Summary
Although transintestinal cholesterol efflux has been identified as an important means of clearing excess sterols, the mechanisms that underlie this process remain poorly understood. Here we show that magro, a direct target of the Drosophila DHR96 nuclear receptor, is required in the intestine to maintain cholesterol homeostasis. Magro encodes a LipA homolog that is secreted from the anterior gut into the intestinal lumen to digest dietary triacylglycerol. Expression of magro in intestinal cells is required to hydrolyze cholesterol esters and promote cholesterol clearance. Restoring magro expression in the intestine of DHR96 mutants rescues their defects in triacylglycerol and cholesterol metabolism. These studies show that the central role of the intestine in cholesterol efflux has been conserved through evolution, that the ancestral function of LipA is to coordinate triacylglycerol and cholesterol metabolism, and that the region-specific activities of magro correspond to the metabolic functions of its upstream regulator, DHR96.
Keywords: nuclear receptors, lipid metabolism, cholesterol, LipA, LXR, cholesterol efflux
Introduction
Coordinate regulation of lipid metabolism is central to human health, with disruption of this process leading to a range of metabolic disorders, including obesity and cardiovascular disease. Normal lipid homeostasis is maintained by balancing dietary lipid uptake and synthesis with lipid catabolism and excretion. Under normal feeding conditions, dietary lipids such as triacylglycerol (TAG) and cholesterol esters are broken down into free fatty acids, monoacylglycerols, and free sterols in the lumen of the intestine. These digested lipids can then be absorbed by the intestinal cells, where TAG is resynthesized and packaged together with cholesterol, cholesterol esters, and carrier proteins to form lipoprotein particles that are trafficked throughout the body. These lipids can be either utilized by cells or deposited in storage tissues such as the adipose and liver. Under conditions of excess lipids, TAG and cholesterol esters are broken down and free fatty acids can be utilized for energy, while excess cholesterol is excreted from the body (Lusis and Pajukanta, 2008; van der Velde et al., 2010).
Nuclear receptors (NRs) are ligand-regulated transcription factors that play essential roles in multiple aspects of lipid homeostasis. Many NRs bind small lipophilic compounds such as fatty acids, sterols, and other metabolic intermediates, and coordinate multiple aspects of metabolism by directing specific changes in gene expression. One example of this is LXRα (NR1H3), which binds oxysterols and promotes the modification and clearance of excess sterols (Kalaany and Mangelsdorf, 2006). In addition, LXRα is required to maintain proper TAG levels, at least in part through the regulation of SREBP-mediated fat synthesis (Schultz et al., 2000). LXRα activity is thus central to both TAG and cholesterol homeostasis, although much remains to be learned about the roles of specific LXR target genes in mediating these key metabolic functions.
We have been studying a Drosophila homolog of LXR, DHR96, as a simple system to understand the physiological and molecular roles for this family of NRs and their target genes. Biochemical and genetic studies of DHR96 have shown that it shares the central metabolic functions of its mammalian counterpart. DHR96 binds cholesterol and is required for normal cholesterol homeostasis, with DHR96 null mutants exhibiting a ~20% increase in whole animal cholesterol levels due, at least in part, to increased npc1b expression (Horner et al., 2009; Bujold et al., 2010). In addition, DHR96 mutants display an ~50% decrease in whole animal TAG levels that can be attributed to an inability to break down dietary TAG due to reduced expression of the intestinal lipase Magro (CG5932) (Sieber and Thummel, 2009). Interestingly, magro transcription is responsive to dietary cholesterol levels and this regulation is dependent on DHR96 function, providing a potential link between cholesterol levels, DHR96, and TAG homeostasis (Horner et al., 2009; Bujold et al., 2010). Moreover, while Magro protein is most similar to mammalian gastric lipase (38% identity, 56% similarity), the second most similar protein is LipA (32% identity 50% similarity), which has both TAG lipase and cholesterol esterase activities (Ameis et al., 1994). Genetic studies have demonstrated a central role for LipA in maintaining proper cholesterol levels in mice (Du et al., 2001). Similar phenotypes are seen in human LipA mutants suffering from Cholesterol Ester Storage Disease (CESD) and Wolman’s disease (Burke and Schubert, 1972). These observations raise the possibility that, in addition to controlling TAG homeostasis, magro may regulate cholesterol homeostasis, and that DHR96 may function through magro to help coordinate TAG and cholesterol metabolism.
In this study we show that loss of magro function leads to an increase in cholesterol levels similar to that seen in DHR96 mutants. We show that, like LipA, Magro has cholesterol esterase activity, and this enzyme is required in intestinal cells to maintain cholesterol homeostasis. In contrast, the TAG lipase activity of Magro arises from the anterior end of the gut and acts in the intestinal lumen to facilitate dietary fat uptake. Restoring magro expression in the intestine of the DHR96 mutant is sufficient to rescue their lean phenotype and elevated levels of cholesterol. Our data support the model that DHR96 functions through magro in the intestine to coordinate both dietary TAG breakdown and the clearance of excess sterols.
Results
magro is required for normal cholesterol homeostasis
The regulation of magro transcription by dietary cholesterol combined with its significant homology to LipA prompted us to test if magro function is required for cholesterol homeostasis. DHR961 null mutants grown on a normal diet display elevated levels of cholesterol compared to genetically matched wild-type controls (Figure 1A), similar to the results seen when DHR96 mutant larvae are subjected to a high cholesterol diet (Horner et al., 2009). Interestingly, ubiquitous RNAi-mediated silencing of magro expression using Act-GAL4, as done previously (Sieber and Thummel, 2009), leads to a similar phenotype (Figure 1A). Taken together with our earlier work, which showed that both DHR96 mutants and magro RNAi animals have significantly lower levels of TAG, these data suggest that DHR96 functions through transcriptional regulation of magro to coordinate TAG and cholesterol homeostasis in Drosophila.
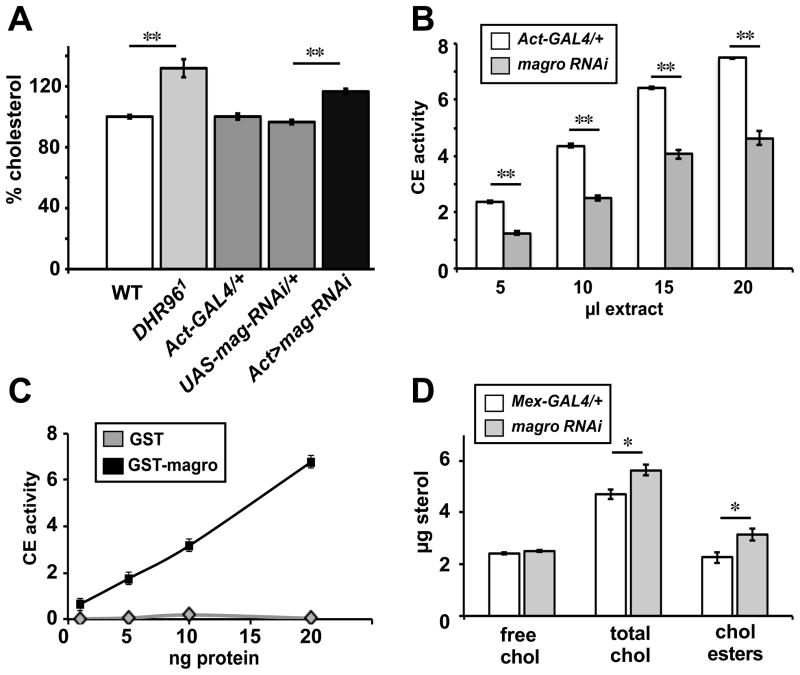
Figure 1. magro maintains proper cholesterol levels and has cholesterol esterase activity.
(A) RNAi for magro results in elevated cholesterol levels similar to those seen in DHR96 mutants. Wild-type (WT), Act-GAL4/+, and UAS-magro-RNAi/+ control flies, along with DHR961 mutants and Act-GAL4/UAS-magro-RNAi animals (Act>mag-RNAi) were assayed for total cholesterol levels. The data were normalized to protein levels and are presented relative to a wild-type level of 100%. (B) RNAi for magro results in reduced intestinal cholesteryl ester hydrolase activity. Intestines dissected from both Act-Gal4/+ control and Act-GAL4/UAS-magro-RNAi (magro RNAi) animals were homogenized and increasing amounts of lysate were tested for cholesteryl ester hydrolase activity (CE) by assaying for the release of free cholesterol from a cholesterol acetate substrate. The y-axis shows μg/ml of free glycerol released. (C) Purified GST-Magro protein has cholesteryl ester hydrolase activity. Recombinant GST and GST-Magro were purified as described (Sieber and Thummel, 2009) and increasing amounts of protein were assayed for CE activity. The y-axis shows μg/ml of free glycerol released. (D) Intestinal-specific RNAi for magro results in elevated levels of esterified cholesterol. Free, esterified, and total cholesterol levels were measured from Mex-GAL4/+ control and Mex-GAL4/UAS-magro-RNAi (magro RNAi) animals. Error bars represent ±SEM. (*p< 0.05, **p< 0.0001)
magro regulates cholesterol homeostasis by breaking down stored cholesterol esters
In order to determine if Magro can act like LipA by cleaving cholesterol esters in addition to its TAG lipase activity, we assayed for cholesterol esterase activity in control and magro RNAi animals (Ameis et al., 1994). While control intestinal lysates exhibit a high level of cholesterol esterase activity in a dose-dependent manner, the lysates from magro RNAi animals display a ~50% decrease in enzymatic function (Figure 1B). Moreover, purified recombinant GST-Magro efficiently cleaves a cholesterol ester substrate in vitro, demonstrating that these effects are a direct result of Magro enzymatic activity (Figure 1C). Taken together with our earlier biochemical studies, this result shows that Magro is a bifunctional enzyme that can act as both a TAG lipase and cholesterol esterase (Sieber and Thummel, 2009).
If decreased intestinal cholesterol esterase activity is the cause of the elevated cholesterol levels seen in the magro RNAi animals, then we should see an increase in stored cholesterol esters when magro expression is specifically silenced in the intestine. Consistent with this proposal, we see a significant increase in the levels of both total cholesterol and cholesterol esters in Mex>magro RNAi animals, while free cholesterol levels remain unchanged (Figure 1D). Elevated cholesterol levels can also be clearly detected in isolated intestines, supporting a role for magro in maintaining cholesterol levels in this tissue (Figure S1). These data support the model that Magro maintains cholesterol homeostasis through its ability to directly break down stored cholesterol esters in the intestine.
magro is expressed throughout the digestive tract
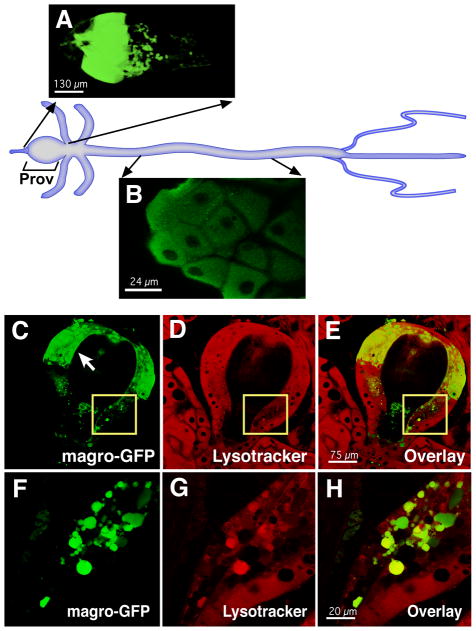
The similarity between lipase sequences complicates our ability to raise specific antibodies against Magro. Accordingly, we determined the pattern of Magro expression using a genomic magro-EGFP transgenic line. This construct contains ~7 kb of genomic DNA spanning the magro locus with 5 kb of upstream promoter sequences and the EGFP gene fused in-frame to the 3′-end of the magro protein coding region. Magro-EGFP is expressed specifically in the intestine with the highest levels of protein accumulation in the anterior region of the proventriculus (Figure 2A). The proventriculus is a bulb shaped structure at the anterior end of the gut that consists of three distinct cell layers (Figure S2). Magro-EGFP is expressed in the anterior half of the outermost layer of cells in the proventriculus (Figures 2C, arrow; S2). In addition, protein is clearly visible in large vesicles that lie posterior to this region of expression (Figure 2C–E, yellow boxes). These are acidic vesicles that stain positive for Lysotracker Red (Figure 2F–H) consistent with the acid lipase activity of Magro and LipA. Interestingly, visualization of vesicles in this region using CD8-EGFP reveals that they move in a posterior direction toward the intestine, providing a potential mechanism to deliver digestive enzymes, such as Magro, into the intestinal lumen (Figure S3). Lower levels of Magro-EGFP protein can also be seen in the major cell type of the intestine, the enterocytes, in a punctate cytoplasmic pattern (Figure 2B). Interestingly, we do not observe Magro-EGFP in large Lysotracker-positive vesicles in these cells. Taken together, these observations suggest that Magro is trafficked differently in different cell types of the digestive tract and raise the possibility that the cholesterol esterase and TAG lipase activities of this enzyme are regionally specified.
Figure 2. Magro is expressed in the proventriculus and midgut.
Magro-EGFP (green) expression in mid-third instar larvae is restricted to the intestine and accumulates to high levels in the anterior region of the proventriculus (Prov) (A, arrow in C). Antibody staining for EGFP also revealed lower levels of punctate expression in the cytoplasm of enterocytes (B). Higher resolution images of the proventriculus revealed Magro-EGFP in large vesicles that extend from the abundant expression at the anterior end of the proventriculus toward the junction with the midgut lumen (yellow boxes in C,D,E, shown in panels F,G,H). Lysotracker Red stains the large acidic vesicles that contain Magro-EGFP protein (D,E,G,H).
magro acts within the intestinal lumen to maintain TAG homeostasis
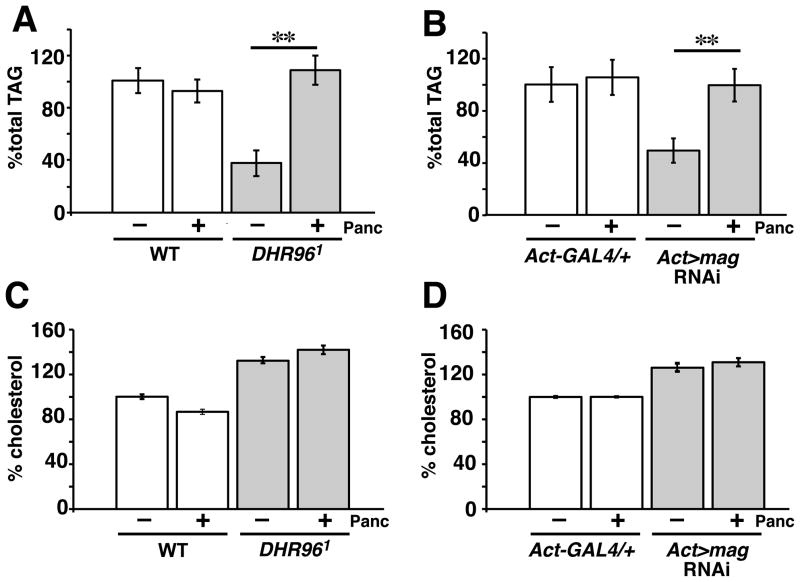
As a first step toward determining if the enzymatic activities of Magro are regionally localized within the intestine, we raised DHR96 mutant and magro RNAi animals on a diet supplemented with pancreatin. This is an enzymatic mixture of pancreatic enzymes, including TAG lipase and cholesterol esterase, that should specifically restore TAG lipase and cholesterol esterase activity in the lumen of the gut. While pancreatin supplementation had no effect on TAG levels in control animals, this diet restored wild-type levels of TAG in both DHR96 and magro RNAi animals (Figure 3A,B). Conversely, pancreatin supplementation had no impact on the elevated cholesterol levels in these animals (Figure 3C,D). These results are consistent with those seen when wild-type flies are treated with Orlistat (tetrahydrolipstatin), which acts as a competitive inhibitor of secreted lipases and cholesterol esterases inside the lumen of the intestine (Borgstrom, 1988). Although Orlistat treatment is sufficient to decrease whole animal TAG levels, as seen previously (Sieber and Thummel, 2009), it has no significant effect on total cholesterol levels (Figure S4). These data confirm our earlier studies indicating that Magro functions in the intestinal lumen to maintain appropriate levels of TAG, and demonstrate that its effects on cholesterol homeostasis are conferred within the cells of the intestinal tract.
Figure 3. Magro enzymatic activity is not required in the intestinal lumen to maintain cholesterol homeostasis.
Wild-type (WT) and Act-GAL4/+ controls, along with DHR961 mutants and Act-GAL4/UAS-magro-RNAi (Act>mag-RNAi) animals, were raised on medium supplemented with 2 mg/mls pancreatin, an enzymatic mixture of pancreatic TAG lipase and cholesterol esterase. Mature adults were collected and assayed for TAG (A,B) and cholesterol (C,D) levels. The data were normalized to protein levels and are presented relative to a wild-type level of 100%. Error bars represent ±SEM. (**p< 0.0001)
magro functions in the proventriculus to promote the breakdown of dietary TAG
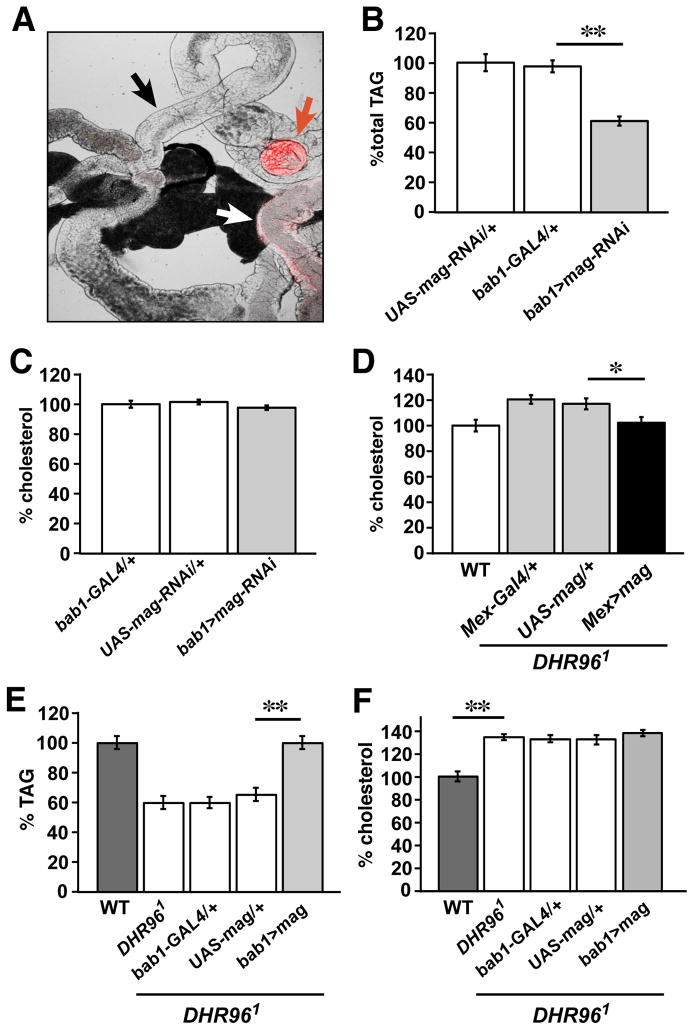
The apparent vesicular trafficking of Magro protein from the proventriculus toward the body of the midgut provides a mechanism to explain its delivery into the lumen of the intestine. If this model is correct, then disrupting magro function specifically in the proventriculus should have an effect on TAG levels, but little or no effect on cholesterol homeostasis. To test this hypothesis, we used the bab1-GAL4 driver, which is expressed highly in the proventriculus and weakly expressed near the midgut-hindgut junction (Figure 4A) (Cabrera et al., 2002). Using this construct to direct magro RNAi resulted in a significant decrease in whole animal TAG levels (Figure 4B), similar to that seen with the pan-intestinal Mex-GAL4 driver (Sieber and Thummel, 2009). In contrast, proventriculus-specific magro RNAi has no effect on total cholesterol levels (Figure 4C). We conclude that the TAG metabolic functions of Magro are restricted to the proventriculus, while its cholesterol regulatory function resides outside the proventriculus in the intestine.
Figure 4. DHR96 regulation of magro in distinct regions of the intestine coordinates TAG and cholesterol homeostasis.
(A) bab1-GAL4 drives high levels of UAS-NLS-DsRed expression in the proventriculus (red arrow) with no detectable expression in the body of the midgut (black arrow) and low levels near the midgut-hindgut junction (white arrow). (B,C) Proventriculus-specific RNAi for magro results in reduced levels of TAG, but has no effect on cholesterol. bab1-GAL4/+ and UAS-magro-RNAi/+ control flies, along with bab1-GAL4/UAS-magro-RNAi (bab1>mag-RNAi) animals were assayed for (B) TAG and (C) cholesterol. The data were normalized to protein levels and are presented relative to a wild-type level of 100%. (D) Midgut-specific expression of magro is sufficient to partially rescue the elevated cholesterol levels in DHR96 mutants. Wild-type (WT) and DHR96 mutants, carrying either the Mex-GAL4 driver alone, UAS-magro transgene alone, or both Mex-GAL4 and UAS-magro (Mex>mag) to express magro in the midgut, were assayed for total cholesterol levels. The data were normalized to protein levels and are presented relative to a wild-type level of 100%. (E,F) Proventriculus-specific expression of magro is sufficient to rescue the lean phenotype of DHR96 mutants, but has no effect on its elevated cholesterol levels. Wild-type (WT) and DHR96 mutants, carrying either the bab1-GAL4 driver alone, UAS-magro transgene alone, or both bab1-GAL4 and UAS-magro (bab1>mag) to express magro in the proventriculus, were assayed for (E) TAG and (F) cholesterol. The data were normalized to protein levels and are presented relative to a wild-type level of 100%. Error bars represent ±SEM (*p< 0.01 **p< 0.0001).
DHR96 regulates major aspects of lipid metabolism through its target gene magro
If decreased magro expression is physiologically relevant to the cholesterol accumulation phenotype seen in DHR96 mutants, then restoring magro function in these animals should rescue their defect in cholesterol homeostasis. Consistent with this hypothesis, expressing wild-type magro specifically in the intestine of the DHR96 mutant is sufficient to reduce the cholesterol levels in these animals (Figure 4D). This rescue, however, is not complete, consistent with the multiple levels of cholesterol metabolism that are regulated by DHR96 (Horner et al., 2009; Bujold et al., 2010). Moreover, specific expression of magro in the proventriculus of DHR96 mutants effectively rescues their lean phenotype (Figure 4E), but has no significant effect on their elevated cholesterol levels (Figure 4F). Taken together with our other data, these results indicate that the region-specific enzymatic activities of Magro correspond to the major lipid metabolic functions of DHR96. DHR96 regulation of magro expression in the proventriculus maintains an appropriate level of TAG lipase activity in the intestinal lumen to facilitate dietary lipid break down, while DHR96 regulation of magro in the body of the intestine promotes the clearance of excess sterols.
Discussion
Magro and LipA share conserved functions in maintaining lipid homeostasis
Relatively little is known about the mechanisms that regulate cholesterol metabolism in Drosophila, with most studies focused on DHR96 and the Niemann-Pick (NPC) disease gene homologs, which play important roles in dietary cholesterol absorption and intracellular cholesterol trafficking (Niwa and Niwa, 2011). In this paper, we identify the intestine as a key tissue for maintaining cholesterol homeostasis and define a central role for the LipA homolog, Magro, in mediating this function. Like LipA, Magro has dual enzymatic activities, cleaving both TAG and cholesterol esters, consistent with their common fatty acid ester bonds (Ameis et al., 1994). Mouse LipA mutants display a lack of stored fat in the form of white adipose tissue along with excess cholesterol esters (Du et al., 2001), reflecting the major defects in magro RNAi animals. Similar phenotypes are seen in human LipA mutants suffering from CESD and Wolman’s disease (Burke and Schubert, 1972). Patients with Wolman’s disease also have digestive dysfunction, which may be related to the defects in lipid uptake that we observe in magro RNAi animals. In addition to these shared phenotypes, however, mammalian LipA mutants display massive accumulations of lipid in the liver, spleen, and intestine – defects that are not apparent in magro RNAi animals (Du et al., 2001). Nonetheless, the parallels between Magro and LipA function in flies and humans establish Drosophila as a system to further our understanding of CESD and Wolman’s disease, and define the ancestral function for this class of acid lipases, demonstrating their central role in the intestine to coordinate TAG and cholesterol homeostasis.
Interestingly, the dual enzymatic functions of Magro appear to arise from distinct regions of the intestine. Disruption of magro function specifically in the proventriculus blocks its TAG lipolytic activity, but has no effect on the levels of cholesterol in these animals (Figure 4B,C). In contrast, magro RNAi throughout the intestine affects both TAG and cholesterol homeostasis (Figure 1D). These region-specific activities are consistent with our dietary supplementation studies with pancreatin and Orlistat (Figure 3,S4). They are also consistent with the expression pattern of Magro-EGFP protein, which provides insights into how the dual functions of this enzyme are manifested. Magro-EGFP is expressed abundantly in the large outer columnar cells in the anterior half of the proventriculus (Figures 2A, S2). We also see a stream of large acidic vesicles that originate from this region and move in a posterior direction toward the lumen of the intestine (Figure 2F–H, S3). This apparent vesicular trafficking of Magro is consistent with the cells at the anterior end of the proventriculus having secretory functions, depositing peritrophic matrix components into the lumen that lies between the outer and inner cell layers of the proventriculus (King, 1988) (Figure S2). The peritrophic matrix is a meshwork of chitin and glycoproteins that provides a protective lining within the gut, much like the mucosal layer of the mammalian intestine (Hegedus et al., 2009). The observation that the Magro-EGFP vesicles reside in the same region of the proventriculus as the developing peritrophic matrix suggests that they are synthesized and exported into the lumen of the gut in a similar manner. This also raises the possibility that the digestive enzymes that are embedded in the peritrophic matrix may originate from vesicular trafficking in the proventriculus. Many genes with predicted digestive functions, including glucosidases, mannosidases, and endopeptidases, are regulated by DHR96 and expressed in the intestine, like magro (Sieber and Thummel, 2009). Several genes that contribute to the peritrophic matrix are also regulated by DHR96. It would be interesting to determine if these proteins are synthesized and secreted in a coordinated manner by the proventriculus.
In addition to its abundant expression in the proventriculus, Magro-EGFP is also present at lower levels throughout the intestine, visible as punctate cytoplasmic staining in the enterocytes (Figure 2B). This expression pattern provides a possible mechanism to explain the role of Magro in maintaining cholesterol homeostasis. We propose that Magro acts as a cholesterol esterase in the enterocytes, breaking down stored cholesterol to facilitate its elimination from the intestine. This model is consistent with the neutral lipid stores that are known to reside in the Drosophila intestine, second only to the fat body. It is also consistent with recent evidence that LipA acts as cholesterol esterase in macrophage foam cells to promote ABCA1-mediated cholesterol efflux (Ouimet et al., 2011). These data suggest that LipA and Magro may break down stored cholesterol esters upstream of reverse cholesterol transport and transintestinal cholesterol efflux to promote the clearance of excess sterols. Tissue-specific studies of LipA function in the pancreas and intestine may provide a clearer understanding of its relationship to the apparent exocrine role of Magro in the proventriculus and its ability to promote cholesterol clearance in the intestine.
Finally, our data provide new directions to understand the roles of LXR family members in lipid metabolism. Both DHR96 mutants and magro RNAi animals display reduced levels of TAG and elevated levels of cholesterol, and restoring magro expression in the intestines of DHR96 mutant animals largely rescues these defects, establishing magro as a key target for DHR96 regulation (Figures 1,4C)(Sieber and Thummel, 2009). These functions for DHR96 parallel those of its mammalian homolog, LXR. LXR activation specifically in the intestine results in a dramatic increase in fecal sterol excretion that correlates with increased expression of the ABCG5/ABCG8 sterol transporter (van der Veen et al., 2009; Lo Sasso et al., 2010). This observation suggests that LXR promotes reverse cholesterol transport in this tissue, which represents the best characterized mechanism for eliminating excess cholesterol from the body. Reverse cholesterol transport involves HDL-mediated transport of cholesterol from peripheral tissues to the hepatobiliary tract, leading to the removal of excess sterol by biliary excretion from the body. Genetic studies of key components in biliary cholesterol excretion, however, such as abcb4 mutants and abcg5/abcg8 double mutants, have challenged the importance of reverse cholesterol transport for cholesterol excretion and have led to the proposal that the intestine plays a more direct role in this process (van der Velde et al., 2010). These studies are shifting the focus of cholesterol efflux toward the intestine and implicate a central role for LXR in regulating intestinal cholesterol clearance not only through regulation of reverse cholesterol transport, but also through novel targets involved in transintestinal cholesterol efflux (Kruit et al., 2005). In addition, our evidence that intestinal cholesterol esterase activity is critical for clearing excess sterol from the body suggests that acid lipases such as LipA may function downstream from LXR to maintain cholesterol homeostasis. Although there is no direct evidence that LXR regulates LipA expression, a recent study has shown that elevated levels of oxidized LDL can repress LipA expression in endothelial cells, and this effect can be reversed by treatment with LXR agonists (Heltianu et al., 2011). Further studies are required to determine if the regulatory links between LXR, LipA, and cholesterol homeostasis have been conserved through evolution, and if Drosophila can be used as a simple model system to better define the mechanisms of transintestinal cholesterol efflux.
Experimental Procedures
Fly stocks
The following stocks were used in this study: Canton S, DHR961 (King-Jones et al., 2006), Mex-Gal4 (Phillips and Thomas, 2006), Act-Gal4/CyO (Bloomington # 25374), UAS-magro (Sieber and Thummel, 2009), UAS-DHR96 (Horner et al., 2009), bab1-GAL4/TM3 (Cabrera et al., 2002). Flies were maintained on Standard Bloomington Stock Center medium with malt at 25°C.
Metabolite assays
Newly eclosed adult male flies were aged 5–7 days prior to use for all experiments. TAG and cholesterol assays were conducted as described (Horner et al., 2009; Sieber and Thummel, 2009) All results shown are derived from 12 samples of five animals collected from each genotype under each condition, and repeated at least three times. A representative experiment is shown in each Figure.
Statistical analyses
Statistical analysis was done for each experiment using an unpaired two-tailed Student’s t-test with unequal variance. All quantitative data are reported as the mean ± SEM. The n and SEM for each data point is derived from the 12 samples of five animals collected from each genotype under each condition.
Supplementary Material
Research Highlights.
The Drosophila LipA homolog, Magro, coordinates TAG and cholesterol homeostasis.
This study implicates the midgut as a key tissue for sterol clearance in Drosophila.
The proventriculus appears to have exocrine digestive function in Drosophila.
DHR96 coordinates lipid metabolism through direct regulation of magro.
Acknowledgments
We thank the Bloomington Stock Center for providing stocks, FlyBase for critical information that made these studies possible, and M. Babst for helpful discussions. We also thank D. Bricker, J. Misra, and J. Tennessen for critical comments on the manuscript. M.S. was supported by an NIH Developmental Biology Predoctoral Training Grant (5T32 HD07491). This research was supported by NIH grant 2R01DK075607.
Footnotes
Supplemental data include Supplemental Experimental Procedures and Supplemental Figures, and can be found with this article online at http://
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ameis D, Merkel M, Eckerskorn C, Greten H. Purification, characterization and molecular cloning of human hepatic lysosomal acid lipase. Eur J Biochem. 1994;219:905–914. doi: 10.1111/j.1432-1033.1994.tb18572.x. [DOI] [PubMed] [Google Scholar]
- Borgstrom B. Mode of action of tetrahydrolipstatin: a derivative of the naturally occurring lipase inhibitor lipstatin. Biochem Biophys Acta. 1988;962:308–316. doi: 10.1016/0005-2760(88)90260-3. [DOI] [PubMed] [Google Scholar]
- Bujold M, Gopalakrishnan A, Nally E, King-Jones K. Nuclear receptor DHR96 acts as a sentinel for low cholesterol concentrations in Drosophila melanogaster. Mol Cell Biol. 2010;30:793–805. doi: 10.1128/MCB.01327-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JA, Schubert WK. Deficient activity of hepatic acid lipase in cholesterol ester storage disease. Science. 1972;176:309–310. doi: 10.1126/science.176.4032.309. [DOI] [PubMed] [Google Scholar]
- Cabrera GR, Godt D, Fang PY, Couderc JL, Laski FA. Expression pattern of Gal4 enhancer trap insertions into the bric a brac locus generated by P element replacement. Genesis. 2002;34:62–65. doi: 10.1002/gene.10115. [DOI] [PubMed] [Google Scholar]
- Du H, Heur M, Duanmu M, Grabowski GA, Hui DY, Witte DP, Mishra J. Lysosomal acid lipase-deficient mice: depletion of white and brown fat, severe hepatosplenomegaly, and shortened life span. J Lipid Res. 2001;42:489–500. [PubMed] [Google Scholar]
- Hegedus D, Erlandson M, Gillott C, Toprak U. New insights into peritrophic matrix synthesis, architecture, and function. Ann Rev Entomology. 2009;54:285–302. doi: 10.1146/annurev.ento.54.110807.090559. [DOI] [PubMed] [Google Scholar]
- Heltianu C, Robciuc A, Botez G, Musina C, Stancu C, Sima A, Simionescu M. Modified low density lipoproteins decrease the activity and expression of lysosomal acid lipase in human endothelial and smooth muscle cells. Cell Biochem Biophys. 2011:1–8. doi: 10.1007/s12013-011-9190-8. [DOI] [PubMed] [Google Scholar]
- Horner MA, Pardee K, Liu S, King-Jones K, Lajoie G, Edwards A, Krause HM, Thummel CS. The Drosophila DHR96 nuclear receptor binds cholesterol and regulates cholesterol homeostasis. Genes Dev. 2009;23:2711–2716. doi: 10.1101/gad.1833609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaany NY, Mangelsdorf DJ. LXRs and FXR: the yin and yang of cholesterol and fat metabolism. Annu Rev Physiol. 2006;68:159–191. doi: 10.1146/annurev.physiol.68.033104.152158. [DOI] [PubMed] [Google Scholar]
- King DG. Cellular organization and peritrophic membrane formation in the cardia (proventriculus) of Drosophila melanogaster. J Morph. 1988;196:253–282. doi: 10.1002/jmor.1051960302. [DOI] [PubMed] [Google Scholar]
- King-Jones K, Horner MA, Lam G, Thummel CS. The DHR96 nuclear receptor regulates xenobiotic responses in Drosophila. Cell Metab. 2006;4:37–48. doi: 10.1016/j.cmet.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Kruit JK, Plosch T, Havinga R, Boverhof R, Groot PH, Groen AK, Kuipers F. Increased fecal neutral sterol loss upon liver X receptor activation is independent of biliary sterol secretion in mice. Gastroenterology. 2005;128:147–156. doi: 10.1053/j.gastro.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Lo Sasso G, Murzilli S, Salvatore L, D’Errico I, Petruzzelli M, Conca P, Jiang ZY, Calabresi L, Parini P, Moschetta A. Intestinal specific LXR activation stimulates reverse cholesterol transport and protects from atherosclerosis. Cell Metab. 2010;12:187–193. doi: 10.1016/j.cmet.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Lusis AJ, Pajukanta P. A treasure trove for lipoprotein biology. Nat Genet. 2008;40:129–130. doi: 10.1038/ng0208-129. [DOI] [PubMed] [Google Scholar]
- Niwa R, Niwa YS. The fruit fly Drosophila melanogaster as a model system to study cholesterol metabolism and homeostasis. Cholesterol. 2011;2011:176802. doi: 10.1155/2011/176802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet M, Franklin V, Mak E, Liao X, Tabas I, Marcel YL. Autophagy regulates cholesterol efflux from macrophage foam cells via Lysosomal Acid Lipase. Cell Metab. 2011;13:655–667. doi: 10.1016/j.cmet.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MD, Thomas GH. Brush border spectrin is required for early endosome recycling in Drosophila. J Cell Sci. 2006;119:1361–1370. doi: 10.1242/jcs.02839. [DOI] [PubMed] [Google Scholar]
- Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, et al. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber MH, Thummel CS. The DHR96 nuclear receptor controls triacylglycerol homeostasis in Drosophila. Cell Metab. 2009;10:481–490. doi: 10.1016/j.cmet.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen JN, van Dijk TH, Vrins CL, van Meer H, Havinga R, Bijsterveld K, Tietge UJ, Groen AK, Kuipers F. Activation of the liver X receptor stimulates trans-intestinal excretion of plasma cholesterol. J Biol Chem. 2009;284:19211–19219. doi: 10.1074/jbc.M109.014860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velde AE, Brufau G, Groen AK. Transintestinal cholesterol efflux. Curr Opin Lipidol. 2010;21:167–171. doi: 10.1097/MOL.0b013e3283395e45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.