Abstract
Context
Advances in antiretroviral therapy (ART) for HIV offer life-extending benefit; however, the side effects associated with ART use negatively impact quality of life and medication adherence among people living with HIV.
Objectives
This study tested the efficacy of mindfulness-based stress reduction (MBSR) for reducing ART symptoms and bother/distress related to ART side effects. Secondary aims were to test the impact of MBSR on medication adherence and psychological functioning.
Methods
Seventy-six people living with HIV who were actively taking ART and reported distress from ART-related side effects were randomly assigned to MBSR or a wait-list control standard care condition. We measured side effects, ART adherence, perceived stress, depression, positive and negative affect, and mindfulness at three time points: baseline, three-month follow-up, and six-month follow-up. Side effects and related distress were assessed separately from other symptoms.
Results
Compared to a wait-list control, participants in the MBSR condition experienced a reduction in the frequency of symptoms attributable to antiretroviral therapies at three months post intervention (mean difference = 0.33; 95% confidence interval [CI] = 0.01, 0.66; t(132) = 2.04, P = 0.044) and at six months post intervention (mean difference = 0.38; 95% CI = 0.05, 0.71; t(132) = 2.27, P = 0.025). MBSR participants also experienced a reduction in distress associated with those symptoms at three months post intervention (mean difference = 0.47; 95% CI = 0.003, 0.94; t(132) = 1.99, P = 0.048) compared with the wait-list control condition.
Conclusion
Mindfulness-based stress reduction is a promising approach for reducing HIV treatment-related side effects.
Keywords: Mindfulness-based stress reduction, HIV, antiretroviral therapy, side effects, symptoms, adherence
Introduction
While the life-extending benefit of antiretroviral therapy (ART) for HIV is well-documented, adverse side effects often accompany drug benefit.1–3 The most commonly reported side effects from ART are gastrointestinal problems such as diarrhea, nausea and vomiting; neuropathic pain; and dermatological problems such as rashes.4 The potential impact of side effects is often cited as a primary deciding factor on when to start ART among HIV+ individuals with middle range CD4 counts (350–500 cells/µl).5
The high adherence demands associated with ART and the accompanying side effects make treatment success challenging,6 with side effects being consistently cited as a reason for nonadherence to ART.7 Finally, there is evidence that potential side effects impact acceptance of offered medication8 and our own research9 suggests that side effect concern is a primary reason for discontinuing ART among HIV+ individuals. While researchers are actively attempting to identify new treatment agents that have fewer side effects, the goal of a completely side effect-free, clinically effective regimen has not yet been developed. As such, people living with HIV will have to face the realities of side effects for the foreseeable future, and interventions to reduce the impact of side effects are needed to optimize benefit from treatment.
Mindfulness-Based Stress Reduction
Mindfulness-based stress reduction (MBSR) is a promising avenue for intervening with side effects. MBSR is a program that provides systematic training in mindfulness meditation as a self-regulation approach to reducing stress and medical and psychological symptoms. 10, 11 Use of MBSR has grown rapidly since it was formulated by Kabat-Zinn at the University of Massachusetts Medical Center in 1979.12, 13 There are an estimated 607 certified MBSR programs now, with more being introduced each year. A number of studies suggest that MBSR is effective in improving psychological and physical well-being among a variety of populations.14,16) Furthermore, several studies suggest that MBSR may act directly to reduce pain and other symptoms. In a randomized controlled trial (RCT) with self-selected community residents, Williams and colleagues found a 46% reduction in medical symptoms.17 An early study of MBSR looking at chronic pain, although uncontrolled, showed 60–72% of patients experienced moderate or great improvement in their pain.10 Recent RCTs have demonstrated greater acceptance of chronic pain after participating in MBSR,18 and reductions in pain intensity lasting up to three years post MBSR.19 Given these findings, addressing HIV treatment side effects through MBSR is a logical yet untested next step.
We performed a randomized, wait-list controlled trial to test the efficacy of MBSR in ameliorating self-reported side effects and side effect-related distress in people with HIV taking ART. The primary aim of the study was to test the effect of MBSR on side effect frequency and related distress among HIV+ adults taking antiretroviral medications. The secondary aim was to test the impact of the intervention on medication adherence and psychological functioning.
Methods
Participants
The FOCUS study was approved by the University of California, San Francisco, Committee for Human Research and all participants included in the study gave signed informed consent. Participants were English-speaking HIV+ adult men and women on ART who reported distress associated with side effects from treatment. We used either a letter from their health care provider or documentation of HIV test results as evidence of HIV infection. Eligibility for the study included currently taking a recognized ART regimen and reporting a level of side effect-related bother for the previous 30 days at or above eight (corresponding to the 40th percentile in another sample) on the side effect and symptom distress scale we used.1, 20 Potential participants were excluded if they were enrolled in another behavioral coping or HIV adherence intervention research study or MBSR program. Severe cognitive impairment, active psychosis, or active substance abuse that would interfere with capacity to participate in MBSR also led to exclusion from the study.
Trial Design and Setting
This study was an RCT of MBSR compared with a wait-list control (WLC) condition receiving standard care. Randomization was performed in blocks of six using the SAS system’s PLAN procedure (SAS Institute, Cary, NC). Groups of six participant IDs were sorted in ascending order and aligned with the treatment output from the procedure. When less than six participants were available, dummy participant IDs were used to complete the block. The MBSR intervention was delivered during the first two to three months following randomization and follow-up assessments occured at three months (immediately after the intervention) and six months post randomization. All members of the standard care (WLC) group underwent exactly the same assessment procedures as the intervention group and were offered the intervention content subsequent to the final six-month follow-up assessment.
We took a multi-pronged approach to recruitment, using advertisements in local publications, outreach to clinics and community-based organizations, and provider referrals. To minimize attrition, incentive payments for all assessments after the baseline interviews were gradually increased over time. This defrayed participants’ transportation costs and helped to ensure high assessment follow-up rates.
Intervention
MBSR draws on centuries-old meditation practices, particularly Buddhist Vipassana and Zen practices, but adapts these practices in a secular form for a Western audience. MBSR aims to teach participants to respond to stressful situations “mindfully” – a state in which one focuses on the present moment, accepting and acknowledging it without getting caught up in thoughts that are about the situation or emotional reactions. This enables people to respond to the situation by making conscious choices to respond instead of reacting automatically. The MBSR course consists of a standardized series of eight weekly sessions of 2.5 to 3 hours. There are daily home assignments of formal and informal mindfulness practice. Examples of formal meditation practices that are taught in the MBSR course include body scan meditation, yoga postures practiced with mindful awareness of the body, and sitting meditation with mindfulness of breath, thoughts, and emotions. Informal mindfulness practices taught in the course include awareness of pleasant and unpleasant events and deliberate awareness of routine activities and events such as eating and interpersonal communications.
The MBSR program consists of the following elements: (1) individual pre-program intake interviews performed by the course instructor with each participant, lasting 30 minutes; (2) eight weekly classes of 2.5 to 3 hours duration; (3) an all-day silent retreat during the sixth week of the program; and (4) daily home assignments including a minimum of 45 minutes per day of formal mindfulness practice and 5–15 minutes of informal practice, six days per week for the entire duration of the course. The total in-class contact is approximately 30 hours, and the total home assignments are a minimum of 42–48 hours. In addition, one to two additional individual interview sessions may be provided, at instructor discretion, to individual participants during the course. As well as teaching mindfulness practices, the course includes didactic presentations that include information on stress physiology and stress reactivity. The course also addresses the effects of perception, appraisal, and attitude on health habits and behavior as well as on interpersonal communication.
The course instructor for our study was an experienced MBSR teacher with a personal mindfulness meditation practice who had undergone formal training in the delivery of MBSR.
Measures
Audio computer-administered self-interview (ACASI) and computer-assisted personal interviewing (CAPI) were used to enhance veracity in self-report. 21–23 In addition to the following measures, detailed demographic and background information such as age, race/ethnicity, education, and length of HIV diagnosis were collected.
CD4 count
also known as T-cell count, is an indicator of the extent of immune damage resulting from conditions such as HIV. The lower the count, the greater the level of immunocompromise. CD4 counts are used as an important consideration for decisions about starting ART. Currently, ART is strongly recommended for persons with counts under 350 and treatment should be offered to those with counts under 500. We collected self-report CD4 counts. In general, persons living with HIV track their CD4 counts and there is evidence that self-reported values are valid when compared to medical records.24
Side Effect Checklist
We used a modified AIDS Clinical Trials Group symptom checklist, in which respondents were asked whether they had experienced each of 25 possible symptoms in the preceding 30 days,7 and whether they attributed these to their ART medications, their HIV infection, or to other causes.25 Symptoms were coded as: 0 = did not experience the symptom vs. 1 = experienced the symptom; the individual symptoms were then summed to create overall counts of symptoms and symptoms attributable to ART medications. To measure severity, participants were asked how much each symptom bothered them (on a Likert-type scale 0 = not present; 1 = present but does not bother me; ranging to 4 = present and bothers me terribly). Average symptom bother scores were computed as the mean of these individual Likert items to quantify the overall average bother and bother attributable to ART medications. Residual plots of the sum of symptoms attributable to ART medications variable appeared skewed and heteroskedastic in our data, so log transformation was applied to this outcome. The residual plots of log-transformed symptoms attributable to ART and their average related bother/distress appeared close to normal and homoskedastic.
The baseline assessment was programmed to provide an overall score of self-reported ART side effect-related distress. Each symptom that was attributed to ART was assigned a value pertaining to the level of reported distress. For example, if a person reported nausea and attributed it to their medications, they were given a point based on how much they reported that the nausea bothered them (0 = did not bother, 1 = bothered a little, 2 = bothered a lot, 3 = bothered terribly). These scores were added across symptoms and a score of 12 or higher was set for eligibility based on prior distributions using the measure. Therefore, a person could meet eligibility by endorsing 12 side effects with minimal bother, 4 side effects with higher reported bother, or a combination of symptoms with varying degrees of bother. Although the items were asked by an interviewer, responses were entered in real-time into the Questionnaire Development System (QDS™, Nova Research Company, Bethesda, MD) interviewing software, which calculated eligibility and notified the interviewer of the eligibility status at the end of the interview. We have previously used this approach in other studies with good success. 20, 26, 27
ART Adherence
The AIDS Clinical Trials Group self-report adherence measure was used to assess pills and doses skipped in the last three days for each ART medication.28 The measure has been widely used, and levels of adherence as indicated by this measure have been meaningfully linked with viral load levels.7, 28–30 For the present study, we calculated percent adherence based on number of pills taken divided by the number of pills respondents reported being expected to take. We also administered the visual analogue scale (VAS) developed by Walsh et al. to assess 30-day adherence.31 This VAS uses a scale anchored by 0% and 100%; adherence for each drug is reported separately. This measure has been validated in relation to other measures of adherence (e.g., electronic monitoring of pill bottle opening).32, 33 The 30-day time frame also has empirical support.34 For the present study, the mean percent adherence was calculated across all drugs in the participant’s ART regimen. For both adherence measures, respondents were classified as having achieved 100% adherence versus lack of full adherence.
Depression
was assessed using the Beck Depression Inventory (BDI).35 The BDI comprises 21 items that are rated on a 4-point scale according to how severely they are experienced (alpha = 0.88). Higher scores indicate more depression.
Perceived stress
was assessed using the Perceived Stress Scale (PSS).36 The PSS is designed for use with community samples and is now the most widely used self-report measure of psychological stress. We used the 10-item version of the scale; participants respond how often (1 = never; 5 = very often) during the past month they experienced thoughts and feelings such as “Felt that you were unable to control the important things in your life,” “Been unable to control irritations in your life” (alpha = 0.77). Perceived stress was scored as the sum of the 10 individual scale items, with four of the items (“Felt confident about your ability to handle your personal problems;” “Felt that things were going your way;” “Been able to control irritations in your life;” and “Felt that you were on top of things”) being reverse scored prior to summation of the 10 items. Higher scores indicate more perceived stress.
Positive and Negative Affect
The Positive and Negative Affect Schedule (PANAS), 37 was used to assess the intensity of positive and negative affect during the previous week. The scale was scored for total positive and negative affect (alpha = 0.88 for positive affect and 0.83 for negative affect). Each item is measured on a 5-point Likert scale (0 = not at all; 4 = extremely) as to how much participants felt each of ten positive (attentive; interested; excited; strong; enthusiastic; determined; proud; inspired; active; alert) and ten negative (afraid; distressed; upset; jittery; guilty; nervous; scared; hostile; ashamed; irritable) emotions in the past week. The Likert item values for the ten positive emotions were summed to create a positive affectivity score and the Likert item values for the ten negative emotions were summed to create a negative affectivity score.
Mindfulness
We assessed four of the five subscales of the Five Factor Mindfulness Questionnaire (FFMQ): 38 observing, describing, awareness/attention, and nonjudging. We examined the four subscales individually and as part of an overall mindfulness construct (alpha = 0.92). Means of the Likert item values for the items in each subscale were calculated and an overall mean was calculated to create a mindfulness score.
Analysis
Baseline characteristics of the MBSR intervention and WLC usual care groups were compared using independent samples t-tests and Chi-square tests. For t-tests, equality of variances was assessed prior to using the pooled variance estimator. Intent-to-treat analyses were conducted to examine intervention effects as follows: Continuous outcome variables were analyzed using a repeated measures comparison of means using PROC MIXED in SAS version 9.2 (SAS Institute, Cary, NC). Each analysis incorporated a categorical group effect for control or intervention group, a categorical time effect (0 vs. 3 vs. 6 months), and a group-by-time interaction effect. Additional planned comparisons were the primary inferences of interest, however. These comprised single degree-of-freedom mean comparisons of the two groups at each follow-up time point. We also considered time-averaged comparisons to quantify the average group difference pooling the three- and six-month follow-up data. All of these comparisons were generated with ESTIMATE statements in PROC MIXED. Because raw mean differences of log-transformed outcomes are difficult to interpret, we also report the percentage change implied in the count of symptoms attributable to being in the MBSR intervention group versus the WLC standard care group.39
The overall sum of ART-related symptoms and bother attributable to symptoms were analyzed using doubly-multivariate repeated measures analysis. In the doubly-multivariate (DM) framework, multiple measures’ scores are incorporated into the same analysis, which enables the analysis to take advantage of the correlated nature of responses arising from the same research participant to maximize statistical power and accuracy of inferences. Thus, the sum of overall symptoms and average bother for those symptoms were pooled in one DM analysis. A second DM analysis pooled sum of symptoms attributable to ART and average bother of the symptoms attributable to ART. A third DM analysis pooled the four mindfulness subscales in the same analysis. For each DM analysis, measure-specific group differences at each time point were computed to further identify which, if any, measures contributed to a post-intervention group difference in means. Perceived stress (by PSS) and depression (by BDI) were considered separately.
Results
Sample Characteristics, Study Completion Rates, and Missing Data
The sample of 76 participants was 84% male and 53% European-American, 20% African American, and 14% Latino(a); mean age was 48 years. Ninety-one percent reported some education beyond high school graduation. The average time since testing positive was 13.7 years and mean self-reported CD4 count at baseline was 434. The mean number of side effects reported at baseline was seven of a possible 25 symptoms and the mean number of disease-related symptoms was five. The most bothersome problems (either side effect or disease-related symptom) were neuropathy, changes in body appearance (including weight gain and lipodystrophy), sleep difficulties, stiff or painful joints, and fatigue. The least bothersome problems, when present, were fever, cough, sore throat, and runny nose. Baseline characteristics of the MBSR intervention group and the WLC standard care group are shown in Table 1. There were no statistically significant differences between the MBSR intervention group and the WLC standard care group on age, gender, race/ethnicity, level of education, self-reported CD4 count, and the time since the participant learned of his or her HIV diagnosis. A higher proportion of participants in the WLC standard care group reported an undetectable HIV RNA viral load (88%) versus the MBSR intervention group (61%, P = 0.01). As a sensitivity analysis, we re-ran the doubly multivariate repeated measures analysis with detectable viral load included as a control variable. Substantive results did not change with viral load included in the analysis.
Table 1.
Sample Characteristics at Baseline
| Wait-List Control | MBSR Group | P-value | |
|---|---|---|---|
| Age — mean (SD) years | 48.2 (9.1) | 47.9 (6.8) | 0.86 |
| Male Gender | 31 (86.1) | 33 (82.5) | 0.76 |
| Race/Ethnicity — n (%) | 0.58 | ||
| African American | 5 (13.9) | 10 (25.0) | |
| European-American | 21 (58.3) | 19 (47.5) | |
| Latino(a) | 6 (16.7) | 5 (12.5) | |
| Other | 4 (11.1) | 6 (15.0) | |
| Education — n (%) | 0.58 | ||
| < HS graduate | 3 (8.3) | 4 (10.0) | |
| HS graduate | 7 (19.4) | 13 (32.5) | |
| Some college or technical training | 13 (36.1) | 13 (32.5) | |
| College graduate | 10 (27.8) | 6 (15.0) | |
| Advanced degree (MA/MS/PhD/JD/MD) | 3 (8.3) | 4 (10.0) | |
| Baseline self-report CD4 count - mean (SD) | 475 (263) | 395 (235) | 0.17 |
| Baseline HIV RNA viral load undetectable | 29 (87.9) | 23 (60.5) | 0.01 |
| Time since learned HIV status - mean (SD) | 13.9 (7.0) | 13.6 (7.6) | 0.88 |
Notes: n = 76 for all variables except HIV viral load (n=71) and CD4 (n=75). Independent samples t-tests were used to determine P-values for age, CD4, and time since learning one’s HIV status. Chi-square tests were used for all other variables; Fisher’s exact P-value was substituted for the Chi-square P-value if any cells were less than 5.
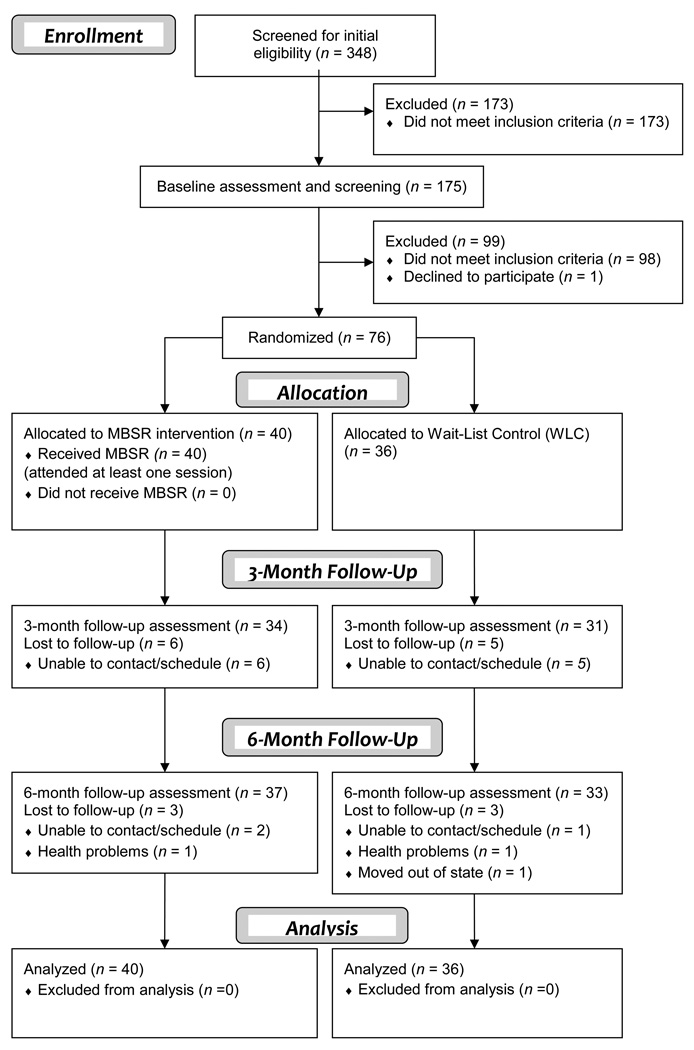
Seventy-six cases were available at baseline for analysis. Of the 272 who were excluded from the trial, 173 (63.6%) did not meet initial phone screen eligibility criteria, 98 (36.0%) did not score above the side effect distress threshold score, and one eligible participant declined to be randomized. Study retention was adequate, with 86% (n = 65) completing three-month follow-up assessments and 93% (n = 71) completing assessments at the six-month follow-up. However, intervention attendance was lower; one-third of the sample completed more than five of eight MBSR sessions. The study participant flowchart is illustrated in Figure 1. Cases with partial data were included in the analysis via full-information maximum likelihood (FIML), which maximizes statistical power for hypothesis testing and generalizability of results under the missing at random (MAR) assumption.40
Figure 1.
Study flow chart.
Primary Outcomes: Side Effects and Related Distress
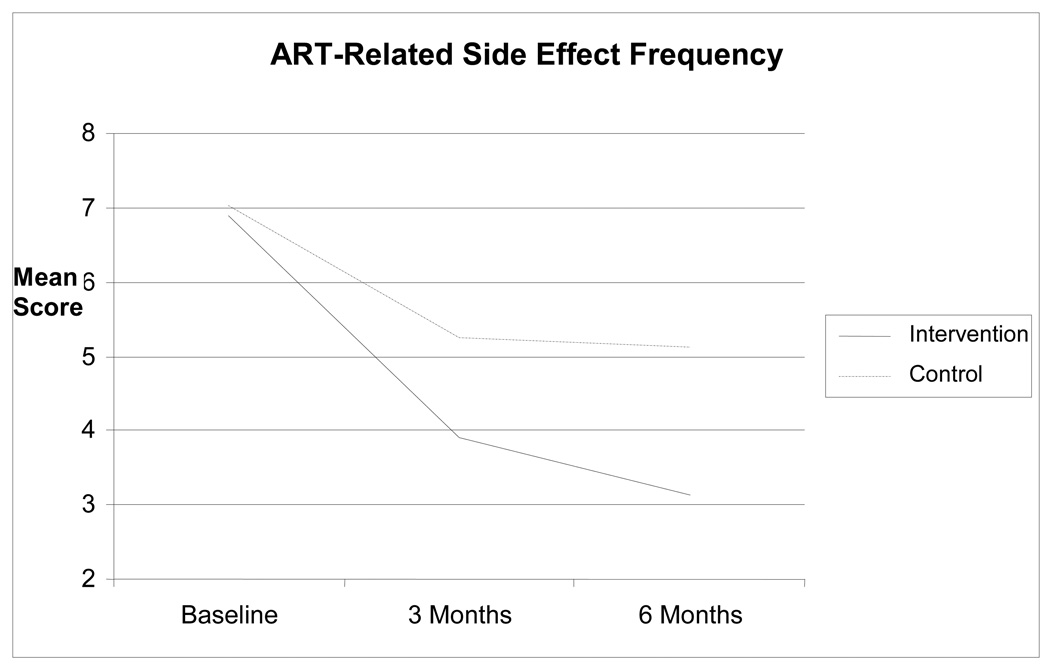
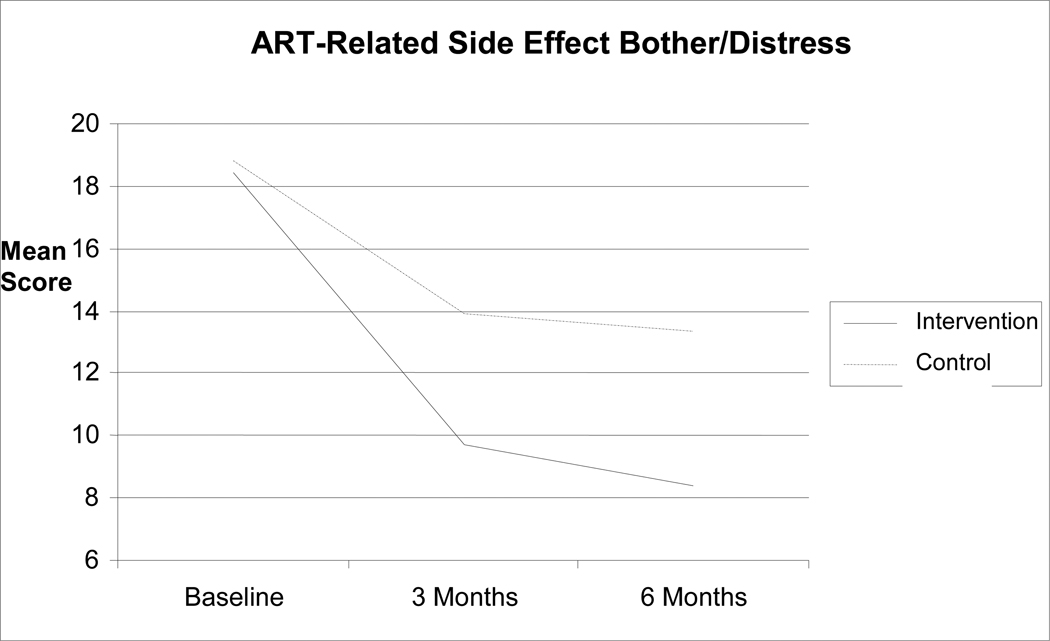
There was a decreasing number of overall symptoms and bother reported over time (F(2, 132) = 21.89, P < 0.0001), but the numbers of symptoms reported were not different between the two study groups (F (2, 132) = 0.12, P = 0.888). For symptoms and bother attributable to ART, however, there was a significant group-by-time interaction (F(2, 132) = 3.87, P = 0.023), such that intervention group participants reported fewer symptoms attributable to ART at three months (mean difference = 0.33; 95% confidence interval [CI] = 0.01, 0.66; t(132) = 2.04, P = 0.044) and at six months (mean difference = 0.38; 95% CI = 0.05, 0.71; t(132) = 2.27, P = 0.025). These raw differences on the natural log scale translate to 39.1% and 46.2% reductions in symptoms reported at three and six months for MBSR participants relative to WLC standard care participants, respectively. Average bother for these symptoms was reduced at three months for intervention group participants (mean difference = 0.47; 95% CI = 0.003, 0.94; t(132) = 1.99, P = 0.048), although this was not statistically significant at six months (mean difference = 0.43; 95% CI = −0.05, 0.90; t(132) = 1.78, trend P = 0.078). The average bother reduction for MBSR intervention participants relative to WLC standard care participants was close to one-half of a scale point on the 5-point Likert scale format used for the average bother measure. Time-averaged comparisons of the pooled three- and six-month data across groups in the full data analyses yielded group differences for both sum of symptoms attributable to ART (mean difference = 0.36; 95% CI = 0.08, 0.63; t(132) = 2.52, P = 0.013; 43.3% reduction in symptoms attributable to ART) and bother attributable to ART (mean difference = 0.45; 95% CI = 0.05, 0.86; t(132) = 2.20, P = 0.029) (Figs. 2 and 3).
Figure 2.
Reductions in side effect frequency.
Figure 3.
Reductions in side effect bother/distress
Note: Sample means are presented above on the original scales of measurement to aid interpretability.
Secondary Outcomes: Adherence and Psychological Functioning
No statistically significant effects were found for adherence. The remainder of the psychological outcomes showed patterns of estimated means consistent with hypotheses. However, the differences between groups on perceived stress, depression, positive and negative affect, and mindfulness were not significant in omnibus tests and there were no significant group differences within time points and specific measures for these outcomes.
Discussion
The intent-to-treat results of this randomized, wait-list controlled trial indicate that MBSR reduced both the frequency of symptoms attributable to antiretroviral therapy and the distress persons living with HIV feel from those side effects. We did not, however, see statistically signifiant effects of the MBSR intervention on our secondary outcomes of adherence and psychological functioning. We recognize that a wait-list, usual care control group design may have limitations in comparison to more active control conditions. For example, we were not able to evaluate directly whether changes in outcomes were attributable to MBSR content rather than the supportive nature of the intervention group setting. This is an innovative application of a complementary and alternative medicine intervention and it is meant to be a proof of concept rather than a definitive demonstration of efficacy or effectiveness; larger scale replication of our results is warranted.
A strength of the study is our attention to assessing side effects separately from other symptoms. The bulk of research describing side effects and disease-related symptoms assess the two types of problems together.41, 42 If a distinction is made, it is based on expert knowledge of what is likely to be the cause of an individual problem without regard for the individual’s causal attributions.6 Given the influence of side effects on adherence to care, it is logical to determine an individual’s perceptions of side effects, even if the attributions are not accurate. In research with older adults with HIV, Siegel et al reported that it is the belief that a medication is causing the problem that is likely to influence adherence.43–45 Unlike disease-related symptoms, side effects may be coupled with a belief that the problems are necessary to stay healthy (i.e., they are inevitably tied to the medication).
Side effects also may be viewed as ultimately controllable, that is, that one has the power to stop taking medication and consequently eliminate side effects. We have found that persons on ART make distinctions between disease-related symptoms and treatment side effects 20 and we used an assessment approach in the current study to assess ART-related causal attributions of symptoms. In our previous work, we found an association between perceptions of personal control over side effects and quality of life.27 Such findings may offer an explanation for the benefits we saw here regarding an MBSR approach to HIV treatment side effects. By practicing mindfulness meditation, including nonjudgmental awareness, participants may have felt greater personal control over the decision to tolerate side effects to obtain the biological benefit of ART.
We did not, however, see a reduction in medical symptoms not attributed to ART. This may be the result of the selection criteria based on level of side effect reporting but not for reporting of symptoms related to HIV disease. In essence, the elevated levels of side effect distress (but not HIV symptom distress) may have allowed for a detection of an intervention effect that was not detected for symptoms. Likewise, it may be that MBSR helped some participants re-evaluate what they previously identified as side effects such that they no longer attributed them to medications. It is possible that some of these symptoms remained but were subsequently appraised as HIV symptoms. Unfortunately, we are not able to disentangle changes in causal attributions for specific symptoms but this may be an avenue for future inquiry.
We likewise did not find effects on our secondary outcomes of adherence and psychological functioning. Because the study did not select participants based on low adherence scores, there was a substantial portion of the sample with high adherence throughout the study. Future investigations that screen for nonadherence may find that MBSR is associated with improvements in adherence among those with adherence difficulties at trial entry. Although patterns of estimated means for the psychological outcomes were in the expected directions, between group and within time comparisons did not yield statistically significant results. It may be that the dosage of MBSR received was insufficient to impact psychological functioning.
Our level of intervention participation was relatively low, with only a third of the sample completing five or more of the eight MBSR class sessions. We suspect that there are several reasons for the low rate of intervention completion. Unlike many trials of MBSR, which often select for participants who are inclined toward meditation and complementary medicine, the current study sought to recruit participants from a specific illness context: adults living with HIV experiencing distress from treatment side effects. The demands of the MBSR curriculum may have been a deterrent in this sample of people who were not explicitly seeking such an intervention and were struggling with health-related concerns. Participants received no incentive payments for attending intervention sessions and transportation to the course was a challenge for some. Future MBSR research with this population could begin with a needs assessment to determine the best location for holding the course and could employ motivational interviewing strategies to improve engagement and address any individual barriers to course participation. Given the potential positive benefit of mindfulness interventions for improving ART side effect-related distress, as we saw here, brief mindfulness interventions such as low-dose MBSR47 also may be considered as an adjunct to HIV care.
Table 2.
Means, Standard Deviations, and Percentages of Outcome Variables for Control and MBSR Intervention Groups
| Outcome | Baseline | 3 Months | 6 Months | |||
|---|---|---|---|---|---|---|
| Control (n=36) | MBSR (n=40) | Control (n=31) a | MBSR (n = 34) | Control (n=34) | MBSR (n=37) a | |
| Side Effects | 15.50 (3.54) | 14.20 (4.20) | 13.84 (4.12) | 12.62 (4.72) | 11.85 (5.39) | 10.84 (4.41) |
| Side Effects Bother | 2.52 (0.34) | 2.61 (0.34) | 2.45 (0.44) | 2.47 (0.42) | 2.44 (0.42) | 2.44 (0.47) |
| ART Side Effects | 7.00 (3.30) | 6.88 (3.37) | 5.23 (3.80) | 43.91 (3.41) | 5.09 (4.68) | 3.14 (2.87) |
| ART Side Effects Bother | 2.68 (0.45) | 2.70 (0.44) | 2.45 (0.88) | 2.01 (1.01) | 2.33 (0.90) | 1.89 (1.23) |
| Depression | 20.50 (8.95) | 20.90 (10.30) | 17.47 (11.08) | 15.71 (11.25) | 19.35 (14.18) | 14.25 (9.27) |
| Perceived Stress | 20.44 (5.60) | 19.45 (6.56) | 20.53 (6.65) | 18.26 (7.60) | 21.06 (8.32) | 18.24 (6.66) |
| Positive Affect | 21.36 (7.12) | 20.58 (8.95) | 22.45 (6.74) | 22.41 (9.29) | 22.15 (8.13) | 24.11 (9.08) |
| Negative Affect | 13.50 (6.81) | 13.43 (8.98) | 12.81 (8.20) | 12.74 (9.15) | 13.15 (10.12) | 10.73 (8.18) |
| Mindfulness: Non-judging | 3.02 (0.80) | 3.11 (0.77) | 2.95 (0.91) | 3.16 (0.93) | 3.16 (0.74) | 3.20 (0.92) |
| Mindfulness: Describing | 3.50 (0.83) | 3,38 (0.77) | 3.73 (0.88) | 3.47 (0.84) | 3.65 (0.78) | 3.48 (0.77) |
| Mindfulness: Observing | 3.42 (0.70) | 3.35 (0.67) | 3.61 (0.68) | 3.51 (0.72) | 3.34 (0.75) | 3.52 (0.64) |
| Mindfulness: Awareness | 2.99 (0.62) | 3.04 (0.71) | 3.20 (0.53) | 3.20 (0.76) | 3.15 (0.73) | 3.25 (0.75) |
| 3-Day Adherence | 72.2% | 70.0% | 67.7% | 70.6% | 73.5% | 75.7% |
| 30-Day Adherence | 25.0% | 37.5% | 13.3% | 14.7% | 11.8% | 16.2% |
ART = Anti-retroviral therapy.
Reported statistics are means (standard deviations), except for 3-day adherence and 30-day adherence, which are the percentage of respondents reporting 100% adherence.
One participant was missing a depression score and a second participant was missing a perceived stress score at 3 months, which resulted in n = 30 each for depression and perceived stress in the control group at 3 months. One participant in the MBSR group was missing a depression score at 6 months, resulting in n = 36 for depression in the MBSR group at the 6-month follow-up measurement point.
Acknowledgments
This study was funded by grant R21 AT003102 from the National Center for Complementary and Alternative Medicine of the National Institutes of Health (NIH). The first author’s role in preparing this paper was supported by NIH grant NCCAM K01 AT005270.
We would like to thank Eunice Stephens and Joey Taylor for project management, the fantastic recruiters, interviewers, facilitators and support staff who carried out the study, and, most of all. the men and women with HIV who participated in the research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this paper were presented by the senior author at the New Developments in the Psychology of Illness meeting held in the Yasawa Islands, Fiji, 2009.
Disclosures
The authors declare no conflicts of interest.
References
- 1.Johnson MO, Charlebois E, Morin SF, et al. Perceived adverse effects of antiretroviral therapy. J Pain Symptom Manage. 2005;29:193–205. doi: 10.1016/j.jpainsymman.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Volberding PA. HIV therapy in 2003: consensus and controversy. AIDS. 2003;17 Suppl 1:S4–S11. doi: 10.1097/00002030-200304001-00002. [DOI] [PubMed] [Google Scholar]
- 3.Johnson SC, Gerber JG. Advances in HIV/AIDS therapy. Adv Intern Med. 2000;45(1):1–40. [PubMed] [Google Scholar]
- 4.Treisman GJ, Kaplin AI. Neurologic and psychiatric complications of antiretroviral agents. AIDS. 2002;16(9):1201–1215. doi: 10.1097/00002030-200206140-00002. [DOI] [PubMed] [Google Scholar]
- 5.Chene G, May M, Costagliola D, et al. Prognosis of HIV-1 infected drug naï ve patients starting potent antiretroviral therapy. ART Cohort Collaboration. Paper presented at the XIV International AIDS Conference; Barcelona, Spain. 2002. [Google Scholar]
- 6.Ammassari A, Murri R, Pezzotti P, et al. Self reported symptoms and medication side effects influence adherence to highly active antiretroviral therapy in persons with HIV infection. J Acquir Immune Defic Syndr. 2001;28:445–449. doi: 10.1097/00042560-200112150-00006. [DOI] [PubMed] [Google Scholar]
- 7.Chesney MA. Factors affecting adherence to antiretroviral therapy. Clin Infect Dis. 2000;30(3) Suppl 2:S171–S176. doi: 10.1086/313849. [DOI] [PubMed] [Google Scholar]
- 8.Wills CE, Moore CF. Judgment processes for medication acceptance: self-reports and configural information use. Med Decis Making. 1994;14(2):137–145. doi: 10.1177/0272989X9401400206. [DOI] [PubMed] [Google Scholar]
- 9.Johnson MO, Chesney MA, Neilands TB, et al. NIMH Healthy Living Project Team. Disparities in reported reasons for not initiating or stopping antiretroviral treatment among a diverse sample of persons living with HIV. J Gen Int Med. 2009;24(2):247–251. doi: 10.1007/s11606-008-0854-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the selfregulation of chronic pain. J Behav Med. 1985;8(2):163–190. doi: 10.1007/BF00845519. [DOI] [PubMed] [Google Scholar]
- 11.Kabat-Zinn J, Massion AO, Kristeller J, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry. 1992;149(7):936–943. doi: 10.1176/ajp.149.7.936. [DOI] [PubMed] [Google Scholar]
- 12.Kabat-Zinn J. Full catastrophe living: Using the wisdom of your mind to face stress, pain and illness. New York: Dell Publishing; 1990. [Google Scholar]
- 13.Kabat-Zinn J. Mindfulness-based interventions in context: past, present, and future. Clin Psychol Sci Prac. 2003;10:144–156. [Google Scholar]
- 14.Bishop SR. What do we really know about mindfulness-based stress reduction? Psychosom Med. 2002;64(1):71–83. doi: 10.1097/00006842-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. J Psychosom Res. 2004;57(1):35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- 16.Carmody J, Baer RA. Relationships between mindfulness practice and levels of mindfulness, medical and psychological symptoms and well-being in a mindfulness-based stress reduction program. J Behav Med. 2008;31(1):23–33. doi: 10.1007/s10865-007-9130-7. [DOI] [PubMed] [Google Scholar]
- 17.Williams KA, Kolar MM, Reger BE, Pearson JC. Evaluation of a wellness-based mindfulness stress reduction intervention: a controlled trial. Am J Health Promot. 2001;15(6):422–432. doi: 10.4278/0890-1171-15.6.422. [DOI] [PubMed] [Google Scholar]
- 18.Morone NE, Greco CM, Weiner DK. Mindfulness meditation for the treatment of chronic low back pain in older adults: a randomized controlled pilot study. Pain. 2008;134(3):310–319. doi: 10.1016/j.pain.2007.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grossman P, Tiefenthaler-Gilmer U, Raysz A, Kesper U. Mindfulness training as an intervention for fibromyalgia: evidence of postintervention and 3-year follow-up benefits in well-being. Psychother Psychosom. 2007;76(4):226–233. doi: 10.1159/000101501. [DOI] [PubMed] [Google Scholar]
- 20.Johnson MO, Stallworth T, Neilands TB. The drugs or the disease? Causal attributions of symptoms held by HIV positive adults on HAART. AIDS Behav. 2003;7(2):109–117. doi: 10.1023/a:1023938023005. [DOI] [PubMed] [Google Scholar]
- 21.Lessler JT, Caspar RA, Penne MA, Barker PR. Developing computer assisted interviewing (CAI) for the National Household Survey on Drug Abuse. J Drug Issues. 2000;30(1):9–34. [Google Scholar]
- 22.Gribble JN, Miller HG, Rogers SM, Turner CF. Interview mode and measurement of sexual behaviors: methodological issues. J Sex Res. 1999;36(1):16–24. doi: 10.1080/00224499909551963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bangsberg DR, Bronstone A, Hofmann R. A computer-based assessment detects regimen misunderstandings and nonadherence for patients on HIV antiretroviral therapy. AIDS Care. 2002;14(1):3–15. doi: 10.1080/09540120220097892. [DOI] [PubMed] [Google Scholar]
- 24.Kalichman SC, Rompa D, Cage M. Reliability and validity of self-reported CD4 lymphocyte count and viral load test results in people living with HIV/AIDS. Int J STD AIDS. 2000;11(9):579–585. doi: 10.1258/0956462001916551. [DOI] [PubMed] [Google Scholar]
- 25.Johnson MO, Stallworth T, Neilands TB. The drugs or the disease? Causal Beliefs of Physical Problems Held by HIV Positive Adults on HAART. AIDS and Behavior. 2003;7(2):109–117. doi: 10.1023/a:1023938023005. [DOI] [PubMed] [Google Scholar]
- 26.Johnson MO, Dilworth SE, Taylor J, Neilands TB. Improving coping skills for selfmanagement of treatment side effects can reduce antiretroviral medication nonadherence among people living with HIV. Ann Behav Med. 2011;41(1):83–91. doi: 10.1007/s12160-010-9230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson MO, Folkman S. Side effect and disease related symptom representations among HIV+ adults on antiretroviral therapy. Psychology, Health – Medicine. 2004;9(2):139–148. [Google Scholar]
- 28.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12(3):255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 29.Chesney MA, Ickovics J. Adherence to combination therapy in AIDS clinical trials. Paper presented at Annual Meeting of the AIDS Clinical Trials Group; Washington, DC.1997. [Google Scholar]
- 30.Hecht FM, Colfax G, Swanson M, Chesney M. Adherence and effectiveness of protease inhibitors in clinical practice [abstract]. Proceedings of the 5th Conference on Retroviruses and Opportunistic Infections; Chicago, IL; 1998. Abstract 151. [Google Scholar]
- 31.Walsh JC, Pozniak AL, Nelson MR, Mandalia S, Gazzard BG. Virologic rebound on HAART in the context of low treatment adherence is associated with a low prevalence of antiretroviral drug resistance. J Acquir Immune Defic Syndr. 2002;30:278–287. doi: 10.1097/00126334-200207010-00003. [DOI] [PubMed] [Google Scholar]
- 32.Walsh JC, Mandalia S, Gazzard BG. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS. 2002;16:269–277. doi: 10.1097/00002030-200201250-00017. [DOI] [PubMed] [Google Scholar]
- 33.Oyugi JH, Byakika-Tusiime J, Charlebois ED, et al. Multiple validated measures of adherence indicate high levels of adherence to generic HIV antiretroviral therapy in a resource-limited setting. J Acquir Immune Defic Syndr. 2004;36:1100–1102. doi: 10.1097/00126334-200408150-00014. [DOI] [PubMed] [Google Scholar]
- 34.Lu M, Safren SA, Skolnik PR, et al. Optimal recall period and response task for self-reported HIV medication adherence. AIDS Behav. 2008;12:86–94. doi: 10.1007/s10461-007-9261-4. [DOI] [PubMed] [Google Scholar]
- 35.Beck AT. Depression: Causes and treatment. Philadelphia, PA: University of Pennsylvania Press; 1967. [Google Scholar]
- 36.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 37.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 38.Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13:27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- 39.Vittinghoff E, Glidden D, Shiboski S, McCulloch CE. Regression methods in biostatistics. New York: Springer; 2005. [Google Scholar]
- 40.Little RJA, Rubin DB. Statistical analysis with missing data. New York: John Wiley and Sons; [Google Scholar]
- 41.Mathews WC, McCutchan JA, Asch S, et al. National estimates of HIV-related symptom prevalence from the HIV Cost and Services Utilization Study. Med Care. 2000;38(7):750–762. doi: 10.1097/00005650-200007000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Vogl D, Rosenfeld B, Breitbart W, et al. Symptom prevalence, characteristics, and distress in AIDS outpatients. J Pain Symptom Manage. 1999;18(4):253–262. doi: 10.1016/s0885-3924(99)00066-4. [DOI] [PubMed] [Google Scholar]
- 43.Siegel K, Dean L, Schrimshaw EW. Symptom ambiguity among late-middle-aged and older adults with HIV. Res Aging. 1999;21(4):595–618. [Google Scholar]
- 44.Siegel K, Schrimshaw EW, Dean L. Symptom interpretation and medication adherence among late middle-age and older HIV-infected adults. J Health Psychol. 1999;4(2):247–257. doi: 10.1177/135910539900400217. [DOI] [PubMed] [Google Scholar]
- 45.Siegel K, Schrimshaw EW, Dean L. Symptom interpretation: implications for delay in HIV testing and care among HIV-infected late middle-aged and older adults. AIDS Care. 1999;11(5):525–535. doi: 10.1080/09540129947686. [DOI] [PubMed] [Google Scholar]
- 46.Klatt MD, Buckworth J, Malarkey WB. Effects of low-dose mindfulness-based stress reduction (MBSR-ld) on working adults. Health Educ Behav. 2009;36(3):601–614. doi: 10.1177/1090198108317627. [DOI] [PubMed] [Google Scholar]