely designed to initiate recruitment in the inpatient setting and continue for 36 weeks through the transition to outpatient treatment. This approach mirrors patients’ transitions among levels and intensities of care in the real world. Many trials complete their intervention in an acute phase and measure outcomes with assessments over the longer term. STRIDE, however, seeks to maximize the effect of study interventions by delivering them for an extended time period. The requirement that sites offer treatment at various levels of intensity for as close to 36 weeks as feasible was a flexible criterion intended to help maintain patients in the study. Other retention solutions could substitute for it.
Finally, since the study site is located on the residential or outpatient unit, sites had to be willing to allow patients to come back to study visits for the duration of the study, regardless of where they were receiving treatment and regardless of their relapse or treatment continuation status. This was a firm requirement.
2.3.2.4. Maximizing the initial dose of study intervention while minimizing restrictiveness of treatment setting after the initial dose to allow the possibility of variability in the outcome
Enrolling study participants while in residential treatment was a feature designed to increase the probability of receiving the initial 3 to 4 week dose of study interventions, maximizing internal validity. A site with a residential program with a length of stay of at about 3 to 4 weeks was therefore needed. However, sites could use flexible methods such as day treatment plus overnight boarding to keep patients in the equivalent of residential treatment if residential treatment was not authorized by a payor for sufficient time.
However, in order to evaluate the effects of study interventions on stimulant abuse access to drugs could not be severely restricted over the course of the period during which drug use primary outcome data is collected. Otherwise, our primary outcome measure, percent of days abstinent, risked not being sensitive enough to identify changes in drug use. This drove the need for a residential length of stay that did not exceed about a month so there would be minimal if any restrictions on community access after the initial 3 to 4 week dosing period during the 12-week acute phase of the trial. It was a firm requirement that the length of stay not be so long that the participant would be enrolled in the study for longer than about a month while in residential treatment.
2.3.2.5. Maximizing recruitment, retention, and study visit attendance of participants over the course of a 36-week study with a high frequency of study visits
The duration and frequency of STRIDE trial interventions is greater than in typical addiction trials. One of the greatest study challenges is keeping participants in the study for 36 weeks with good adherence to study visit attendance. Several selection criteria addressed this challenge.
First, to successfully enroll 2–3 subjects a month, we estimated that a site needed a patient flow of 10–12 newly admitted stimulant-abusing patients per month who lived in close enough proximity to the study site to reasonably expect them to come back to 60 scheduled study visits. Participants were expected to continue in treatment at or close to the study site post-discharge and were likely to remain in the area over the course of the study. This target would allow exclusion of those not meeting other study criteria or not interested in the time and effort required, while leaving enough participants to safely meet the enrollment targets. This target was flexible if a site could otherwise demonstrate that at least 2–3 participants per month could be enrolled in the study with fewer admissions per month or had a realistic strategy for increasing the pool of patients that could be considered for study entry.
Second, we reasoned that strong retention in the residential program (≥80%), in the transition from residential to outpatient treatment, and in the outpatient program would be good indicators of expected study retention and visit attendance since study visits would be scheduled in conjunction with treatment visits where feasible.
Third, if the outpatient program, residential treatment, and study site were at the same physical location or in sufficient physical proximity that participants could easily come for their study visit in conjunction with their outpatient treatment appointment, this would assist with study attendance.
Fourth, if the residential and outpatient programs were part of the same organization it would allow access to a consistent group of staff and administration at these programs with whom to liaison to coordinate appointment times between the study and treatment and to help identify the locations of missing participants. The last three sets of criteria were flexible since alternate retention and coordination plans could be considered.
2.3.2.6. Access to medical evaluation and maximal exercise testing for participants
All patients receive medical screening and a specialized evaluation, maximal exercise testing, prior to randomization, to ensure safety to exercise. A site needed easy access, within 24–48 hours, to a medical facility for this evaluation. If patients could not be screened quickly, too much time in the patients’ residential treatment period would be lost prior to study entry.
2.3.2.7. Internet access for the Web-based behavioral adherence monitoring program
The study includes an intensive Web-based adherence program with data about adherence to exercise or health education interventions entered by participants or research staff and participant-specific adherence prescriptions generated by these data. Although paper based adherence monitoring is available for participants without Web access, a site had to provide internet access for the exercise and health education facilitators to conduct the adherence program in addition to Internet access for study electronic data collection.
2.3.2.8. Administrative support for the specific study after understanding its burden on the site
Administrative support at the levels of the organization accountable for allocation of resources in the residential and outpatient treatment programs, as well as support throughout the treatment team who would be involved with the study research team was essential. While the study provides funds for personnel and costs, running a large and complex study adds burden to a site that cannot be quantified and reimbursed. The leadership and staff had to be willing to allow a study treatment that varied from their typical interventions and be able to support the specific study intervention. Site staff’s participation in identifying potential study participants and helping coordinate appointments between treatment visits and study visits had to be permitted and supported. With competing demands for space and staff time the facility leadership had to, after understanding the implications of implementing the study at the site based on discussion with the study team, express interest in the study, commitment to its success and willingness to support this priority with site staff.
2.3.2.9. Strong minority representation
Although complex systems have been designed to engage minority patients [14], selecting sufficient sites with good access to large minority populations [15] can be sufficient to meet minority recruitment goals as we identified in prior multisite trials [16]. This is a firm requirement of any generalizable clinical trial although it applies to adequate representation within the group of sites selected as opposed to within any specific site.
3. Results and Discussion
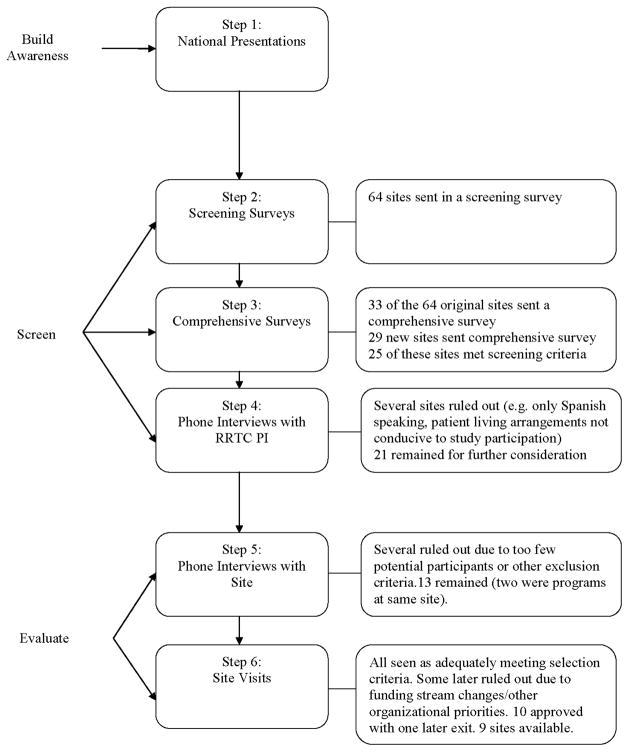
We knew the site selection process would not be easy. Figure 1 describes the steps in site selection: 1) building awareness among potential sites about the study; 2) screening with initial site selection screening surveys, follow-up comprehensive site surveys and interviews with node PIs; 3) evaluating candidate sites to select those that appeared best positioned to conduct the study successfully through site phone interviews and site visits, 4) identification of proposed sites and 5-) final review of proposed sites by study and NIDA oversight committees.
Figure 1.
STRIDE Site Selection Steps
3.1. Building awareness among potential sites about the study
Early in the process of study finalization we addressed awareness of the study thorough presentations about the study rationale and design at CTN meetings where sites had representation, national CTN conference calls, and conversations with the node PIs. This generated interest in the study and readiness to begin to recruit interested potential study sites from this national network of sites
3.2. Screening candidate sites
3.2.1. Initial site selection screening surveys
An initial screening survey was forwarded to the node PIs or their delegates to invite their partner sites to apply for consideration. Guidance about key site selection criteria as well as criteria for participant eligibility was sent to sites to allow them to evaluate their own match with the protocol (see Table 2). The screening survey included questions such as number of admissions of patients to residential treatment and percent admissions by drug of abuse; length of stay and retention rate for the residential program; availability, location, and length of stay for affiliated outpatient programs; percentage of patients that continue in outpatient care at the same location after discharge; and availability of resources such as space for exercising. Sixty-four sites responded to the initial screening questionnaire.
Table 2.
Sample Guidance Sent to Potential Sites for Self Evaluation
| The main challenge is to identify settings where we are most likely to retain participants in the study over the course of 12 weeks, and ideally the next 6 months as well. The following features in a CTP may make it a good candidate to be successful in this study: |
|
|
|
|
|
|
|
|
|
At this juncture it was important to confirm that sufficient sites would be available to conduct the study based on initial firm screening requirements—space for the study, residential stays of about 3 or 4 weeks, outpatient care at the same or a very close site or within the same organization; and patients transitioned into the community after about 21–30 days. A comprehensive database was built from the surveys received.
3.2.2. Comprehensive site selection surveys
As the study design was finalized we forwarded comprehensive surveys to the node PIs for completion by sites that responded to the screening surveys and were still interested. We also continued to recruit interested new sites. The comprehensive survey included detailed questions addressing all criteria (see Table 3 for sample questions). Many sites kept sufficient data to respond to survey questions while others provided estimates and collected specific data following our request. We received 33 comprehensive surveys from the 64 residential treatment programs that previously submitted screening surveys, reflecting their continued interest. Following several additional waves of site recruitment, we also received 29 submissions from new sites. Based on the screening requirements, 25 of these 62 sites were identified for further consideration.
Table 3.
Sample Questions from the Comprehensive Survey
| |
|
typical length of stay/duration_____ typical length of stay/duration_____ typical length of stay/duration_____ |
|
| |
| |
|
| |
| |
|
| |
| |
|
| |
| |
|
| |
| |
| 2 weeks? | 1 month? |
|
| |
| |
|
| |
| |
|
| |
| |
|
| |
| |
3.2.3. Interviews with node PIs
We discussed the strengths of each applicant site and the challenges the study would pose at that site with each site’s node PI. This focused site-specific issues to be addressed in later calls with the sites. Several sites were eliminated from consideration for reasons such as only having Spanish-speaking patients (assessments were not validated in Spanish), or treating populations with transient living arrangements and very poor likelihood of remaining in the study.
3.3. Evaluating Candidate Sites
3.3.1. Site Phone Interviews
Sites were then scheduled for a phone interview with the study team, including as feasible a site administrator who could commit resources, program leaders for residential and outpatient programs, the anticipated site PI and any research staff members, the node PI and oversight staff. A one-hour standardized interview was developed with specific questions individualized for each site based on the site’s responses to the comprehensive questionnaire (see Table 4 for sample questions). The interviews confirmed the accuracy of the information forwarded in the surveys, any areas where firm or flexible site criteria did not appear to be met and alternate solutions for the flexible indicators. For example, one site had an average admission rate of only 6–8 stimulant-abusing patients per month, below the expectation of 10–12. However, the facility’s director was willing to open up additional beds until the study enrollment goals were met. Since most patients admitted to the site abused stimulants, this was a good alternative solution. Another site did not have a strong retention rate in the transition from residential to outpatient treatment but had a research team very experienced with retaining participants in addiction studies and was able to list a variety of retention strategies.
Table 4.
Sample Phone Interview Questions
|
|
|
|
|
|
|
|
|
|
|
|
Several sites were eliminated due to too few admissions of stimulant abusing patients with no alternative solution to allow confidence that 2–3 participants a month could still be enrolled or other challenges in meeting the selection criteria that became clear during the calls.
3.3.2. Site Visits
Sites that met the firm criteria and met or had good alternative solutions for the flexible criteria were scheduled for a site visit.
At the visit, the site’s fit with all criteria was confirmed again. The visit focused on the challenges to successful study completion identified earlier and clarifying administrative support for the study. We also evaluated the acceptability of the identified space, addressed how study visits could dovetail treatment visits, reviewed prior experiences with research including challenges posed and their resolution, and gave the site the opportunity to evaluate their interest in working with the investigators.
3.4. Identification of proposed sites
Far from having multiple sites from which selections would have to be made, we were able to identify only 13 sites, two of which were men and women’s programs in the same organization, that were a match for the study. All criteria needed to be met to select the site since each one was identified a priori as important to the successful conduct of STRIDE. While an approach that weights criteria may be developed over time, at this point no data were available to support weighting.
Several sites subsequently excluded themselves due to funding stream changes that reduced the pool of available candidates for study participation or determining there were other organizational priorities. Ten sites were selected and approved with no viable site refused. Of these one additional site dropped out of the pool due to late funding stream changes. The identification of this relatively small number out of the large initial pool of sites was in part due to the complexity of the study, which had a number of requirements for both the residential and outpatient treatment settings. It was also related to having such comprehensive site criteria and an iterative process that permitted sites and investigators to rule sites in or out. The primary reasons for site exclusion included insufficient numbers of monthly residential treatment admissions of stimulant abusing patients, lack of availability of an on-site or very closely located outpatient treatment program, or insufficient patients who lived close enough to the facility to continue treatment in their outpatient program at discharge from residential treatment.
3.5. Review of sites by a study oversight committee and NIDA’s oversight committee
The final step was review of the sites selected for the first and then second wave of the study by peers on the STRIDE Executive Committee, the committee charged to oversee development and implementation of the study. This was followed by peer review by the CTN Executive Committee, the CTN’s oversight committee.
4. CONCLUSIONS
Despite the impact of site selection on the successful conduct of clinical trials there has been little guidance available for use by researchers in selecting sites.
We have presented a simple model for site selection. It includes (1) selecting study sites based on identifying a comprehensive set of basic criteria essential to most trials as well as criteria specific to the study design, (2) determining which are firm and which are flexible, and (3) using the criteria in an organized, sometimes iterative, multistep method to complete site selection. This could maximize the chance that selected sites will implement a study successfully and trial aims can be achieved.
Several pragmatic lessons evolved from our experience:
Comprehensiveness of site selection criteria is important. Criteria must be firm if the requirements are clearly needed to conduct a study or they may be flexible such as those that project information about how a site might perform in a study. For example, for STRIDE, absent safety issues, study participants had to be permitted to return to the study site, generally located in the treatment facility, even if they relapsed, or the study would fail to retain these participants. However, indicators that support study recruitment, retention or visit attendance are flexible and can be addressed with any alternate solution that meets the original intent of the criterion.
Data provided in a site survey questionnaire do not necessarily reflect what a study leadership team hopes to understand. On more than one occasion, despite carefully constructed questions, sites answered survey questions with assumptions different than the ones intended or data changed over the course of time. It is important to check the accuracy of and assumptions behind the responses to key questions.
A thorough process that allows a site to have several opportunities to carefully evaluate their match with a study themselves in addition to evaluation by the study team is important.
The timing of site selection is a consideration. In the real-world factors affecting the functioning of an organization such as administrations, funding sources, referral sources, or organizational priorities and cultures can change. Evaluating the match of a site and study close to the time of study initiation makes it more likely the match still exists at the onset of the study. Changes occurred in our site pool over the evaluation time period, changing the viability of some sites. Even after finalization of 10 sites a site withdrew from implementing the study due to funding issues. A search for additional sites did not result in any new additions to the pool and STRIDE was implemented at nine sites.
However promising a site may appear based on criteria, the study may not work well at that site. It is therefore essential to choose back-up study sites.
Some of the unique features of STRIDE, such as the need for a residential length of stay of about 3 to 4 weeks but not longer, an outpatient program in close physical proximity, and a patient flow of about 10–12 stimulant users a month made it challenging to identify study sites. This was due to variability in residential program lengths, admission rates for stimulant users, and availability of outpatient treatment close to the residential program and used by its population. Study feature-driven criteria such as these are not specifically generalizable to other studies. However, the principle of utilizing important study features to drive the site selection process is generalizable.
The success of study recruitment, retention and data collection in the sites identified with these selection criteria and comprehensive approach to site selection has yet to be determined. However, to fill the gap in the literature about the science of site selection, models and tools based on this and other trialists’ experiences are needed. It would be useful to see reports on this issue from other researchers.
Acknowledgments
This work is supported by the National Institute on Drug Abuse through the Clinical Trials Network for the Stimulant Reduction Intervention using Dosed Exercise (STRIDE) study, Madhukar H. Trivedi, M.D., Lead Investigator (U10DA020024).
Glossary
- IRB
Institutional Review Board
- STRIDE
Stimulant Reduction Intervention using Dosed Exercise
- CTN
Clinical Trials Network
- CTP
Community Treatment Program
- PI
Principal Investigator
Footnotes
DISCLOSURES
Diane Warden, Ph.D., M.B.A. has owned stock in Bristol Myers Squibb and Pfizer, Inc. in the last 5 years and has received funding from the National Alliance for Research in Schizophrenia and Depression.
Bruce D. Grannemann, M.A. has no disclosures to report.
Tracy Greer, Ph.D. has received research support from the National Alliance for Research in Schizophrenia and Depression.
Viviana E. Horigian, M.D. has no disclosures to report.
Tiffany Kyle, Ph.D. has no disclosures to report.
Edward Nunes, M.D. has received funding from NIDA for grants K24DA022412 (PI: Nunes) and U10DA13035 (PI: Nunes).
Kolette Ring, B.A. has no disclosures to report.
Eugene Somoza, M.D. has no disclosures to report.
Jose Szapocznik, Ph.D. has no disclosures to report.
Madhukar H. Trivedi, M.D. is a consultant to or on speaker bureaus for Abbott Laboratories, Inc., Abdi Ibrahim, Akzo (Organon Pharmaceuticals Inc.), AstraZeneca, Bristol-Myers Squibb Company, Cephalon, Inc., Cyberonics Inc., Eli Lilly & Company, Evotec, Fabre Kramer Pharmaceuticals, Inc., Forest Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica Products, LP, Johnson & Johnson PRD, Meade Johnson, Medtronic, Neuronetics, Otsuka Pharmaceuticals, Parke-Davis Pharmaceuticals, Inc., Pfizer Inc., Sepracor, SHIRE Development, Solvay Pharmaceuticals, VantagePoint, and Wyeth-Ayerst Laboratories. He receives research support from the Agency for Healthcare Research and Quality (AHRQ), Corcept Therapeutics, Inc., Cyberonics, Inc., Merck, National Alliance for Research in Schizophrenia and Depression, National Institute of Mental Health, National Institute on Drug Abuse, Novartis, Pharmacia & Upjohn, Predix Pharmaceuticals (Epix), Solvay Pharmaceuticals, Inc., and Targacept.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maggon K. Investigator and site selection and performing GCP clinical studies in India. Control Clin Trials. 2004;25(4):366–77. doi: 10.1016/j.cct.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Ferrantelli F, Buttò S, Cafaro A, Wahren B, Ensoli B. Building collaborative networks for HIV/AIDS vaccine development: the AVIP experience. Springer Semin Immunopathol. 2006;28(3):289–301. doi: 10.1007/s00281-006-0026-3. [DOI] [PubMed] [Google Scholar]
- 3.Demeter J. Selecting sites and investigators. An approach for Central and Eastern Europe. Applied Clinical Trials. 2002;11(3):56–66. [Google Scholar]
- 4.Green LA, Lutz LJ. Notions about networks: primary care practices in pursuit of improved primary care. In: Mayfield J, Grady ML, editors. Primary care research: an agenda for the 90s. Rockville (MD): Agency on Health Care and Research; 1990. pp. 125–35. [Google Scholar]
- 5.Bleyer WA. The US pediatric cancer clinical trials programmes: international implications and the way forward. Eur J Cancer. 1997;33(9):1439–47. doi: 10.1016/s0959-8049(97)00249-9. [DOI] [PubMed] [Google Scholar]
- 6.Topol EJ, Califf RM, Van de Werf F, Simoons M, Hampton J, Lee KL, White H, Simes J, Armstrong PW for the Virtual Coordinating Center for Global Collaborative Cardiovascular Research (VIGOUR) Group. Perspectives on large-scale cardiovascular clinical trials for the new millennium. Circulation. 1997;95(4):1072–82. doi: 10.1161/01.cir.95.4.1072. [DOI] [PubMed] [Google Scholar]
- 7.Rush AJ, Trivedi M, Fava M. Depression IV: STAR*D treatment trial for depression. Am J Psychiatry. 2003;160(2):237. doi: 10.1176/appi.ajp.160.2.237. [DOI] [PubMed] [Google Scholar]
- 8.Sachs GS, Thase ME, Otto MW, Bauer M, Miklowitz D, Wisniewski SR, Lavori P, Lebowitz B, Rudorfer M, Frank E, Nierenberg AA, Fava M, Bowden C, Ketter T, Marangell L, Calabrese J, Kupfer D, Rosenbaum JF. Rationale, design, and methods of the systematic treatment enhancement program for bipolar disorder (STEP-BD) Biol Psychiatry. 2003;53(11):1028–42. doi: 10.1016/s0006-3223(03)00165-3. [DOI] [PubMed] [Google Scholar]
- 9.Schneider LS, Ismail MS, Dagerman K, Davis S, Olin J, McManus D, Pfeiffer E, Ryan JM, Sultzer DL, Tariot PN. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE): Alzheimer’s disease trial. Schizophr Bull. 2003;29(1):57–72. doi: 10.1093/oxfordjournals.schbul.a006991. [DOI] [PubMed] [Google Scholar]
- 10.Tai B, Straus MM, Liu D, Sparenborg S, Jackson R, McCarty D. The first decade of the National Drug Abuse Treatment Clinical Trials Network: Bridging the gap between research and practice to improve drug abuse treatment. J Subst Abuse Treat. 2010;38 (Suppl1):S4–S13. doi: 10.1016/j.jsat.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wisniewski SR, Rush AJ, Nierenberg AA, Gaynes BN, Warden D, Luther JF, McGrath PJ, Lavori PW, Thase ME, Fava M, Trivedi MH. Can phase III trial results of antidepressant medications be generalized to clinical practice? A STAR*D report. Am J Psychiatry. 2009;166(5):599–607. doi: 10.1176/appi.ajp.2008.08071027. [DOI] [PubMed] [Google Scholar]
- 12.Baquet CR, Mack KM, Mishra SI, Bramble J, Deshields M, Datcher D, Savoy M, Brooks SE, Boykin-Brown S, Hummel K. Maryland’s Special Populations Network. A model for cancer disparities research, education, and training. Cancer. 2006;107(8 Suppl):2061–70. doi: 10.1002/cncr.22158. [DOI] [PubMed] [Google Scholar]
- 13.Mahony L, Sleeper LA, Anderson PA, Gersony WM, McCrindle BW, Minich LL, Newburger JW, Saul JP, Vetter VL, Pearson GD Pediatric Heart Network Investigators. The pediatric heart network: A primer for the conduct of multicenter studies in children with congenital and acquired heart disease. Pediatr Cardiol. 2006;27(2):191–8. doi: 10.1007/s00246-005-1151-9. [DOI] [PubMed] [Google Scholar]
- 14.Gorelick PB, Richardson D, Hudson E, Perry C, Robinson D, Brown N, Harris Y. Establishing a community network for recruitment of African Americans into a clinical trial. The African-American Antiplatelet Stroke Prevention Study (AAASPS) experience. J Natl Med Assoc. 1996;88(11):701–4. [PMC free article] [PubMed] [Google Scholar]
- 15.Cook ED, Moody-Thomas S, Anderson KB, Campbell R, Hamilton SJ, Harrington JM, Lippman SM, Minasian LM, Paskett ED, Craine S, Arnold KB, Probstfield JL. Minority recruitment to the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Clin Trials. 2005;2(5):436–42. doi: 10.1191/1740774505cn111oa. [DOI] [PubMed] [Google Scholar]
- 16.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]