Abstract
Data from laboratory studies, observational research, and/or secondary prevention trials suggest that vitamin D and marine omega-3 fatty acids may reduce risk for cancer or cardiovascular disease (CVD), but primary prevention trials with adequate dosing in general populations (i.e., unselected for disease risk) are lacking. The ongoing VITamin D and OmegA-3 TriaL (VITAL) is a large randomized, double-blind, placebo-controlled, 2×2 factorial trial of vitamin D (in the form of vitamin D3 [cholecalciferol], 2000 IU/day) and marine omega-3 fatty acid (Omacor® fish oil, eicosapentaenoic acid [EPA] + docosahexaenoic acid [DHA], 1 g/day) supplements in the primary prevention of cancer and CVD among a multi-ethnic population of 20,000 U.S. men aged ≥50 and women aged ≥55. The mean treatment period will be 5 years. Baseline blood samples will be collected in at least 16,000 participants, with follow-up blood collection in about 6000 participants. Yearly follow-up questionnaires will assess treatment compliance (plasma biomarker measures will also assess compliance in a random sample of participants), use of non-study drugs or supplements, occurence of endpoints, and cancer and vascular risk factors. Self-reported endpoints will be confirmed by medical record review by physicians blinded to treatment assignment, and deaths will be ascertained through national registries and other sources. Ancillary studies will investigate whether these agents affect risk for diabetes and glucose intolerance; hypertension; cognitive decline; depression; osteoporosis and fracture; physical disability and falls; asthma and other respiratory diseases; infections; rheumatoid arthritis, systemic lupus erythematosus, thyroid diseases, and other autoimmune disorders.
Keywords: Cancer, cardiovascular disease, cholecalciferol, primary prevention, omega-3 fatty acids, vitamin D, randomized controlled trial
1. Introduction
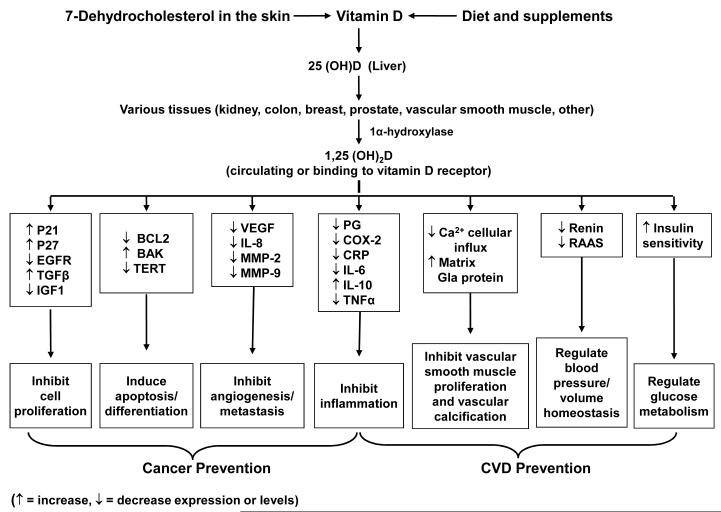
Although there have been marked advances in our understanding of the potential role of vitamin D and omega-3 fatty acids in the prevention of cancer and cardiovascular disease (CVD) in recent years, clear gaps in knowledge remain. Data from laboratory studies [1-3], ecologic studies [4-7], epidemiologic investigations [8-19], and secondary analyses of small randomized trials [20-23] suggest a protective effect for vitamin D against cancer and CVD. Mechanisms by which vitamin D may prevent these diseases [1-3] are shown in Figure 1. The vitamin D receptor is expressed in most tissues. Vitamin D may promote cell differentiation, inhibit cancer-cell proliferation, and exhibit anti-inflammatory, proapototic, and antiangiogenic properties; it may also inhibit vascular smooth muscle proliferation and vascular calcification and control blood pressure and glucose metabolism. One large trial—the Women’s Health Initiative calcium-vitamin D trial, in which 36,282 postmenopausal women were randomly assigned to a daily combination of calcium (1000 mg) and low-dose vitamin D3 (400 IU) or to placebo and followed for a mean of 7 years—found that the intervention did not reduce risk for cancer, CHD, or stroke [24, 25], but its effect on blood levels of 25-hydroxyvitamin D [25(OH)D], the major circulating vitamin D metabolite, was small [8]. However, there are no large randomized trials of supplemental vitamin D in doses adequate to produce meaningful changes in 25(OH)D levels or designed to assess cancer or CVD as primary prespecified outcomes. Although there is a lack of consensus on the definition and prevalence of vitamin D insufficiency in the United States [26, 27], it is concerning that some estimates suggest that >1/2 of middle-aged and older women and >1/3 of similarly aged men have such insufficiency [28, 29]. African-American (black) individuals are particularly vulnerable, in part because darkly pigmented skin is less able to synthesize vitamin D in response to solar radiation and because blacks tend to have lower dietary and supplemental vitamin D intakes than whites [30, 31]. Obese individuals are also at above-average risk, presumably because of decreased bioavailability of this fat-soluble vitamin [32, 33]. Given the aging population and soaring obesity prevalence [34], low vitamin D status is an increasingly important public health issue.
Figure 1.
Mechanisms by which vitamin D may lower cancer and cardiovascular risk. COX-2, cyclooxygenase-2; CRP, C-reactive protein; EFGR, epidermal growth factor receptor; IGF-1, insulin-like growth factor-1; IL-6, interleukin-6; IL-8, interleukin-8; IL-10, interleukin-10; MMP-9, matrix metalloproteinase-9; PG, prostaglandin; RAAS, renin-angiotensin-aldosterone system; TERT, telomerase reverse transcriptase; TGFß, transforming growth factor-ß; TNFα, tumor necrosis factor-α; VEGF, vascular endothelial growth factor.
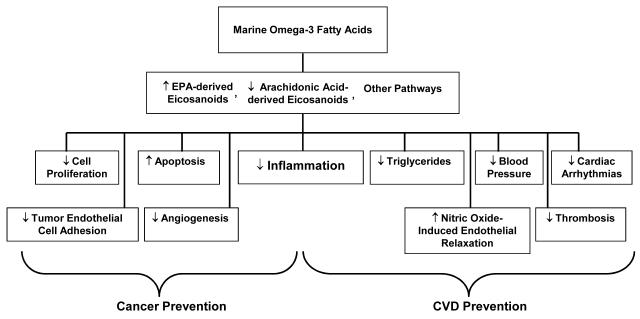
The marine omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which are found in fish and fish-oil supplements, have shown considerable promise for the prevention of CVD in laboratory [35-41] and observational studies [42-46]; large randomized trials in secondary prevention [47] or high-risk settings [48] have also found benefit. Data on marine omega-3 fatty acids for cancer prevention have been suggestive but inconsistent [48-53]. Mechanisms by which marine omega-3 fatty acids may reduce the risk for CVD [35-41] and cancer [54] are shown in Figure 2. However, there are no trials of marine omega-3 fatty acid supplements for the primary prevention of these diseases in a general population that has been selected only on the basis of age and not on vascular risk factors such as diabetes or dyslipidemia. It is important to clarify these relationships.
Figure 2.
Mechanisms by which marine omega-3 fatty acids may lower cancer and cardiovascular risk.
2. Materials and Methods
2.1 Overview of study design
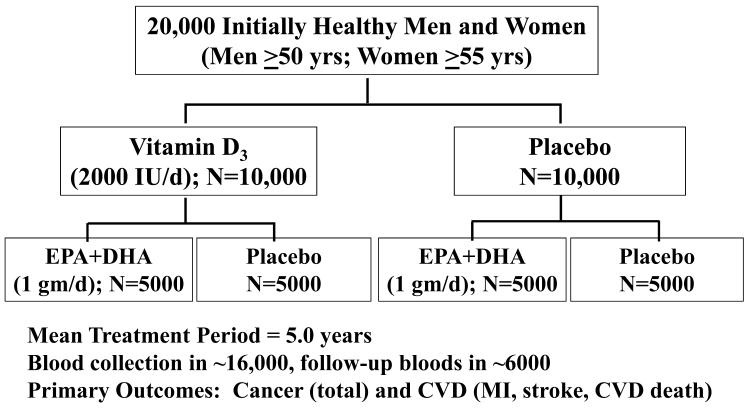
To address the role of vitamin D and marine omega-3 fatty acids in the primary prevention of cancer and CVD, we are conducting the VITamin D and OmegA-3 TriaL (VITAL), a randomized, double-blind, placebo-controlled clinical trial among 20,000 U.S. men and women without cancer or CVD at baseline, who are selected on age only (men aged ≥50 and women aged ≥55), with an oversampling of blacks. In a 2×2 factorial design, participants will be randomized to vitamin D3 (cholecalciferol; 2000 IU/day) and marine omega-3 fatty acids (Omacor® fish oil, EPA + DHA, 1 g/d) supplements (or placebos) independently. The mean treatment period will be 5 years. Baseline blood samples will be collected in at least 80% of participants (n=16,000), with follow-up blood collection in about 6000 participants. Yearly follow-up questionnaires will assess treatment compliance (plasma biomarker measures will also assess compliance in a random sample of participants), use of non-study drugs or supplements, occurence of endpoints, and cancer and vascular risk factors. Self-reported endpoints will be confirmed by medical record review by a committee of physicians blinded to treatment assignment and deaths will be ascertained through the National Death Index-Plus and other sources. A summary of the study design is provided in Figure 3.
Figure 3.
The VITamin D and OmegA-3 TriaL (VITAL) design.
2.2 Aims
The primary aims of the trial are to test whether vitamin D3 or marine omega-3 fatty acid supplementation reduces the risk for total cancer and major CVD events (a composite endpoint of myocardial infarction [MI], stroke, and cardiovascular mortality). The secondary aims are to test whether vitamin D3 or marine omega-3 fatty acid supplementation reduces the risk for site-specific cancers, including incident colorectal cancer, breast cancer (in women), and prostate cancer (in men); total cancer mortality; an expanded composite cardiovascular endpoint of MI, stroke, cardiovascular mortality, and coronary revascularization (coronary artery bypass grafting [CABG] or percutaneous coronary intervention [PCI]); and the individual components of the primary cardiovascular endpoint, particularly total CVD mortality. The tertiary aims are to explore whether vitamin D3 and marine omega-3 fatty acid supplementation exhibit synergistic or additive effects on the risk for total cancer, major CVD events, and the secondary endpoints specified above, and to explore whether the effect of vitamin D3 or marine omega-3 fatty acid supplementation on cancer and CVD risk varies by baseline blood levels of these nutrients, race/skin pigmentation (for vitamin D3), and body mass index (BMI) (for vitamin D3). Blacks are at higher risk of vitamin D deficiency and are also at higher risk for certain cancers (e.g., prostate cancer) [55] and cardiovascular events (e.g., stroke) [56], as well as mortality from cancer [55] and CVD [56], so it is critical to test the effect of vitamin D supplementation in this group.
2.3 Sponsors
The primary sponsor of VITAL is the National Cancer Institute, and the secondary sponsor is the National Heart, Lung and Blood Institute. The Office of Dietary Supplements, the National Institute of Neurologic Disorders and Stroke, and the National Center for Complementary and Alternative Medicine are also cosponsors of the study. Several other NIH institutes are sponsors of VITAL ancillary studies. Pharmavite LLC of Northridge, California (vitamin D3) and Pronova BioPharma of Norway (Omacor® fish oil) are donating the agents, matching placebos, and packaging in the form of calendar packs. Because the trial (with the exception of some ancillary studies [Section 2.12]) is being conducted by mail and is utilizing a 2×2 factorial design to test the independent and synergistic effects of two promising interventions, it is extremely cost effective. VITAL has been approved by the Institutional Review Board of Partners Healthcare/Brigham and Women’s Hospital, and the study agents have received Investigational New Drug Approval from the U.S. Food and Drug Administration. VITAL is registered at clinicaltrials.gov (NCT01169259), and the study website is www.vitalstudy.org.
2.4 Interventions
2.4.1 Vitamin D supplement
VITAL is testing a vitamin D3 dose of 2000 IU/day. A careful review of the literature suggests that this dose provides the best balance of efficacy and safety.
Efficacy
We seek to obtain a large-enough difference in vitamin D status between the treatment and placebo groups to detect benefits for the primary endpoints of cancer and CVD. VITAL was designed in 2008, when the recommended dietary allowances (RDA), which are set by the Institute of Medicine (IOM), were 400 IU/day for adults aged 50-70 and 600 IU/day for adults aged >70 [57]. In 2011, the IOM raised the RDAs for these age groups to 600 IU/day and 800 IU/day, respectively [26]. These RDAs correspond to a serum 25(OH)D level of 50 nmol/L and are sufficient for the maintenance of bone health in at least 97.5% of the North American population. Nevertheless, accumulating data suggest that vitamin D intakes above these RDAs may be necessary for maximal health benefits. In a review of studies of serum 25(OH)D in relation to various outcomes, including colorectal cancer, falls, fractures, physical functioning, and dental health, Bischoff-Ferrari et al. [58] found that advantageous 25(OH)D levels began at 75 nmol/L, and optimal levels were between 90-100 nmol/L. The average older individual requires an oral vitamin D3 intake of at least 800-1000 IU/day to achieve a serum 25(OH)D of 75 nmol/L [59]. Among postmenopausal women in the Women’s Health Initiative (a population similar to that of VITAL), 400 IU/day of vitamin D3 was estimated to have raised median plasma 25(OH)D from 42.3 to only 54.1 nmol/L [8, 24]. Also, a study by Aloia et al. [60] showed a nonlinear dose-response relation between serum 25(OH)D and vitamin D intake, with the rate of increase in serum levels slowing at higher levels of intake. Extrapolation of the Women’s Health Initiative data, along with consideration of the Aloia et al. findings, suggest that 2000 IU of vitamin D3 would be required to reach the postulated optimal value of 90 nmol/L in the active vitamin D group in VITAL. The difference in achieved 25(OH)D levels between the active treatment and placebo groups is expected to be approximately 50 nmol/L.
Safety
The vitamin D study pills have undergone extensive quality control testing for stability of nutrient content and other parameters at a range of temperatures and humidity levels. There appear to be few safety issues associated with the selected treatment dose of 2000 IU/day. Because we are excluding from the trial persons who report supplemental vitamin D intakes of more than 800 IU/day (see Section 2.5) and we can estimate an average of 200 IU/day from diet [61], few if any participants assigned to the active vitamin D group will be consuming a dose above 3000 IU/day, which is well below the tolerable upper intake level of 4000 IU/day set by the IOM (26) and the no-observed-adverse-effect level of 4000 IU/day specified by the European Commission Scientific Committee on Food [62]. Moreover, participants assigned to the placebo group should not become vitamin D-deficient because we are allowing background intake at RDA levels. Potential side effects of vitamin D are rare and include gastrointestinal (GI) symptoms (nausea, constipation, or diarrhea), hypercalcemia, and kidney stones. To minimize risk for the latter two outcomes, we are requiring that participants limit calcium intake from supplemental sources to 1200 mg/day, and we are excluding from the trial persons with a history of hypercalcemia or sarcoidosis. As a further safety precaution, blood levels of calcium, parathyroid hormone, and kidney function will be monitored in a random subsample of participants.
Exclusion of calcium from the intervention
We have not included calcium as a component of the intervention, for several reasons. First, to test the effects of vitamin D alone, calcium alone, and calcium-plus-vitamin D would require a factorial design with a much larger sample size and a much higher cost than a trial of vitamin D alone. Second, the Women’s Health Initiative calcium-vitamin D trial reported a statistically significant 17% increase in the risk for kidney stones with combined supplementation [24]. Third, supplemental calcium (calcium citrate, 1 g/day) was associated with a significant doubling in risk for MI and a borderline significant 47% increase in risk for major CVD events in a 5-year trial among 1471 initially healthy older women [63]. Fourth, the high prevalence of calcium supplement use in women [64] would reduce the pool of eligible female participants. Finally, some studies have reported a direct association between intake of calcium or milk and incidence of prostate cancer [65-67].
2.4.2 Marine omega-3 fatty acid supplement
VITAL is testing a total marine omega-3 fatty acid dose of 1 g/day (EPA+DHA, in the ratio of 1.3 to 1). A careful review of the literature suggests that a total dose of 1 g/day provides the best balance of efficacy and safety.
Efficacy
We seek to obtain a large-enough difference in omega-3 fatty acid status between the treatment and placebo groups to detect health benefits. Health authorities’ recommendations vary from 400 mg to 1 g/day for cardioprotection [61]. For VITAL, we selected a total dose of marine omega-3 fatty acids of 1 g/day, which is recommended by the American Heart Association (AHA) and was demonstrated to be beneficial, with minimal side effects, in a large secondary prevention trial [47]. Because the optimal ratio of EPA to DHA is unknown [39, 68], we chose an EPA-to-DHA ratio close to 1-to-1, specifically 1.3-to-1. The 1 g/day dose in a single capsule and 1.3-to-1 ratio is available in an FDA-approved product (Omacor®). On a related note, the ratio of omega-3 to omega-6 fatty acid intake may also be important for disease prevention. This ratio is between 1:10 and 1:20 in most Western countries, including the U.S., whereas the optimal ratio has been hypothesized to be closer to 1:1 or 1:2 [69, 70], although this is controversial [71]. Indeed, there is growing consensus that the absolute intake of omega-3 is a more important predictor of health than is the ratio of omega-3 to omega-6 intake, at least for cardiovascular outcomes [71-74]. However, given that the average intake of EPA+DHA is 100-200 mg/day among U.S. adults [61], the intervention of 1 g/day is expected to increase the average participant’s omega-3 intake by a factor of 5 to 10. Assuming no concurrent change in omega-6 intake, the omega-3 dose would thus have the effect of achieving the purported optimal omega-3 to omega-6 ratio and providing intakes associated with benefits in previous studies. Safety: The chosen omega-3 fatty acid supplement, Omacor® fish oil, has undergone an extensive purification process and is free of environmental toxins (e.g., methylmercury, polychlorinated biphenyls [PCBs], and dioxins) found in some fish. It has also undergone extensive quality control testing for stability of nutrient content and other parameters at a range of temperatures and humidity levels. Omacor® contains no vitamin D, ensuring that participants are not given higher vitamin D doses than intended and that VITAL has the ability to test the separate effects of the two agents (vitamin D and marine omega-3 fatty acids) under study.
There appear to be few safety issues associated with the selected treatment dose of 1 g/day. The FDA has concluded that marine omega-3 fatty acid doses of up to 3 g/day are “Generally Recognized as Safe” [75]. Although omega-3 fatty acids have potential antithrombotic effects, systematic reviews of data from small trials suggest that omega-3 fatty acid supplements at doses of up to 4 g/day do not increase the risk of clinically significant bleeding, even in combination with anticoagulant medications such as aspirin or warfarin [76, 77]. One large trial [48] did report an increase in bleeding events with 1.8 g/day of EPA (1.1%) as compared with placebo (0.6%; p=0.0006), but this dose is higher than that being tested in VITAL. Other concerns include a fishy aftertaste and GI disturbance (e.g., nausea or diarrhea), which may contribute to patient intolerance [35, 77]. In addition, some evidence suggests that fish-oil supplements may worsen glycemia in patients with impaired glucose tolerance and increase LDL cholesterol in patients with hypertriglyceridemia [35]. However, the AHA has concluded that these risks are very low or low at doses of up to 1 g/day and low to moderate at doses of 1-3 g/day [35]. Ancillary studies will provide opportunities to assess such effects.
2.5 Trial eligibility
VITAL will be conducted among 20,000 apparently healthy adults—10,000 of whom are men aged ≥50 and 10,000 of whom are women aged ≥55, ages at which chronic disease rates increase substantially. Participants are being recruited throughout the United States, and blacks are being oversampled (our goal is a study population that is 25% black). As this is a primary prevention trial, participants are required to have no history of cancer (except non-melanoma skin cancer), MI, stroke, transient ischemic attack (TIA), angina pectoris, or coronary revascularization (CABG or PCI). In addition, participants are required to limit consumption of supplemental vitamin D to no more than 800 IU/day from all supplemental sources combined (stand-alone vitamin D supplements, calcium+vitamin D supplements, medications containing vitamin D [e.g., Fosamax Plus D] and multivitamins), to limit consumption of supplemental calcium to no more than 1200 mg/day from all supplemental sources combined, and to forego the use of fish-oil supplements during the run-in and randomized treatment periods. Safety exclusions are as follows: renal failure or dialysis, hypercalcemia, hypo- or hyperparathyroidism, severe liver disease (cirrhosis), or sarcoidosis or other granulomatous diseases such as active chronic tuberculosis or Wegener’s granulomatosis; allergy to soy (which is in the vitamin D placebo pill) or fish (for the marine omega-3 fatty acid intervention); or other serious illness that would preclude participation. Participants who are willing to participate, as evidenced by signing the informed consent form and demonstrating good compliance in pill taking, defined as taking ≥ 2/3 of the study pills during the run-in period, are eligible for enrollment.
In a 2×2 factorial design, willing and eligible participants will be randomized to 5 years of vitamin D3 and marine omega-3 fatty acid supplements (or placebos) independently. Participants will be instructed to discontinue their study pills if, during follow-up, they receive a diagnosis of hypercalcemia, sarcoidosis, or other safety-exclusion condition specified above. However, all participants will be included in intention-to-treat analyses.
2.6 Recruitment and randomization of the study population
2.6.1 Source of participants
We are recruiting potential participants from a master mailing tape of names and addresses assembled from commercially available U.S. mailing lists of professional organizations (e.g., those for licensed health professionals and business professionals) and other organizations (e.g., AARP), as well as subscription lists of magazines that cater or appeal to individuals likely to be eligible for the trial. The master mailing tape includes mailing lists of professional organizations and magazines for black individuals. In addition, we are recruiting potential participants via direct appeals in articles and advertisements in newspapers and magazines, and we are also inviting participants in our previously completed trials [78-80] to consider participating in VITAL. We will also employ targeted recruitment efforts in the black community, including the creation and distribution of specialized information about the study supplements and the burden of cancer and CVD in blacks, to achieve our goal of a study population that is 25% black.
2.6.2 Enrollment
Potential participants receive the following materials by postal mail: (1) an invitational letter that explains the rationale for VITAL, outlines what participation entails, and provides sources for further information on relevant scientific issues; (2) an informed consent form; (3) brief questionnaires with items on demographics (age, gender, race/ethnicity, education, occupation, income); medical history (cancer, CVD, kidney stones, hypercalcemia, kidney failure, sarcoidosis, other major illnesses); allergy to fish or soy; current use of supplements containing vitamin D or fish oil; current use of other supplements or medications; dietary intake of vitamin D and consumption of fish; cancer and vascular risk factors (e.g., smoking, height, weight, blood pressure, cholesterol, diabetes, alcohol use, physical activity, and family history of cancer and CVD); and potential effect modifiers such as skin pigmentation and sunlight exposure; and (4) pre-paid envelopes for returning study forms. Questionnaire responses will be evaluated to determine respondents’ eligibility for the trial. Our goal is to identify 40,000 individuals who are willing and eligible to enter the run-in phase of the trial.
2.6.3. Run-in
Prior experience in the conduct of large randomized trials has demonstrated the utility of a run-in period for selecting excellent compliers for long-term follow-up, which increases the trial’s power [78, 80-82]. In VITAL, there will be a 3-month run-in during which all participants will take one placebo vitamin D pill and one placebo fish-oil pill per day. It would not be scientifically appropriate to use active agent in the run-in and then randomize to placebo because some effects of the interventions on cancer risk may be chronic. A placebo-only run-in also permits the clearest detection of any side effects during the randomized treatment period. If the active intervention were to be used during the run-in, potential participants may drop out not only because of poor compliance but also because of side effects of treatment, and thus the true rate of side effects may be underestimated among those ultimately randomized into the trial. For ease of pill-taking, study pills will be packaged in 31-day calendar packs (2 pills per day).
2.6.4. Randomization
Initially willing and eligible participants will be randomized into the trial if they (1) demonstrate good compliance in pill taking, defined as taking ≥2/3 of the study pills during the run-in; (2) report no new history of cancer (except non-melanoma skin cancer), MI, stroke, TIA, angina pectoris, CABG, PCI, hypercalcemia, sarcoidosis, or other serious illness during the run-in; and (3) remain willing to comply with limits on non-study use of supplemental vitamin D and calcium and fish oil (Section 2.5). We estimate that 50% of the 40,000 individuals (n=20,000) who enter the run-in will comply with pill-taking and remain willing and eligible for randomization. Within 5-year age groups, randomized treatment assignments (using a computer-generated table of random numbers) will be made in blocks of eight individuals, with two individuals in each of the four treatment combinations. The use of age stratification during randomization ensures balance and increases statistical efficiency.
2.6.5. Ethnicity/race of study population
The anticipated racial/ethnic distribution of the randomized study population is as follows: 5000 (25%) non-Hispanic black, 1400 (7%) Hispanic, 500 (2.5%) Asian, 400 (2%) American Indian, 80 (0.4%) Pacific Islander, and 12,620 (63.1%) non-Hispanic white participants.
2.7 Blood collection and assays
2.7.1 Blood collection
We will collect fasting blood samples at baseline (i.e., during the run-in, prior to randomization) from as many participants as are willing to provide them (expected response rate is 80%, or n=16,000). Fasting blood samples will also be collected at trial years 1, 2, and 4 from a randomly selected subset of about 6000 participants who provide baseline samples. The main reason for the baseline blood collection is to assess whether treatment effects are modified by baseline blood levels of 25(OH)D (for vitamin D) and EPA+DHA (for the marine omega-3 fatty acids). The main reasons for the follow-up blood collection are to assess (a) pill-taking compliance, (b) changes in biomarkers with treatment, and (c) in the placebo group, the effect of changing trends in background intakes of vitamin D and marine omega-3 fatty acids. The follow-up blood samples will be particularly important for determining how 25(OH)D levels change in response to vitamin D supplementation in black individuals, an understudied area of investigation [83]. In addition, changes in blood calcium and parathyroid hormone levels will be measured to assess possible hypercalcemia, a potential side effect of high vitamin D intake. The blood samples will also be used to evaluate whether the interventions affect biomarkers related to lipids, glucose tolerance, inflammation, endothelial dysfunction, thrombosis, insulin, and insulin-like growth factor pathways. Finally, the samples will allow for future explorations of other biochemical and genetic hypotheses in a well-characterized cohort.
During the run-in period, participants will be mailed a blood collection kit, including a gel-filled freezer pack and overnight courier air bill. We anticipate that most participants will have their blood drawn by their own healthcare provider or at a local blood-drawing facility. Some participants will have their blood drawn in their homes by a company that provides phlebotomy services. Participants will be instructed to return the fasting blood sample to our blood laboratory in the freezer pack within 24 hours of venipuncture. Upon receipt, the samples will be centrifuged to separate plasma, serum, red blood cells, and buffy coat; these components will be stored in nitrogen freezers (−170 °C) within 30-36 hours of venipuncture. Identical procedures will be used for the follow-up blood collection.
2.7.2 Blood assays
Baseline and follow-up blood levels of 25(OH)D and EPA+DHA, as well as calcium and parathyroid hormone, will be assayed in a subset of participants who provide a sample at baseline and all participants who provide a blood sample at follow-up. The biochemical assays will be performed by established laboratories with extensive experience in clinical chemistry and the conduct of these assays.
Serum 25(OH)D
Measurement of serum 25(OH)D will be performed at the Clinical and Translational Science Center (CTSC) laboratory at Harvard. Circulating 25(OH)D will determined by radioimmunoassay [84, 85], using reagents from the DiaSorin Corporation (Stillwater, NM) that recognize and quantify 25(OH)D2 and 25(OH)D3 equally. The intra- and interassay coefficients of variation (CV) in the ranges expected are <10%. The VITAL study will participate in the National Institute of Standards and Technology/Office of Dietary Supplements quality assurance program for measurement of 25(OH)D2 and 25(OH)D3 in human blood samples (86).
Omega-3 fatty acids (EPA and DHA) in red blood cells
Measurement of omega-3 fatty acids in red blood cells (RBC) will be performed by Dr. William Harris at the University of South Dakota. RBC samples will be analyzed by gas chromatography. The CV for EPA+DHA as a percent of total RBC fatty acids (our metric of primary interest) is 5.0% for a mean value of 10.9% (SD 0.5%) and 5.3% for a mean value of 3.8% (SD 0.2%).
Calcium, parathyroid hormone, phosphorus
Measurement of calcium, parathyroid hormone, and phosphorus will be performed in the Harvard CTSC laboratory. All CVs are <10%.
2.8 Assessment of dietary and non-study supplemental intakes of vitamin D and marine omega-3 fatty acids
Classifying participants by background intakes—at baseline and during the course of the trial—of vitamin D, marine omega-3 fatty acids, and other nutrients will allow an evaluation of whether the study agents’ effects are influenced by these variables. For example, it may be that partipants with the lowest baseline intakes of vitamin D will benefit the most from the vitamin D intervention. Participants will be asked to complete a self-administered semi-quantitative food frequency questionnaire (FFQ) at baseline, 2 years, and at trial’s end. This questionnaire is an efficient, reliable, and accurate instrument for categorizing individuals’ nutrient intake, including intake of vitamin D and marine omega-3 fatty acids [87-92]. Respondents estimate their average intake over the past year of various foods, beverages, and supplements that contain vitamin D, marine omega-3 fatty acids, and other nutrients. Additional questions on use of non-study supplements or drugs containing vitamin D or marine omega-3 fatty acids will be asked at baseline, 6 months, and on yearly follow-up questionnaires. We will ascertain and analyze nutrient intakes from food alone, supplements alone, and the two sources combined, to determine whether the interventions’ effects vary according to these variables.
2.9 Follow-up and endpoint determination procedures
The primary method of follow-up will be mailed questionnaires and review of medical records to confirm study endpoints. Participants will receive follow-up questionnaires at 6 months and 1 year after randomization and annually thereafter. The questionnaires include items on compliance with randomized treatments, use of nonstudy supplements of vitamin D and marine omega-3 fatty acids, dietary intakes of vitamin D and fish, development of major illnesses, risk factors for cancer and CVD, and potential side effects of the study agents. For vitamin D, side effects include GI symptoms and physician diagnosis of hypercalcemia or kidney stones. For marine omega-3 fatty acids, side effects include GI upset or bleeding, skin eruptions, and physician diagnosis of atrial fibrillation or other irregular rhythms. Non-responders will receive two additional requests by mail and then be telephoned to collect study data. At a minimum, vital status will be ascertained. At 6-month intervals between the annual follow-ups, participants will receive a short questionnaire limited to items on the development of primary endpoints (cancer, MI, and stroke), difficulties with pill compliance, and address changes. This will allow us to address compliance issues and collect medical records for endpoint confirmation in a timely fashion.
Participants who report a cancer or cardiovascular endpoint of interest will be asked to sign a medical release for relevant hospital and physician records. An Endpoints Committee of physicians who are blinded to the randomized treatment assignment will review the records to confirm or disconfirm the case by applying a defined protocol. Cancer diagnoses will be confirmed with histologic or cytologic data, or, if these are unavailable, strong clinical evidence accompanied by radiologic evidence or laboratory markers; the histologic type, grade, and stage of cancer will also be recorded [93]. MI will be confirmed using Joint European Society of Cardiology/American College of Cardiology Foundation/American Heart Association/World Heart Federation Task Force for the Redefinition of Myocardial Infarction criteria [94]. Stroke will be confirmed and categorized according to Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria [95]. Cardiovascular deaths will be confirmed by convincing evidence of a CVD event from all available sources, including death certificates, hospital records, autopsy reports, and, for deaths outside the hospital, observer accounts.
For deaths reported by family members, the next-of-kin will be asked to provide medical records and a copy of the death certificate. If the latter is not provided, a copy will be obtained from the state vital records bureau where the participant died. The Endpoints Committee will review the records and assign a cause of death. If records are not available (or if participants are lost to follow-up), we will search the National Death Index Plus (NDI-Plus) to obtain an Interational Classification of Disease-coded cause of death based on death-certificate information.
2.10 Assessment of compliance
The primary measure of compliance with pill-taking will be participants’ responses to questionnaire items asking about adherence. Our experience with large trials indicates that although most participants strive to take their pills as assigned, those who do not willingly admit not doing so. Thus, blood levels and self-reported adherence data have been strongly correlated in previous trials [96]. Nevertheless, to obtain an objective measure of compliance, we will visit a group of randomly sampled local participants (50 per year, or a total of 250 participants during the course of the trial) to draw an on-the-spot blood sample for determination of 25(OH)D and EPA+DHA levels. (A separate consent form, to be administered by the phlebotomist at the time of the draw, will be used for these blood collections.) The distribution of these values will be compared between the active treatment and placebo groups, and compared with the questionnaire data on adherence, as a check on the validity of the latter. In addition, during years 1, 2, and 4 of the trial, about 6000 participants will provide follow-up blood samples to allow an assessment of changes in levels of 25(OH)D, EPA+DHA, and other biomarkers in the treatment group, as well as changes in the placebo group that may result from changes in background intakes (Section 2.8).
2.11 Analysis plan and statistical power
2.11.1 Analysis plan
Analyses of treatment effects will be based on the intention-to-treat principle. An initial analysis will compare baseline demographic, medical, and lifestyle characteristics of the study population by randomized treatment assignment to ensure that balance was achieved by the randomization. The large sample size and successful balance of known potential confounders will provide assurance that unmeasured or unknown potential confounders are also equally distributed across randomized treatment groups.
The primary analyses will compare the main effects of intention-to-treat with vitamin D and with marine omega-3 fatty acids (assigned independently in a 2×2 factorial design) on the cancer and CVD endpoints specified in Aims (Section 2.2). Use of the Cox proportional hazards model will allow for variable follow-up lengths [97] and estimation of hazard ratios for each intervention while adjusting for the second intervention, age, and gender. Because the cohort will consist of older individuals, competing risks due to deaths from other causes will be considered. This will be done by estimating the cause-specific hazard and the hazard ratio comparing intervention groups for each outcome of interest by censoring individuals with deaths due to competing causes. To estimate the cumulative incidence function, the subdistribution of each endpoint will be plotted over time [98, 99]. Although we will consider the alternative Fine and Gray model [100], the proportional hazards approach will be our primary analysis.
We will also explore interactions between the vitamin D and the omega-3 fatty acid intervention; between the vitamin D intervention and baseline serum levels of 25(OH)D; and between the the marine omega-3 fatty acid intervention and baseline RBC levels of EPA+DHA. We hypothesize that the intervention effects may be larger among those with below-median baseline levels; we will examine treatment effects by quartiles of these biomarkers. Blood levels of 25(OH)D and EPA+DHA will be assayed in the final 2 years of the study in a case-cohort design on a subset of participants who provide a blood sample at baseline and all participants who provide one at follow-up. A case-cohort design allows an efficient and unbiased estimation of hazard ratios as well as absolute risks for individuals [101]. With this design, a common subcohort sample can serve as the reference risk set for more than one outcome—in this instance, total cancer and total CVD. Cancer and CVD cases will accrue during 5 years of follow-up; a subcohort sample that is approximately twice the size of the case sample for each outcome will be selected, stratifying by gender and baseline age (within 5-year groups) to frequency-match the distribution in the total case group. The biomarker data will be analyzed using proportional hazards regression [102] using appropriate age and gender stratum-specific weighting of the observations [103]. We will also examine effects of the vitamin D intervention on cancer and CVD outcomes within groups defined by race/skin pigmentation and by BMI. In exploratory analyses, we will evaluate effect modification by age, gender, sunlight exposure, calcium and phosphorus intakes estimated from the FFQ (as these nutrients affect vitamin D bioavailability [104]); and baseline risk factors for cancer and CVD. The latter interaction effects will be interpreted cautiously, as hypothesis generating. Finally, we will examine whether treatment effects of the two interventions vary over time and duration of treatment by examining survival plots and interactions with time. There may be a latent effect on cancer incidence, depending on the stage of carcinogenesis during which these agents act.
We will also compare the incidence of potential side effects in the active v. placebo groups for each agent, including the incidence of kidney stones with vitamin D assignment and the incidence of GI symptoms and bleeding with marine omega-3 fatty acid assignment.
2.11.2 Statistical power
Careful attention to issues of statistical power is necessary to ensure the success of a large clinical trial—i.e., achieve definitive results. VITAL was designed to have excellent statistical power to test the primary hypotheses and adequate statistical power to test the secondary hypotheses. The following assumptions were made for the power calculations: (1) a 2×2 factorial trial in 10,000 men aged >50 and 10,000 women aged >55 at baseline; (2) independent and equal allocation of participants to each treatment (achieved by randomization); (3) an age distribution based on that observed at baseline in our past trials for men aged ≥50 and women aged ≥55, but limited to 30% in the youngest age groups (50-59 years in men and 55-64 years in women); (4) age-specific event rates based on the observed rates in the first 5 years of follow-up in our trials with similarly aged populations; (5) a target of 25% blacks, with a corresponding increase in rates of CVD [105] and cancer [106]; (6) a trial follow-up period of 5 years, with little loss to follow-up as achieved in our past trials; and (7) compliance (80%) similar to that in published trials upon which our estimated rate ratio (RR) reductions are based. The cited reductions are thus the observed effects we would see in the trial. The corresponding ‘true’ RR is given assuming an average compliance of 80%. Power was computed for a two-sided test using a logrank analysis [107] with a significance level of 0.05.
Assuming that only one agent is effective, there will be 86% power to detect an observed rate ratio (RR) of 0.85 for the primary cancer endpoint of total cancer incidence (Table 1) and 89% power to detect an observed RR of 0.80 for the primary cardiovascular endpoint—a composite of MI, stroke, and cardiovascular mortality (Table 2). With regard to secondary endpoints, there will be adequate power to detect risk reductions of 25-40%. If both agents are effective in reducing risk for disease but act independently, power would be reduced slightly due to a smaller number of endpoints. If the agents interact to influence risk for disease, power will be affected to the extent of the interaction. Should the agents act synergistically, power would increase, as illustrated in Table 3 for the endpoint of total cancer. For example, if the effect of each agent alone is a risk reduction of 10%, but the effect is stronger in combination, with an additional 10% decrease, the RR comparing the group assigned to active vitamin D plus active marine omega-3 fatty acids with the group assigned to vitamin D placebo and marine omega-3 fatty acid placebo would be 0.73, as opposed to 0.81 with additive effects (on the multiplicative scale). Power for the main effect of each agent would then increase to 81% (or higher with greater synergy). Should the agents interact in a subadditive fashion, power would be reduced.
Table 1.
Power for effects of a single agent on cancer in VITAL, a 2×2 factorial trial of 10,000 men aged ≥50 and 10,000 women aged ≥55, with 5 years of follow-up
| Observed RRa |
True RRb |
Total Cancer |
Cancer Mortality |
Colorectal Cancer |
Breast Cancer (Women) |
Prostate Cancer (Men) |
|---|---|---|---|---|---|---|
| 0.90 | 0.875 | 52.1 | - | - | - | - |
| 0.85 | 0.812 | 86.3 | - | - | - | - |
| 0.80 | 0.750 | 98.5 | 42.3 | 26.3 | 32.8 | 67.5 |
| 0.75 | 0.687 | 99.9 | 60.9 | 39.0 | 48.4 | 86.4 |
| 0.70 | 0.625 | >99.9 | 77.7 | 53.4 | 64.7 | 96.1 |
| 0.65 | 0.560 | >99.9 | 89.7 | 67.8 | 79.0 | 99.3 |
| 0.60 | 0.500 | >99.9 | 96.2 | 80.3 | 89.4 | 99.9 |
Observed RR=intent-to-treat RR, including noncompliant participants. Compliance is assumed to be 80%.
True RR=that with perfect compliance.
RR, rate ratio.
Table 2.
Power for effects of a single agent on CVD in VITAL, a 2×2 factorial trial of 10,000 men aged ≥50 and 10,000 women aged ≥55, with 5 years of follow-up
| Observed RRa | True RRb | Major CVDc |
Total CVDd |
CVD Mortality | MI | Stroke |
|---|---|---|---|---|---|---|
| 0.90 | 0.875 | 34.6 | 52.8 | - | - | - |
| 0.85 | 0.812 | 66.1 | 86.9 | - | - | - |
| 0.80 | 0.750 | 89.4 | 98.6 | 45.1 | 50.9 | 51.0 |
| 0.75 | 0.687 | 98.2 | >99.9 | 64.4 | 71.0 | 71.1 |
| 0.70 | 0.625 | 99.9 | >99.9 | 80.9 | 86.4 | 86.5 |
| 0.65 | 0.560 | >99.9 | >99.9 | 91.9 | 95.2 | 95.2 |
| 0.60 | 0.500 | >99.9 | >99.9 | 97.3 | 98.8 | 98.8 |
Observed RR=intent-to-treat RR, including noncompliant participants. Compliance is assumed to be 80%.
True RR=that with perfect compliance.
Major CVD=myocardial infarction, stroke, and CVD mortality
Total CVD=myocardial infarction, stroke, CVD mortality, and coronary revascularization (coronary artery bypass grafting or percutaneous coronary intervention)
CVD, cardiovascular disease; MI, myocardial infarction; RR, rate ratio.
Table 3. Power for interaction effects on total cancer in VITAL, a 2×2 factorial trial of 10,000 men aged ≥50 and 10,000 women aged ≥55, with 5 years of follow-up
| RR | RR | Power | ||
|---|---|---|---|---|
| Single agenta | Interactionb | Both agents | Main Effect | Interaction |
| 0.90 | 1.0 | 0.81 | 49.9 | |
| 0.9 | 0.73 | 80.6 | ||
| 0.8 | 0.65 | 95.9 | 51.5 | |
| 0.7 | 0.57 | 99.6 | 87.6 | |
| 0.85 | 1.0 | 0.72 | 83.4 | |
| 0.9 | 0.65 | 96.2 | ||
| 0.8 | 0.58 | 99.5 | 48.8 | |
| 0.7 | 0.51 | >99.9 | 85.2 | |
| 0.80 | 1.0 | 0.64 | 97.4 | |
| 0.9 | 0.58 | 99.6 | ||
| 0.8 | 0.51 | >99.9 | 46.0 | |
| 0.7 | 0.45 | >99.9 | 82.5 | |
RR = intent-to-treat RR, including noncompliant participants (assuming 80% compliance). Represents the effect among those not assigned to the other intervention and assumes the same effect for both agents.
The interaction is the RR for the combined group divided by the product of risks for the two separate groups—i.e., RRint=RRboth/(RR vitamin D alone*RR omega-3 fatty acids alone). An interaction=1 implies additive effects (no interaction).
RR, rate ratio.
2.12 Ancillary studies and the Clinical and Translational Science Center subcohort
Although the primary goal of VITAL is to test whether vitamin D or omega-3 fatty acids reduce the risk for cancer and CVD, the trial will also advance our understanding of the role of these agents in other major health outcomes through the integration of ancillary studies. These studies will examine whether the interventions can prevent diabetes and glucose intolerance; hypertension; age-related cognitive decline; late-life depression; osteoporosis and fracture; physical disability and fall; chronic knee pain symptoms; asthma and other respiratory diseases; infections; periodontal disease; rheumatoid arthritis, systemic lupus erythematosus, thyroid diseases, and other autoimmune disorders. Other ancillary studies using non-invasive imaging techniques are planned among participants available for in-person visits (see next paragraph), including dual energy x-ray absorptiometry scans to measure bone density and body composition; mammography to assess breast tissue density, a predictor of breast cancer risk; and vascular imaging—ultrasound to assess carotid intima-media thickness, cardiac multi-detector computed tomography to assess coronary artery calcification, and Doppler echocardiography to assess left ventricular function—to clarify mechanisms by which the interventions may influence CVD risk.
A key feature of VITAL is the establishment of a subcohort of 1000 participants who will be evaluated at Clinical and Translational Science Centers (CTSCs) in Boston. The CTSC subcohort will be identified toward the end of the placebo run-in, prior to randomization. In addition to fulfilling eligibility criteria for the main trial, CTSC participants must live within driving distance of the Boston CTSC sites and be able to provide informed consent for the CTSC evaluation. The CTSC subcohort is expected to reflect the diverse racial/ethnic composition of the VITAL study population and will be randomized equally into the four treatment groups created by the factorial design (i.e., 250 participants per treatment group).
CTSC participants will visit the CTSC sites for detailed health assessments prior to randomization and again two years later. The two visits will use the same protocol to gather basic clinical data (e.g., medical history and physical exam, including measurement of height, weight, other anthropometric indices, and blood pressure) and data on variables related to aims of the ancillary studies (e.g., glucose tolerance testing, physical performance batteries, lung function exams, cognitive assessments, and structured interviews to diagnose mood and other mental disorders). Blood samples will be drawn not only for glucose tolerance testing but also for assays of 25(OH)D and EPA+DHA levels. The timing of the second CTSC visit will be matched by month to the initial visit to minimize variability in seasonal sun exposure, a major source of within-person variation in 25(OH)D levels. The CTSC visits provides a valuable opportunity for face-to-face contact with a subset of the VITAL study population, allowing for detailed phenotyping and in-person validation of the remote assessment methods used in the main trial and ancillary studies. For example, in-person assessments of cognitive function at the CTSC visit will be used to validate telephone-based assessments in the cognitive function ancillary study, and in-person structured diagnostic interviews for clinical depression at the CTSC visit will be used to validate clinical depression cases identified by screening checklists in the depression ancillary study.
2.13 Trial monitoring
An independent Data and Safety Monitoring Board (DSMB) consisting of representatives from the National Institutes of Health (NIH) and other experts in clinical trials, epidemiology, biostatistics, and relevant clinical areas of cancer and CVD meets annually to review the progress of VITAL and the unblinded data on study endpoints and possible adverse effects in order to recommend continuation, modifications to the study design, or early termination of the trial. Evaluation of interim results will be guided by Haybittle-Peto rules [108, 109], which appropriately require strong evidence for early stopping and allow for assessments at convenient intervals without inducing statistical complexity [110]. These rules apply to the main endpoints of cancer and CVD. However, because VITAL will also assess the overall balance of benefits and risks of the interventions in the primary prevention of these diseases, other outcomes that may critically affect the benefit-risk balance will be considered. Decisions regarding the trial’s continuation will also be informed by relevant scientific data (e.g., from other trials of the study agents) that may become available during the planned 5-year treatment period.
3. Discussion
VITAL has many strengths. This study is testing two very promising nutritional agents (vitamin D and marine omega-3 fatty acids) for the prevention of two major chronic diseases (cancer and CVD) in a multi-ethnic population in an extremely cost-effective fashion—i.e., utilizing a mail-based, large simple trial design. The trial has excellent power to detect small to moderate effects of the interventions on the primary endpoints of interest. The trial will include the collection and storage of baseline blood samples in the majority of the cohort to allow assessment of effect modification by baseline 25(OH)D and EPA+DHA levels, and the collection and storage of follow-up samples in a subgroup of participants to allow assessment of pill-taking compliance; changes in biomarkers with treatment; and, in the placebo group, the effect of food fortification and changing background intakes of vitamin D and marine omega-3 fatty acids. VITAL will also further our understanding of the role of the interventions in relation to many other major health outcomes through well-integrated ancillary studies, including currently funded and future investigations of multiple clinical, biochemical, and genetic hypotheses. The study also has some limitations. It will test only one dose of each agent rather than examining multiple doses to determine the dose-response relationship. However, the dose for each agent was chosen on the basis of an extensive and careful review of available evidence, with the goal of optimizing the balance of safety and efficacy. Because the trial population is older, the results may not be generalizable to younger individuals. However, older populations have higher disease rates, allowing the trial to be completed in a shorter time period, at greater cost efficiency. Finally, latency of effect may be an issue, especially for cancer outcomes. However, several lines of evidence suggest that the agents of interest, particularly vitamin D [2, 20, 111], may act at later stages of carcinogenesis (including effects on tumor invasion and metastasis), suggesting that benefits could be observed with 5 years of treatment.
The purported health benefits of vitamin D and marine omega-3 fatty acids are receiving increasing attention in both the medical literature and the popular press. Sales of fish-oil supplements are rising, and an increasing number of foods are omega-3 fortified [112]. Many clinicians now include vitamin D blood tests as part of routine lab work and recommend vitamin D supplements to patients. Indeed, sales of such supplements have skyrocketed in recent years [113]. However, in a report published earlier this year, the IOM critically reviewed nearly 1000 studies of vitamin D in relation to a wide variety of health outcomes and concluded that although there is clear evidence that vitamin D—at doses of 600 to 800 IU/day—confers bone benefits, current data are inconclusive as to whether higher vitamin D intakes reduce risk for cancer, CVD, and other chronic diseases [26]. Because of this uncertainty, the IOM called for more research—especially large randomized clinical trials—to determine whether high-dose vitamin D supplements can lower the risk for nonskeletal illnesses and whether they pose any health risks. Similarly, the conclusion from a 2004 NIH workshop [114] was that “… the body of evidence is consistent with the hypothesis that intake of omega-3 fatty acids reduces CVD but … a definitive trial is needed.” Rigorous trials of many other single-agent nutritional interventions—such as certain antioxidant vitamins, selenium, B-vitamins, and calcium—have disproved some health claims and even uncovered health risks that may not have otherwise been detected [115-119]. Indeed, recent observational data suggest that not only very low but also very high 25(OH)D levels may contribute to the development of CVD [9, 16] and certain cancers [120]. The growing enthusiasm for vitamin D and marine omega-3 fatty acid supplements underscores the need for a timely initiation of a large randomized trial such as VITAL to test these agents rigorously, before their use becomes so prevalent as to render participant recruitment and hypothesis testing impossible. The results of VITAL are expected to inform individual decisions, clinical recommendations, and public health guidelines regarding the use of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and CVD.
Acknowledgments
VITAL is supported by grant U01 CA138962, which includes support from the National Cancer Institute, National Heart, Lung and Blood Institute (NHLBI), Office of Dietary Supplements, National Institute of Neurological Disorders and Stroke, and the National Center for Complementary and Alternative Medicine. The ancillary studies are supported by grants R01 DK088078 and R01 DK088762 from the National Institute of Diabetes and Digestive and Kidney Diseases; R01 HL101932 and R01 HL102122 from NHLBI; R01 AG036755 from the National Institute on Aging; R01AR059086 and R01AR060574 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases; and R01 MH091448 from the National Institute of Mental Health. Members of the Data and Safety Monitoring Board include Lawrence S. Cohen, Theodore Colton, Mark A. Espeland, I. Craig Henderson, Alice H. Lichtenstein, Rebecca A. Silliman, and Nanette K. Wenger (chair), and Josephine Boyington, Cindy D. Davis, Rebecca B. Costello, Lawrence Fine, and Peter Greenwald (ex-officio members).
Footnotes
Article category
Study design, statistical design, study protocols
References
- 1.Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol. 2011;51:311–36. doi: 10.1146/annurev-pharmtox-010510-100611. [DOI] [PubMed] [Google Scholar]
- 2.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 3.Manson JE, Bassuk SS. Vitamin D and cardiovascular disease. Menopause Management. 2009;18:28–31. [Google Scholar]
- 4.Grant WB. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer. 2002;94:1867–75. doi: 10.1002/cncr.10427. [DOI] [PubMed] [Google Scholar]
- 5.Grant WB. Ecologic studies of solar UV-B radiation and cancer mortality rates. Recent Results Cancer Res. 2003;164:371–7. doi: 10.1007/978-3-642-55580-0_27. [DOI] [PubMed] [Google Scholar]
- 6.Mizoue T. Ecological study of solar radiation and cancer mortality in Japan. Health Phys. 2004;87:532–8. doi: 10.1097/01.hp.0000137179.03423.0b. [DOI] [PubMed] [Google Scholar]
- 7.Zittermann A, Schleithoff SS, Koerfer R. Putting cardiovascular disease and vitamin D insufficiency into perspective. Br J Nutr. 2005;94:483–92. doi: 10.1079/bjn20051544. [DOI] [PubMed] [Google Scholar]
- 8.Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–9. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 9.Freedman DM, Looker AC, Chang SC, Graubard BI. Prospective study of serum vitamin D and cancer mortality in the United States. J Natl Cancer Inst. 2007;99:1594–602. doi: 10.1093/jnci/djm204. [DOI] [PubMed] [Google Scholar]
- 10.Pilz S, Dobnig H, Winklhofer-Roob B, Riedmuller G, Fischer JE, Seelhorst U, et al. Low serum levels of 25-hydroxyvitamin D predict fatal cancer in patients referred to coronary angiography. Cancer Epidemiol Biomarkers Prev. 2008;17:1228–33. doi: 10.1158/1055-9965.EPI-08-0002. [DOI] [PubMed] [Google Scholar]
- 11.Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, et al. Vitamin D and prevention of colorectal cancer. J Steroid Biochem Mol Biol. 2005;97:179–94. doi: 10.1016/j.jsbmb.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, et al. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta-analysis. Am J Prev Med. 2007;32:210–6. doi: 10.1016/j.amepre.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Bertone-Johnson ER, Chen WY, Holick MF, Hollis BW, Colditz GA, Willett WC, et al. Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1991–7. doi: 10.1158/1055-9965.EPI-04-0722. [DOI] [PubMed] [Google Scholar]
- 14.Lowe LC, Guy M, Mansi JL, Peckitt C, Bliss J, Wilson RG, et al. Plasma 25-hydroxy vitamin D concentrations, vitamin D receptor genotype and breast cancer risk in a UK Caucasian population. Eur J Cancer. 2005;41:1164–9. doi: 10.1016/j.ejca.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Abbas S, Linseisen J, Slanger T, Kropp S, Mutschelknauss EJ, Flesch-Janys D, et al. Serum 25-hydroxyvitamin D and risk of post-menopausal breast cancer--results of a large case-control study. Carcinogenesis. 2008;29:93–9. doi: 10.1093/carcin/bgm240. [DOI] [PubMed] [Google Scholar]
- 16.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giovannucci E, Liu Y, Hollis BW, Rimm EB. A prospective study of 25-hydroxyvitamin D and risk of myocardial infarction in men. Arch Intern Med. 2008;168:1174–80. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–9. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, et al. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. 2010;106:963–8. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 20.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–91. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 21.Lappe JM, Heaney RP. Calcium supplementation: Results may not be generalisable. BMJ. 2008;336:403. doi: 10.1136/bmj.39493.476667.1F. author reply 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326:469. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prince RL, Austin N, Devine A, Dick IM, Bruce D, Zhu K. Effects of ergocalciferol added to calcium on the risk of falls in elderly high-risk women. Arch Intern Med. 2008;168:103–8. doi: 10.1001/archinternmed.2007.31. [DOI] [PubMed] [Google Scholar]
- 24.Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O’Sullivan MJ, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–96. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 25.Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–83. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 26.Institute of Medicine . Dietary Reference Intakes for Calcium and Vitamin D. The National Academies Press; Washington DC: 2011. [PubMed] [Google Scholar]
- 27.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771–7. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 29.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 30.Harris SS. Vitamin D and African Americans. J Nutr. 2006;136:1126–9. doi: 10.1093/jn/136.4.1126. [DOI] [PubMed] [Google Scholar]
- 31.Moore CE, Murphy MM, Holick MF. Vitamin D intakes by children and adults in the United States differ among ethnic groups. J Nutr. 2005;135:2478–85. doi: 10.1093/jn/135.10.2478. [DOI] [PubMed] [Google Scholar]
- 32.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–65. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 33.Harris SS, Dawson-Hughes B. Reduced sun exposure does not explain the inverse association of 25-hydroxyvitamin D with percent body fat in older adults. J Clin Endocrinol Metab. 2007;92:3155–7. doi: 10.1210/jc.2007-0722. [DOI] [PubMed] [Google Scholar]
- 34.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 35.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–57. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 36.Robinson JG, Stone NJ. Antiatherosclerotic and antithrombotic effects of omega-3 fatty acids. Am J Cardiol. 2006;98:39i–49i. doi: 10.1016/j.amjcard.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 37.Reiffel JA, McDonald A. Antiarrhythmic effects of omega-3 fatty acids. Am J Cardiol. 2006;98:50i–60i. doi: 10.1016/j.amjcard.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 38.Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ. Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis. 2008;197:12–24. doi: 10.1016/j.atherosclerosis.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Lee JH, O’Keefe JH, Lavie CJ, Marchioli R, Harris WS. Omega-3 fatty acids for cardioprotection. Mayo Clin Proc. 2008;83:324–32. doi: 10.4065/83.3.324. [DOI] [PubMed] [Google Scholar]
- 40.Mori TA, Beilin LJ. Omega-3 fatty acids and inflammation. Curr Atheroscler Rep. 2004;6:461–7. doi: 10.1007/s11883-004-0087-5. [DOI] [PubMed] [Google Scholar]
- 41.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–99. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 42.He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, et al. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation. 2004;109:2705–11. doi: 10.1161/01.CIR.0000132503.19410.6B. [DOI] [PubMed] [Google Scholar]
- 43.Whelton SP, He J, Whelton PK, Muntner P. Meta-analysis of observational studies on fish intake and coronary heart disease. Am J Cardiol. 2004;93:1119–23. doi: 10.1016/j.amjcard.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 44.Lemaitre RN, King IB, Mozaffarian D, Kuller LH, Tracy RP, Siscovick DS. n-3 Polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: the Cardiovascular Health Study. Am J Clin Nutr. 2003;77:319–25. doi: 10.1093/ajcn/77.2.319. [DOI] [PubMed] [Google Scholar]
- 45.Iso H, Kobayashi M, Ishihara J, Sasaki S, Okada K, Kita Y, et al. Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: the Japan Public Health Center-Based (JPHC) Study Cohort I. Circulation. 2006;113:195–202. doi: 10.1161/CIRCULATIONAHA.105.581355. [DOI] [PubMed] [Google Scholar]
- 46.He K, Rimm EB, Merchant A, Rosner BA, Stampfer MJ, Willett WC, et al. Fish consumption and risk of stroke in men. JAMA. 2002;288:3130–6. doi: 10.1001/jama.288.24.3130. [DOI] [PubMed] [Google Scholar]
- 47.Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–55. [PubMed] [Google Scholar]
- 48.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–8. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 49.MacLean CH, Newberry SJ, Mojica WA, Khanna P, Issa AM, Suttorp MJ, et al. Effects of omega-3 fatty acids on cancer risk: a systematic review. JAMA. 2006;295:403–15. doi: 10.1001/jama.295.4.403. [DOI] [PubMed] [Google Scholar]
- 50.Norat T, Bingham S, Ferrari P, Slimani N, Jenab M, Mazuir M, et al. Meat, fish, and colorectal cancer risk: the European Prospective Investigation into Cancer and Nutrition. J Natl Cancer Inst. 2005;97:906–16. doi: 10.1093/jnci/dji164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kato I, Akhmedkhanov A, Koenig K, Toniolo PG, Shore RE, Riboli E. Prospective study of diet and female colorectal cancer: the New York University Women’s Health Study. Nutr Cancer. 1997;28:276–81. doi: 10.1080/01635589709514588. [DOI] [PubMed] [Google Scholar]
- 52.Hall MN, Chavarro JE, Lee IM, Willett WC, Ma J. A 22-year prospective study of fish, n-3 fatty acid intake, and colorectal cancer risk in men. Cancer Epidemiol Biomarkers Prev. 2008;17:1136–43. doi: 10.1158/1055-9965.EPI-07-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hooper L, Thompson RL, Harrison RA, Summerbell CD, Ness AR, Moore HJ, et al. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ. 2006;332:752–60. doi: 10.1136/bmj.38755.366331.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004;79:935–45. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 55.American Cancer Society . Cancer Facts & Figures for African Americans 2009-2010. American Cancer Society; Atlanta: 2009. [Google Scholar]
- 56.Harris SS. Does vitamin D deficiency contribute to increased rates of cardiovascular disease and type 2 diabetes in African Americans? Am J Clin Nutr. doi: 10.3945/ajcn.110.003491. e-pub 2011 Mar 2. [DOI] [PubMed] [Google Scholar]
- 57.Institute of Medicine Food and Nutrition Board . Dietary Reference Intakes: Calcium, Phosphorous, Magnesium, Vitamin D and Fluoride. National Academies Press; Washington DC: 1999. [Google Scholar]
- 58.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 59.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–6. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 60.Aloia JF, Patel M, Dimaano R, Li-Ng M, Talwar SA, Mikhail M, et al. Vitamin D intake to attain a desired serum 25-hydroxyvitamin D concentration. Am J Clin Nutr. 2008;87:1952–8. doi: 10.1093/ajcn/87.6.1952. [DOI] [PubMed] [Google Scholar]
- 61.Harris WS. International recommendations for consumption of long-chain omega-3 fatty acids. J Cardiovasc Med (Hagerstown) 2007;8(Suppl 1):S50–2. doi: 10.2459/01.JCM.0000289274.64933.45. [DOI] [PubMed] [Google Scholar]
- 62.Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr. 2007;85:6–18. doi: 10.1093/ajcn/85.1.6. [DOI] [PubMed] [Google Scholar]
- 63.Bolland MJ, Barber PA, Doughty RN, Mason B, Horne A, Ames R, et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ. 2008;336:262–6. doi: 10.1136/bmj.39440.525752.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999-2000. Am J Epidemiol. 2004;160:339–49. doi: 10.1093/aje/kwh207. [DOI] [PubMed] [Google Scholar]
- 65.Stacewicz-Sapuntzakis M, Borthakur G, Burns JL, Bowen PE. Correlations of dietary patterns with prostate health. Mol Nutr Food Res. 2008;52:114–30. doi: 10.1002/mnfr.200600296. [DOI] [PubMed] [Google Scholar]
- 66.Ahn J, Albanes D, Peters U, Schatzkin A, Lim U, Freedman M, et al. Dairy products, calcium intake, and risk of prostate cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2007;16:2623–30. doi: 10.1158/1055-9965.EPI-07-0601. [DOI] [PubMed] [Google Scholar]
- 67.Park Y, Mitrou PN, Kipnis V, Hollenbeck A, Schatzkin A, Leitzmann MF. Calcium, dairy foods, and risk of incident and fatal prostate cancer: the NIH-AARP Diet and Health Study. Am J Epidemiol. 2007;166:1270–9. doi: 10.1093/aje/kwm268. [DOI] [PubMed] [Google Scholar]
- 68.Agency for Healthcare Research and Quality [Accessed March 24, 2011];Effects of Omega-3 Fatty Acids on Cardiovascular Disease. Evidence Report/Technology Assessment Number 94. AHRQ Pub. No. 04-E009-1. Available at: http://www.ahrq.gov/clinic/epcsums/o3cardsum.pdf.
- 69.Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother. 2006;60:502–7. doi: 10.1016/j.biopha.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 70.Kris-Etherton PM, Taylor DS, Yu-Poth S, Huth P, Moriarty K, Fishell V, et al. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71:179S–88S. doi: 10.1093/ajcn/71.1.179S. [DOI] [PubMed] [Google Scholar]
- 71.Harris WS. The omega-6/omega-3 ratio and cardiovascular disease risk: uses and abuses. Curr Atheroscler Rep. 2006;8:453–9. doi: 10.1007/s11883-006-0019-7. [DOI] [PubMed] [Google Scholar]
- 72.Stanley JC, Elsom RL, Calder PC, Griffin BA, Harris WS, Jebb SA, et al. UK Food Standards Agency Workshop Report: the effects of the dietary n-6:n-3 fatty acid ratio on cardiovascular health. Br J Nutr. 2007;98:1305–10. doi: 10.1017/S000711450784284X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harris WS, Poston WC, Haddock CK. Tissue n-3 and n-6 fatty acids and risk for coronary heart disease events. Atherosclerosis. 2007;193:1–10. doi: 10.1016/j.atherosclerosis.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 74.Mozaffarian D, Ascherio A, Hu FB, Stampfer MJ, Willett WC, Siscovick DS, et al. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation. 2005;111:157–64. doi: 10.1161/01.CIR.0000152099.87287.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Department of Health and Human Services. U.S. Food and Drug Administration . Federal Register. 108. Vol. 62. Jun 5, 1997. Substances affirmed as generally recognized as safe: menhaden oil; pp. 30751–30757. 21 CFR Part 184 [Docket No. 86G-0289], 1997. [Google Scholar]
- 76.Harris WS. Expert opinion: omega-3 fatty acids and bleeding--cause for concern? Am J Cardiol. 2007;99:44C–46C. doi: 10.1016/j.amjcard.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 77.Bays HE. Safety considerations with omega-3 fatty acid therapy. Am J Cardiol. 2007;99:35C–43C. doi: 10.1016/j.amjcard.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 78.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 79.Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, MacFadyen J, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2009;301:52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, et al. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women’s Antioxidant Cardiovascular Study. Arch Intern Med. 2007;167:1610–8. doi: 10.1001/archinte.167.15.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Steering Committee of the Physicians’ Health Study Research Group Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129–35. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 82.Lang JM, Buring JE, Rosner B, Cook N, Hennekens CH. Estimating the effect of the run- in on the power of the Physicians’ Health Study. Stat Med. 1991;10:1585–93. doi: 10.1002/sim.4780101010. [DOI] [PubMed] [Google Scholar]
- 83.Talwar SA, Aloia JF, Pollack S, Yeh JK. Dose response to vitamin D supplementation among postmenopausal African American women. Am J Clin Nutr. 2007;86:1657–62. doi: 10.1093/ajcn/86.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hollis BW, Napoli JL. Improved radioimmunoassay for vitamin D and its use in assessing vitamin D status. Clin Chem. 1985;31:1815–9. [PubMed] [Google Scholar]
- 85.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem. 1993;39:529–33. [PubMed] [Google Scholar]
- 86.National Institute of Standards and Technology [Accessed March 24, 2011];Vitamin D Metabolites Quality Assurance Program. Available at http://www.nist.gov/mml/analytical/vitdqap.cfm.
- 87.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–26. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 88.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–6. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 89.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18:858–67. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 90.Jacques PF, Sulsky SI, Sadowski JA, Phillips JC, Rush D, Willett WC. Comparison of micronutrient intake measured by a dietary questionnaire and biochemical indicators of micronutrient status. Am J Clin Nutr. 1993;57:182–9. doi: 10.1093/ajcn/57.2.182. [DOI] [PubMed] [Google Scholar]
- 91.Feskanich D, Willett WC, Colditz GA. Calcium, vitamin D, milk consumption, and hip fractures: a prospective study among postmenopausal women. Am J Clin Nutr. 2003;77:504–11. doi: 10.1093/ajcn/77.2.504. [DOI] [PubMed] [Google Scholar]
- 92.Hunter DJ, Rimm EB, Sacks FM, Stampfer MJ, Colditz GA, Litin LB, et al. Comparison of measures of fatty acid intake by subcutaneous fat aspirate, food frequency questionnaire, and diet records in a free-living population of US men. Am J Epidemiol. 1992;135:418–27. doi: 10.1093/oxfordjournals.aje.a116302. [DOI] [PubMed] [Google Scholar]
- 93.Fritz AG, Percy C, Jack A, Shanmugaratham K, Sobin LH, Parkin MD, et al. International Classification of Diseases for Oncology (ICD-O) Third Edition World Health Organization; Geneva: 2000. [Google Scholar]
- 94.Thygesen K, Alpert JS, White HD, Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction Universal definition of myocardial infarction. Circulation. 2007;116:2634–53. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 95.Adams HP, Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 96.Satterfield S, Greco PJ, Goldhaber SZ, Stampfer MJ, Swartz SL, Stein EA, et al. Biochemical markers of compliance in the Physicians’ Health Study. Am J Prev Med. 1990;6:290–4. [PubMed] [Google Scholar]
- 97.Cox DR. Regression models and life-tables. J Royal Statist Soc B. 1972;34:187–220. [Google Scholar]
- 98.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 99.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 100.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 101.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73:1–11. [Google Scholar]
- 102.Barlow WE. Robust variance estimation for the case-cohort design. Biometrics. 1994;50:1064–72. [PubMed] [Google Scholar]
- 103.Borgan O, Langholz B, Samuelsen SO, Goldstein L, Pogoda J. Exposure stratified case-cohort designs. Lifetime Data Anal. 2000;6:39–58. doi: 10.1023/a:1009661900674. [DOI] [PubMed] [Google Scholar]
- 104.Holick MF. Vitamin D for health and in chronic kidney disease. Semin Dial. 2005;18:266–75. doi: 10.1111/j.1525-139X.2005.18402.x. [DOI] [PubMed] [Google Scholar]
- 105.Hozawa A, Folsom AR, Sharrett AR, Chambless LE. Absolute and attributable risks of cardiovascular disease incidence in relation to optimal and borderline risk factors. Arch Intern Med. 2007;167:573–9. doi: 10.1001/archinte.167.6.573. [DOI] [PubMed] [Google Scholar]
- 106.Surveillance Research Program, National Cancer Institute [Accessed on March 24, 2011];Fast Stats: An interactive tool for access to SEER cancer statistics. http://seer.cancer.gov/faststats.
- 107.Freedman LS. Tables of the number of patients required in clinical trials using the logrank test. Stat Med. 1982;1:121–9. doi: 10.1002/sim.4780010204. [DOI] [PubMed] [Google Scholar]
- 108.Haybittle JL. Repeated assessment of results in clinical trials of cancer treatment. Br J Radiol. 1971;44:793–7. doi: 10.1259/0007-1285-44-526-793. [DOI] [PubMed] [Google Scholar]
- 109.Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976;34:585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pocock SJ. Current controversies in data monitoring for clinical trials. Clin Trials. 2006;3:513–21. doi: 10.1177/1740774506073467. [DOI] [PubMed] [Google Scholar]
- 111.Welsh J. Vitamin D and prevention of breast cancer. Acta Pharmacol Sin. 2007;28:1373–82. doi: 10.1111/j.1745-7254.2007.00700.x. [DOI] [PubMed] [Google Scholar]
- 112.Stipp D. Fish-oil doses can be hard to swallow. Wall Street Journal. 2008 January 8;:D1–D2. [Google Scholar]
- 113.Parker-Pope T. [Accessed on February 24, 2011];The miracle of vitamin D: sound science, or hype? Well blog of the New York Times. 2010 Feb 1; < http://well.blogs.nytimes.com/2010/02/01/the-miracle-of-vitamin-d-sound-science-or-hype/>. [Google Scholar]
- 114.NIH Office of Dietary Supplements, National Heart Lung and Blood Institute . Working Group Report on Future Clinical Research Directions on Omega-3 Fatty Acids and Cardiovascular Disease. Bethesda MD: Jun 2, 2004. Available at: http://www.nhlbi.nih.gov/meetings/workshops/omega-3/omega-3-rpt.htm on March 24, 2011. [Google Scholar]
- 115.Kris-Etherton PM, Lichtenstein AH, Howard BV, Steinberg D, Witztum JL. Antioxidant vitamin supplements and cardiovascular disease. Circulation. 2004;110:637–41. doi: 10.1161/01.CIR.0000137822.39831.F1. [DOI] [PubMed] [Google Scholar]
- 116.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 117.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–57. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 118.Byers T. Anticancer vitamins du jour--The ABCED’s so far. Am J Epidemiol. 2010;172:1–3. doi: 10.1093/aje/kwq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. 2010;341:c3691. doi: 10.1136/bmj.c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Toner CD, Davis CD, Milner JA. The vitamin D and cancer conundrum: aiming at a moving target. J Am Diet Assoc. 2010;110:1492–500. doi: 10.1016/j.jada.2010.07.007. [DOI] [PubMed] [Google Scholar]