Abstract
Type-2 diabetes is growing at epidemic proportions world-wide. This report describes the effect of a novel, synthetic, small molecule 2-(3, 4-dihydro-2H-pyrrolium-1-yl)-3oxoindan-1-olate (DHPO), on metabolic abnormalities in genetic and dietary mouse models of type-2 diabetes. DHPO (20mg/kg/d i.p. for 21d) attenuated fasting blood glucose, improved glucose disposal and corrected dyslipidemia in genetic (leptin deficient, ob/ob) and dietary (high-fat-fed) mouse models of insulin resistance. In addition, DHPO augmented 2-deoxy-D-glucose (2DG) uptake in gastrocnemius muscles of wild-type mice and in cultured myotubes. The increase in 2DG-uptake was associated with an increase in the phosphorylation of AMPK (Thr-172) and its downstream effector acetyl-CoA carboxylase without any changes in the phosphorylation of Akt of insulin receptor. The AMPK inhibitor, compound C attenuated DHPO-induced glucose uptake whereas the PI3-kinase inhibitor Wortmannin was less effective. In addition, DHPO failed to augment glucose up-take in the gastrocnemius muscle from AMPK-α2-transgenic (kinase-dead) mice. Taken together, these results suggest that DHPO is a novel small molecule that alleviates impaired glucose tolerance and lipid abnormalities associated with type 2 diabetes.
1. Introduction
Insulin resistance concomitant with obesity, type II diabetes, hypertension, and other features of the metabolic syndrome is a major risk factor for cardiovascular diseases, the leading cause of mortality and morbidity [1]. The pathogenesis of type 2 diabetes involves progressive development in insulin resistance associated with a defect in insulin secretion, leading to overt hyperglycemia. The molecular mechanisms involved in the process leading type-2 diabetes remain elusive. Indeed, disruptions in skeletal muscle glucose up-take, insulin signaling, and glycogen synthesis are evident in insulin resistant subjects up to two decades before their developing diabetes [2]. Insulin resistance is characterized by impairment of postprandial glucose up-take by muscle with endogenously secreted insulin. In patients with this condition, insulin levels are 2–4 folds higher than in nondiabetics [3]. Defects in the insulin receptor function, signaling, glucose transport, and glycogen synthesis are believed to contribute to insulin resistance [4].
Although management of insulin resistance and type 2 diabetes by diet and exercise helps delay or prevent the onset of diabetes [5], for many patients, achievement of tight glucose control is difficult, requiring therapy with drugs which augment the action of insulin. However, compounds which improve insulin sensitivity and glucose intolerance are somewhat limited warranting the discovery and characteriziation of novel molecules targeting various pathways involved in the pathogenesis of type-2 diabetes. During our screening for lipoxygenase inhibitors we serendipitously identified DHPO, 2-(3,4-dihydro-2H-pyrrolium-1-yl)-3oxoindan-1-olate (DHPO) as a lead compound with potent antioxidant properties [6]. The objective of this study was to evaluate the impact DHPO on cellular and animal models of insulin resistance. Our studies reveal that DHPO markedly improved carbohydrate and lipid abnormalities associated with insulin resistant conditions. Furthermore, investigations into cellular signaling mechanisms suggest that DHPO may be causing its effects via the augmentation of the AMP-activated protein kinase (AMPK) signaling pathway.
2. Materials and Methods
2.1. Synthesis of DHPO
To a solution of ninhydrin (2.0 mmol) in methanol, proline (2.0 mmol) was added and the product obtained was separated by vacuum filtration, washed and dried. DHPO was also synthesized by solid state reaction by triturating proline (2.0 mmol) and ninhydrin (2.0 mmol) on a water bath at 40°C. The completion of the reaction was ascertained by TLC (Silica gel G F254 plate; solvent system: 5% v/v methanol in carbon tetrachloride) by monitoring the disappearance of ninhydrin at 254mm. The structure was characterized by elemental and spectral analysis [6] (Online Fig. 1).
2.2 Animals and treatment protocol
All animal treatment procedures described in this study were approved by the Animal Care and Use Committee at University of Wyoming (Laramie, WY). Animals were housed under well-controlled conditions of temperature (22 ± 2°C), humidity (55 ± 5%) and a 12 h/12 h light-dark cycle with access to food and water ad libitum. For the genetic model, homozygous B6.V lep<ob>/J male mice (5-weeks old) were obtained from the Jackson Laboratory (Bar Harbor, ME). For the dietary model, 3-month-old male C57/bL6 mice were randomly assigned to low-fat (10% of total calorie) or high-fat (45% of total calorie) diets (Research Diets, New Brunswick, NJ) for 16 weeks. Both ob/ob (n=20) and the high-fat diet fed mice (n=20) were randomly assigned to receive DHPO or vehicle via intraperitoneal injection at a dose of 20mg/kg/day for 14 and 21 days respectively. The dosage was determined by pharmacokinetic studies described below.
2.2. DPHO pharmacokinetics
Pharmacokinetic studies were performed in overnight fasted C57BL/6 mice and the serum concentrations of DHPO were determined by liquid chromatography-mass spectrometry (LCMS). Briefly, aliquots of serum were extracted with acetonitrile, and injected into the LC/MS: mobile phase: 0.3% HCHO and acetonitrile (40:60); stationary phase: Waters C18 column (100 × 4.6mm) 0.5µm; Flow-rate: 0.3 ml/min; retention time: 0.51 min; capillary voltage 3.35 kV; cone voltage 40V; extractor voltage 3V; RF lens voltage 1.6 V. The source and desolvation temperatures were 110 and 40°C respectively, whereas the desolvation and cone gas flow were 800 and 20 L/hr, respectively. The selected mass-to-charge ratio (m/z) transition of DHPO ions [M-2]− used in the single ion reaction was 212.3, at a dwell time set at 500ms.
2.3. Intraperitoneal glucose tolerance test
At the end of treatment period both ob/ob mice and high-fat fed mice were subjected to intraperitoneal glucose tolerance test (GTT) as described previously [7]. Briefly, the mice were fasted (~12h), and glucose challenge was initiated with intraperitoneal injection of glucose (1g/kg). Glucose levels were determined in blood drops obtained by clipping the tail of the mice immediately before glucose challenge, as well as at 30, 60, and 120 min intervals using AUCC-CHEK Advantage Glucose Analyzer (Roche Diagnostics, IN).
2.4. Determination of serum lipids
Serum levels of triglycerides, total cholesterol and high-density-lipoprotein (HDL) were measured using commercial kits (from Equal Diagnostics, Exton, PA) per manufacturer’s protocol.
2.5. Cell culture and treatment
The skeletal muscle cell line C2C12 from adult mouse hind-legs (American Type Culture Collection) was grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin under a humidified atmosphere of 5%CO2 in air and maintained at low confluence. Differentiation was induced in sub-confluent cells by replacing the media with one that contained low serum as reported previously [8]. After differentiation myotubes were rendered quiescent by incubating them with serum-free medium for 12h and during the last 1h of quiescence, myotubes were subjected to DHPO-treatment. At the end of the treatment period, the cells were lysed with RIPA buffer containing protease and phosphatase inhibitors, sonicated, centrifuged (12000g for 20 min at 4 °C), protein concentration of the supernatant determined, and subjected to Western blotting.
2.6. 2-deoxy-glucose up-take assay
A 2-deoxy-glucose uptake assay was performed as previously described [9,10]. Briefly, after serum starvation for 4 hours, C2C12 myotubes were washed with Krebs-Ringer phosphate HEPES buffer (KRPH buffer; 10 mM phosphate buffer, pH 7.4, 1 mM MgSO4, 1 mM CaCl2, 136 mM NaCl, 4.7 mM KCl, 10 mM HEPES (pH 7.6)) and then incubated without or with DHPO in KRPH buffer for 30 min at 37°C in the presence of 2-deoxy-[3H]-glucose (0.2 µCi). At the end of the incubation period, the cells were washed three times with ice-cold PBS. The cells were then lysed in PBS containing 0.2M NaOH, and glucose uptake was assessed by scintillation counting. For ex-vivo glucose-uptake assay, gastrocnemius muscles were dissected out from mice, incubated for 30min in Krebs Henseleit buffer supplemented with 8mM glucose and 0.1% bovine serum albumin in the presence or absence of DPHO in an atmosphere containing 95%O2-5% CO2. Glucose uptake was measured as described above except that the muscle strips were freeze-dried, weighed, treated with 1N NaOH prior to determination of the radioactivity count.
2.7. Western blot analysis
The expression and phosphorylation of each protein were analyzed by western blot analysis [7]. In brief, the harvested cell lysates were subjected to 7.5% SDS-polyacrylamide gel electrophoresis (1: 30 bis: acrylamide). Proteins were transferred to a nitrocellulose membrane, and the membranes were then blocked for 1 hour at room temperature with 5% non-fat dry milk in Tris buffered saline (TBS) containing 0.1% Tween 20. Immunostaining to detect each protein was achieved with an over-night incubation (4 °C) with a 1:1000 dilution of anti-phospho (Ser-473) Akt (1:1000), anti-phospho (thr-308) Akt (1:1000), Akt (1:1000), IRβ (1:1000), anti-IRS-1 (1:1000), anti-GLUT-4 (1:1000), anti-phospho (thr-172) AMPK (1:1,000), AMPK (1:1,000) and anti-beta-actin (1:5,000). Proteins were visualized after subsequent incubation with a 1:5000 dilution of anti-mouse or rabbit IgG conjugated to horseradish peroxidase and a SuperSignal Chemiluminescence detection procedure (Pierce Biotechnology Inc.). All antibodies used in this study were from Cell Signaling Technology Inc. (Beverly, MA). Protein concentrations were determined using a bicinchoninic acid assay (BCA) (Pierce Biotech. Inc.). Three independent experiments were performed for each condition and the intensity of immune-reactive bands was analyzed with a Bio-Rad calibrated densitometer.
2.8. Glucose transporter-4 (GLUT-4) determination
Skeletal muscle membrane proteins were extracted using a membrane protein extraction kit (Biovision) as described by us previously [9]. The membrane protein was subsequently used for Western blot analysis of glucose transporter-4.
2.9. AMPK kinase activity determination
AMPK kinase activity was determined using AMPK KinEASE™ FP Fluorescein Green Assay kit (Millipore). AMPK active enzyme (BIOMOL, Plymouth Meeting, PA) was incubated with different concentrations of DHPO and activity was determined as per the protocol supplied by the manufacturer.
2.10. Data analysis
Data are expressed as mean ± SEM and statistically evaluated using One-way ANOVA followed by Bonferroni post-hoc test using Graph Pad Prism (Graph Pad Software, Inc. San Diego, CA, USA). A ‘p’ value of less than 0.05 was considered to be statistically significant.
3. Results
3.1. DHPO pharmacokinetics
Bioavailability of DHPO in mice was determined following intraperitoneal injections of DHPO (20mg/kg body weight) and assessing serum DHPO concentrations by HPLC/MS. Pharmacokinetic parameters of DHPO including half-life (t1/2), volume of distribution (Vd), clearance, and area under the plasma concentration–time graph (AUC) are given in Online Table 1. The percentage bioavailability of DHPO was 47.7 ± 4.3 following intraperitoneal administration.
3.2. Effect of DPHO on body mass, blood glucose and serum insulin in obese and high-fat-fed mice
As anticipated body, heart, liver, and kidney masses were significantly higher in ob/ob mice compared to lean mice (Online table 2). In addition, obese mice had higher fasting blood glucose and serum insulin levels compared to the lean counterparts indicating the presence of insulin resistance. In contrast, obese mice treated with DPHO had significantly lower serum insulin levels compared to the vehicle treated obese mice. However, fasting blood glucose concentrations did not differ significantly between the DPHO and vehicle-treated obese mice. In the dietary model, high-fat-feeding resulted in elevated body mass compared to the mice that received the standard-chow (Table 1) without any significant changes in the masses of various organs (heart, liver, kidney and spleen) following high-fat-feeding. DHPO-treatment resulted in a significant reduction in the overall body mass gain caused by high-fat-feeding. This decrease in weight-gain was not associated with a decrease in food-intake (data not shown). In addition, DPHO treatment lowered the elevated basal blood glucose and serum insulin levels in high-fat-diet-fed mice. In obese mice, in contrast, there were no changes in the basal blood-glucose levels.
Table 1.
General parameters of vehicle and DHPO-treated high-fat-fed-mice.
| Standard Chow | HF Diet (Vehicle) |
HF Diet (DHPO) |
|
|---|---|---|---|
| Body mass (g) | 26.3 ± 0.9 | 35.7 ± 5.3* | 27.0 ± 1.8# |
| Heart mass (g) | 0.16 ± 0.0 | 0.19 ± 0.01 | 0.17 ± 0.01 |
| Liver mass (g) | 1.64 ± 0.07 | 1.78 ± 0.20 | 1.64 ± 0.20 |
| Kidney mass (g) | 0.32 ± 0.03 | 0.38 ± 0.08 | 0.34 ± 0.01 |
| Spleen mass (g) | 0.13 ± 0.01 | 0.12 ± 0.03 | 0.25 ± 0.11 |
| Blood glucose (mmol/L) | 7.11 ± 0.58 | 10.47 ± 0.74* | 6.10 ± 0.77# |
| Serum insulin (ng/mL) | 0.34 ± 0.02 | 1.69 ± 0.16* | 0.74 ± 0.19# |
Data are means ± SEM.
p<0.05 versus standard chow,
p<0.01 versus high-fat-diet-fed mice.
3.3. Effect of DHPO on whole-body glucose tolerance
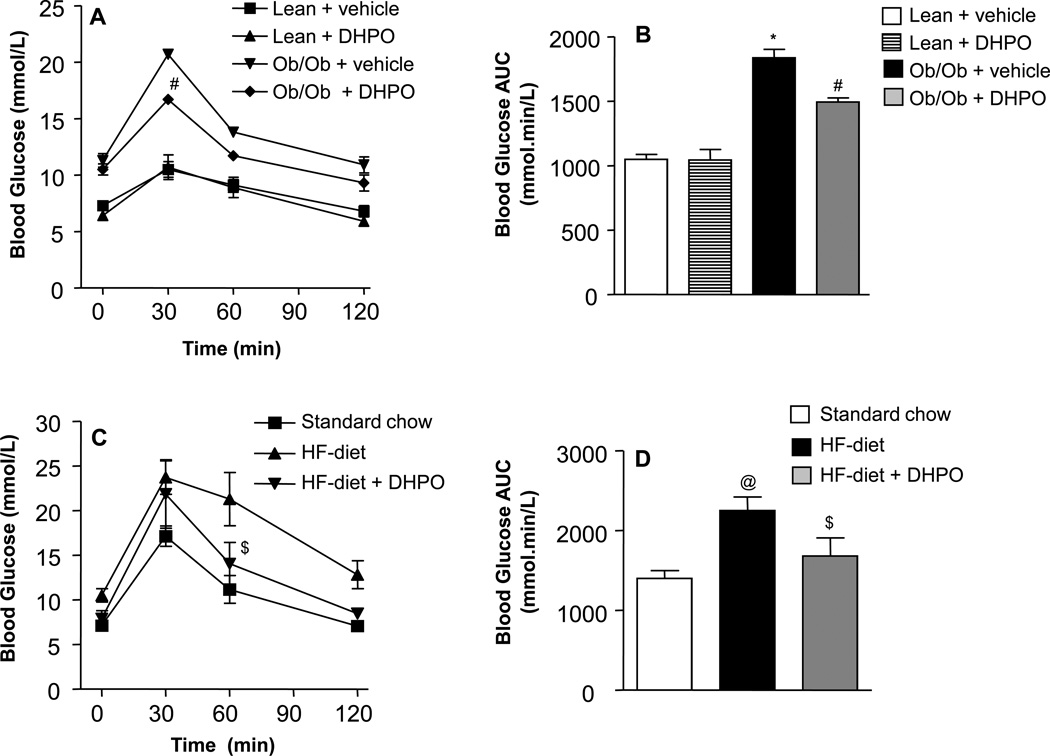
Following an acute intraperitoneal glucose-challenge, blood glucose concentrations in lean mice began to drop after peaking at 30 min and returned to nearly baseline values after 120 min (Fig. 1A). In contrast, obese mice exhibited severe hyperglycemia and impaired glucose tolerance as evidenced by the high post-challenge blood glucose concentrations. Treatment with DHPO facilitated glucose clearance in obese mice with lower post-challenge blood glucose concentrations at all time points compared to the vehicle-treated mice. The total incremental area-under the curve (AUC) for blood glucose was significantly higher in the obese mice compared to the lean mice whereas the AUC was significantly attenuated in obese mice that received DHPO (Fig. 1B). Similarly, high-fat-fed mice exhibited sever hyperglycemia and impaired glucose tolerance upon administration of glucose as evidenced by the high post-challenge blood glucose levels (Fig. 1C). As was the case with obese mice, DPHO-treated mice showed lower blood glucose levels. Whereas DHPO showed maximum glucose lowering effect at the 30 min time-point following glucose challenge in obese mice, in the high-fat-fed mice the maximum glucose lowering effect was observed at 60 min post-glucose challenge. The total AUC for post-challenge blood glucose was significantly higher in the high fat-fed mice compared to the mice that received normal chow, which was significantly attenuated by DHPO (Fig. 1D).
Figure 1.
Effect of DHPO on whole-body glucose-uptake in genetic and dietary mouse models of diabetes. Mice received DPHO (20mg/Kg/day) intraperitoneally for a period of 3-weeks following which blood glucose levels in lean and ob/ob mice (A) and in standard chow and high-fat-diet-fed mice (C) at different time points following intraperitoneal glucose challenge (1g/kg) were assessed. Panels B and D illustrate the corresponding incremental AUC0–120. Data are represented as means ± SEM, and *p<0.05 versus lean control, #p<0.001 versus ob/ob control, @p<0.05 versus standard chow, $p<0.05 versus high-fat-fed mice.
3.4. Effect of DHPO on serum lipids
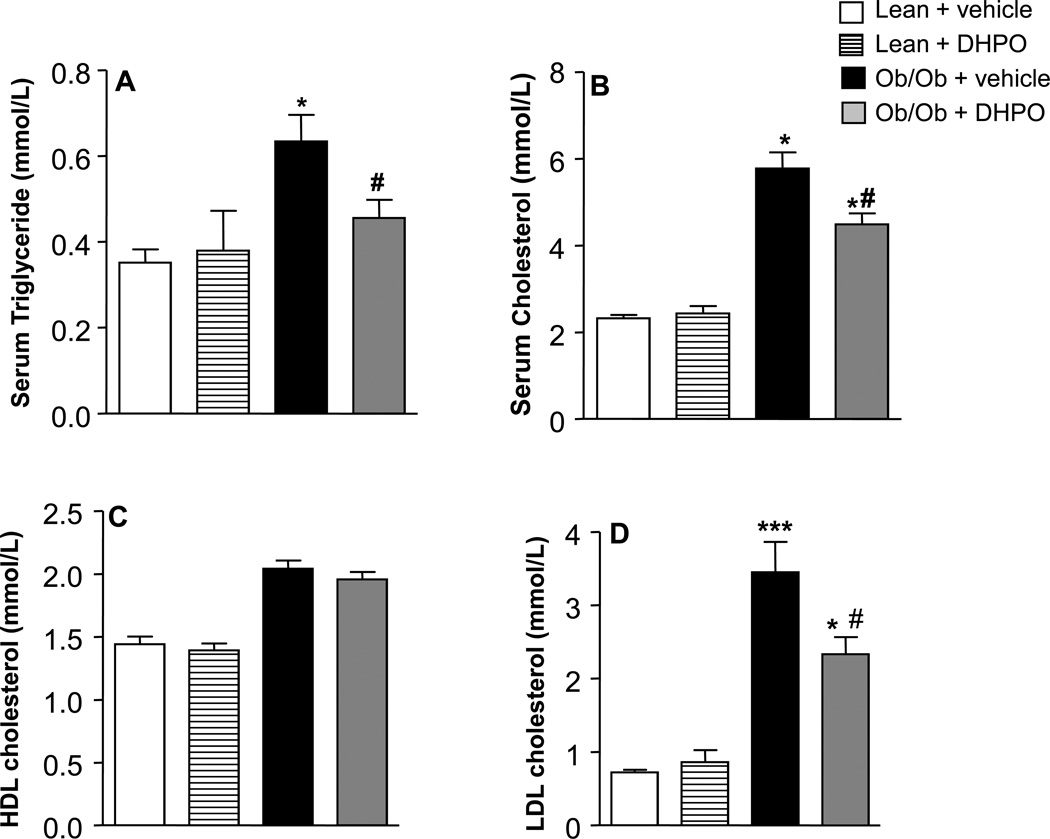
Obese mice exhibited severe dyslipidemia as evidenced by the elevated serum cholesterol, triglycerides (TG), and low-density lipoproteins (LDL)-cholesterol mice compared to lean mice. Interestingly, mice that received DHPO had all these elevated lipid parameters reverted back significantly (Fig. 2A–D). In contrast, serum high-density lipoprotein (HDL) levels were unaltered in all the groups. DHPO-treatment had qualitatively similar effects on serum lipid levels in high-fat-fed animals (Online Fig. 2A–D).
Figure 2.
Effect of DHPO on serum triglyceride (A), cholesterol (B), high-density lipoprotein (C) and low-density-lipoprotein (D) in ob/ob and lean mice following intraperitoneal administration of DPHO (20mg/Kg/day intraperitoneal for 3 weeks). Data are represented as means ± SEM, and *p<0.05 compared to lean control, ***p<0.001 versus lean control, #p<0.05 versus ob/ob control.
3.5. Effect of in-vivo treatment with DHPO on skeletal muscle glucose up-take
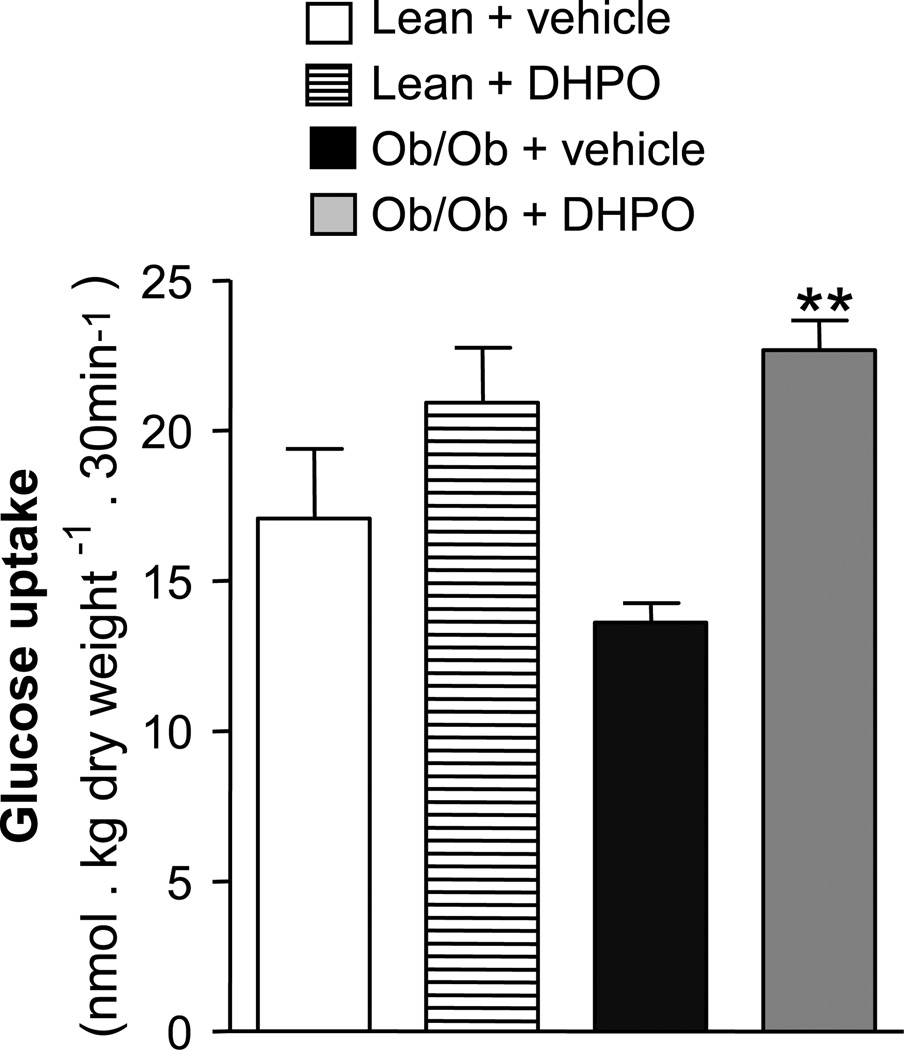
Since skeletal muscle is the major peripheral tissue which plays an important role in energy balance, glucose up-take and disposal, we next sought to investigate the effect of DHPO-treatment on glucose up-take in the gastrocnemius muscles isolated from lean and obese mice. As anticipated, basal glucose up-take was significantly blunted in the gastrocnemius muscles of obese mice compared to lean mice (Fig. 3). Interestingly, intraperitoneal injections with DHPO for 14 days reversed significantly the effects of obesity on skeletal muscle glucose up-take as evidenced by higher levels of glucose up-take compared to the vehicle-treated mice. A trend towards increased muscle-glucose up-take was also observed in the lean mice treated with DHPO but it failed to reach significance.
Figure 3.
Effect of DHPO on glucose-uptake in isolated gastrocnemius muscle. Glucose uptake in gastrocnemius muscle isolated from ob/ob and lean-mice treated with DPHO (20 mg/Kg/day, intraperitoneal for 3 weeks). Data are represented as means ± SEM, **p<0.01 versus ob/ob control.
3.6. Effect of DHPO on glucose up-take in cultured, differentiated myotubes
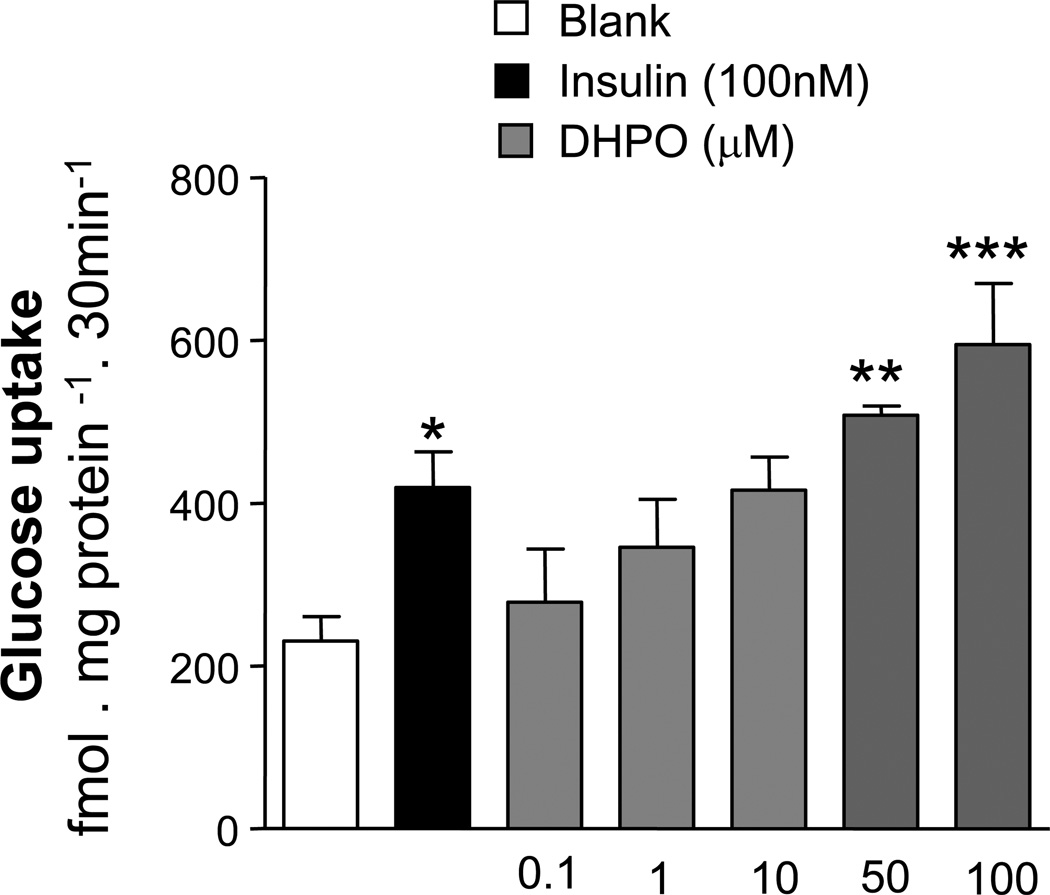
To investigate whether DHPO directly acts on the skeletal muscles to cause glucose up-take, we next studied the effect of DHPO on glucose up-take in cultured myotubes. DHPO-treatment resulted in a concentration dependent increase in glucose up-take in cultured myotubes with a 2-fold increase over basal glucose up-take was observed at a concentration of 100µM of DHPO (Fig. 4). Interestingly, the glucose up-take stimulated by a 10µM concentration of DHPO was comparable to that observed with 100nM insulin. DHPO at the concentrations used in the experiments did not cause any overt cellular damage (data not shown).
Figure 4.
Effect of DHPO on basal glucose-uptake in cultured C2C12 myoblasts. Values are means ± SEM (n=3–4 independent experiments). *p<0.05, **p<0.01 and ***p<0.001 versus vehicle. Insulin (100nM) was used as a positive control.
3.7. Effect of DHPO on insulin and AMPK-signaling cascade
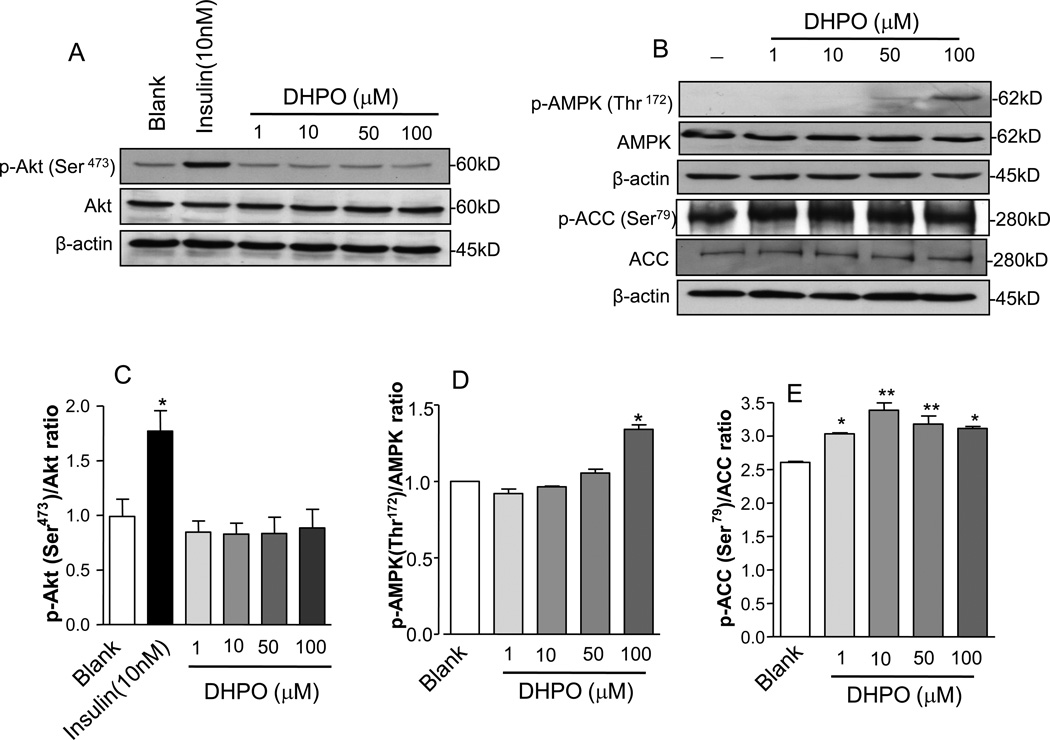
To understand the mechanisms of DHPO-stimulated glucose up-take we investigated the effect of DHPO-treatment on key cellular mediators of insulin signaling. To this end, we treated cultured myotubes with DHPO and assessed the phosphorylation levels of Akt, a key downstream effector of insulin signaling. As illustrated in Fig. 5A, treatment with DHPO failed to induce phosphorylation of Akt (thr-473) at the concentrations used whereas treatment with insulin resulted in a pronounced phosphorylation of Akt. Neither did treatment of DHPO cause phosphorylation of the insulin receptor beta (data not shown).
Figure 5.
Effect of DHPO on Akt, AMPK, and ACC phosphorylation in differentiated C2C12 myoblast. C2C12 myotubes were stimulated with the indicated doses of DHPO and were Western blotted against antibodies that recognize phospho-Akt, Akt, beta-actin phospho-AMPK, phospho-ACC, AMPK, and ACC. Panel A is a representative Western blot for p-Akt, Akt and beta-actin and pane B is a representative Western blot for p-AMPK, AMPK, p-ACC, ACC and beta-actin. Relative densities of respective phosphorylated to total protein and/or beta-actin are shown in lower panels (C, D and E). Values are represented as mean ± SEM, n= 4–6. *p<0.05 versus control.
Since muscle cells can uptake glucose independent of insulin signaling, via the activation of AMPK, we determined the phosphorylation (hence activity) of AMPK at the thr-172 residue in cultured myotubes following DHPO-treatment. Interestingly, in treatment with DHPO resulted in a small but significant phosphorylation of AMPK thr-172 as shown in Fig. 5B. In addition to AMPK phosphorylation, DHPO-treatment also resulted in the phosphorylation of acetyl co-A-carboxylase (ACC) a down-stream substrate of AMPK at the Ser-79 residue. The phosphorylation of ACC was observed at lower concentrations of DHPO compared to that required to cause AMPK phosphorylation.
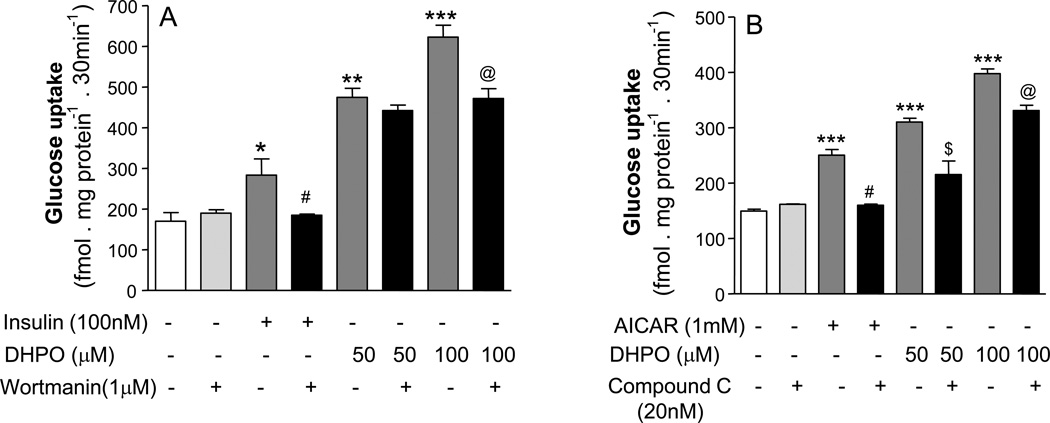
We next used pharmacological inhibitors of the PI3-kinase pathway and the AMPK-pathway Wortmannin and compound C respectively in an effort to elucidate the mechanism of DHPO. As expected, Wortmannin at concentration of 1 µM completely inhibited insulin (100nM)-stimulated glucose up-take in myotubes (Fig. 6A) whereas it failed to inhibit DHPO-stimulated glucose up-take. When the concentration of DHPO was doubled to 100µM a partial, but significant inhibition of DHPO-stimulated glucose up-take was observed. In contrast, treatment with Compound C caused a 58% inhibition of DHPO (50 µM)-stimulated glucose up-take (Fig. 6B). When the DHPO concentration was raised to 100µM, the inhibition caused by Compound C reduced to 41% indicating competition kinetics. As expected, C completely blocked the glucose up-take stimulated by 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR), a pharmacological activator of AMPK.
Figure 6.
Effect of Wortmanin and Compound C on DHPO-stimulated basal glucose-uptake in cultured C2C12 myoblasts. A. Wortmanin (1mM) significantly (p<0.01) reduced DHPO (100mM)-stimulated glucose-uptake. *p<0.05, **p<0.01, ***p<0.001 versus basal; #p<0.01 versus insulin 100nM; @p<0.05 versus DHPO 100µM. B. Compound C (20mM) significantly reduced DHPO-induced glucose-uptake. ***p<0.001 versus basal; #p<0.01 versus AICAR (1mM); $p<0.01 versus DHPO (50µM); @p<0.05 versus DHPO (100µM). Values are represented as mean ± SEM, n = 3–4 independent experiments.
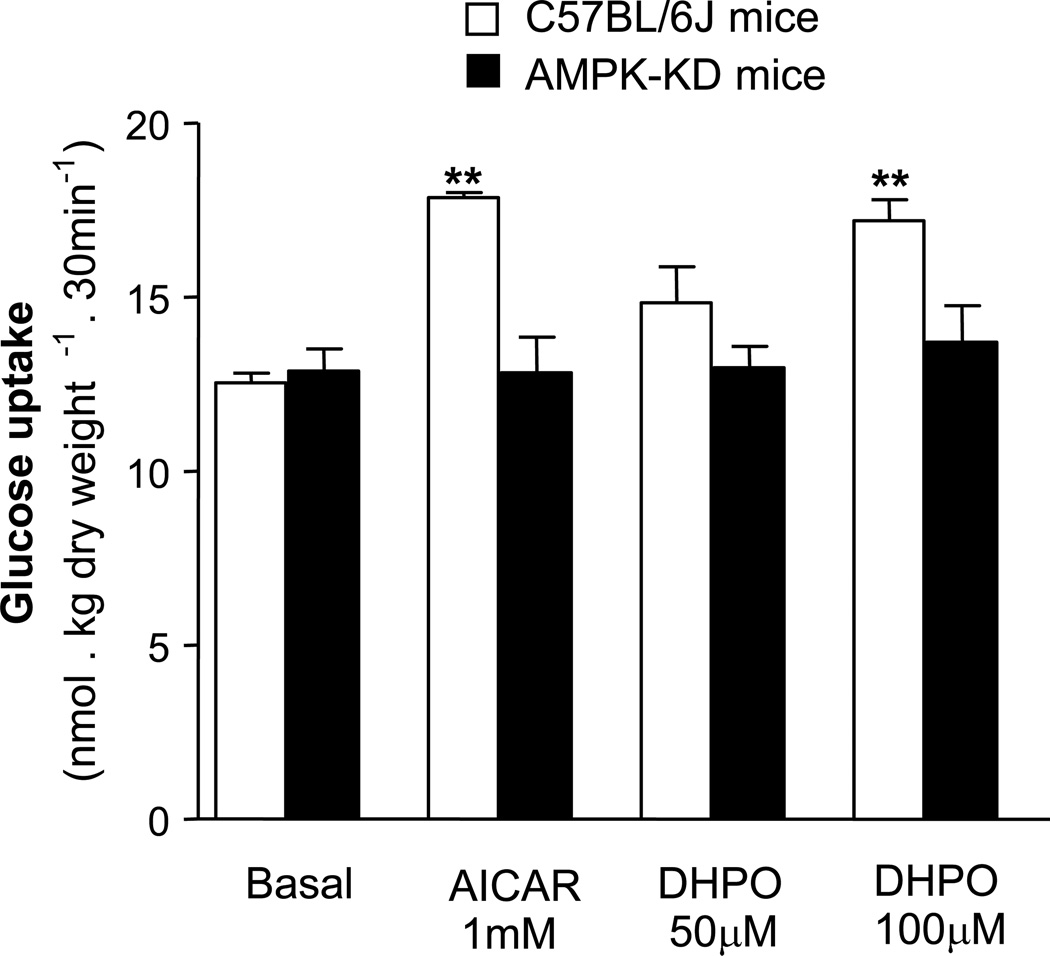
3.8. Effect of DHPO on skeletal muscle glucose-uptake in transgenic, AMPK-kinase dead mice
To ascertain the role of AMPK in DHPO-induced glucose-uptake, we next investigated the effects of DHPO on glucose uptake in the gastrocnemius muscles of wild-type mice and transgenic mice with muscle-specific over-expression of a kinase-dead (KD) from of the catalytic α-2 subunit of AMPK [11]. To ascertain the KD-phenotype, we used AICAR to stimulated muscle glucose-uptake in the isolated gastrocnemius muscle of these transgenic mice. Data presented in figure 7 demonstrate that incubation with AICAR failed to stimulate muscle glucose up-take in ex-vivo preparations of gastrocnemius muscle from the transgenic AMPK-KD mice. Interestingly, similar to AICAR, incubation with DHPO failed to stimulate muscle glucose up-take in the KD mice, while it stimulated glucose up-take in the muscles from the wild-type, C57/BL6 mice.
Figure 7.
Effect of DHPO on glucose-uptake in isolated gastrocnemius muscle in AMPK-KD mice. DHPO-stimulated glucose-uptake in gastrocnemius muscle from C57/BL6 mice, but failed to show similar effect in muscle preparations from AMPK-KD mice. Values are represented as mean ± SEM, n = 4–5 mice in each group. **p<0.01 versus basal glucose-uptake.
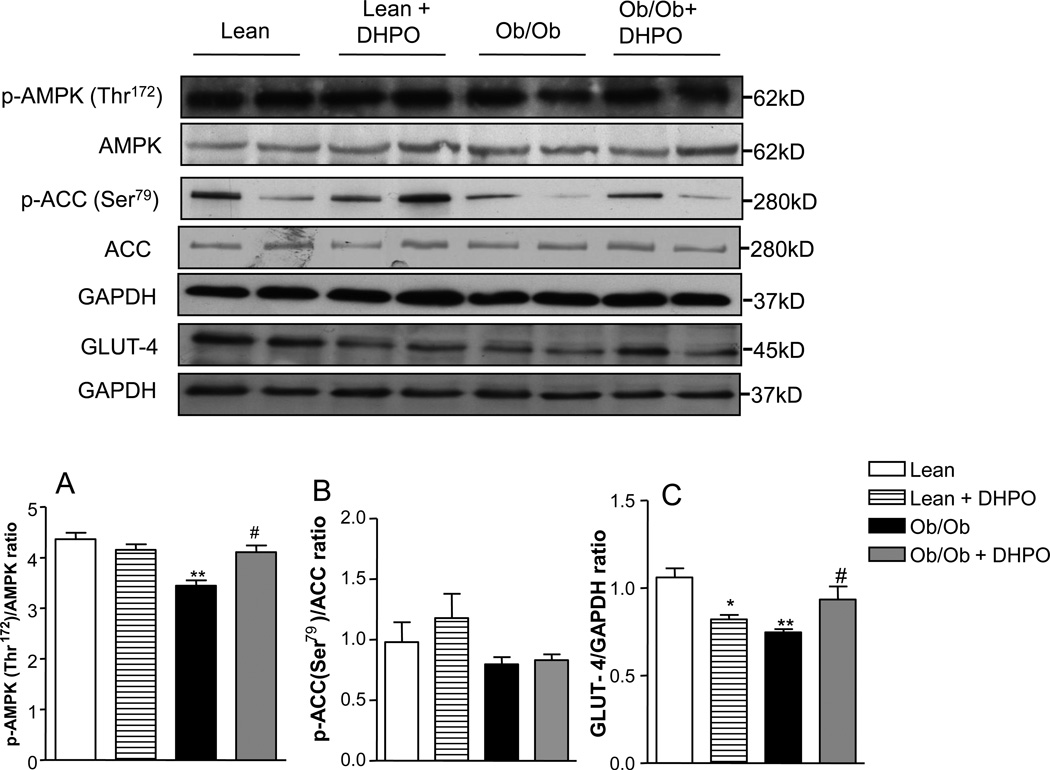
3.9. Effect of DHPO treatment on skeletal muscle AMPK signaling and GLUT-4 translocation
Because we found changes augmentation of AMPK signaling in cultured cells treated with DHPO we were interested in investigating the in-vivo effects of DHPO on these pathways. As shown in Fig. 8 the skeletal muscles of obese mice had lower levels of phosphorylated-AMPK compared to lean mice. Obese mice receiving DHPO had higher levels of phospho-AMPK which did not differ significantly from that observed in lean mice. However, no significant changes were seen in the phospho-ACC levels in any of the groups. We also measured the extent of Glut-4 translocation to the membrane in the skeletal muscle in both the obese and lean mice. Glut-4 levels were significantly lower in the obese mice compared to lean mice. DHPO-treated obese mice had elevated membrane Glut-4 levels compared to vehicle-treated obese mice.
Figure 8.
Effect of DHPO on skeletal muscle AMPK signaling and membrane associated GLUT-4 in ob/ob and lean mice following intraperitoneal administration of DPHO (20mg/Kg/day intraperitoneal for 3 weeks). Upper panel is a representative Western blot and lower panels are relative densities of phosphorylated to total AMPK, phosphorylated ACC to total ACC and membrane associated GLUT-4. Glyceraldehyde 3-phosphate dehydrogenase was used as a loading control. Values are represented as mean ± SEM, n= 4–6.*p<0.05, ***p<0.01 versus Lean and #p<0.05 verses Ob/Ob.
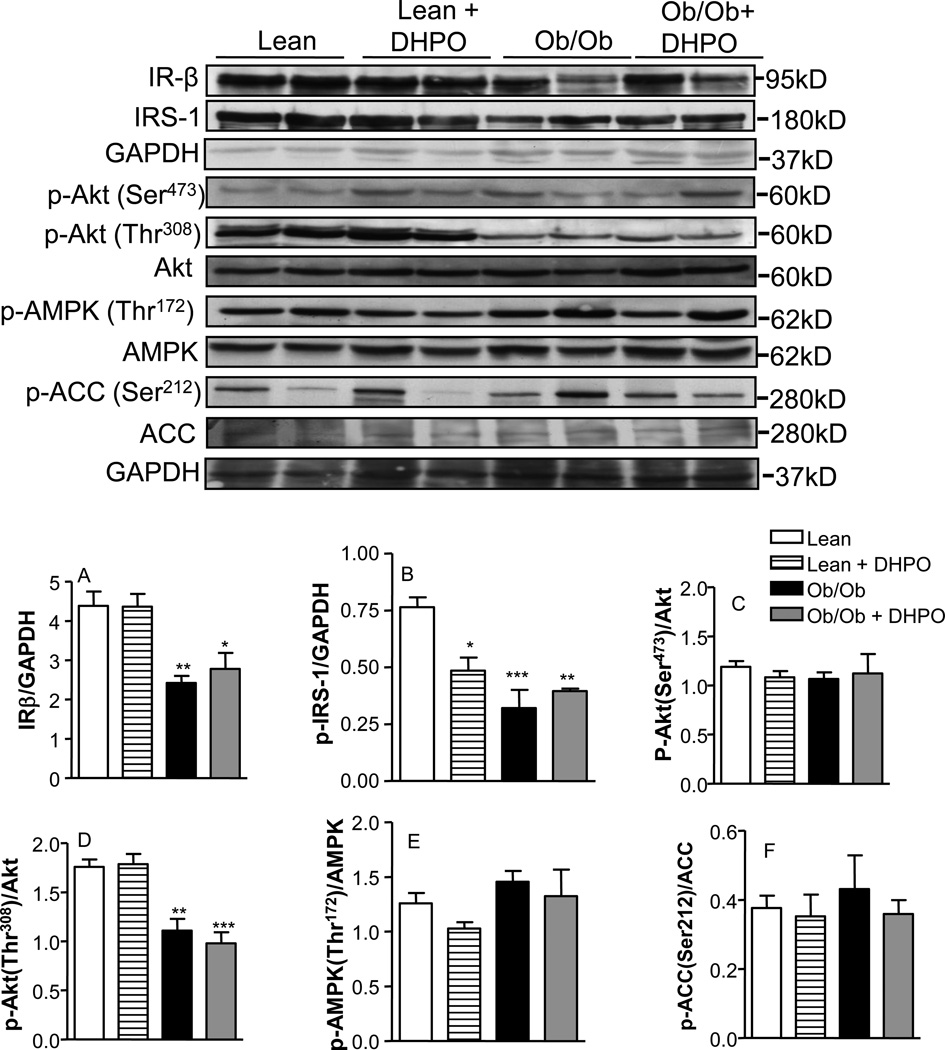
3.10. Effect of DHPO treatment on liver insulin signaling and AMPK pathways
We also assessed the effect of DHPO-treatment on insulin and AMPK-signaling in the liver of lean and obese mice following treatment with DHPO. As shown in Fig. 9, obesity markedly reduced protein levels of IRβ, phospho-IRS-1 and phospho (thr-308)-Akt in the liver of obese mice compared to lean controls. No changes were seen in Akt (Ser-473), phospho-AMPK or phospho-ACC. DHPO-treatment did not alter the phosphorylation status of any of these markers in the livers or either obese or lean mice. Interestingly, DHPO-treated lean mice reduced IRS-phosphorylation levels in the lean mice without altering it in the obese mice.
Figure 9.
Effect of DHPO on liver Insulin and AMPK-signaling in ob/ob and lean mice following intraperitoneal administration of DPHO (20mg/Kg/day intraperitoneal for 3 weeks). Upper panel is a representative Western blot and lower panels are relative densities of IR-β, phospho-IRS-1, phosphorylated Ser473 and Thr308 to total-Akt, phosphorylated to total AMPK and phosphorylated ACC to total ACC. Glyceraldehyde 3-phosphate dehydrogenase was used as a loading control. Values are represented as mean ± SEM, n= 4–6.*p<0.05, **p<0.01, ***p<0.01 versus lean.
3.11. Effect of DHPO on AMPK kinase activity
Next we sought to investigate whether DHPO is a direct activator of AMPK in an in-vitro assay by incubating DHPO with recombinant AMPK and estimating the fluorescence of an AMPK-peptide substrate. We first prepared standard curves to ascertain -concentration and -time-dependent phosphorylation of the peptide substrate by AMPK (data not shown). Based on these standard curves, 5ng of AMPK was chosen and the incubation period was set for 60 min. As shown in online Fig. 3, DHPO had no effect on AMPK-mediated phosphorylation of the substrate peptide at the concentration range of 0–10 µM. Neither did preincubating DHPO with AMPK for different time periods (0–60 min) affect the phosphorylation of the peptide substrate (data not shown).
4. Discussion
The major findings of our study are that a novel, synthetic small molecule, DHPO alleviates impaired glucose intolerance in genetic (leptin deficient ob/ob) and dietary (high-fat fed) mouse models of insulin resistance. DHPO-treatment markedly improved the impaired glucose-uptake in the gastrocnemius muscles in obese mice, suggesting that DPHO-treatment augments peripheral glucose utilization. Interestingly, DHPO treatment was also associated with a reduction in the gain in body mass in the high-fat-fed mice. In addition to improving carbohydrate metabolism, DHPO-treatment corrected the lipid abnormalities associated with obesity and high-fat diet. Skeletal muscles from obese mice showed significantly attenuated glucose uptake compared to lean mice which was reversed in mice that were treated with DHPO. Of great interest are the experiments that demonstrate the lack of effect of DHPO on glucose-uptake in AMPK-KD mice. These results strongly suggest that DHPO may be mediating its effects by modulating the AMPK-signaling pathway.
To further ascertain the role of AMPK and insulin signaling pathways in DHPO–mediated augmentation of glucose uptake we investigated the effects of DHPO on glucose transport in cultured myotubes in the presence of compound C and Wortmanin. As expected, in cultured myotubes DHPO augmented glucose-uptake in a concentration-dependent manner. There was however a discrepancy in the concentrations mediating the in-vivo and in-vitro activity. Whereas in-vivo serum concentrations of DHPO ranged in the nanomolar levels, micromolar concentrations were necessary for in-vitro activity. Although we are unable to reconcile this difference a potential explanation may be the accumulation of the drug on chronic treatment and/or the presence of active metabolites of DHPO that may be responsible for the activity. In addition to augmenting glucose uptake, DHPO caused an increase in phosphorylation of AMPK and its downstream substrate ACC. However, contrary to our expectations both compound C and Wortmanin only modestly inhibited DHPO's effect, while these compounds completely blocked the effects of insulin and AICAR. In addition, DHPO failed to alter the activity of AMPK in an in-vitro kinase assay. Taken together, these results seem to suggest that DHPO may not be mediating its activity by directly activating AMPK, although it is likely that AMPK is necessary for its actions. It is also plausible that other unknown mechanisms in addition to augmenting the AMPK signaling may contribute to the observed biological effects of DHPO.
Insulin mediates it’s action by binding to the e insulin receptor (IR), a disulfide-bonded heterotetrameric membrane protein [12]. Binding of insulin to the alpha-subunit of IR causes conformational changes that lead to autophosphorylation of the membrane-spanning beta-subunit and activation of the receptor’s intrinsic tyrosine kinase activity [13]. Thus activated insulin receptor transphosphorylates several downstream substrates (on Tyr residues) including insulin receptor substrate (IRS) proteins, causing the activation of Akt and subsequent translocation of Glut-4 glucose transporter vesicles to the cell-surface resulting in glucose uptake. In our studies DHPO failed to augment any of these pathways suggesting that DHPO may not affect the insulin signaling pathway. Interestingly however, we did observe an augmentation of Glut-4 translocation in response to DHPO treatment.
Recent studies have shown that in skeletal muscle, glucose uptake can be induced in an insulin-independent manner via the activation of AMPK [14], which plays a pivotal role in the regulation of carbohydrate and fat metabolism [15,16]. Consequently, AMPK has emerged as a novel and attractive therapeutic target to treat or prevent diseases and symptoms associated with impaired carbohydrate and fat metabolism. Muscle-specific expression of a kinase-dead form of the catalytic α2-subunit of AMPK in mice results in an insulin resistant phenotype whereas, overexpression of this subunit results in lowering of blood glucose and increased fatty acid oxidation [17]. At the cellular level, AMPK is thought to mediated cellular-glucose uptake by increasing glut-4 translocation to the cell surface [18]. Interestingly, insulin sensitizing activity of the antidiabetic drugs such as metformin and thiazolidinediones has been attributed, at least in part, to their stimulation of AMPK activity. The ability of DHPO to cause Glut-4 translocation independent of insulin and apparent lack of glucose-uptake in skeletal muscle of AMPK-KO mice suggest that DHPO may be mediating its effects, at least in part, by activating the AMPK-pathway.
AMPK is activated by known antidiabetic drugs such as metformin and rosiglitazone [19] and by a variety of structurally unrelated molecules that include, AICAR [20], A-769662 [21], resveratrol [22,23] and caffeic acid phenylethyl ester [24]. Cool and coworkers have characterized novel thienopyridones as selective activators of AMPK, and have demonstrated that these molecules alleviate metabolic symptoms and dyslipidemia in mice [21,25]. More recently, Pang and coworkers have characterized a novel small-molecule activator of AMPK called PT1 capable of docking with AMPK near the auto-inhibitory domain and directly relieve the auto-inhibition [26]. Activation of AMPK requires phosphorylation of Thr172 in the catalytic α-subunit activation T-loop which is regulated by several upstream kinases and downstream phosphatases [27]. The diversity in the chemical structure of compounds that have been reported thus far to cause specific activation of AMPK suggests that these molecules may be working by different mechanisms or at multiple targets in the AMP-signaling cascade. Both compound C (to a greater extent) and Wortmannin (to a lesser extent) suppressed glucose up-take induced by DHPO in myotubes although both compounds failed to completely negate the effects of DHPO suggesting that DHPO may be mediating its effect by targeting multiple pathways. On the other hand, the increase in phosphorylation of Thr-172 and lack of phosphorylation of Akt, in cells treated with DHPO suggest that AMPK-pathway may be playing a predominant role in mediating the effects of DHPO, although contributions from other unknown pathways cannot be ruled out.
In the present study we also found that treatment with DHPO results in a robust attenuation of serum LDL and triglyceride levels which are elevated in obese mice. The role AMPK-ACC pathway in lipid metabolisms has been well documented [28, 29]. ACC catalyzes the biosynthesis of malonyl-CoA, the primary substrate for fatty acid biosynthesis as well as a potent inhibitor of carnitine palmitoyltransferase I, the rate-limiting step for mitochondrial fatty acid oxidation. Previous studies have demonstrated that the lipid lowering properties of leptin and metformin may be mediated via the activation of AMPK [30,31]. However, hepatic p-ACC levels did not differ significantly (although a trend towards reduction was observed) between obese mice treated with vehicle and DHPO suggesting that the reduction in LDL-levels caused by DHPO may be mediated by other factors beyond ACC phosphorylation that affect fatty acid synthesis or oxidation, such as sterol regulatory element-binding proteins. Interestingly, there were no changes in serum HDL-levels following treatment with DHPO in both the ob/ob and high-fat fed mice. Ob/ob mice used in this study may not be an ideal model to study HDL owing to the fact that the basal HDL-cholesterol levels are significantly higher in these animals compared to the lean controls [32]. It is however interesting that high-fat feeding did not show a significant reduction of HDL in our experiments. It is likely that the short duration of feeding may be the reason for this lack of change in serum HDL levels. Further extensive studies, with long duration feeding of high-fat diet will be needed to understand the effects of DHPO on HDL levels and to reveal the critical mediators of the lipid-lowering effect of DHPO via AMPK.
Although DHPO altered AMPK pathway in the peripheral tissues, treatment with DHPO failed to change insulin signaling pathway or AMPK signaling pathway in the liver -the phosphorylation status of AMPK, ACC and IRβ remained unchanged following DHPO treatment in the hepatic tissues of obese and lean mice. This suggests that DHPO may not be affecting insulin/AMPK signaling in the liver and may be mediating its effect on glucose uptake by acting predominantly in the periphery. A discrepancy however is the IRS-1 phosphorylation in the liver tissue which was significantly lowered by DHPO in lean mice compared to vehicle treatment. This attenuation of IRS-1 phosphorylation did not alter the phosphorylation status of its downstream effector Akt and therefore the significance of the reduced IRS-1 phosphorylation in response to DHPO-treatment is difficult to explain.
In summary, these results suggest that DHPO may be used as a template to develop newer, more potent and selective pharmacological agents to treat or control insulin resistance observed with obesity and type 2 diabetes. It also supports the view that activation of AMPK may represent a potential therapeutic option in treatment of diabetes and its complications.
Supplementary Material
Online Figure 1. Chemical structure of DHPO [2-(3, 4-dihydro-2H-pyrrolium-1-yl)-3oxoindan-1-olate]
Online Figure 2. Effect of DHPO on serum lipids in high-fat-diet-fed mice. A. triglyceride B. cholesterol C. serum HDL D. serum LDL. Data are represented as mean ± SEM, and *p<0.05 versus standard chow, #p<0.05 versus high-fat diet.
Online Figure 3. Effect of DHPO on AMPK kinase activity. Data are represented as mean ± SEM of relative fluorescence. Open bar is the blank (no enzyme, no DHPO); solid bar is enzyme alone and shaded bars are enzyme in the presence of DHPO.
Acknowledgments
This work was supported by grants from ADA (AMDIAB47595), NCRR and the Wyoming INBRE (P20RR016474). Parts of this work were presented at the American Diabetic Association, 69th scientific sessions, New Orleans, LA, USA, 2009.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplemental data
Supplemental data associated with this article can be found in the online version.
References
- 1.DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88:787–835. doi: 10.1016/j.mcna.2004.04.013. ix. [DOI] [PubMed] [Google Scholar]
- 2.Karlsson HK, Ahlsen M, Zierath JR, Wallberg-Henriksson H, Koistinen HA. Insulin signaling and glucose transport in skeletal muscle from first-degree relatives of type 2 diabetic patients. Diabetes. 2006;55:1283–1288. doi: 10.2337/db05-0853. [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo RA, Ferrannini E, Simonson DC. Fasting hyperglycemia in non-insulin-dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism. 1989;38:387–395. doi: 10.1016/0026-0495(89)90129-7. [DOI] [PubMed] [Google Scholar]
- 4.Storgaard H, Song XM, Jensen CB, Madsbad S, Bjornholm M, Vaag A, Zierath JR. Insulin signal transduction in skeletal muscle from glucose-intolerant relatives of type 2 diabetic patients [corrected] Diabetes. 2001;50:2770–2778. doi: 10.2337/diabetes.50.12.2770. [DOI] [PubMed] [Google Scholar]
- 5.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prabhakar KR, et al. Identification and evaluation of antioxidant, analgesic/anti-inflammatory activity of the most active ninhydrin-phenol adducts synthesized. Bioorg Med Chem. 2006;14:7113–7120. doi: 10.1016/j.bmc.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 7.Sreejayan N, Dong F, Kandadi MR, Yang X, Ren J. Chromium alleviates glucose intolerance, insulin resistance, and hepatic ER stress in obese mice. Obesity (Silver Spring) 2008;16:1331–1337. doi: 10.1038/oby.2008.217. [DOI] [PubMed] [Google Scholar]
- 8.Nedachi T, Kanzaki M. Regulation of glucose transporters by insulin and extracellular glucose in C2C12 myotubes. Am J Physiol Endocrinol Metab. 2006;291:E817–E828. doi: 10.1152/ajpendo.00194.2006. [DOI] [PubMed] [Google Scholar]
- 9.Dong F, Kandadi MR, Ren J, Sreejayan N. Chromium (D-phenylalanine) 3 supplementation alters glucose disposal, insulin signaling, and glucose transporter-4 membrane translocation in insulin-resistant mice. J Nutr. 2008;138:1846–1851. doi: 10.1093/jn/138.10.1846. [DOI] [PubMed] [Google Scholar]
- 10.Kurowski TG, Lin Y, Luo Z, Tsichlis PN, Buse MG, Heydrick SJ, Ruderman NB. Hyperglycemia inhibits insulin activation of Akt/protein kinase B but not phosphatidylinositol 3-kinase in rat skeletal muscle. Diabetes. 1999;48:658–663. doi: 10.2337/diabetes.48.3.658. [DOI] [PubMed] [Google Scholar]
- 11.Miller EJ, Li J, Leng L, McDonald C, Atsumi T, Bucala R, Young LH. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature. 2008;451:578–582. doi: 10.1038/nature06504. [DOI] [PubMed] [Google Scholar]
- 12.Rosen OM. Banting lecture 1989. Structure and function of insulin receptors. Diabetes. 1989;38:1508–1511. doi: 10.2337/diab.38.12.1508. [DOI] [PubMed] [Google Scholar]
- 13.Hubbard SR. Crystal structure of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog. Embo J. 1997;16:5572–5581. doi: 10.1093/emboj/16.18.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilder TL, Baer LA, Fuller PM, Fuller CA, Grindeland RE, Wade CE, Graves LM. Insulin-independent pathways mediating glucose uptake in hindlimb-suspended skeletal muscle. J Appl Physiol. 2005;99:2181–2188. doi: 10.1152/japplphysiol.00743.2005. [DOI] [PubMed] [Google Scholar]
- 15.Lage R, Dieguez C, Vidal-Puig A, Lopez M. AMPK: a metabolic gauge regulating whole-body energy homeostasis. Trends Mol Med. 2008;14:539–549. doi: 10.1016/j.molmed.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Viana AY, et al. Role of hepatic AMPK activation in glucose metabolism and dexamethasone-induced regulation of AMPK expression. Diabetes Res Clin Pract. 2006;73:135–142. doi: 10.1016/j.diabres.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Holmes BF, Kurth-Kraczek EJ, Winder WW. Chronic activation of 5'-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J Appl Physiol. 1999;87:1990–1995. doi: 10.1152/jappl.1999.87.5.1990. [DOI] [PubMed] [Google Scholar]
- 19.Fryer LG, Parbu-Patel A, Carling D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 20.Iglesias MA, Ye JM, Frangioudakis G, Saha AK, Tomas E, Ruderman NB, Cooney GJ, Kraegen EW. AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat-fed rats. Diabetes. 2002;51:2886–2894. doi: 10.2337/diabetes.51.10.2886. [DOI] [PubMed] [Google Scholar]
- 21.Cool B, et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403–416. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Breen DM, Sanli T, Giacca A, Tsiani E. Stimulation of muscle cell glucose uptake by resveratrol through sirtuins and AMPK. Biochem Biophys Res Commun. 2008;374:117–122. doi: 10.1016/j.bbrc.2008.06.104. [DOI] [PubMed] [Google Scholar]
- 23.Park CE, Kim MJ, Lee JH, Min BI, Bae H, Choe W, Kim SS, Ha J. Resveratrol stimulates glucose transport in C2C12 myotubes by activating AMP-activated protein kinase. Exp Mol Med. 2007;39:222–229. doi: 10.1038/emm.2007.25. [DOI] [PubMed] [Google Scholar]
- 24.Lee ES, et al. CAPE (caffeic acid phenethyl ester) stimulates glucose uptake through AMPK (AMP-activated protein kinase) activation in skeletal muscle cells. Biochem Biophys Res Commun. 2007;361:854–858. doi: 10.1016/j.bbrc.2007.07.068. [DOI] [PubMed] [Google Scholar]
- 25.Sanders MJ, Ali ZS, Hegarty BD, Heath R, Snowden MA, Carling D. Defining the mechanism of activation of AMP-activated protein kinase by the small molecule A-769662, a member of the thienopyridone family. J Biol Chem. 2007;282:32539–32548. doi: 10.1074/jbc.M706543200. [DOI] [PubMed] [Google Scholar]
- 26.Pang T, et al. Small molecule antagonizes autoinhibition and activates AMP-activated protein kinase in cells. J Biol Chem. 2008;283:16051–16060. doi: 10.1074/jbc.M710114200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 28.Hardie DG, Carling D. The AMP-activated protein kinase--fuel gauge of the mammalian cell? Eur J Biochem. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- 29.Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- 30.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 31.Zang M, et al. AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J Biol Chem. 2004;279:47898–47905. doi: 10.1074/jbc.M408149200. [DOI] [PubMed] [Google Scholar]
- 32.Yang X, Li SY, Dong F, Ren J, Sreejayan N. Insulin-sensitizing and cholesterol-lowering effects of chromium (D-Phenylalanine)3. J Inorg Biochem. 2006;100:1187–1193. doi: 10.1016/j.jinorgbio.2006.01.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Figure 1. Chemical structure of DHPO [2-(3, 4-dihydro-2H-pyrrolium-1-yl)-3oxoindan-1-olate]
Online Figure 2. Effect of DHPO on serum lipids in high-fat-diet-fed mice. A. triglyceride B. cholesterol C. serum HDL D. serum LDL. Data are represented as mean ± SEM, and *p<0.05 versus standard chow, #p<0.05 versus high-fat diet.
Online Figure 3. Effect of DHPO on AMPK kinase activity. Data are represented as mean ± SEM of relative fluorescence. Open bar is the blank (no enzyme, no DHPO); solid bar is enzyme alone and shaded bars are enzyme in the presence of DHPO.