Abstract
Endocytosis in the budding yeast Saccharomyces cerevisiae involves the ordered recruitment, activity and disassembly of nearly 60 proteins at distinct sites on the plasma membrane. Two-color live-cell fluorescence microscopy has proven to be invaluable for in vivo analysis of endocytic proteins: identifying new components, determining the order of protein arrival and dissociation, and revealing even very subtle mutant phenotypes. Yeast genetics and functional genomics facilitate identification of complex interaction networks between endocytic proteins and their regulators. Quantitive datasets produced by these analyses have made theoretical modeling possible. Here, we discuss recent findings on budding yeast endocytosis that have advanced our knowledge of how ~60 endocytic proteins are recruited, regulated by lipid and protein modifications, and disassembled with remarkable regularity.
ENDOCYTOSIS IN YEAST: PAST, PRESENT AND FUTURE
The yeast endocytosis field stretches back decades (Box 1). Striking parallels between yeast and mammalian endocytosis allow discoveries in one system to be applied to the other. Yeast endocytosis occurs at sites on the plasma membrane marked by the ordered recruitment of endocytic proteins, culminating in the rapid accumulation of actin directly before the scission event. These cortical actin patches were first visualized by immunofluorescence and rhodamine-phalloidin staining [1, 2] in budding yeast, where they are polarized to the growing bud (Figure 1). A growing body of work suggested the involvement of actin in endocytosis [3, 4].
TEXT BOX 1. Historical Perspectives.
Yeast endocytosis occurs at plasma membrane subdomains called actin patches that were first identified in the mid 1980s by immunofluorescence and rhodamine-phalloidin staining as polarized, plasma membrane-localized actin punctae of unknown function [1, 2]. The intersection of actin and endocytosis in yeast was discovered in 1993 [3]. Using immuno-EM, plasma membrane invaginations were observed at sites of actin density suggesting that these could be sites of endocytosis [4]. Evidence built that actin-regulating proteins affected endocytosis, but direct evidence that actin patches are sites of endocytosis was not presented until the process was analyzed using two-color real-time fluorescence microscopy in the early 2000s [5].
Alpha factor pheromone and its receptor were cargos of choice for early endocytic studies [90]. Radio-labeled alpha factor was the first cargo whose entry into the cell could be tracked during receptor-mediated endocytosis, and is still used today for quantitative assays. The growing popularity of fluorescent imaging popularized the use of commerical dyes like Lucifer Yellow [91] as a marker of fluid-phase endocytosis and FM4-64 [92] as a marker for the plasma membrane and internalized lipids. Two decades later, fluorescently tagged alpha factor was introduced [7]. Many permeases can be followed as endocytic cargos because they are expressed highly when their substrates are removed from the media, and then are rapidly internalized when their substrates are added back in excess, dramatically increasing endocytic activity [93]. Ste6p, the a-factor exporter, and Pdr5p, a multi-drug resistance pump, represent another class of cargos, which are rapidly and constitutively endocytosed under normal conditions [94–96].
Live-cell imaging showed that internalizing fluorescent alpha-factor and FM4-64 co-localize with actin patches, confirming that patches are sites of endocytosis [7, 8]. Live-cell two-color imaging revealed the highly regular, dynamic nature of endocytosis and the recruitment of actin directly prior to scission and internalization [5, 6, 31]. The highly regular ordered recruitment of fluorescently tagged proteins facilitated determining the order of arrival of proteins at endocytic sites and detection of subtle changes in dynamics, protein localization, scission, and initial vesicle movement caused by mutations or chemical inhibitors [6, 12]. Although most initial work occurred in budding yeast, work in fission yeast now makes unique contributions and allows for comparisons across the vast evolutionary distance separating these yeasts [39, 97].
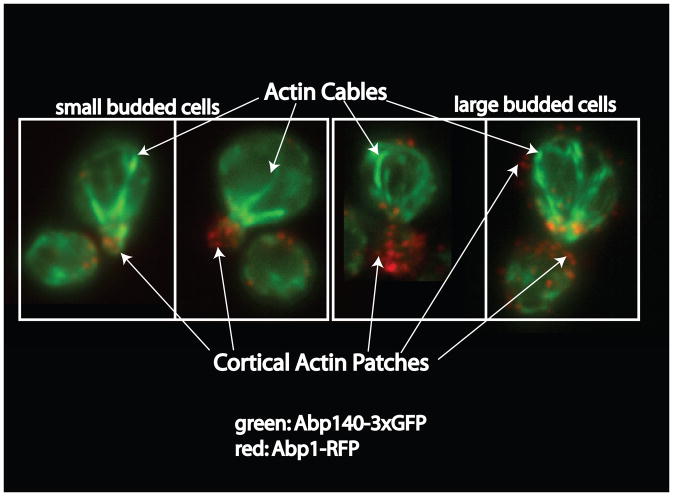
Figure 1. Actin structures in budding yeast.
In budding yeast actin forms three structures: the cytokinetic ring (not shown) responsible for separating the mother and daughter cell cytoplasm; actin cables, which are parallel bundles of short actin filaments running the length of the cell, nucleated by formins and serving as tracks for myosin-based motility (green); and cortical actin patches, composed of branched filaments nucleated by the Arp2/3 complex, and which are the sites of endocytosis (red). Cables and patches are visualized by imaging Abp140-3xGFP and Abp1-RFP, respectively.
The spread of simultaneous two-color imaging and total internal reflection microscopy resulted in rapid advances in the study of endocytosis. These new techniques demonstrated that the previously observed partial colocalization of actin and endocytic proteins was a result of taking snapshots of a highly dynamic process. Upon full spatiotemporal visualization, endocytosis clearly involved the ordered arrival and dissociation of many proteins. The excellent genetics of Saccharomyces cerevisiae and the existence of tools such as GFP-tagged libraries, combined with modern microscopy, enabled researchers to rapidly screen for proteins that localize to endocytic sites and to observe their dynamics in mutant yeast strains [5, 6]. The first definitive demonstration that actin patches are sites of endocytosis was attained when two-color live-cell fluorescence microscopy demonstrated colocalization of actin patches with internalizing alpha-factor, a historically important model cargo, and FM4-64, a fluorescent lipid marker [7, 8].
Studies of endocytosis in yeast continue to make important contributions even after several decades of intense research. Newly developed tools and techniques continue to drive the field forward. Mass spectrometry enables sensitive detection of post-translational modifications and low-affinity binding partners [9]. Synthetic genetic arrays and other genomic approaches expand the knowledge of the complex network of interactions and identify novel interacting proteins [10, 11]. Studies of post-translational modifications have benefited from the development of analog-sensitive kinases, which allow for a rapid, isothermic, reversible and precise inhibition [12]. These approaches all augment the techniques used to launch the field, including two-color fluorescence microscopy, co-immunoprecipitation and double-mutant analysis. These technological advances allow researchers to identify potential endocytic proteins via interactions revealed by genomic screens, and to then rapidly identify binding partners and potential regulatory modifications by mass spectrometry, and to perform sensitive localization assays using fluorescently tagged proteins under normal conditions and in mutants of interacting proteins.
While the field has advanced in great leaps and bounds there are still many questions to be answered in the coming years. Active fields of study include determining the detailed mechanisms behind endocytic site selection, cargo recruitment, actin assembly, scission and uncoating, and the role of lipid and post-translational protein modifications in regulating the timing of recruitment, activation and dissociation of the endocytic machinery.
This review will cover recent advances in the study of budding yeast endocytosis, starting from the early stages of coat protein and cargo recruitment, through the maturation of the patch to the polymerization of actin and scission. The advances provided through ultrastructural analysis and mathematical modeling will be discussed along with modes of regulation and the relationship between yeast and mammalian endocytosis.
EARLY STAGES OF ENDOCYTOSIS, INCLUDING CARGO SELECTION
How endocytic sites are initiated is a mystery. Mutations that partially disturb cellular actin affect the polarization of endocytic patches in daughter cells, but this observation is a bit mysterious as actin does not appear at the endocytic site until late in the pathway, after the endocytic site has been selected [13]. Perhaps the polarization of endocytic sites to daughter cells is facilitated by actin cables (Figure 1), or perhaps actin is present at an undetectable level at patches before the rapid polymerization late in the pathway (discussed below).
Among the earliest arriving proteins detected at yeast endocytic sites are the ubiquitin-binding protein Ede1p (Eps15), which is important for the recruitment of many later arriving proteins, and the F-BAR protein Syp1p (FCHo1/SGIP1) (Figure 2) [14, 15]. (Throughout this review, the first mention of a yeast protein will be followed in parentheses by the mammalian homologue, when known. For a complete listing, see Table 1.) Syp1p is known to promote the formation of endocytic sites in a polarized fashion at the bud neck, but deletion of SYP1 does not prevent patch formation in buds, while EDE1 deletion reduces the number of endocytic sites but does not change their polarization [15]. Clearly some additional factors are involved in the selection and initiation of endocytic sites (Box 2).
Figure 2. Timeline for endocytic vesicle formation and modular organization of proteins.
Endocytic proteins are dynamically recruited in a highly predictable order. The “early” and “early coat” proteins are present at the cell surface for a variable amount of time. After a transition point, possibly defined by cargo recruitment, the lifetimes of endocytic proteins are quite regular. Actin polymerization and BAR domain proteins bend the membrane into an extended tubule, which is pinched off by the combined actions of BAR domain proteins, actin polymerization and possibly by a lipid phase separation. The newly formed vesicle is uncoated by the combined actions of the Ark1p/Prk1p kinases, the Sjl2p lipid-phosphatase and the actions of Arf3p/Gts1p/Lsb5p.
Table 1.
S. cerevisiae endocytic proteins and their mammalian homologs
| Yeast Protein | Mammalian Protein |
|---|---|
| EARLY | |
| Ede1p | Eps15 |
| Syp1p | FCho1/2 |
| EARLY COAT | |
| Chc1p | Clathrin heavy chain |
| Clc1p | Clathrin light chain |
| Yap1801/2p | AP180 |
| Pal1p | Fungi only |
| Apl1p | AP2 complex beta subunit |
| Apl3p | AP2 complex alpha subunit |
| Apm4p | AP2 complex mu subunit |
| Aps2p | AP2 complex sigma subunit |
| INTERMEDIATE COAT | |
| Sla2p | Hip1R |
| Ent1/2p | Epsin |
| LATE COAT | |
| Pan1p | Intersectin |
| Sla1p | Intersectin/CIN85 |
| End3p | Eps15* |
| Lsb3p | SH3YL1* |
| Lsb4p | SH3YL1* |
| Lsb5p | Fungi Only |
| Gts1p | SMAP2 (small ArfGAP2)* |
| WASP/MYO | |
| Las17p | WASP/N-WASP |
| Vrp1p | WIP/WIRE |
| Bzz1p | syndapin |
| Scd5p | Saccharomycetales only |
| Myo3p | myosin-1E (Type 1 Myosin) |
| Myo5p | myosin-1E (Type 1 Myosin) |
| Bbc1p | Fungi only |
| Aim21p | Saccharomycetales only |
| ACTIN | |
| Act1p | Actin |
| Arc15/18/19/35/40p & Arp2/3p | Arp2/3 complex |
| Abp1p | ABP1 |
| Cap1p | Capping protein alpha |
| Cap2p | Capping protein beta |
| Sac6p | fimbrin |
| Scp1p | transgelin |
| Twf1p | twinfilin |
| Crn1p | coronin |
| Ark1p/Prk1p/Akl1p | BMP2 inducible kinase/AP2 associated kinase 1/AAK1 |
| Cof1p | cofilin |
| Aip1p | Aip1 |
| Bsp1p | no related proteins identified |
| Pfy1p | profilin |
| Aim3p | Saccharomycetales only |
| SCISSION | |
| Rvs161p | amphiphysin |
| Rvs167p | amphiphysin/endophilin |
| App1p | Fungi Only |
| Sjl2p | synaptojanin-1 |
Physical homology, but no functional homology identified.
TEXT BOX 2. Are MCC/Eisosomes Sites of Endocytosis?
Eisosomes, also called MCCs (Membrane Compartments of Can1p), are static plasma membrane structures associated with long furrows in EM [98]. These furrows were first described in 1963 [99] though they weren’t associated with a specific set of proteins or a function until much later [98]. Whether eisosomes play a direct role in endocytosis is controversial. Mutations in proteins important for eisosome structure were reported to cause endocytic phenotypes [100], but whether these are direct effects due to eisosomes being sites of endocytosis or indirect effects due to changes in membrane composition or cargo sorting and modification is not clear. Eisosomes do not colocalize with known endocytic proteins by fluorescence microscopy, and cargo localized to the MCC may relocalize out of the eisosome and into the rest of the plasma membrane prior to endocytosis [101, 102]. While some cargos localize to the MCC, their stimulated internalization is not affected by loss of a structural MCC protein [102]. Endocytosis is a very dynamic process, and some cargos can undergo massive, rapid, increases in rates of endocytosis, while eisosomes are very static structures, both in location and protein composition [100]. While immuno-EM has been used to localize MCC and eisosome components to long furrows [98], the same techniques reveal that endocytic proteins localize to tubular invaginations [19]. Correlative EM has demonstrated that actin and endocytic proteins localize to finger-like invaginations [81]. Eisosomes are most often found on mother cells or in cells that are in the stationary phase of growth [99, 103], while endocytosis is thought to be involved in membrane homeostasis and to occur predominantly in locations of high membrane turnover such as growing buds. Endocytosis can be blocked by inhibiting actin assembly in patches or by mutations in actin patch proteins [104].
The ability of both Syp1p and Ede1p to bind to membranes - Syp1p via its F-BAR domain and Ede1p in a ubiquitin-dependent manner [16] - suggests that patch initiation may occur via a lipid signal, although no such signal has been identified in yeast. While PIP2 may be important for initiation in mammalian endocytosis via FCHo proteins [17], temperature-sensitive mutations of the lipid kinase Mss4p do not inhibit actin patch initiation [18]. While the F-BAR domain of Syp1p can bind and tubulate membranes [14], electron microscopy (EM) suggests that the membrane remains relatively flat during the initial stages of the endocytic pathway (C. Buser and D. Drubin, unpublished) [19], indicating that Syp1p might be binding without tubulation.
The arrival of the early proteins is quickly followed by the accumulation of cargo molecules at the nascent patch (Figure 2). Historically, the difficulties of imaging cargo accumulating in patches prevented solving the long-standing chicken and egg question of the endocytosis field: does the clustering of cargo recruit the endocytic machinery, or is cargo concentrated at a pre-formed endocytic site? Studies using a fluorescent derivative of alpha factor demonstrated that cargo arrives after the appearance of Ede1p, but before the appearance of Sla1p (intersectin/CIN85) (Figure 2) [7]. This timing and apparent differences in bud vs mother cell endocytic patch dynamics [20] support the hypothesis that the variable lifetimes of the early proteins (Ede1p, Syp1p, Clc1p (clathrin light chain), Chc1p (clathrin heavy chain), Yap1801/2p (AP180), Pal1p, AP2 complex) compared to the regular lifetimes of later proteins like Sla1p might be due to a cargo checkpoint that pauses the pathway after the arrival of early proteins until cargo loading is complete, and that the very regular timing of the later events of the pathway is the result of an endocytic site being fully loaded with cargo (Figure 2). Such a checkpoint also has been postulated for mammalian cells [21]. While alpha factor arrives after early proteins, it is possible that other cargos may be recruited later, after the arrival of intermediate or late coat, or may cluster before being recognized by the endocytic machinery [22].
In mammalian cells, the AP2 complex is an important cargo adaptor whose knockdown impairs internalization of several cargos including the well-studied transferrin receptor [23]. In yeast, no endocytic phenotype had been observed in AP2 subunit knockouts until studies on the yeast killer toxin K28 demonstrated that AP2 knockouts are resistant to the toxin, and thus AP2 is likely involved in K28 internalization [24]. Other reported cargo-specific adaptors include Yap1801/2p as an adaptor for Snc1p [25], and Syp1p as an adaptor for Mid2p [14].
Ubiquitin has long been recognized as a signal for internalization of membrane proteins, and several early and coat proteins, including Ede1p, Sla1p and Ent1/2p (epsin), have ubiquitin-binding domains [16, 26] (Table 2). It has become clear, however, that while these ubiquitin-binding domains may be important for cargo binding, they also are likely important for regulating protein-protein interactions within the endocytic network. Extensive analysis of mutations in the ubiquitin-binding domains of Ent1/2p and Ede1p demonstrate that in the absence of these domains both ubiquitinated and non-ubiquitinated cargos are internalized equally, though at a reduced rate compared to wild type, suggesting that the ubiquitin-interaction motifs (UIM) regulate a general, rather than a ubiquitinated-receptor specific, step in endocytosis [27]. Because many endocytic proteins are likely to be ubiquitinated (Table 2), the ubiquitin-binding domains, along with the EH-NPF interactions (see Glossary), are likely to stabilize interactions between some of the ~60 proteins that localize to endocytic patches. Similar mutational analyses define a role for the Yap1801/2p endocytic adaptors as functioning redundantly with Ent1/2p, likely by stabilizing the endocytic protein network via NPF-EH and UIM-ubiquitin interactions [28].
Table 2.
Ubiquitin binding and ubiquitinated endocytic proteins
| Ubiquitin binding [16, 27, 65, 108] | Ubiquitinated by Rsp5p [27, 77, 106] | Ubiquitinated in vivo[107] | |
|---|---|---|---|
| EARLY | |||
| Ede1p | + | + | + |
| EARLY COAT | |||
| Clc1p | n.d. | — | + |
| INTERMEDIATE COAT | |||
| Ent1p | + | — | + |
| Ent2p | + | + | + |
| Sla2p | n.d. | — | + |
| LATE COAT | |||
| Lsb5p | + | — | − |
| Sla1p | + | — | + |
| WASP/MYO | |||
| Myo3p | n.d. | — | + |
| ACTIN | |||
| Abp1p | n.d. | — | + |
| Arc18p | n.d. | — | + |
| Arc35p | n.d. | — | + |
| Arc40p | n.d. | — | + |
| Arp2p | n.d. | — | + |
| Arp3p | n.d. | — | + |
| Sac6p | n.d. | — | + |
| SCISSION | |||
| Rvs167p | n.d. | + | + |
In vivo ubiquitination data comes from genomic screens and may not be relevant for endocytosis. Negative signs for “Ubiquitinated by Rsp5p” indicate “not detected,” n.d. indicates “not done”.
Early arriving endocytic proteins are candidates to both regulate and facilitate the early steps of patch initiation, patch polarization and cargo recruitment. They may also regulate later steps, however. In addition to its adaptor and endocytic site-specification roles [14, 15], Syp1p is implicated as a regulator of Arp2/3 activity late in the endocytic pathway, just before Syp1p leaves the patch [29]. Many endocytic proteins are large, multi-domain proteins with multiple separable functions, and may therefore act at multiple steps during endocytosis.
The early arriving endocytic proteins are integral for defining how many endocytic sites are formed and where they form, but they are not the only players involved. These early proteins bind to cargo and to each other, forming the nascent endocytic protein network. These are the proteins that act in an early temporally variable phase before an apparent cargo-triggered transition point that marks the beginning of the extremely temporally regular later stages of endocytosis (Figure 2).
COAT MATURATION
After the proposed cargo-triggered transition point, other coat proteins join the endocytic site in regular temporal fashion. The next set of proteins to be recruited to the endocytic site, Sla2p (Hip1R) and Ent1/2p, have domains that specifically bind to phosphatidylinositol 4,5-bisphosphate (PIP2) [16, 30]. The UIMs of Ent1/2p were thought to bind ubiquitinated cargo, but recent work suggests they are more important for promoting protein-protein interactions within the endocytic machinery, as discussed above [27, 28].
The Pan1p (intersectin)/End3p (Eps15)/Sla1p complex is related to, and may serve a similar function as, the mammalian endocytic protein intersectin [31]. While Pan1p has nucleation promoting factor (NPF) activity, its most important role may be to serve as a scaffold to recruit other proteins to endocytic sites. The stability of the Pan1p/End3p/Sla1p complex is regulated by the Ark1p/Prk1p kinases (AAK1), which phosphorylate the complex, promoting disassembly, as discussed below [32, 33].
The other proteins in the late coat module arrive along with members of the WASP/Myo module, but are grouped as coat proteins because, in contrast to the WASP/Myo proteins, they are internalized with the newly formed vesicle (Figure 2). Lsb5p and Gts1p (small ArfGAP2) have roles in uncoating [33], while Lsb3p (SH3YL1) and Lsb4p/Ysc84p (SH3YL1) promote actin polymerization and bundling [34].
The later arriving components of the endocytic coat are likely involved in linking together the early coat, which recruits cargo and begins to shape the membrane, with the actin and scission machinery.
WASP/MYOSIN RECRUITMENT
Las17p (WASP/N-WASP) and Myo3/5p (myosin-1E) are the most important NPFs in vivo, based on patch internalization movements in mutants; specific mutation of their NPF domains results in patches that fail to internalize [35], similar to what is observed in latrunculin A-treated cells [5]. Abp1p (ABP1) and Pan1p can stimulate Arp2/3 activity in vitro, but removal of their NPF domains in vivo causes less severe phenotypes than similar mutations in either Las17p or Myo3/5p, demonstrating that they play lesser roles in Arp2/3-mediated actin polymerization during endocytosis [35, 36].
The yeast Arp2/3 complex has a higher basal activity than its mammalian counterpart, although NPFs still strongly stimulate its nucleation activity. The myosin activator Vrp1p (WIP/WIRE) arrives after Las17p, and is followed by Myo3/5p, which arrive just before actin is detected (Figure 2) [35]. Regulation of Las17p NPF activity is not fully understood. Las17p arrives ~20s before actin is detectable (Figure 2) [6]. During this time, the Arp2/3 complex might be held in an inactive state by the earlier arriving proteins Syp1p and Sla1p [29, 37]. At the onset of internalization, Syp1p departs and Sla1p moves inward with the coat, while Las17p remains closer to the plasma membrane, which may relieve the inhibition of the Arp2/3 complex [35]. As Sla1p internalizes, the Las17p inhibitor Bbc1p localizes to the patch [6, 37]. How Bbc1p contributes to Las17p regulation is not understood. Combining null mutants of the inhibitors Sla1p and Bbc1p results in long actin tails associated with deep membrane invaginations (Figure 3A) [6]. The F-BAR protein Bzz1p (syndapin) can stimulate activity of Las17p/Sla1p and it arrives slightly before actin [35]. It is possible that the Bzz1p F-BAR domain might recognize curvature induced by Syp1p, also an F-BAR protein, and thus be recruited to help relieve Sla1p inhibition at endocytic sites. Las17p has many interacting partners that bind to its proline-rich regions via SH3 domains, but the exact roles of many of these proteins are poorly understood [38]. The role of Ysc84p as a Las17p activating protein was recently reported while its homolog Lsb3p remains relatively uncharacterized [34], so keys to WASP/Myo activation and repression may reside in interactions of NPFs with relatively unstudied proteins.
Figure 3. Endocytic mutants affecting actin polymerization.
A. In sla1Δ bbc1Δ mutants, Las17p inhibition is greatly reduced, resulting in excessive actin polymerization. The connection between actin and the endocytic coat is intact, so deep invaginations are formed. B. In sla2Δ mutants the connection between actin and the endocytic coat is missing, resulting in long, treadmilling actin tails that continuously assemble proximal to the plasma membrane, and flat membranes. Coat proteins and NPFs remain at the plasma membrane while all the actin-associated proteins normally associated with endocytosis localize to the comet tails.
Particle-tracking of fluorescently tagged members of the WASP/Myo module reveal that these proteins do not internalize with the newly formed vesicle but rather stay at the plasma membrane (Figure 2) [6]. Immuno-EM studies revealed a second population of Myo5p at the invagination tip, as well as Las17p localized on the sides of tubules [19]. Fluorescence recovery after photobleaching (FRAP) analysis of actin tails in sla2Δ cells (Figure 3B) suggests that actin is polymerized at the plasma membrane and moves inwards [5], so what is the role of Myo5p at the tip of an invagination? The myosin could be acting as a bridge by simultaneously binding to actin filaments and coat proteins, it could be nucleating filaments for a reason that is presently obscure, or it could be acting as a motor generating force through contact with actin filaments. The latter possibility is supported by the observation that motor domain mutants prevent inward movement, but do not prevent actin polymerization, which suggests that the NPF and motor functions are separable, but that both are important [35].
Despite the identification of a large number of associated players, how the Arp2/3 complex is regulated during endocytosis remains incompletely understood. Additional factors, perhaps unidentified proteins or post-translational and lipid modifications, are likely to act as switches for activating the NPFs in order to promote actin polymerization, membrane invagination and ultimately vesicle scission. Interestingly, it was recently suggested that in fission yeast, actin filaments from one endocytic structure can activate actin assembly at another endocytic site by contacting it [39]. The authors speculate that pre-existing actin filaments in the first structure trigger autocatalytic Arp2/3 activation. This phenomenon has also been observed in vitro on NPF-coated beads and rods where actin polymerization does not occur until a short F-actin primer makes contact with the NFP-coated surface [40]. These results suggest the possibility that endocytic sites are primed to assemble actin and await initiation of the autocatalytic assembly process.
ACTIN POLYMERIZATION
Actin assembly is required for yeast endocytosis. This requirement can be partially overcome by providing osmotic support, which suggests that actin polymerization is needed to counter the turgor pressure at the plasma membrane [41]. A similar phenomenon in mammalian cells may explain discrepancies in the reported dependency on actin polymerization of mammalian clathrin-dependent endocytosis [42]. Severing the attachment between the actin network and the endocytic membrane in sla2Δ cells or inhibiting polymerization by latrunculin A sequestration of monomers, stops endocytosis [5]. The organization and physical properties of the actin network are also important for force generation; deletion of bundling proteins Sac6p (fimbrin) and Scp1p (transgelin) results in nonproductive endocytic sites, although actin can still polymerize at the plasma membrane [6, 43].
In order to provide force to pull a vesicle out from the planar plasma membrane, the expanding actin network must be tightly connected to the internalizing invagination. In sla2Δ cells, which may lack this connection, actin polymerizes into long “comet tails” that continue to undergo flux while endocytosis is unproductive (Figure 3B) [5]. Fluorescence microscopy reveals that while coat proteins remain at the plasma membrane and are not internalized, actin-associated proteins are recruited to the long tails in sla2Δ cells [5]. Electron microscopy confirmed this conclusion as these mutant cells have very few invaginations (C. Buser and D. Drubin, unpublished). Together, these data suggest that Sla2p may be integral in connecting the coat to the polymerizing actin. Sla2p can bind to PIP2 in membranes via its ANTH domain and can interact with other coat proteins including Clc1p, Ede1p and Sla1p, as well as with actin, Las17p, and the Arp2/3 complex [30, 44–49]. These many interactions suggest that Sla2p may act as an adaptor, connecting the clathrin coat and plasma membrane to actin filaments, which lets the growing network exert force on the membrane, deforming the membrane and ultimately helping to pinch off a vesicle.
The roles of the Arp2/3 complex and its NPF, Las17p, have been investigated using Las17p-coated microbeads added to yeast extract [50]. These beads stimulate actin polymerization and recruit a large number of endocytic proteins. A cloud of polymerized actin forms and a symmetry-breaking event often follows, resulting in bead motility. Mass spectrometry of the bead-associated actin networks identified actin regulating proteins (e.g. Cap1/2p (capping protein alpha/beta), Sac6p, the Arp2/3 complex) and endocytic specific proteins (e.g. Sla1p and Syp1p). These results demonstrate that an NPF (Las17p) is sufficient to form an actin network of biologically relevant composition. The exact mechanism for creating a branched dendritic network, like that found at endocytic patches [51, 52], versus a parallel bundled network, as exists in cables, is not known, but recent reports from S. pombe implicate fimbrin (Sac6p) as an important factor for excluding tropomyosin from endocytic actin filaments via fimbrin’s actin binding activity, yet also confirm that its crosslinking activity is important for endocytic function; a truncated fimbrin mutant, which lacks actin bundling activity while preserving actin binding activity, localizes to endocytic sites and excludes tropomyosin, but otherwise phenocopies a fimbrin null [53, 54].
As polymerization is turned off, disassembly of the actin network ensues, and the action of cofilin/Aip1/coronin (Cof1p/Aip1p/Crn1p) becomes dominant. Cof1p binds to older, ADP-bound actin filaments with the aid of Crn1p while being inhibited from binding to younger ATP/ADP+Pi-actin by Crn1p [55]. The Cof1p induces a twist, causing filaments to break apart [55]. Aip1p is important in breaking down short actin filaments produced by Cof1p into monomers [56]. Intriguingly, actin oligomer-based polymerization can occur, and this oligomer assembly pathway is enhanced by the loss of Aip1p, suggesting that oligomer annealing occurs in vivo at actin patches [56].
Roles for actin after uncoating have been suggested. There are reports that vesicles appear to move along actin cables [7, 8]. Some imaging suggests that endosomes move into close proximity with internalizing vesicles, facilitating fusion [7]. Observing actin cables in EM is very difficult, but immuno-EM results support the existence of a link between cables and endocytic sites [4].
The importance of actin in yeast endocytosis is unquestionable. Actin assembly provides force necessary for membrane deformation and scission, and likely plays a role in moving the newly formed vesicles away from the plasma membrane. Open questions remain about the regulation of actin assembly, the signals that control the NPFs and initiate polymerization as well as the signals that turn off polymerization to allow for disassembly and the eventual uncoating of the vesicle. However, many of the key players seem to have been identified.
SCISSION AND UNCOATING
In the final steps of endocytosis, the vesicle pinches off from the plasma membrane, moves inward toward the cell center, and the endocytic coat disassembles from the newly formed vesicle. While in mammalian cells the GTPase dynamin is essential for scission of clathrin-coated vesicles, the role of dynamin in yeast endocytosis is unsettled. While at least one study suggested that dynamin does not participate, recent reports have suggested that the dynamin Vps1p might be involved in endocytosis (Box 3) [57–59]. However, the involvement of Vps1p in multiple important intracellular trafficking events has prevented definitive conclusions from being drawn.
TEXT BOX 3. A Role for Dynamin in Yeast Endocytosis?
Dynamin plays a crucial role in vesicle scission for clathrin-dependent endocytosis in metazoans, but whether it has a role in yeast endocytosis is controversial. The yeast genome encodes three dynamin-like proteins, two of which, Dnm1p and Mgm1p, are involved in mitochondrial fusion and fission, while Vps1p is involved in fusion and fission reactions at other organelles including the Golgi, vacuole, endosomes and peroxisomes. While some recent reports find evidence for a role for Vps1p in endocytosis [57, 58, 105], other reports found no such connection and, in contrast to the situation in metazoan cells, Vps1p does not have an obligate endocytic function and is at most detected at only a subset of endocytic sites. The roles of Vps1p in other trafficking steps may explain some of the apparent endocytic phenotypes, as, for example, recycling of the endocytic machinery may be impaired in trafficking mutants. It has been noted that Vps1p lacks the PH domain and C-terminal proline-rich domain found in conventional endocytic dynamins [57]. Similarly, the fission yeast dynamin Vps1p does not appear to be localized to actin patches [97].
In order for scission to occur, the two membrane bilayers must be brought into close proximity, at a distance of ~10 nm or less, at which point they can fuse and a vesicle can form [60]. To perform this action, yeast take advantage of the membrane tubulating activities of the BAR and F-BAR proteins. While the Rvs161/167p (amphiphysin) heterodimeric BAR protein complex is capable of deforming membranes, this activity, combined with the force derived from actin polymerization, may not be sufficient to pinch off the membrane [60]. Where does the rest of the force come from? One possible source is a line tension generated by lipid phase separation, as discussed below. The combination of the line tension, tubulation from BAR proteins and actin polymerization pushing on coat proteins at the bud and perhaps squeezing the tubule are proposed to provide the scission force [61, 62].
This model provides a plausible explanation for why the lipid phosphatase synaptojanin is important for scission [63]. Along with creating a proposed lipid phase separation by having higher activity on the bud then on the tubule, Sjl2p (synaptojanin) has a role in vesicle uncoating. This is not surprising as a number of endocytic coat proteins that are internalized with the forming vesicle have PIP2 binding domains (Sla2p, Ent1/2p), and thus the action of Sjl2p reduces their affinity for the vesicle as it destroys the PIP2 [33]. At the same time, the regulatory kinases Ark1p and Prk1p are responsible for phosphorylating a variety of coat proteins including Pan1p, Sla1p and Ent1/2p, and enabling their disassembly (Figure 4). Arf3p/Lsb5p/Gts1p also promote disassembly of the Pan1p/Sla1p/End3p complex, yet at the same time arf3 mutants provide evidence for an Arf3p role in modulation of PIP2 levels [64, 65]. These results suggest that a wide variety of factors contribute to the dissociation of coat proteins in a coordinated fashion in which multiple pathways converge to dissociate certain key proteins leading to the disassembly of the entire coat. Weakening of the coat-actin connection is expected to briefly relax tension on the membrane, which may be required for the final vesicle scission event [61].
Figure 4. Scission and uncoating of endocytic vesicles.
Scission is accomplished by constriction of the bud neck, driven by BAR protein-driven membrane deformation, actin-generated force and the proposed Sjl2p-imposed line tension created by lipid phase separation. After scission, the Pan1 complex proteins Sla1p, Pan1p and End3p are phosphorylated by Ark1p/Prk1p resulting in their dissociation. Ark1p/Prk1p are also responsible for turning off actin polymerization, allowing Cof1p/Aip1p/Crn1p to disassemble the actin filaments. Sjl2p is responsible for dephosphorylating PIP2, reducing the affinity of Sla2p and Ent1/2p for the vesicles. Arf3p, along with Gts1p and Lsb5p, are also involved in the dissociation of the Pan1 complex.
While a number of uncoating factors are known, other proteins specific for removing different endocytic proteins, or shutting off actin polymerization, may exist. The signals that turn on or recruit uncoating factors are likewise unclear, but membrane curvature, actin filaments and lipid composition are likely candidates.
PHOSPHORYLATION, UBIQUITINATION AND LIPID MODIFICATION
Endocytosis is regulated by a variety of signals including phosphorylation, ubiquitination and lipid modification. The plasma membrane is a complex sea of proteins and lipids of various types including: phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositols, phosphatidylserine, sphingolipids and ergosterol [66]. These lipids play a poorly understood role in regulating endocytosis. Adding another layer of complexity, the inner and outer leaflets of the plasma membrane are asymmetric, with the inner leaflet being enriched for phosphatidylserine and PIP2. Fluorescent probes combining GFP and lipid-binding protein domains, mutants of enzymes responsible for producing or modifying lipids, and the ability to directly grow yeast in or on media containing exogenous lipids has facilitated studies of how lipids contribute to endocytic regulation.
The importance of PIP2 in yeast endocytosis has been demonstrated genetically; mutants of Mss4p, which produces PIP2 from PIP, and of Sjl1p and Sjl2p, the PIP2 phosphatases, have endocytic defects [63, 67]. Using the PIP2-binding ANTH domain of Sla2p, it was demonstrated that PIP2 is concentrated at endocytic sites as the endocytic coat assembles, and disappears upon scission, soon after Sjl2p is recruited [63]. It should be noted that while PIP2 appears to be concentrated at endocytic sites, Mss4p is more broadly distributed on the plasma membrane, which suggests that PIP2 may be concentrated by PIP2-binding coat proteins such as Sla2p, Ent1/2p and Rvs161/167p.
The phenotypes of synaptojanin mutants suggest multiple roles for PIP2 in endocytosis. Disassembly of PIP2-binding proteins upon vesicle formation is delayed in these mutants, suggesting that Sjl2p hydrolysis of PIP2 is important for uncoating of Sla2p and Ent1/2p [33, 63]. Secondly, long extended invaginations with coat proteins at the tips form in sjl1Δ sjl2Δ cells suggesting a role for PIP2 regulation in scission [63]. Thirdly, these mutant cells inappropriately initiate invaginations on invaginations, suggesting a role for PIP2 as a signal for formation of endocytic sites [63]. Mathematical modeling led to the proposal that PIP2’s scission role might be in development of a line tension between an area of low PIP2 concentration - the tip of the invagination where Sjl2p is active - and the extended tubule where PIP2 is protected from Sjl2p by the BAR domain proteins [61, 63]. The observed phenotype fits this hypothesis; when Sjl2p is missing, a concentration gradient cannot form, and so scission does not occur. Experimental support for this model was provided by studies in mammalian cells on dynamin-independent scission [68].
While the role of PIP2 in endocytosis may be best studied among the lipids, genetic analyses suggest that sterols and sphingolipids may also be important for endocytosis [69–71]. Deletions of ergosterol biosynthetic enzymes ERG2 or ERG6 result in endocytic defects that are exacerbated when combined with each other or with deletions of ERG3 [71, 72]. Temperature-sensitive mutations in LCB1/END8, whose product catalyzes the first step in sphingolipid synthesis, are deficient in endocytosis, and this block can be overcome by addition of exogenous sphingoid bases [69]. As new tools for the detection and modulation of other specific lipids are developed, our understanding of their contributions to endocytosis will be expanded.
Ubiquitination and phosphorylation have long-studied roles in regulating the internalization of transmembrane cargo molecules. In the canonical model, a receptor gets phosphorylated upon ligand binding, the phosphorylation signals for ubiquitination – in the form of either mono-, multiple mono-, or K63-linked poly-ubiquitinations – and this leads to internalization mediated by ubiquitin-binding coat molecules. Ubiquitination of cargo molecules is carried out by the WW domain HECT-type ubiquitin ligase Rsp5p assisted by a number of adaptor proteins including the recently described family of arrestins [73]. Mutations in Rsp5p’s individual WW domains, which mediate protein-protein interactions, affect the internalization of specific cargos, while temperature-sensitive mutations in this protein generally reduce fluid-phase endocytosis [74].
It is possible that Rsp5p also regulates the endocytic machinery directly. Mammalian homologs of Ede1p and Ent1/2p - Eps15 and epsin, respectively - are ubiquitinated in vivo, and are thought to be regulated by binding to their own modifications [75]. Rsp5p also may regulate endocytosis via effects on the actin cytoskeleton [76]. The BAR domain protein Rvs167p has been reported to be ubiquitinated by Rsp5p, but no phenotype was observed in the K->R mutant, in which the lysine proposed to be ubiquitinated is mutated to arginine (Table 2) [77].
Phosphorylation of Pan1p by Prk1p prevents binding of Pan1p to F-actin, a prerequisite for its activation of the Arp2/3 complex [78]. A role for Ark1p and Prk1p as negative regulators of endocytic actin assembly is supported by the observation that ark1Δ prk1Δ cells have large clumps of actin, presumably due to uncontrolled actin polymerization [12, 79]. These data suggest that Prk1p activity is important both for uncoating of internalized vesicles and cessation of actin polymerization. Prk1p autophosphorylation provides a mechanism for regulation of its kinase activity. Prk1p phosphorylates multiple targets, including Sla1p. These phosphoproteins then stimulate Prk1p autophosphorylation which reduces its kinase activity [80]. This mechanism may both prevent hyperphosphorylation of targets and turn off Prk1p activity.
Despite recent advances, many questions about how yeast endocytosis is regulated still need to be addressed: Do lipids in addition to PIP2 play important regulatory roles? What is the nature of the signal that specifies endocytic site selection? Is the endocytic machinery ubiquitinated as a regulatory mechanism? Does phosphorylation only regulate the endocytic machinery during late steps in endocytosis? Dissection of regulatory mechanisms is an exciting and fertile area of current research.
ULTRASTRUCTURAL ANALYSIS
Fluorescence microscopy has proven to be an invaluable tool for studying endocytosis in yeast. With a practical resolution limit of ~200nm, conventional fluorescence microscopy cannot resolve important details such as the shape of the invagination, where proteins localize along the invagination, or the orientation and organization of the actin network. These details can only be resolved using electron microscopy and possibly one day by super-resolution fluorescence microscopy. Unfortunately, there are several complications to studying endocytosis via electron microscopy. Firstly, as endocytosis is a highly dynamic process and EM is performed on fixed cells, one can only capture snapshots and it may be unclear exactly what step in the process an image reveals. Secondly, the more highly curved the cell, the harder it is to section perpendicularly to the plasma membrane so that the invagination is in the section and is oriented properly. Sites of endocytosis are concentrated in the more highly curved small bud (Figure 1). Thirdly, the cytoplasm of yeast is especially dense and filled with ribosomes, making it suboptimal for viewing actin filaments, which are notoriously hard to view by electron microscopy in the first place.
Immuno-EM was used to demonstrate precise localization of various endocytic proteins [19]. These results validated many of the proposed localizations of proteins based on fluorescence microscopy studies, such as the BAR domain protein Rvs167p localizing to the sides of tubules. However, the appearance of Myo5p not only at the base but also at the tips of invaginations was an unexpected result, as was the localization of Las17p at both the base of the invagination and partway up the tubule [5, 19, 35]. These observations demonstrate the importance of ultrastructural analysis in providing critical information on the localization of proteins that could not be acquired by fluorescence microscopy alone [4, 19].
Recent innovative use of correlative light-electron microscopy has allowed researchers to tackle the problem of determining which transient stage of endocytosis has been captured in an electron micrograph. An elegant correlative technique was developed in which fixed samples are imaged first by fluorescence microscopy, and then by electron microscopy [81]. Using fluorescent microbeads as fiduciary marks, the authors could image the same endocytic sites using both techniques. By using two fluorescent markers that localize to endocytic sites at different times, the authors classified the sites into two groups, those with Abp1-RFP only and those with both Abp1-RFP and Rvs167-GFP, a scission protein. Since approximately half of the double-labeled sites had vesicles and half had intact invaginations, the authors concluded that scission must occur halfway through the ~10s period during which the two proteins colocalize. This technique holds promise for such applications as determining exactly when invaginations begin to form relative to recruitment of specific proteins and which proteins are first involved in constricting the neck of an invagination [81].
For this correlative light-electron microscopy study, high-pressure freezing with freeze-substitution was used, which is best able to preserve the fine structure of dynamic cellular features such as endocytic invaginations. In the future, combining high-pressure freezing/freeze-substitution with immunostaining promises to both faithfully preserve structural features and provide precise localization of specific proteins. Modulating the stains used in the freeze-substitution steps can enhance the contrast between membranes and ribosome-rich yeast cytoplasm creating even clearer images [82].
One crucial challenge for ultrastructural analysis is to elucidate the exact geometry of the actin network that provides much of the force that drives invagination and scission. Important unanswered questions that could potentially be addressed by ultrastructural analysis include: Where do the filaments attach to the invagination and with what orientation? Where do they contact the plasma membrane? Does the network enclose the entire invagination or is the tip left exposed? Where exactly are the assembly nucleators? Are they all at the base of the invagination or are some at the tip or on the sides? Further technical innovation will be required to tackle the crucial but very challenging problem of reliably visualizing actin in EM of yeast. Analysis of unroofed yeast cells is one promising avenue to overcoming the challenge presented by the dense cytoplasm of yeast [51].
THEORETICAL MODELING
Mathematical modeling is an important tool for exploring the mechanical and physical aspects of biological processes. If experimentalists can provide theoreticians with sufficient high quality quantitative data, theoretical models can be developed. Such models allow researchers to understand how the process can and cannot work, they provide testable hypotheses, and they identify areas in which our understanding is incomplete, stimulating further experimentation, which in turn inspires new iterations of the models.
In an attempt to develop a comprehensive model for the endocytic pathway, over 20 measured parameters from a variety of studies were used to model the recruitment of endocytic proteins, membrane deformation and scission [61]. The most important notion introduced in this model is that membrane curvature is a signal source for biochemical reactions involved in membrane scission. The model accurately predicted phenotypes of some mutations, such as the retraction seen in BAR domain knockouts, validating the model’s predictive powers [61].
The model also established that much of the scission force could be generated by a lipid phase separation. The calculated force of actin polymerization was found to be too small to drive membrane deformation and scission to completion. The authors speculated that scission might be driven by an interfacial force from lipid phase separation created when PIP2 is concentrated in the tubule and depleted in the bud. The model proposed that a pair of feedback loops drive this process (Figure 5). First, binding of BAR domain proteins on the tubule deforms the membrane, thus promoting recruitment of additional BAR domain proteins. These BAR proteins were proposed to protect PIP2 from dephosphorylation by the synaptojanin Sjl2p, the phosphatase responsible for PIP2 dephosphorylation. Second, BAR protein-induced curvature along the tubule was predicted to drive recruitment and activation of Sjl2p at the unprotected tubule-bud interface. As PIP2 is hydrolyzed, the phase boundary becomes more pronounced, and a line tension at the interface increases curvature, further increasing Sjl2p’s recruitment and activity. The model proposes that these two positive feedback loops rapidly generate the force necessary to drive scission [61].
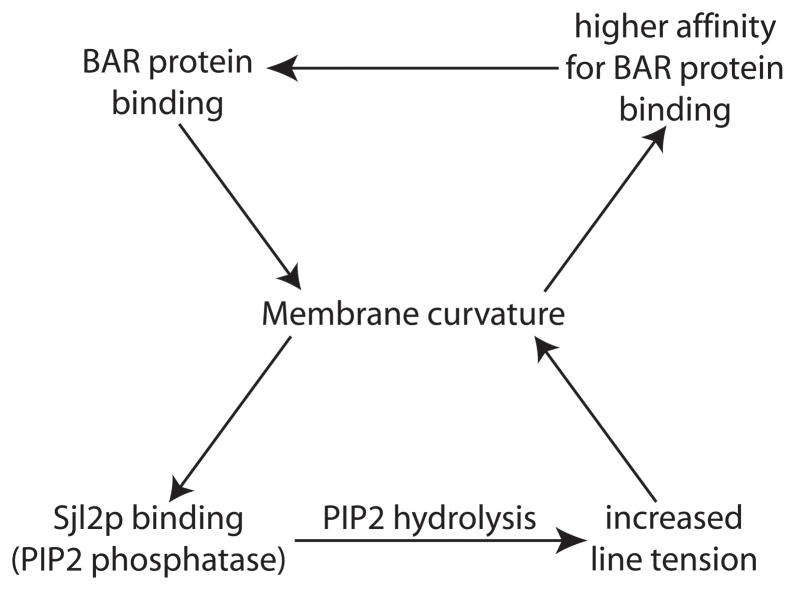
Figure 5. Positive feedback loops involved in scission.
During scission, two positive feedback loops are proposed to drive the increase in membrane curvature that leads to pinching off of the vesicle. Firstly, BAR domain proteins bind to areas of high membrane curvature generated by actin polymerization forces, and through their binding enhance the membrane curvature, recruiting more BAR domain proteins. Secondly, Sjl2p, the PIP2 phosphatase, is more active on curved membranes [83]. The activity of Sjl2p on the curved neck decreases levels of PIP2 on the unprotected bud while the PIP2 of the neck is shielded by the BAR domain proteins. The lipid phase separation produces a line tension, which further increases membrane curvature, enhancing the activity of Sjl2p.
The notion that Sjl2p’s activity might be stimulated by increasing membrane curvature recently gained experimental support when it was shown in a cell-free system that synaptojanin-1 is more efficient at dephosphorylating PIP2 in more highly curved membranes, especially when a BAR domain protein is added to create very highly curved membranes [83]. This observation validates a major requirement of the model.
This model highlights one of the main differences between mammalian and yeast clathrin-mediated endocytosis: the apparent lack in yeast of dynamin participation in scission. It was long assumed that the requirement for actin in yeast endocytosis indicated that the primary scission force was provided by actin polymerization, but this model suggests that the phase separation of PIP2 also contributes necessary force.
Another recent mathematical model focused on force generation by actin polymerization during fission yeast endocytosis [84]. Using constants derived from in vitro biochemical studies and quantification of local protein concentrations by fluorescence microscopy in vivo, the authors developed a model focused on actin polymerization and depolymerization at endocytic sites. Among the novel conclusions drawn from this study were: (i) in vivo binding of capping protein and Arp2/3 complex are faster than in vitro data suggest, and (ii) disassembly happens faster than can be explained solely by pointed-end depolymerization, which suggests that severing proteins may produce small fragments of actin that diffuse away and depolymerize away from the patch. In vitro, Aip1p enhances the depolymerization of short fragments produced by cofilin severing [56].
RELATIONSHIP TO MAMMALIAN ENDOCYTOSIS
Of the ~60 yeast proteins known to be localized to sites of endocytosis, ~85% have homology to mammalian proteins also involved in endocytosis (Table 1). There are several striking examples wherein a single, modular mammalian protein has homology to several yeast proteins which each contain a subset of the modules. For example, mammalian intersectin has two Eps15 homology domains and 5 SH3 domains and is believed to play a similar role to the yeast proteins Pan1p and Sla1p. Together, these proteins have two Eps15 homology domains (Pan1p) and three SH3 domains (Sla1p) and are known to function in a complex together with End3p, which has another two Eps15 homology domains. The functional and domain homology suggests that while individual proteins may not be perfectly conserved, the network of interactions that hold the endocytic machinery together is conserved [85].
The dynamic, ordered recruitment of endocytic proteins is similar between yeast and mammals [85]. Clathrin and adaptor proteins arrive early, followed by additional coat proteins, then actin and scission factors, and ending with uncoating proteins. Although several publications have emphasized differences in the dynamics of endocytosis in yeast and mammals, to what extent apparent differences were due to how the process was being studied was not clear. For yeast, but not mammalian cell studies, fluorescent endocytic proteins are typically expressed from their native promoters without overexpression. Using zinc-finger nucleases to edit the genomes of mammalian cell lines, tagged clathrin light chain and dynamin were expressed at endogenous levels and the observed dynamics of mammalian endocytosis were described to be more similar to those of yeast than had previously been appreciated [86].
Interestingly, in contrast to mammalian cells, endocytosis in yeast still occurs in both clc1Δ and chc1Δ cells, establishing that while important, clathrin is not essential for yeast endocytosis [87, 88]. While clathrin-mediated endocytosis is conserved from yeast to mammals, no yeast counterparts for mammalian clathrin-independent pathways such as caveolin-mediated endocytosis have been identified. This relative simplicity enhances the power of yeast genetics because it is possible to focus on a single pathway without the confounding endocytosis of cargo by multiple pathways.
One potential major difference between yeast and mammalian endocytosis concerns the function of dynamin during scission. The yeast genome encodes three dynamin-related GTPases. Two are associated with mitochondria while the third, Vps1p, is crucial for intracellular trafficking events, but is not essential for endocytosis. Strikingly, the physical appearance of clathrin-coated pits in dynamin knockout mouse cells examined by electron microscopy strongly resemble the tubular invaginations present in yeast cells. Like yeast cells, the mammalian tubular invaginations were formed in an actin-dependent manner [89].
A third major difference is that mammalian but not yeast endocytosis relies heavily on the AP2 complex as a cargo adaptor; several well-studied cargos including the transferrin receptor show reduced internalization in AP2 knockdowns [23]. In yeast, the only known role for the AP2 complex is in endocytosis of the killer toxin K28; internalization of most cargo molecules is not affected in cells lacking the AP2 complex [24].
Because of the extensive homology between proteins and dynamics of yeast and mammalian clathrin-mediated endocytosis, findings in one system are often applicable in the other. Comparisons provide an avenue toward distilling fundamental mechanisms of endocytosis.
CONCLUDING REMARKS
The combination of traditional genetic approaches with the introduction of two-color, live-cell fluorescence microscopy, modern genomic techniques and mass spectrometry has resulted in a plethora of new discoveries about yeast endocytosis. Nearly 60 proteins are known to localize to sites of endocytosis and functions have been identified for many of these proteins. Yet open questions exist for all steps of the endocytic process (Box 4). How sites are initiated, how activities of the ~60 proteins are coordinated, and how actin polymerization and uncoating are regulated are examples of active areas of research, which promise to further our understanding of this vital process in the coming years.
Box 4. Outstanding questions.
While many great advances have been made, there are still many important outstanding questions in the field of budding yeast endocytosis, including:
The nature of the signal governing endocytic site initiation is unknown, as is the role of actin in site initiation.
The nature of the signal that causes a switch from the initial variable lifetime stage to the later regular lifetime stage is not known but may involve a cargo checkpoint.
How proteins are recruited to endocytic sites and how recruitment timing is controlled are both poorly understood.
How all of the Las17p activators and inhibitors function together to regulate actin polymerization remains a mystery.
The relative contributions of the type I myosins as Arp2/3 activators and motor proteins is unclear.
The scission mechanism is still unclear.
The roles of actin and other proteins involved in uncoating and endocytic site disassembly are relatively unstudied.
Whether lipids in addition to PIP2 have strong specific contributions to endocytosis needs to be investigated.
Roles for post-translational modifications, including ubiquitination and phosphorylation, need to be explored.
Finally, how actin filaments are organized and attach to the coat, how much of the invagination is covered with actin filaments, as well as the exact locations of many endocytic proteins need to be determined at an ultrastructural level.
Acknowledgments
We thank Alphée Michelot, Yidi Sun, Yonsong Miao and Christa Cortesio for comments on the manuscript. This work was supported by National Institutes of Health Grants (GM R01 42759) and (GM R01 50399) to DGD.
Glossary
- ANTH domain
AP180 N-terminal homology domain
- EH-NPF
interaction between Eps15 homology domain and Asparagine-Proline-Phenylalanine motif (not to be confused with a nucleation promoting factor)
- HECT ligase
E3 ubiquitin ligase with a HECT (homologous to the E6-AP Carboxyl terminus domain). These ligases form a covalent bond with the activated ubiquitin before transferring it directly to the substrate
- Line tension
the interfacial energy, or sum of the free energy, between two surfaces. This energy may be minimized by separating a mixture into two distinct phases
- UIM
ubiquitin-interacting motif
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Adams AE, Pringle JR. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J Cell Biol. 1984;98(3):934–45. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kilmartin JV, Adams AE. Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J Cell Biol. 1984;98(3):922–33. doi: 10.1083/jcb.98.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kubler E, Riezman H. Actin and fimbrin are required for the internalization step of endocytosis in yeast. EMBO J. 1993;12(7):2855–62. doi: 10.1002/j.1460-2075.1993.tb05947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulholland J, et al. Ultrastructure of the yeast actin cytoskeleton and its association with the plasma membrane. J Cell Biol. 1994;125(2):381–91. doi: 10.1083/jcb.125.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaksonen M, Sun Y, Drubin DG. A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell. 2003;115(4):475–87. doi: 10.1016/s0092-8674(03)00883-3. [DOI] [PubMed] [Google Scholar]
- 6.Kaksonen M, Toret CP, Drubin DG. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell. 2005;123(2):305–20. doi: 10.1016/j.cell.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Toshima JY, et al. Spatial dynamics of receptor-mediated endocytic trafficking in budding yeast revealed by using fluorescent alpha-factor derivatives. Proc Natl Acad Sci U S A. 2006;103(15):5793–8. doi: 10.1073/pnas.0601042103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huckaba TM, et al. Live cell imaging of the assembly, disassembly, and actin cable-dependent movement of endosomes and actin patches in the budding yeast, Saccharomyces cerevisiae. J Cell Biol. 2004;167(3):519–30. doi: 10.1083/jcb.200404173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toshima J, et al. Negative regulation of yeast Eps15-like Arp2/3 complex activator, Pan1p, by the Hip1R-related protein, Sla2p, during endocytosis. Mol Biol Cell. 2007;18(2):658–68. doi: 10.1091/mbc.E06-09-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friesen H, et al. Characterization of the yeast amphiphysins Rvs161p and Rvs167p reveals roles for the Rvs heterodimer in vivo. Mol Biol Cell. 2006;17(3):1306–21. doi: 10.1091/mbc.E05-06-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tonikian R, et al. Bayesian modeling of the yeast SH3 domain interactome predicts spatiotemporal dynamics of endocytosis proteins. PLoS Biol. 2009;7(10):e1000218. doi: 10.1371/journal.pbio.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sekiya-Kawasaki M, et al. Dynamic phosphoregulation of the cortical actin cytoskeleton and endocytic machinery revealed by real-time chemical genetic analysis. J Cell Biol. 2003;162(5):765–72. doi: 10.1083/jcb.200305077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong MH, et al. Vrp1p-Las17p interaction is critical for actin patch polarization but is not essential for growth or fluid phase endocytosis in S. cerevisiae. Biochim Biophys Acta. 2010;1803(12):1332–46. doi: 10.1016/j.bbamcr.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Reider A, et al. Syp1 is a conserved endocytic adaptor that contains domains involved in cargo selection and membrane tubulation. EMBO J. 2009;28(20):3103–16. doi: 10.1038/emboj.2009.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stimpson HE, et al. Early-arriving Syp1p and Ede1p function in endocytic site placement and formation in budding yeast. Mol Biol Cell. 2009;20(22):4640–51. doi: 10.1091/mbc.E09-05-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguilar RC, Watson HA, Wendland B. The yeast Epsin Ent1 is recruited to membranes through multiple independent interactions. J Biol Chem. 2003;278(12):10737–43. doi: 10.1074/jbc.M211622200. [DOI] [PubMed] [Google Scholar]
- 17.Henne WM, et al. FCHo proteins are nucleators of clathrin-mediated endocytosis. Science. 2010;328(5983):1281–4. doi: 10.1126/science.1188462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Homma K, et al. Phosphatidylinositol-4-phosphate 5-kinase localized on the plasma membrane is essential for yeast cell morphogenesis. J Biol Chem. 1998;273(25):15779–86. doi: 10.1074/jbc.273.25.15779. [DOI] [PubMed] [Google Scholar]
- 19.Idrissi FZ, et al. Distinct acto/myosin-I structures associate with endocytic profiles at the plasma membrane. J Cell Biol. 2008;180(6):1219–32. doi: 10.1083/jcb.200708060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Layton AT, et al. Modeling vesicle traffic reveals unexpected consequences for Cdc42p-mediated polarity establishment. Curr Biol. 2011;21(3):184–94. doi: 10.1016/j.cub.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loerke D, et al. Cargo and dynamin regulate clathrin-coated pit maturation. PLoS Biol. 2009;7(3):e57. doi: 10.1371/journal.pbio.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Pietro SM, et al. Regulation of clathrin adaptor function in endocytosis: novel role for the SAM domain. EMBO J. 2010;29(6):1033–44. doi: 10.1038/emboj.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motley A, et al. Clathrin-mediated endocytosis in AP-2-depleted cells. J Cell Biol. 2003;162(5):909–18. doi: 10.1083/jcb.200305145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carroll SY, et al. A yeast killer toxin screen provides insights into a/b toxin entry, trafficking, and killing mechanisms. Dev Cell. 2009;17(4):552–60. doi: 10.1016/j.devcel.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burston HE, et al. Regulators of yeast endocytosis identified by systematic quantitative analysis. J Cell Biol. 2009;185(6):1097–110. doi: 10.1083/jcb.200811116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hicke L, Riezman H. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell. 1996;84(2):277–287. doi: 10.1016/s0092-8674(00)80982-4. [DOI] [PubMed] [Google Scholar]
- 27.Dores MR, et al. The function of yeast epsin and Ede1 ubiquitin-binding domains during receptor internalization. Traffic. 2010;11(1):151–60. doi: 10.1111/j.1600-0854.2009.01003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maldonado-Baez L, et al. Interaction between Epsin/Yap180 adaptors and the scaffolds Ede1/Pan1 is required for endocytosis. Mol Biol Cell. 2008;19(7):2936–48. doi: 10.1091/mbc.E07-10-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boettner DR, et al. The F-BAR protein Syp1 negatively regulates WASp-Arp2/3 complex activity during endocytic patch formation. Curr Biol. 2009;19(23):1979–87. doi: 10.1016/j.cub.2009.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Y, et al. Interaction of Sla2p’s ANTH domain with PtdIns(4,5)P2 is important for actin-dependent endocytic internalization. Mol Biol Cell. 2005;16(2):717–30. doi: 10.1091/mbc.E04-08-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaksonen M, Toret CP, Drubin DG. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2006;7(6):404–14. doi: 10.1038/nrm1940. [DOI] [PubMed] [Google Scholar]
- 32.Zeng G, Yu X, Cai M. Regulation of yeast actin cytoskeleton-regulatory complex Pan1p/Sla1p/End3p by serine/threonine kinase Prk1p. Mol Biol Cell. 2001;12(12):3759–72. doi: 10.1091/mbc.12.12.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toret CP, et al. Multiple pathways regulate endocytic coat disassembly in Saccharomyces cerevisiae for optimal downstream trafficking. Traffic. 2008;9(5):848–59. doi: 10.1111/j.1600-0854.2008.00726.x. [DOI] [PubMed] [Google Scholar]
- 34.Robertson AS, et al. The WASP homologue Las17 activates the novel actin-regulatory activity of Ysc84 to promote endocytosis in yeast. Mol Biol Cell. 2009;20(6):1618–28. doi: 10.1091/mbc.E08-09-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Y, Martin AC, Drubin DG. Endocytic internalization in budding yeast requires coordinated actin nucleation and myosin motor activity. Dev Cell. 2006;11(1):33–46. doi: 10.1016/j.devcel.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 36.Galletta BJ, Chuang DY, Cooper JA. Distinct roles for Arp2/3 regulators in actin assembly and endocytosis. PLoS Biol. 2008;6(1):e1. doi: 10.1371/journal.pbio.0060001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodal AA, et al. Negative regulation of yeast WASp by two SH3 domain-containing proteins. Curr Biol. 2003;13(12):1000–8. doi: 10.1016/s0960-9822(03)00383-x. [DOI] [PubMed] [Google Scholar]
- 38.Madania A, et al. The Saccharomyces cerevisiae homologue of human Wiskott-Aldrich syndrome protein Las17p interacts with the Arp2/3 complex. Mol Biol Cell. 1999;10(10):3521–38. doi: 10.1091/mbc.10.10.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basu R, Chang F. Characterization of dip1p reveals a switch in arp2/3-dependent actin assembly for fission yeast endocytosis. Curr Biol. 2011;21(11):905–16. doi: 10.1016/j.cub.2011.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Achard V, et al. A “primer”-based mechanism underlies branched actin filament network formation and motility. Curr Biol. 2010;20(5):423–8. doi: 10.1016/j.cub.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 41.Aghamohammadzadeh S, Ayscough KR. Differential requirements for actin during yeast and mammalian endocytosis. Nat Cell Biol. 2009;11(8):1039–42. doi: 10.1038/ncb1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boulant S, et al. Actin dynamics counteract membrane tension during clathrin-mediated endocytosis. Nat Cell Biol. 2011 doi: 10.1038/ncb2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodman A, et al. The Saccharomyces cerevisiae calponin/transgelin homolog Scp1 functions with fimbrin to regulate stability and organization of the actin cytoskeleton. Mol Biol Cell. 2003;14(7):2617–29. doi: 10.1091/mbc.E03-01-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gourlay CW, et al. An interaction between Sla1p and Sla2p plays a role in regulating actin dynamics and endocytosis in budding yeast. J Cell Sci. 2003;116(Pt 12):2551–64. doi: 10.1242/jcs.00454. [DOI] [PubMed] [Google Scholar]
- 45.McCann RO, Craig SW. Functional genomic analysis reveals the utility of the I/LWEQ module as a predictor of protein:actin interaction. Biochem Biophys Res Commun. 1999;266(1):135–40. doi: 10.1006/bbrc.1999.1776. [DOI] [PubMed] [Google Scholar]
- 46.Gavin AC, et al. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature. 2002;415(6868):141–7. doi: 10.1038/415141a. [DOI] [PubMed] [Google Scholar]
- 47.Newpher TM, et al. Novel function of clathrin light chain in promoting endocytic vesicle formation. Mol Biol Cell. 2006;17(10):4343–52. doi: 10.1091/mbc.E06-07-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newpher TM, Lemmon SK. Clathrin is important for normal actin dynamics and progression of Sla2p-containing patches during endocytosis in yeast. Traffic. 2006;7(5):574–88. doi: 10.1111/j.1600-0854.2006.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boettner DR, et al. Clathrin light chain directs endocytosis by influencing the binding of the yeast Hip1R homologue, Sla2, to F-actin. Mol Biol Cell. 2011 doi: 10.1091/mbc.E11-07-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Michelot A, et al. Reconstitution and protein composition analysis of endocytic actin patches. Curr Biol. 2010;20(21):1890–9. doi: 10.1016/j.cub.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodal AA, et al. Actin and septin ultrastructures at the budding yeast cell cortex. Mol Biol Cell. 2005;16(1):372–84. doi: 10.1091/mbc.E04-08-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Young ME, Cooper JA, Bridgman PC. Yeast actin patches are networks of branched actin filaments. J Cell Biol. 2004;166(5):629–35. doi: 10.1083/jcb.200404159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skau CT, Kovar DR. Fimbrin and tropomyosin competition regulates endocytosis and cytokinesis kinetics in fission yeast. Curr Biol. 2010;20(16):1415–22. doi: 10.1016/j.cub.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skau CT, et al. Actin filament bundling by fimbrin is important for endocytosis, cytokinesis and polarization in fission yeast. J Biol Chem. 2011 doi: 10.1074/jbc.M111.239004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gandhi M, et al. Coronin switches roles in actin disassembly depending on the nucleotide state of actin. Mol Cell. 2009;34(3):364–74. doi: 10.1016/j.molcel.2009.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okreglak V, Drubin DG. Loss of Aip1 reveals a role in maintaining the actin monomer pool and an in vivo oligomer assembly pathway. J Cell Biol. 2010;188(6):769–77. doi: 10.1083/jcb.200909176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smaczynska-de R, II, et al. A role for the dynamin-like protein Vps1 during endocytosis in yeast. J Cell Sci. 2010;123(Pt 20):3496–506. doi: 10.1242/jcs.070508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nannapaneni S, et al. The yeast dynamin-like protein Vps1:vps1 mutations perturb the internalization and the motility of endocytic vesicles and endosomes via disorganization of the actin cytoskeleton. Eur J Cell Biol. 2010;89(7):499–508. doi: 10.1016/j.ejcb.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 59.Yu X, Cai M. The yeast dynamin-related GTPase Vps1p functions in the organization of the actin cytoskeleton via interaction with Sla1p. J Cell Sci. 2004;117(Pt 17):3839–53. doi: 10.1242/jcs.01239. [DOI] [PubMed] [Google Scholar]
- 60.Liu J, et al. Endocytic vesicle scission by lipid phase boundary forces. Proc Natl Acad Sci U S A. 2006;103(27):10277–82. doi: 10.1073/pnas.0601045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu J, et al. The mechanochemistry of endocytosis. PLoS Biol. 2009;7(9):e1000204. doi: 10.1371/journal.pbio.1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Youn JY, et al. Dissecting BAR domain function in the yeast Amphiphysins Rvs161 and Rvs167 during endocytosis. Mol Biol Cell. 2010;21(17):3054–69. doi: 10.1091/mbc.E10-03-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun Y, et al. PtdIns(4,5)P2 turnover is required for multiple stages during clathrin- and actin-dependent endocytic internalization. J Cell Biol. 2007;177(2):355–67. doi: 10.1083/jcb.200611011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smaczynska-de R, II, Costa R, Ayscough KR. Yeast Arf3p modulates plasma membrane PtdIns(4,5)P2 levels to facilitate endocytosis. Traffic. 2008;9(4):559–73. doi: 10.1111/j.1600-0854.2008.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Costa R, Warren DT, Ayscough KR. Lsb5p interacts with actin regulators Sla1p and Las17p, ubiquitin and Arf3p to couple actin dynamics to membrane trafficking processes. Biochem J. 2005;387(Pt 3):649–58. doi: 10.1042/BJ20041729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van der Rest ME, et al. The plasma membrane of Saccharomyces cerevisiae: structure, function, and biogenesis. Microbiol Rev. 1995;59(2):304–22. doi: 10.1128/mr.59.2.304-322.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singer-Kruger B, et al. Synaptojanin family members are implicated in endocytic membrane traffic in yeast. J Cell Sci. 1998;111(Pt 22):3347–56. doi: 10.1242/jcs.111.22.3347. [DOI] [PubMed] [Google Scholar]
- 68.Romer W, et al. Actin dynamics drive membrane reorganization and scission in clathrin-independent endocytosis. Cell. 2010;140(4):540–53. doi: 10.1016/j.cell.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 69.Zanolari B, et al. Sphingoid base synthesis requirement for endocytosis in Saccharomyces cerevisiae. EMBO J. 2000;19(12):2824–33. doi: 10.1093/emboj/19.12.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Friant S, et al. Sphingoid base signaling via Pkh kinases is required for endocytosis in yeast. EMBO J. 2001;20(23):6783–92. doi: 10.1093/emboj/20.23.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Munn AL, et al. Specific sterols required for the internalization step of endocytosis in yeast. Mol Biol Cell. 1999;10(11):3943–57. doi: 10.1091/mbc.10.11.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heese-Peck A, et al. Multiple functions of sterols in yeast endocytosis. Mol Biol Cell. 2002;13(8):2664–80. doi: 10.1091/mbc.E02-04-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin CH, et al. Arrestin-related ubiquitin-ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell. 2008;135(4):714–25. doi: 10.1016/j.cell.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 74.Gajewska B, et al. WW domains of Rsp5p define different functions: determination of roles in fluid phase and uracil permease endocytosis in Saccharomyces cerevisiae. Genetics. 2001;157(1):91–101. doi: 10.1093/genetics/157.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oldham CE, et al. The ubiquitin-interacting motifs target the endocytic adaptor protein epsin for ubiquitination. Curr Biol. 2002;12(13):1112–6. doi: 10.1016/s0960-9822(02)00900-4. [DOI] [PubMed] [Google Scholar]
- 76.Kaminska J, et al. Rsp5p, a new link between the actin cytoskeleton and endocytosis in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 2002;22(20):6946–8. doi: 10.1128/MCB.22.20.6946-6958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stamenova SD, et al. The Rsp5 ubiquitin ligase binds to and ubiquitinates members of the yeast CIN85-endophilin complex, Sla1-Rvs167. J Biol Chem. 2004;279(16):16017–25. doi: 10.1074/jbc.M313479200. [DOI] [PubMed] [Google Scholar]
- 78.Toshima J, et al. Phosphoregulation of Arp2/3-dependent actin assembly during receptor-mediated endocytosis. Nat Cell Biol. 2005;7(3):246–54. doi: 10.1038/ncb1229. [DOI] [PubMed] [Google Scholar]
- 79.Cope MJ, et al. Novel protein kinases Ark1p and Prk1p associate with and regulate the cortical actin cytoskeleton in budding yeast. J Cell Biol. 1999;144(6):1203–18. doi: 10.1083/jcb.144.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang B, et al. Negative regulation of the actin-regulating kinase Prk1p by patch localization-induced autophosphorylation. Traffic. 2009;10(1):35–41. doi: 10.1111/j.1600-0854.2008.00842.x. [DOI] [PubMed] [Google Scholar]
- 81.Kukulski W, et al. Correlated fluorescence and 3D electron microscopy with high sensitivity and spatial precision. J Cell Biol. 2011;192(1):111–9. doi: 10.1083/jcb.201009037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mobius W. Cryopreparation of biological specimens for immunoelectron microscopy. Ann Anat. 2009;191(3):231–47. doi: 10.1016/j.aanat.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 83.Chang-Ileto B, et al. Synaptojanin 1-mediated PI(4,5)P2 hydrolysis is modulated by membrane curvature and facilitates membrane fission. Dev Cell. 2011;20(2):206–18. doi: 10.1016/j.devcel.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berro J, Sirotkin V, Pollard TD. Mathematical modeling of endocytic actin patch kinetics in fission yeast: disassembly requires release of actin filament fragments. Mol Biol Cell. 2010;21(16):2905–15. doi: 10.1091/mbc.E10-06-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taylor MJ, Perrais D, Merrifield CJ. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol. 2011;9(3):e1000604. doi: 10.1371/journal.pbio.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Doyon JB, et al. Rapid and efficient clathrin-mediated endocytosis revealed in genome-edited mammalian cells. Nat Cell Biol. 2011;13(3):331–7. doi: 10.1038/ncb2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Newpher TM, et al. In vivo dynamics of clathrin and its adaptor-dependent recruitment to the actin-based endocytic machinery in yeast. Dev Cell. 2005;9(1):87–98. doi: 10.1016/j.devcel.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 88.Tan PK, et al. Clathrin facilitates the internalization of seven transmembrane segment receptors for mating pheromones in yeast. J Cell Biol. 1993;123(6 Pt 2):1707–16. doi: 10.1083/jcb.123.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ferguson SM, et al. Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev Cell. 2009;17(6):811–22. doi: 10.1016/j.devcel.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jenness DD, Spatrick P. Down regulation of the alpha-factor pheromone receptor in S. cerevisiae. Cell. 1986;46(3):345–53. doi: 10.1016/0092-8674(86)90655-0. [DOI] [PubMed] [Google Scholar]
- 91.Riezman H. Endocytosis in yeast: several of the yeast secretory mutants are defective in endocytosis. Cell. 1985;40(4):1001–9. doi: 10.1016/0092-8674(85)90360-5. [DOI] [PubMed] [Google Scholar]
- 92.Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128(5):779–92. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Volland C, et al. Endocytose and degradation of the uracil permease of S. cerevisiae under stress conditions: possible role of ubiquitin. Folia Microbiol (Praha) 1994;39(6):554–7. doi: 10.1007/BF02814106. [DOI] [PubMed] [Google Scholar]
- 94.Berkower C, Loayza D, Michaelis S. Metabolic instability and constitutive endocytosis of STE6, the a-factor transporter of Saccharomyces cerevisiae. Mol Biol Cell. 1994;5(11):1185–98. doi: 10.1091/mbc.5.11.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kolling R, Hollenberg CP. The ABC-transporter Ste6 accumulates in the plasma membrane in a ubiquitinated form in endocytosis mutants. EMBO J. 1994;13(14):3261–71. doi: 10.1002/j.1460-2075.1994.tb06627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Egner R, Kuchler K. The yeast multidrug transporter Pdr5 of the plasma membrane is ubiquitinated prior to endocytosis and degradation in the vacuole. FEBS Lett. 1996;378(2):177–81. doi: 10.1016/0014-5793(95)01450-0. [DOI] [PubMed] [Google Scholar]
- 97.Sirotkin V, et al. Quantitative analysis of the mechanism of endocytic actin patch assembly and disassembly in fission yeast. Mol Biol Cell. 2010;21(16):2894–904. doi: 10.1091/mbc.E10-02-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stradalova V, et al. Furrow-like invaginations of the yeast plasma membrane correspond to membrane compartment of Can1. J Cell Sci. 2009;122(Pt 16):2887–94. doi: 10.1242/jcs.051227. [DOI] [PubMed] [Google Scholar]
- 99.Moor H, Muhlethaler K. Fine Structure in Frozen-Etched Yeast Cells. J Cell Biol. 1963;17(3):609–28. doi: 10.1083/jcb.17.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Walther TC, et al. Eisosomes mark static sites of endocytosis. Nature. 2006;439(7079):998–1003. doi: 10.1038/nature04472. [DOI] [PubMed] [Google Scholar]
- 101.Grossmann G, et al. Plasma membrane microdomains regulate turnover of transport proteins in yeast. J Cell Biol. 2008;183(6):1075–88. doi: 10.1083/jcb.200806035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brach T, Specht T, Kaksonen M. Reassessment of the role of plasma membrane domains in the regulation of vesicular traffic in yeast. J Cell Sci. 2011;124(Pt 3):328–37. doi: 10.1242/jcs.078519. [DOI] [PubMed] [Google Scholar]
- 103.Moreira KE, et al. Pil1 controls eisosome biogenesis. Mol Biol Cell. 2009;20(3):809–18. doi: 10.1091/mbc.E08-03-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ayscough KR, et al. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J Cell Biol. 1997;137(2):399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mishra R, et al. Expression of Vps1 I649K a self-assembly defective yeast dynamin, leads to formation of extended endocytic invaginations. Commun Integr Biol. 2011;4(1):115–7. doi: 10.4161/cib.4.1.14206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gupta R, et al. Ubiquitination screen using protein microarrays for comprehensive identification of Rsp5 substrates in yeast. Mol Syst Biol. 2007;3:116. doi: 10.1038/msb4100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peng J, et al. A proteomics approach to understanding protein ubiquitination. Nat Biotechnol. 2003;21(8):921–6. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- 108.Stamenova SD, et al. Ubiquitin binds to and regulates a subset of SH3 domains. Mol Cell. 2007;25(2):273–84. doi: 10.1016/j.molcel.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]