Abstract
Background
Alcohol dependence is associated with inhibitory control deficits, possibly related to abnormalities in frontoparietal cortical and midbrain function and connectivity.
Methods
We examined functional connectivity and microstructural fiber integrity between frontoparietal and midbrain structures using a Stroop Match-to-Sample task with functional MRI and diffusion tensor imaging in 18 alcoholics and 17 controls. Manipulation of color cues and response repetition sequences modulated cognitive demands during Stroop conflict.
Results
Despite similar lateral frontoparietal activity and functional connectivity in alcoholics and controls when processing conflict, controls deactivated the posterior cingulate cortex (PCC), whereas alcoholics did not. Posterior cingulum fiber integrity predicted the degree of PCC deactivation in controls but not alcoholics. Also, PCC activity was modulated by executive control demands: activated during response switching and deactivated during response repetition. Alcoholics showed the opposite pattern: activation during repetition and deactivation during switching. Here, in alcoholics, greater deviations from the normal PCC activity correlated with higher amounts of lifetime alcohol consumption. A functional dissociation of brain network connectivity between the groups further showed that controls exhibited greater corticocortical connectivity between middle cingulate, posterior cingulate, and medial prefrontal cortices than alcoholics. By contrast, alcoholics exhibited greater midbrain-orbitofrontal cortical network connectivity than controls. Degree of microstructural fiber integrity predicted robustness of functional connectivity.
Conclusion
Thus, even subtle compromise of microstructural connectivity in alcoholism can influence modulation of functional connectivity and underlie alcohol-related cognitive impairment.
Keywords: Functional connectivity, functional MRI, diffusion tensor imaging, white matter fiber tractography, Stroop task, Alcohol Use Disorder
Introduction
Neuroadaptive changes occur in the brain’s response to chronic alcohol consumption. The circuits involved in these neuroadaptive changes are anchored in midbrain areas with dopaminergic and glutamatergic projections to cortical and striatal sites: ventral striatum for cue-induced alcohol-seeking (1), striato-pallado-thalamic loops for automaticity of behavior (2), and prefrontal cortices for attentional selection and executive control of pre-potent responses (3–4). In alcohol abusers, neuroadaptation of reward circuits interferes with normal functional connectivity between midbrain, striato-thalamic, and prefrontal nodes involved in mediating attention (5) and decision making (6). Despite these clues, it is not yet fully understood how chronic alcoholism affects dynamic functional connectivity of brain systems while engaged in tasks.
Even minimal disruption of neural connectivity can have a significant impact on the interaction of brain circuits that regulate reward, attention, and executive control (7–8). Functional connectivity is typically defined in terms of synchrony between brain regions demonstrating spontaneous fluctuations in hemodynamic activity measured in the “resting state” that is, in the absence of externally-driven task demands, and are considered intrinsic activity of the brain (9). Alcoholism may affect intrinsic networks (10), including the default mode network (DMN) linking posterior cingulate and medial prefrontal cortices, that are normally decoupled while engaged in a task, presumably for efficient processing, but highly connected during rest (11). Consequently, primary nodes of the DMN such as the posterior cingulate cortex (PCC) (12–13) may play a role for the brain’s balance between activation and deactivation to task demands (14) for resource maximization. The modulation of functional network connectivity by different task demands (15) may be affected in alcoholism. Because efficient communication between brain regions depends on white matter fiber tract integrity (16–17), compromised structural integrity may further alter functional connectivity between brain regions (12,18).
Excessive alcohol consumption can adversely affect white matter fibers, thereby disrupting transmission of information between brain sites (17–19). Even minor disruption of brain structural connectivity without frank lesions may pose the need for compensatory task-specific functional connectivity adjustments. Functional recovery in abstinent alcoholics may reflect recovery of structural connectivity or, alternatively, a strengthened functional connectivity to unaffected nodes forming a compensatory network (20). Thus, executive control, such as the successful inhibition of a pre-potent response, probably requires both intact structural and robust functional connectivity between multiple task-specific regions (19).
To investigate the effects of chronic alcoholism on microstructural and functional brain networks that underlie inhibitory control function, we addressed automaticity of responses arising from repetition learning, a mechanism that likely plays a key role in situations when inhibition of over-learned behavior is required, such as abstaining from alcohol. We used task-related functional magnetic resonance imaging (fMRI) obtaining measures of functional activation and connectivity among midbrain, limbic, and prefrontal regions and between dorsolateral prefrontal and parietal cortices. This conflict resolution task varied visual attentional demands by using visual cues that either matched or did not match the color of the upcoming Stroop stimulus (21). Executive control demands were manipulated by requiring subjects either to switch responses or to repeat the same response several times in a row. In addition, MR diffusion tensor imaging (DTI)-based fiber tracking quantified the microstructural integrity of cingulate fiber bundles, which connects frontal and posterior brain regions relevant to attentional control processes assessed with the Stroop task using fMRI and functional connectivity (fcMRI). We tested two principal hypotheses: 1) the levels of functional activity (fMRI) and connectivity (fcMRI), indexed as correlated time courses in task-related activation between cortical and subcortical network nodes, would be related to the degree of integrity of inter-nodal network microstructural connectivity of cingulate bundles (DTI tractography); 2) chronic alcoholism would exhibit compromised structural (DTI tractography) and functional connectivity (fcMRI) between distributed attention and executive control network nodes enabling efficient inhibitory control.
Methods and Materials
Participants
Groups comprised 18 alcoholic men and 17 control men. Alcoholics were recruited from local rehabilitation programs; controls were volunteers from the local community. All subjects were screened with the Structural Clinical Interview for DSM-IV (SCID) (22) and a clinical examination to rule out other Axis I diagnoses or non-alcohol substance abuse. All alcoholics met DSM-IV criteria for alcohol dependence; none had current dependence; 5 alcoholics met criteria for sustained full remission, and 13 alcoholics met criteria for early full remission. Subject groups were matched in age, handedness, and other demographic variables (Table 1; Supplement).
Table 1.
| Group | Age | Education | NART IQ | Handedness CROVITZ | Visual Acuity | Lifetime Alcohol Consumption (kg) | Length of Sobriety (days) |
|---|---|---|---|---|---|---|---|
| ALC | 51 (6.6) | 15 (3.1) | 110 (7.6) | 21 (5.5) | 1.3 (0.6) | 1549 (620.7) | 257.7(219) |
| CTL | 50 (14.9) | 16 (2.5) | 115 (6.9) | 21 (8.9) | 1.8 (0.9) | 29 (45.1) | - |
|
| |||||||
| P | 0.7 | 0.15 | 0.06 | 0.9 | 0.08 | 0.0001 | |
Means and standard deviations (SD) of demographic data of the two study groups: Chronic alcoholics (ALC) and controls (CTL). Between group t-tests: significance at p < 0.05, 2-tailed.
All participants gave written informed consent to participate in this study, which was approved by the Institutional Review Boards at SRI International and Stanford University School of Medicine.
Stimuli and experimental design
Study participants underwent cognitive testing and additional behavioral testing during fMRI image acquisition. Structural and functional MR imaging data were acquired using a clinical whole-body GE 3T scanner. Using a back-projection system during the fMRI session, participants viewed stimuli on a mirror attached to the head coil.
Stroop Match-to-Sample Task
Stimuli were created and presented with PsyScope software and were synchronized with MRI acquisition using a PsyScope button box as interface (23). Subjects matched the color of a cue stimulus displayed for 450 ms in the center of the screen to the color of a Stroop target stimulus that appeared for 1100 ms after an inter-stimulus interval of 300 milliseconds. Cue and target colors were red, green, or blue. Total trial duration was 3.3 sec. The color cue either matched or did not match the color of the Stroop target, which was either congruent (word blue written in blue font color) or incongruent (word blue written in red font color). The Stroop effect is defined as the difference in reaction time (RT) to incongruent and congruent stimuli. For incongruent-nonmatch conditions, the cue’s color always matched the word’s content (e.g., red cue, word RED written in green font color) (Figure 1) (21, 24–26).
Figure 1.
(Top) Stroop Match-to-Sample paradigm, illustrating four conditions: (1) congruent-match, (2) incongruent-match, (3) congruent-nonmatch, and (4) incongruent-nonmatch. A color cue (XXXX) presented for 450 ms was followed by an incongruent or congruent Stroop target stimulus that appeared for 1100 ms after an inter-stimulus interval (ISI) of 300 ms. The inter-trial interval (ITI) was 1450 ms. Subjects matched the color (red, green or blue) of the cue to the ink color of the Stroop stimulus. (Bottom) fMRI block design illustrated for 8 blocks: Stroop stimuli in each block were either congruent (word BLUE written in blue font) or incongruent (word BLUE written in red font). Four blocks contained mixed YES- and NO responses: 2 incongruent with match and non-match trials (incongruent, INC), 2 congruent with match and nonmatch trials (congruent, CON). In addition, four same-response blocks were presented: congruent-match, congruent-nonmatch, incongruent-match, incongruent-nonmatch. Trials presented in same- and mixed-response blocks were the same, only the order of trials differed, i.e., in same response blocks, either six match trials or six non-match trials were presented in a row, whereas in mixed-response blocks, three match and three nonmatch trials were presented in random order. Each block consisted of 6 trials, and lasted for of 19.8 s. In response-repetition blocks, cue-target color either matched or did not match, in response-switching blocks, match and nonmatch trials were mixed. In total 32 blocks were presented in pseudo-random order ensuring that each condition was equally often represented (24).
Thus, Debriefing after fMRI scanning revealed that none of our subjects was aware about the blocked nature of the task.
Subjects pressed a YES-key for cue-target color matches and a NO-key for nonmatches using their right hand, yielding accuracy and RT measures. To test the effect of repetitive behavior on cognitive control and conflict resolution, we presented trials in two block types: mixed and same response blocks (Figure 1). In mixed-response blocks, both match and non-match trials were presented and required switching between YES and NO responses, whereas same-response blocks contained either match (YES-responses) or nonmatch trials (NO-responses) and did not require response switches. To quantity Stroop effects within a block design, incongruent and congruent trials were never mixed within a block. Mixed-response and same-response blocks comprised the same number of trials. Total number of trials was 96 per run. Two runs were presented with 16 blocks each (1 block = 9TRs or 6 trials; TR=2.2sec). Test instructions were reviewed with the subject in a practice session before entering the scanner and again through the scanner’s intercom system before each run. Subjects had a short break between run 1 and run 2, but remained in the scanner.
Magnetic Resonance Imaging (MRI)
Data acquisition and analyses
Whole-brain functional MRI and DTI data were acquired with an 8-channel head coil at a 3T GE whole body scanner. Detailed description of fMRI (21) and diffusion tensor imaging (DTI) (19) data acquisition and analyses are provided in the Supplement.
For fMRI analysis, standard preprocessing and statistical analyses were performed using the SPM8 software package (Wellcome Department of Cognitive Neurology), and for functional connectivity analysis (fcMRI), the “conn” toolbox was used as implemented SPM8. Analyses were carried out with a joint-expected probability threshold of p=0.01 for height and p=0.05 for extent family-wise-error (FWE) corrected for the whole brain volume (27). Montreal Neurological Institute (MNI) coordinates are reported in the text, and fMRI and fcMRI result tables provided in the Supplement. DTI-based fiber tracking of cingulum tracts was performed with the software by Gerig et al. (28), based on the method of Mori and colleagues (29–31). Mean fractional anisotropy (FA), longitudinal diffusivity (λl = λ1) and transverse diffusivity (λt = [λ2 +λ3]/2) for each of the three sections of the cingulate bundle (superior, posterior, inferior) were the units of analysis.
Statistical analyses
For behavioral data analysis, repeated measures analysis of variance (ANOVA) was used to test for group effects (alcoholics, controls) as between-subjects factor and to test for Stroop effects (incongruent/congruent), color match effects (nonmatch/match), and response block effects (mix/same) as within-subject factors. For DTI data analysis, group differences in FA and diffusivity measures (λl and λt) were tested with analysis of variance (ANOVA). To examine the relationships predicted between DTI measures (FA and diffusivity), task-related functional regional brain activation and the behavioral measures of Stroop performance, we used 2-tailed Pearson correlation in each group separately. We used 1-tailed Pearson correlation to test directional hypothesis on the effects of lifetime alcohol consumption on behavior and brain function. The alpha level was set to 0.05 for all statistical tests, including hypotheses-driven analyses of structure-function relationships. Bonferroni corrected p-levels were applied for multiple comparisons where applicable.
Results
Behavioral results
Stroop task performance
Overall reaction time (RT) during Stroop-task performance did not differ between alcoholics and controls (F(1,33)=1.92, p=0.18). An ANOVA on RT yielded a 4-way interaction involving group (ALC, CTL), Stroop (incongruent/congruent), color-match (nonmatch/match), and response block (repetition/switching) (F(1,33)=5.09, p=0.031) (Figure 2). Following up on the 4-way interaction, post-hoc analyses compared group differences for Stroop effects (RTINC – RTCON) from each condition (match/nonmatch; response-repetition/response-switching). Alcoholics showed smaller Stroop effects than controls for response-repetition-match (F(1,33)=6.12, p=0.019) but not response-repetition-nonmatch (F(1,33)=0.01, p=0.9), response-switching-match (F(1,33)=0.01, p=0.9), or response-switching-nonmatch (F(1,33)=0.48, p=0.5) conditions. These smaller Stroop effects in alcoholics than controls resulted from less benefit from response-repetitions and valid color cueing in congruent trials.
Figure 2.
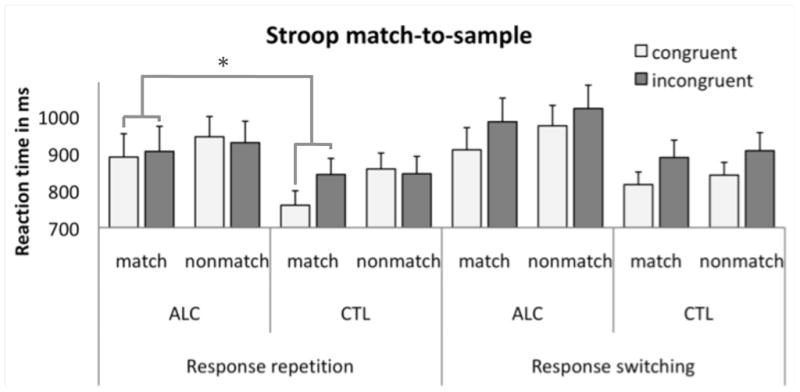
Mean reaction times (ms) and standard errors for each condition (congruent-match, incongruent-match, congruent-nonmatch, and incongruent-nonmatch) and response block (response repetition, response switching) for alcoholics (ALC) and controls (CTL).
In addition, the smaller Stroop-match effects during response-repetition (less benefit) in alcoholics correlated significantly with greater lifetime alcohol consumption (Figure 3). Smaller Stroop effects during response-repetition further correlated with younger age at onset of alcohol dependency (r=.61, p=0.004; Rho=.61, p=0.003). Length of sobriety was not related to performance.
Figure 3.
Pearson correlation between lifetime alcohol consumption (kg) and the behavioral Stroop-match effects (RTincongruent – RTcongruent) for response repetition trials for alcoholics (ALC) and controls (CTL). In ALC, higher amounts of lifetime alcohol consumption were associated with smaller Stroop-match effects during response repetition (less benefit).
Functional MRI results
With task-activated functional MRI data, we first asked which brain regions in alcoholics and controls were activated and which were deactivated during Stroop processing, i.e., when comparing incongruent and congruent trials. Given the 4-way interaction, we further asked whether Stroop activation would differ between groups as a function of response-repetition or color-cueing.
Stroop (INC>CON)
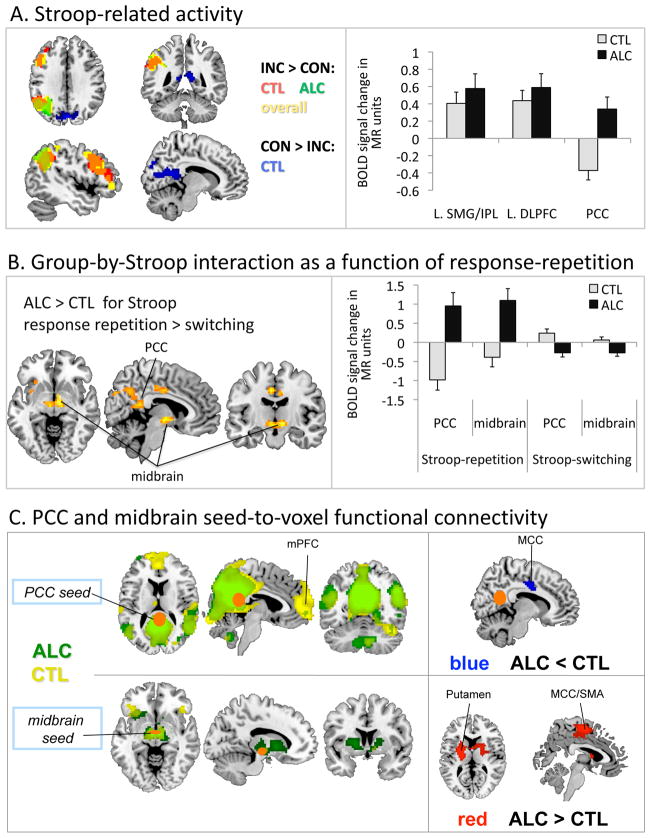
Over all subjects, incongruent Stroop targets (e.g., the word RED printed in blue) activated left middle [x=−48;y=14;z=40] and inferior frontal [x=−51;y=20;z=22] cortical regions and left inferior parietal lobe [x=−51;y=−55;z=34] more than congruent Stroop targets (e.g., the word RED printed in red). Groups did not significantly differ in left fronto-parietal activation pattern, but alcoholics activated midbrain regions [x=9;y=−4;z=−5] during Stroop conflict processing more than controls. The opposite contrast (CON>INC) revealed that alcoholics did not show the Stroop-related deactivation in the cuneus [x=0;y=−79;z=34] and posterior cingulate cortex (PCC) [x=−12;y=−52;z=10] seen in controls (Figure 4A; Table S1 in the Supplement).
Figure 4.
Functional magnetic resonance imaging (fMRI): (A) Stroop-related brain activation (incongruent, INC > congruent, CON) (left) and deactivation (CON > INC) (right) merged over response blocks; alcoholic subjects (ALC), control subjects (CTL). (B) Group-by-Stroop interaction (left) showing higher activation in the posterior cingulate cortex (PCC) and a ventral midbrain cluster (vMB) for response repetition than switching in ALC relative to CTL, and bar graphs (right) illustrating the extracted mean signal change (INC – CON) from the PCC and vMB clusters for each response block (repetition, switching) and for each group. (C) Functional connectivity MRI: Seed-to-voxel synchronous activation for the PCC seed (upper panel) and the ventral midbrain (vMB) seed (lower panel) for each response block and group. BOLD, blood oxygen level dependent; MR, magnetic resonance; L. SMG, left supramarginal gyrus; IPL, inferior parietal lobe; L. DLPFC, left dorsolateral prefrontal cortex; mPFC, medial prefrontal cortex; MCC, middle cingulate cortex; SMA, supplementary motor area.
Interaction between groups and Stroop response-repetition
Two regions showed a significant group-by-Stroop-response-block interaction: Relative to controls, alcoholics showed greater activation of bilateral PCC [x=0;y=−40;z=19] and midbrain areas (substantia nigra/subthalamic nucleii) [x=12;y=−13;z=−5] during response-repetition than switching (Figure 4B; Table S1 in the Supplement).
Interaction between groups and Stroop for color cueing
The block design allowed analyses of color-cueing effects separately for response-repetition blocks. PCC [x=−9;y=−49;z=13] and hippocampal [x=−27;y=−16;z=−14] activity differed between groups as a function of color-cue validity. When a valid cue correctly predicted the Stroop word’s color (Stroop-match), alcoholics deactivated PCC and cuneus [x=0;y=−79;z=34] less than controls (between-groups contrast). The within-group contrast showed that controls deactivated the PCC [x=0;y=−40;z=19] more during incongruent-match trials (INC>CON); the other face of this contrast indicates that controls activated the PCC during congruent-match (CON>INC) response-repetition blocks. For Stroop (INC>CON) processing with invalid, nonmatching color cues (Stroop-nonmatch), alcoholics activated the left hippocampus [x=−27;y=−16;z=−14] less than controls (Table S1 in the Supplement).
Lifetime alcohol consumption and Stroop activation
Based on the assumption that lifetime alcohol consumption would predict group differences in brain activation, we used correlational analyses to test the relation between alcohol consumption and activity in PCC and midbrain during Stroop response-repetition and switching (Figure 2B). In support of our hypothesis, the amount of lifetime alcohol consumption predicted the deviant activation and deactivation patterns observed in alcoholics: During Stroop response-repetition, higher amounts of alcohol consumption predicted greater PCC (r=.50, p=0.003; Rho=.58, p<0.0001, n=33) and midbrain activation (r=.47, p=0.006; Rho=.44, p=0.01, n=33). During Stroop response-switching, higher amounts of alcohol consumption predicted lower PCC (r=−.49, p=0.004; Rho=−.51, p=0.002, n=33) and midbrain activation (r=−.43, p=0.013; Rho=−.41, p=0.017, n=33) (Figure 5). Correlations were significant with Bonferroni-correction for multiple comparisons, which required p<0.025 for directional hypotheses.
Figure 5.
Pearson correlation between lifetime alcohol consumption (kg) and activity in PCC and midbrain for response-repetition and response-switching blocks for alcoholics (ALC) and controls (CTL). In ALC, higher amounts of lifetime alcohol consumption predicted the degree to which this brain activity was deviant from the normal modulation.
Functional connectivity
ROI-to-voxel functional connectivity
For each group, we derived functional connectivity maps, indexing ROI-to-voxel synchronous activity, via time series correlations of activity over 324 time points for the regions showing significant group-by-task activation contrasts: PCC/cuneus and midbrain. Using the cortical PCC as seed region, both groups showed extensive functional connectivity to motor, supplementary motors areas, and bilateral temporal cortices. Controls showed extensive PCC connectivity to medial prefrontal cortices that was not as widespread in alcoholics. However, group comparison revealed that alcoholics had less synchronized activity than controls, mainly between posterior (PCC) and middle cingulate cortices (MCC) [x=12;y=−4;z=37] (Figure 4C; Table S2 in the Supplement).
Using the midbrain as the seed region, both groups showed synchronous time series with bilateral inferior frontal and orbitofrontal regions. Alcoholics showed more midbrain connectivity to the middle cingulate cortex [x=9;y=−1;z=37] and striatal regions [x=−30;y=−1;z=7] than controls (Figure 4C; Table S2 in the Supplement).
Brain microstructure-function relationships
Cingulate fiber bundles
Separate group-by-regional cingulated bundle sector ANOVAs were calculated for FA, λl, and λt with group (alcoholics, controls) as between-subjects factor and the cingulate fiber bundle sectors (superior, posterior, inferior) and hemisphere as within-subject factors. Significant 3-way interactions occurred for FA (F(1,31)=5.63, p=0.01); although λt showed a trend (F(1,33)=2.63, p=0.06), λl did not (F(1,33)=0.03, p=0.89). Overall, cingulate bundle integrity was not affected in alcoholics except for statistically significant, albeit subtle, compromise in left-hemispheric posterior cingulate fibers.
Fiber integrity and brain activation
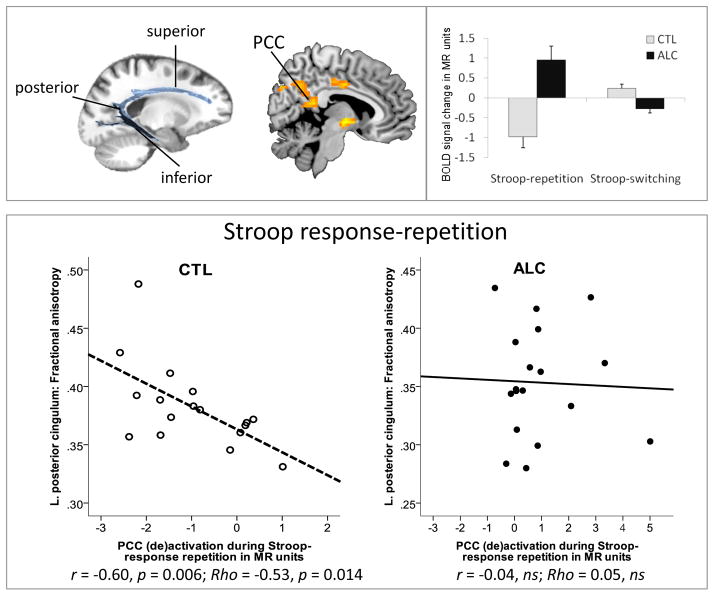
Using correlation analyses, we tested for group differences in the relationship between left-hemispheric posterior cingulum fiber integrity (FA) and PCC activation during Stroop response-repetition. In controls, but not alcoholics (r=−.04, ns), posterior cingulum fiber integrity, indexed by higher FA, predicted more pronounced PCC deactivations (r=−.60, p=0.006) (Figure 6). This posterior cingulate function–microstructure correlation differed significantly between alcoholics and controls (z=1.75, p<0.05) (r to zr transformation [zr=0.5 loge(1 + r) (1 − r)]) (32).
Figure 6.
(TOP LEFT) DTI-based visualization of cingulate fibers divided into three sectors: superior, posterior, and inferior bundles. (TOP MIDDLE) Illustration of posterior cingulate cortex (PCC) deactivation during Stroop processing and (TOP RIGHT) bar graphs illustrating the extracted mean signal change (INC – CON) from the PCC cluster for each response block (repetition, switching) and group (ALC, CTL). (BOTTOM) Pearson correlation between fiber integrity, indexed as high fractional anisotropy (FA), of the posterior cingulate fiber sector and Stroop-related deactivation of the posterior cingulate cortex during response repetition blocks (LEFT) and response switching blocks (RIGHT) for each group: controls (CTL), alcoholics (ALC).
Fiber integrity and functional connectivity
To explore whether functional connectivity differences in alcoholics and controls were related to microstructural degradation in alcoholics, we correlated left posterior cingulum FA with the time-series correlation values (z-transformed) for posterior and middle cingulate cortex (PCC-MCC) connectivity (Table S1 in the Supplement) and midbrain-putamen and midbrain-MCC functional connectivity (Table S1 in the Supplement). In alcoholics, greater left posterior cingulum fiber degradation (lower FA) predicted stronger midbrain-MCC functional connectivity for Stroop processing during more difficult response-switching conditions (r=−.56, p=0.016). By contrast, in controls, greater fiber integrity (higher FA) predicted stronger functional midbrain-putamen connectivity for Stroop processing during easier response-repetition conditions (r=.70, p=0.002). Correlations were significant when Bonferroni-corrected for multiple comparisons, which required p<0.017 for undirectional hypotheses.
Fiber integrity and brain activation as predictors of performance
Behavioral results indicated reduced benefits from valid color cues in congruent-match trials resulting in smaller Stroop effects (RTINC>RTCON) in alcoholics than controls, specifically in response-repetition blocks (Figure 2). To test whether this effect was predicted by brain function and its microstructure, we used multiple linear regression analyses and entered PCC activation during Stroop-match response-repetition (Table 2C), left posterior cingulum fiber integrity (FA), and lifetime alcohol consumption (kg) as predictors of Stroop-match performance. For alcoholics, all variables together accounted for 65% of the total variance of behavioral Stroop-match effects (F(3,17)=8.8, p=0.002), with posterior cingulum FA (t=3.1, p=0.008) and lifetime alcohol consumption (t=3.5, p=0.003) contributing independently over and above the contribution of PCC activity (t=−1.5, ns). The regression model was not significant for controls (F(3,14)=0.05, ns).
Discussion
Using multimodal neuroimaging this study revealed substantial diagnosis-specific differences in the neural substrates invoked to process Stroop conflict, i.e., when matching the font color of an incongruent color-word stimulus to the color of a cue. Although alcoholics and controls performed equally well overall on the Stroop Match-to-Sample task and showed similar regional brain activation patterns in response to incongruent relative to congruent color-word information, five critical differences between the groups emerged: 1) the only test condition more challenging for alcoholics than controls was the easiest; 2) level of posterior cingulate cortex (PCC) deactivation was less and midbrain activation was more in alcoholics than controls; 3) PCC and midbrain activity was modulated by executive control demands and differed between the two groups with the degree of this modulation being predicted by the amount of lifetime alcohol consumption; 4) alcoholics showed greater midbrain connectivity to striatal and middle cingulate cortex (MCC) regions but less PCC-MCC connectivity than controls; and 5) this enhanced midbrain-MCC connectivity, particularly for more difficult response-switching conditions, was related to white matter structural compromise of posterior cingulum fiber bundles. Further, better posterior cingulate white matter fiber integrity in controls predicted the degree of PCC deactivation during Stroop processing.
First, performance differences were observed only for the easiest Stroop condition. When a valid color cue predicted the Stroop word’s color correctly during response repetition blocks, alcoholics showed smaller Stroop effects, suggesting that the benefit from repetitive congruent-match trials typically seen in healthy adults was attenuated in alcoholics (cf., 21). When a stimulus sequence follows an underlying regularity, adaptation from implicit learning can occur (33), and one would expect less cognitive effort processing regular stimulus–response sequences (34–36). That alcoholics derived less benefit from repetitive valid cueing could indicate compromised implicit learning for repetitive, more automatic stimulus-response mappings (37).
Second, despite the fact that both groups activated lateral frontoparietal regions well known to be associated with voluntary, top-down attention (38–40) and conflict resolution (21, 41–42), the groups differed in regional deactivation and interaction of brain systems that regulate reward, attention, and executive control. Alcoholics, in contrast to controls, did not deactivate the PCC in the easiest task condition, i.e., repetitive Stroop-match trials. It can be speculated that the task-dependent balance of activation and deactivation allows maximization of resources (14). The PCC has been associated with an intrinsic task-independent network, is considered a hub in the default mode network (DMN) because of its functional interconnectedness to other DMN regions, and appears to be preferentially deactivated during cognitive tasks (14,43) and active during rest, i.e., when individuals are not focused on external environments (13,44–45). In our study, activity of the PCC was modulated by task demands, suggesting that regions that are part of the DMN also play an active role during task processing. The normal DMN deactivation pattern was disturbed in chronic alcoholism, possibly via alcohol-related neuroadaptation of mesostriatal-cortical pathways. For example, a recent study combining positron emission tomography (PET) and fMRI showed that striatal dopamine transporter (DAT) availability can modulate task-related DMN deactivation (46). Also, similar to a recent fMRI study in cirrhotic patients (47), absence of PCC deactivation during a Stroop task may predict or be a marker of a greater need in alcoholics to use reserve network resources to achieve comparable performance levels to that of controls.
Third, in addition to the enhanced PCC activation, alcoholics also activated a midbrain cluster that involved substantia nigra and subthalamic nucleus and extended to the red nucleus and ventral tegmental area during response-repetition in contrast to response-switching, whereas controls deactivated these regions. That the amount of lifetime alcohol consumption predicted the degree to which this modulation of brain activity was deviant from the normal modulation suggests that the observed group differences do, at least to some extent, result from heavy drinking and not solely from differences predating the onset of alcoholism. Recently, an fMRI study demonstrated that blood-oxygen-level-dependent (BOLD) responses in the ventral tegmental area reflect midbrain dopaminergic signals (48). In healthy adults, dopamine transporters in striatum (measured with PET) modulated BOLD neuronal activity in the precuneus and cingulate gyrus (46). Furthermore, cocaine abusers have shown enhanced midbrain activation to drug cues and disrupted midbrain-thalamo-cortical functional connectivity suggesting drug-related neuroadaptions of dopaminergic midbrain systems (49). Considering the multiple roles of midbrain regions in reward (2, 50), sensorimotor integration (51) and motor-based learning (52–53), we speculate that greater midbrain activation during Stroop response-repetition than switching in alcoholics may reflect poor down-regulation of midbrain responsiveness to repetition learning, or relative to controls, up-regulation of midbrain responsiveness to low cognitive demand ((21) for less cognitive conflict with repetition in healthy subjects). This group difference in activation pattern cannot be explained by task difficulty per se because, although same-response blocks were easier than mixed-response blocks, as indicated by faster reaction times for response-repetitions than switches, this advantage was similar in both groups.
Fourth, up-regulation of midbrain activity occurred in alcoholics. Also present were larger midbrain–striatal and midbrain–middle cingulate cortex functional connectivity and lower corticocortical posterior–middle cingulate cortex connectivity in alcoholics than controls. The middle cingulate cortex is associated with processes of response selection (54) and decision-making (55–57). Its enhanced synchrony with activity in the striatum and midbrain but dampened synchrony with activity in the posterior cingulate cortex (PCC) in alcoholics may reflect difficulty in adapting functional network activity to upcoming executive task demands. In particular, the PCC is considered a ‘hub’ for multiple networks involving both rest and task-related networks that function together to support complex behavior (58). Within the Stroop task-related network, the posterior and middle cingulate cortices appear to mediate functional connections between voluntary (top-down) attention, executive control network regions, and midbrain nodes of the reward network associated with automatic (bottom-up) attention and behavioral conditioning to optimize utilization of resources for response selection (59–61). It can be speculated that functional recovery occurs through network adaptation or reorganization (62). In the present study, abstinent alcoholics did not significantly differ from controls in overall task performance and Stroop-related BOLD response but did so in the functional connectivity of network nodes mediating executive control demands. We interpret this functional difference as reflecting neuro-functional compensation, similar to recruitment of additional brain regions, beyond those normally needed to accomplish the task (20, 63–64).
In alcoholics, we further observed task-related activation synchrony between limbic (hippocampus, amygdala) and midbrain regions. An association between reward-related fMRI activation of dopaminergic midbrain with hippocampus-dependent long-term memory formation has been recently found in healthy subjects (65). Stroop stimulus processing may involve association learning where past events are integrated into mnemonic representations to enhance integrative encoding for upcoming events and to reduce effort, especially during repeated trials. For example, in a recent fMRI study using an association-learning task, coupled changes in the learning-phase activity between hippocampus and midbrain (ventral tegmental area/substantia nigra) predicted successful behavioral generalization for future events (66). In alcoholics and controls, the midbrain cluster was also functionally connected to orbitofrontal regions possibly conveying the learning-behavior translation for repetitive stimulus-response mappings (52).
The fifth finding was a subtle microstructural deficit in posterior cingulum fiber tracts of alcoholics. The cingulate bundle of the limbic system has long and short fibers that course along cingulate cortex and parahippocampal gyrus (67–68). Its anterior extent is connected to amygdala, nucleus accumbens, thalamus, and dorsolateral prefrontal cortex, and the posterior extent to temporal association, medial temporal, parietal, and orbitofrontal cortices. In alcoholics, poorer posterior cingulate fiber integrity predicted greater midbrain-MCC connectivity for more difficult Stroop response-switching conditions, whereas in controls greater fiber integrity predicted greater midbrain-striatal connectivity for the more automatic Stroop response-repetition conditions. In other words, when executive control demands were high, alcoholics engaged a midbrain-MCC network. This alternative network engagement closely depended on the degree of microstructural fiber compromise likely forming a compensatory network supporting normal performance.
One of the first combined structure-function connectivity studies reported that resting-state functional connectivity reflects structural white matter fiber connectivity (69). Our results add to this finding by demonstrating that in both groups task-related functional connectivity in a cingulate-midbrain network was associated with the condition of cingulate white matter fibers. As hypothesized, greater microstructural integrity predicted greater functional connectivity, suggesting that even subtle compromise of structural connectivity in alcoholism has potential to modulate functional connectivity.
Supplementary Material
Acknowledgments
This work was supported by NIAAA Grants AA012388, AA018022, AA010723, AA005965, and AA017168. We thank Margaret Rosenbloom for critical reading of and valuable comments on this manuscript. We also thank Shara Vinco for excellent technical experimental assistance, and Dr. Stephanie Sassoon for clinical screening of study participants.
Footnotes
Financial disclosures:
All authors report no biomedical financial interest or potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Millan EZ, Furlong TM, McNally GP. Accumbens shell-hypothalamus interactions mediate extinction of alcohol seeking. J Neurosci. 2010;31:4626–35. doi: 10.1523/JNEUROSCI.4933-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcohol Clin Exp Res. 2001;25:236–245. [PubMed] [Google Scholar]
- 4.Bowirrat A, Oscar-Berman M. Relationship between dopaminergic neurotransmission, alcoholism, and Reward Deficiency syndrome. Am J Med Genet B Neuropsychiatr Genet. 2005;132B:29–37. doi: 10.1002/ajmg.b.30080. [DOI] [PubMed] [Google Scholar]
- 5.Hermann D, Smolka MN, Wrase J, Klein S, Nikitopoulos J, Georgi A, Braus DF, Flor H, Mann K, Heinz A. Blockade of cue-induced brain activation of abstinent alcoholics by a single administration of amisulpride as measured with fMRI. Alcohol Clin Exp Res. 2006;30:1349–1354. doi: 10.1111/j.1530-0277.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 6.Park SQ, Kahnt T, Beck A, Cohen MX, Dolan RJ, Wrase J, Heinz A. Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. J Neurosci. 2010;30:7749–7753. doi: 10.1523/JNEUROSCI.5587-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim E, Ku J, Namkoong K, Lee W, Lee KS, Park JY, Lee SY, Kim JJ, Kim SI, Jung YC. Mammillothalamic functional connectivity and memory function in Wernicke’s encephalopathy. Brain. 2009;132:369–376. doi: 10.1093/brain/awn311. [DOI] [PubMed] [Google Scholar]
- 9.Northoff G, Qin P, Nakao T. Rest-stimulus interaction in the brain: a review. Trends Neurosci. 2010;33:277–284. doi: 10.1016/j.tins.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Chanraud S, Pitel AL, Pfefferbaum A, Sullivan EV. Disruption of functional connectivity of the default-mode network in alcoholism. Cereb Cortex. 2011;21:2272–2281. doi: 10.1093/cercor/bhq297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;25:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damoiseaux JS, Greicius MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct Funct. 2009;213:525–533. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- 13.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomasi D, Ernst T, Caparelli EC, Chang L. Common deactivation patterns during working memory and visual attention tasks: an intra-subject fMRI study at 4 Tesla. Hum Brain Mapp. 2006;27:694–705. doi: 10.1002/hbm.20211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newton AT, Morgan VL, Gore JC. Task demand modulation of steady-state functional connectivity to primary motor cortex. Hum Brain Mapp. 2007;28:663–672. doi: 10.1002/hbm.20294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turken A, Whitfield-Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JD. Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. Neuroimage. 2008;42:1032–44. doi: 10.1016/j.neuroimage.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfefferbaum A, Rosenbloom MJ, Fama R, Sassoon SA, Sullivan EV. Transcallosal white matter degradation detected with quantitative fiber tracking in alcoholic men and women: selective relations to dissociable functions. Alcohol Clin Exp Res. 2010;34:1201–1211. doi: 10.1111/j.1530-0277.2010.01197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chanraud S, Reynaud M, Wessa M, Penttilä J, Kostogianni N, Cachia A, Artiges E, Delain F, Perrin M, Aubin HJ, Cointepas Y, Martelli C, Martinot JL. Diffusion tensor tractography in mesencephalic bundles: relation to mental flexibility in detoxified alcohol-dependent subjects. Neuropsychopharmacology. 2009;34:1223–1232. doi: 10.1038/npp.2008.101. [DOI] [PubMed] [Google Scholar]
- 19.Schulte T, Müller-Oehring EM, Rohlfing T, Pfefferbaum A, Sullivan EV. White matter fiber degradation attenuates hemispheric asymmetry when integrating visuomotor information. J Neurosci. 2010;30:12168–12178. doi: 10.1523/JNEUROSCI.2160-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parks MH, Greenberg DS, Nickel MK, Dietrich MS, Rogers BP, Martin PR. Recruitment of additional brain regions to accomplish simple motor tasks in chronic alcohol-dependent patients. Alcohol Clin Exp Res. 2010;34:1098–109. doi: 10.1111/j.1530-0277.2010.01186.x. [DOI] [PubMed] [Google Scholar]
- 21.Schulte T, Müller-Oehring EM, Vinco S, Hoeft F, Pfefferbaum A, Sullivan EV. Double dissociation between action-driven and perception-driven conflict resolution invoking anterior versus posterior brain systems. Neuroimage. 2009;48:381–390. doi: 10.1016/j.neuroimage.2009.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
- 23.Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: A new graphic interactive environment for designing psychology experiments. Behavioral Research Methods, Instruments, and Computers. 1993;25:257–271. [Google Scholar]
- 24.Schulte T, Müller-Oehring EM, Chanraud S, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Age-related reorganization of functional networks for successful conflict resolution: A combined functional and structural MRI study. Neurobiol Aging. 2011;32:2075–2090. doi: 10.1016/j.neurobiolaging.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schulte T, Müller-Oehring EM, Javitz H, Pfefferbaum A, Sullivan EV. Callosal Compromise Differentially Affects Conflict Processing and Attentional Allocation in Alcoholism, HIV, and Their Comorbidity. Brain Imaging Behav. 2008;2:27–38. doi: 10.1007/s11682-007-9014-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulte T, Müller-Oehring EM, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Differential effect of HIV infection and alcoholism on conflict processing, attentional allocation, and perceptual load: evidence from a Stroop Match-to-Sample task. Biol Psychiatry. 2005;57:67–75. doi: 10.1016/j.biopsych.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 27.Poline JB, Worsley KJ, Evans AC, Friston KJ. Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage. 1997;5:83–96. doi: 10.1006/nimg.1996.0248. [DOI] [PubMed] [Google Scholar]
- 28.Gerig G, Corouge I, Vachet C, Krishnan KR, MacFall JR. Quantitative analysis of diffusion properties of white matter fiber tracts: a validation study. 13th Proceedings of the International Society for Magnetic Resonance in Medicine; Miami, FL: ISMRM; 2005. [Google Scholar]
- 29.Mori S, van Zijl PC. Fiber tracking: principles and strategies — a technical review. NMR Biomed. 2002;15:468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- 30.Xue R, van Zijl PC, Crain BJ, Solaiyappan M, Mori S. In vivo three-dimensional reconstruction of rat brain axonal projections by diffusion tensor imaging. Magn Reson Med. 1999;42:1123–1127. doi: 10.1002/(sici)1522-2594(199912)42:6<1123::aid-mrm17>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 31.Xu D, Mori S, Solaiyappan M, van Zijl PC, Davatzikos C. A framework for callosal fiber distribution analysis. Neuroimage. 2002;17:1131–1143. doi: 10.1006/nimg.2002.1285. [DOI] [PubMed] [Google Scholar]
- 32.Walker H, Lev J. Statistical Inference. New York, NY: Holt; 1953. [Google Scholar]
- 33.Nissen MJ, Bullemer PT. Attentional requirements for learning: Evidence from performance measures. Cognitive Psychology. 1987;19:1–32. [Google Scholar]
- 34.Mayr U, Awh E, Laurey P. Conflict adaptation effects in the absence of executive control. Nat Neurosci. 2003;6:450–452. doi: 10.1038/nn1051. [DOI] [PubMed] [Google Scholar]
- 35.Hommel B. Coloring an action: intending to produce color events eliminates the Stroop effect. Psychol Res. 2004;68:74–90. doi: 10.1007/s00426-003-0146-5. [DOI] [PubMed] [Google Scholar]
- 36.Verguts T, Notebaert W. Hebbian learning of cognitive control: dealing with specific and nonspecific adaptation. Psychol Rev. 2008;115:518–525. doi: 10.1037/0033-295X.115.2.518. [DOI] [PubMed] [Google Scholar]
- 37.Ullsperger M, Bylsma LM, Botvinick MM. The conflict adaptation effect: it’s not just priming. Cogn Affect Behav Neurosci. 2005;5:467–472. doi: 10.3758/cabn.5.4.467. [DOI] [PubMed] [Google Scholar]
- 39.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 39.Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Oscar-Berman M, Marinković K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vanderhasselt MA, De Raedt R, Baeken C. Dorsolateral prefrontal cortex and Stroop performance: tackling the lateralization. Psychon Bull Rev. 2009;16:609–612. doi: 10.3758/PBR.16.3.609. [DOI] [PubMed] [Google Scholar]
- 42.Morishima Y, Okuda J, Sakai K. Reactive mechanism of cognitive control system. Cereb Cortex. 2010;20:2675–2683. doi: 10.1093/cercor/bhq013. [DOI] [PubMed] [Google Scholar]
- 43.Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, Pillon B, Le Bihan D, Dubois B. The neural system that bridges reward and cognition in humans: an fMRI study. Proc Natl Acad Sci USA. 2002;99:5669–5674. doi: 10.1073/pnas.082111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain’s default network. Proc Natl Acad Sci U S A. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomasi D, Volkow ND, Wang R, Telang F, Wang GJ, Chang L, Ernst T, Fowler JS. Dopamine transporters in striatum correlate with deactivation in the default mode network during visuospatial attention. PLoS One. 2009;4:e6102. doi: 10.1371/journal.pone.0006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang LJ, Yang G, Yin J, Liu Y, Qi J. Abnormal default-mode network activation in cirrhotic patients: a functional magnetic resonance imaging study. Acta Radiol. 2007;48:781–787. doi: 10.1080/02841850701422161. [DOI] [PubMed] [Google Scholar]
- 48.D’Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;29:1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- 49.Tomasi D, Volkow ND, Wang R, Carrillo JH, Maloney T, Alia-Klein N, Woicik PA, Telang F, Goldstein RZ. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS One. 2010;5:e10815. doi: 10.1371/journal.pone.0010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wise RA. Roles for nigrostriatal — not just mesocorticolimbic — dopamine in reward and addiction. Trends in Neurosciences. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dypvik A, Bland B. Functional connectivity between the red nucleus and the hippocampus supports the role of the hippocampal formation in sensorimotor integration. Journal of Neuropsychology. 2004;92:2040–2050. doi: 10.1152/jn.01081.2003. [DOI] [PubMed] [Google Scholar]
- 52.Bédard P, Sanes JN. On a basal ganglia role in learning and rehearsing visual-motor associations. Neuroimage. 2009;47:1701–1710. doi: 10.1016/j.neuroimage.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheth SA, Abuelem T, Gale JT, Eskandar EN. Basal Ganglia Neurons Dynamically Facilitate Exploration during Associative Learning. J Neurosci. 2011;31:4878–4885. doi: 10.1523/JNEUROSCI.3658-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paus T, Petrides M, Evans AC, Meyer E. Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: A positron emission tomography study. J Neurophysiol. 1993;70:453–469. doi: 10.1152/jn.1993.70.2.453. [DOI] [PubMed] [Google Scholar]
- 55.Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rushworth MF, Behrens TE, Rudebeck PH, Walton ME. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn Sci. 2007;11:168–176. doi: 10.1016/j.tics.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 57.Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp. 2009;30:2731–2745. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci. 2011;31:3217–3224. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mohanty A, Gitelman DR, Small DM, Mesulam MM. The spatial attention network interacts with limbic and monoaminergic systems to modulate motivation-induced attention shifts. Cereb Cortex. 2008;18:2604–2613. doi: 10.1093/cercor/bhn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Potts GF, Martin LE, Burton P, Montague PR. When things are better or worse than expected: the medial frontal cortex and the allocation of processing resources. J Cogn Neurosci. 2006;18:1112–1119. doi: 10.1162/jocn.2006.18.7.1112. [DOI] [PubMed] [Google Scholar]
- 61.Hazy TE, Frank MJ, O’Reilly RC. Neural mechanisms of acquired phasic dopamine responses in learning. Neurosci Biobehav Rev. 2010;34:701–720. doi: 10.1016/j.neubiorev.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schafer RJ, Lacadie C, Vohr B, Kesler SR, Katz KH, Schneider KC, Pugh KR, Makuch RW, Reiss AL, Constable RT, Ment LR. Alterations in functional connectivity for language in prematurely born adolescents. Brain. 2009;132:661–670. doi: 10.1093/brain/awn353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Desmond JE, Chen SH, DeRosa E, Pryor MR, Pfefferbaum A, Sullivan EV. Increased frontocerebellar activation in alcoholics during verbal working memory: an fMRI study. Neuroimage. 2003;19:1510–1520. doi: 10.1016/s1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- 64.Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV. Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. Neuroimage. 2001;14:7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- 65.Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze HJ, Düzel E. Reward-related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron. 2005;45:459–467. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 66.Shohamy D, Wagner AD. Integrating memories in the human brain: hippocampal-midbrain encoding of overlapping events. Neuron. 2008;60:378–389. doi: 10.1016/j.neuron.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vogt BA, Rosene DL, Pandya DN. Thalamic and cortical afferents differentiate anterior from posterior cingulate cortex in the monkey. Science. 1979;204:205–207. doi: 10.1126/science.107587. [DOI] [PubMed] [Google Scholar]
- 68.Goldman-Rakic PS. Topography of cognition: Parallel distributed networks in primate association cortex. Annu Rev Neurosci. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- 69.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.