Abstract
The bioactive sphingolipid metabolite, sphingosine-1-phosphate (S1P), is now recognized as a critical regulator of many physiological and pathophysiological processes, including cancer, atherosclerosis, diabetes and osteoporosis. S1P is produced in cells by two sphingosine kinase isoenzymes, SphK1 and SphK2. Many cells secrete S1P, which can then act in an autocrine or paracrine manner. Most of the known actions of S1P are mediated by a family of five specific G protein-coupled receptors. More recently, it was shown that S1P also has important intracellular targets involved in inflammation, cancer and Alzheimer’s disease. This suggests that S1P actions are much more complex than previously thought, with important ramifications for development of therapeutics. This review highlights recent advances in our understanding of mechanisms of action of S1P and its roles in disease.
S1P: a signaling molecule
Like glycerophospholipids, sphingolipids are ubiquitous components of mammalian membranes that are metabolized to form signaling molecules. It is now recognized that sphingolipid metabolites play important roles in regulation of many cellular processes important for health and disease. One of the most important of these metabolites is sphingosine-1-phosphate (S1P, Figure 1). It has been 20 years since the discovery that S1P is a signaling molecule that regulates cell growth 1 and suppresses apoptosis 2, suggesting that it may play a role in cancer. Later, it was shown that S1P acts through a family of cell surface receptors, subsequently shown to be critical for migration of immune cells throughout the body. Indeed, one of the hallmarks of S1P involvement in disease is its control of cell trafficking 3. New tools, such as specific agonists and antagonists and the generation of targeted knockouts has led to a surge of interest in the role of S1P in numerous diseases. Remarkable progress has been made in understanding its mechanism of action, though many questions are still unanswered. In this review, we will discuss how S1P is generated, how it signals, and briefly summarize its involvement in several diseases.
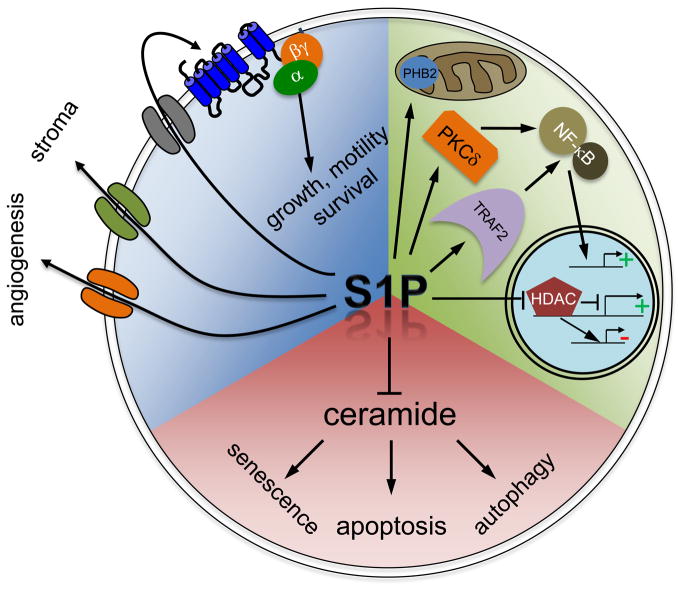
Figure 1. Intracellular and extracellular actions of S1P.
S1P produced intracellularly can inhibit functions of its pro-apoptotic precursor ceramide. Ceramide is implicated in growth arrest, apoptosis and autophagy (red quadrant). S1P also has intracellular targets (green quadrant) or can be exported out of cells to act in autocrine and/or paracrine manners through the S1P receptors (blue quadrant).
Localized production of S1P
S1P is formed by the phosphorylation of sphingosine – the backbone of sphingolipids – by two kinases, sphingosine kinase 1 and 2 (SphK1 and SphK2). S1P levels are tightly controlled both by the enzymes that produce its substrate sphingosine, by the SphKs themselves, and by the enzymes that degrade S1P, which include S1P lyase (SPL), two S1P-specific phosphatases, and three lipid phosphate phosphatases.
Numerous agonists activate SphK1, including growth factors, hormones, pro-inflammatory cytokines, lipopolysaccharide, ligation of the IgE and IgG receptors, and many GPCR ligands, and this activation is critical for their full actions. Many of these agonists induce extracellular signal regulated kinase (ERK)-dependent phosphorylation at serine 225 of SphK1, leading to its translocation from the cytosol to the plasma membrane where its substrate sphingosine resides4. The SphK1-S225A mutant acts like a dominant negative, even though it retains full kinase activity. Targeting this mutant to the plasma membrane reverses the inhibitory phenotype, however, and even transforms quiescent fibroblasts 4. Translocation of SphK1 to the plasma membrane may also be mediated by interaction with other proteins. For example, it was recently shown that calcium and integrin-binding protein 1 bind SphK1 in a calcium-dependent manner resulting in its translocation to the plasma membrane through a calcium-myristoyl switch 5. Several other proteins that interact with SphK1 also directly increase its activity 4. One interesting recent example is elongation factor 1A (eEF1a). Although well known for its role in translation, eEF1a is a G protein that activates SphK1 only in its GDP-bound state 6.
In contrast to SphK1, which is mainly localized in the cytosol, SphK2 is present in several intracellular compartments, depending on cell type. Its functions in these compartments have only recently begun to be elucidated. Consistent with its nuclear localization signal, SphK2 in the nucleus regulates gene transcription, at least in part by producing S1P, which acts as an endogenous inhibitor of histone deacetylases 7. Although lacking an identifiable mitochondrial targeting signal, SphK2 is also present in mitochondria where it is required for correct assembly of the cytochrome oxidase complex 8. SphK2 also is postulated to bind to phosphatidylinositol monophosphates through an N-terminal domain, targeting it to intracellular membranes 9.
Compared to SphK1, much less is known about activation of SphK2. Both EGF and phorbol ester activate SphK2 through ERK1-dependent phosphorylation 7, 10, and phosphorylation by protein kinase D promotes its nuclear export 11. Hypoxia activates SphK2 in A549 lung cancer cells 12 and brain microvasculature 13, producing S1P that protected against apoptosis and promoted ischemic tolerance, respectively.
In sum, the diverse compartment-specific localizations of the SphKs indicate that the specific microenvironment in which S1P is produced dictates its functions.
S1P acts extracellularly though cell surface receptors
There are five specific cell surface G protein-coupled receptors for S1P, termed S1PR1–5, all with low nM Kd’s. Differential signaling induced by binding of S1P to these receptors is due to distinct, though sometimes overlapping, coupling to diverse heterotrimeric G proteins (Figure 1). These receptors have been implicated in a variety of developmental and disease-related processes. For example, S1PR1 activation plays a critical role in the trafficking of immune cells 14. The pro-drug FTY720, approved by the FDA for treatment of the autoimmune disease multiple sclerosis 15, is phosphorylated in vivo by SphK2 and induces downregulation and degradation of S1PR1, but not other S1PRs, on lymphocytes. Loss of S1PR1 prevents lymphocytes from sensing the S1P gradient from the lymphoid organs into the blood, blocking egress and inducing lymphopenia 16. The role of this S1P/S1PR axis in the trafficking of immune cells is becoming paradigmatic 3, as it is also important for B cell migration through the lymph node follicle 17. Furthermore, T-bet-dependent S1PR5 expression is required for natural killer T cell egress from lymph organs 18, and S1PR4 plays a role in neutrophil trafficking 19. S1P also may be important for stem cell recruitment, differentiation, and maintenance in a pluripotent state 20.
S1P/S1PR axes also are important in controlling vascular tone and permeability. S1P in the circulation regulates basal and inflammation-induced vascular leak via S1PR1 21. TNF-α induces vasoconstriction of the spiral modiolar artery, the sole provider of blood to the cochlea, by activating SphK1 and producing S1P that stimulates S1PR2, whose downstream signaling leads to vasoconstriction 22. This finding provides a link between inflammation- and vasculopathy-induced hearing loss and may explain why S1PR2 receptor null mice are deaf 23, 24.
These, together with many other studies, raise a broader issue with agonist-induced activation of SphK1 leading to S1PR signaling: SphK1 is a cytosolic enzyme, and yet the S1PRs bind S1P on the exoplasmic leaflet. How does the S1P get outside the cell? Several transporters are now implicated in exporting S1P, where it can locally activate S1PRs. These include the ATP binding cassette transporters ABCA1 25, ABCC1, and ABCG2 26,27, as well as the putative transporter, Spinster 2 28. This process – whereby agonists activate SphK1 and induce its recruitment to the plasma membrane, producing S1P that is then locally secreted to activate S1PRs – is called “inside-out signaling” and explains the astounding variety of autocrine and paracrine actions of S1P (Figure 1).
Intriguingly, the S1P-S1PR signaling axis can also be controlled by the activity of S1P lyase (SPL), an ER-localized enzyme. Inhibition or genetic ablation of SPL increases circulating S1P and induces lymphopenia, reminiscent of the effects of FTY720 19, 29. Others have suggested that extracellular S1P-mediated motility of lung endothelial cells is dependent on intracellular S1P levels, which are regulated by both SphK1 and SPL 30. Moreover, it has been suggested that intracellular localization of SphK1 determines access to sphingosine substrate pools but does not affect the degradative fate of S1P 31. These studies not only reinforce the importance of “inside-out” signaling but also highlight SPL as a potential therapeutic target 32.
S1P is an intracellular messenger
Many lines of evidence point to an intracellular role for S1P. S1P counteracts apoptosis mediated by its pro-apoptotic precursor ceramide and SphK1 is suggested to play a critical role in this “sphingolipid rheostat” (reviewed in 33, Figure 1). Moreover, early studies demonstrated that S1P could induce calcium release from the ER, 34–36, though no target has yet been conclusively identified. For nearly two decades, no bona fide intracellular targets of S1P had been identified. Recently, this changed with the demonstration that S1P binds and alters the function of several disparate intracellular proteins.
HDAC
Although SphK2 is present in the nucleus of many cells, its function there was unknown. Recently, it was shown for the first time that nuclei contain significant amounts of both S1P and sphingosine. Moreover, SphK2 is in a repressor complex with histone H3 and histone deacetylases (HDACs), producing S1P that regulates histone acetylation at specific lysine residues and gene transcription 7. Although S1P has no effect on histone acetyl transferases, it binds to and inhibits both HDAC1 and its close homolog, HDAC2. Inhibition of HDAC1/2 by S1P is physiologically relevant, as SphK2 was detected at the promoters of the cyclin-dependent kinase inhibitor p21, and the transcriptional regulator c-fos, where it regulates histone acetylation and enhances their transcription 7. These results indicate that S1P produced in the nucleus by SphK2 influences the dynamic balance of histone acetylation and thus the epigenetic regulation of specific target genes.
TRAF2
TNF receptor-associated factor 2 (TRAF2) is an adaptor protein containing a RING domain that is implicated in the regulatory ubiquitination of RIP1, a critical event in activation of NF-κB in response to TNF-α. All attempts to demonstrate that TRAF2 was the E3 ligase for RIP1 failed, however. TRAF2 is known to bind SphK1 and stimulate its activity 37, though the role of S1P in the canonical NF-κB pathway was unclear. Recently, it was discovered that S1P is a missing cofactor required for the E3 ligase activity of TRAF2 and, consequently, Lys-63-linked polyubiquitination of RIP1 and NF-κB activation 38. Interestingly, only S1P, and not dihydro-S1P, which lacks the double bond in S1P, binds to and activates TRAF2. This explains why, although S1P and dihydro-S1P are equally potent ligands for the five S1P receptors, only S1P suppresses apoptosis 38. These data provide the first mechanistic explanation for the numerous observations of the importance of SphK1 and S1P in cytoprotective, inflammatory, and immune responses.
PKCδ
SphK1 plays a key role in a number of immune responses, including sepsis 39. Investigation of the molecular mechanism revealed a role for protein kinase C delta (PKCδ) in mediating endotoxin-induced activation of NF-κB. Moreover, S1P, or recombinant SphK1 incubated with sphingosine and ATP to produce S1P, activates recombinant human PKCδ in vitro 39. Although direct binding was not determined, these data suggest that S1P binds and activates PKCδ in vivo, and implicate SphK1 as a critical component and therapeutic target for septic shock.
PHB2
Prohibitin 2 (PHB2), a highly conserved protein that regulates mitochondrial assembly and function, was recently shown to bind S1P in vitro and in vivo 8. PHB2 predominantly localizes to the inner mitochondrial membrane where it is thought to form a large, macromolecular complex with PHB1 that is involved in mitochondrial biogenesis and metabolism 40. This study showed for the first time that SphK2 also localizes to the mitochondria, where it produces the majority of mitochondrial S1P. Mitochondria respiration was reduced in SphK2 knockout mice due to aberrant assembly and reduced activity of complex IV (cytochrome oxidase) of the electron transport chain, suggesting that interaction of S1P with PHB2 is important for cytochrome-c oxidase assembly and mitochondrial respiration. Further work is needed to elucidate the role of mitochondria S1P in mitochondrial function during oxidative stress and aging.
BACE1
A recent study showed that the activity of β-site amyloid precursor protein (APP) cleaving enzyme-1 (BACE1), the rate-limiting enzyme for amyloid-β peptide (Aβ) production, is modulated by S1P in neurons 41. Inhibition or downregulation of SphK1, or overexpression of S1P-degrading enzymes, all decreased BACE1 activity and Aβ production 41. S1P also specifically bound to BACE1 in vitro and increased its proteolytic activity, suggesting that cellular S1P directly modulates BACE1 activity. Importantly, SphK2 was upregulated in the brains of patients with Alzheimer’s disease (AD). These results suggest a unique link between S1P, SphK2, and AD.
S1P in disease
As summarized above, the SphKs/S1P/S1PRs axis is implicated in regulation of many physiological processes, drawing attention to their potential functions in pathophysiology and diseases (Figure 2). The important and rapidly emerging area of targeting S1PRs in inflammation and multiple sclerosis, has been extensively reviewed recently 3, 15, 42. Therefore, we will focus on selected recent advances in understanding the role of S1P in cancer, atherosclerosis, diabetes, and osteoporosis.
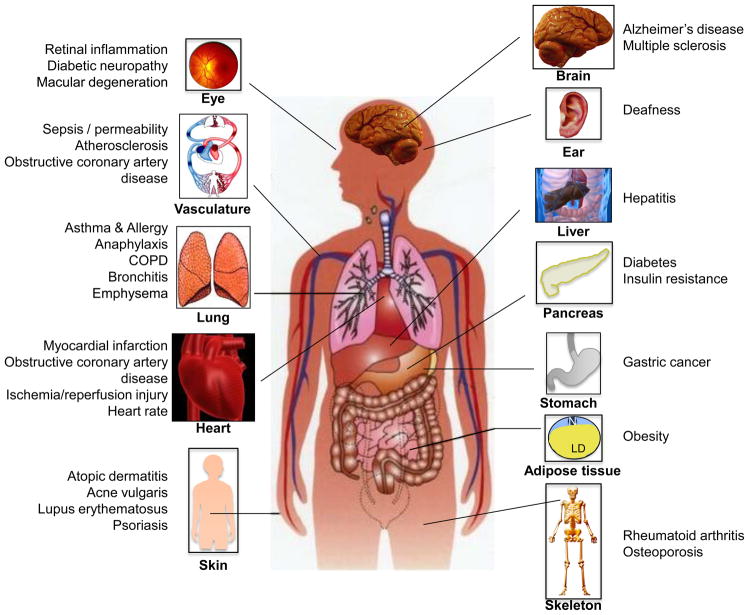
Figure 2. The pathobiology of S1P.
S1P is implicated in numerous pathophysiological conditions and diseases that affect almost every organ in the human body. Because S1P is also linked to almost every type of cancer, only a few are shown.
Cancer
Numerous studies have shown that SphK1 and production of S1P promotes tumor growth, resistance to apoptosis, tumor angiogenesis and metastasis (reviewed in 43). SphK1 message and protein levels are often upregulated in cancerous tissue and expression is correlated with chemo- and radio-resistance and poor prognosis. Consistent with these observations, overexpression of SphK1 promotes, while its inhibition reduces, tumor growth, angiogenesis and chemoresistance in numerous xenograft models 43, 44.
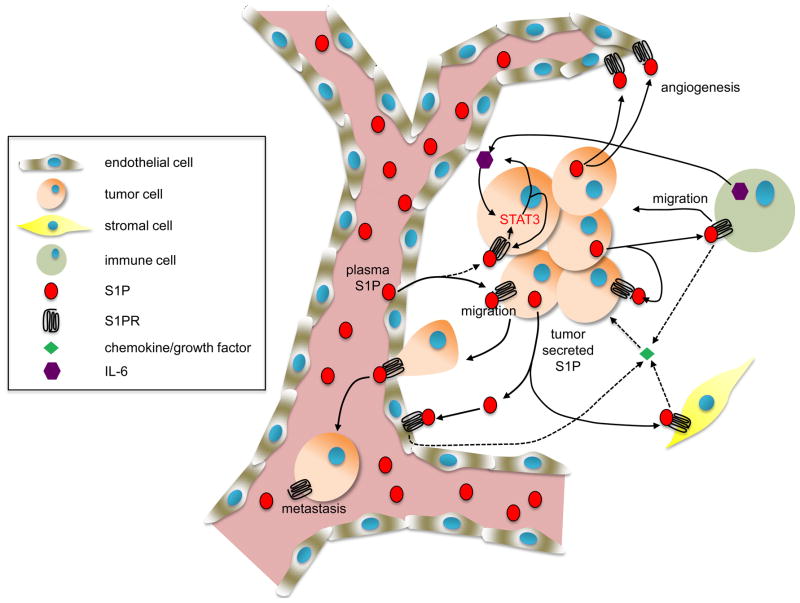
S1P secreted from tumor cells can act through S1PRs either in an autocrine manner to promote growth, survival, motility, and metastasis 45, 46 or in a paracrine manner to induce endothelial adhesion molecules, angiogenesis and regulate tumor-stromal interactions as well as immune cells 47 (Figure 3). As discussed above, S1P may also promote cancer progression via intracellular targets, such as HDAC1/2 and NF-κB. Finally, S1P can promote resistance of cancer cells to therapy by counteracting the pro-apoptotic effects of ceramide 2 (Figure 1). An intriguing study recently identified S1PR1 as a key element involved in persistent activation of signal transducer and activator of transcription-3 (STAT3) in tumor cells and the tumor microenvironment 48. STAT3 is a transcription factor for S1pr1 and, conversely, enhanced S1pr1 expression activates STAT3 and upregulates IL-6 expression, a pro-inflammatory cytokine crucial for STAT3 activation and inflammatory cell-mediated transformation and tumor progression. Thus, this is a new feed-forward mechanism that explains persistent STAT3 activation in cancer cells and the tumor microenvironment that is important for malignant progression and metastasis 48.
Figure 3. Involvement of S1P in cancer.
As described in the text, S1P regulates many processes in cancer cells and the tumor microenvironment that are important for malignant progression, including growth, survival and metastasis of the tumor, migration of stromal cells, and angiogenesis. S1P is also involved in recruitment of inflammatory cells and secretion of cytokines and chemokines that are important for inflammation and tumorigenesis.
Because of the potential importance of SphK1 in cancer and other diseases, much emphasis is now on development of isotype-specific inhibitors of SphK1 (Table 1). First-generation SphK inhibitors lacked isoform specificity, although one of these, safingol, is in phase 1 clinical trials 49. One SphK1-specific inhibitor, known as SK1-I, inhibits the growth of acute myelogenous leukemia 50 and glioblastoma tumors 51 in xenografts by affecting multiple survival signaling pathways. Another inhibitor, SKI-II, enhances the sensitivity of non-small cell lung cancer cells to chemotherapeutics both in vitro and in vivo 52. Surprisingly, SKI-II also induces proteosomal degradation of SphK1 53 and binds directly to the antagonist ligand-binding domain of the estrogen receptor, inhibiting estrogen receptor-stimulated transcriptional activity in human breast cancer cells 54. Recently, progress was reported in the design of specific SphK1 inhibitors based on a homology model of SphK1 trained with a library of amidine-based compounds. Inhibitors with nM Ki’s for SphK1 were developed and found to significantly reduce endogenous S1P levels in leukemia U937 cells 55. It will be interesting to see the results of in vivo studies with these compounds.
Table 1.
Small molecules targeting S1P.
| Name | Mechanism | Diseases | References |
|---|---|---|---|
| FTY720 (Fingolimod, Gilenya) 2-amino-2-[2-(4-octylphenyl)ethyl]propane-1,3-diol | • Prodrug; phosphorylated by SphK2 | • FDA approved for multiple sclerosis* | [15] |
| • S1PR1, S1PR3–5 agonist | • Dermatitis | [82] | |
| • Downregulates S1P1 | • Arthritis | [83] | |
| • Allergy | [84] | ||
|
| |||
| SK1-I (BML-258) (2R,3S,4E)-N-methyl-5-(4′-pentylphenyl)-2-aminopent-4-ene-1,3-diol | • SphK1 inhibitor | • Glioblastoma | [51] |
| • Leukemia | [50] | ||
|
| |||
| Safingol L-threo-dihydrosphingosine | • pan SphK inhibitor | • Solid tumors* (Phase 1) | [49] |
|
| |||
| SKI 2-(p-hydroxyanilino)-4-(p-chlorophenyl) thiazole | • SphK1 inhibitor | • Pancreatic cancer | [85] |
| • Induces proteasomal and lysosomal degradation of SphK1 | • Leukemia | [86] | |
| • Asthma | [87] | ||
|
| |||
| ABC294640 3-(4-chlorophenyl)-adamantane-1-carboxylic acid (pyridinyl-4-methyl) amide | • SphK2 inhibitor | • Cancer | [59] |
| • Estrogen receptor agonist | • Rheumatoid arthritis | [88] | |
| • Inflammatory bowel disease | [89] | ||
|
| |||
| ABC294735 3-(4-chlorophenyl)adamantane-1-carboxylic acid [2-(3,4-dihydroxyphenyl)ethyl]amide | • pan SphK inhibitor | • Cancer | [90] |
|
| |||
| SKI-II (SKI-2, SPHK I2) 4-[4-(4-chlorophenyl)-thiazolyl-2-amino]-phenol | • SphK1 inhibitor | • Asthma | [91] |
|
| |||
| THI 2-acetyl-4(5)-[1(R),2(S),3(R),4-tetrahydroxybutyl]-imidazole | • S1P Lyase inhibitor | • Ischemia/reperfusion injury | [92] |
| • Lung injury | [93] | ||
|
| |||
| LX2931 (E)-1-(4-((1R,2S,3R)-1,2,3,4-tetrahydroxybutyl)-1H-imidazol-2-yl)ethanone oxime | • S1P Lyase inhibitor | • Rheumatoid arthritis* (Phase II) | [32] |
|
| |||
| 5c 2,2-dimethyl-4S-(1-oxo-2-hexadecyn-1-yl)-1,1-dimethylethyl ester-3-oxazolidinecarboxylic acid | • SphK1 inhibitor | • Sepsis | [39] |
|
| |||
| SEW2871 5-[4-phenyl-5-(trifluoromethyl)-2-thienyl]-3-[3-(trifluoromethyl) phenyl]-1,2,4-oxadiazole | • S1PR1 agonist | • Diabetic nephropathy | [94] |
| • Renal protection | [95] | ||
| • Emphysema | [96] | ||
| • Neuroprotection | [97] | ||
| • Spontaneous autoimmune polyneuropathy | [98] | ||
|
| |||
| AAL(R) 2-amino-4-(4-heptyloxyphenyl)-2-methylbutanol | • Prodrug; phosphorylated by SphK2 | • Influenza | [99] |
| • S1PR1/S1PR3 agonist | |||
|
| |||
| AUY954 | • S1PR1 agonist | • Sepsis | [100] |
| • Transplanation | [101] | ||
| • Experimental autoimmune neuritis | [102] | ||
|
| |||
| JTE013 | • S1PR2 antagonist | • Anaphylaxis | [103] |
| • Atherosclerosis | [65] | ||
| • Cancer | [45] | ||
Effects in humans. All other compounds have only been tested in animal models.
In contrast, the connection between SphK2 and cancer is still not well defined. SphK2 downregulation was more effective than SphK1 downregulation in inhibiting growth of glioblastoma cells 56. Downregulation of SphK2 in MCF7 cells also decreased G2-M arrest and markedly enhanced apoptosis induced by doxorubicin, probably due to effects on p21 expression 57. SphK2-deficient breast cancer cells have impaired growth in a mouse tumor model 58. A proposed SphK2-specific inhibitor, ABC294640, inhibited the proliferation of a variety of cancer cells in culture and reduced the S1P content and growth of mammary tumors in nude mice 59. Interpretation of these results is complicated by the recent demonstration that this inhibitor also has anti-estrogenic effects and binds to the estrogen receptor 60. The demonstration that S1P produced by SphK2 is an endogenous HDAC inhibitor 7 also suggests that the role of SphK2 in cancer progression may be dependent on tissue-specific and perhaps tumor-specific properties.
Atherosclerosis
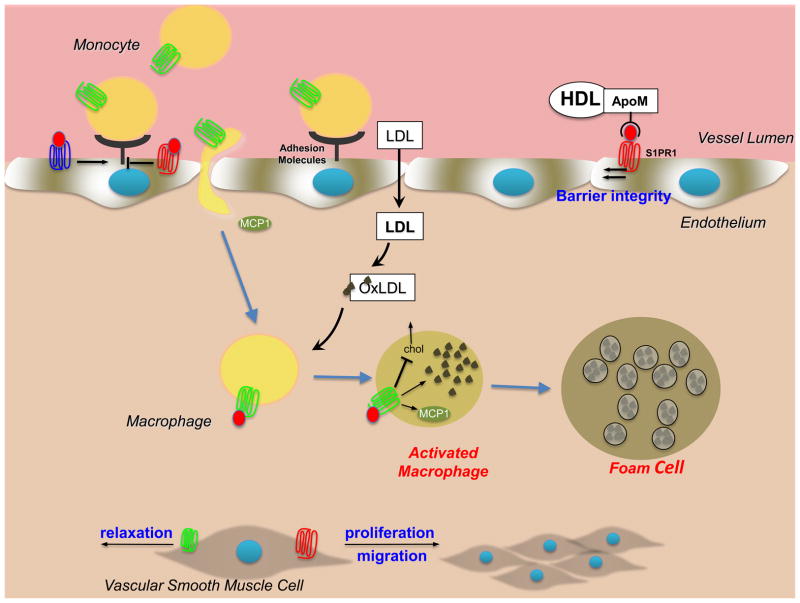
Atherosclerosis is a chronic inflammatory condition, a hallmark of which is the extensive accumulation of lipids and immune cells, called plaques, in subendothelial regions, causing the arterial lumen to narrow and thrombose after rupture of the plaque. Interest in S1P in the pathogenesis of atherosclerosis stems from observations that S1P is in high concentrations in the circulation, where it is largely bound to high density lipoprotein (HDL) and albumin. S1P has diverse effects on a variety of cell types central to the development of atherosclerosis, including monocyte attachment and migration, proliferation of smooth muscle cells, vascular tone, and stimulation of the NF-κB pathway leading to production of pro-inflammatory cytokines 61. S1P actions are complex and it is still not clear whether S1P is pro- or anti-atherogenic. The stimulatory pathway is mediated mainly by NF-κB activation and adhesion molecule expression, probably via S1PR3, and the inhibitory pathway is mediated mainly by activation of endothelial nitric oxide synthase and nitric oxide-dependent vasorelaxation by activation of S1PR1 62 (Figure 4). Although there are no major differences in atherosclerotic lesions and lipid volume in the aorta of apolipoprotein E (ApoE)−/− S1PR3−/− double knockout mice, there is significant reduction in macrophage and smooth muscle content of the lesions 63. These results suggest that S1PR3 promotes inflammatory monocyte/macrophage recruitment and alters smooth muscle cell behavior. Because proliferation of smooth muscle cells in vitro was unaffected by S1PR3 deletion, further studies with targeted deletions of this receptor are needed to confirm its role. Both pharmacologic and genetic approaches to silence S1PR2 in ApoE−/− mice attenuated atherosclerotic lesion formation as shown by reduced plaque area, inhibition of macrophage accumulation in the aorta and increased cholesterol efflux in macrophages 64, 65. These effects were attributed to lower S1PR2-dependent macrophage retention and/or transmigration 65 and decreased ROCK and NF-κB activities, leading to decreased expression of pro-inflammatory cytokines, adhesion molecules and the chemokine MCP-1 64 (Figure 4). Nevertheless, the anti-atherogenic actions of HDL independent of cholesterol metabolism are suggested to be mediated by HDL-associated S1P 62. Indeed, a recent study showed that HDL-associated S1P is bound solely to apolipoprotein M (ApoM), which is known to have anti-atherogenic effects 66. Hence, in addition to increasing cholesterol efflux from macrophage foam cells, and pre-β-HDL formation and its antioxidative effects, ApoM is vasculoprotective by delivering S1P to S1PR1 on endothelial cells 66, and S1P-ApoM may well be anti-atherogenic. It is possible that binding of S1P to ApoM in HDL is one factor that determines the specificity of the physiological effects of S1P.
Figure 4. Role of S1P and its receptors in atherogenesis.
Binding of S1P to distinct S1P receptors regulates vascular tone and barrier function, monocyte attachment and migration, macrophage infiltration and retention, proliferation of smooth muscle cells, and activation of NF-κB leading to production of pro-inflammatory cytokines and adhesion molecules. S1PR1, red; S1PR2, green; S1PR3, blue.
Interestingly, FTY720 markedly reduced atherosclerotic lesions in both low density lipoprotein receptor-null and ApoE-null mice 67, 68. This is likely due to FTY720-mediated downregulation of S1PR1 on lymphocytes and resulting lymphopenia, as well as to shifting classical inflammatory M1 macrophages to anti-inflammatory M2 types 67. FTY720 conferred atheroprotective effects in human primary macrophages by reducing transport of scavenged lipoprotein cholesterol to the ER and facilitating its release 69. Surprisingly, however, this effect was independent of S1PRs. Rather, FTY720 stimulated production of 27-hydroxycholesterol from cholesterol, providing an additional explanation for its atheroprotective effects and identifying a novel mechanism for the actions of FTY720 69.
Diabetes and obesity
Diabetes is a chronic disease affecting hundreds of millions of people worldwide. Sphingolipid metabolism is altered in diabetic conditions, however, focus to date in this area has been on ceramide 70, 71 and only a few studies have examined the involvement of S1P. Fatty acid (palmitate)-induced stimulation of de novo ceramide synthesis is required, but not sufficient, for the onset of insulin resistance and ceramide is linked to insulin resistance, in part due to inhibition of Akt 70, 71. Moreover, inhibition of sphingolipid synthesis increased insulin sensitivity, resolved hepatic steatosis, and prevented the onset of diabetes in obese rodents.
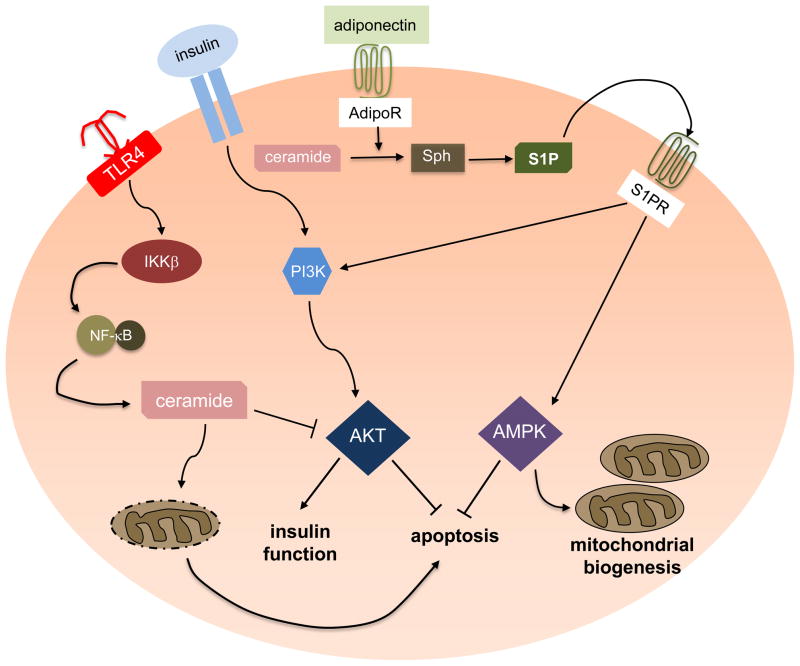
Inflammatory responses in obesity exacerbate insulin resistance and development of diabetes. Activation of toll-like receptor 4 (TLR4) by saturated fatty acids is one pathway of innate immunity contributing to increased inflammation associated with obesity. An intriguing study recently demonstrated that TLR4 stimulates IKKβ and NF-κB, which increases the transcription of genes involved in ceramide synthesis 72. These genes drive ceramide elevation, inhibiting Akt, which is required for insulin signaling (Figure 5). Interestingly, increased ceramide production was dispensable for TLR4-dependent induction of inflammatory cytokines, but required for TLR4-dependent insulin resistance 72. Altogether, this work suggests that ceramide is a key mediator that links lipid-induced inflammatory pathways to insulin resistance and diabetes.
Figure 5. Role of S1P in diabetes and obesity.
Saturated fatty acid-induced insulin resistance is mediated by the proinflammatory receptor TLR4, which stimulates IKKβ and leads to NF-κB activation. This results in the upregulation of biosynthetic genes involved in de novo ceramide formation. Ceramide, in turn, inhibits Akt to induce apoptosis and suppress insulin function. Binding of adiponectin to its receptors enhances deacylation of ceramide to sphingosine, which can then be phosphorylated to form S1P. Inside-out signaling via S1PRs can activate AMPK, important for mitochondria biogenesis. S1P also stimulates Akt and prevents apoptosis.
Another intriguing link was recently uncovered between adipocyte-derived adiponectin – a hormone with insulin sensitizing, anti-inflammatory and anti-apoptotic functions – and ceramide and its metabolites with insulin sensitivity 73. Binding of adiponectin to its two receptors, AdipoR1 and AdipoR2, stimulates ceramidase activity (deacylation to sphingosine) and formation of its anti-apoptotic metabolite S1P. S1P may then bind to its receptors, leading to activation of AMPK. These actions of adiponectin via S1P might promote survival of pancreatic beta cells, nutrient uptake, nutrient utilization and mitochondrial proliferation 73 (Figure 5).
Plasma S1P levels are elevated in two animal models of type 1 diabetes (streptozotocin-induced diabetic rats and Ins2 Akita diabetic mice), yet no changes in levels were detected in the livers of these animals 74, suggesting other sources of S1P. An S1PR2 antagonist prevented the onset of diabetes in a streptozotocin diabetes mouse model and S1PR2−/− mice displayed lower blood glucose levels and reduced beta cell apoptosis together with higher insulin/glucose ratios (an index of relative insulin deficiency) 75. Glucose induces a rapid and sustained increase in islet sphingosine kinase activity that is dependent on glucose phosphorylation, but independent of ATP generation and protein biosynthesis. Moreover, glucose-supported beta cell growth appears to be, in part, mediated by this increased activity. Although several studies suggest that S1P might be pro-survival for pancreatic beta cells, it is still not clear whether this is mediated by intracellular or extracellular actions of S1P, nor do we know the mechanism or which S1PRs are involved.
S1P in osteoporosis
Osteoporosis is a systemic skeletal disorder and one of the most common types of metabolic bone diseases 76. In the past few years there has been increasing interest in the role of S1P in normal bone homeostasis as well as in the pathogenesis of bone destructive disorders. S1P has multiple functions in osteoclasts, including stimulating their motility, providing dynamic control over the migration of osteoclast precursors, and maintaining bone mineral homeostasis and increased osteoclastogenesis 77–80. S1P stimulates osteoblast migration and promotes their survival, and thus secretion of S1P at sites of bone resorption may be important for osteogenesis 77. Osteoclasts might recruit osteoprogenitors to the site of bone remodeling through the SIP/S1PR1 axis and BMP6, and stimulate bone formation through increased activation of Wnt/BMP pathways 78.
Low concentrations of S1P are chemotactic for osteoclast precursors, while high concentrations inhibit their movement 79. This suggests that these precursors, which express both S1PR1 and S1PR2, are kept in the vicinity of the bone by the S1P gradient: S1PR2 prevents S1PR1-induced migration of these bone-resorbing cells away from bone. S1PR2 null mice develop osteopetrosis due to a decrease in bone resorption 80. Likewise, in ovariectomized mice, the S1PR2 antagonist JTE013 prevented bone loss by decreasing osteoclast attachment to bone 80. Conversely, osteoclast-specific ablation of S1PR1 induced osteoporosis by increasing their attachment to bone 79. Thus, the balance between the S1PRs and their sensing of local S1P concentrations plays a critical physiological role in bone homeostasis and likely in osteoporosis, although this remains to be examined. Similarly, in a TNF transgenic mouse model for inflammatory arthritis, knockout of SphK1 significantly reduced arthritis symptoms as well as bone erosive disease 81. Moreover, the synovial joints had fewer osteoclasts, suggesting that that SphK1 plays a key role in TNF-induced inflammatory arthritis by regulating synovial inflammation and osteoclast number.
Concluding remarks
The sum of a plethora of in vitro and in vivo studies in the decades since S1P was first discovered to be a second messenger has taught us much about its mechanisms of action. We now understand why S1P is so important for regulation of many normal and pathophysiological processes. The successful development of the sphingosine analogue FTY720, a pro-S1P mimetic, as a useful drug for treatment of multiple sclerosis has proven that it is possible and beneficial to specifically target S1P signaling in humans without serious side effects. Inhibitors of S1P metabolism and its receptors are effective in many animal models of human disease. There is also now tantalizing evidence, summarized briefly here, that dysregulation of S1P correlates with progression of diseases such as cancer and autoimmune disorders. Thus, the time is ripe for translating this knowledge into a new class of sphingolipid-centric therapeutics.
Acknowledgments
We apologize to authors whose work has not been cited here owing to space limitations. This work was supported by grants from the US National Institutes of Health R01CA61774, R37GM043880, R01AI50094, 1U19AI077435 (to S.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olivera A, Spiegel S. Sphingosine-1-phosphate as a second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- 2.Cuvillier O, et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 3.Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11:403–415. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pitson SM. Regulation of sphingosine kinase and sphingolipid signaling. Trends Biochem Sci. 2010;36:97–107. doi: 10.1016/j.tibs.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Jarman KE, et al. Translocation of sphingosine kinase 1 to the plasma membrane is mediated by calcium- and integrin-binding protein 1. J Biol Chem. 2010;285:483–492. doi: 10.1074/jbc.M109.068395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leclercq TM, et al. Guanine nucleotides regulate sphingosine kinase 1 activation by eukaryotic elongation factor 1A and provide a mechanism for eEF1A-associated oncogenesis. Oncogene. 2011;30:372–378. doi: 10.1038/onc.2010.420. [DOI] [PubMed] [Google Scholar]
- 7.Hait NC, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strub GM, et al. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J. 2010 doi: 10.1096/fj.10-167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Don AS, Rosen H. A lipid binding domain in sphingosine kinase 2. Biochem Biophys Res Commun. 2009;380:87–92. doi: 10.1016/j.bbrc.2009.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hait NC, et al. Sphingosine kinase type 2 activation by ERK-mediated phosphorylation. J Biol Chem. 2007;282:12058–12065. doi: 10.1074/jbc.M609559200. [DOI] [PubMed] [Google Scholar]
- 11.Ding G, et al. Protein kinase D-mediated phosphorylation and nuclear export of sphingosine kinase 2. J Biol Chem. 2007;282:27493–27502. doi: 10.1074/jbc.M701641200. [DOI] [PubMed] [Google Scholar]
- 12.Schnitzer SE, et al. Hypoxia enhances sphingosine kinase 2 activity and provokes sphingosine-1-phosphate-mediated chemoresistance in A549 lung cancer cells. Mol Cancer Res. 2009;7:393–401. doi: 10.1158/1541-7786.MCR-08-0156. [DOI] [PubMed] [Google Scholar]
- 13.Wacker BK, et al. Hypoxic preconditioning-induced cerebral ischemic tolerance: role of microvascular sphingosine kinase 2. Stroke. 2009;40:3342–3348. doi: 10.1161/STROKEAHA.109.560714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 15.Brinkmann V, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 16.Pham TH, et al. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity. 2008;28:122–133. doi: 10.1016/j.immuni.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cyster JG. B cell follicles and antigen encounters of the third kind. Nat Immunol. 2010;11:989–996. doi: 10.1038/ni.1946. [DOI] [PubMed] [Google Scholar]
- 18.Jenne CN, et al. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J Exp Med. 2009;206:2469–2481. doi: 10.1084/jem.20090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allende ML, et al. Sphingosine-1-phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J Biol Chem. 2010 doi: 10.1074/jbc.M110.171819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitson SM, Pebay A. Regulation of stem cell pluripotency and neural differentiation by lysophospholipids. Neurosignals. 2009;17:242–254. doi: 10.1159/000231891. [DOI] [PubMed] [Google Scholar]
- 21.Camerer E, et al. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest. 2009;119:1871–1879. doi: 10.1172/JCI38575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scherer EQ, et al. Tumor necrosis factor-alpha enhances microvascular tone and reduces blood flow in the cochlea via enhanced sphingosine-1-phosphate signaling. Stroke. 2010;41:2618–2624. doi: 10.1161/STROKEAHA.110.593327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kono M, et al. Deafness and stria vascularis defects in S1P2 receptor null mice. J Biol Chem. 2007;282:10690–10696. doi: 10.1074/jbc.M700370200. [DOI] [PubMed] [Google Scholar]
- 24.Maclennan AJ, et al. The S1P(2) sphingosine 1-phosphate receptor is essential for auditory and vestibular function. Hear Res. 2006;220:38–48. doi: 10.1016/j.heares.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Sato K, et al. Critical role of ABCA1 transporter in sphingosine 1-phosphate release from astrocytes. J Neurochem. 2007;103:2610–2619. doi: 10.1111/j.1471-4159.2007.04958.x. [DOI] [PubMed] [Google Scholar]
- 26.Mitra P, et al. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci USA. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takabe K, et al. Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. J Biol Chem. 2010;285:10477–10486. doi: 10.1074/jbc.M109.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawahara A, et al. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323:524–527. doi: 10.1126/science.1167449. [DOI] [PubMed] [Google Scholar]
- 29.Schwab SR, et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 30.Berdyshev EV, et al. Intracellular S1P generation is essential for S1P-induced motility of human lung endothelial cells: role of sphingosine kinase 1 and S1P lyase. PLoS One. 2011;6:e16571. doi: 10.1371/journal.pone.0016571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siow DL, et al. Intracellular localization of sphingosine kinase 1 alters access to substrate pools but does not affect the degradative fate of sphingosine-1-phosphate. J Lipid Res. 2010;51:2546–2559. doi: 10.1194/jlr.M004374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bagdanoff JT, et al. Inhibition of sphingosine 1-Phosphate lyase for the treatment of rheumatoid arthritis: discovery of (E)-1-(4-((1R,2S,3R)-1,2,3,4-tetrahydroxybutyl)-1H-imidazol-2-yl)ethanone Oxime (LX2931) and (1R,2S,3R)-1-(2-(Isoxazol-3-yl)-1H-imidazol-4-yl)butane-1,2,3,4-tetraol (LX2932) J Med Chem. 2010;53:8650–8662. doi: 10.1021/jm101183p. [DOI] [PubMed] [Google Scholar]
- 33.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 34.Ghosh TK, et al. Intracellular calcium release mediated by sphingosine derivatives generated in cells. Science. 1990;248:1653–1656. doi: 10.1126/science.2163543. [DOI] [PubMed] [Google Scholar]
- 35.Ghosh TK, et al. Sphingosine 1-phosphate generated in the endoplasmic reticulum membrane activates release of stored calcium. J Biol Chem. 1994;269:22628–22635. [PubMed] [Google Scholar]
- 36.Mattie M, et al. Sphingosine-1-phosphate, a putative second messenger, mobilizes calcium from internal stores via an inositol trisphosphate-independent pathway. J Biol Chem. 1994;269:3181–3188. [PubMed] [Google Scholar]
- 37.Xia P, et al. Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-alpha signaling. J Biol Chem. 2002;277:7996–8003. doi: 10.1074/jbc.M111423200. [DOI] [PubMed] [Google Scholar]
- 38.Alvarez SE, et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465:1084–1088. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puneet P, et al. SphK1 regulates proinflammatory responses associated with endotoxin and polymicrobial sepsis. Science. 2010;328:1290–1294. doi: 10.1126/science.1188635. [DOI] [PubMed] [Google Scholar]
- 40.Artal-Sanz M, Tavernarakis N. Prohibitin and mitochondrial biology. Trends Endocrinol Metab. 2009;20:394–401. doi: 10.1016/j.tem.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Takasugi N, et al. BACE1 Activity Is Modulated by Cell-Associated Sphingosine-1-Phosphate. J Neurosci. 2011;31:6850–6857. doi: 10.1523/JNEUROSCI.6467-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chi H. Sphingosine-1-phosphate and immune regulation: trafficking and beyond. Trends Pharmacol Sci. 2011;32:16–24. doi: 10.1016/j.tips.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- 44.Guan H, et al. Sphingosine Kinase 1 Regulates the Akt/FOXO3a/Bim Pathway and Contributes to Apoptosis Resistance in Glioma Cells. PLoS One. 2011;6:e19946. doi: 10.1371/journal.pone.0019946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salas A, et al. Sphingosine kinase-1 and sphingosine 1-phosphate receptor 2 mediate Bcr-Abl1 stability and drug resistance by modulation of protein phosphatase 2A. Blood. 2011;117:5941–5952. doi: 10.1182/blood-2010-08-300772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim ES, et al. Sphingosine 1-phosphate regulates matrix metalloproteinase-9 expression and breast cell invasion through S1P3-G{alpha}q coupling. J Cell Sci. 2011;124(Pt 13):2220–2230. doi: 10.1242/jcs.076794. [DOI] [PubMed] [Google Scholar]
- 47.Anelli V, et al. Role of sphingosine kinase-1 in paracrine/transcellular angiogenesis and lymphangiogenesis in vitro. FASEB J. 2010;24:2727–2738. doi: 10.1096/fj.09-150540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee H, et al. STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nat Med. 2010;16:1421–1428. doi: 10.1038/nm.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dickson MA, et al. A phase I clinical trial of safingol in combination with cisplatin in advanced solid tumors. Clin Cancer Res. 2011;17:2484–2492. doi: 10.1158/1078-0432.CCR-10-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paugh SW, et al. A selective sphingosine kinase 1 inhibitor integrates multiple molecular therapeutic targets in human leukemia. Blood. 2008;112:1382–1391. doi: 10.1182/blood-2008-02-138958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kapitonov D, et al. Targeting sphingosine kinase 1 inhibits Akt signaling, induces apoptosis, and suppresses growth of human glioblastoma cells and xenografts. Cancer Res. 2009;69:6915–6923. doi: 10.1158/0008-5472.CAN-09-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song L, et al. Sphingosine kinase-1 enhances resistance to apoptosis through activation of PI3K/Akt/NF-kappaB pathway in human non-small cell lung cancer. Clin Cancer Res. 2011;17:1839–1849. doi: 10.1158/1078-0432.CCR-10-0720. [DOI] [PubMed] [Google Scholar]
- 53.Loveridge C, et al. The sphingosine kinase 1 inhibitor 2-(p-hydroxyanilino)-4-(p-chlorophenyl)thiazole induces proteasomal degradation of sphingosine kinase 1 in mammalian cells. J Biol Chem. 2010;285:38841–38852. doi: 10.1074/jbc.M110.127993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antoon JW, et al. Pharmacological inhibition of sphingosine kinase isoforms alters estrogen receptor signaling in human breast cancer. J Mol Endocrinol. 2011;46:205–216. doi: 10.1530/JME-10-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kennedy AJ, et al. Development of amidine-based sphingosine kinase 1 nanomolar inhibitors and reduction of sphingosine 1-phosphate in human leukemia cells. J Med Chem. 2011;54:3524–4358. doi: 10.1021/jm2001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Brocklyn JR, et al. Sphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme: roles of sphingosine kinase isoforms in growth of glioblastoma cell lines. J Neuropathol Exp Neurol. 2005;64:695–705. doi: 10.1097/01.jnen.0000175329.59092.2c. [DOI] [PubMed] [Google Scholar]
- 57.Sankala HM, et al. Involvement of sphingosine kinase 2 in p53-independent induction of p21 by the chemotherapeutic drug doxorubicin. Cancer Res. 2007;67:10466–10474. doi: 10.1158/0008-5472.CAN-07-2090. [DOI] [PubMed] [Google Scholar]
- 58.Weigert A, et al. Sphingosine kinase 2 deficient tumor xenografts show impaired growth and fail to polarize macrophages towards an anti-inflammatory phenotype. Int J Cancer. 2009;125:2114–2121. doi: 10.1002/ijc.24594. [DOI] [PubMed] [Google Scholar]
- 59.French KJ, et al. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J Pharmacol Exp Ther. 2010;333:129–139. doi: 10.1124/jpet.109.163444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Antoon JW, et al. Antiestrogenic effects of the novel sphingosine kinase-2 inhibitor ABC294640. Endocrinology. 2010;151:5124–5135. doi: 10.1210/en.2010-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Daum G, et al. Sphingosine 1-phosphate: a regulator of arterial lesions. Arterioscler Thromb Vasc Biol. 2009;29:1439–1443. doi: 10.1161/ATVBAHA.108.175240. [DOI] [PubMed] [Google Scholar]
- 62.Sato K, Okajima F. Role of sphingosine 1-phosphate in anti-atherogenic actions of high-density lipoprotein. World J Biol Chem. 2010;1:327–337. doi: 10.4331/wjbc.v1.i11.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keul P, et al. Sphingosine-1-Phosphate Receptor 3 Promotes Recruitment of Monocyte/Macrophages in Inflammation and Atherosclerosis. Circ Res. 2011;108:314–323. doi: 10.1161/CIRCRESAHA.110.235028. [DOI] [PubMed] [Google Scholar]
- 64.Wang F, et al. Sphingosine-1-phosphate receptor-2 deficiency leads to inhibition of macrophage proinflammatory activities and atherosclerosis in apoE-deficient mice. J Clin Invest. 2010;120:3979–3995. doi: 10.1172/JCI42315. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Skoura A, et al. Sphingosine-1-phosphate receptor-2 function in myeloid cells regulates vascular inflammation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:81–85. doi: 10.1161/ATVBAHA.110.213496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Christoffersen C, et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc Natl Acad Sci USA. 2011;108:9613–9618. doi: 10.1073/pnas.1103187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nofer JR, et al. FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2007;115:501–508. doi: 10.1161/CIRCULATIONAHA.106.641407. [DOI] [PubMed] [Google Scholar]
- 68.Keul P, et al. The sphingosine-1-phosphate analogue FTY720 reduces atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:607–613. doi: 10.1161/01.ATV.0000254679.42583.88. [DOI] [PubMed] [Google Scholar]
- 69.Blom T, et al. FTY720 stimulates 27-hydroxycholesterol production and confers atheroprotective effects in human primary macrophages. Circ Res. 2010;106:720–729. doi: 10.1161/CIRCRESAHA.109.204396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Summers SA. Sphingolipids and insulin resistance: the five Ws. Curr Opin Lipidol. 2010;21:128–135. doi: 10.1097/MOL.0b013e3283373b66. [DOI] [PubMed] [Google Scholar]
- 71.Deevska GM, Nikolova-Karakashian MN. The twists and turns of sphingolipid pathway in glucose regulation. Biochimie. 2011;93:32–38. doi: 10.1016/j.biochi.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holland WL, et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest. 2011;121:1858–1870. doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holland WL, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fox TE, et al. Circulating sphingolipid biomarkers in models of type 1 diabetes. J Lipid Res. 2011;52:509–517. doi: 10.1194/jlr.M010595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Imasawa T, et al. Blockade of sphingosine 1-phosphate receptor 2 signaling attenuates streptozotocin-induced apoptosis of pancreatic beta-cells. Biochem Biophys Res Commun. 2010;392:207–211. doi: 10.1016/j.bbrc.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ryu J, et al. Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EMBO J. 2006;25:5840–5851. doi: 10.1038/sj.emboj.7601430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pederson L, et al. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci USA. 2008;105:20764–20769. doi: 10.1073/pnas.0805133106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ishii M, et al. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 2009;458:524–528. doi: 10.1038/nature07713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ishii M, et al. Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. J Exp Med. 2010;207:2793–2798. doi: 10.1084/jem.20101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baker DA, et al. Genetic sphingosine kinase 1 deficiency significantly decreases synovial inflammation and joint erosions in murine TNF-alpha-induced arthritis. J Immunol. 2010;185:2570–2579. doi: 10.4049/jimmunol.1000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reines I, et al. Topical application of sphingosine-1-phosphate and FTY720 attenuate allergic contact dermatitis reaction through inhibition of dendritic cell migration. J Invest Dermatol. 2009;129:1954–1962. doi: 10.1038/jid.2008.454. [DOI] [PubMed] [Google Scholar]
- 83.Tsunemi S, et al. Effects of the novel immunosuppressant FTY720 in a murine rheumatoid arthritis model. Clin Immunol. 2010;136:197–204. doi: 10.1016/j.clim.2010.03.428. [DOI] [PubMed] [Google Scholar]
- 84.Kurashima Y, et al. Sphingosine 1-phosphate-mediated trafficking of pathogenic Th2 and mast cells for the control of food allergy. J Immunol. 2007;179:1577–1585. doi: 10.4049/jimmunol.179.3.1577. [DOI] [PubMed] [Google Scholar]
- 85.Guillermet-Guibert J, et al. Targeting the sphingolipid metabolism to defeat pancreatic cancer cell resistance to the chemotherapeutic gemcitabine drug. Mol Cancer Ther. 2009;8:809–820. doi: 10.1158/1535-7163.MCT-08-1096. [DOI] [PubMed] [Google Scholar]
- 86.Ricci C, et al. In vitro anti-leukaemia activity of sphingosine kinase inhibitor. Br J Haematol. 2009;144:350–357. doi: 10.1111/j.1365-2141.2008.07474.x. [DOI] [PubMed] [Google Scholar]
- 87.Nishiuma T, et al. Inhalation of sphingosine kinase inhibitor attenuates airway inflammation in asthmatic mouse model. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1085–L1093. doi: 10.1152/ajplung.00445.2007. [DOI] [PubMed] [Google Scholar]
- 88.Fitzpatrick LR, et al. Attenuation of arthritis in rodents by a novel orally-available inhibitor of sphingosine kinase. Inflammopharmacology. 2011;19:75–87. doi: 10.1007/s10787-010-0060-6. [DOI] [PubMed] [Google Scholar]
- 89.Chumanevich AA, et al. Suppression of colitis-driven colon cancer in mice by a novel small molecule inhibitor of sphingosine kinase. Carcinogenesis. 2010;31:1787–1793. doi: 10.1093/carcin/bgq158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beljanski V, et al. Combined anticancer effects of sphingosine kinase inhibitors and sorafenib. Invest New Drugs. 2011 doi: 10.1007/s10637-010-9452-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chiba Y, et al. SKI-II, an inhibitor of sphingosine kinase, ameliorates antigen-induced bronchial smooth muscle hyperresponsiveness, but not airway inflammation, in mice. J Pharmacol Sci. 2010;114:304–310. doi: 10.1254/jphs.10202fp. [DOI] [PubMed] [Google Scholar]
- 92.Bandhuvula P, et al. S1P lyase: a novel therapeutic target for ischemia-reperfusion injury of the heart. Am J Physiol Heart Circ Physiol. 2011;300:H1753–H1761. doi: 10.1152/ajpheart.00946.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao Y, et al. Protection of LPS-Induced Murine Acute Lung Injury by Sphingosine-1-Phosphate Lyase Suppression. Am J Respir Cell Mol Biol. 2011;45:426–435. doi: 10.1165/rcmb.2010-0422OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Awad AS, et al. Chronic sphingosine 1-phosphate 1 receptor activation attenuates early-stage diabetic nephropathy independent of lymphocytes. Kidney Int. 2011;79:1090–1098. doi: 10.1038/ki.2010.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bajwa A, et al. Activation of sphingosine-1-phosphate 1 receptor in the proximal tubule protects against ischemia-reperfusion injury. J Am Soc Nephrol. 2010;21:955–965. doi: 10.1681/ASN.2009060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Diab KJ, et al. Stimulation of sphingosine 1-phosphate signaling as an alveolar cell survival strategy in emphysema. Am J Respir Crit Care Med. 2010;181:344–352. doi: 10.1164/rccm.200906-0826OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hasegawa Y, et al. Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke. 2010;41:368–374. doi: 10.1161/STROKEAHA.109.568899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim HJ, et al. Fingolimod and related compounds in a spontaneous autoimmune polyneuropathy. J Neuroimmunol. 2009;214:93–100. doi: 10.1016/j.jneuroim.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marsolais D, et al. A critical role for the sphingosine analog AAL-R in dampening the cytokine response during influenza virus infection. Proc Natl Acad Sci USA. 2009;106:1560–1565. doi: 10.1073/pnas.0812689106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Niessen F, et al. Endogenous EPCR/aPC-PAR1 signaling prevents inflammation-induced vascular leakage and lethality. Blood. 2009;113:2859–2866. doi: 10.1182/blood-2008-12-192385. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 101.Pan S, et al. A monoselective sphingosine-1-phosphate receptor-1 agonist prevents allograft rejection in a stringent rat heart transplantation model. Chem Biol. 2006;13:1227–1234. doi: 10.1016/j.chembiol.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 102.Zhang ZY, et al. AUY954, a selective S1P(1) modulator, prevents experimental autoimmune neuritis. J Neuroimmunol. 2009;216:59–65. doi: 10.1016/j.jneuroim.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 103.Oskeritzian CA, et al. Essential roles of sphingosine-1-phosphate receptor 2 in human mast cell activation, anaphylaxis, and pulmonary edema. J Exp Med. 2010;207:465–474. doi: 10.1084/jem.20091513. [DOI] [PMC free article] [PubMed] [Google Scholar]