Abstract
A Mannich-type multicomponent assembly process/1,3-dipolar cycloaddition strategy has been developed for the rapid and efficient construction of a parent tetrahydroisoquinoline fused isoxazolidine scaffold, which was subsequently functionalized using well-established protocols to access a diverse 70-membered library of novel 2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinoline-2-amine derivatives.
Keywords: Combinatorial chemistry, dipolar cycloaddition, heterocycles, Mannich, multicomponent reaction
INTRODUCTION
Modern day drug discovery relies on the identification of potent and selective modulators of biological systems, either as probes for the functional and mechanistic study of these systems, or as drug leads. Once initial leads are identified, their properties can be fine-tuned through selective modification of functional groups and substituents to achieve the desired physiochemical attributes. According to Lipinski’s rule of five, compounds with a molecular weight of 500 or less, a clogP of 5 or less, 5 or less hydrogen bond donors, and 10 or less hydrogen bond acceptors are more likely to be successful candidates than compounds violating more than one of these rules.1 Although these criteria are certainly not absolute, they provide medicinal chemists with a reliable guideline for rational library design. In the context of lead compound identification, various strategies have been developed for generating small molecule libraries2 that are then evaluated for their biological properties by high-throughput screening (HTS).
Some years ago we developed a novel approach to the total synthesis of (±)-tetrahydroalstonine. A pivotal step in this synthesis was a Mannich-type multicomponent assembly process (MCAP) that allowed facile access to a key aldehyde intermediate that was further elaborated via an intramolecular Diels-Alder reaction to a pentacyclic intermediate, refunctionalization of which delivered the natural product in a mere five chemical operations from tryptamine.3 We have since developed this reaction into a four-component process,4,5 and have demonstrated its utility for the diversity oriented synthesis (DOS) of unique heterocycles comprising the benzodiazepine,6 tetrahydropyridine,7 2-aryl piperidine,8 and tetrahydroisoquinoline ring systems.9,10
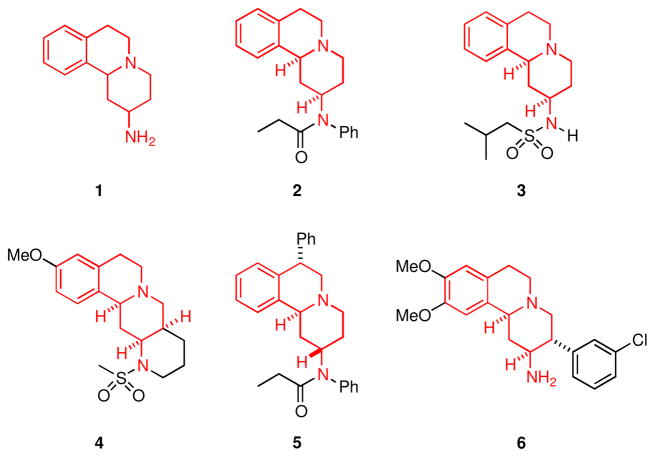
The tetrahydroisoquinoline ring system is present in a variety of natural products and pharmaceutical agents that exhibit a wide array of biological properties including antihypertensive,11 antitumor,12 and antimalarial activities.13 Moreover, compounds containing the 2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinoline-2-amine scaffold (1) are documented as α2-adrenoceptor antagonists (2–4),14 opioid receptor antagonists (5),15 and dipeptidyl peptidase IV (DPP-IV) inhibitors (6)16 (Figure 1). We have recently reported a method for the rapid and efficient assembly of the scaffold comprising 1 via an MCAP/1,3-dipolar cycloaddition strategy.9 In order to demonstrate the utility of this chemistry in the synthesis of libraries of small molecules, we prepared a diverse 70-membered library of 2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinoline-2-amine (1) derivatives from a readily accessible scaffold by exploiting well-established palladium-catalyzed cross-coupling protocols, and simple N-functionalization reactions. We now report the details of these studies.
Figure 1.
Biologically active compounds containing the 2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinoline-2-amine scaffold.
RESULTS AND DISCUSSION
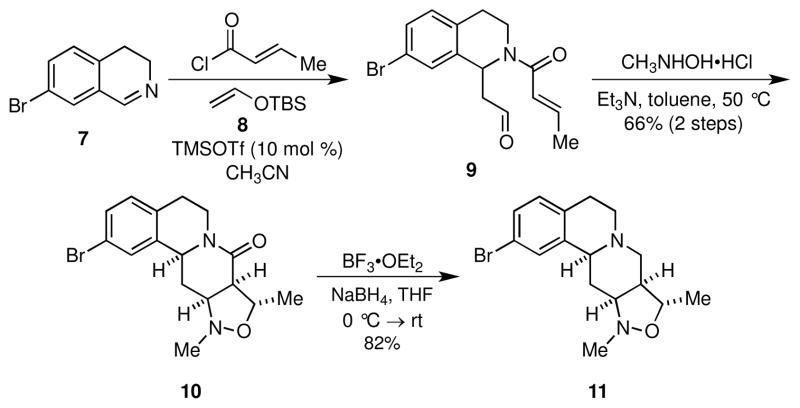
The library synthesis commenced with the construction of the parent tetrahydroisoquinoline fused isoxazolidine scaffolds 10 and 11 from readily available 7-bromodihydroisoquinoline (7) (Scheme 1).17 In the event, treatment of imine 7 with trans-crotonoyl chloride and silyl enol ether 8 in the presence of catalytic amounts of TMSOTf at room temperature furnished aldehyde 9, which upon condensation with N-methylhydroxylamine gave an intermediate nitrone that underwent facile 1,3-dipolar cycloaddition to provide the isoxazolidine 10 in 66% yield from 7; no other stereoisomers were detected. The relative stereochemistry of 10 was determined unambiguously by single crystal X-ray analysis.9d Notably, 10 is easily prepared from 7 on a multi-gram scale, without the need for column chromatography. Reduction of the lactam moiety in 10 with freshly prepared borane gave tertiary amine 11 in 82% yield. It was necessary to use borane for this transformation, because reaction with lithium aluminum hydride was unselective and resulted in reductive cleavage of the N,O-bond of the isoxazolidine ring as well as reduction of the lactam moiety.
Scheme 1.
Synthesis of isoxazolidine scaffolds 10 and 11.
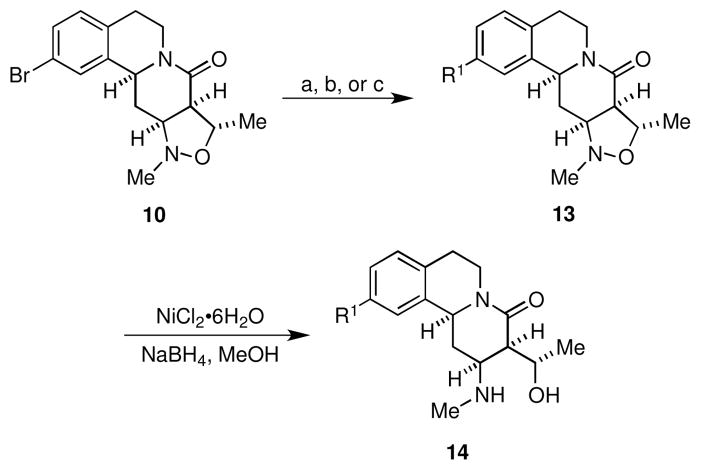
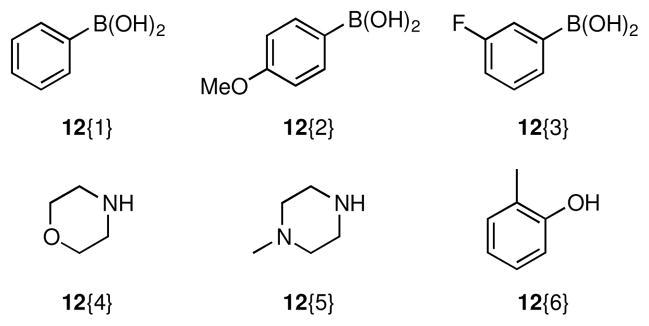
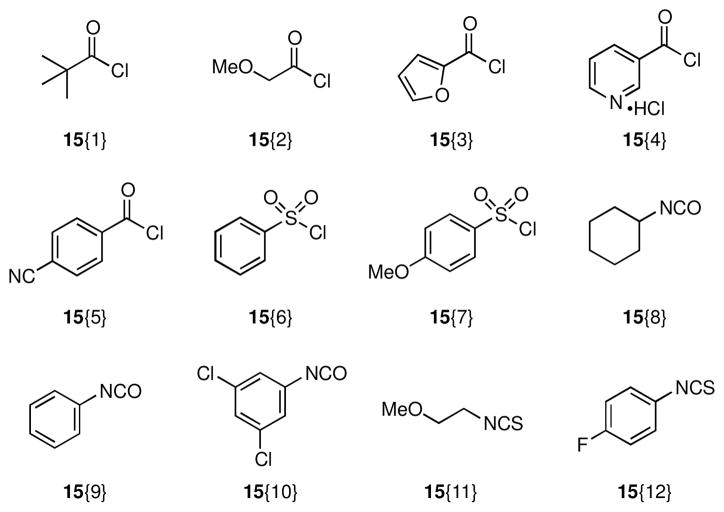
With scaffolds 10 and 11 in hand, we prepared the corresponding libraries of lactams and amines, respectively. Accordingly, reaction of 10 under standard Suzuki18 or Buchwald-Hartwig19 cross-coupling conditions provided chemset 13 in moderate to excellent yields (Scheme 2, Figure 2, Table 1). For Suzuki reactions, arylboronic acids were chosen such that electron neutral (12{1}), electron rich (12{2}), and electron deficient (12{3}) groups with varied substitution patterns were incorporated in the biaryl products in order to enable exploration of structure-activity relationships (SAR) during biological screening. Subsequent N,O-bond cleavage mediated by nickel(II) boride that was generated in situ proceeded smoothly to furnish chemset 14 in 81–92% yields.20 This transformation could also be achieved with Zn/AcOH, however this reductive method was typically lower yielding.
Scheme 2.
Cross-coupling reactions of lactam 10 and N,O-bond cleavage of isoxazolidines 13.
Conditions: (a) 12{1–3}, Pd[P(t-Bu)3]2 (1 mol %), Cs2CO3, 1,4-dioxane, 100 °C (b) 12{4,5}, Pd(OAc)2 (5 mol %), JohnPhos (5 mol %), NaOt-Bu, toluene, 100 °C (c) 12{6}, Pd(OAc)2 (5 mol %), JohnPhos (5 mol %), K3PO4, toluene, 100 °C
Figure 2.
Reagents used for cross-coupling reactions of lactam 10.
Table 1.
Preparation of 13 via palladium-catalyzed cross-couplings and 14 via N,O-bond cleavage
| Entry | Cross-Coupling Reagent | Product | Yield (%) | Product | Yield (%) |
|---|---|---|---|---|---|
| 1 | 12{1} | 13{1} | 99 | 14{1} | 87 |
| 2 | 12{2} | 13{2} | 94 | 14{2} | 88 |
| 3 | 12{3} | 13{3} | 99 | 14{3} | 83 |
| 4 | 12{4} | 13{4} | 99 | 14{4} | 81 |
| 5 | 12{5} | 13{5} | 88 | 14{5} | 90 |
| 6 | 12{6} | 13{6} | 57 | 14{6} | 92 |
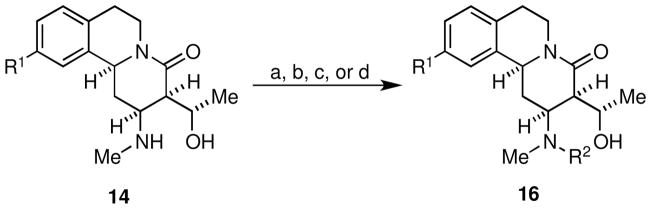
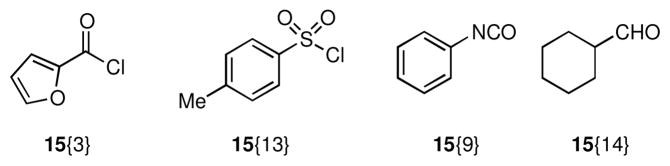
The secondary amine functionality resident in chemset 14 was an obvious embarkation point for derivatization, and as such we sought to exploit it for rapid access to novel derivatives of 1. Reaction of chemset 14 with the N-functionalizing reagents 15 under standard conditions provided chemset 16 (Scheme 3, Figure 3, Table 2) in moderate to excellent yields. A wide array of N-functionalizing reagents was chosen including alkyl, heteroaryl, and aryl substituents with varied electronics and substitution patterns to gain useful SAR data. Notably, these reactions were selective for reaction on nitrogen, and products of O-functionalization were only seldom detected in trace quantities. Although the biological profiles of compounds related to 1 are well documented, lactam derivatives such as those embodied in chemset 16 have not been thoroughly studied.
Scheme 3.
N-Functionalization of secondary amines 14.
Conditions: (a) 15{1–5}, Et3N, CH2Cl2, 0 °C → rt (b) 15{6,7}, Et3N, CH2Cl2 (c) 15{8–10}, CH2Cl2 (d) 15{11,12}, CH2Cl2
Figure 3.
N-Functionalizing reagents.
Table 2.
Data for compounds 16 synthesized via N-functionalizations
| Entry | Secondary Amine | N-functionali zing Reagent | Product | Yield (%) |
|---|---|---|---|---|
| 1 | 14{2} | 15{1} | 16{2,1} | 76 |
| 2 | 14{3} | 15{1} | 16{3,1} | 65 |
| 3 | 14{4} | 15{1} | 16{4,1} | 67 |
| 4 | 14{1} | 15{2} | 16{1,2} | 64 |
| 5 | 14{5} | 15{2} | 16{5,2} | 84 |
| 6 | 14{6} | 15{2} | 16{6,2} | 79 |
| 7 | 14{1} | 15{3} | 16{1,3} | 44 |
| 8 | 14{5} | 15{3} | 16{5,3} | 69 |
| 9 | 14{6} | 15{3} | 16{6,3} | 85 |
| 10 | 14{1} | 15{4} | 16{1,4} | 81 |
| 11 | 14{5} | 15{4} | 16{5,4} | 99 |
| 12 | 14{6} | 15{4} | 16{6,4} | 80 |
| 13 | 14{1} | 15{5} | 16{1,5} | 74 |
| 14 | 14{5} | 15{5} | 16{5,5} | 89 |
| 15 | 14{6} | 15{5} | 16{6,5} | 88 |
| 16 | 14{2} | 15{6} | 16{2,6} | 64 |
| 17 | 14{3} | 15{6} | 16{3,6} | 92 |
| 18 | 14{4} | 15{6} | 16{4,6} | 61 |
| 19 | 14{1} | 15{7} | 16{1,7} | 78 |
| 20 | 14{5} | 15{7} | 16{5,7} | 55 |
| 21 | 14{6} | 15{7} | 16{6,7} | 75 |
| 22 | 14{1} | 15{8} | 16{1,8} | 72 |
| 23 | 14{5} | 15{8} | 16{5,8} | 62 |
| 24 | 14{6} | 15{8} | 16{6,8} | 78 |
| 25 | 14{2} | 15{9} | 16{2,9} | 69 |
| 26 | 14{3} | 15{9} | 16{3,9} | 86 |
| 27 | 14{4} | 15{9} | 16{4,9} | 74 |
| 28 | 14{1} | 15{10} | 16{1,10} | 73 |
| 29 | 14{5} | 15{10} | 16{5,10} | 61 |
| 30 | 14{6} | 15{10} | 16{6,10} | 48 |
| 31 | 14{1} | 15{11} | 16{1,11} | 42 |
| 32 | 14{5} | 15{11} | 16{5,11} | 65 |
| 33 | 14{6} | 15{11} | 16{6,11} | 87 |
| 34 | 14{1} | 15{12} | 16{1,12} | 75 |
| 35 | 14{5} | 15{12} | 16{5,12} | 74 |
| 36 | 14{6} | 15{12} | 16{6,12} | 68 |
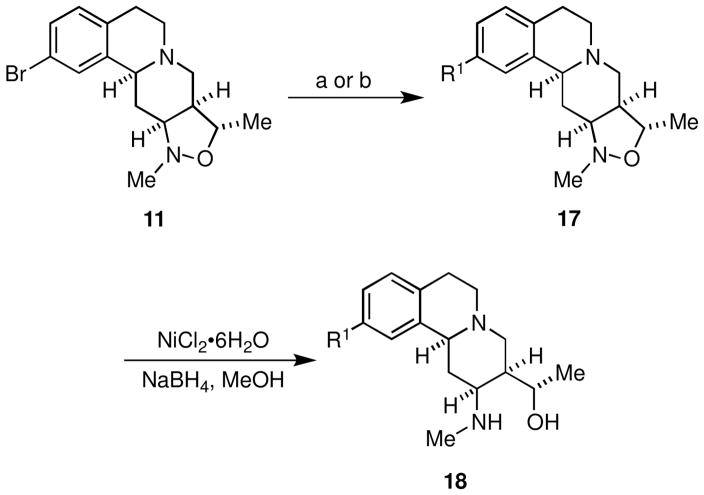
Analogous to the chemistry outlined in Scheme 2, cross-coupling of amine 11 with the reagents in chemset 12 under Suzuki21 or Buchwald-Hartwig22 conditions provided chemset 17 in 81–95% yield (Scheme 4, Figure 4, Table 3). Perhaps because of the tertiary amine functionality present in 11, we found that catalyst systems different from those used to promote the related cross-couplings of lactam 10 (Scheme 2) gave better yields of product. Subsequent N,O-bond cleavage proceeded without event to furnish chemset 18 in good yields.
Scheme 4.
Cross-coupling reactions and N,O-bond cleavage of amine 11.
Conditions: (a) 12{7,8}, [PdCl2(dppf)]•CH2Cl2 (5 mol %), CsF, toluene, 110 °C (b) 12{4}, Pd(OAc)2 (10 mol %), (±)-BINAP (12 mol %), Cs2CO3, toluene, 100 °C
Figure 4.
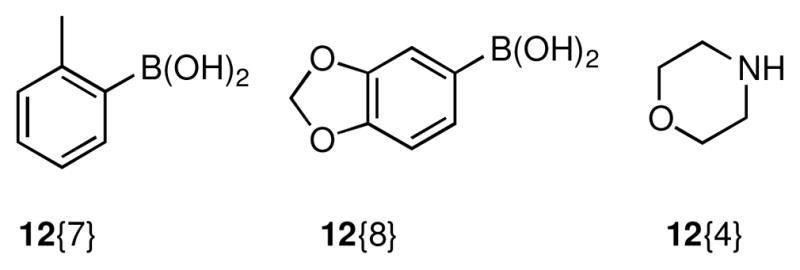
Reagents used for cross-coupling reactions.
Table 3.
Preparation of 17 via palladium-catalyzed cross-couplings and 18 via N,O-bond cleavage
| Entry | Cross-Coupling Reagent | Product | Yield (%) | Product | Yield (%) |
|---|---|---|---|---|---|
| 1 | 12{7} | 17{7} | 95 | 18{7} | 90 |
| 2 | 12{8} | 17{8} | 82 | 18{8} | 84 |
| 3 | 12{4} | 17{4} | 81 | 18{4} | 90 |
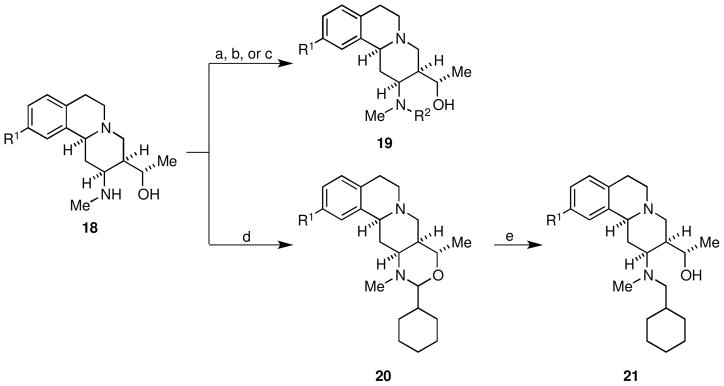
The secondary amine functionality present in chemset 18 was exploited to rapidly prepare derivatives of 1. Chemset 19 was readily accessed through reaction of amines 18 with 15{3}, 15{13}, and 15{5} under standard conditions (Scheme 5, Figure 5, Table 4). Attempted reductive amination of amines 18 under standard conditions resulted in mixtures of N,O-acetals 20 and tertiary amines 21. Because the N,O-acetals 20 proved markedly stable, a two-step procedure was employed to access tertiary amines 21. Accordingly, amines 18 were condensed with cyclohexane carboxaldehyde (15{14}) to give N,O-acetals 20, which underwent facile reduction with sodium cyanoborohydride in the presence of acetic acid to provide tertiary amines 21 in good overall yield.
Scheme 5.
N-Functionalization of secondary amines 18.
Conditions: (a) 15{3}, Et3N, CH2Cl2 (b) 15{13}, Et3N, CH2Cl2 (c) 15{5}, CH2Cl2 (d) 15{14}, DCE, 84 °C (e) NaCNBH3, AcOH, DCE
Figure 5.
N-Functionalizing reagents.
Table 4.
Data for compounds 19–21 synthesized via N-functionalizations
| Entry | Secondary Amine | N-functionali zing Reagent | Product | Yield (%) |
|---|---|---|---|---|
| 1 | 18{7} | 15{3} | 19{7,3} | 77 |
| 2 | 18{8} | 15{3} | 19{8,3} | 67 |
| 3 | 18{4} | 15{3} | 19{4,3} | 99 |
| 4 | 18{7} | 15{13} | 19{7,13} | 62 |
| 5 | 18{8} | 15{13} | 19{8,13} | 55 |
| 6 | 18{4} | 15{13} | 19{4,13} | 73 |
| 7 | 18{7} | 15{9} | 19{7,9} | 55 |
| 8 | 18{8} | 15{9} | 19{8,9} | 70 |
| 9 | 18{4} | 15{9} | 19{4,9} | 99 |
| 10 | 18{7} | 15{14} | 20{7,14} | 99 |
| 11 | 18{8} | 15{14} | 20{8,14} | 84 |
| 12 | 18{4} | 15{14} | 20{4,14} | 81 |
| 13 | -- | -- | 21{7,14} | 83 |
| 14 | -- | -- | 21{8,14} | 70 |
| 15 | -- | -- | 21{4,14} | 64 |
SUMMARY
We have prepared a 70-membered library of derivatives of the pyrido[2,1-a]isoquinoline 1 utilizing a sequential MCAP/1,3-dipolar cycloaddition process to generate functionalized scaffolds that were readily diversified. It is noteworthy that only three members of this library violate Lipinski’s rule of five. Thus, the vast majority of the members of this novel library are worthy lead candidates having favorable physiochemical properties (see table in Supporting Information). These compounds have been submitted to the NIH Molecular Libraries Small Molecule Repository (MLSMR) for distribution to HTS centers within the Molecular Libraries Probe Production Centers Network (MLPCN) and subsequent evaluation of their biological properties. Moreover, selected compounds have been sent to the National Institute of Mental Health’s Psychoactive Drug Screening Program (NIMH PDSP). Further applications of this and related approaches to the synthesis of compound libraries are in progress, and the results of these investigations and the biological activities of representative members will be reported in due course.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (GM 86192 and GM 24539) and the Robert A. Welch Foundation (F-0652) for their generous support of this work. We also thank Dr. Karin Keller (The University of Texas at Austin) for her assistance with LCMS experiments.
Footnotes
Experimental procedures, spectral data for all new compounds, full characterization data for representative compounds, LCMS data for representative compounds, and tabulated Lipinski’s rule parameters. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv Drug Deliv Rev. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 2.(a) Brenner S, Lerner RA. Encoded Combinatorial Chemistry. Proc Natl Acad Sci USA. 1992;89:5381–5383. doi: 10.1073/pnas.89.12.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Njardarson JT, Gaul C, Shan D, Huang X-Y, Danishefsky SJ. Discovery of Potent Cell Migration Inhibitors through Total Synthesis: Lessons from Structure-Activity Studies of (+)-Migrastatin. J Am Chem Soc. 2004;126:1038–1040. doi: 10.1021/ja039714a. [DOI] [PubMed] [Google Scholar]; (c) Kaiser M, Wetzel S, Kumar K, Waldmann H. Biology-Inspired Synthesis of Compound Libraries. Cell Mol Life Sci. 2008;65:1186–1201. doi: 10.1007/s00018-007-7492-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Schreiber SL. Target-Oriented and Diversity-Oriented Organic Synthesis in Drug Discovery. Science. 2000;287:1964–1969. doi: 10.1126/science.287.5460.1964. [DOI] [PubMed] [Google Scholar]
- 3.Martin SF, Benage B, Hunter JE. A Concise Strategy for the Syntheses of Indole Alkaloids of the Heteroyohimboid and Corynatheioid Families. Total Syntheses of (±)-Tetrahydroalstonine, (±)-Cathenamine and (±)-Geissoschizine. J Am Chem Soc. 1988;110:5925–5927. [Google Scholar]
- 4.For a review of such strategies, see: Sunderhaus JD, Martin SF. Applications of Multicomponent Reactions to the Synthesis of Diverse Heterocyclic Scaffolds. Chem Eur J. 2009;15:1300–1308. doi: 10.1002/chem.200802140.
- 5.(a) Sunderhaus JD, Dockendorff C, Martin SF. Applications of Multicomponent Reactions for the Synthesis of Diverse Heterocyclic Scaffolds. Org Lett. 2007;9:4223–4226. doi: 10.1021/ol7018357. [DOI] [PubMed] [Google Scholar]; (b) Sunderhaus JD, Dockendorff C, Martin SF. Synthesis of Diverse Heterocyclic Scaffolds via Tandem Additions to Imine Derivatives and Ring-Forming Reactions. Tetrahedron. 2009;65:6454–6469. doi: 10.1016/j.tet.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donald JR, Martin SF. Synthesis and Diversification of 1,2,3-Triazole-Fused Benzodiazepine Scaffolds. Org Lett. 2011;13:852–855. doi: 10.1021/ol1028404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahn JJ, Su JY, Martin SF. Facile and Unified Approach to Skeletally Diverse, Privileged Scaffolds. Org Lett. 2011;13:2590–2593. doi: 10.1021/ol200709h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardy S, Martin SF. Multicomponent Assembly and Diversification of Novel Heterocyclic Scaffolds Derived from 2-Arylpiperidines. Org Lett. 2011;13:3102–3105. doi: 10.1021/ol201010s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Granger BA, Kaneda K, Martin SF. Multicomponent Assembly Strategies for the Synthesis of Diverse Tetrahydroisoquinoline Scaffolds. Org Lett. 2011;13:4542–4545. doi: 10.1021/ol201739u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.For a review of such strategies, see: Donald JR, Granger BA, Hardy S, Sahn JJ, Martin SF. Applications of Multicomponent Assembly Processes to the Facile Syntheses of Diversely Functionalized Nitrogen Heterocycles. Heterocycles. 2012;84 doi: 10.3987/COM-11-S(P)92.
- 11.Gavras I, Vlahakos D, Melby JC, Gavras H. Pilot Study of the Effects of the Angiotensin-Converting Enzyme CI-906 on Patients with Essential Hypertension. J Clin Pharm. 1984;24:343–350. doi: 10.1002/j.1552-4604.1984.tb02786.x. [DOI] [PubMed] [Google Scholar]
- 12.Asaoka T, Yazawa K, Mikami Y, Takahashi K. A New Saframycin, Saframycin R. J Antibiot. 1982;35:1708–1710. doi: 10.7164/antibiotics.35.1708. [DOI] [PubMed] [Google Scholar]
- 13.Francois G, Bringmann G, Phillipson JD, Boyd MR, Assi LA, Schneider C, Timperman G. Antimalarial Naphthylisoquinoline Alkaloids and Pharmaceutical Compositions and Medical Uses Thereof. 5,639,761. U S Patent No. 1994
- 14.(a) Van Dyke JW, Jr, Havera HJ, Johnson RD, Vidrio H, Viveros A. Cardiovascular Activity of Some Substituted 2-Aminobenzoquinolizines. J Med Chem. 1971;15:91–94. doi: 10.1021/jm00271a025. [DOI] [PubMed] [Google Scholar]; (b) Ward TJ, White JF, Lattimer N, Rhodes KF, Sharma S, Waterfall JF. Synthesis and Structure-Activity Relationships of 2-Sulfonamido-1,3,4,6,7,11ba-hexahydro-2H-benzo[a]quinolizines as α2-Adrenoceptor Antagonists. J Med Chem. 1988;31:1421–1426. doi: 10.1021/jm00402a029. [DOI] [PubMed] [Google Scholar]; (c) Clark RD, Repke DB, Kilpatrick AT, Brown CM, MacKinnon AC, Clague RU, Spedding M. (8aa, 12aa, 13aa)-5,8,8a,9,10,11,12,12a,13,13a-Decahydro-3-methoxy-12-(methylsulfonyl)-6H-isoquino[2,1-g][1,6]naphthyridine, a Potent and Highly Selective α2-Adrenoceptor Antagonist. J Med Chem. 1989;32:2034–2036. doi: 10.1021/jm00129a002. [DOI] [PubMed] [Google Scholar]
- 15.Maryanoff BE, McComsey DF, Taylor RJ, Jr, Gardocki JF. Synthesis and Stereochemistry of 7-Phenyl-2-propionanilidobenzo[a]quinolizidine Derivatives. Structural Probes of Fentanyl Analgesics. J Med Chem. 1981;24:79–88. doi: 10.1021/jm00133a017. [DOI] [PubMed] [Google Scholar]
- 16.Lübbers T, Böhringer M, Gobbi L, Hennig M, Hunziker D, Kuhn B, Löffler B, Mattei P, Narquizion R, Peters J-U, Ruff Y, Wessel HP, Wyss P. 1,3-Disubstituted 4-Aminopiperidines as Useful Tools in the Optimization of the 2-Aminobenzo[a]quinolizine Dipeptidyl Peptidase IV Inhibitors. Bioorg Med Chem Lett. 2007;17:2966–2970. doi: 10.1016/j.bmcl.2007.03.072. [DOI] [PubMed] [Google Scholar]
- 17.(a) Worrall DE. Nitrostyrene. Org Synth. 1929;9:66. [Google Scholar]; (b) Schumacher RW, Davidson BS. Synthesis of Didemnolines A-D, N-9-Substituted β-Carboline Alkaloids from the Marine Ascidian Didemnum sp. Tetrahedron. 1999;55:935–942. [Google Scholar]; (c) Mjalli AMM, Gohimmukkula DR, Tyagi S. Aryl and Heteroaryl Compounds, Compositions, and Methods of Use. 7,208,601. U S Patent No. 2005
- 18.Littke AF, Dai C, Fu GC. Versatile Catalysts for the Suzuki Cross-Coupling of Arylboronic Acids with Aryl and Vinyl Halides and Triflates under Mild Conditions. J Am Chem Soc. 2000;122:4020–4028. [Google Scholar]
- 19.Wolfe JP, Buchwald SL. A Highly Active Catalyst for the Room-Temperature Amination and Suzuki Coupling of Aryl Chlorides. Angew Chem, Int Ed Engl. 1999;38:2413–2416. doi: 10.1002/(sici)1521-3773(19990816)38:16<2413::aid-anie2413>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 20.Jones AD, Knight DW, Thornton SR. On the Lewis Acid-Induced [1,3]-Dipolar Cycloaddition of Allylic and Homoallylic Alcohols to N-Methyl-C-phenyl Nitrone. J Chem Soc, Perkin Trans. 1999;1:3337–3344. [Google Scholar]
- 21.Ishiyama T, Murata M, Miyaura N. Palladium(0)-Catalyzed Cross-Coupling Reactions of Alkoxydiboron with Haloarenes: A Direct Procedure for Arylboronic Esters. J Org Chem. 1995;60:7508–7510. [Google Scholar]
- 22.Wolfe JP, Wagaw S, Buchwald SL. An Improved Catalyst System for Aromatic Carbon-Nitrogen Bond Formation: The Possible Involvement of Bis(Phosphine) Palladium Complexes as Key Intermediates. J Am Chem Soc. 1996;118:7215–7216. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.