Abstract
Background
Somatostatin is a pleiotropic peptide, exerting a variety of effects through its receptor subtypes. Recently, somatostatin has been shown to act as a chemoattractant for hematopoietic progenitor cells and hepatic oval cells (HOC) via receptor subtype 2 and subtype 4 (SSTR4), respectively.
Aims
we investigated the in vivo effect of somatostatin/ SSTR4 on HOC migration in the injured liver model of rats and the type of signaling molecules associated with the chemotactic function.
Methods
Migration assay, HOC transplantation and PI3K signaling were assessed with or without somatostatin and an analogue of somatostatin (TT232) that specifically binds to SSTR4.
Results
TT232 was shown to have an anti-migratory action on HOC induced by somatostatin in vitro. In HOC transplantation experiments, a lower number of donor-derived cells was detected in TT232-treated animals, as compared to control animals. Activation of PI3K was observed in HOC exposed to somatostatin, and this activation was suppressed by either anti-SSTR4 antibody or TT232-pretreatment. In addition, a PI3K inhibitor abrogated the motility of HOC.
Conclusion
Together, these data suggest that somatostatin stimulates the migration of HOC within injured liver through SSTR4, and this action appears to be mediated by the PI3K pathway.
Keywords: somatostatin, hepatic oval cell, homing, somatostatin receptor type 4, PI3K
Introduction
Hepatic oval cells (HOC) are activated to proliferate and differentiate when the regenerative capacity of terminally differentiated hepatocytes is compromised (1). In HOC-mediated liver regeneration, tissue damage leads to a dramatic increase in the level of secreted chemokines, cytokines, and proteolytic enzymes that impact stem cell migration and repopulation (2). Of the known factors that control stem cell migration, stem cell factor (SCF), hepatocyte growth factor (HGF), granulocyte colony-stimulating factor (GCSF) and stromal cell-derived factor-1(SDF-1) are some of the best characterized (3-7). However, there is relatively little information regarding the biological factors that influence HOC.
Somatostatin is a pleiotropic hormone, exerting a variety of systemic effects, including control of hormone secretion and influencing the proliferation, motility and development of a wide variety of cells (8-10). Somatostatin has also been shown to act as a chemoattractant for hematopoietic progenitor cells, HOC, and immature neurons (11-13). Physiological effects of somatostatin are mediated through a family of seven transmembrane spanning G-protein coupled receptors (GPCR) (8). Increasing evidence has shown that the distant effects on cell response elicited by the individual receptor types were correlated with activation of the various intracellular signaling pathways (14).
Given the effects of somatostatin, several analogues have been recently developed for clinical applications. Among these, TT232, a stable analogue with a highest binding affinity for SSTR4 (8, 15), has been shown to have tumor-selective, anti-proliferative activity without anti-secretory effects (16). These anti-proliferative properties are dose-dependent and apoptosis-inducing, independent of SSTR activity (17). In order to clarify the effects of TT232, we investigated the effects of TT232 on HOCs in this study.
The phosphatidylinositol-3-kinase (PI3K) signaling pathway is crucial for many aspects of cell growth and survival (18, 19). PI3K signaling is a key regulator of cell migration and invasion in response to biological factors (18). Akt, an essential downstream protein of PI3K, is involved in a variety of biological functions, including angiogenesis, glycogen synthesis, gene expression, inhibition of apoptosis, cell cycle arrest, endocytosis, vesicular trafficking, and cell transformation (18), while p21 activated kinase (PAK) 1 has been identified as a downstream molecule of activated Cdc21/Rac or Akt (19). PAK activity is regulated by different classes of membrane receptors, including GPCR, tyrosine kinase receptors, and cytokine receptors (19). Activation of PAK1 has been shown to induce formation of motility structures (19, 20), although it remains unclear how signaling molecules are involved in mediating the effects of PAK in forming these structures. In addition, the PI3K system has recently been shown to be an essential step in mediating the migration of stem cell (18, 19). Thus, the PI3K pathway and its various components, including the key effectors, Akt and PAK1, are important for controlling the survival and proliferation of stem cells , similar to their roles in mature cell systems (21).
In our previous studies, we showed increased expression of somatostatin in oval cell-mediated liver regeneration with the increase in somatostatin expression correlating to HOC migration but not proliferation. We also demonstrated that SSTR4 was expressed by HOCs only and that SSTR4 is involved in the chemotactic role of somatostatin on HOCs in vitro. SSTR4 is coupled to G-proteins, and somatostatin was known to modulate the PI3K signaling pathway (22). Therefore, we hypothesized that somatostatin would mediate the migration of HOCs through a SSTR4-coupled, G-protein/PI3K signaling pathway.
In the current study, the role of somatostatin in the migration of HOC through SSTR4 was examined in an in vivo setting. Also, the signaling molecules regulating HOC migration by somatostatin /SSTR4 were investigated. Observed data demonstrate that somatostatin /SSTR4 stimulates HOC migration within the injured liver, and these effects appear to be mediated by the intracellular PI3K signaling pathway.
Methods
Animals
Dipeptidyl peptidase IV deficient (DPPIV-) female F344 breeding animals were house bred and maintained on standard laboratory chow with daily cycles of alternating 12 hours of light and dark. They were used at approximately 8-10 weeks of age and 150-180 g of weight. Normal male DPPIV+ F344 rats (age 8-10 weeks, weight 180-220 g) were purchased from Charles River Laboratories and were used as donor animals for all transplantation studies. All animal work was conducted under protocols approved by the IACUC at the University of Florida.
HOC preparation and transplantation
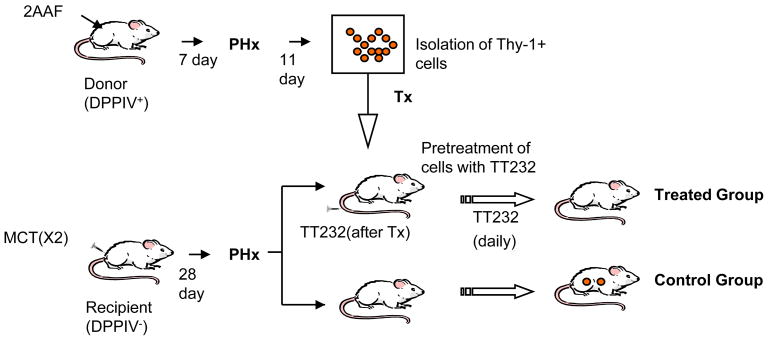
The standard protocol for oval cell activation, 2-acetyl-aminofluorene (2AAF)/partial hepatectomy (PHx), was used. HOC were isolated as described by Jung et al (12). For transplantation experiments, DPPIV- female recipients were treated with two I.P. injections of monocrotaline (MCT) with PHx performed as previously described (23). Donor HOC (2.4 × 105 cells /rat) from DPPIV+ males were injected intrasplenically. Donor HOC were pre-treated with 5 μM of TT232 (generous gift from Biostatin, Budapest, Hungary) for 15 min, and then transplanted into recipient animals (treated group). The treated group was injected intravenously daily with 3 μg/kg of TT232. Animals were sacrificed on days 13 and 24 post-transplantation for tissue collection and histological examination.
Immunohistochemistry
DPPIV staining was performed as described by Dabeva et al (24).
Cell apoptosis experiment
TUNEL staining was performed according to the manufacturer's instructions (BD Biosciences). Briefly, cells were seeded in 6-well plates (4.5 × 104 cell/well) and grown in DMEM supplemented with 10 % FBS at 37 oC (5% CO2). After 48 h, the media was replaced with serum-free DMEM for 16 h. The cells were subsequently cultured with media alone, or medium supplemented with TT232 at concentrations of 1, 5, 10, 25, 50 μM for 24 h.
Migration Assay
Migration assays were performed as described by Jung et al. (12) in transwell culture plates. The motility assay was conducted by transferring the entire transwell chamber to new a cluster plate well containing 100 nM of somatostatin-14 (synthesized in ICBR, University of Florida) or TT232 (1 and 5 μM) in migration buffer. Plates were maintained for 4 h. In some experiments, cells were pretreated for 30 min with 5 μM TT232. As a control, cells were incubated without somatostatin or TT232. At the end of the experiment, cells were fixed and stained as described by Stolz et al (25). Cells that had migrated to the bottom of the transwell filter were enumerated by counting each transwell chamber, at 4× magnification. Each migration assay was performed a minimum of three-times.
Stimulation and Western Blot assay
Quiescent HOC were incubated for 10, 30, and 60 min at 37°C in the presence of 100 nM somatostatin. In the inhibition experiment, HOC were pretreated with either 5 μM TT232, 5 μg/ml anti-SSTR4 antibody, 20 μM LY294002 (LC lab., Woburn, MA) or 0.08% DMSO for 30 min, then incubated with somatostatin at the aforementioned times, and then washed in PBS. HOC were homogenized in Triton Lysis Buffer (with 20 mM Tris, pH 7.4, 137 mM NaCl, 10% glycerol, 1% Triton X-100, 2 mM EDTA, 1 mM PMSF, 10 mM NaF, 5 μg/ml aprotinin, 20 μM Leupeptin, and 1 mM Sodium ortho-vanadate) and centrifugated at 10,000× g for 15 min. Protein concentrations were measured using the Lowry assay. Immunoblot was performed using anti-pAKT (Cell Signaling), anti-AKT (Cell Signaling), anti-pPAK1(Cell Signaling), anti-PAK1(Cell Signaling) antibodies. Membranes were developed by chemiluminescence (Amersham). The blots were scanned and an ROI around the band of interest was defined. Band intensities were calculated by the pixel density from the Histogram measurements in Adobe Photoshop (version 5.5) (26).
Statistical analysis
All results are expressed as the mean ± SD. Statistical differences were determined by Student's t-test. P values of <0.05 were considered statistically significance.
Results
The somatostatin analogue, TT232, suppresses the migration of HOC
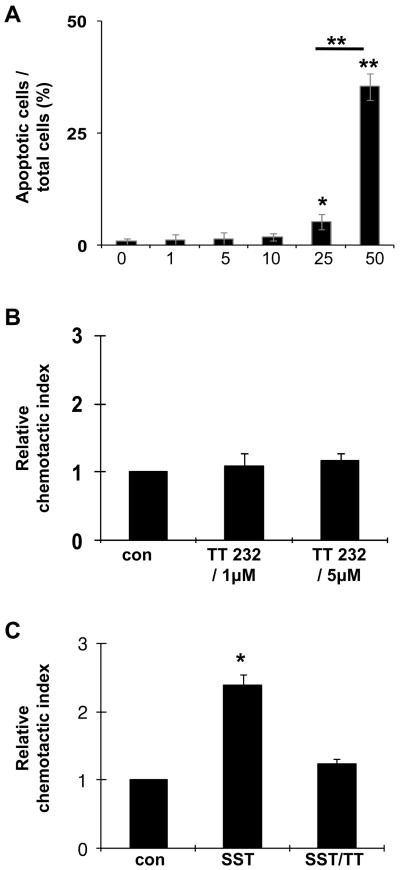
Several somatostatin analogues have been developed for clinical applications. TT232 is a stable somatostatin analogue with the highest binding affinity for SSTR4 (8, 15) and has been shown to exert an anti-proliferative effect on several tumor cell lines and animal models by inducing apoptosis (16). This effect has been shown to be very specific and is concentration-dependent. In these experiments, high doses of above 30μM are required for inducing apoptosis, which is independent from SSTR activity (17, 27). As a specific somatostatin analogue for SSTR4, the dose extent of TT232 was tested (8), and this concentration was shown to have no effect on cell proliferation (28). To clarify the dose-dependent effect of TT232 on HOC, we investigated apoptotic cell death induced by TT232 at different concentrations. A range of 1 μM to 50 μM was tested, and 5 and 35% of apoptosis were observed in 25 μM and 50 μM of TT232, respectively. However, HOC treated with 1, 5, or 10 μM of TT232 exhibited significantly low level of apoptosis (below the 2% of apoptosis) (Fig. 1A). The number of apoptotic cells seen at the lower doses was similar to the number of apoptotic cells observed in the control (0 μM of TT232). These data demonstrated that TT232-induced HOC apoptosis occurs in a dose-dependent manner, suggesting that the somatostatin analogue did not induce significant HOC apoptosis at physiologic concentrations where SSTR4 binding occurs (<10 μM).
Figure 1. Effect of Somatostatin analogues (TT232) on HOC migration.
(A) TUNEL assay was performed in order to examine apoptotic cells. A higher number of apoptotic cells are observed in HOC treated with high dose (50μM) TT232, compared with other treated groups. Data shown represent the results of three separate experiments. (B) Migration assay with TT232 on HOC. HOC were seeded in the top chamber of transwell plates with 1 and 5 μM of TT232 in the bottom chamber. Controls contained no TT232 in either chamber. (C) HOC were subjected to chemotaxis assays with 100 nM somatostatin (SST). Cells were treated with 5 μM TT232 for 30 minutes and cultured in 100 nM somatostatin – containing medium for 4 hours (TT/SST). Controls (con) were performed with neither TT232 nor somatostatin. Data represents mean value ± SD of three independent experiments. Data were normalized for each independent experiment with respect to control migration. (*P<0.01, relative chemotactic index vs control).
Our previous study revealed that the somatostatin /SSTR4 pathway was involved in the migratory response of HOC (12). Here, we examined the effect of TT232 on HOC motility in vitro. At concentrations of 1 μM or 5μM of TT232 in the lower chamber, HOC failed to migrate toward TT232 (Fig. 1B). However, pretreatment of HOC with TT232 abrogated the somatostatin -mediated motility, whereas somatostatin -stimulated HOC showed an approximately two and a half-fold increase in motility toward somatostatin (Fig. 1C). These results indicated that TT232 suppressed the chemotactic action of somatostatin on HOC migration.
Somatostatin affects HOC homing through SSTR4 in the injured liver
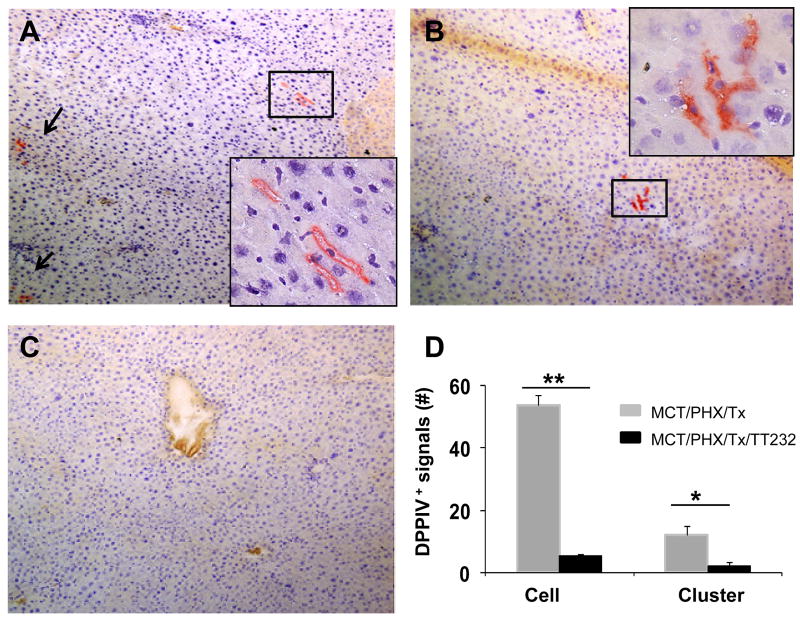
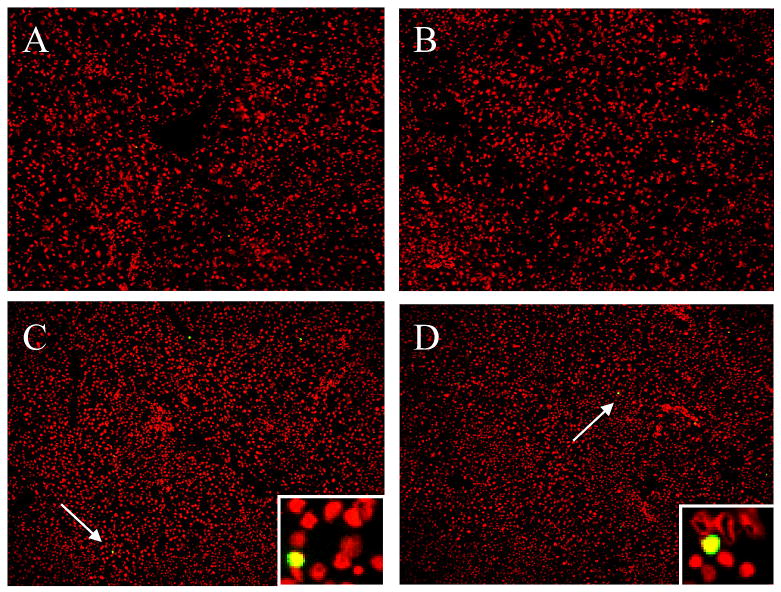
To detemine if the effect of somatostatin /SSTR4 on HOC migration was a functional consequence of the injured liver model, we designed a cell transplantation model (Fig. 2). In this model, DPPIV+ HOC were transplanted into DPPIV- female rats which were pretreated with MCT/PHx as described by Witek et al (23). The reaction product of DPPIV appears as diffuse orange staining of the bile canalicular membrane of hepatocytes. No staining is observed in DPPIV- animals, so the transplanted cells are easily distinguished from recipient cells (29). The inhibitory function of TT232 on the chemotactic action of somatostatin was expected to suppress motility of HOC in the transplantation model. Cytochemical staining for DPPIV-positive cells showed the donor-originated DPPIV+ cells in recipient liver sections at 24 days post-transplantation (Fig. 3). As expected, a high number of DPPIV+ cells and clusters were observed in the liver of saline injected control group, whereas a significantly lower number of DPPIV+ cells and cluster were observed in TT232-treated livers. It is possible that daily injection of TT232 could induce apoptosis in transplanted cells, and for that reason, a lower number of donor cells could be observed in TT232-treated group. To test this possibility, TUNEL assay on liver sections from the recipient group was performed (Fig. 4). Apoptotic cells were rarely detected in both groups (day 13 and 24 post-transplantation), confirming that there was little to no apoptosis induced by TT232 exposure. These results demonstrated the inhibitory effects of TT232 on somatostatin /SSTR4 – medicated HOC migration within the damaged liver
Figure 2. The plan for experiment is described.
The DPPIV- female rats received two doses of 30mg/kg MCT then underwent PHx. HOC were isolated from DDPIV+ rats which were treated with 2AAF/PHx. HOCs from the treated group were pretreated with TT232 (5 μM) for 15 minutes and transplanted by splenic injection into DPPIV- female rats at 7 days post-PHx. The treated group was injected intravenously with 3 μg/kg of TT232 on a daily basis. Both groups were sacrificed at 13 and 24 days post-transplantation.
Figure 3. DPPIV staining for transplanted cells in DPPIV- rats at 24 days post-transplantation.
(A-B) Liver sections from HOC-transplanted rats without TT232 treatment (Arrows indicate DPPIV+ cells). (C) Liver sections from HOC-transplanted and TT232-treated rats. Data shown are representative sections of observed slides. Representative slides were viewed at 20×, and insert viewed originally at 20× magnification and enlarged. (D) To quantify DPPIV staining, ten randomly chosen fields/section (20× magnification) were evaluated from each animal. The number of DPPIV+ cells and clusters were added from each of 10 slides from two liver lobes from recipient animals and are depicted graphically as the total number.
Data represents the mean value ± SD of three independent experiments (*P<0.05, **P<0.01).
Figure 4. Apoptosis assay on recipient liver section using TUNEL.
(A-B) Liver sections from untreated or TT232-treated recipient rats, respectively (day 13 post-transplantation. (C-D) Liver sections from untreated or TT232-treated recipient rats, respectively (day 24 post-transplantation). (Arrows indicate apoptotic cells. Data shown are representative of TUNEL-stained slides from recipient group). Propidium iodide (red) was used for nuclear staining. Representative slides were viewed at 10× magnification, and insert viewed originally at 20× magnification.
Somatostatin activates intracellular PI3 kinase pathway
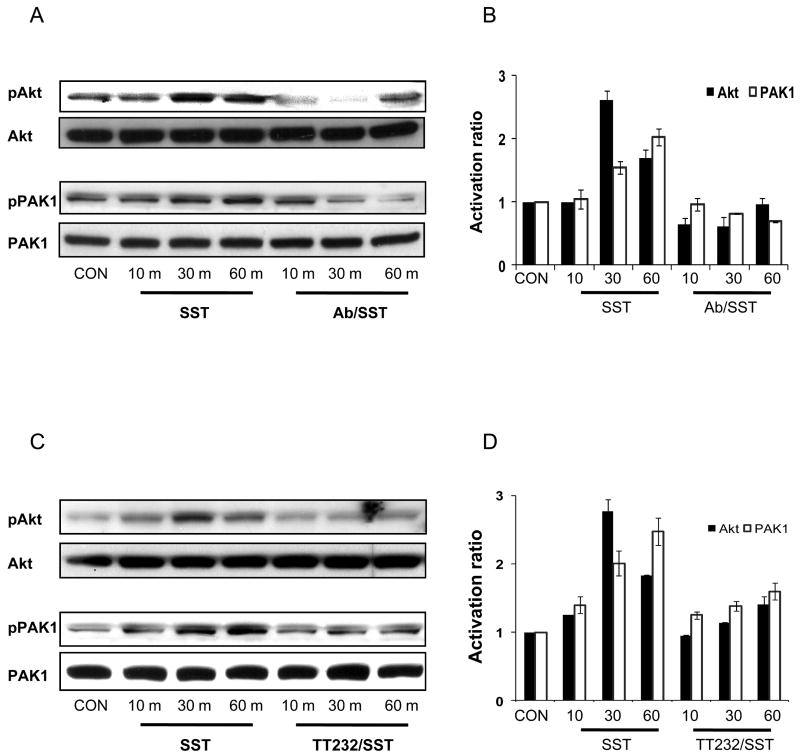
Recent studies have confirmed the need for the PI3K pathway activation in cell migration (18, 19). The activation of Akt and PAK1 was examined in order to determine if somatostatin activates PI3K signaling in HOC migration. Akt is a known PI3K effector while PAK1 is a known effector of Akt or direct PI3K effector (19). Somatostatin stimulated activation of Akt after 30 min and PAK1 after 60 min, whereas somatostatin -induced phosphorylation of Akt and PAK1 were suppressed by an anti-SSTR4 antibody (Figure 5A & B). Furthermore, TT232 decreased the activation of Akt and PAK1 (Fig. 5C & D). These inhibitory effects of anti-SSTR4 antibody and TT232 on somatostatin -mediated phosphorylation of Akt and PAK1 confirmed that TT232 impeded signaling events elicited by somatostatin, in a similar manner to anti-SSTR4 antibody exposure. The activation of Akt and PAK1 suggested that PI3K signaling might be involved in migration of HOC via the somatostatin /SSTR4 pathway.
Figure 5. Somatostatin/SSTR4-induced effects are mediated by PI3K pathway.
(A) Somatostatin (SST) induced phosphorylation of Akt and PAK1 with a maximum effect at 30 and 60 minutes, respectively. Somatostatin-induced activation of Akt and PAK1 was suppressed by 5μg/ml anti-SSTR4 antibody (Ab/SST). (B) Densitometric analysis to evaluate the relative activation of Akt and PAK1. The histogram function of Adobe Photoshop was used to approximate the density of each band. (C) The effects of somatostatin on phosphorylations of Akt and PAK1 were decreased by 5μM TT232 (TT232/SST). (D) Densitometry analysis to show the relative activation of Akt and PAK1. The histogram function of Adobe Photoshop was used to approximate the density of each band. Figure 4A &C are representative of three independent experiments. Data shown in figure 4B & D represent mean value ± SD of three independent experiments. Data were normalized for each independent experiment with respect to control band density
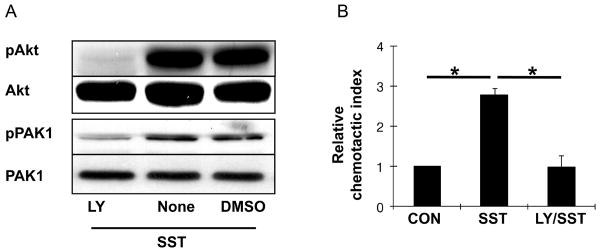
In order to investigate whether PI3K is essential for HOC migration, we employed a PI3K inhibitor, LY294002, in a somatostatin migration assay. Western blot analysis showed that the action of PI3K on Akt and PAK1 was significantly blocked, when LY294002 was present. DMSO, the vehicle used for LY294002, did not affect either Akt and PAK1 activation (Fig. 6A) The migration assay confirmed that pretreatment with LY294002 abrogated HOC migration toward somatostatin, whereas somatostatin increased the number of migrating HOC by approximately a threefold increase in migrating cell number (Fig. 6B). Our results suggested that intracellular PI3K pathway activation is essential for HOC migration mediated by somatostatin.
Figure 6. PI3K plays an important role in Somatostatin-stimulated HOC migration.
(A) HOC were pretreated with PI3K inhibitor (LY294002), DMSO (solvent for LY294002), and normal media for 30 minutes. These cells were then stimulated with 100 nM somatostatin (SST). Western blot analysis for Akt and PAK1 was performed to determine if PI3K was blocked by LY294002. Data shown is representative of three separate experiments. (B) Chemotaxis assays were performed using HOC with 100 nM somatostatin only (SST), and somatostatin after 30 minutes of pretreatment with the PI3K inhibitor (LY/SST). Control contained no LY or somatostatin (con). Data represents the mean value ± SD of three independent experiments. Data were normalized for each independent experiment with respect to control migration. (*P<0.01, relative chemotactic index vs control).
Discussion
After cytotoxic therapy and administration of growth factors, stem cells are mobilized to the location of injury but still retain the ability to selectively repopulate a tissue system. Trafficking, mobilization, and homing of stem cells are a multifactorial process that is regulated by adhesion molecules and cytokines as well as chemotactic factors (30). In hematopoietic stem cells, recent studies have provided evidence that several chemotactic factors, particularly SDF-1 and its receptor chemokine receptor 4 (CXCR4) play significant roles in regulating mobilization and homing of hematopoietic stem cells (7, 31). Thus, homing is the hallmark of hematopoietic stem cells, for which the mechanism and cytokines involved have been a thoroughly studied and well-characterized. However, there is relatively little information regarding factors that influence HOC homing. In this context, a major challenge is presented in the identification of the chemotactic function of somatostatin /SSTR4 on HOC migration in the injured rat liver and the mechanism inducing this effect of somatostatin.
In the present study, we investigated the effect of TT232 on HOC and found a dose-dependent effect. At higher doses (25 and 50 μM) of TT232, apoptosis of HOC was induced (Fig 1A), whereas migration of HOC was abrogated by lower doses of TT232 (1 and 5 μM) (Fig. 1B and C). Low dose TT232 represents an appropriate concentration for SSTR4 binding (8). These inhibitory effects of TT232 for somatostatin /SSTR4 also resulted in functional consequences in HOC migration, showing the anti-migratory effect on HOC within the damaged liver. The inhibitory effects of TT232 were similar to that of anti-SSTR4 antibody on suppressing somatostatin -induced activation of Akt and PAK1 (Fig. 5), confirming that TT232 binds to SSTR4, which then blocks the signaling pathway triggered by somatostatin.
From HOC-transplantation experiments, the data indicates a significant difference in DPPIV+ cells and cluster numbers between the non-treated and TT232-treated animals. A few DPPIV+ cells in the TT232-treated group are likely to result from incomplete inhibition of SSTR4 by TT232. Although it is already verified that TT232 specifically binds to SSTR4, inhibition of receptor subtypes by TT232 is dependent on the concentration of TT232 (8). Following the report described by Weckbecker, et al.(8), we used different concentrations to block the function of SSTR4 and injected low concentrations which would neither affect other receptor subtypes nor induce apoptosis. Indeed, it is worth considering that oligomerization between SSTR4 and SSTR2 could occur (32). A cellular interaction between SSTR4 and SSTR2 could further affect the function of individual receptors (33). Thus, there is a possibility that SSTR2 can compensate for the loss of function of SSTR4. Recently, two studies have provided evidence that SDF-1/CXCR4 might be involved in HOC-aided liver regeneration and HOC migration (34, 35). Consequently, it is conceivable that somatostatin may have a synergistic effect on migration of HOC with other cytokines, such as SDF-1. When the response of SSTR4 to somatostatin is blocked, HOC escaping from the suppressive effect of TT232 or having decreased motility due to SSTR4-inhibition may respond to SDF-1, and migrate to the damaged liver.
We did not observed the significant difference of liver weight between two groups (TT232-treated group and TT232-nontreated group which is a control group), although we observed the higher number of donor-originated HOC in control group. The migration of stem cell is regulated by various chemokines rather than by a single chemokine (30). And, it is assumable that other chemotactic factors might be engaged in repairing the damaged liver. For example, G-CSF (granulocyte-colony stimulating factor) was shown to induce the migration of bone marrow (BM)-derived progenitors to damaged liver (36). It is possible that G-CSF might induce the migration BM-derived progenitors of recipient to damaged liver, because MCT inhibit the proliferation of liver cell, not BM-derived cells of recipient(23). Hence, G-SCF or other chemotactic factors could balance the diminished regenerating abilities of liver by restrained chemotactic action of somatostatin /SSTR4 in TT232-greated group. However, it still remains unclear how somatostatin interacts with other chemokine factors.
SSTR4 belongs to the family of seven α-helical transmembrane domain GPCR, which are coupled to heterotrimeric G-proteins (8). This receptor can be linked to the activation of PI3K which is directly stimulated by the G-protein βγ subunits released from α-subunit when the latter is activated by the binding of GTP (19). The downstream effector, PI3K, has been implicated in the migration of several cell types (18, 19, 37). Western blot assays in this study have shown that the activity of PI3K was suppressed by anti-SSTR4 antibody or TT232. The motility of HOC has been shown to be abrogated by anti-SSTR4 antibody and TT232. These results demonstrate that SSTR4 is involved in the activation of the PI3K signaling pathway. These results further suggest that PI3K activation is essential in HOC migration when somatostatin /SSTR4 is activated. Migration assay with a PI3K inhibitor confirmed that somatostatin stimulates HOC migration in a PI3K-dependent manner.
Since the anti-proliferative effect of TT232 in cancer cell lines has been shown to be induced through p38 (28), we examined whether anti-proliferation via p38 or cell cycle arrest though p21 (5) caused anti-migratory effects in HOC. In these experiments, the p21 and p38 expression were not observed by Western blot analysis (data not shown). In addition, the anti-proliferative property of TT232 was shown to have no effect on bone marrow cells (38). Accordingly, this inhibitory action of TT232 appeared to be selective within certain cancer models.
In the current study, we found that treating cells with somatostatin resulted in Akt activation, peaking at 30 minutes, and subsequently PAK1 activation, peaking at 60 minutes. Although PI3K was also shown to induce the activation of PAK1 directly (19), the sequential activation of Akt and PAK1 suggests a potential signal transduction pathway (PI3K→Akt→PAK1) in HOC migration stimulated by somatostatin /SSTR4. Additionally, exposure to anti-SSTR4 antibody inhibited the activation of PAK1, while TT232 appears to delay it, although these differences were subtle. It is assumed that anti-SSTR4 antibody binds predominantly to SSTR4 than somatostatin, thereby blocking the SSTR4 response to somatostatin, whereas TT232 inactivated SSTR4-coupled signaling molecule, PI3K. However, both consequently induced inactivation of PI3K, and finally abrogated motility of HOC toward somatostatin.
In conclusion, our findings suggest that somatostatin and SSTR4 could influence HOC migration in the injured rat liver model, and this effect appears to be mediated by the intracellular PI3K pathway. However, more detailed studies about the relationship with other chemokines are necessary to clarify the mechanism of homing and/or migration in this unique hepatic cell population.
Acknowledgments
Financial support: National Institute of Health Grants DK 058614 and DK 065096 awarded to Bryon E Petersen funded this research. Also, this research was supported by Basic Sciences Research Program through NRF funded by the MEST (2010-0022056) and Pusan National University Research Grant 2010
The abbreviations used are
- 2AAF
2-acetyl-aminofluorene
- DPPIV
Dipeptidyl peptidase IV
- HOC
hepatic oval cell
- MCT
monocrotaline
- PAK1
p21 activated kinase 1
- PHx
partial hepatectomy
- PI3K
phosphatidylinositol-3-kinase
- SSTR4
somatostatin receptor type 4
Footnotes
Conflict of Interest: None declared
References
- 1.Oh SH, Hatch HM, Petersen BE. Hepatic oval 'stem' cell in liver regeneration. Semin Cell Dev Biol. 2002;13(6):405–9. doi: 10.1016/s1084952102001271. [DOI] [PubMed] [Google Scholar]
- 2.Libbrecht L, Desmet V, Van Damme B, Roskams T. Deep intralobular extension of human hepatic 'progenitor cells' correlates with parenchymal inflammation in chronic viral hepatitis: can 'progenitor cells' migrate? J Pathol. 2000;192(3):373–8. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH700>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 3.Bonig H, Priestley GV, Nilsson LM, Jiang Y, Papayannopoulou T. PTX-sensitive signals in bone marrow homing of fetal and adult hematopoietic progenitor cells. Blood. 2004;104(8):2299–306. doi: 10.1182/blood-2004-04-1605. [DOI] [PubMed] [Google Scholar]
- 4.Papayannopoulou T, Craddock C, Nakamoto B, Priestley GV, Wolf NS. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci U S A. 1995;92(21):9647–51. doi: 10.1073/pnas.92.21.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forte G, Minieri M, Cossa P, et al. Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells. 2006;24(1):23–33. doi: 10.1634/stemcells.2004-0176. [DOI] [PubMed] [Google Scholar]
- 6.Abkowitz JL, Robinson AE, Kale S, Long MW, Chen J. Mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood. 2003;102(4):1249–53. doi: 10.1182/blood-2003-01-0318. [DOI] [PubMed] [Google Scholar]
- 7.Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283(5403):845–8. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 8.Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C. Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Discov. 2003;2(12):999–1017. doi: 10.1038/nrd1255. [DOI] [PubMed] [Google Scholar]
- 9.Solomou K, Ritter MA, Palmer DB. Somatostatin is expressed in the murine thymus and enhances thymocyte development. Eur J Immunol. 2002;32(6):1550–9. doi: 10.1002/1521-4141(200206)32:6<1550::AID-IMMU1550>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 10.Bertherat J, Bluet-Pajot MT, Epelbaum J. Neuroendocrine regulation of growth hormone. Eur J Endocrinol. 1995;132(1):12–24. doi: 10.1530/eje.0.1320012. [DOI] [PubMed] [Google Scholar]
- 11.Oomen SP, Van Hennik PB, Antonissen C, et al. Somatostatin is a selective chemoattractant for primitive (CD34(+)) hematopoietic progenitor cells. Exp Hematol. 2002;30(2):116–25. doi: 10.1016/s0301-472x(01)00772-x. [DOI] [PubMed] [Google Scholar]
- 12.Jung Y, Oh SH, Zheng D, Shupe TD, Witek RP, Petersen BE. A potential role of somatostatin and its receptor SSTR4 in the migration of hepatic oval cells. Lab Invest. 2006;86(5):477–89. doi: 10.1038/labinvest.3700410. [DOI] [PubMed] [Google Scholar]
- 13.Yacubova E, Komuro H. Stage-specific control of neuronal migration by somatostatin. Nature. 2002;415(6867):77–81. doi: 10.1038/415077a. [DOI] [PubMed] [Google Scholar]
- 14.Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20(3):157–98. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- 15.Szolcsanyi J, Bolcskei K, Szabo A, et al. Analgesic effect of TT-232, a heptapeptide somatostatin analogue, in acute pain models of the rat and the mouse and in streptozotocin-induced diabetic mechanical allodynia. Eur J Pharmacol. 2004;498(1-3):103–9. doi: 10.1016/j.ejphar.2004.07.085. [DOI] [PubMed] [Google Scholar]
- 16.Keri G, Erchegyi J, Horvath A, et al. A tumor-selective somatostatin analog (TT-232) with strong in vitro and in vivo antitumor activity. Proc Natl Acad Sci U S A. 1996;93(22):12513–8. doi: 10.1073/pnas.93.22.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JU, Hosotani R, Wada M, et al. Antiproliferative activity induced by the somatostatin analogue, TT-232, in human pancreatic cancer cells. Eur J Cancer. 2002;38(11):1526–34. doi: 10.1016/s0959-8049(02)00101-6. [DOI] [PubMed] [Google Scholar]
- 18.Qian Y, Zhong X, Flynn DC, et al. ILK mediates actin filament rearrangements and cell migration and invasion through PI3K/Akt/Rac1 signaling. Oncogene. 2005;24(19):3154–65. doi: 10.1038/sj.onc.1208525. [DOI] [PubMed] [Google Scholar]
- 19.Papakonstanti EA, Stournaras C. Association of PI-3 kinase with PAK1 leads to actin phosphorylation and cytoskeletal reorganization. Mol Biol Cell. 2002;13(8):2946–62. doi: 10.1091/mbc.02-01-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniels RH, Bokoch GM. p21-activated protein kinase: a crucial component of morphological signaling? Trends Biochem Sci. 1999;24(9):350–5. doi: 10.1016/s0968-0004(99)01442-5. [DOI] [PubMed] [Google Scholar]
- 21.Heasley LE, Petersen BE. Signalling in stem cells: meeting on signal transduction determining the fate of stem cells. EMBO Rep. 2004;5(3):241–4. doi: 10.1038/sj.embor.7400098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Theodoropoulou M, Zhang J, Laupheimer S, et al. Octreotide, a somatostatin analogue, mediates its antiproliferative action in pituitary tumor cells by altering phosphatidylinositol 3-kinase signaling and inducing Zac1 expression. Cancer Res. 2006;66(3):1576–82. doi: 10.1158/0008-5472.CAN-05-1189. [DOI] [PubMed] [Google Scholar]
- 23.Witek RP, Fisher SH, Petersen BE. Monocrotaline, an alternative to retrorsine-based hepatocyte transplantation in rodents. Cell Transplant. 2005;14(1):41–7. doi: 10.3727/000000005783983278. [DOI] [PubMed] [Google Scholar]
- 24.Dabeva MD, Hwang SG, Vasa SR, et al. Differentiation of pancreatic epithelial progenitor cells into hepatocytes following transplantation into rat liver. Proc Natl Acad Sci U S A. 1997;94(14):7356–61. doi: 10.1073/pnas.94.14.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stolz DB, Michalopoulos GK. Synergistic enhancement of EGF, but not HGF, stimulated hepatocyte motility by TGF-beta 1 in vitro. J Cell Physiol. 1997;170(1):57–68. doi: 10.1002/(SICI)1097-4652(199701)170:1<57::AID-JCP7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 26.Ultanir SK, Kim JE, Hall BJ, Deerinck T, Ellisman M, Ghosh A. Regulation of spine morphology and spine density by NMDA receptor signaling in vivo. Proc Natl Acad Sci U S A. 2007;104(49):19553–8. doi: 10.1073/pnas.0704031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keri G, Racz G, Magyar K, et al. Pro-apoptotic and anti-apoptotic molecules affecting pathways of signal transduction. Ann N Y Acad Sci. 2003;1010:109–12. doi: 10.1196/annals.1299.018. [DOI] [PubMed] [Google Scholar]
- 28.Vantus T, Keri G, Krivickiene Z, et al. The somatostatin analogue TT-232 induces apoptosis in A431 cells: sustained activation of stress-activated kinases and inhibition of signalling to extracellular signal-regulated kinases. Cell Signal. 2001;13(10):717–25. doi: 10.1016/s0898-6568(01)00194-2. [DOI] [PubMed] [Google Scholar]
- 29.Slehria S, Rajvanshi P, Ito Y, et al. Hepatic sinusoidal vasodilators improve transplanted cell engraftment and ameliorate microcirculatory perturbations in the liver. Hepatology. 2002;35(6):1320–8. doi: 10.1053/jhep.2002.33201. [DOI] [PubMed] [Google Scholar]
- 30.Mohle R, Bautz F, Denzlinger C, Kanz L. Transendothelial migration of hematopoietic progenitor cells. Role of chemotactic factors. Ann N Y Acad Sci. 2001;938:26–34. doi: 10.1111/j.1749-6632.2001.tb03571.x. discussion 34-5. [DOI] [PubMed] [Google Scholar]
- 31.Naiyer AJ, Jo DY, Ahn J, et al. Stromal derived factor-1-induced chemokinesis of cord blood CD34(+) cells (long-term culture-initiating cells) through endothelial cells is mediated by E-selectin. Blood. 1999;94(12):4011–9. [PubMed] [Google Scholar]
- 32.Patel RC, Kumar U, Lamb DC, et al. Ligand binding to somatostatin receptors induces receptor-specific oligomer formation in live cells. Proc Natl Acad Sci U S A. 2002;99(5):3294–9. doi: 10.1073/pnas.042705099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moneta D, Richichi C, Aliprandi M, et al. Somatostatin receptor subtypes 2 and 4 affect seizure susceptibility and hippocampal excitatory neurotransmission in mice. Eur J Neurosci. 2002;16(5):843–9. doi: 10.1046/j.1460-9568.2002.02146.x. [DOI] [PubMed] [Google Scholar]
- 34.Kollet O, Shivtiel S, Chen YQ, et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112(2):160–9. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hatch HM, Zheng D, Jorgensen ML, Petersen BE. SDF-1alpha/CXCR4: a mechanism for hepatic oval cell activation and bone marrow stem cell recruitment to the injured liver of rats. Cloning Stem Cells. 2002;4(4):339–51. doi: 10.1089/153623002321025014. [DOI] [PubMed] [Google Scholar]
- 36.Piscaglia AC, Shupe TD, Oh SH, Gasbarrini A, Petersen BE. Granulocyte-colony stimulating factor promotes liver repair and induces oval cell migration and proliferation in rats. Gastroenterology. 2007;133(2):619–31. doi: 10.1053/j.gastro.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirchmair R, Egger M, Walter DH, et al. Secretoneurin, an angiogenic neuropeptide, induces postnatal vasculogenesis. Circulation. 2004;110(9):1121–7. doi: 10.1161/01.CIR.0000139884.81390.56. [DOI] [PubMed] [Google Scholar]
- 38.Li H, Jiang X, Howell SB, Goodman M. Synthesis, conformational analysis and bioactivity of Lan-7, a lanthionine analog of TT-232. J Pept Sci. 2000;6(1):26–35. doi: 10.1002/(SICI)1099-1387(200001)6:1<26::AID-PSC231>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]