Abstract
Inwardly rectifying potassium (Kir) channels are essential for maintaining normal potassium homeostasis and the resting membrane potential. As a consequence, mutations in Kir channels cause debilitating diseases ranging from cardiac failure to renal, ocular, pancreatic, and neurological abnormalities. Structurally, Kir channels consist of two trans-membrane domains, a pore-forming loop that contains the selectivity filter and two cytoplasmic polar tails. Within the cytoplasmic structure, clusters of amino acid sequences form regulatory domains that interact with cellular metabolites to control the opening and closing of the channel. In this review, we present an overview of Kir channel function and recent progress in the characterization of selected Kir channel mutations that lie in and near a C-terminal cytoplasmic ‘hotspot’ domain. The resultant molecular mechanisms by which the loss or gain of channel function leads to organ failure provide potential opportunities for targeted therapeutic interventions for this important group of channelopathies.
Keywords: Inwardly rectifying potassium channel (Kir), Channelopathy, Andersen-Tawil syndrome, Bartter syndrome, DEND syndrome, EAST/SeSAME syndrome, Phosphoinositides, KCNJ, cytoplasmic bPbbb cluster, retinopathy
Introduction
In multi-cellular organisms, cells are characterized by energetically favorable gradient moving potassium from the intracellular to the extracellular environment. Inwardly rectifying potassium-selective (Kir), channels encoded by the KCNJ gene family are constitutively active and favor the influx of potassium more readily than its efflux from the cells, thereby maintaining potassium homeostasis. Kir channels are also known as IRK or KCNJ channels. Fifteen mammalian KCNJ gene products have been described which result in seven distinct Kir channels [1, 2]. These channels are located within the plasma membrane of most cell types, where they regulate membrane potential and potassium homeostasis (Table 1). Kir channels contribute to functions such as the repolarization of cardiac action potentials, trans-epithelial transport, and the maintainance of the voltage gradient across the cell membrane. These functions are achieved by regulating the opening and closing (i.e., gating) of Kir channels [2]. For this reason, genetic alterations in Kir channels underlie many of the hereditary ion channel diseases known as channelopathies, and which affect the function of multiple organ systems [3, 4].
Table 1.
Kir gene, protein and tissue distribution.
| Gene | Protein & Other Identifiers |
Chromosome Location |
Main Tissue Localization |
References |
|---|---|---|---|---|
| KCNJ1 | Kir1.1, ROMK, ROMK1 | 11q24 | Kidney | 34 |
| KCNJ2 | Kir2.1, HHIRK1,IRK1 | 17q23.1–q24.2 | Heart, Skeletal Muscle | 39, 40, 41 |
| KCNJ10 | Kir4.1 | 1q23.2 | Glia (Retinal Müller cells), Kidney, Cochlea | 69, 70 |
| KCNJ11 | Kir6.2, BIR | 11p15.1 | Beta cells, Neurons, Endocrine & Muscle cells | 90 |
| KCNJ13 | Kir7.1 | 2q37 | Retina, Small Intestine, Stomach, Kidney | 107 |
Kir channel structure and the intracellular regulatory “hotspot”
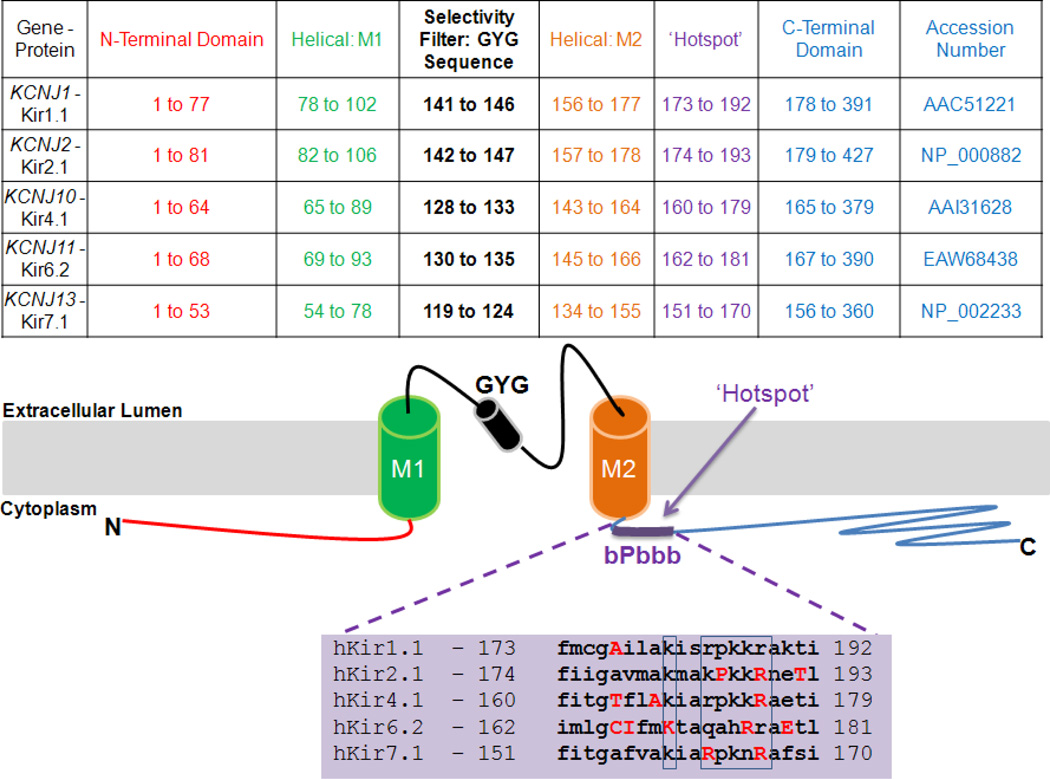
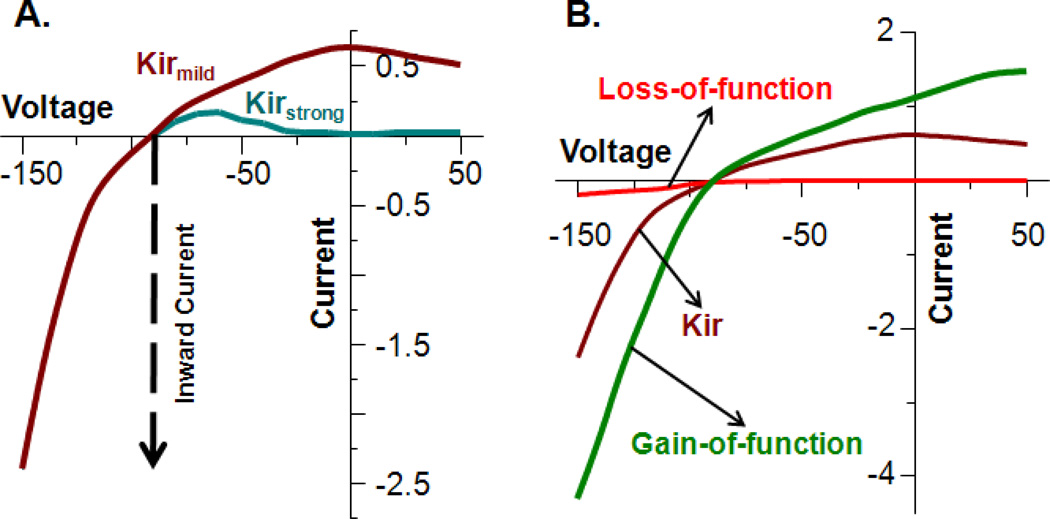
High-resolution structural characterization of Kir channels predicts that their protein subunits consist of an N-terminal cytoplasmic domain followed by a trans-membrane domain, then by a pore-forming, P-loop sequence that includes the selectivity filter, followed in turn by a second trans-membrane domain, and lastly, by a C-terminal cytoplasmic domain [5, 6] (Figure 1). Four such subunits interact to form a tetramer that creates a single-pore channel. The channel may be either homo- or hetero-tetrameric [7]. A subgroup of Kir channels conduct K+ ions into the cells most effectively (named strong inward rectifiers) whereas others more modestly facilitate the efflux of K+ (mild inward rectifiers: Kir4.1 and Kir7.1) additionally (Figure 2). The crystal structure of a eukaryotic Kir channel (Chicken Kir 2.2) showed that in the case of the strong inward rectifiers, binding of polyvalent cations like Mg2+ and polyamines to concentric rings of acidic amino acids on the inner face of the pore block K+ efflux out of the cell [6]. The cytoplasmic portion of the channel thus serves as a site for regulatory modifications that result in the opening or closing of the channel [6, 8]. Cytoplasmic sequences of Kir channels possess multiple binding sites for intracellular regulators such as H+, Mg2+, ATP, phosphoinositides, membrane cholesterol, long chain acyl Coenzyme A, polyamines, and protein kinases A and C [9–22]. Trans-Golgi trafficking and signal sequences [23] are also found primarily in the cytoplasmic distal C-terminal sequence. Several genetic mutations have been reported to affect Kir channel conductance, either through a gain-of-function or a loss-of-function, thereby affecting potassium conductance and resulting in alterations in the current-voltage relationship (Figure 2) affecting cellular physiology.
Figure 1. Kir channel topology.
The predicted amino acid positions of the cytoplasmic, trans-membrane, and extracellular domains of the selected Kir channel subunits. The membrane topology illustrating the localization of two cytoplasmic (N and C terminal), two trnasmembrane (M1 and M2), and extracellular GYG selectivity loop with reference to plasma-membrane is represented. The cytoplasmic ‘hotspot’ is highlighted with sequence homology amongst human Kir channels compared. Highlighted aminoacids within and nearby the ‘hotspot’ are shown that represent disease-causing mutations.
Figure 2. Inward rectification properties of Kir channels.
Current amplitude in response to membrane voltage is shown by representative current-voltage (I–V) relationships of Kir channels with both strong inward rectifiers (A. aqua trace), or mild inward rectifiers illustrated (A. dark red trace). Current in the negative direction (inward current) is indicated by a downward arrow and current in the positive direction is the outward current. For strong inward rectifiers, the outward current is completely blocked by intracellular factors affecting the I–V relationship as compared to the persistent outward current demonstrated by mild inward rectifiers. B) I–V relationship model of a mildly inward rectifier channel (B. dark red trace, as in A.) showing predicted changes in both inward and outward current due to either a gain-of-function (B. green trace) or loss-of-function (B. red trace) due to genetic mutation(s).
Phosphoinositides, e.g. PIP2, are important regulators of Kir channel function [24–27]. PIP2 is found in the cytoplasmic leaflet of the plasma membrane. The distribution of this inositol phosphate is dynamic, and is precisely controlled by lipid kinases, phospholipases and phosphatases [28]. D’Avanzo and colleagues have recently demonstrated that PIP2 in the eukaryotic cell membrane serves as an evolutionary adaptation for the direct activation of Kir channels by PIP2 [29]. A cluster of positively charged amino acid residues in the C-terminal cytoplasmic domain creates a site that supports an electrostatic interaction between the Kir channel and the PIP2 head group [24] (Figure 1). This cytoplasmic ‘hotspot’ is defined by a cluster of basic amino acids known as the bPbbb cluster, wherein b represents a basic amino acid residue and P represents proline, a polar uncharged residue. This ‘hotspot’ is found near the inner plasma membrane leaflet at the beginning of the C-terminal cytoplasmic domain, immediately following the second trans-membrane domain (Figure 1). Although mutations in any aspect of the protein structure may result in channel dysfunction, in this review we will focus on those reported mutations that lie in or near to the bPbbb hotspot (Table 2).
Table 2.
Genetic correlation between Kir channel hotspot mutations and disease.
| Gene-Protein | Mutation | Disease | Inheritance | References |
|---|---|---|---|---|
| KCNJ1- Kir1.1 | Ala(A) 177 Thr(T) | Hyperprostaglandin E Syndrome/antenatal Bartter Syndrome | Autosomal Recessive - Homozygous Variant | 37 |
| KCNJ2 - Kir2.1 | Pro(P) 186 Leu(L) | Andersen-Tawil Syndrome | Autosomal Dominant | 45, 46 |
| Arg(R) 189 Ile(I) | 40, 45 | |||
| Thr(T) 192 Ala(A) | 47, 48 | |||
| KCNJ10 -Kir4.1 | Thr(T) 164 Ile(I) | SeSAME Syndrome (Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance) | Autosomal Recessive - Homozygous Variant | 72 |
| Ala(A) 167 Val(V) | Autosomal Recessive - Compound heterozygous | 72 | ||
| Arg(R) 175 Gln(Q) | EAST Syndrome (Epilepsy, Sensorineural Deafness, Tubulopathy) - same as SeSAME | Autosomal Recessive | 73 | |
| KCNJ11 -Kir6.2 | Cys(C) 166 Phe(F) | DEND Syndrome (Developmental Delay, Epilepsy, and Neonatal Diabetes) - Gain-of-Function mutation | Sporadic, de novomutation - Heterozygous activating mutation | 94 |
| Ile(I) 167 Leu(L) | 96 | |||
| Lys(K) 170 Arg(R) | Permanent Neonatal Diabetes Mellitus (PNDM) | 97 | ||
| Lys(K) 170 Asn(N) | 97 | |||
| Arg(R) 176 Cys(C) | 98 | |||
| Glu(E) 179 Ala(A) | Transient Neonatal Diabetes Mellitus (TNDM) | 102 | ||
| KCNJ13 -Kir7.1 | Arg(R) 162 Trp(W) | Snowflake Vitreoretinal Degeneration | Autosomal Dominant | 112 |
| Arg(R) 166 Trp(W) | Leber's Congenital Amaurosis | Homozygous nonsense | 114 |
The various members of the Kir family can be subdivided into three distinct groups based upon their sensitivity to PIP2 regulation of channel function, with low (Kir3.1 and Kir6.1), intermediate (Kir1.1 and Kir7.1) and high (Kir2.1 and 4.1) sensitivity defined by phosphoinositide binding specificity [30]. Phosphoinositide specificity cannot be predicted by the amino acid signature of the positively charged ‘hotspot’, nor does phosphoinositide binding specificity determine the degree of inward rectification. For example, Kir2.1 is a strong inward rectifier whereas Kir4.1 is a weak inward rectifier, but both are highly sensitive to the regulatory effects of PIP2 [31]. The prokaryotic bacterial Kir channel KirBac 1.1 is inhibited by PIP2 and lacks the regulatory residues that are conserved in the transmembrane-cytoplasmic linkers of eukaryotes whose displacement upon electrostatic interaction with PIP2 gates eukaryotic Kir channels [29]. The Kir family and its modifiers therefore provide a sensitive and specific partnership that contributes to the regulation of a number of metabolic pathways.
In this review, we will focus on the correlation between genetic alterations that lie within and around the cytoplasmic ‘hotspot’ cluster of positively charged residues (Figure 1), and the metabolic consequences of the resultant Kir-associated channelopathies.
Defects in Kir channel function due to mutations within the ‘hotspot’
Kir1.1 (KCNJ1)
The KCNJ1 gene encodes Kir1.1, also known as the Renal Outer Medullary K+ channel (ROMK) [1, 32, 33]. Kir 1.1 is localized to kidney epithelial cells [34] and plays a crucial role in reabsorbing salt via potassium recycling in the kidney at the level of the thick ascending limb (TAL) of Henle’s loop [35] (Figure 3). Kir1.1 works in conjunction with a Na+-K+-2Cl− co-transporter (NKCC2) to ensure proper salt and water transport between cells of the TAL and the lumen of the renal tubule. Mutations in genes encoding NKCC2 and Kir1.1 result in altered function of these ion channels and are a cause of Hyperprostaglandin E syndrome, an autosomal recessive disorder also known as Bartter syndrome (Table 2). Bartter syndrome has both neonatal and classic forms, with the classic form typically presenting in school-age children. Bartter syndrome is characterized by hypokalemic alkalosis, hyperprostaglandinuria, and hypercalciuria associated with nephrocalcinosis. The neonatal form is associated with polyhydramnios which may lead to premature birth. Affected infants suffer severe postnatal salt and water losses that can lead to life-threatening dehydration [36].
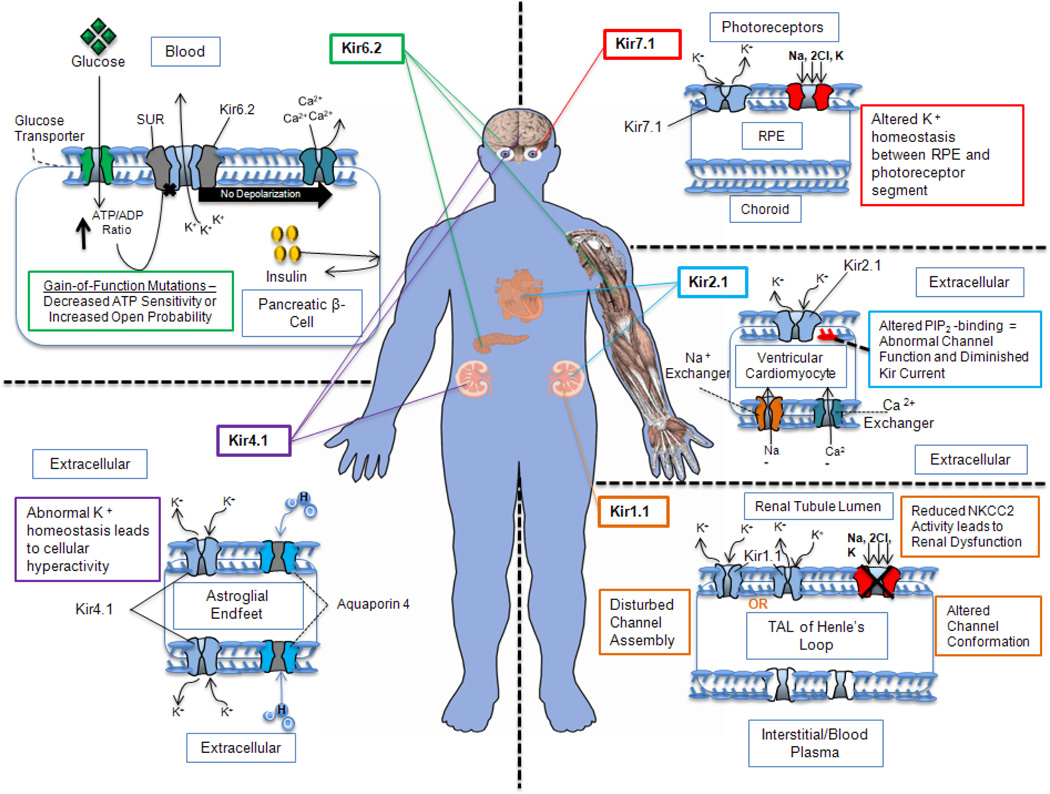
Figure 3. Tissue distribution of Kir channel subunits.
The tissue-specific distribution of the Kir channels suggests that they play an important role in ion homeostasis and disease. Kir channel subunits are indicated by light blue within the membrane structure. All other possible associated channels, transporters and regulatory molecules are also shown in the membrane that controls cellular physiology. Kir channels tissue distribution along with their respective physiopathology are color-coded (Kir1.1- orange; Kir2.1- blue; Kir4.1- purple; Kir6.2- green and Kir7.1- red). Abbreviations: Kir, inwardly rectifying potassium channel; SUR, regulatory suramine subunit; ATP, adenosine tri-phosphate; ADP, adenosine di-phosphate; RPE, retinal pigment epithelium; PIP2, phosphatidylinositol (4,5)-bisphosphate; TAL, thick ascending limb.
One mutation in the KCNJ1 gene that has been associated with Bartter syndrome lies within the C-terminal cytoplasmic domain of the Kir1.1 protein, slightly upstream from the ‘hotspot’, and results in a nonpolar, hydrophobic Alanine at position 177 being changed to a polar Threonine residue (A177T). Electrophysiological analyses show a decrease in K+ conductance by the mutant channel that is either due to disrupted channel assembly or due to altered channel conformation within the pore-forming domain [37] (Figure 3). Mutations within the ‘hotspot’ of the Kir1.1 channel affect PIP2 binding but have not been associated with a disease phenotype to-date [27, 31, 38].
Kir2.1 (KCNJ2)
The KCNJ2 gene encodes the protein subunits of the Kir2.1 channel [1]. Kir 2.1 is highly expressed in cardiac and skeletal muscle in addition to neural tissue [39] (Figure 3). It plays a crucial role in determining the resting membrane potential and in controlling the duration of action potentials in excitable cells [40, 41]. In cardiac myocytes, the steep inwardly rectifying K+-current via the Kir2.1 channel is responsible for the terminal, phase 3 repolarization of the action potential [42]. Loss of Kir2.1 current lengthens the ventricular action potential and prolongs the QT interval, putting affected individuals at risk of developing ventricular tachyarrhythmias [41]. Mutations within the ‘hotspot’ in the KCNJ2 gene are associated with Andersen-Tawil syndrome (ATS) [43], a rare autosomal dominant disease [44] that is characterized by cardiac arrhythmias, periodic paralysis, short stature, and dysmorphic features that include cleft palate, low-set ears, and limb abnormalities (syndactyly, bradydactyly, clinodactyly) [41, 45, 46] (Figure 3). No genotype-phenotype correlations have been described between the mutations and the various clinical features of ATS.
Three loss-of-function mutations have been described in the KCNJ2 gene. All three occur in and around the highly conserved PIP2-binding domain and result in an amino acid with an electron dense R-group being converted to a non-polar group. The altered electrostatic interactions between the channel and PIP2 result in abnormal channel function. Two of the mutations lie within the C-terminal cytoplasmic ‘hotspot’ of the Kir2.1 protein and exhibit decreased affinity of Kir2.1 for PIP2, which may explain the loss of channel function [Table 2] [40].
KCNJ2 mutation T192A converts a polar, hydrophilic Threonine to a nonpolar, hydrophobic Alanine at amino acid position 192, slightly downstream from the cytoplasmic ‘hotspot’ [32]. Heteromeric channels (possessing both wild-type and T192A mutant subunits) have partial levels of Kir2.1-mediated current, whereas homomeric T192A Kir2.1 channels have a complete loss of function [47]. It is likely that the clinical findings in ATS in its most severe form are due to abolished Kir2.1-PIP2 interactions and a nonfunctional channel, whereas mutations that simply result in reduced activity of Kir2.1 lead to the less severe forms of ATS [48].
When there is complete loss of Kir2.1 channel activity, an increased frequency of spontaneous action potentials is seen that is likely triggered by altered function of the Na+/Ca2+ exchanger [48] (Figure 3). Reduction in potassium current in ventricular myocytes leads to spontaneous ventricular activity. The cardiac arrhythmia observed in ATS results when the cardiac action potential is prolonged due to a reduction or absence of the repolarizing current normally attributed to Kir2.1 activity [49]. In skeletal muscle, reduced Kir2.1 activity depolarizes the resting membrane potential through inactivation of Na+ channel function and results in the periodic paralysis experienced by some individuals with KCNJ2 mutations [49].
Kir3.1 (KCNJ3)
The KCNJ3 gene encodes the Kir3.1 channel subunit [1, 50, 51]. The Kir3.1 channel is a G protein-coupled inward rectifier K+ channel (GIRK1) [52, 53] activated by serotonin, muscarinic and opioid signaling of G protein subunits [54–59]. Kir3.1 channels form a heteromeric complex with other Kir3 channels [60] and are present in a variety of human tissues, including brain, heart, eye and muscle tissue [61–67]. Like other Kir channels, the C-terminus of Kir3 channel containing the cytoplasmic ‘hotspot’ is also proposed to interact with PIP2 to regulate channel function [68], but there have been no reports of a mutation within the ‘hotspot’ that has been associated with a channelopathy.
Kir4.1 (KCNJ10)
The KCNJ10 gene encodes the Kir4.1 channel subunit. Kir4.1 is expressed in glial cells of the central nervous system, Müller cells of the retina, and in cochlea [1, 69, 70] (Figure 3). Kir4.1 also plays a crucial role in facilitating salt reabsorption in the distal convoluted tubule of the kidney, where it has been hypothesized to recycle potassium by transporting salts down an electrochemical gradient from the tubular lumen into the cell, in combination with the sodium-potassium pump (Na+/K+-ATPase) [71, 72] (Figure 3).
The tissue distribution of Kir4.1 explains why loss-of-function mutations in KCNJ10 have been associated with the autosomal recessive SeSAME syndrome (Seizures, Sensorineural deafness, Ataxia, Mental retardation, and Electrolyte imbalance), also known as EAST syndrome (Epilepsy, Ataxia, Sensorineural deafness, and Tubulopathy)) [73–75] (Table 2). Loss of Kir4.1 channel function in the brain or spinal cord induces astrocyte depolarization, loss of K+ clearance, and a reduced seizure threshold as seen in the case of reactive gliosis (Figure 3) [76, 77]. In contrast, epithelial transport abnormalities occur when Kir4.1 function is lost in the kidney or in the cochlea. Loss of cochlear function contributes to the generation of an abnormal endocochlear potential and consequent hearing loss [78]. Loss of Kir4.1 function in the kidney leads to abnormal salt reabsorption in the distal convoluted tubule which leads to serum electrolyte abnormalities [74]. Several members of the Kir channel family are expressed in the retina [79], and although there have been no clinically significant vision abnormalities described, the mutations associated with SeSAME/EAST syndrome do result in altered retinal physiology [80].
Two SeSAME/EAST-associated mutations have been described in the KCNJ10 gene that are found immediately upstream of the C-terminal cytoplasmic ‘hotspot’ (Table 2). A third mutation lies directly within the ‘hotspot’ and results in a polar threonine at position 164 changing to a hydrophobic isoleucine (T164I). The isoleucine substitution prevents the formation of a hydrogen bond with the lysine residue at amino acid position 67 that likely controls both the pH and PIP2 gating [81]. The loss of the hydrogen bond alters the channel response to pH, and this results in a loss of channel function as demonstrated by patch-clamp electrophysiology [72, 75, 82–84]. Another hotspot mutation is located within the second transmembrane domain (A167V) and is also linked with defects in channel gating [82]. Expression of this mutation leads to the autosomal recessive finding of decreased Kir channel current when compared to the wild type [72, 75, 82–84]. Cells expressing hotspot mutation R175Q have impaired channel function as measured by reduced current, negligible inward rectification, channel-open probabilities in the 10–15% range, and reduced pH and PIP2 sensitivity [73].
Kir5.1 (KCNJ16)
The KCNJ16 gene encodes the inwardly rectifying potassium channel 5.1 (Kir5.1) [1]. Kir5.1 channels influence the function of Kir4.1 (as in kidney) [85–87] or Kir4.2 [88] channels. Mutations in Kir5.1 likely regulate the overall K+ conductance by affecting other Kir subunits with which they assemble [89]. No specific mutations in the hotspot region have been described that are associated with a channelopathy.
Kir6.2 (KCNJ11)
The KCNJ11 gene encodes the Kir6.2 channel, also known as an ATP-sensitive potassium channel (KATP) [1, 2]. Kir6.2 regulates electrical signaling in a variety of cell types, including brain, heart, skeletal muscle, and the pancreas [2, 90], and acts by coupling K+ movement with various aspects of cellular metabolic activity [2] (Figure 3). KATP channels are made up of four pore-forming Kir6.2 subunits and four sulfonylurea receptor (SUR) subunits which respond to the absolute concentrations of ATP and ADP in the cell [91]. Kir6.2 channels are activated by ADP and are inhibited by ATP [2].
In the brain, Kir6.2 responds to the serum glucose concentration and contributes to mechanisms that protect against seizures. In skeletal muscle, it influences muscular tone. Kir6.2 protects against ischemic stress in the heart [92]. In pancreatic beta cells, Kir6.2 channels trigger insulin secretion when high levels of glucose in the blood increases the intracellular ATP concentration. The increased concentration of ATP inhibits KATP channel activity which stimulates an increase in cytosolic Ca2+ leading to the release of insulin [93]. In contrast, when glucose levels are low, ADP concentrations increase, leading to Kir6.2 channel activation resulting in a decrease in cytosolic Ca2+ and an inhibition of insulin release.
Abnormalities in insulin secretion that are present in the newborn period are associated with defects in Kir6.2 function. The mutations fall within two functional categories: 1) loss of channel function, and 2) abnormal biosynthesis or trafficking of Kir6.2 channels resulting in absent or reduced expression of the channel at the cellular membrane [94]. In other instances, gain-of-function mutations lead to Permanent or Transient Neonatal Diabetes Mellitus (PNDM or TNDM). Developmental delay and Epileptic episodes in association with Neonatal Diabetes (DEND syndrome) occur when Kir6.2 channels become less responsive to ATP inhibition, resulting in persistent hyperpolarization and decreased insulin secretion. DEND syndrome can also be the result of altered channel biosynthesis which, in the absence of ATP inhibition, increases the stability of the open state [95]. Five de novo heterozygous activating mutations have been described that occur near, and a sixth mutation is found within, the C-terminal cytoplasmic ‘hotspot’ of the Kir6.2 protein (Table 2). Genotype-phenotype correlations have shown that two of these mutations are linked to DEND syndrome (C166F, I167L) three of the mutations are associated with PNDM (K170R, K170N, R176C), and one mutation is associated with TNDM (E179A) (Table 2). The effect of these mutations lies in stark contrast to the effect of mutations located distal to the hotspot region (Y12X and L147P) that result in excessive insulin secretion and have been associated with Persistent Hyperinsulinemic Hypoglycemia of Infancy (PHHI, nesidioblastosis) [4].
The C166F mutation in the KCNJ11 gene is associated with DEND syndrome and results in the substitution of a polar, hydrophilic cysteine residue at amino acid position 166 with a nonpolar, aromatic phenylalanine [96]. The Kir6.2 C166F mutant channel has a marked increase in the probability of being in the open state, as well as a reduced sensitivity to ATP [96]. The proband exhibited severe intrauterine growth retardation, postnatal feeding problems, and at 3 months of age was diagnosed with diabetes mellitus which was confirmed by the presence of polyuria, polydipsia, hyperglycemia and ketosis. In addition, the proband experienced seizures, hypsarrhythmia, neurologic deterioration, diffuse hypotonia and had dysmorphic features.
The I167L mutation is another cause of DEND syndrome and results in the substitution of a nonpolar isoleucine with a hydrophobic leucine residue [97]. In this instance, the proband exhibited persistent hyperglycemia within hours after birth and had seizures by two weeks of age. By 3.5 years of age, the child was severely delayed, with a developmental age of 6 months. The I167L mutation increases the probability of the channel being in the open state, thereby indirectly reducing ATP inhibition which may impact normal pore gating [97].
Other mutations in or near to the ‘hotspot’ region in the KCNJ11 gene also lead to Permanent Neonatal Diabetes Mellitus (PNDM). Barbetti and colleagues studied two individuals with mutations in this region, both at residue 170, that are associated with PNDM [54] (Table 2). The first mutation, K170R, alters the basic lysine residue to a positively charged basic arginine. The second mutation, K170N, converts the basic lysine residue to a polar, hydrophilic asparagine [98]. Both probands were diagnosed with neonatal diabetes and ketoacidosis prior to 3 months of age. Although functional assays have not been performed on either the K170R or K170N mutant channels, it is known that a K170C mutation results in a nonfunctional Kir6.2 channel [98].
A third KCNJ11 mutation of interest lies directly within the ‘hotspot’ region and results in the substitution of a basic arginine with a cysteine residue at amino acid position 176 (R176C) [99]. Residue 176 is involved in PIP2 binding to the KATP channel and induction of channel opening. When PIP2 binding to the channel is decreased, it leads to channel closure and the absence of Kir6.2 activity [100–102]. The proband was diagnosed with Type 1 diabetes mellitus [99].
Transient neonatal diabetes (TNDM) typically undergoes remission during infancy, with a potential for relapse in early childhood and adolescence. Most TNDM cases result from defects in imprinting at chromosome 6q24, but surprisingly mutations in the KCNJ11 gene (which is located at 11p15.1) can also lead to this transient disease [103]. TNDM mutation E179A results in a hydrophilic glutamic acid residue being changed to a hydrophobic alanine at amino acid position 179. The E179A proband exhibited reduced birth weight, diabetes diagnosed within the first few months of life, and the remission of diabetes during infancy. Although functional assays have not been completed for this Kir6.2 mutation, the clinical manifestations suggest that mutant Kir6.2 channels in pancreatic beta-cells result in altered release of insulin [103] (Figure 3).
Kir 7.1 (KCNJ13)
The KCNJ13 gene encodes the Kir7.1 channel subunit which plays an important role in retinal physiology [1, 104]. Kir7.1 channels are mildly inwardly-rectifying [105, 106] and are expressed in multiple tissues, including kidney, intestine, stomach, thyroid, spinal cord, brain, and eye [107] (Figure 3). Partnered with NKCC transporters and the Na+-K+-ATPase in the retinal pigment epithelium (RPE) apical membrane [108], the Kir7.1 channels help to maintain the electrical potential necessary for driving trans-epithelial fluid transport [109, 110] (Figure 3). The apical aspects of RPE cells have an abundance of Kir7.1 and interdigitate with, and help to maintain potassium homeostasis around the photoreceptor outer segments (POS). Tight regulation of these channels by membrane PIP2 [111] may contribute to the light response that is mediated by RPE cells. For example, receptor-activated (P2Y) depletion of PIP2 reduces Kir7.1 channel activity in the apical membrane. The same signaling pathway also increases intracellular Ca2+ concentration through the activation of IP3 mediated release. A rise in intracellular Ca2+ concentration leads to the activation of the basal membrane Cl− conductance and results in a net depolarization of the RPE cell. This effect is typically recorded as a delayed light response originating within the RPE cell [110]. Thus, regulation of Kir7.1 channel function in the apical membrane is coupled to basal membrane conductance, and influences RPE cell physiology.
The R162W mutation within the bPbbb ‘hotspot’ in the KCNJ13 gene converts a basic arginine residue to a bulky tryptophan at Kir7.1 amino acid position 162 and is associated with Snowflake Vitreoretinal Degeneration (SVD) [112, 113] (Table 2). In the rat, a similar mutation affecting Kir7.1 results in a non-selective leaky channel and might thereby lead to premature depolarization of the RPE cells [66]. The resultant lack of regulation of transport across the RPE may contribute to the deposition of cellular metabolites as debris on the retina that is visible on the clinical fundus examination of SVD patients. Our group has recently demonstrated that a human Kir7.1 mutant clone is non-functional when is ectopically expressed in a heterologous expression system. Co-expression with the wild type Kir7.1 subunit revealed that the mutant Kir7.1 subunit has a dominant-negative effect on the heteromeric Kir7.1 channel function (Pattnaik and Pillers, unpublished results).
Another loss-of-function homozygous mutation in the hotspot domain, R166X, was recently identified in a patient suffering from Leber Congenital Amaurosis (LCA) [114] (Table 2). R166X results in an early stop codon and thereby produces a truncated Kir7.1 protein lacking most of the cytoplasmic C-terminal sequence. The C-terminal sequence is critical for the membrane trafficking of the translated protein [71]. The truncated protein likely does not successfully localize to the RPE membrane domain where Kir channels normally mediate potassium influx.
Summary
Deregulation of Kir channel function may be one of the earliest cellular events that lead to the complex multi-organ findings that are associated with Kir channelopathies. Kir channels are typically comprised of either homo- or heterotetrameric structures whose function is highly sensitive to genetic alterations in the cytoplasmic domain. Although mutations in any domain may influence channel function, in this review we have focused solely on those mutations that lie within or near to the bPbbb cluster hotspot. The importance of the ‘hotspot’ is that it plays a role as a receptor in Kir channel regulation by intracellular metabolites, such as PIP2. The C-terminal cytoplasmic ‘hotspot’ domain is critical to normal Kir channel function and thereby, mutations in this region lead to altered organ function. Given the contribution of the cytoplasmic ‘hotspot’ to Kir channel regulation, any interactions between the channel and its metabolic regulators could be important targets for the development of novel therapeutic interventions for Kir channelopathies.
Highlights.
-
*
Inwardly rectifying potassium (Kir) channels are ubiquitous.
-
*
They control cellular events from neurotransmitter release to epithelial transport.
-
*
We will focus on genetic and molecular understanding of various disease-related Kir channel mutations.
-
*
These mutations are positioned within a regulatory cytoplasmic "hotspot".
-
*
Various metabolic regulation pathways provide insight into phenotypic heterogeneity
Acknowledgements
Supported by the UW-Madison School of Medicine and Public Health, Graduate School, and the Department of Pediatrics (DMP), UW-Medical School research project (BRP), the Rebecca Meyer Brown Professorship of the UW-Eye Research Institute (Retina Research Foundation) (BRP), Meriter Hospital and the Meriter Foundation (BRP and DMP), and supported by grant 1UL1RR025011 (BRP) from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources (NCRR), NIH. The authors thank Robert Gorden for graphics and Laura Hagan for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions:
BRP, MPA, and DMP wrote this manuscript. RS wrote specific sections. All authors have approved this manuscript.
References
- 1.Sharman JL, Mpamhanga CP, Spedding M, Germain P, Staels B, Dacquet C, Laudet V, Harmar AJ. IUPHAR-DB: new receptors and tools for easy searching and visualization of pharmacological data. Nucleic Acids Res. 2011;39:D534–D538. doi: 10.1093/nar/gkq1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 3.Neusch C, Weishaupt JH, Bahr M. Kir channels in the CNS: emerging new roles and implications for neurological diseases. Cell Tissue Res. 2003;311:131–138. doi: 10.1007/s00441-002-0669-x. [DOI] [PubMed] [Google Scholar]
- 4.Abraham MR, Jahangir A, Alekseev AE, Terzic A. Channelopathies of inwardly rectifying potassium channels. Faseb J. 1999;13:1901–1910. doi: 10.1096/fasebj.13.14.1901. [DOI] [PubMed] [Google Scholar]
- 5.Loussouarn G, Rose T, Nichols CG. Structural basis of inward rectifying potassium channel gating. Trends Cardiovasc Med. 2002;12:253–258. doi: 10.1016/s1050-1738(02)00170-6. [DOI] [PubMed] [Google Scholar]
- 6.Tao X, Avalos JL, Chen J, MacKinnon R. Crystal structure of the eukaryotic strong inward-rectifier K+ channel Kir2.2 at 3.1 A resolution. Science. 2009;326:1668–1674. doi: 10.1126/science.1180310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bichet D, Haass FA, Jan LY. Merging functional studies with structures of inward-rectifier K(+) channels. Nat Rev Neurosci. 2003;4:957–967. doi: 10.1038/nrn1244. [DOI] [PubMed] [Google Scholar]
- 8.Clarke OB, Caputo AT, Hill AP, Vandenberg JI, Smith BJ, Gulbis JM. Domain reorientation and rotation of an intracellular assembly regulate conduction in Kir potassium channels. Cell. 2010;141:1018–1029. doi: 10.1016/j.cell.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Oliver D, Baukrowitz T, Fakler B. Polyamines as gating molecules of inward-rectifier K+ channels. Eur J Biochem. 2000;267:5824–5829. doi: 10.1046/j.1432-1327.2000.01669.x. [DOI] [PubMed] [Google Scholar]
- 10.Ruppersberg JP. Intracellular regulation of inward rectifier K+ channels. Pflugers Arch. 2000;441:1–11. doi: 10.1007/s004240000380. [DOI] [PubMed] [Google Scholar]
- 11.Rosenhouse-Dantsker A, Leal-Pinto E, Logothetis DE, Levitan I. Comparative analysis of cholesterol sensitivity of Kir channels: role of the CD loop. Channels (Austin) 2010;4:63–66. doi: 10.4161/chan.4.1.10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Logothetis DE, Petrou VI, Adney SK, Mahajan R. Channelopathies linked to plasma membrane phosphoinositides. Pflugers Arch. 2010;460:321–341. doi: 10.1007/s00424-010-0828-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahlmann A, Li M, Gao Z, McGarrigle D, Sackin H, Palmer LG. Regulation of Kir channels by intracellular pH and extracellular K(+): mechanisms of coupling. J Gen Physiol. 2004;123:441–454. doi: 10.1085/jgp.200308989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh DK, Rosenhouse-Dantsker A, Nichols CG, Enkvetchakul D, Levitan I. Direct regulation of prokaryotic Kir channel by cholesterol. J Biol Chem. 2009;284:30727–30736. doi: 10.1074/jbc.M109.011221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du X, Zhang H, Lopes C, Mirshahi T, Rohacs T, Logothetis DE. Characteristic interactions with phosphatidylinositol 4,5-bisphosphate determine regulation of kir channels by diverse modulators. J Biol Chem. 2004;279:37271–37281. doi: 10.1074/jbc.M403413200. [DOI] [PubMed] [Google Scholar]
- 16.Cheng WW, D'Avanzo N, Doyle DA, Nichols CG. Dual-mode phospholipid regulation of human inward rectifying potassium channels. Biophys J. 2011;100:620–628. doi: 10.1016/j.bpj.2010.12.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enkvetchakul D, Jeliazkova I, Nichols CG. Direct modulation of Kir channel gating by membrane phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2005;280:35785–35788. doi: 10.1074/jbc.C500355200. [DOI] [PubMed] [Google Scholar]
- 18.Kurata HT, Cheng WW, Nichols CG. Polyamine block of inwardly rectifying potassium channels. Methods Mol Biol. 2011;720:113–126. doi: 10.1007/978-1-61779-034-8_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallazzini M, Karim Z, Bichara M. Regulation of ROMK (Kir 1.1) channel expression in kidney thick ascending limb by hypertonicity: role of TonEBP and MAPK pathways. Nephron Physiol. 2006;104:126–135. doi: 10.1159/000095855. [DOI] [PubMed] [Google Scholar]
- 20.Gallazzini M, Attmane-Elakeb A, Mount DB, Hebert SC, Bichara M. Regulation by glucocorticoids and osmolality of expression of ROMK (Kir 1.1), the apical K channel of thick ascending limb. Am J Physiol Renal Physiol. 2003;284:F977–F986. doi: 10.1152/ajprenal.00255.2002. [DOI] [PubMed] [Google Scholar]
- 21.Karle CA, Zitron E, Zhang W, Wendt-Nordahl G, Kathofer S, Thomas D, Gut B, Scholz E, Vahl CF, Katus HA, Kiehn J. Human cardiac inwardly-rectifying K+ channel Kir(2.1b) is inhibited by direct protein kinase C-dependent regulation in human isolated cardiomyocytes and in an expression system. Circulation. 2002;106:1493–1499. doi: 10.1161/01.cir.0000029747.53262.5c. [DOI] [PubMed] [Google Scholar]
- 22.Schulte U, Fakler B. Gating of inward-rectifier K+ channels by intracellular pH. Eur J Biochem. 2000;267:5837–5841. doi: 10.1046/j.1432-1327.2000.01671.x. [DOI] [PubMed] [Google Scholar]
- 23.Tateno T, Nakamura N, Hirata Y, Hirose S. Role of C-terminus of Kir7.1 potassium channel in cell-surface expression. Cell Biol Int. 2006;30:270–277. doi: 10.1016/j.cellbi.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Xie LH, John SA, Ribalet B, Weiss JN. Activation of inwardly rectifying potassium (Kir) channels by phosphatidylinosital-4,5-bisphosphate (PIP2): interaction with other regulatory ligands. Progress in biophysics and molecular biology. 2007;94:320–335. doi: 10.1016/j.pbiomolbio.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Logothetis DE, Jin T, Lupyan D, Rosenhouse-Dantsker A. Phosphoinositide-mediated gating of inwardly rectifying K(+) channels. Pflugers Arch. 2007;455:83–95. doi: 10.1007/s00424-007-0276-5. [DOI] [PubMed] [Google Scholar]
- 26.Logothetis DE, Lupyan D, Rosenhouse-Dantsker A. Diverse Kir modulators act in close proximity to residues implicated in phosphoinositide binding. J Physiol. 2007;582:953–965. doi: 10.1113/jphysiol.2007.133157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbetagamma. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 28.Stansfeld PJ, Hopkinson R, Ashcroft FM, Sansom MS. PIP(2)-binding site in Kir channels: definition by multiscale biomolecular simulations. Biochemistry. 2009;48:10926–10933. doi: 10.1021/bi9013193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D'Avanzo N, Cheng WW, Doyle DA, Nichols CG. Direct and specific activation of human inward rectifier K+ channels by membrane phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2010;285:37129–37132. doi: 10.1074/jbc.C110.186692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rohacs T, Lopes CM, Jin T, Ramdya PP, Molnar Z, Logothetis DE. Specificity of activation by phosphoinositides determines lipid regulation of Kir channels. Proc Natl Acad Sci U S A. 2003;100:745–750. doi: 10.1073/pnas.0236364100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopes CM, Zhang H, Rohacs T, Jin T, Yang J, Logothetis DE. Alterations in conserved Kir channel-PIP2 interactions underlie channelopathies. Neuron. 2002;34:933–944. doi: 10.1016/s0896-6273(02)00725-0. [DOI] [PubMed] [Google Scholar]
- 32.Yano H, Philipson LH, Kugler JL, Tokuyama Y, Davis EM, Le Beau MM, Nelson DJ, Bell GI, Takeda J. Alternative splicing of human inwardly rectifying K+ channel ROMK1 mRNA. Mol Pharmacol. 1994;45:854–860. [PubMed] [Google Scholar]
- 33.Boim MA, Ho K, Shuck ME, Bienkowski MJ, Block JH, Slightom JL, Yang Y, Brenner BM, Hebert SC. ROMK inwardly rectifying ATP-sensitive K+ channel. II. Cloning and distribution of alternative forms. The American journal of physiology. 1995;268:F1132–F1140. doi: 10.1152/ajprenal.1995.268.6.F1132. [DOI] [PubMed] [Google Scholar]
- 34.Nusing RM, Pantalone F, Grone HJ, Seyberth HW, Wegmann M. Expression of the potassium channel ROMK in adult and fetal human kidney Histochem. Cell Biol. 2005;123:553–559. doi: 10.1007/s00418-004-0742-5. [DOI] [PubMed] [Google Scholar]
- 35.Shuck ME, Piser TM, Bock JH, Slightom JL, Lee KS, Bienkowski MJ. Cloning and characterization of two K+ inward rectifier (Kir) 1.1 potassium channel homologs from human kidney (Kir1.2 and Kir1.3) J Biol Chem. 1997;272:586–593. doi: 10.1074/jbc.272.1.586. [DOI] [PubMed] [Google Scholar]
- 36.Simon D, Karet F, Rodriguez-Soriano J, Hamdan J, DiPietro A, Trachtman H, Sanjad S, Lifton R. Genetic heterogeneity of Bartter's syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet. 1996;14:152–156. doi: 10.1038/ng1096-152. [DOI] [PubMed] [Google Scholar]
- 37.Peters M, Ermert S, Jeck N, Derst C, Pechmann U, Weber S, Schlingmann K, Seyberth H, Waldegger S, Konrad M. Classification and rescue of ROMK mutations underlying hyperprostaglandin E syndrome/antenatal Bartter syndrome. Kidney Int. 2003;64:923–932. doi: 10.1046/j.1523-1755.2003.00153.x. [DOI] [PubMed] [Google Scholar]
- 38.Dong K, Tang L, MacGregor GG, Hebert SC. Localization of the ATP/phosphatidylinositol 4,5 diphosphate-binding site to a 39-amino acid region of the carboxyl terminus of the ATP-regulated K+ channel Kir1.1. J Biol Chem. 2002;277:49366–49373. doi: 10.1074/jbc.M208679200. [DOI] [PubMed] [Google Scholar]
- 39.Raab-Graham KF, Radeke CM, Vandenberg CA. Molecular cloning and expression of a human heart inward rectifier potassium channel. Neuroreport. 1994;5:2501–2505. doi: 10.1097/00001756-199412000-00024. [DOI] [PubMed] [Google Scholar]
- 40.Donaldson M, Jensen J, Tristani-Firouzi M, Tawil R, Bendahhou S, Suarez W, Cobo A, Poza J, Behr E, Wagstaff J, Szepetowski P, Pereira S, Mozaffar T, Escolar D, Fu Y, Ptácek L. PIP2 binding residues of Kir2.1 are common targets of mutations causing Andersen syndrome. Neurology. 2003;60:1811–1816. doi: 10.1212/01.wnl.0000072261.14060.47. [DOI] [PubMed] [Google Scholar]
- 41.Ma D, Tang X, Rogers T, Welling P. An andersen-Tawil syndrome mutation in Kir2.1 (V302M) alters the G-loop cytoplasmic K+ conduction pathway. J Biol Chem. 2007;282:5781–5789. doi: 10.1074/jbc.M608776200. [DOI] [PubMed] [Google Scholar]
- 42.Anumonwo JM, Lopatin AN. Cardiac strong inward rectifier potassium channels. J Mol Cell Cardiol. 2010;48:45–54. doi: 10.1016/j.yjmcc.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ricardo Perez Riera A, Ferreira C, Dubner SJ, Schapachnik E. Andersen syndrome: the newest variant of the hereditary-familial long QT syndrome. Annals of noninvasive electrocardiology : the official journal of the International Society for Holter and Noninvasive Electrocardiology, Inc. 2004;9:175–179. doi: 10.1111/j.1542-474X.2004.92552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donaldson MR, Yoon G, Fu YH, Ptacek LJ. Andersen-Tawil syndrome: a model of clinical variability, pleiotropy, and genetic heterogeneity. Annals of medicine. 2004;36 Suppl 1:92–97. doi: 10.1080/17431380410032490. [DOI] [PubMed] [Google Scholar]
- 45.Bendahhou S, Donaldson M, Plaster N, Tristani-Firouzi M, Fu Y, Ptácek L. Defective potassium channel Kir2.1 trafficking underlies Andersen-Tawil syndrome. J Biol Chem. 2003;278:51779–51785. doi: 10.1074/jbc.M310278200. [DOI] [PubMed] [Google Scholar]
- 46.Tristani-Firouzi M, Jensen JL, Donaldson MR, Sansone V, Meola G, Hahn A, Bendahhou S, Kwiecinski H, Fidzianska A, Plaster N, Fu YH, Ptacek LJ, Tawil R. Functional and clinical characterization of KCNJ2 mutations associated with LQT7 (Andersen syndrome) The Journal of clinical investigation. 2002;110:381–388. doi: 10.1172/JCI15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ai T, Fujiwara Y, Tsuji K, Otani H, Nakano S, Kubo Y, Horie M. Novel KCNJ2 mutation in familial periodic paralysis with ventricular dysrhythmia. Circulation. 2002;105:2592–2594. doi: 10.1161/01.cir.0000019906.35135.a3. [DOI] [PubMed] [Google Scholar]
- 48.Tristani-Firouzi M, Etheridge S. Kir 2.1 channelopathies: the Andersen-Tawil syndrome. Pflugers Arch. 2010;460:289–294. doi: 10.1007/s00424-010-0820-6. [DOI] [PubMed] [Google Scholar]
- 49.Plaster N, Tawil R, Tristani-Firouzi M, Canún S, Bendahhou S, Tsunoda A, Donaldson M, Iannaccone S, Brunt E, Barohn R, Clark J, Deymeer F, George AJ, Fish F, Hahn A, Nitu A, Ozdemir C, Serdaroglu P, Subramony S, Wolfe G, Fu Y, Ptácek L. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen's syndrome. Cell. 2001;105:511–519. doi: 10.1016/s0092-8674(01)00342-7. [DOI] [PubMed] [Google Scholar]
- 50.Schoots O, Voskoglou T, Van Tol HH. Genomic organization and promoter analysis of the human G-protein-coupled K+ channel Kir3.1 (KCNJ3/HGIRK1) Genomics. 1997;39:279–288. doi: 10.1006/geno.1996.4495. [DOI] [PubMed] [Google Scholar]
- 51.Stoffel M, Espinosa R, 3rd, Powell KL, Philipson LH, Le Beau MM, Bell GI. Human G-protein-coupled inwardly rectifying potassium channel (GIRK1) gene (KCNJ3): localization to chromosome 2 and identification of a simple tandem repeat polymorphism. Genomics. 1994;21:254–256. doi: 10.1006/geno.1994.1253. [DOI] [PubMed] [Google Scholar]
- 52.He C, Yan X, Zhang H, Mirshahi T, Jin T, Huang A, Logothetis DE. Identification of critical residues controlling G protein-gated inwardly rectifying K(+) channel activity through interactions with the beta gamma subunits of G proteins. J Biol Chem. 2002;277:6088–6096. doi: 10.1074/jbc.M104851200. [DOI] [PubMed] [Google Scholar]
- 53.Zylbergold P, Ramakrishnan N, Hebert T. The role of G proteins in assembly and function of Kir3 inwardly rectifying potassium channels. Channels (Austin) 2010;4:411–421. doi: 10.4161/chan.4.5.13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jaen C, Doupnik CA. Neuronal Kir3.1/Kir3.2a channels coupled to serotonin 1A and muscarinic m2 receptors are differentially modulated by the "short" RGS3 isoform. Neuropharmacology. 2005;49:465–476. doi: 10.1016/j.neuropharm.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 55.Zhao Q, Kawano T, Nakata H, Nakajima Y, Nakajima S, Kozasa T. Interaction of G protein beta subunit with inward rectifier K(+) channel Kir3. Mol Pharmacol. 2003;64:1085–1091. doi: 10.1124/mol.64.5.1085. [DOI] [PubMed] [Google Scholar]
- 56.Bettahi I, Marker CL, Roman MI, Wickman K. Contribution of the Kir3.1 subunit to the muscarinic-gated atrial potassium channel IKACh. J Biol Chem. 2002;277:48282–48288. doi: 10.1074/jbc.M209599200. [DOI] [PubMed] [Google Scholar]
- 57.Styer AM, Mirshahi UL, Wang C, Girard L, Jin T, Logothetis DE, Mirshahi T. G protein {beta}{gamma} gating confers volatile anesthetic inhibition to Kir3 channels. J Biol Chem. 2010;285:41290–41299. doi: 10.1074/jbc.M110.178541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kawano T, Zhao P, Floreani CV, Nakajima Y, Kozasa T, Nakajima S. Interaction of Galphaq and Kir3, G protein-coupled inwardly rectifying potassium channels. Mol Pharmacol. 2007;71:1179–1184. doi: 10.1124/mol.106.032508. [DOI] [PubMed] [Google Scholar]
- 59.Rebois RV, Robitaille M, Gales C, Dupre DJ, Baragli A, Trieu P, Ethier N, Bouvier M, Hebert TE. Heterotrimeric G proteins form stable complexes with adenylyl cyclase and Kir3.1 channels in living cells. J Cell Sci. 2006;119:2807–2818. doi: 10.1242/jcs.03021. [DOI] [PubMed] [Google Scholar]
- 60.Ishihara K, Yamamoto T, Kubo Y. Heteromeric assembly of inward rectifier channel subunit Kir2.1 with Kir3.1 and with Kir3.4. Biochem Biophys Res Commun. 2009;380:832–837. doi: 10.1016/j.bbrc.2009.01.179. [DOI] [PubMed] [Google Scholar]
- 61.Yang D, Zhang X, Hughes BA. Expression of inwardly rectifying potassium channel subunits in native human retinal pigment epithelium. Exp Eye Res. 2008;87:176–183. doi: 10.1016/j.exer.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gaborit N, Le Bouter S, Szuts V, Varro A, Escande D, Nattel S, Demolombe S. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J Physiol. 2007;582:675–693. doi: 10.1113/jphysiol.2006.126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huttmann K, Yilmazer-Hanke D, Seifert G, Schramm J, Pape HC, Steinhauser C. Molecular and functional properties of neurons in the human lateral amygdala. Mol Cell Neurosci. 2006;31:210–217. doi: 10.1016/j.mcn.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 64.Leaney JL. Contribution of Kir3.1, Kir3.2A and Kir3.2C subunits to native G protein-gated inwardly rectifying potassium currents in cultured hippocampal neurons. Eur J Neurosci. 2003;18:2110–2118. doi: 10.1046/j.1460-9568.2003.02933.x. [DOI] [PubMed] [Google Scholar]
- 65.Dobrev D, Friedrich A, Voigt N, Jost N, Wettwer E, Christ T, Knaut M, Ravens U. The G protein-gated potassium current I(K,ACh) is constitutively active in patients with chronic atrial fibrillation. Circulation. 2005;112:3697–3706. doi: 10.1161/CIRCULATIONAHA.105.575332. [DOI] [PubMed] [Google Scholar]
- 66.Voigt N, Trausch A, Knaut M, Matschke K, Varro A, Van Wagoner DR, Nattel S, Ravens U, Dobrev D. Left-to-right atrial inward rectifier potassium current gradients in patients with paroxysmal versus chronic atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:472–480. doi: 10.1161/CIRCEP.110.954636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jo HY, Kim SY, Lee S, Jeong S, Kim SJ, Kang TM, Lee KY. Kir3.1 channel is functionally involved in TLR4-mediated signaling. Biochem Biophys Res Commun. 2011;407:687–691. doi: 10.1016/j.bbrc.2011.03.076. [DOI] [PubMed] [Google Scholar]
- 68.Rogalski SL, Chavkin C. Eicosanoids inhibit the G-protein-gated inwardly rectifying potassium channel (Kir3) at the Na+/PIP2 gating site. J Biol Chem. 2001;276:14855–14860. doi: 10.1074/jbc.M010097200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ito M, Inanobe A, Horio Y, Hibino H, Isomoto S, Ito H, Mori K, Tonosaki A, Tomoike H, Kurachi Y. Immunolocalization of an inwardly rectifying K+ channel, K(AB)-2 (Kir4.1), in the basolateral membrane of renal distal tubular epithelia. FEBS Lett. 1996;388:11–15. doi: 10.1016/0014-5793(96)00502-9. [DOI] [PubMed] [Google Scholar]
- 70.Takumi T, Ishii T, Horio Y, Morishige K, Takahashi N, Yamada M, Yamashita T, Kiyama H, Sohmiya K, Nakanishi S, et al. A novel ATP-dependent inward rectifier potassium channel expressed predominantly in glial cells. J Biol Chem. 1995;270:16339–16346. doi: 10.1074/jbc.270.27.16339. [DOI] [PubMed] [Google Scholar]
- 71.Bockenhauer D, Feather S, Stanescu H, Bandulik S, Zdebik A, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross J, van't Hoff W, Al Masri O, Tullus K, Yeung S, Anikster Y, Klootwijk E, Hubank M, Dillon M, Heitzmann D, Arcos-Burgos M, Knepper M, Dobbie A, Gahl W, Warth R, Sheridan E, Kleta R. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med. 2009;360:1960–1970. doi: 10.1056/NEJMoa0810276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scholl UI, Choi M, Liu T, Ramaekers VT, Hausler MG, Grimmer J, Tobe SW, Farhi A, Nelson-Williams C, Lifton RP. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci U S A. 2009;106:5842–5847. doi: 10.1073/pnas.0901749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reichold M, Zdebik A, Lieberer E, Rapedius M, Schmidt K, Bandulik S, Sterner C, Tegtmeier I, Penton D, Baukrowitz T, Hulton S, Witzgall R, Ben-Zeev B, Howie A, Kleta R, Bockenhauer D, Warth R. KCNJ10 gene mutations causing EAST syndrome (epilepsy, ataxia, sensorineural deafness, and tubulopathy) disrupt channel function. Proc Natl Acad Sci U S A. 2010;107:14490–14495. doi: 10.1073/pnas.1003072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scholl U, Choi M, Liu T, Ramaekers V, Häusler M, Grimmer J, Tobe S, Farhi A, Nelson-Williams C, Lifton R. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci U S A. 2009;106:5842–5847. doi: 10.1073/pnas.0901749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sala-Rabanal M, Kucheryavykh LY, Skatchkov SN, Eaton MJ, Nichols CG. Molecular mechanisms of EAST/SeSAME syndrome mutations in Kir4.1 (KCNJ10) J Biol Chem. 2010;285:36040–36048. doi: 10.1074/jbc.M110.163170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Butt AM, Kalsi A. Inwardly rectifying potassium channels (Kir) in central nervous system glia: a special role for Kir4.1 in glial functions. Journal of cellular and molecular medicine. 2006;10:33–44. doi: 10.1111/j.1582-4934.2006.tb00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Higashimori H, Sontheimer H. Role of Kir4.1 channels in growth control of glia. Glia. 2007;55:1668–1679. doi: 10.1002/glia.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rozengurt N, Lopez I, Chiu CS, Kofuji P, Lester HA, Neusch C. Time course of inner ear degeneration and deafness in mice lacking the Kir4.1 potassium channel subunit. Hear Res. 2003;177:71–80. doi: 10.1016/s0378-5955(02)00799-2. [DOI] [PubMed] [Google Scholar]
- 79.Kofuji P, Biedermann B, Siddharthan V, Raap M, Iandiev I, Milenkovic I, Thomzig A, Veh RW, Bringmann A, Reichenbach A. Kir potassium channel subunit expression in retinal glial cells: implications for spatial potassium buffering. Glia. 2002;39:292–303. doi: 10.1002/glia.10112. [DOI] [PubMed] [Google Scholar]
- 80.Thompson DA, Feather S, Stanescu HC, Freudenthal B, Zdebik AA, Warth R, Ognjanovic M, Hulton SA, Wassmer E, van't Hoff W, Russell-Eggitt I, Dobbie A, Sheridan E, Kleta R, Bockenhauer D. Altered electroretinograms in patients with KCNJ10 mutations and EAST syndrome. J Physiol. 2011;589:1681–1689. doi: 10.1113/jphysiol.2010.198531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rapedius M, Paynter JJ, Fowler PW, Shang L, Sansom MS, Tucker SJ, Baukrowitz T. Control of pH and PIP2 gating in heteromeric Kir4.1/Kir5.1 channels by H-Bonding at the helix-bundle crossing. Channels (Austin) 2007;1:327–330. doi: 10.4161/chan.5176. [DOI] [PubMed] [Google Scholar]
- 82.Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross JH, van't Hoff W, Al Masri O, Tullus K, Yeung S, Anikster Y, Klootwijk E, Hubank M, Dillon MJ, Heitzmann D, Arcos-Burgos M, Knepper MA, Dobbie A, Gahl WA, Warth R, Sheridan E, Kleta R. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. The New England journal of medicine. 2009;360:1960–1970. doi: 10.1056/NEJMoa0810276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reichold M, Zdebik AA, Lieberer E, Rapedius M, Schmidt K, Bandulik S, Sterner C, Tegtmeier I, Penton D, Baukrowitz T, Hulton SA, Witzgall R, Ben-Zeev B, Howie AJ, Kleta R, Bockenhauer D, Warth R. KCNJ10 gene mutations causing EAST syndrome (epilepsy, ataxia, sensorineural deafness, and tubulopathy) disrupt channel function. Proc Natl Acad Sci U S A. 2010;107:14490–14495. doi: 10.1073/pnas.1003072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Williams DM, Lopes CM, Rosenhouse-Dantsker A, Connelly HL, Matavel A, J OU, McBeath E, Gray DA. Molecular basis of decreased Kir4.1 function in SeSAME/EAST syndrome. J Am Soc Nephrol. 2010;21:2117–2129. doi: 10.1681/ASN.2009121227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Paulais M, Lourdel S, Teulon J. Properties of an inwardly rectifying K(+) channel in the basolateral membrane of mouse. TAL Am J Physiol Renal Physiol. 2002;282:F866–F876. doi: 10.1152/ajprenal.00238.2001. [DOI] [PubMed] [Google Scholar]
- 86.Lourdel S, Paulais M, Cluzeaud F, Bens M, Tanemoto M, Kurachi Y, Vandewalle A, Teulon J. An inward rectifier K(+) channel at the basolateral membrane of the mouse distal convoluted tubule: similarities with Kir4-Kir5.1 heteromeric channels. J Physiol. 2002;538:391–404. doi: 10.1113/jphysiol.2001.012961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lachheb S, Cluzeaud F, Bens M, Genete M, Hibino H, Lourdel S, Kurachi Y, Vandewalle A, Teulon J, Paulais M. Kir4.1/Kir5.1 channel forms the major K+ channel in the basolateral membrane of mouse renal collecting duct principal cells. Am J Physiol Renal Physiol. 2008;294:F1398–F1407. doi: 10.1152/ajprenal.00288.2007. [DOI] [PubMed] [Google Scholar]
- 88.Lam HD, Lemay AM, Briggs MM, Yung M, Hill CE. Modulation of Kir4.2 rectification properties and pHi-sensitive run-down by association with Kir5.1. Biochim Biophys Acta. 2006;1758:1837–1845. doi: 10.1016/j.bbamem.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 89.Paulais M, Bloch-Faure M, Picard N, Jacques T, Ramakrishnan SK, Keck M, Sohet F, Eladari D, Houillier P, Lourdel S, Teulon J, Tucker SJ. Renal phenotype in mice lacking the Kir5.1 (Kcnj16) K+ channel subunit contrasts with that observed in SeSAME/EAST syndrome. Proc Natl Acad Sci U S A. 2011;108:10361–10366. doi: 10.1073/pnas.1101400108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haider S, Antcliff JF, Proks P, Sansom MS, Ashcroft FM. Focus on Kir6.2: a key component of the ATP-sensitive potassium channel. J Mol Cell Cardiol. 2005;38:927–936. doi: 10.1016/j.yjmcc.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 91.Tarasov A, Dusonchet J, Ashcroft F. Metabolic regulation of the pancreatic beta-cell ATP-sensitive K+ channel: a pas de deux. Diabetes. 2004;53(Suppl 3):S113–S122. doi: 10.2337/diabetes.53.suppl_3.s113. [DOI] [PubMed] [Google Scholar]
- 92.Proks P, Antcliff J, Lippiat J, Gloyn A, Hattersley A, Ashcroft F. Molecular basis of Kir6.2 mutations associated with neonatal diabetes or neonatal diabetes plus neurological features. Proc Natl Acad Sci U S A. 2004;101:17539–17544. doi: 10.1073/pnas.0404756101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pinney S, MacMullen C, Becker S, Lin Y, Hanna C, Thornton P, Ganguly A, Shyng S, Stanley C. Clinical characteristics and biochemical mechanisms of congenital hyperinsulinism associated with dominant KATP channel mutations. J Clin Invest. 2008;118:2877–2886. doi: 10.1172/JCI35414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huopio H, Shyng S, Otonkoski T, Nichols C. K(ATP) channels and insulin secretion disorders. Am J Physiol Endocrinol Metab. 2002;283:E207–E216. doi: 10.1152/ajpendo.00047.2002. [DOI] [PubMed] [Google Scholar]
- 95.Winkler M, Lutz R, Russ U, Quast U, Bryan J. Analysis of two KCNJ11 neonatal diabetes mutations, V59G and V59A, and the analogous KCNJ8 I60G substitution: differences between the channel subtypes formed with SUR1. J Biol Chem. 2009;284:6752–6762. doi: 10.1074/jbc.M805435200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gloyn A, Siddiqui J, Ellard S. Mutations in the genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum Mutat. 2006;27:220–231. doi: 10.1002/humu.20292. [DOI] [PubMed] [Google Scholar]
- 97.Shimomura K, Hörster F, de Wet H, Flanagan S, Ellard S, Hattersley A, Wolf N, Ashcroft F, Ebinger F. A novel mutation causing DEND syndrome: a treatable channelopathy of pancreas and brain. Neurology. 2007;69:1342–1349. doi: 10.1212/01.wnl.0000268488.51776.53. [DOI] [PubMed] [Google Scholar]
- 98.Massa O, Iafusco D, D'Amato E, Gloyn A, Hattersley A, Pasquino B, Tonini G, Dammacco F, Zanette G, Meschi F, Porzio O, Bottazzo G, Crinó A, Lorini R, Cerutti F, Vanelli M, Barbetti F, Diabetology EODSGotISoPEa. KCNJ11 activating mutations in Italian patients with permanent neonatal diabetes. Hum Mutat. 2005;25:22–27. doi: 10.1002/humu.20124. [DOI] [PubMed] [Google Scholar]
- 99.Edghill E, Gloyn A, Gillespie K, Lambert A, Raymond N, Swift P, Ellard S, Gale E, Hattersley A. Activating mutations in the KCNJ11 gene encoding the ATP-sensitive K+ channel subunit Kir6.2 are rare in clinically defined type 1 diabetes diagnosed before 2 years. Diabetes. 2004;53:2998–3001. doi: 10.2337/diabetes.53.11.2998. [DOI] [PubMed] [Google Scholar]
- 100.Fan Z, Makielski JC. Anionic phospholipids activate ATP-sensitive potassium channels. J Biol Chem. 1997;272:5388–5395. doi: 10.1074/jbc.272.9.5388. [DOI] [PubMed] [Google Scholar]
- 101.John SA, Weiss JN, Ribalet B. Regulation of cloned ATP-sensitive K channels by adenine nucleotides and sulfonylureas: interactions between SUR1 and positively charged domains on Kir6.2. J Gen Physiol. 2001;118:391–405. doi: 10.1085/jgp.118.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shyng SL, Cukras CA, Harwood J, Nichols CG. Structural determinants of PIP(2) regulation of inward rectifier K(ATP) channels. J Gen Physiol. 2000;116:599–608. doi: 10.1085/jgp.116.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Flanagan S, Patch A, Mackay D, Edghill E, Gloyn A, Robinson D, Shield J, Temple K, Ellard S, Hattersley A. Mutations in ATP-sensitive K+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes. 2007;56:1930–1937. doi: 10.2337/db07-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Derst C, Doring F, Preisig-Muller R, Daut J, Karschin A, Jeck N, Weber S, Engel H, Grzeschik KH. Partial gene structure and assignment to chromosome 2q37 of the human inwardly rectifying K+ channel (Kir7.1) gene (KCNJ13) Genomics. 1998;54:560–563. doi: 10.1006/geno.1998.5598. [DOI] [PubMed] [Google Scholar]
- 105.Shimura M, Yuan Y, Chang JT, Zhang S, Campochiaro PA, Zack DJ, Hughes BA. Expression and permeation properties of the K(+) channel Kir7.1 in the retinal pigment epithelium. J Physiol. 2001;531:329–346. doi: 10.1111/j.1469-7793.2001.0329i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Doring F, Derst C, Wischmeyer E, Karschin C, Schneggenburger R, Daut J, Karschin A. The epithelial inward rectifier channel Kir7.1 displays unusual K+ permeation properties. J Neurosci. 1998;18:8625–8636. doi: 10.1523/JNEUROSCI.18-21-08625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nakamura N, Suzuki Y, Sakuta H, Ookata K, Kawahara K, Hirose S. Inwardly rectifying K+ channel Kir7.1 is highly expressed in thyroid follicular cells, intestinal epithelial cells and choroid plexus epithelial cells: implication for a functional coupling with Na+,K+-ATPase. Biochem J. 1999;342(Pt 2):329–336. [PMC free article] [PubMed] [Google Scholar]
- 108.Hughes BA, Takahira M. Inwardly rectifying K+ currents in isolated human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1996;37:1125–1139. [PubMed] [Google Scholar]
- 109.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 110.Wimmers S, Karl MO, Strauss O. Ion channels in the RPE. Prog Retin Eye Res. 2007;26:263–301. doi: 10.1016/j.preteyeres.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 111.Pattnaik BR, Hughes BA. Regulation of Kir channels in bovine retinal pigment epithelial cells by phosphatidylinositol 4,5-bisphosphate. Am J Physiol Cell Physiol. 2009;297:C1001–C1011. doi: 10.1152/ajpcell.00250.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hejtmancik J, Jiao X, Li A, Sergeev Y, Ding X, Sharma A, Chan C, Medina I, Edwards A. Mutations in KCNJ13 cause autosomal-dominant snowflake vitreoretinal degeneration. Am J Hum Genet. 2008;82:174–180. doi: 10.1016/j.ajhg.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jiao X, Ritter R, 3rd, Hejtmancik JF, Edwards AO. Genetic linkage of snowflake vitreoretinal degeneration to chromosome 2q36. Invest Ophthalmol Vis Sci. 2004;45:4498–4503. doi: 10.1167/iovs.04-0722. [DOI] [PubMed] [Google Scholar]
- 114.Sergouniotis PI, Davidson AE, Mackay DS, Li Z, Yang X, Plagnol V, Moore AT, Webster AR. Recessive Mutations in KCNJ13, Encoding an Inwardly Rectifying Potassium Channel Subunit, Cause Leber Congenital Amaurosis. Am J Hum Genet. 2011;89:183–190. doi: 10.1016/j.ajhg.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]