Abstract
Background/Aim
Recent evidence suggests that endoplasmic reticulum (ER) stress provoked under diabetic conditions augments the expression of scavenger receptors on macrophages, promoting the uptake of oxidized low-density lipoprotein (ox-LDL) uptake and atherogenesis. The aim of the present study was to test the hypothesis that the chemical chaperone tauroursodeoxycholic acid (TUDCA) attenuates lipid accumulation in macrophages subjected to ER stress.
Methods
Cultured human macrophages were subjected to ER-stress by treating them with tunicamycin. Lipid-uptake by macrophages subjected to ER-stress in the presence or absence of TUDCA was assessed by oil red O staining and by assessing the cellular uptake of Dil-ox-LDL by fluorescence measurement. Protein levels and phosphorylation status of ER stress markers, insulin-signalling molecules and scavenger receptor were assessed by Western blotting.
Results
Treatment of cultured human macrophages with the ER-stressor tunicamycin caused an increase in the protein levels of CD-36, and augmentation of lipid-uptake both of which were inhibited by TUDCA. TUDCA-treatment inhibited tunicamycin-induced ER-stress as evidenced by the attenuation of phosphorylation of eukaryotic translation initiation factor-2α and glucose reactive protein-78. In addition, TUDCA improved insulin signaling in macrophages by augmenting Akt-phosphorylation and blunting c-Jun N-terminal kinase activity.
Conclusion
Inhibition of macrophage ER-stress may represent a potential strategy in preventing atherogenesis under diabetic conditions.
Keywords: atherosclerosis, macrophages, insulin resistance, ER stress, tauroursodeoxycholic acid
INTRODUCTION
Accelerated atherosclerosis is the primary cause of death in subjects with type-2 diabetes.1–3 Hyperinsulinemia and hyperglycemia associated with insulin resistance are thought to play a pivotal role in vascular complications seen in diabetic individuals.4, 5 Insulin resistance can lead to dyslipidemia,6 hypercoagulable state7 and endothelial dysfunction8 all of which change the milieu of the arterial wall and prompt production of a number of adhesion molecules and chemokines.9 Subsequently, recruitment, activation and transmigration of the monocytes and their differentiation into tissue macrophages set the stage of early lesions.10 Tissue macrophages, the most prominent cell type in the atherosclerotic lesions, ingest oxidized low-density lipoproteins (ox-LDL) and become lipid-laden macrophages.11 This uptake of ox-LDL is mediated by scavenger receptors expressed on the macrophage cell surface.12 Recent studies have indicated that insulin resistant conditions lead to an increase in scavenger receptors on the macrophages, predisposing them to foam cell formation. In addition to providing a molecular explanation for the increased incidence of atherosclerosis in diabetic patients, the aforementioned observation suggests that targeting insulin resistance in the macrophage may represent an attractive strategy to prevent atherogenesis under insulin resistant conditions.
Although the mechanisms leading to insulin resistance are not well established, several recent studies have implicated endoplasm reticulum (ER)-stress as a common pathway in the etiology of insulin resistance and type-2 diabetes.13–15 The ER is a cellular organelle that plays a critical role in protein biosynthesis, folding and trafficking. Accumulation of unfolded proteins or by excessive protein trafficking overwhelms the ER resulting in ER stress16. Elevated ER-stress has been observed in adipocytes and liver of genetically obese and high-fat fed insulin-resistant mice.17 Impairment of insulin signal transduction by ER-stress is believed to be mediated via the activation of c-Jun N-terminal kinase (JNK) that phosphorylates, a serine residue (307) on insulin receptor substrate-1 (IRS-1), leading to the suppression of insulin signaling 17, 18. Consequently, molecules that counter ER stress may have potential benefits in the treatment of diabetes19 and atherosclerosis.20
Tauroursodeoxycholic acid (TUDCA) is an endogenous, hydrophilic bile acid credited with pluripotent properties,21 and is clinically used in the treatment of cholestatic liver diseases.22 Recent studies have shown that TUDCA, functions as a chemical chaperone, and helps attenuate ER stress.19, 23 Consequently, TUDCA augmented insulin signaling and improved glucose tolerance in animal models of type-2 diabetes.19 However, there have been no studies that investigated the effects of TUDCA on lipid-uptake and insulin resistance in macrophages. Therefore, in the present study, we tested the hypothesis that TUDCA inhibits ox-LDL uptake in ER-stressed macrophages.
MATERIALS AND METHODS
Chemicals
Phorbol 12-myristate 13-acetate (PMA), Oil red O, tauroursodeoxycholic acid (TUDCA), 1,1’-Dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate (Dil), RPMI-1640 cell growth media and mercaptoethanol were purchased from Sigma Chemical Co (St. Louis, MO). Human ox-LDL was from Kalen Biomedical (Savage, MD). Tunicamycin was obtained from Calbiochem (Gibbstown, NJ). Antibodies to c-Jun, phospho-c-Jun, Akt, phospho-AKT, phospho-eukaryotic translation initiation factor-2α (EIF2α) subunit and β-actin were purchased from Cell Signaling Technology (Boston, MA). Monoclonal antibodies to glucose reactive protein (GRP)-78 and CD36 were from Santa Cruz Biotechnology (Santa Cruz, CA). QuantiTect SYBR Green real-time PCR kit was purchased from Bio-Rad Laboratories (Hercules, CA). Fetal bovine serum (FBS) was obtained from Invitrogen Corporation (Carlsbad, CA).
Cell culture
Human monocyte cell line (THP-1) was obtained from the American Type Culture Collection (Manassas, VA) and were grown in RPMI-1640 culture medium supplemented with FBS (10%) and 2-mercaptoethanol (0.05 mM). Cells were maintained at 37° C in a humidified atmosphere with 5% CO2. PMA (100ng/mL) was added to the media and the incubation was continued for a further 48–72 hours to allow differentiation of the monocytes to macrophages24. Once differentiated the serum supplemented RPMI media was replaced with serum free for indicated periods as specified below.
Lipid uptake assay
Two different methods were used to assess the uptake of lipid by macrophages. In the first assay oil red O stain was used to stain intracellular lipid deposits in the macrophages25. Briefly, macrophages were incubated with or without tunicamycin (0.5µg/mL), TUDCA (500µg/mL) and ox-LDL (50µg/mL) for 24 hours. Following incubation, the media was removed and replaced with formaldehyde buffer (10%, pH 7.0) and incubated for 5 min. The formaldehyde buffer was then discarded and replaced with fresh formaldehyde buffer and left for 1–2 days. Following fixation, cells were washed with 60% isopropanol, dried, and treated with oil red O working solution (a mixture of 0.5% (wt/vol) oil red O in 2-propanol and distilled water at a 3:2 ratio) for 10 min. Cells were rinsed four times with PBS and examined by light microscopy (magnification, ×200; Olympus, Japan, CK40-F100). For the second method, macrophages were incubated with fluorescent labeled ox-LDL and the extent of lipid uptake was quantitated by fluorescence measurement. Briefly ox-LDL (4:1, v/v) was labeled with with Dil (as a 1.07 mM solution in dimethyl sulfoxide) overnight at 37° C.26 Dil-ox-LDL (50 µg) was incubated with macrophages in the presence or absence of tunicamycin (0.5 µg/mL) and TUDCA (500 µg/mL) for 24 hours at 37 °C. Following treatment, cells were washed twice with phosphate buffered saline (PBS), and then lysed in 200 µL lysis buffer. The protein concentration of each sample was measured using BCA assay. Dil fluorescence was detected following at excitation at 520 nm and emission at 590 nm using a 590 nm cut-off filter (Molecular Devices Gemini XS microplate fluorometer). Fluorescence standard curve was prepared by diluting 0, 1, 2, 3, 4, 5, 10 and 20 µg Dil-ox-LDL in lysis buffer. Dil-ox-LDL uptake by macrophages was calculated as amount of Dil-ox-LDL per mg of cellular protein as reported previously.27
Western blot analysis
ER-stress was induced by treating the macrophages with tunicamycin (0.5µg/mL) in the presence or absence of TUDCA for 5h. During the final 10 min of the incubation, insulin (100nM) was added to the cells to stimulate the insulin signal pathway immediately following which the cells were lysed with RIPA buffer containing protease and phosphatase inhibitor cocktail. Cell lysates were sonicated for 15 sec and centrifuged at 12,000 × g for 20 min at 4°C. The protein concentration of the supernatant was evaluated using the Micro BCA reagent. Equivalent amounts (~25–50 µg protein/lane) of lysates and pre-stained molecular weight markers were separated on 10% SDS-polyacrylamide gels and transferred onto nitrocellulose membranes. Membranes were incubated for 1 hr in a blocking solution containing 5% non-fat milk in Tris-buffered saline (TBS), washed in TBS and incubated overnight at 4°C with appropriate primary antibodies: anti-phospho-JNK (1:1,000), anti-total JNK(1:1,000), anti-Akt (1:1,000), anti-phospho-Akt (1:1,000), phospho-EIFα, GRP78 (1:2000), CD3(1:2000), anti-beta-actin (1:1000) antibody. After incubation with the primary antibody, blots were incubated with an anti-rabbit IgG HRP-linked antibody at a dilution of 1:5,000 for 1h at room temperature. Immunoreactive bands were detected by enhanced chemiluminescence autoradiography per manufacturer’s instructions. The intensity of the bands was quantitated with a scanning densitometer (Model GS-800; Bio-Rad) coupled with Bio-Rad PC analysis software.
Assessment of mRNA expression by quantitative real-time PCR
Total RNA was extracted from macrophages and was reverse transcribed to cDNA. Quantitative real-time reverse-transcription (qRT-PCR) was performed for activating transcription factor 6 (ATF6α), CCAAT/enhancer-binding protein (C/EBP) homologous protein (CHOP) and 18S (used as the housekeeping gene) with a QuantiTect SYBR Green PCR Kit (Qiagen). The primer (Integrated DNA Technologies, Coralville, IA) sequences of ATF6 α were, forward: 5’-GCC TTT ATT GCT TCC AGC AG-3’, reverse: 5’-TCT TCG CTT TGG ACT AGG GAC-3’28, for CHOP were, forward: 5’-TGA GGA GAG AGT GTT CAA GAA G-3’, reverse: 5’-TCC AGG AGG TGA AAC ATA GG-3’29 and for 18s were, forward: 5’-AGT GAC AAG AAA TAA CAA TAC AGG-3’, reverse: 5’-CCT GCT TTA AGC ACT CTA ATT TTC-3.30 The experiments were performed in triplicate.
Statistical analysis
Data are presented as mean ± S.E.M and statistically evaluated using ANOVA method using Sigma Plot statistical software (Jandel Scientific, San Rafael, CA). P<0.05 was considered to be statistically significant.
RESULTS
TUDCA alleviates tunicamycin-induced lipid uptake in macrophages
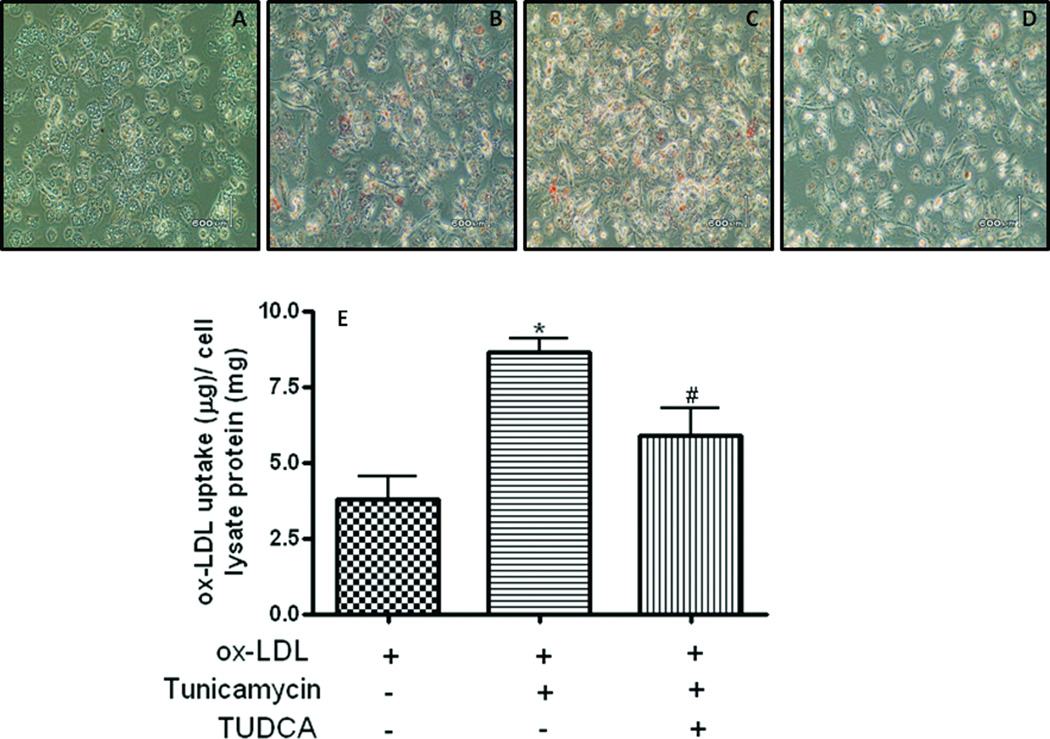
Macrophage binding and uptake of ox-LDL is the event that triggers initiation of atherosclerotic lesion development, the formation of lipid-laden foam cells. Since insulin resistance has been shown to enhance the capacity of ox-LDL-uptake by macrophages we examined the effect of ER stress on ox-LDL uptake by macrophages and the effect of TUDCA on this process. As shown in Fig. 1 macrophages treated with tunicamycin had higher levels of intracellular lipids compared to the vehicle treated control macrophages. Treatment of macrophages with TUDCA attenuated the tunicamycin-induced lipid accumulation by the ER-stressed macrophages. TUDCA however did not have any effect on the basal levels of lipid-uptake.
Fig 1.
TUDCA inhibits tunicamycin-induced increase of ox-LDL uptake in macrophages. Macrophages were treated with tunicamycin (0.5µg/mL, for 5h) in the presence or absence of TUDCA (500µg/mL) and extent of lipid loading was assessed by Oil red O staining. Panels A to D are) oil red O stain pictures for control, ox-LDL, ox-LDL plus tunicamycin and ox-LDL plus tunicamycin plus TUDCA groups, respectively. The picture is a representative of four independent experiments. Panel E shows Dil-ox-LDL fluorescence intensity in cells incubated with tunicamycin and TUDCA. Data are mean ± SEM of Dil-ox-LDL fluorescence intensity to cell lysate protein ratio. The experiments were repeated four times. *P < 0.05 versus ox-LDL group, #P<0.05 versus ox-LDL plus tunicamycin group.
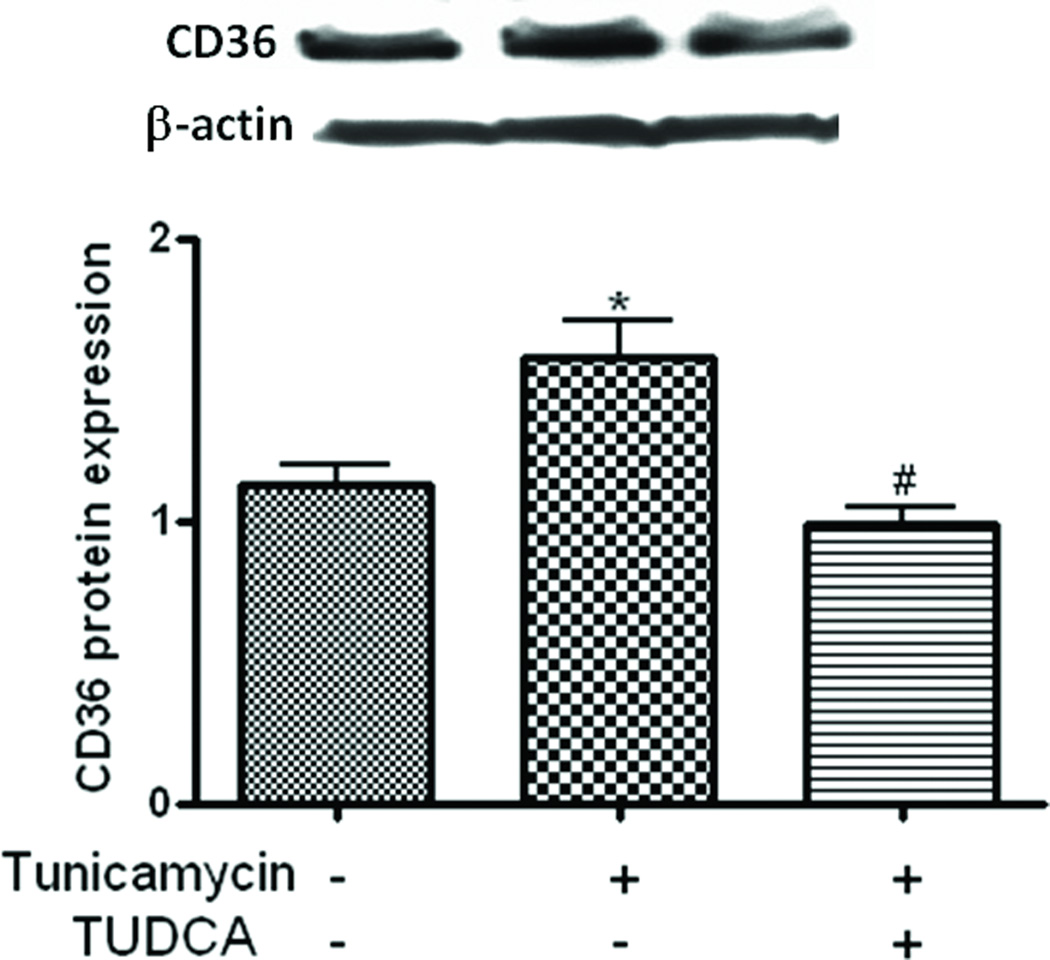
TUDCA inhibits tunicamycin-induced overexpression of CD-36 on macrophages
CD36 is one of several oxLDL receptors responsible for the uptake of oxLDL by macrophages and accounts for about 60–70% of macrophage foam cell formation.31, 32 To understand the potential mechanisms involved in the inhibition of macrophage LDL-uptake by TUDCA we quantitated the scavenger receptor CD-36 that mediates the LDL-uptake by macrophages. As shown in Fig. 2, CD-36 levels were elevated following treatment of the macrophages with tunicamycin which was inhibited by TUDCA. Once again TUDCA did not affect the basal levels of CD-36 as was the case with ox-LDL uptake.
Fig 2.
TUDCA inhibits tunicamycin-induced upregulation of CD36. Macrophages were treated with tunicamycin (0.5µg/mL, for 5h) in the presence or absence of TUDCA (500µg/mL) and protein levels of CD36 was assessed by Western blotting. Upper panels are representative Western blots and lower panels are mean ± SEM of the densities of the protein bands from 3 independent experiments. *P < 0.05 versus control group, #P<0.05 versus tunicamycin group.
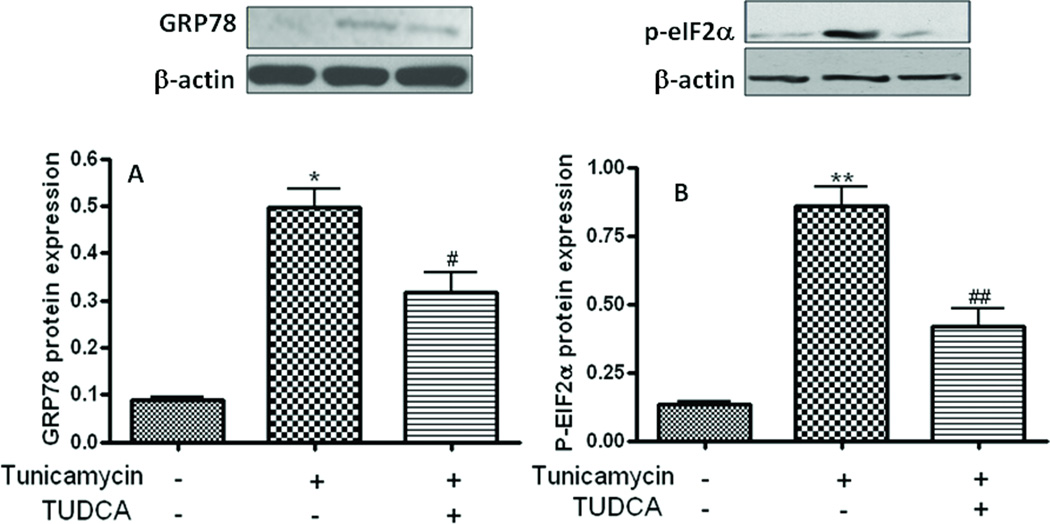
TUDCA attenuates tunicamycin-induced macrophage ER-stress
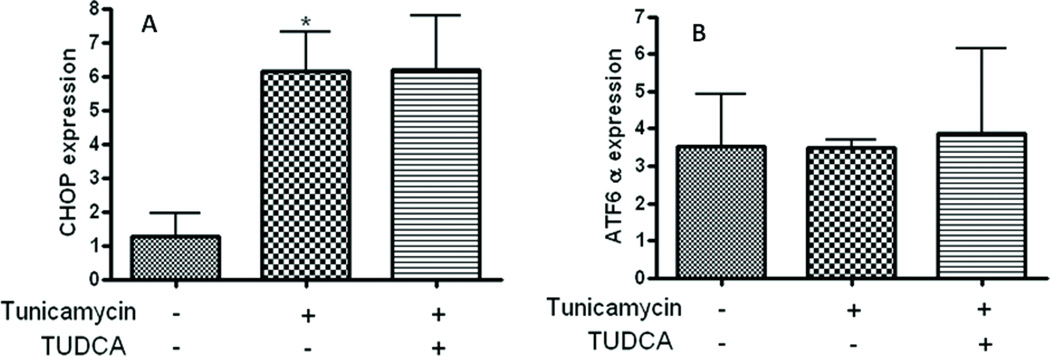
We sought to examine whether the ability of TUDCA to inhibit LDL-uptake is related to its ability to augment ER-stress. To this end, we investigated whether the N-glycosylation inhibitor tunicamycin induces ER stress in macrophages and studied the effect of TUDCA treatment on ER-stress by assessing the extent of phosphorylation markers eIF2α and GRP-78 as markers of ER-stress. Consistent with previous reports from other cell types, treatment with tunicamycin (0.5µg/mL, for 5h) induced ER-stress as evidenced by an elevation in the phosphorylation levels of eIF2a (Fig. 3A) and protein levels of GRP-78 (Fig. 3B). In contrast, macrophages treated with TUDCA (500 µg/mL) exhibited a robust inhibition of tunicamycin-induced phosphorylation of both these ER-stress markers (Figs 3A & B). Treatment with tunicamycin also caused a significant increase in the mRNA levels of the transcription factors C/EBP homologous protein (CHOP). Interestingly however, TUDCA treatment failed to attenuate tunicamycin-stimulated induction of CHOP mRNA (Fig 4). Furthermore, unlike with the case eiF2α, GRP-78 and CHOP stress markers tunicamycin failed to augment the levels of activating transcription factor 6 (ATF6). Neither did TUDCA have any effect on ATF-6 levels.
Fig 3.
TUDCA inhibits tunicamycin-induced ER-stress in human macrophages. Macrophages were treated with tunicamycin (0.5µg/mL, for 5h) in the presence or absence of TUDCA (500 µg/mL) and the extent of phosphorylation of eIF2α (A) and protein levels of GRP-78 (B) were assessed by Western blotting. Upper panels are representative Western blots whereas the lower panels are mean ± SEM of the densities of the protein bands from 3 independent experiments. *p<0.05 versus control group, #p<0.05 versus tunicamycin group, **p<0.01 versus control group, ##p<0.01 versus tunicamycin group.
Fig 4.
Monocyte-derived macrophages were treated with or without tunicamycin (0.5µg/mL) and TUDCA (500µg/mL). Cells treated with TUDCA failed to inhibit the elevated CHOP mRNA levels induced by tunicamycin. Panel A is CHOP mRNA expression and panel B is ATF6 α mRNA expression. n=3–5. *P < 0.05 versus control group.
TUDCA restores insulin-signalling in macrophages treated with tunicamycin
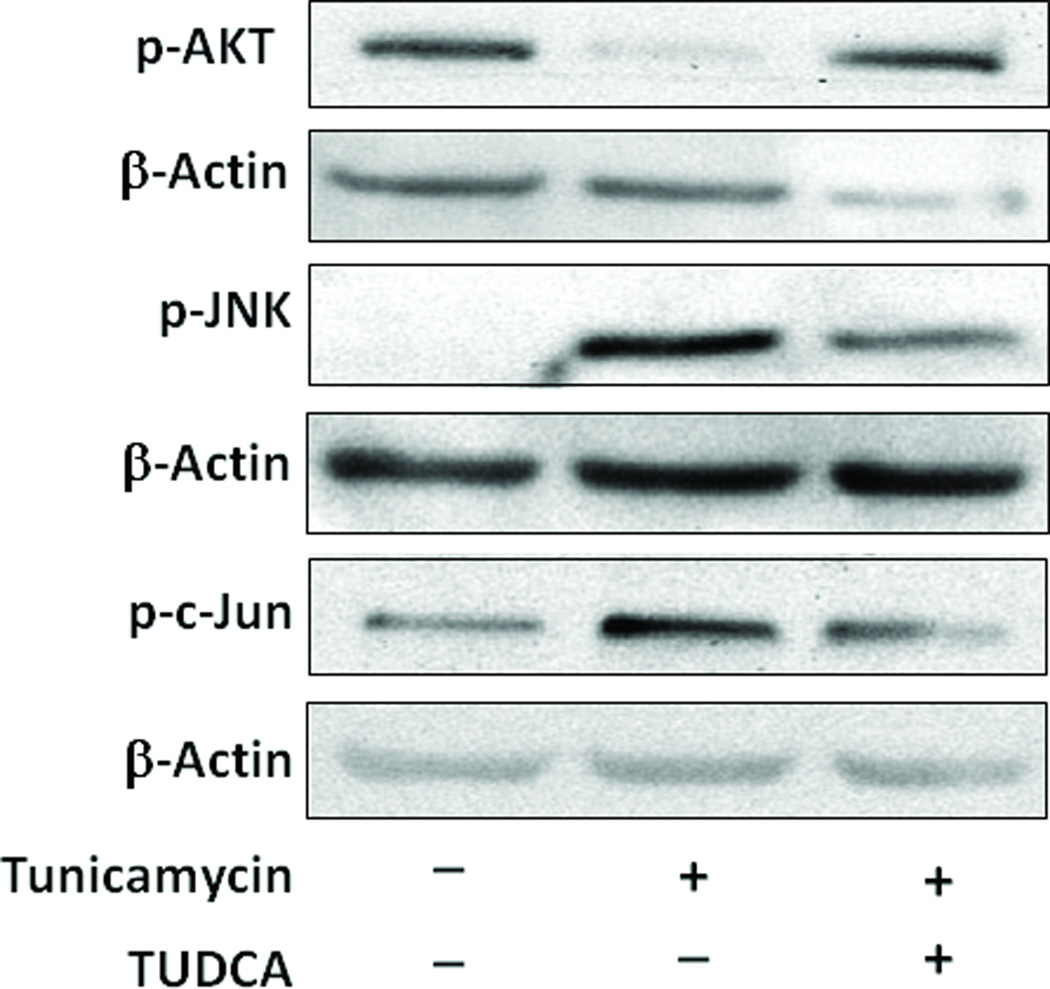
Phosphorylation of Akt, an important kinase downstream of the insulin receptor, in response to insulin stimulation is a pivotal event in insulin-signal transduction and the metabolic effects 33. Consequently, decreased insulin-stimulated Akt kinase activity has been recognized as an important component underlying insulin resistance.34 Macrophages treated with tunicamycin exhibited extreme insulin resistance as evidenced by the complete inhibition of insulin-stimulated phosphorylation of Akt, a major downstream effector of insulin (Fig. 5). Akt-phosphorylation which was abrogated following tunicamycin treatment was fully restored in the presence of TUDCA (Fig. 5). Relative densities are shown in Supplemental Figure 1.
Fig 5.
TUDCA reverses the blunting of insulin signaling by tunicamycin in cultured macrophages. Macrophages were treated with tunicamycin (0.5µg/mL, for 5h) in the presence or absence of TUDCA (500µg/mL) and during the final 10 min of incubation were challenged with insulin (100 nM). The extent of phosphorylation of Akt (threonine 308), JNK and c-Jun site were assessed by Western blotting. Representative Western blots are shown here. Online figure 1 represent mean ± SEM of the densities of the protein bands from 3 independent experiments. *P<0.05 versus insulin group, #P<0.05 versus insulin with tunicamycin group.
Insulin resistance and obesity are associated with activation of the c-Jun/JNK pathway. Activated JNK phosphorylates a serine (307) residue IRS-1 that negatively regulates insulin signaling.18, 35 The transcriptional activity of c-Jun (a component of AP-1 transcription factor complex) is also enhanced by the phosphorylation on the N-terminus by JNK.36 Phosphorylation of c-Jun increases its transcriptional activity and inhibits its ubiquitination and degradation.37 Macrophages treated with tunicamycin had elevated levels of both phospho-JNK and phospho-c-Jun (Fig 4). Treatment of macrophages with TUDCA inhibited the ER-stress induced elevation of phosphorylation of these markers of insulin resistance.
Inhibition of JNK results in downregulation of CD-36
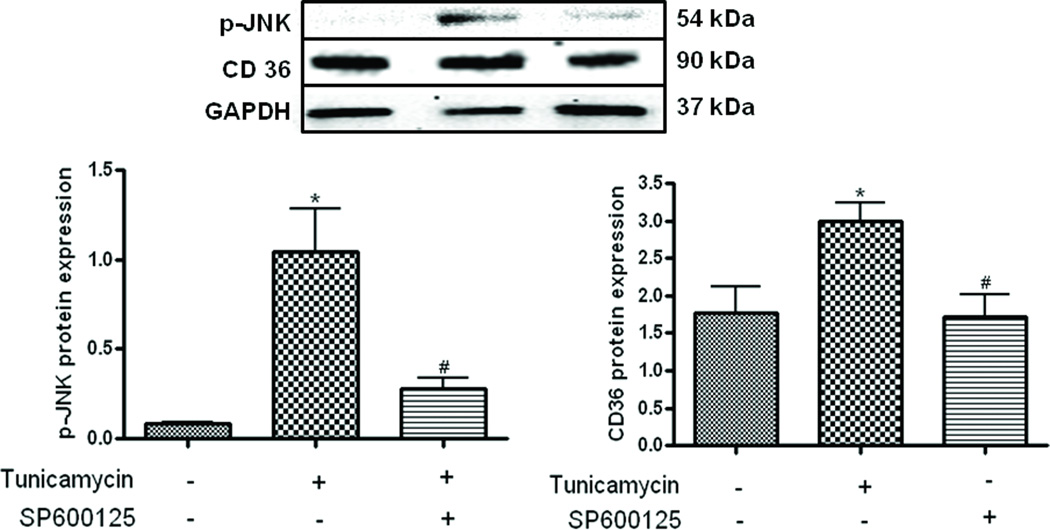
To understand ascertain the cross-talk between JNK and CD-36, we used the JNK inhibitor SP600125 to inhibit JNK in the monocyte derived macrophages. As seen in figure 6, treatment with tunicamycin caused a significant elevation in JNK which was significantly attenuated by treating the cells with SP600125. This pharmacological inhibition of JNK also caused the inhibition of tunicamycin induced upregulation of CD-36 (Fig. 6.) indicating a cross-talk between the insulin signaling pathway and CD-36.
Fig 6.
Monocyte-derived macrophages were treated with or without tunicamycin (0.5 µg/mL) and SP600125 (20 µM). SP600125 treatment blunted enhancement of tunicamycin-induced JNK phosphorylation and CD36 expression. Upper panel is a representative blot, n=3, *P<0.05 versus control group, #P<0.05 versus tunicamycin group.
DISCUSSION
The major finding of the present study is that the hydrophilic bile acid, TUDCA inhibits ER-stress-induced uptake of ox-LDL in macrophages, the primary step in foam cell formation and atherogenesis. This inhibition was associated with a lowering of cell surface ox-LDL scavenger receptor, CD36. The inhibitory activity of TUDCA may be attributed to its ability to alleviate ER-stress and augment insulin signaling in the macrophages.
In mammals, ER stress responses can be triggered by activation of three distinct ER membrane-spanning signalling molecules, namely PKR-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1) and activating transcription factor 6 (ATF6).38, 39 These three ER-spanning molecules interact with BiP via their luminal domain. ER stress removes BiP from the interacting domain allowing these proteins to initiate signaling. PERK, activated by phosphorylation causes the phosphorylation of its target eIF2α that triggers the inhibition of protein synthesis. Active PERK also allows the translation ATF4 mRNA. During this process ATF6 gets released from which eventually translocates to the nucleus and binds to ER-stress elements to induce genes that encode ER chaperones such as BiP and GRP as well as the transcription factor CHOP. Our study showed that tunicamycin selectively activates GRP-78, EI2α and CHOP without altering AT6. Interestingly, TUDCA only inhibited GRP-78, EI2α and did not have any effect on CHOP levels.
Macrophages represent the major cell type in the atherosclerotic plaque. Early studies have demonstrated that defective insulin receptor tyrosine kinase activity in human monocytes contributes to the development of insulin resistance.40 Monocytes isolated from diabetic subjects had impaired binding of insulin to the its insulin receptors compared to those obtained from non-diabetic subjects.41 This decreased binding was attributed to the decrease in the number of insulin receptors on the monocytes 41. Interestingly, no decrease in insulin binding was observed in erythrocytes from the same subjects suggesting the macrophages may have a bigger role to play in the pathophysiology of insulin resistance.41 Grunberger and co-workers found that mononuclear blood cells form a patient with insulin resistance exhibited defects in the insulin-stimulated tyrosine kinase activity, suggesting that the defects are distal (beta-subunit) to the insulin-binding site on the insulin receptor.42 Chronic insulin and hyperglycemic conditions have also been shown to inhibit downstream targets such as phospho-inositol-3-kinase in macrophages.43 In addition, peritoneal macrophages from diabetic mice had reduced IRS-2-associated PI3-kinase activity and elevated IRS-2 Ser/Thr phosphorylation compared to control mice.43 Collectively, these studies imply a major role for macrophage insulin signalling in the pathophysiology of insulin resistance and type-2 diabetes.
Subsequent studies by Lian and coworkers show that macrophages from obese mice exhibited marked insulin resistance, elevated CD36 and increased capacity to uptake ox-LDL, demonstrating the potential link between macrophage insulin resistance and atherosclerosis.44 Another study evaluated the effects of macrophage insulin resistance on atherogenesis in LDL-receptor knock-out mice following transplantation with either insulin receptor+/+ or insulin receptor−/− bone marrow.45 In these studies macrophages devoid of insulin receptors exhibit impaired Akt-phosphorylation and exaggerated ER-stress, substantiating the view that insulin resistance predisposes to foam cell formation and atherosclerotic cardiovascular disease.
TUDCA is a hydrophilic bile acid present in low concentrations in human bile (3%) was originally isolated from the bile of bears in China and has been used for centuries in traditional Chinese medicine. Currently, it is used clinically to treat a variety of liver diseases including the dissolution of gallstone and hepatitis C viral infection (reviewed in21, 22). The molecular mechanism by which TUDCA imparts its beneficial effects is unclear, although there have been several hypotheses proposed. TUDCA has been show to exhibit cytoprotective, anti-apoptotic and immunomodulatory effects.21, 22 At the molecular level it attenuates oxidative stress and inhibits nuclear-factor-kappa-B activation both in-vivo and in-vitro.46, 47 Recent reports suggest that TUDCA, by virtue of its inhibition of ER stress prevents advanced glycation-end product induced podocyte-death,48 inhibits apoptosis in hepatoma cell lines49 and more importantly, improves insulin signalling and whole body glucose tolerance in obese mice. To our knowledge, this is the first study to show that TUDCA inhibits ER-stress in human macrophages and that this inhibition is associated with an attenuation of ox-LDL-uptake. TUDCA may mediate these effects by improving insulin signalling via suppression of ER stress or inhibition of JNK activation.
In summary, the data presented here suggest that TUDCA attenuates the uptake of ox-LDL by macrophage that is augmented under insulin resistant conditions. TUDCA may be mediating these effects by augmenting insulin signal transduction and inhibiting ER stress. Based on these observations, it is tempting to speculate that TUDCA may have beneficial effects in halting or preventing atherogenesis in subjects with insulin resistance.
Supplementary Material
Relative densities of pAkt, JNK and pc-JUN, obtained from the ratio of the densitometric scans of the respective Western bots. Values are mean + SEM (n=3 or 4), *P<0.05
Acknowledgment
Parts of this work was funded by grants from NCRR and the Wyoming INBRE (P20RR016474).
REFERENCES
- 1.Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet. 2008;371:1800–1809. doi: 10.1016/S0140-6736(08)60768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 3.Jeppesen J, Hansen TW, Rasmussen S, Ibsen H, Torp-Pedersen C, Madsbad S. Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease: a population-based study. J Am Coll Cardiol. 2007;49:2112–2119. doi: 10.1016/j.jacc.2007.01.088. [DOI] [PubMed] [Google Scholar]
- 4.Plutzky J, Viberti G, Haffner S. Atherosclerosis in type 2 diabetes mellitus and insulin resistance: mechanistic links and therapeutic targets. J Diabetes Complications. 2002;16:401–415. doi: 10.1016/s1056-8727(02)00202-7. [DOI] [PubMed] [Google Scholar]
- 5.Haffner SM. Lipoprotein disorders associated with type 2 diabetes mellitus and insulin resistance. Am J Cardiol. 2002;90:55i–61i. doi: 10.1016/s0002-9149(02)02634-6. [DOI] [PubMed] [Google Scholar]
- 6.Bloomgarden ZT. Insulin resistance, dyslipidemia, and cardiovascular disease. Diabetes Care. 2007;30:2164–2170. doi: 10.2337/dc07-zb08. [DOI] [PubMed] [Google Scholar]
- 7.Aras R, Sowers JR, Arora R. The proinflammatory and hypercoagulable state of diabetes mellitus. Rev Cardiovasc Med. 2005;6:84–97. [PubMed] [Google Scholar]
- 8.Hartge MM, Unger T, Kintscher U. The endothelium and vascular inflammation in diabetes. Diab Vasc Dis Res. 2007;4:84–88. doi: 10.3132/dvdr.2007.025. [DOI] [PubMed] [Google Scholar]
- 9.Yeh ET, Anderson HV, Pasceri V, Willerson JT. C-reactive protein: linking inflammation to cardiovascular complications. Circulation. 2001;104:974–975. doi: 10.1161/01.cir.104.9.974. [DOI] [PubMed] [Google Scholar]
- 10.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 11.Matsuura E, Hughes GR, Khamashta MA. Oxidation of LDL and its clinical implication. Autoimmun Rev. 2008;7:558–566. doi: 10.1016/j.autrev.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Shashkin P, Dragulev B, Ley K. Macrophage differentiation to foam cells. Curr Pharm Des. 2005;11:3061–3072. doi: 10.2174/1381612054865064. [DOI] [PubMed] [Google Scholar]
- 13.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 14.Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, Matsuoka TA, Ozawa K, Ogawa S, Hori M, Yamasaki Y, Matsuhisa M. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem. 2005;280:847–851. doi: 10.1074/jbc.M411860200. [DOI] [PubMed] [Google Scholar]
- 15.Hotamisligil GS. Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes. 2005;54 Suppl 2:S73–S78. doi: 10.2337/diabetes.54.suppl_2.s73. [DOI] [PubMed] [Google Scholar]
- 16.Schröder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 17.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 18.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 19.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robertson LA, Kim AJ, Werstuck GH. Mechanisms linking diabetes mellitus to the development of atherosclerosis: a role for endoplasmic reticulum stress and glycogen synthase kinase-3. Can J Physiol Pharmacol. 2006;84:39–48. doi: 10.1139/Y05-142. [DOI] [PubMed] [Google Scholar]
- 21.Ikegami T, Matsuzaki Y. Ursodeoxycholic acid: Mechanism of action and novel clinical applications. Hepatol Res. 2008;38:123–131. doi: 10.1111/j.1872-034X.2007.00297.x. [DOI] [PubMed] [Google Scholar]
- 22.Beuers U. Drug insight: Mechanisms and sites of action of ursodeoxycholic acid in cholestasis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:318–328. doi: 10.1038/ncpgasthep0521. [DOI] [PubMed] [Google Scholar]
- 23.Xie Q, Khaoustov VI, Chung CC, Sohn J, Krishnan B, Lewis DE, Yoffe B. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology. 2002;36:592–601. doi: 10.1053/jhep.2002.35441. [DOI] [PubMed] [Google Scholar]
- 24.Auwerx JH, Deeb S, Brunzell JD, Peng R, Chait A. Transcriptional activation of the lipoprotein lipase and apolipoprotein E genes accompanies differentiation in some human macrophage-like cell lines. Biochemistry. 1988;27:2651–2655. doi: 10.1021/bi00408a003. [DOI] [PubMed] [Google Scholar]
- 25.Tong J, Zhu MJ, Underwood KR, Hess BW, Ford SP, Du M. AMP-activated protein kinase and adipogenesis in sheep fetal skeletal muscle and 3T3-L1 cells. J Anim Sci. 2008;86:1296–1305. doi: 10.2527/jas.2007-0794. [DOI] [PubMed] [Google Scholar]
- 26.Nakano E, Taiwo FA, Nugent D, Griffiths HR, Aldred S, Paisi M, Kwok M, Bhatt P, Hill MH, Moat S, Powers HJ. Downstream effects on human low density lipoprotein of homocysteine exported from endothelial cells in an in vitro system. J Lipid Res. 2005;46:484–493. doi: 10.1194/jlr.M400339-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Luan Y, Griffiths HR. Ceramides reduce CD36 cell surface expression and oxidised LDL uptake by monocytes and macrophages. Arch Biochem Biophys. 2006;450:89–99. doi: 10.1016/j.abb.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Thuerauf DJ, Morrison L, Glembotski CC. Opposing roles for ATF6alpha and ATF6beta in endoplasmic reticulum stress response gene induction. J Biol Chem. 2004;279:21078–21084. doi: 10.1074/jbc.M400713200. [DOI] [PubMed] [Google Scholar]
- 29.Chan SW, Egan PA. Hepatitis C virus envelope proteins regulate CHOP via induction of the unfolded protein response. Faseb J. 2005;19:1510–1512. doi: 10.1096/fj.04-3455fje. [DOI] [PubMed] [Google Scholar]
- 30.Morgan U, Constantine C, Forbes DT, R Differentiation between human and animal isolates of cryptosporidium parvum using rDNA sequencing and direct PCR analysis. J. Parasitol. 1997;83:825–830. [PubMed] [Google Scholar]
- 31.Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- 32.Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, Koehn S, Rhee JS, Silverstein R, Hoff HF, Freeman MW. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 33.Katome T, Obata T, Matsushima R, Masuyama N, Cantley LC, Gotoh Y, Kishi K, Shiota H, Ebina Y. Use of RNA interference-mediated gene silencing and adenoviral overexpression to elucidate the roles of AKT/protein kinase B isoforms in insulin actions. J Biol Chem. 2003;278:28312–28323. doi: 10.1074/jbc.M302094200. [DOI] [PubMed] [Google Scholar]
- 34.Shao J, Yamashita H, Qiao L, Friedman JE. Decreased Akt kinase activity and insulin resistance in C57BL/KsJ-Leprdb/db mice. J Endocrinol. 2000;167:107–115. doi: 10.1677/joe.0.1670107. [DOI] [PubMed] [Google Scholar]
- 35.Lee YH, Giraud J, Davis RJ, White MF. c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J Biol Chem. 2003;278:2896–2902. doi: 10.1074/jbc.M208359200. [DOI] [PubMed] [Google Scholar]
- 36.Janulis M, Silberman S, Ambegaokar A, Gutkind JS, Schultz RM. Role of mitogen-activated protein kinases and c-Jun/AP-1 trans-activating activity in the regulation of protease mRNAs and the malignant phenotype in NIH 3T3 fibroblasts. J Biol Chem. 1999;274:801–813. doi: 10.1074/jbc.274.2.801. [DOI] [PubMed] [Google Scholar]
- 37.Fuchs SY, Dolan L, Davis RJ, Ronai Z. Phosphorylation-dependent targeting of c-Jun ubiquitination by Jun N-kinase. Oncogene. 1996;13:1531–1535. [PubMed] [Google Scholar]
- 38.Zhang K, Kaufman RJ. Signaling the unfolded protein response from the endoplasmic reticulum. J Biol Chem. 2004;279:25935–25938. doi: 10.1074/jbc.R400008200. [DOI] [PubMed] [Google Scholar]
- 39.Lai E, Teodoro T, Volchuk A. Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology (Bethesda) 2007;22:193–201. doi: 10.1152/physiol.00050.2006. [DOI] [PubMed] [Google Scholar]
- 40.Frittitta L, Grasso G, Munguira ME, Vigneri R, Trischitta V. Insulin receptor tyrosine kinase activity is reduced in monocytes from non-obese normoglycaemic insulin-resistant subjects. Diabetologia. 1993;36:1163–1167. doi: 10.1007/BF00401061. [DOI] [PubMed] [Google Scholar]
- 41.Naidoo C, Jialal I, Dunn RD, Govender T, Joubert SM. Insulin binding to circulating monocytes and erythrocytes in patients with non-insulin-dependent diabetes in the young. Diabetes Res. 1987;4:35–38. [PubMed] [Google Scholar]
- 42.Grunberger G, Zick Y, Gorden P. Defect in phosphorylation of insulin receptors in cells from an insulin-resistant patient with normal insulin binding. Science. 1984;223:932–934. doi: 10.1126/science.6141638. [DOI] [PubMed] [Google Scholar]
- 43.Hartman ME, O'Connor JC, Godbout JP, Minor KD, Mazzocco VR, Freund GG. Insulin receptor substrate-2-dependent interleukin-4 signaling in macrophages is impaired in two models of type 2 diabetes mellitus. J Biol Chem. 2004;279:28045–28050. doi: 10.1074/jbc.M404368200. [DOI] [PubMed] [Google Scholar]
- 44.Liang CP, Han S, Okamoto H, Carnemolla R, Tabas I, Accili D, Tall AR. Increased CD36 protein as a response to defective insulin signaling in macrophages. J Clin Invest. 2004;113:764–773. doi: 10.1172/JCI19528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han S, Liang CP, DeVries-Seimon T, Ranalletta M, Welch CL, Collins-Fletcher K, Accili D, Tabas I, Tall AR. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 2006;3:257–266. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Sreejayan N, von Ritter C. Effect of bile acids on lipid peroxidation: the role of iron. Free Radic Biol Med. 1998;25:50–56. doi: 10.1016/s0891-5849(98)00045-8. [DOI] [PubMed] [Google Scholar]
- 47.Sreejayan N, Von Ritter C. Lipid peroxidation in bile: the role of hydrophobic bile acids and the effect on biliary epithelial cell function. Pathophysiol. 1999;8:225–232. [Google Scholar]
- 48.Chen Y, Liu CP, Xu KF, Mao XD, Lu YB, Fang L, Yang JW, Liu C. Effect of Taurine-Conjugated Ursodeoxycholic acid on endoplasmic reticulum stress and apoptosis induced by advanced glycation end products in cultured mouse podocytes. Am J Nephrol. 2008;28:1014–1022. doi: 10.1159/000148209. [DOI] [PubMed] [Google Scholar]
- 49.Sohn J, Khaoustov VI, Xie Q, Chung CC, Krishnan B, Yoffe B. The effect of ursodeoxycholic acid on the survivin in thapsigargin-induced apoptosis. Cancer Lett. 2003;191:83–92. doi: 10.1016/s0304-3835(02)00624-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relative densities of pAkt, JNK and pc-JUN, obtained from the ratio of the densitometric scans of the respective Western bots. Values are mean + SEM (n=3 or 4), *P<0.05