Abstract
Background
Clinical observation and research findings show that acute traumatic coagulopathy (ATC) is a major factor that must be addressed in the care of severely injured patients. In this review article, we discuss the incidence and causes of ATC, the potential means of early risk stratification for it, and recommendations for its treatment.
Methods
We selectively reviewed the pertinent literature and retrospectively analyzed data from the Trauma Registry of the German Trauma Society (Traumaregister der Deutschen Gesellschaft für Unfallchirurgie, TR-DGU) relating to the incidence, causes, and outcome of ATC. We provide an overview of current treatment recommendations, supplemented by our own findings regarding the ratio of packed red blood cell concentrate (pRBC) to fresh-frozen plasma (FFP) transfusion and regarding coagulation-factor-based treatments for coagulopathy in the acute phase after trauma.
Results and conclusion
ATC, a condition associated with increased morbidity and mortality, is seen on admission in one out of four patients with major trauma. The main causes of ATC are tissue damage, hypoperfusion, hemodilution, hypothermia, acidosis, and inflammation. It may be possible to identify patients at risk for ATC early on through the use of rapidly calculable, predictive numerical scales (McLaughlinScore, TASH, and ABC), laboratory tests, and imaging studies (FAST and CT). Acute treatment is focused on the control of bleeding and support of the coagulation system according to the current guidelines. Patients at high risk may benefit from a balanced transfusion strategy. Innovative strategies currently under study include point-of-care-guided treatment and coagulation-factor-concentrate-based treatment.
Ten percent of deaths worldwide are caused by trauma (1). In Europe alone, almost a million people each year die as the result of an injury (1). Apart from head injury, the most frequent cause of death in the acute post-traumatic period is uncontrolled hemorrhage. Of the patients who die in the acute phase after trauma, 30 to 40% bleed to death (2).
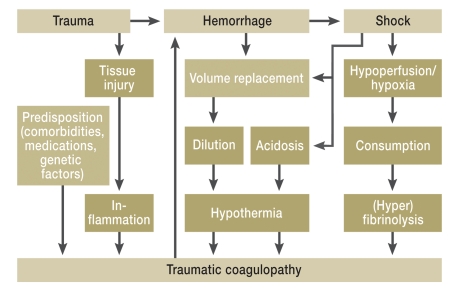
Clinical observation and recent research findings underline the key part played by acute traumatic coagulopathy in the care of severely injured patients. Acute traumatic coagulopathy is now viewed as an independent entity and as a problem regularly encountered immediately after trauma (3). Multiple factors combine to cause acute traumatic coagulopathy. Six factors have been proposed as “drivers”: tissue damage/-trauma, hypoperfusion, hemodilution, hypothermia, acidosis, and inflammation (3, 4) (Figure 1). Above all, the combination of hypotension, acidosis, and hypothermia results in a vicious circle that leads to exacerbation of the coagulopathy (5). Mortality from acute traumatic coagulopathy can be lowered by early detection and aggressive management (6, 7). This requires timely risk assessment and standardized treatment protocols.
Figure 1.
Possible mechanisms of acute traumatic coagulopathy.
Besides dilution coagulopathy, hemorrhage induces shock, followed by acidosis and hypothermia, and this “lethal triad” reinforces the coagulopathy. Moreover, the traumatic shock leads to hypoperfusion and hypoxia and further aggravates the coagulopathy via consumption of coagulation materials and hyperfibrinolysis. The role of inflammation in the development of acute traumatic coagulopathy has not yet been completely clarified (modified from [3]). In the summarized TR-DGU data, acute traumatic coagulopathy was independently associated with severity of injury (ISS), prehospital volume replacement, body temperature ≤35°C, and shock status (4).
Method
This article is based on a selective review of the recent literature, together with retrospective analysis of data on severely injured patients from the Trauma Registry of the German Trauma Society (Traumaregister der Deutschen Gesellschaft für Unfallchirurgie, TR-DGU).
Pertinent publications were retrieved from the Medline (PubMed) and Cochrane databases using various combinations of relevant search terms (e.g., “bleeding/hemorrhage,” “coagulopathy,” “management,” “mortality,” “outcome,” “transfusion,” and “trauma”). The search was restricted to the past 10 years due to the topicality of the subject. We also considered the current, recently revised and published European guidelines.
Clinical incidence
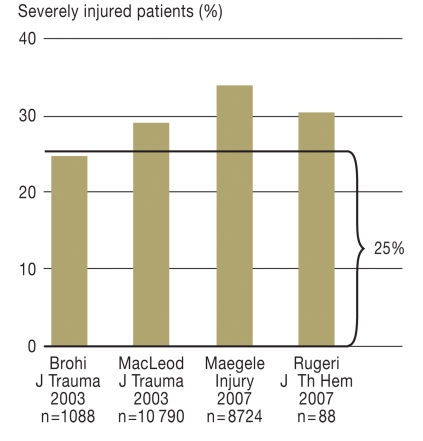
On average, every fourth severely injured patient already has an acute trauma-related coagulation disorder at the time of admission to the shock room (8– 10, 12) (Figure 2). Depending on definition, rates of up to 60% have been reported (11). In our retrospective study of 8724 sets of data from the TR-DGU, the rate was 34.2% (12). For the purposes of that investigation, coagulopathy was defined in terms of clinical signs of active hemorrhage (visible bleeding, pulse ↑ and/or blood pressure ↓ see “ACS-ATLS classification of blood loss based on initial patient presentation” [13]), together with Quick score <70% and/or thrombocyte count <100 000/µL. The coagulation disorder begins as soon as the injury occurs (11) and increases with the severity of injury (8, 12). In our investigation, 84% of the patients with coagulopathy had an Injury Severity Score (ISS) of ≥ 16 at the time of admission (12). The ISS is a classification system for estimating the overall severity of trauma suffered on the basis of the threat to survival posed by the individual injuries.
Figure 2.
Incidence of acute traumatic coagulopathy in four large studies (8–10, 12). On average, every fourth severely injured patient arrives at the shock room with an acute traumatic disorder of the coagulation system
Increasing incidence with greater prehospital volume replacement
According to TR-DGU data, the frequency and extent of acute traumatic coagulopathy are associated with the amount of volume replacement during prehospital care. Coagulopathy was observed in over 40% of severely injured patients who received 2 liters of fluid, in over 50% of those who received 3 liters, and in over 70% of those who received 4 liters (12). In a recent report, Wafaisade et al. (4) identified prehospital fluid replacement of ≥3000 mL and a colloid/crystalloid ratio of ≥1:2 in the early phase of volume replacement as independent risk factors for early coagulopathy following trauma.
Coagulopathy combined with shock
A central role in the emergence of acute traumatic coagulopathy is now thought to be played by hypotension and the resulting tissue hypoperfusion (3, 14). Base excess (BE) and serum lactate are employed as parameters for hypoperfusion in the case of a shock event and for the extent of bleeding after severe injury (evidence level 1B) (13). In one study, for example, 2% of severely injured patients with BE more than 6 mEq/L had coagulopathy, compared with 20% of those with BE less than 6 mEq/L (14). Niles et al. (15) reported that only patients with a markedly lowered BE showed a higher degree of coagulopathy with increasing severity of injury. The TR-DGU data confirm increasing severity of acute coagulopathy with decreasing BE. Forty-one percent of patients with elevated lactate values (>2.2 mmol/L) had coagulopathy, versus 25% of those with normal lactate levels.
Mortality and morbidity
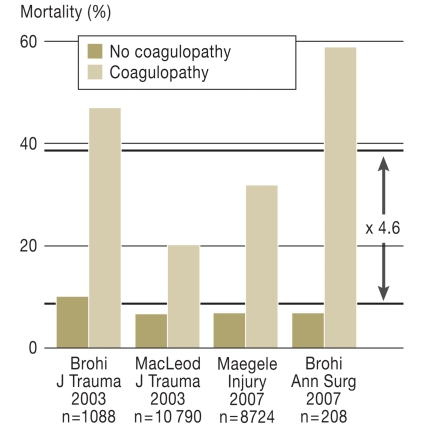
Macleod et al. (9) described acute coagulopathy as an independent risk factor for mortality after severe trauma. Our group pointed out at an early stage the prognostic value of the Quick test with regard to survival after trauma. Particularly in the early phase (<48 h), uncontrollable hemorrhage is the second most common cause of death after the consequences of head injury (2). Figure 3 shows the mortality rates of trauma patients with and without coagulopathy at the time of admission to the shock room from four observational studies. In stark contrast to patients without coagulopathy, the relative risk (RR) of death is 4.6 times higher in severely injured patients with acute coagulopathy, and their absolute risk of death is 37.7%. Surviving patients with early coagulopathy subsequently develop multiple organ failure almost 3 times as often as those without coagulopathy (30% vs. 12%) (12).
Figure 3.
The relative risk (RR) of death is 4.6 times higher in patients who have an acute traumatic disorder of the coagulation system at the time of admission to the shock room (8– 10, 12).
Early detection
The basic precondition for adequate management of a bleeding or coagulation problem in the acute phase after trauma is timely recognition that a problem exists (13). The usual laboratory parameters (Quick/PT/INR, aPTT, fibrinogen, and thrombocytes) are employed to this end, with the addition of thrombelastometry to characterize the coagulopathy and guide treatment (evidence level 2C) (13). It is problematic, however, that the conventional global parameters are often not available until 30 to 40 minutes after admission to the shock room and are sometimes hard to interpret. The amount of bleeding is estimated from the mechanism of injury, the current physiological status, the anatomical damage, and the patient’s response to initial volume replacement (1C) (13). In the case of injury to the torso, free intra-abdominal fluid is demonstrated by diagnostic imaging (FAST and/or CT), depending on hemodynamic stability (1B) (13).
Predictive scoring systems for early detection and risk stratification
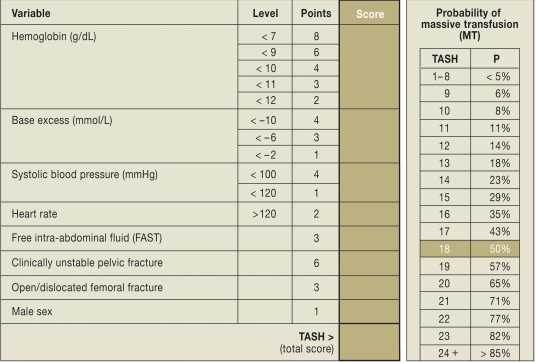
Predictive scoring systems are valuable for early identification of risk patients with bleeding and coagulation problems (16– 18). These models were developed using military (16) and civilian (17, 18) databases. Based on >6000 sets of data from the TR-DGU, our own group presented the Trauma Associated Severe Hemorrhage (TASH) score (17). This system uses the probability of massive transfusion as surrogate for a persisting bleeding problem (Figure 4). The variables that are taken into account refer to the pattern of injury and to the physiology, and were extracted from the TR-DGU with the aid of uni- and multivariate regression analyses. Early and individual risk stratification on the basis of increasing scores can help to place the acute coagulopathy in the focus of treatment, initiate aggressive management of coagulopathy according to the recommendations described below, activate blood bank resources in good time, and, if treatment cannot be continued locally, organize transfer to a trauma center.
Figure 4.
Trauma Associated Severe Hemorrhage (TASH) score (from [17]). All the information needed for calculation of the TASH score can be obtained within 15 minutes of the patient’s arrival at the shock room.
Nunez et al. (18) recently compared the performance of the three best-known prediction models (McLaughlin score [16], TASH score [17], and ABC score [18]) on their local trauma data and found that they all possessed comparable good predictive power with regard to massive transfusion (area under ROC [AUROC] as measure of test quality: McLaughlin 0.846; TASH 0.842; ABC: 0.842). This study represented the first external validation of the TASH score, which had previously only been validated internally (AUROC 0.905).It should be mentioned, however, that the TASH score was developed in patients with blunt trauma (>90%) and is merely a surrogate for persisting bleeding, not a direct indicator. Furthermore, the TASH score was developed retrospectively, so it is unclear whether the transfusions noted in the patients’ records represented proactive or reactive interventions.
Current recommendations
Various recommendations for the control of acute traumatic hemorrhage have been published in recent years. The European consensus guidelines are currently being revised (13). Operative interventions for acute control of bleeding include:
Stabilization/closure of the pelvic girdle (in the acute phase, this may be achieved temporarily by use of a pelvic binder or clamp) (1B)
Intra-abdominal surgical hemostasis with abdominal packing and vascular embolization (1B/C)
Damage control measures (e.g., for early repositioning and fracture stabilization of long tubular bones and control of internal organ injury/hemorrhage) (1C)
Local hemostasis combined with other surgical procedures or packing in the case of venous or moderate arterial hemorrhages with parenchymal injury (1B)
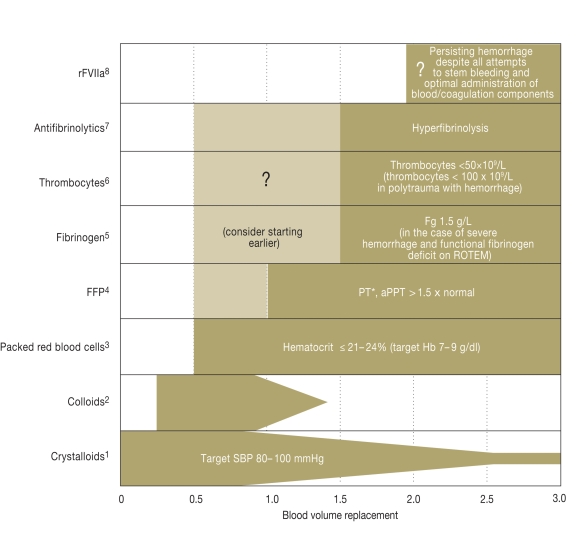
The time between the injury and whatever operation is necessary should be kept short (1A). Patients with demonstrated significant intra-abdominal fluid collection and hemodynamic instability should undergo immediate emergency intervention (1A). Tourniquets can be used to control open, profusely bleeding injuries of the extremities (1C). The current recommendations for acute support of coagulatory function are outlined in Figure 5. The coagulatory support measures should be initiated as early as possible and monitored (1C).
Figure 5.
Current recommendations (according to [13]) for support of coagulation in the early phase after trauma.
Additional measures include normothermia (evidence level 1C), monitoring of the ionized Ca2+ level during massive transfusion (1C), and possibly substitution if levels are low or if there are ECG changes (2C).
*1Target blood pressure 80 to 100 mmHg until achievement of hemostasis in the initial phase after trauma without head injury (1C) / crystalloids as initial treatment for hemorrhaging patients (1B) / hypertonic solutions may also be considered in the initial phase of treatment (2B);
*2Administration within the recommended limits for each individual substance in the case of hemodynamic instability (2C);
*3Administration of erythrocyte concentrates with a target Hb of 7 to 9 g/dL (1C);
*4Early administration of frozen fresh plasma concentrates in patients with massive transfusions, initially at 10 to 15 mL/kg, then according to need (1C);
*5Administration of fibrinogen in the case of severe hemorrhage and simultaneous signs of functional fibrinogen deficit on thrombelastometry (ROTEM) or fibrinogen levels ≤1.5 g/L, initial fibrinogen dose 3 to 4 g, then monitoring by thrombelastometry and fibrinogen level (2C);
*6Administration of thrombocyte concentrates to keep thrombocyte levels above 50×109/L (1C) or above 100×109/L in the case of multiple injury with severe hemorrhage and head injury (2C), initial administration of four to eight bags of thrombocyte concentrate and one apheresis pack (2C);
*7Administration of antifibrinolytics can be considered in bleeding trauma patients (2C; see also CRASH-2 study [20]), monitoring of fibrinolysis by thrombelastometry recommended in all patients, in the case of hyperfibrinolysis administration of antifibrinolytics (1B), initially tranexamic acid 10 to 15 mg/kg followed by infusion 1 to 5 mg/kg/h or -aminocapronic acid 100 to 150 mg/kg followed by 15 mg/kg/h until bleeding is controlled (2C);
*8rFVIIa can be considered if hemorrhage persists despite all efforts to control it and optimal use of blood/coagulation components (2C), before administration of rFVIIa: thrombocytes >50×109/L, fibrinogen >1g/L, hematocrit >24, and pH >7.2
Results of CONTROL and CRASH-2
The results of the two largest randomized treatment studies on traumatic hemorrhage to date were published in 2010. While the CONTROL study on the use of recombinant factor VIIa (rFVIIa) as adjunct to standard therapy in refractory trauma patients with active bleeding was discontinued after preliminary analysis showed no advantage for patients in the verum arm (19), the CRASH-2 study (“Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage 2”) showed a benefit for the early administration of the antifibrinolytic agent tranexamic acid (20). Altogether, CRASH-2 embraced 20 211 patients at 274 centers in 40 countries (Germany was not one of them), all of whom had severe blood loss (SBP <90 mmHg and/or heart rate >110/min) or were at risk of severe hemorrhage. The patients received either an early infusion (within 8 h of trauma) containing tranexamic acid (bolus 1 g in 10 min, followed by infusion of 1 g over 8 h) or placebo. Tranexamic acid significantly reduced overall mortality; compared with placebo, the relative risk of death was reduced by 10%. The relative risk of bleeding to death was lowered by 15%. The frequency of transfusions and operations was comparable in the two groups. No undesired/serious side effects were observed, and the incidence of non-fatal vascular occlusions was comparable. Although many critics of CRASH-2 have drawn attention to what they see as methodological limitations, it is a broad-based study that has described a therapeutic benefit for a demonstrably low-risk and above all low-cost drug.
Administration of blood products
The experience of the US armed forces has shown that a more balanced transfusion ratio of packed red blood cell concentrates (pRBC) and the early administration of frozen fresh plasma concentrates (FFP) is associated with reduced mortality in severely injured patients with massive transfusion (6). While some investigations have meanwhile shown a survival benefit for the direct transfusion of FFP and pRBC in the ratio 1:1, other studies point to a critical ratio between 1:2 and 1:3 (21). The results of a large multicenter study have now underlined the importance of early administration of FFP and pRBC in high ratios with regard to survival and total pRBC use in patients receiving massive transfusions (22). In contrast, one of the few prospective studies on this topic showed no advantage of early, aggressive FFP administration, so overall the data are inconclusive (23). Criticism of the studies published to date concerns first their mostly retrospective nature and second the period of time over which the blood products were given. Particularly, it has been speculated whether the survival benefit observed for 1:1 administration of FFP and pRBC might not simply reflect the fact that the 1:1 patients survived longer, or long enough to receive transfusions in this ratio (survival bias).
In a retrospective study covering 713 sets of data from the TR-DGU, we investigated whether a strategy of early, aggressive transfusion with pRBC and FFP administered in a ratio of 1:1 between shock room and intensive care unit was associated with a survival benefit (7). The patients were divided into three groups:
Group 1: pRBC:FFP >1.1
Group 2: pRBC:FFP = 0.9 to 1.1
Group 3: pRBC:FFP <0.9
There were no differences among the groups regarding severity of injury, physiology, laboratory findings, type and intensity of interventions in the shock room, or volume replacement. The mean time that elapsed between admission to the shock room and transfer to the intensive care unit was 4.5 h across all groups. Acute mortality (<24 h) and 30-day mortality were lower in patients with an pRBC:FFP ratio of 0.9 to 1.1 (1:1) than in the group with a ratio >1.1, and lowest of all in the group with an pRBC:FFP ratio <0.9, where the mortality rates were 11.3% and 24.3% respectively. Mortality for the first 6 h after admission was 24.6% in group 1, 9.6% in group 2, and 3.5% in group 3 (p<0.0001). The incidence of septic complications and organ failure was higher for patients with pRBC:FFP ratios of 0.9 to 1.1 than for those with ratios <0.9. The duration of ventilation, length of stay in intensive care, and total length of stay in hospital were longest in patients with an pRBC:FFP transfusion ratio <0.9.
The results of our study suggest that possibly not all severely injured patients benefit to the same degree from this transfusion regimen. On the other hand, it could be argued that the reduction in mortality compared to the “control group” may result from survival of patients whose initial prognosis, based on the trauma they suffered, was less favorable than that of the survivors of the “control group”. Prospective studies remain necessary to establish the optimal ratio of blood products and the ideal timing of their administration (currently, for example, the PROPPR [Prospective Randomized Optimum Platelet and Plasma Ratios] study investigating different blood product ratios for trauma patients at risk of massive transfusion).
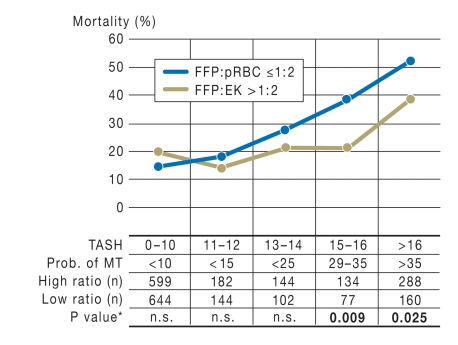
Survival advantage for 1:1 transfusions at TASH scores ≥15
Administration of high amounts of FFP can lead to volume overloading, infectious diseases, allergic reactions, and transfusion-related acute lung injury (TRALI). In a further step, the attempt was made to use the TASH score to identify specific patient populations who would benefit from aggressive coagulation management with 1:1 transfusion and to demarcate these patients from those more likely to be harmed by 1:1 transfusion. The severely injured patients with massive transfusion were retrospectively divided into those with FFP:pRBC ratios >1:2 (the median FFP:pRBC was 1:1) and those with FFP:pRBC ratios ≤1:2, The TASH score was calculated for each individual patient and plotted against mortality. Patients with TASH scores ≥15 were significantly more likely to survive if they received FFP and pRBC in a ratio >1:2 (Figure 6). A TASH value of 15 was taken as cut-off and patients with a score of ≥15 were defined as high-risk patients. The overall hospital mortality was 34.8% for patients with FFP:pRBC ratios >1:2, versus 47.7% for those with ratios ≤1:2. For low-risk patients (TASH score <15) mortality was comparable in the two transfusion groups. Morbidity was higher, however, in patients with FFP:pRBC ratios >1:2; the length of stay, both in the intensive care unit and overall, and the incidence of multiple organ failure were higher and more days of ventilation were documented than for patients with FFP:pRBC ratios ≤1:2. The risk stratification by TASH score showed that particularly high-risk patients with a TASH score ≥15 can benefit from transfusion of FFP and pRBC in a ratio >1:2.
Figure 6.
Hospital mortality by TASH risk group for patients receiving transfusions with FFP and pRBC in high ratios (>1:2) and low ratios (≤1:2). This retrospective study showed an absolute reduction of 17% in hospital mortality in the high ratio group (FFP:pRBC >1:2). Prob. of MT, Probability of massive transfusion
Factor-concentrate-based management of coagulation
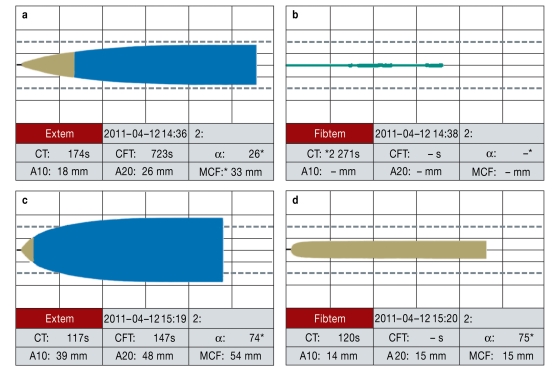
An alternative concept is based on the targeted substitution of coagulation factor concentrates via point-of-care systems (24). Factor deficiencies can be detected and treated with the aid of rotational thrombelastometry (ROTEM), so that in some cases there is no need for fresh plasma (Figure 7). In contrast to FFP, coagulation factor concentrates are immediately available. In a patient with dilution coagulopathy, fibrinogen, before all other coagulation factors and thrombocytes, reaches a critical level that indicates substitution is required. Retrospectively, Schoechl et al. (24) showed that ROTEM-guided coagulation therapy and factor substitution with fibrinogen concentrate as first-line treatment, backed up by prothrombin concentrate (factors II, VII, IX, and X, proteins C and S), reduced mortality below the prognosticated rate. However, they did not take account of confounding variables. A recent retrospective matched-pairs analysis of data sets from the TR-DGU with strict inclusion criteria compared severely injured and bleeding patients who were treated either according to the 1:1 concept or the ROTEM-guided factor concentrate approach. Owing to the low case numbers no survival benefit was shown for either treatment, but factor-concentrate-based management was associated with much lower usage of allogeneic blood products, a lower complication rate, and a shorter stay in the intensive care unit (25). These findings require confirmation by controlled prospective studies.
Figure 7.
Example of point-of-care-guided and factor-concentrate-based coagulation management in a hemorrhaging polytraumatized patient. Thrombelastometry (ROTEM) analysis (a, c: EXTEM; b, d: FIBTEM) before (a, b) and after (c, d) administration of 10 g fibrinogen concentrate, 1800 units of prothrombin concentrate (factors II, VII, IX, and X, proteins C and S), and 2 g tranexamic acid. After this treatment there was swift pronounced shortening of coagulation time (EXTEM clotting time [CT] 117 s versus 174 s; FIBTEM CT 120 s versus not measurable and also significantly improved clot firmness (EXTEM MCF [Maximum Clot Firmness] 54 mm versus 33 mm; FIBSTEM MCF 15 mm versus not measurable). EXTEM measures activation of coagulation by thromboplastin (tissue factor) with factors VII, X, V, and I, thrombocytes, and fibrinolysis. FIBTEM records the activation as for EXTEM with the addition of the thrombocyte blocker cytochalasin. The resultant clot is thus formed purely by fibrin formation and fibrin polymerization; the fibrin level and the degree of fibrin polymerization can therefore be evaluated. (Reproduced by kind permission of H. Schoechl, Salzburg)
Key Messages.
Every fourth severely injured patient arrives at the shock room with an acute traumatic disorder of the coagulation system, associated with increased morbidity and mortality.
Tissue damage, hypoperfusion, hemodilution, hypothermia, acidosis, and inflammation have been proposed as initiators of acute traumatic coagulopathy.
Alongside the conventional laboratory parameters, thrombelastometry to determine the type of coagulopathy and guide treatment, and imaging procedures (FAST and CT), scoring systems aid early detection and risk stratification.
The essential treatment principles are embodied in the recently revised European guidelines. High-risk patients may benefit from aggressive coagulation management with balanced transfusion of FFP and pRBC in a 1:1 ratio.
A new concept for the control of acute traumatic coagulopathy, currently under evaluation, is the more targeted substitution of coagulation factor concentrates via point-of-care systems.
Acknowledgments
Translated from the original German by David Roseveare.
Footnotes
Conflict of interest statement
Prof. Maegele has received reimbursement of expenses from Pfizer, CSL Behring, and Novo Nordisk; remuneration for lecturing from Roche Pharma and for conducted commissioned clinical studies from Novo Nordisk and Pfizer; and funding from Novo Nordisk, Siemens, and Pentapharm.
Prof. Bouillon has received remuneration for consulting from Novo Nordisk, De Puy Trauma, Biomet, and Behring.
Dr. Paffrath has received remuneration for consulting from EKK eG and for lecturing from De Puy Trauma, J&J Ethicon, and ATLS.
References
- 1.Peden M, McGee K, Sharma G. Geneva: World Health Organization; 2002. The injury chart book: A graphical overview of the global burden of injuries. [Google Scholar]
- 2.Evans JA, van Wessem KJ, McDougall D, et al. Epidemiology of traumatic deaths: comprehensive population-based assessment. World J Surg. 2010;34:158–163. doi: 10.1007/s00268-009-0266-1. [DOI] [PubMed] [Google Scholar]
- 3.Hess J, Brohi K, Dutton R, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65:748–754. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 4.Wafaisade A, Wutzler S, Lefering R, et al. Drivers of acute coagulopathy after severe trauma: a multivariate analysis of 1987 patients. Emerg Med J. 2010;27:934–939. doi: 10.1136/emj.2009.088484. [DOI] [PubMed] [Google Scholar]
- 5.Spahn D, Rossaint R. Coagulopathy and blood component transfusion in trauma. Br J Anaesth. 2005;95:130–139. doi: 10.1093/bja/aei169. [DOI] [PubMed] [Google Scholar]
- 6.Borgman M, Spinella P, Perkins J, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 7.Maegele M, Lefering R, Paffrath T, et al. Red-blood-cell to plasma ratios transfused during massive transfusion are associated with mortality in severe multiple injury: a retrospective analysis from the Trauma Registry of the Deutsche Gesellschaft fur Unfallchirurgie. Vox Sang. 2008;95:112–119. doi: 10.1111/j.1423-0410.2008.01074.x. [DOI] [PubMed] [Google Scholar]
- 8.Brohi K, Singh J, Heron M, et al. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 9.MacLeod J, Lynn M, McKenney M, et al. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55:39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 10.Rugeri L, Levrat A, David J, et al. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J Thromb Haemost. 2007;5:289–295. doi: 10.1111/j.1538-7836.2007.02319.x. [DOI] [PubMed] [Google Scholar]
- 11.Floccard B, Rugerib L, Faurea A, et al. Early coagulopathy in trauma patients: an on-scene and hospital admission study. Injury. 2010 doi: 10.1016/j.injury.2010.11.003. doi:10.1016/j.injury.2010.11.003 (in press) [DOI] [PubMed] [Google Scholar]
- 12.Maegele M, Lefering R, Yucel N, et al. Early coagulopathy in multiple injury: an analysis from the German Trauma Registry on 8724 patients. Injury. 2007;38:298–304. doi: 10.1016/j.injury.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Rossaint R, Bouillon B, Cerny V, et al. Management of bleeding following major trauma: an updated European guideline. Crit Care. 2010;14 doi: 10.1186/cc8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brohi K, Cohen M, Davenport R. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care. 2007;13:680–685. doi: 10.1097/MCC.0b013e3282f1e78f. [DOI] [PubMed] [Google Scholar]
- 15.Niles S, McLaughlin D, Perkins J, et al. Increased mortality associated with the early coagulopathy of trauma in combat casualties. J Trauma. 2008;64:1459–1463. doi: 10.1097/TA.0b013e318174e8bc. [DOI] [PubMed] [Google Scholar]
- 16.McLaughlin DF, Niles S, Salinas J, et al. A predictive model for massive transfusion in combat casualty patients. J Trauma. 2008 64;(Suppl 2):57–63. doi: 10.1097/TA.0b013e318160a566. [DOI] [PubMed] [Google Scholar]
- 17.Maegele M, Lefering R, Wafaisade A, et al. Revalidation and update of the TASH-Score: a scoring system to predict the probability for massive transfusion as a surrogate for life-threatening haemorrhage after severe injury. Vox Sang. 2010;100(2):231–238. doi: 10.1111/j.1423-0410.2010.01387.x. [DOI] [PubMed] [Google Scholar]
- 18.Nunez TC, Voskresensky IV, Dossett LA, et al. Early prediction of massive transfusion in trauma: Simple as ABC (Assessment of blood consumption)? J Trauma. 2009;66:346–352. doi: 10.1097/TA.0b013e3181961c35. [DOI] [PubMed] [Google Scholar]
- 19.Hauser C, Boffard K, Dutton R, et al. Results of the CONTROL trial: efficacy and safety of recombinant activated Factor VII in the management of refractory traumatic hemorrhage. J Trauma. 2010;69:489–500. doi: 10.1097/TA.0b013e3181edf36e. [DOI] [PubMed] [Google Scholar]
- 20.Shakur H, Roberts I, Bautista R, et al. CRASH-2 trial collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376:23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 21.Spinella P, Holcomb J. Resuscitation and transfusion principles for traumatic hemorrhagic shock. Blood Rev. 2009;23:231–240. doi: 10.1016/j.blre.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zink KA, Sambasivan CN, Holcomb JB, et al. A high ratio of plasma and platelets to packed red blood cells in the first 6 hours of massive transfusion improves outcomes in a large multicenter study. Am J Surg. 2009;197:565–570. doi: 10.1016/j.amjsurg.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Scalea T, Bochicchio K, Lumpkins K, et al. Early aggressive use of fresh frozen plasma does not improve outcome in critically injured trauma patients. Ann Surg. 2008;248:578–584. doi: 10.1097/SLA.0b013e31818990ed. [DOI] [PubMed] [Google Scholar]
- 24.Schoechl H, Nienaber U, Hofer G, et al. Goal-directed coagulation management of major trauma patients using rotation thromboelastometry (ROTEM)-guided administration of fibrinogen and prothrombin complex concentrate. Crit Care. 2010;14 doi: 10.1186/cc8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nienaber U, Innerhofer P, Westernmann I, et al. The impact of fresh frozen plasma versus coagulation factor concentrates on morbidity and mortality in trauma-associated hemorrhage and massive transfusion. Injury. 2011;42:697–701. doi: 10.1016/j.injury.2010.12.015. [DOI] [PubMed] [Google Scholar]