Abstract
PURPOSE
Hypothalamic-pituitary axis (HPA) control may be impaired in Type 2 diabetes (T2DM). Glucocorticoids increase consumption of low quality foods high in calories, sugar and fat. We explored the relationship between cortisol levels, poor blood glucose control, and food quality choice in T2DM.
METHODS
Twenty-seven healthy controls were age-, gender- and education-matched to 27 T2DM participants. Standard clinical blood tests and cortisol values were measured from fasting blood samples. Participants recorded all consumed food and drink items in a consecutive three-day food diary. Diaries were analyzed for “high quality” and “low quality” foods using a standardized method with high reliability (0.97 and 0.86, respectively).
RESULTS
Controlling for education, body mass index (BMI) and hemoglobin A1C (HbA1C), log-transformed cortisol (LogC) predicted the percent of low quality foods (R2=.092, β=.360, p<.05), but not the percent of high quality foods chosen. Controlling for education, BMI and LogC, HbA1C significantly predicted both the percent of low quality foods (ΔR2 = .079, β=.348, p=.024) and high quality foods chosen (ΔR2=.085, β=-.362, p=.022). The relationship between HbA1C and low quality food choice may be mediated by cortisol, controlling for BMI and education (p<.01). HbA1C displayed both an indirect (cortisol-mediated) effect (p<.05) and direct effect on low quality food choice (p<.05).
CONCLUSION
The relationship between HbA1C and low quality food choice may be partially mediated by cortisol. Poor blood glucose control may cause HPA axis disruption, increased consumption of low quality foods.
Keywords: HPA, Cortisol, Food diary, Hemoglobin A1C, Food choice, Type II Diabetes
Introduction
In the next forty years, the number of people with newly diagnosed diabetes will almost triple, from 11 million to 29 million. It is estimated the United States, in 2007 alone, spent more than $174 billion on diabetes care [1]. Cardiovascular complications are the leading cause of morbidity and mortality in Type 2 Diabetes Mellitus (T2DM) [2]. Poor lifestyle choices, namely over-consumption of energy and decreased physical activity, increase the risk of developing insulin resistance and T2DM [3]. Moreover, lifestyle modifications in diet and physical activity are crucial to improved T2DM control and slowing the progression of diabetes-related complications [4]. While it is known a healthy diet and exercise help prevent T2DM, the direction of the relationship between T2DM and food choice remain unclear [5]. Behavior modification strategies in diabetes would greatly benefit from improving our understanding of the driving forces behind T2DM dietary control.
Established factors associated with food choice include socioeconomic status (SES), waist circumference, and cortisol [6–8]. People suffering from chronic stress may demonstrate increases in intake of “snack-type foods” [7]. Glucocorticoids (GCs) such as cortisol increase salience of satisfying activities, notably the consumption of food items with high sugar and fat content [9]. Likewise, a study by George et al. (2009) observes increases in total caloric and total food consumption following corticotrophin releasing hormone (CRH) stimulation of the HPA axis [10]. Contrary to the protective effects of acute GC elevations, chronic GC elevations and high nocturnal cortisol levels have been associated with hippocampal damage [11, 12]. In T2DM, impaired glucose regulation is associated with chronic hypercortisolism [13]. In a previous study using Dexamethasone (DEX) suppression tests, we show individuals with T2DM display reductions in hypothalamic-pituitary axis (HPA) axis feedback inhibition. The reduction in hippocampal volume with accompanying declarative memory impairments and HPA hyperactivity suggest abnormal HPA feedback sensitivity in T2DM [14].
Given that abnormal HPA feedback sensitivity dysregulates cortisol secretion, it is expected that food choice among individuals with T2DM may also be affected. This study explored the association between high cortisol levels and poor food quality choice in T2DM.
Method
Participants
This study involved 54 participants selected from larger ongoing studies on normal aging. Participants ranged from 45–78 years of age, who, at minimum, completed high school. Exclusion criteria included any signs, symptoms, or diagnoses of neurological, medical (other than T2DM and associated hypertension and dyslipidemia), and/or psychiatric disorders (including depression, alcohol and/or substance abuse). Other exclusion criteria were a history of significant head trauma, stroke, hydrocephalus, lacunar infarcts, extensive white matter disease, or the use of corticosteroids or drugs that could affect cortisol levels (e.g., phenytoin, androgens). All participants provided informed written consent and were compensated for their time and inconvenience. All research protocols were approved by the Institutional Review Board of the New York University School of Medicine.
Classification of participants
Classification into the diabetic group required having at least one of three criteria: 1) fasting glucose greater than 125 mg/dL in two separate measurements; 2) two-hour glucose value over 200 mg/dL during a glucose tolerance test (75g glucose); 3) previous T2DM diagnosis coupled with prescribed treatment by hypoglycemic agents and/or diet and exercise.
Procedure
All participants were evaluated by medical examinations, endocrine measurements, neuropsychological and psychiatric evaluations, as well as a consecutive three-day food record collection.
Glucose, Insulin, glycosylated hemoglobin (HbA1c)
Following an overnight fast, glucose, insulin and HbA1c were measured. A glucose oxidase method (VITROS 950 AT; Ortho-Clinical Diagnostics, Inc., Amersham, UK) measured glucose, immunoassay with chemiluminesence readout measured insulin (Advia Centaur; Bayer Corp., Leverkusen, Germany), and an automated high-performance liquid chromatography (HPLC) method measured HbA1c (Tosoh Corp., Kanagawa, Japan). All methods were certified by the National Glycohemoglobin Standardization Program.
HPA axis endocrine measures
Basal cortisol and glucose
Intravenous catheters were placed 45 minutes to 1 hour prior to the procedures to ensure that the stress of catheter insertions did not influence our findings. Prior to infusing a weight-adjusted dose of glucose for an intravenous glucose tolerance test (IVGTT) at 0930 h, two baseline blood samples (2 minutes apart) were collected from the sampling catheter from all study participants. Glucose and cortisol values were measured from both samples, and values were averaged to approximate basal glucose and basal cortisol.
Modified DEX/CRH test
We used a shortened-version of the DEX/CRH assessment test to quantify HPA axis feedback. The details of this method were previously published [14]. Total cortisol was measured with an enzyme immunoassay (EIA; IBL, Hamburg, Germany) with a sensitivity of 0.1 g/dL.
Three-day Food Diary
All participants were given clear instructions to record all food and drink (other than water) items consumed within a consecutive three-day period. Participants were provided with the forms to record their food intake and instructed to return them to the laboratory for analysis upon their next visit.
Statistical methods
To compare group demographics, we used independent t-tests to compare means between groups. Baseline cortisol and cortisol after DEX levels were base-10 log-transformed prior to analysis to obtain a normal distribution.
One rater, JIC, a Ph.D. in Nutrition, quantified all food diary values. To measure inter-rater reliability, MD, a doctoral student in Nutrition and Dietetics, independently analyzed 30 food diaries. Both the rater and the double-scorer were blinded to the participants’ group classification. This dietary assessment method demonstrated high reliability with inter-class correlation coefficients (ICC) for high and low quality foods of 0.97 and 0.86, respectively. Food items were classified a priori into the following groups: fruits and vegetables, meat, fish, dairy, junk food, soda, whole grains, fried foods, nuts, fast food, simple carbohydrates, and miscellaneous. Quantification of each food item was determined by serving sizes established by the American Dietetic Association. Fruits and vegetables, fish, whole grains, and nuts were classified as high quality foods. Additionally, meat, junk food, soda, fried foods, fast food, and simple carbohydrates were classified as low quality foods. As dietary patterns high in meat are associated with chronic diseases and conditions such as diabetes, obesity and hypertension, meat was classified within the low quality category [15, 16]. Low quality dietary patterns (such as the Western diet) high in meat, saturated fat, and refined sugars are linked to obesity, diabetes, reduced cognitive function, hippocampal volumes, and Alzheimer’s disease [17]. Therefore, we specifically focused on measuring intake dietary patterns (low quality and high quality) and not caloric intake. Miscellaneous foods (i.e. tofu), alcohol, and dairy were omitted due to large variability in documentation and inability to determine its content (e.g., fat-free yogurt vs. ice cream). Low quality percent was calculated as the percentage of low quality foods divided by the total number of foods consumed, which included alcohol, miscellaneous and dairy items. Conversely, high quality percent was calculated as the percentage of high quality foods divided by the total number of foods consumed.
Multivariate linear regression models were used to determine the variance in the choice of food quality (high or low) explained by cortisol and HbA1c. Percentage of food quality (high or low) was set as the dependent variable. We controlled for education and body mass index (BMI) in the first step. Education is indicative of SES, and both SES and BMI have been associated with dietary quality [18–20]. To examine the amount of variance in food quality choice determined by blood glucose control controlling for cortisol, the log-transformed cortisol was added as the second step and HbA1c as the last step. To ascertain the independent contributions of glucose control and cortisol level in explaining the food quality, we inverted the order of entry for the second and third steps. To test whether cortisol had a mediating relationship in the association between glucose control and low dietary quality, we employed a Sobel test using the Ohio State INDIRECT macro (http://www.comm.ohiostate.edu/ahayes/spss%20programs/indirect.htm) [22]. The Sobel test evaluates whether a mediating relationship by cortisol statistically exists after controlling for education and BMI. For more information on this method, please refer to Appendix A. We entered cortisol as the potential mediator with HbA1c as the independent variable, and education and BMI as covariates. Confidence intervals were set to 95%, with alpha levels at 0.05.
Results
Demographic variables and group descriptors
Statistical analysis of the demographic data showed no significant differences in age, gender, education (years), or height (in) between lean control (n=27) and diabetic groups (n=27). Individuals with diabetes were significantly higher in weight (lb), BMI (kg/m2), HbA1c%, fasting glucose (mg/dL), cortisol (µg/dL), and cortisol after the DEX suppression test (µg/dL) (Table 1). The T2DM group consumed significantly more servings of low quality foods and significantly less servings of high quality foods than the control group (p<.01). Moreover, the total number of food servings did not differ between groups, only the percentage of low quality and high quality food servings within the diet was significantly different. The total number of food servings was defined as all food items including alcohol, miscellaneous, and dairy items (P>.05) (Table 2).
Table 1.
Description of the control and T2DM groups.
| Controls (n=27) | T2DM (n=27 | |
|---|---|---|
| Age (yr) | 60.44 ± 8.69 | 60.32 ± 7.66 |
| No. of females/males | 14/13 | 13/14 |
| Education (yr) | 15.70 ± 2.13 | 15.20 ± 2.25 |
| Time from diagnosis of T2DM (yr) | N/A | 6.76 ± 6.76 |
| No. on antidiabetic medication* | 0 | 22 |
| Height (in) | 67.17 ± 3.80 | 66.31 ± 3.61 |
| Weight (lb)* | 164.50 ± 27.58 | 206.57 ± 46.74 |
| BMI (kg/m2)* | 25.43 ± 3.99 | 32.75 ± 7.01 |
| HbA1C(%)* | 5.28 ± .47 | 7.39 ± 1.53 |
| Glucose (mg/dL)* | 81.48 ± 8.09 | 130 ± 45.48 |
| Cortisol (µg/dL)* a | 18.13 ± 21.57 | 70.24 ± 60.36 |
| Cortisol after DEX (µg/dL) * a,b | 2.62 ± 3.52 | 11.94 ± 14.14 |
Unless noted, values are expressed as mean ± SD.
Significant group differences (P<0.05).
Reported significance based on base 10-logarithmically transformed values.
DEX = dexamethasone suppression test
Table 2.
Comparison of food quality choice between control and T2DM groups.
| Controls (n=27) | T2DM (n=27) | |
|---|---|---|
| %Low quality foods*a | 0.378 ± 0.150 | 0.544 ± 0.177 |
| %High quality foods*a | 0.408 ± 0.166 | 0.279 ± 0.166 |
| Total items consumed b | 13.89 ± 3.47 | 12.90 ± 4.55 |
Unless noted, values are expressed as mean ± SD.
Significant group differences (p<.01).
Low quality of high quality items divided by total foods consumed.
All foods, including alcohol, dairy and miscellaneous items.
Multivariate linear regression
Our regression analyses ascertained how cortisol variables and/or glucose control contributed to food choice after accounting for possible confounders. Results indicated both cortisol and glucose control contributed independently to the variance of the quality of food consumed. Education (p=.563) and BMI (p=.331) were not significant predictors of low quality or high quality food choice in both models, taking the effect of either cortisol or HbA1c in the second step. After controlling for BMI and education and accounting for cortisol, the positive association between HbA1c and % low quality food servings significantly explained an additional 7.9% of the variance of % low quality foods (ΔR2 = .079, β=.348, p=.024). Additionally, after accounting for cortisol, HbA1c was negatively associated with % high quality food servings in the diet and this explained 8.5% of the model (ΔR2=.085, β=−.362, p=.022). Reversing the order of the regression analysis allowed for the determination of additional variance explained by cortisol after accounting for HbA1c. In this reversed analysis, cortisol significantly explained an additional 9.2% of the variance in % low quality food servings, and displayed a positive association with % low quality food servings (ΔR2=.092, β=.360, p=.016). Cortisol also explained 6% of the variance in, and presented a negative association with, % high quality food servings after accounting for HbA1c (ΔR2=.060, β=−.291, p=.052). The multivariate linear regression showed food quality was predicted by poor glucose control and cortisol.
INDIRECT mediation model
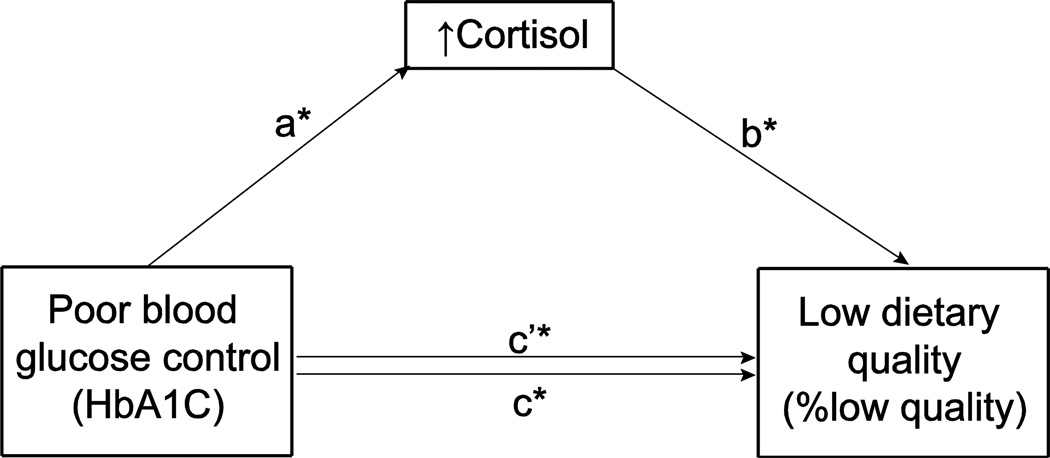
We examined the mediation by log-cortisol on the relationship between T2DM and % low quality food servings with a Sobel test using the macro INDIRECT. Controlling for education and BMI, cortisol partially mediates the relationship between poor blood glucose control (high HbA1c) and low dietary quality. All coefficients, fulfilling requirements for partial mediation, were significant (as depicted by an asterisk in Figure 1): a=0.3066 (between HbA1c and cortisol), b=0.0602 (between cortisol and % low quality food servings), and c’=0.0417 (direct effect of HbA1c on % low quality food servings including mediation by cortisol). The total effect of blood glucose control (HbA1c) with the cortisol mediator on low dietary quality, or c, is the sum of c’ and the product of aXb = 0.0602. R2 for the total effect mediation model was 0.3130 (p=0.0012, 95% CI: 0.0026–0.0479). The positive relationship between poor blood glucose control and low dietary quality was partially and significantly mediated by cortisol (p<.05).
Figure 1.
Cortisol partially mediated the relationship between poor blood glucose control and low dietary quality controlling for education and BMI. The “a” coefficient indicates the direct relationship between poor blood glucose control (HbA1C) and cortisol. The “b” coefficient illustrates the direct relationship between cortisol and low quality food choice. The “c” and “c’” coefficient denote the indirect and direct relationship, respectively, between poor blood glucose control and low quality food choice (%low quality food servings). Asterisks denote significant coefficients (p<.05): a (.3066), b (.0602), c’ (.0417).
Discussion
In this study, we found individuals with Type 2 diabetes displayed significantly higher cortisol levels and impaired HPA axis feedback (evaluated as cortisol after DEX) compared to age-, gender-, and education-matched healthy controls. Individuals with diabetes, although not reporting to consume more food overall than controls, reported to consume significantly less servings of high quality foods, and significantly more servings of low quality foods. Furthermore, both poor blood glucose control and cortisol explained the group differences in the percentage of low quality food servings. Differences in the percentage of high quality food servings were attributed to poor blood glucose control alone. Lastly, we found cortisol partially, but significantly, mediates the predictive relationship between poor glucose control and low quality food choices. Though this does not necessarily prove causation, it lends some support to the causal theory in which poor blood glucose control causes hippocampal damage and consequently HPA axis disruption, which in turn may be followed by increased appetite for low quality foods. Although this proposed causal direction is likely possible, the reverse may also be true; a greater consumption of low quality foods may lead to HPA axis dysfunction. The imbalanced intake of low quality foods high in sugar and fat may predisposes one to become overweight or obese, which in conjunction with HPA axis dysfunction may lead to the development of Type 2 Diabetes. The hippocampus, an important regulator of the HPA axis, and hippocampal-based cognitive function may be influenced by diet choice. Specifically, overconsumption of the Western Diet, a dietary pattern marked by high intake of saturated fat and refined simple carbohydrates, may negatively impact hippocampal integrity. This hippocampal impairment may be directly related to weight gain resulting from Western diet overconsumption [17].The partial mediation by cortisol between T2DM and low quality food choice found in our study is well-supported in the literature. Firstly, we previously established that HPA axis dysfunction occurs in T2DM, and that this dysfunction is reflected in increased basal cortisol levels and decreased responses to DEX suppression [14]. Secondly, many studies describe the increase in food intake, particularly of more palatable foods, following increases in stress, cortisol, and CRH. This second line of support is further augmented by BOLD fMRI studies indicating that reward signaling and reward sensitivity are significantly lower under stress, and correlate to high energy intake. These changes in reward sensitivity may be attributed to changes in putamen activation [21]. Additionally, pharmacological stimulation of the HPA axis with CRH leads to increased food intake, which supports the theory that the HPA axis is highly tied to appetite [10]. Although the concept of glucocorticoids increasing the consumption of low quality foods is not new[9, 22, 23], what is novel is the possibility that the HPA axis is a intermediary between T2DM and the consumption of a low quality diet.
Quality of glucose control, estimated in this study by HbA1c, predicted both the percent of low quality and high quality food consumed. Our results suggest that T2DM itself may impact appetite, and that in addition to the effect mediated by cortisol, it also has a direct effect. These results suggest a mechanism in which blood glucose control may directly impact appetite. This position is in part supported by rodent studies where insulin alone can modulate how the reward system in the brain responds to foods that are high in fat and sugar [24]. Furthermore, obese rodents need to consume larger quantities of low quality food in order to experience the same hedonic signal [25].
It is possible that cortisol controls a physiological response that only influences the choice to consume more palatable foods. In other words, cortisol may only control appetite for low quality foods but not high quality foods. Low quality foods, high in calories, sugar and fat, may relieve stress by stimulating the anterior portion of the nucleus accumbens and generating a reward (pleasure) signal. Furthermore, under instances of chronic stress, levels of glucocorticoids and insulin are elevated, which may promote intake of low quality foods. As a result, increased caloric intake promotes centrally distributed fat storage, which also decreases feed-forward to the HPA stress system [22].
In contrast to low quality foods, high quality intake may be controlled by inhibitory behavioral control, namely the ability to withstand the impulse to consume foods rich in sugars and fat, which are likely to be highly palatable. The Stroop test is a neuropsychological measure that can be used to estimate behavioral inhibition (the ability to check automatic responses to impulses). In this study, individuals with T2DM displayed a significantly lower mean Stroop score than controls (−3.586, SD 8.78 vs. 1.594, SD 7.14, p<.05, respectively). This difference in the Stroop score indicates that our individuals with T2DM may have decreased behavioral control, and this may have further contributed to the observed higher intake of low quality food servings. However, the Stroop test scores were not directly associated with the percent of low or high quality foods consumed, making a loss of behavioral inhibitory control as measured by the Stroop not the direct explanation.
In summary, we demonstrate that in 2 diabetes an increased low quality foods that may be mediated by increased cortisol. In addition, our data lends preliminary partial support to a possible causal order in which poor blood glucose control may disrupt HPA axis feedback (perhaps mediated by the hippocampal atrophy that is associated with T2DM), which in turn may lead to increased appetite for foods high in sugar and fat.
Table 3.
Associations between food quality, basal cortisol, and HbA1C for all subjects, derived from linear regression analyses.
| Step 1 (education/BMI) |
Step 2 (log cortisol) |
Step 3 (HbA1C) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ΔR2 | β | P value | ΔR2 | β | P value | ΔR2 | β | P value | SE | |
| %Low Quality | .023 | .088/−.117 | .563 | .211 | .360 | .001 | .079 | .348 | .024 | .160 |
| %High Quality | .044 | −.246/.065 | .331 | .160 | −.291 | .003 | .085 | −.362 | .022 | .229 |
| Step 1 (education/BMI) |
Step 2 (HbA1C) |
Step 3 (log cortisol) |
||||||||
| ΔR2 | β | P value | ΔR2 | β | P value | ΔR2 | β | P value | ||
| %Low Quality | .023 | .088/−.117 | .563 | .198 | .348 | .001 | .092 | .360 | .016 | .160 |
| %High Quality | .044 | −.246/.065 | .331 | .185 | −.362 | .001 | .060 | −.291 | .052 | .154 |
Steps of the regression are shown separated by the columns; the bottom block shows the order between HbA1 c and log Cortisol inverted. ΔR2 is the change in R2, β (standardized beta) value, P value for the ΔR2, and SE is the standard error of the estimate of the final model.
Acknowledgements
This study was supported by a grant from the National Institutes of Health DK064087 and supported in part by grant1UL1RR029893 from the National Center for Research Resources.
Contributor Information
Michelle Duong, Email: michelle.duong@nyu.edu.
Jessica I. Cohen, Email: cohenj25@nyumc.org.
Antonio Convit, Email: antonio.convit@med.nyu.edu.
References
- 1.Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States. 2007. [Google Scholar]
- 2.Waltenberger J. Impaired collateral vessel development in diabetes: potential cellular mechanisms and therapeutic implications. Cardiovasc. Res. 2001;49:554–560. doi: 10.1016/s0008-6363(00)00228-5. [DOI] [PubMed] [Google Scholar]
- 3.Hu FB, Manson, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N. Engl.J. Med. 2001;345:790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 4.Magkos F, Yannakoulia M, Chan JL, Mantzoros CS. Management of the metabolic syndrome and type 2 diabetes through lifestyle modification. Annu. Rev. Nutr. 2009;29:223–256. doi: 10.1146/annurev-nutr-080508-141200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker KZ, O'Dea K, Gomez M, Girgis S, Colagiuri R. Diet and exercise in the prevention of diabetes. J Hum Nutr Diet. 2010;23:344–352. doi: 10.1111/j.1365-277X.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- 6.Halkjaer J, Tjønneland A, Overvad K, Sørensen TIA. Dietary predictors of 5-year changes in waist circumference. J Am Diet Assoc. 2009;109:1356–1366. doi: 10.1016/j.jada.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Oliver G, Wardle J. Perceived effects of stress on food choice. Physiol. Behav. 1999;66:511–515. doi: 10.1016/s0031-9384(98)00322-9. [DOI] [PubMed] [Google Scholar]
- 8.Wardle J, Steptoe A. Socioeconomic differences in attitudes and beliefs about healthy lifestyles. J Epidemiol Community Health. 2003;57:440–443. doi: 10.1136/jech.57.6.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of"comfort food". Proc. Natl. Acad. Sci. U.S.A. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George SA, Khan S, Briggs H, Abelson JL. CRH-stimulated cortisol release and food intake in healthy, non-obese adults. Psychoneuroendocrinology. 2010;35:607–612. doi: 10.1016/j.psyneuen.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knoops AJG, Gerritsen L, van der Graaf Y, Mali WPTM, Geerlings MI. Basal hypothalamic pituitary adrenal axis activity and hippocampal volumes: the SMART-Medea study. Biol. Psychiatry. 2010;67:1191–1198. doi: 10.1016/j.biopsych.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 12.McEwen B. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886(1–2):172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- 13.Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing's syndrome. Lancet. 2006;367:1605–1617. doi: 10.1016/S0140-6736(06)68699-6. [DOI] [PubMed] [Google Scholar]
- 14.Bruehl H, Rueger M, Dziobek I, Sweat V, Tirsi A, Javier E, Arentoft A, Wolf OT, Convit A. Hypothalamic-pituitary-adrenal axis dysregulation and memory impairments in type 2 diabetes. J. Clin. Endocrinol. Metab. 2007;92:2439–2445. doi: 10.1210/jc.2006-2540. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy ET, Bowman SA, Spence JT, Freedman M, King J. Popular diets: correlation to health, nutrition, and obesity. J Am Diet Assoc. 2001;101:411–420. doi: 10.1016/S0002-8223(01)00108-0. [DOI] [PubMed] [Google Scholar]
- 16.Maffeis C, Pinelli L. Teaching children with diabetes about adequate dietary choices. Br. J. Nutr. 2008;99 Suppl 1:S33–S39. doi: 10.1017/S0007114508892495. [DOI] [PubMed] [Google Scholar]
- 17.Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: Links to hippocampal dysfunction and obesity. Physiol. Behav. 2011;103:59–68. doi: 10.1016/j.physbeh.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Govil SR, Weidner G, Merritt-Worden T, Ornish D. Socioeconomic status and improvements in lifestyle, coronary risk factors, and quality of life: the Multisite Cardiac Lifestyle Intervention Program. Am J Public Health. 2009;99:1263–1270. doi: 10.2105/AJPH.2007.132852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubowitz T, Heron M, Bird CE, Lurie N, Finch BK, Basurto-Dávila R, Hale L, Escarce JJ. Neighborhood socioeconomic status and fruit and vegetable intake among whites, blacks, and Mexican Americans in the United States. Am.J. Clin. Nutr. 2008;87:1883–1891. doi: 10.1093/ajcn/87.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mela DJ. Determinants of food choice: relationships with obesity and weight control. Obes. Res. 2001;9 Suppl 4:249S–255S. doi: 10.1038/oby.2001.127. [DOI] [PubMed] [Google Scholar]
- 21.Born JM, Lemmens SGT, Rutters F, Nieuwenhuizen AG, Formisano E, Goebel R, Westerterp-Plantenga MS. Acute stress and food-related reward activation in the brain during food choice during eating in the absence of hunger. Int J Obes (Lond) 2010;34:172–181. doi: 10.1038/ijo.2009.221. [DOI] [PubMed] [Google Scholar]
- 22.Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav. Immun. 2005;19:275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- 24.Fulton S. Appetite and reward. Front Neuroendocrinol. 2010;31:85–103. doi: 10.1016/j.yfrne.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]