Abstract
We investigated whether cortical glutamatergic and GABAergic release machineries can be differentiated on the basis of the nature and amount of proteins they express, by performing a quantitative analysis of the degree of co-localization of synaptotagmin (SYT) 1 and 2, synaptic vesicle protein 2 (SV2) A and B, and Rab3a and c in VGLUT1+, VGLUT2+, and VGAT+ terminals and synaptic vesicles (SVs) in rat cerebral cortex. Co-localization studies showed that VGLUT1 puncta had high levels of SV2A and B and of Rab3c, intermediate levels of SYT1, and low levels of SYT2 and Rab3c; VGLUT2 puncta exhibited intermediate levels of all presynaptic proteins studied; whereas vesicular GABA transporter (VGAT) puncta had high levels of SV2A and SYT2, intermediate levels of SYT1, Rab3a, and Rab3c, and low levels of SV2B. Since SV2B is reportedly expressed by glutamatergic neurons and we observed SV2B expression in VGAT puncta, we performed electron microscopic studies and found SV2B positive axon terminals forming symmetric synapses. Immunoisolation studies showed that the expression levels of the protein isoforms varied in the three populations of SVs. Expression of SYT1 was highest in VGLUT1–SVs, while SYT2 expression was similar in the three SV groups. Expression of SV2A was similarly high in all three SV populations, except for SV2B levels that were very low in VGAT SVs. Finally, Rab3a levels were similar in the three SV groups, while Rab3c levels were highest in VGLUT1–SVs. These quantitative results extend our previous studies on the differential expression of presynaptic proteins involved in neurotransmitter release in GABAergic and glutamatergic terminals and indicate that heterogeneity of the respective release machineries can be generated by the differential complement of SV proteins involved in distinct stages of the release process.
Keywords: VGAT, VGLUT1, VGLUT2, synaptotagmin, SV2, Rab3
Introduction
The possibility that glutamatergic and GABAergic release machineries can be differentiated on the basis of the proteins they express has attracted considerable interest (e.g., Sugino et al., 2006; Micheva et al., 2010). In previous studies, we have approached this question by investigating quantitatively the localization of synapsin I and II (SYNI and II), synaptophysin I and II (SYPI and II), synaptosomal-associated protein (SNAP)-25 and SNAP-23, synaptogyrin (SGYR) 1 and 3, synaptobrevin/vesicle-associated membrane protein (VAMP) 1 and 2, and syntaxin 1A and 1B (STX1A and B) in vesicular GABA transporter (VGAT)-positive (+) GABAergic and vesicular glutamate transporter VGLUT1+ and VGLUT2+ glutamatergic axon terminals (AT) in cerebral cortex (Bragina et al., 2007, 2010). The results show that the expression of these presynaptic proteins in neocortex varies both between glutamatergic and GABAergic terminals and between VGLUT1+ and VGLUT2+ glutamatergic terminals (Bragina et al., 2007, 2010).
To further define the complement of proteins participating in transmitter release in GABAergic and glutamatergic terminals, we performed a quantitative analysis of the localization of synaptotagmin (SYT) 1 and 2, synaptic vesicle protein 2 (SV2) A and B, and Rab3a and c in VGLUT1+, VGLUT2+, and VGAT+ terminals and synaptic vesicles (SVs) of the cerebral cortex of adult rats. SYT1 and 2 are the main SYT isoforms present in SVs (Sudhof, 2002; Xu et al., 2007). They are known Ca2+ sensors for fast synchronous release and exhibit distinct expression patterns and properties (Geppert et al., 1994b; Fernandez-Chacon et al., 2001; Pang et al., 2006a,b; Xu et al., 2007), and SYT2 appears to be associated to inhibitory neurons and to operate at fast signaling synapses (Geppert et al., 1994b; Pang et al., 2006a,b; Sun et al., 2007). SV2 is a component of all vertebrate SVs (Buckley and Kelly, 1985); it plays a crucial role in the trafficking of SYT to SVs and regulates the effectiveness of calcium in inducing vesicle fusion. SV2A is expressed ubiquitously in the brain, whereas SV2B expression is restricted to forebrain and seems to be lacking in GABAergic neurons (Bajjalieh et al., 1993, 1994; Gronborg et al., 2010). Small GTPases of the Rab family are thought to confer membrane specificity in intracellular fusion reactions (Zerial and McBride, 2001; Pfeffer and Aivazian, 2004), and to control release probability, with implications for synaptic plasticity (Schluter et al., 2004, 2006). Rab3a is the most robustly expressed Rab protein in the brain (Geppert et al., 1994a), and an abundant SVs protein (Fischer von Mollard et al., 1990); Rab3c has also been localized to SVs in most brain areas, although at variable levels (Schluter et al., 2002).
Here we report that in the cerebral cortex of adult rats SYT2, SV2B, and Rab3c exhibit significant differences both in the distribution and expression levels between glutamatergic and GABAergic AT and SVs, and that some GABAergic AT express SV2B.
Materials and Methods
Animals and tissue preparation
Adult male Sprague-Dawley rats (190–220 g; Charles River, Milan, Italy) were used. All studies were performed in accordance with the E.C. Council Directive 86/609 (November 24, 1986) and were approved by the local authority veterinary service. Animals were kept under a dark–light cycle of 12 h and permitted food and water ad libitum.
For Western blotting, rats were anesthetized with chloral hydrate (300 mg/kg i.p.) and decapitated. Homogenization of neocortex, membrane preparation, protein determination, and SDS-PAGE analysis and immunoblotting were performed as described (Bragina et al., 2006). Precast gels (Tris–HCl; BioRad, Hercules, CA, USA) were used at 15% polyacrylamide concentration for Rab3 isoforms and at 7.5% for the remaining proteins. For immunocytochemistry, rats were anesthetized with chloral hydrate (300 mg/kg i.p.), and perfused through the ascending aorta with saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4). Brains were postfixed for 2 h (immunofluorescence) or 24 h (electron microscopy) at 4°C in the same fixative, cut with a Vibratome into 50 μm thick sections, and processed.
Antibodies
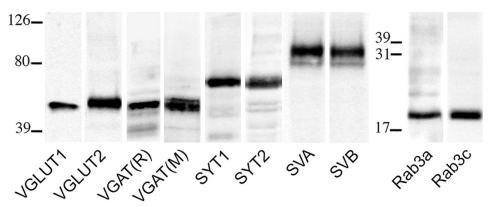
Primary antibodies used in these studies are listed in Table 1. Western blots were performed to verify antibodies specificity; nitrocellulose filters were probed with antibodies to VGLUT1, VGLUT2, VGAT, SYT1 and 2, SV2A and B, and Rab3a and c at the dilutions reported in Table 1. After exposure to the appropriate peroxidase-conjugated antibodies (Vector; Burlingame, CA, USA), immunoreactive bands were visualized by BioRad Chemidoc and Quantity One software (BioRad, Hemel Hempstead, UK) using the SuperSignal West Pico (Rockford, IL, USA) chemiluminescent substrate. In cortical crude membrane fractions, all antibodies recognized bands of predicted molecular mass (Figure 1; Matteoli et al., 1991; Bajjalieh et al., 1993; Ullrich et al., 1994; Bellocchio et al., 1998; Chaudhry et al., 1998; Schluter et al., 2002; Varoqui et al., 2002).
Table 1.
Primary antibodies.
| Antibodies | Host° | Dilution* | Source | Characterization |
|---|---|---|---|---|
| VGAT | Rabbit | 1:500 (IF) | Synaptic system/131003 | Takamori et al. (2000) |
| 1:1000 (WB) | ||||
| 1:500 (II) | ||||
| VGAT | Mouse | 1:50 (IF) | Synaptic system/131011 | Bogen et al. (2006), Tafoya et al. (2006) |
| 1:500 (WB) | ||||
| VGLUT1 | Guinea pig | 1:2000 (IF and WB) | Chemicon/AB5905 | Melone et al. (2005) |
| VGLUT1 | Rabbit | 1:500 (II) | Synaptic system/135303 | Takamori et al. (2001) |
| VGLUT2 | Guinea pig | 1:2000 (IF and WB) | Chemicon/AB5907 | Cubelos et al. (2005), Liu et al. (2005) |
| VGLUT2 | Rabbit | 1:500 (II) | Synaptic system/135403 | Takamori et al. (2001) |
| SYT1 | Mouse | 1:1500 (IF and WB) | Synaptic system/105011 | Brose et al. (1992), Von Kriegstein et al. (1999) |
| SYT2 | Rabbit | 1:800 (IF) | Synaptic system/105123 | Johnson et al. (2010) |
| 1:1000 (WB) | ||||
| SV2A | Rabbit | 1:1500 (IF) | Synaptic system/119002 | Janz and Sudhof (1999) |
| 1:80.000 (IF-TSA) | ||||
| 1:1000 (WB) | ||||
| SV2B | Rabbit | 1:1500 (IF) | Synaptic system/119102 | Janz and Sudhof (1999) |
| 1:80.000 (IF-TSA) | ||||
| 1:1000(WB and EM) | ||||
| Rab3a | Mouse | 1:1500 (IF) | Synaptic system/107111 | Matteoli et al. (1991) |
| 1:1000 (WB) | ||||
| Rab3c | Rabbit | 1:1500 (IF) | Synaptic system/107203 | Cai et al. (2008) |
| 1:1000 (WB) |
°GP, guinea pig; M, mouse; R, rabbit; *IF, immunofluorescence; IF-TSA, immunofluorescence with tyramide signal amplification; WB, western blotting; II, immunoisolation; EM, electron microscopy. Western blotting studies for VGLUT1, SYT1, SYT2, SV2A, and SV2B were performed with 5 μg of protein, those for VGAT, VGLUT2, Rab3a, and Rab3c with 10 μg.
Figure 1.
VGLUT1, VGLUT2, VGAT (R, rabbit; M, mouse), SYT1, SYT2, SV2A, SV2B, Rab3a, and Rab3c antibodies recognized bands of ~55, 60, 57, 65, 65, 100, 95, 25, and 25 kDa in the order, in crude membrane fractions of rat cerebral cortex.
Co-localization studies
Vibratome sections from rat brains were incubated for 1 h in normal goat serum (NGS; 10% in PB), and overnight at room temperature in a solution containing a mixture of the primary antibodies (see Table 2). The next day, sections were incubated in 10% NGS (30 min) and in a mixture of the appropriate secondary fluorescent antibodies (1 h; Table 2). Sections were then mounted, air-dried, and cover slipped using Vectashield mounting medium (H-1000; Vector). For all experimental series (i.e., the vesicular transporters and a presynaptic proteins), the VGLUT1, VGLUT2, and VGAT series were run in parallel to minimize the variability of experimental conditions.
Table 2.
Dilutions of antibodies in double-labeling studies.
| Primary antibodies | Secondary antibodies | Dilutions |
|---|---|---|
| VGLUT1/SYT1 | FITC–GAPA/TRITC–GAMC | 1:100/1:100 |
| VGLUT1/SYT2 | FITC–GAPA/TRITC–GARB | 1:100/1:100 |
| VGLUT1/SV2A | FITC–GAPA/TRITC–GARB | 1:100/1:100 |
| VGLUT1/SV2B | FITC–GAPA/TRITC–GARB | 1:100/1:100 |
| VGLUT1/Rab3a | FITC–GAPA/TRITC–GAMC | 1:100/1:100 |
| VGLUT1/Rab3c | FITC–GAPA/TRITC–GARB | 1:100/1:100 |
| VGLUT2/SYT1 | FITC–GAPA/TRITC–GAMC | 1:100/1:100 |
| VGLUT2/SYT2 | FITC–GAPA/TRITC–GARB | 1:100/1:100 |
| VGLUT2/SV2A | FITC–GAPA/TRITC–GARB | 1:100/1:100 |
| VGLUT2/SV2B | FITC–GAPA/TRITC–GARB | 1:100/1:100 |
| VGLUT2/Rab3a | FITC–GAPA/TRITC–GAMC | 1:100/1:100 |
| VGLUT2/Rab3c | FITC–GAPA/TRITC–GARB | 1:100/1:100 |
| VGAT/SYT1 | FITC–GARD/TRITC–GAMC | 1:100/1:100 |
| VGAT/SYT2 | FITC–GAME/TRITC–GARB | 1:100/1:100 |
| VGAT/SV2A | FITC–GAME/TRITC–GARB | 1:100/1:100 |
| VGAT/SV2B | FITC–GAME/TRITC–GARB | 1:100/1:100 |
| VGAT/Rab3a | FITC–GARD/TRITC–GAMC | 1:100/1:100 |
| VGAT/Rab3c | FITC–GAME/TRITC–GARB | 1:100/1:100 |
| SYT1/SYT2 | FITC–GAME/TRITC–GARB | 1:100/1:100 |
| SV2A/SV2B | bGAR (FITC)F/TRITC–GARB | 1:200 (1:50)/1:100 |
| SV2B/SV2A | bGAR (FITC)F/TRITC–GARB | 1:200 (1:50)/1:100 |
| Rab3a/Rab3c | FITC–GAME/TRITC–GARB | 1:100/1:100 |
AFluorescein isothiocyanate-conjugated goat anti-guinea-pig IgG (FI-7000, Vector; Burlingame, CA, USA); Btetramethylrhodamine isothiocyanate-conjugated goat anti-rabbit IgG (T-2769, Molecular Probes; Poort Gebouw, The Netherlands); Ctetramethylrhodamine isothiocyanate-conjugated goat anti-mouse IgG (T-2762, Molecular Probes); Dfluorescein isothiocyanate-conjugated goat anti-rabbit IgG (FI-1000, Vector); Efluorescein goat anti-mouse IgG (F-2761, Molecular probes);Ffluorescein-conjugated tyramide (TSA system).
Double-labeled sections were examined using a Leica TCS-SP2 confocal laser microscope equipped with an argon (488 nm) and a helium/neon (543 nm) laser for excitation of FITC and TRITC, respectively. Green and red immunofluorescence were imaged sequentially. Control experiments with single-labeled sections and sections incubated either with two primary antibodies and one secondary antibody, or with one primary antibody and two secondary antibodies revealed no appreciable FITC/TRITC bleed-through or antibody cross-reactivity.
Images from all experimental series were from the parietal cortex and were acquired from randomly selected subfields in layers II–VI (at least four to six per layer; two to four sections per animal; 14 rats). Layer I was not sampled because it hardly contains VGAT+ puncta (Chaudhry et al., 1998; Minelli et al., 2003). Images were acquired using a ×60 oil immersion lens (numerical aperture 1.4; pinhole 1.0 and image size 1024 × 1024 pixels, yielding a pixel size of 0.06 μm) from a plane in which the resolution of both stains was optimal and never >1.8 μm from the surface (Melone et al., 2005). Signal acquisition was optimized through the “Q LUT” button, which permitted direct visualization of pixel saturation; photomultiplier gain was set so that the brightest pixels were just slightly below saturation, and the offset such that the darkest pixels were just above zero. To avoid bleed-trough between green and red fluorescence, images were acquired sequentially. To improve the signal/noise ratio, 15 frames/image were averaged.
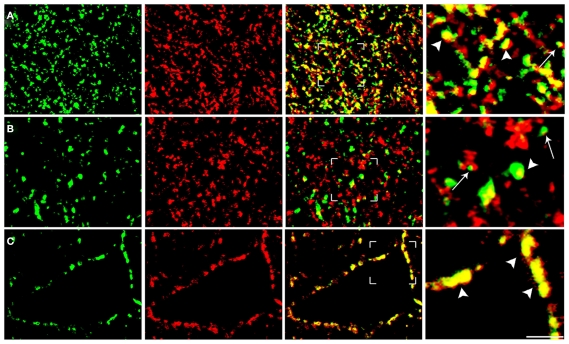
Quantitative analysis was performed in ~8,000 randomly selected subfields measuring about 25 μm × 25 μm from the 1024 × 1024 pixel images. In order to minimize fusion of puncta, contrast of each image was manually adjusted within the maximum range of levels for each color channel. Preliminary studies (not shown) showed that gain/contrast changes within the spectrum used did not alter significantly the percentage of co-localized puncta (Bragina et al., 2010). Then, without reducing the image resolution, each channel was examined separately to identify and manually count immunopositive puncta; the two channels were then merged and the number of co-localizing puncta was counted manually. Puncta were considered double-labeled when overlap was complete or occupied most of the immunopositive puncta and they exhibited morphological similarity (Bragina et al., 2007, 2010; Figure 2).
Figure 2.
Analysis of confocal microscopy images of cortical sections. The figure shows examples from VGLUT1/SYT1 [row(A)], VGLUT2/Rab3a [row (B)], and VGAT/SV2A series [row (C)]. To minimize the fusion of puncta, the contrast of each image was manually adjusted within the maximum range of levels for each color channel, which was examined separately to identify and count manually immunopositive puncta (first and second columns). Following merging, puncta were considered double-labeled when the overlap was complete or it occupied most of the area of the puncta and they were morphologically similar (arrowheads in fourth columns). Puncta not meeting these criteria (e.g., those indicated by arrows) were not considered double-labeled. Bars: 2 μm.
Synaptic vesicles immunoisolation studies
Eupergit C1Z methacrylate microbeads (1 μm in diameter; Röhm Pharmaceuticals, Darmstadt, Germany) were either blocked with glycine or conjugated with affinity-purified goat anti-rabbit antibodies (IgG; Sigma, Milan, Italy) as previously described (Burger et al., 1991). Affinity-purified anti-VGAT (R), anti-VGLUT1 (R), or anti-VGLUT2 (R) antibodies (Table 1) were conjugated with the covalently bound secondary antibodies, generating IgG-coated anti-VGAT, anti-VGLUT1, or anti-VGLUT2 beads. Beads coated only with glycine or secondary antibodies were used as negative controls (mock beads). Aliquots of the LS1 fraction obtained from osmotic lysis of adult rat cortical synaptosomes (150–300 μg protein/sample; Huttner et al., 1983) was incubated for 2–4 h at 4°C in phosphate-buffered saline (PBS) with the various bead preparations (50–70 μl settled beads in 500 μl final volume) under constant rotation. After centrifugation at 1000 × g for 1 min and repeated washes in PBS, corresponding amounts of bead pellets and supernatant fractions were solubilized in sample buffer and subjected to SDS-PAGE on 7 and 12% polyacrylamide gels. Gels were electrophoretically transferred to nitrocellulose membranes and immunoblotted with polyclonal or monoclonal SYT1/SYT2, SV2A/SV2B, and Rab3a/Rab3c antibodies (Table 1). Specific immunoreactivity was revealed using peroxidase-conjugated secondary antibodies and the chemiluminescence detection system, as described (Bragina et al., 2007). Quantification of recovered immunoreactivity was performed by densitometric analysis of the fluorograms and by data interpolation into a standard curve of rat brain LS1 fraction run in parallel with the unknown samples. The amounts of synaptic proteins associated with the immunoisolated vesicles were expressed in percent of the respective the total input of LS1 fraction added to the samples. Experiments were repeated at least five times.
Electron microscopy
Four sections/rat (n = 2) were processed for immunoperoxidase technique and prepared for electron microscopy as described in previous studies (Bragina et al., 2007, 2010). After completion of the immunoperoxidase procedure, sections were flat-embedded in Epon–Spurr (Bragina et al., 2007); then small blocks of tissue containing layer V were selected by light microscope inspection, glued to blank epoxy, and sectioned with an ultramicrotome. The most superficial ultrathin sections were examined with a Philips EM 208 electron microscope (Eindhoven, The Netherlands) coupled to a MegaView-II high resolution CCD camera (Soft Imaging System; Munster, Germany). Identification of profiles was based on established morphological criteria (Peters et al., 1991).
Results
The distribution of VGLUT1, VGLUT2, VGAT, SYT1, SYT2, SV2A, SV2B, Rab3a, and Rab3c immunoreactivities was as previously described (Moya et al., 1992; Bajjalieh et al., 1994; Ullrich et al., 1994; Bellocchio et al., 1998; Chaudhry et al., 1998; Kaneko et al., 2002; Minelli et al., 2003; Alonso-Nanclares et al., 2004; Conti et al., 2005). All VGAT+ puncta (e.g., Chaudhry et al., 1998; Minelli et al., 2003) and the vast majority of VGLUT1+ and VGLUT2+ puncta (Kaneko et al., 2002) are AT. Since some astrocytic processes express VGLUT1 or VGLUT2 (Bezzi et al., 2004; Montana et al., 2004) and some of the presynaptic proteins analyzed here (Montana et al., 2006), it is possible that some VGLUT1+ and VGLUT2+ puncta may include rare astrocytic processes. However, considering the paucity of VGLUTs expressed in astrocytes and the elevated number of puncta studied in present studies, the possible bias appears negligible.
We also preliminarily studied the degree of co-localization between pairs of isoforms (SYT1/SYT2, SV2A/SV2B, and Rab3a/Rab3c) in 12 sections from two animals. Co-localization was detected in 21.9% of SYT1+ and 33.4% of SYT2+ puncta; in 27.6% of SV2A+ and 48.6% of SV2B+ puncta; and in 34.1% of Rab3a+ and 64.2% of Rab3c+ puncta. Since all pairs of protein isoforms were not highly co-localized, we investigated immunocytochemically whether SYT1 and 2, SV2A and B, Rab3a and c are differentially expressed in VGLUT1+, VGLUT2+, and VGAT+ AT.
Expression of SYT1, SYT2, SV2A, SV2B, Rab3a, and Rab3c in cortical VGLUT1+, VGLUT2+, and VGAT+ axon terminals and synaptic vesicles
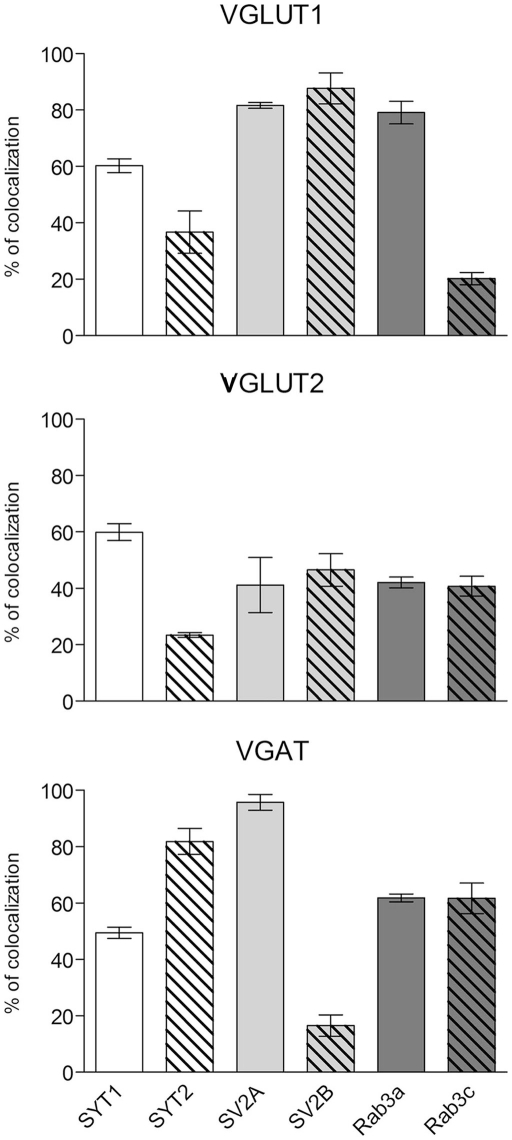
Expression of presynaptic proteins in VGLUT1+ cortical AT was studied in 38 sections from nine rats. Results showed that 60.2% of VGLUT1 were SYT1+, 36.7% were SYT2+, 81.7% SV2A+, 87.7% SV2B+, 79.1% Rab3a+, and 20.2% Rab3c+ (Figure 3; Table 3 for details). Analysis of VGLUT2+ cortical terminals (40 sections from 10 animals) showed that 59.9% of them were SYT1+, 23.4% SYT2+, 41.1% SV2A+, 46.5% SV2B+, 42% Rab3a+, and 40.7% Rab3c+ (Figure 3; Table 3 for details). Analogous analysis of VGAT+ terminals (44 sections from 10 rats) revealed that 49.4% of them were SYT1+, 81.8% SYT2+, 95.7% SV2A+, 16.5% SV2B+, 61.8% Rab3a+, and 61.7% Rab3c+ (Figure 3; Table 3 for details). The degree of co-localization between vesicular transporters and presynaptic proteins studied here did not exhibit any differential laminar distribution.
Figure 3.
Co-localization of SYT1, SYT2, SV2A, SV2B, Rab3a, and Rab3c in VGLUT1+, VGLUT2+, and VGAT+ axon terminals in cerebral cortex. Values (means ± SEM) refer to the percentages of total puncta positive for the respective protein isoform for each of the three terminal populations identified based on the specific vesicular transporter.
Table 3.
SYT1 and 2, SV2A and B, and Rab3a and c in VGLUT1, VGLUT2, and VGAT puncta.
| VT | Puncta (#) | Co-localization (%) | PP |
|---|---|---|---|
| VGLUT1 | 4640 | 60.2 ± 2.4 | SYT1 |
| 5087 | 36.7 ± 7.5 | SYT2 | |
| 6707 | 81.7 ± 1.0 | SV2A | |
| 4750 | 87.7 ± 5.5 | SV2B | |
| 6351 | 79.1 ± 4.0 | Rab3a | |
| 3770 | 20.2 ± 2.2 | Rab3c | |
| VGLUT2 | 2026 | 59.9 ± 2.9 | SYT1 |
| 3150 | 23.4 ± 0.8 | SYT2 | |
| 3254 | 41.1 ± 9.8 | SV2A | |
| 2665 | 46.5 ± 5.8 | SV2B | |
| 3345 | 42.0 ± 1.9 | Rab3a | |
| 1914 | 40.7 ± 3.5 | Rab3c | |
| VGAT | 1558 | 49.4 ± 2.0 | SYT1 |
| 1758 | 81.8 ± 4.6 | SYT2 | |
| 1106 | 95.7 ± 2.8 | SV2A | |
| 1793 | 16.5 ± 3.8 | SV2B | |
| 1855 | 61.8 ± 1.4 | Rab3a | |
| 849 | 61.7 ± 5.4 | Rab3c |
VT, vesicular transporter; PP, presynaptic protein.
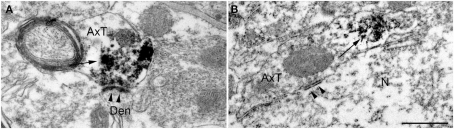
Previous studies reported that in neocortex SV2B is preferentially expressed at glutamatergic neurons (Bajjalieh et al., 1994). The observation that SV2B was present in some VGAT+ fluorescent terminals was therefore unexpected. To verify whether known GABAergic cortical synapses expressed SV2B, we performed electron microscopy studies in pre-embedded immunoperoxidase material. Our analysis was limited to AT forming symmetric synapses on pyramidal cell bodies in layer V, which are GABAergic and express VGAT (Ribak, 1978; Houser et al., 1984; Chaudhry et al., 1998). Besides confirming that numerous asymmetric synapses are SV2B+, these studies showed that in some cases AT forming perisomatic symmetric synapses were indeed SV2B+ (Figures 4A,B).
Figure 4.
Pre-embedding electron microscopy studies show that SV2B immunoreactivity [arrows in (A,B)] is present in axon terminals forming both asymmetric [arrowheads in (A); layer V] and symmetric [arrowheads in (B); layer V] synaptic contacts. AxT, axon terminal; Den, dendrite; N, pyramidal neuron. Scale bar: 500 nm (A,B).
These studies provide information on whether a given protein participating in neurotransmitter release is expressed above the threshold for immunocytochemical detection in a subpopulation of identified AT; if this occurs, that terminal is considered to express that protein. However, the percentages reported above result from an all-or-none evaluation of the presence/absence of a given protein in a given terminal and do not provide any information on the expression levels of this protein in glutamatergic and GABAergic SVs. We therefore set up a study to investigate the complement of SYT1, SYT2, SV2A, SV2B, Rab3a, and Rab3c in immunoisolated VGLUT1, VGLUT2, and VGAT SV populations from the rat neocortex. Immunoisolation studies showed that the expression levels of the protein isoforms varied in the three populations of SVs.
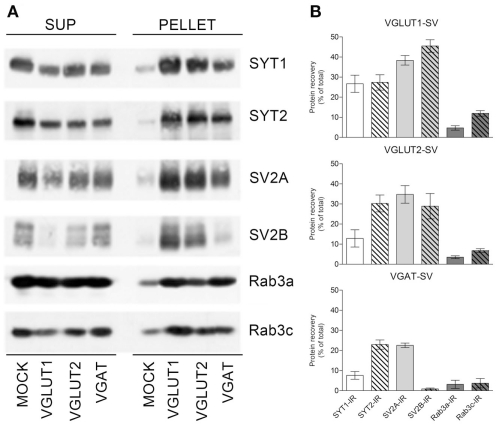
Expression levels of SYT1 was highest in VGLUT1–SVs (26.7 ± 4.3% of total immunoreactivity), while its levels were lower in VGLUT2–SV (12.9 ± 4.3%) and VGAT SVs (7.6 ± 1.9%), while SYT2 expression was similar in the three SV groups (27.3 ± 3.8, 30.2 ± 4.2, and 23.0 ± 2.3% for VGLUT1, VGLUT2, and VGAT SV populations, respectively). Expression levels of SV2A was similarly high in all three SV populations (38.4 ± 2.4, 34.8 ± 4, and 22.6 ± 1.1% for VGLUT1, VGLUT2, and VGAT SVs, respectively), while SV2B levels were similar to those of SV2 in glutamatergic SVs (45.5 ± 3.1 and 28.9 ± 6.3% in VGLUT1 and VGLUT2 SVs, respectively), but very low in VGAT SVs (0.8 ± 0.43%). Finally, Rab3a levels were similar in the three SV groups (4.7 ± 1.1, 3.5 ± 0.7, and 3.1 ± 2.1% for VGLUT1, VGLUT2, and VGAT SVs, respectively), while Rab3c levels were higher in VGLUT1–SVs (11.9 ± 1.4%) than in VGLUT2 (6.8 ± 1.0%) or VGAT (3.7 ± 2.3%) SVs (Figure 5).
Figure 5.
Expression of SYT1, SYT2, SV2A, SV2B, and Rab3a and c in VGLUT1+, VGLUT2+, and VGAT+ SVs. (A) Glutamatergic and GABAergic SVs were immunoisolated from the LS1 fraction of rat cerebral cortex using beads coupled with either rabbit VGLUT1, VGLUT2, or VGAT antibodies. After immunoisolation, corresponding amounts of pellet and supernatant (SUP) fractions were subjected to immunoblotting with anti-SYT1 and -SYT2 antibodies, anti-SV2A/SV2B antibodies, or anti-Rab3a/Rab3c antibodies. (B) Quantification of the recovered immunoreactivities was carried out by densitometric scanning and interpolation of the data into a standard curve of rat brain LS1 fraction, and expressed as percent of the total input of LS1 added to the samples. The percentage of SYT1/SYT2, SV2A/SV2B, and Rab3a/Rab3c immunoreactivities (IR) detected in SVs immunoisolated with anti-VGLUT1 (VGLUT1–SV; upper left/right panel), anti-VGLUT2 (VGLUT2–SV; lower left/right panel), or anti-VGAT (VGAT SV; upper right/right panel) beads are shown as means (±SEM) of five independent experiments.
Discussion
The quantitative studies reported here showed that SYT1/2, SV2A/B, and Rab3a/c are differentially expressed in glutamatergic and GABAergic terminals, and that in VGLUT1, VGLUT2, and VGAT terminals the levels of these proteins associated with were also variable.
Under our experimental conditions, SYT1 distribution was similar at glutamatergic and GABAergic terminals, whereas SYT2 expression was more robust in GABAergic terminals. The latter finding extend quantitatively the results of previous studies showing an association between SYT2 and GABAergic neurons (Pang et al., 2006a; Fox and Sanes, 2007). As synapses expressing SYT2 display a faster transmitter release than those expressing SYT1 (Xu et al., 2007), it is conceivable that expression of a given SYT isoform contributes to specific release properties. As far as SV2A and B distribution in the three classes of terminals is concerned, Bajjalieh et al. (1994) and Gronborg et al. (2010) showed that SV2B is preferentially localized to glutamatergic neurons. Our light and electron microscopic immunocytochemical studies add the observation that some VGAT+ terminals express SV2B. However, immunoisolation studies showed very low levels of SV2B in VGAT+ vesicles. The most likely explanation for this discrepancy is presumably correlated to the paucity of SV2B expression in GABAergic terminals. Finally, in our hands Rab3a distribution was more widespread than that of Rab3c in VGLUT1+ terminals, whereas it was similar in VGLUT2 and VGAT+ terminals.
Besides distribution frequency in the nerve terminal populations, the SV protein isoforms displayed also remarkably different expression levels in VGLUT1, VGLUT2, and VGAT immunoisolated SVs, with SYT2 levels higher than SYT1 levels in VGLUT2 and VGAT SVs and SV2B levels very low compared to SV2A levels in VGAT SVs.
In our previous studies on the heterogeneity of glutamatergic and GABAergic release machinery in neocortex, we demonstrated quantitatively remarkable differences in the expression of presynaptic proteins participating in transmitter release between glutamatergic and GABAergic AT and between VGLUT1 and VGLUT2 glutamatergic terminals, with SYNI, SYNII, SYPI, SNAP-25, and STX1A highly expressed in VGLUT1+ AT, SNAP-23 highly expressed in VGLUT2+ AT and STX1B and VAMP1 more expressed in VGAT+ AT (Bragina et al., 2007, 2010). The present observation that other presynaptic proteins involved in transmitter release exhibit differences between glutamatergic and GABAergic AT, with SV2B preferentially expressed in VGLUT1+ terminals, and SYT2 and Rab3c in VGAT+ ones, strengthens the notion that glutamatergic and GABAergic exocytotic machineries greatly differ in their complement of presynaptic proteins.
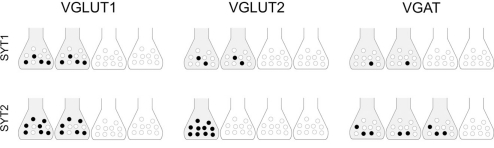
Comparison between double-labeling studies, which yields an all-or-none evaluation of expression of a given presynaptic protein in AT, and SV immunoisolation studies, which provide quantitative information on protein expression in homogeneous populations of SVs, allows the definition of a more complete picture of the differential expression of key actors of exocytosis and adds to the issue of molecular heterogeneity of glutamatergic and GABAergic terminals. For example, the VGLUT1 series indicate that in about 50% of VGLUT1+ terminals ~40% of SVs expressed SYT1 and ~70% expressed SYT2 (Figure 6), that in most of them about 50% of SVs expressed SV2A and B, and that in the majority of VGLUT1+ terminals few SVs expressed Rab3a while most of them expressed Rab3c. Thus, the data support the concept that molecular heterogeneity within glutamatergic terminals and between these terminals and GABAergic ones is achieved not only by the presence/absence of a given protein, but also by the relative abundance of SVs expressing a given protein and the number of copies of the protein sorted to each SV (see also Micheva et al., 2010).
Figure 6.
Hypothetical distribution of SYT1/2 in VGLUT1+ and VGLUT2+ glutamatergic and VGAT+ GABAergic cortical terminals. Based on a correlative analysis of confocal and immunoisolation data, the scenario proposed is highly schematic (e.g., the total amount of a SV-related protein is equally distributed among all positive terminals), and does not take into account the number of proteins/vesicles, but it emphasizes the concept that the amount of a SV-related protein is variable in positive terminals. Shaded terminals indicate terminals expressing a given protein; black circles indicate levels of expression of the protein in immunoisolated SVs. Four terminals make up 100%; similarly, 10 vesicles makes up 100%.
In present and previous studies (Bragina et al., 2007, 2010), we reported on the heterogeneous expression of SNARE, calcium sensor, and regulatory proteins at VGLUT1, VGLUT2, and VGAT+ cortical AT. Glutamate- and GABA-operated synapses exhibit different forms of frequency-dependent short-term synaptic plasticity (Galarreta and Hestrin, 1998; Varela et al., 1999; Thomson, 2000), and there is evidence that different isoforms of presynaptic proteins regulate SVs availability at glutamate and GABA synapses (Moulder et al., 2007). Functional differences exist also between VGLUT1 and VGLUT2 terminals: the first ones appear to be associated with low release probability synapses, whereas the latter ones participate in synapses exhibiting higher release probability (Gil et al., 1999; Fremeau et al., 2001). Notwithstanding the known role of other proteins in determining release efficiency (Weston et al., 2011), the present results support the notion that heterogeneous expression levels of presynaptic proteins may contribute to differences in release probability and plasticity (Staple et al., 1997, 2000). Our observation that two types of glutamatergic and GABAergic AT expressed different patterns of presynaptic proteins are in line with this scenario and may contribute to explain functional differences. Whether the different patterns of presynaptic protein expression in the two types of glutamatergic and in GABAergic AT are stable throughout the animal’s life or are regulated during development and/or aging will prove a challenge of great interest for future studies.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by research grants from the Italian Ministry of University and Research (PRIN to Fiorenzo Conti, Fabio Benfenati, and Silvia Giovedí), the UNIVPM (to Fiorenzo Conti, Luca Bragina, Giorgia Fattorini, and Marcello Melone), and Telethon-Italy (Grant GGP09134 to Fabio Benfenati).
References
- Alonso-Nanclares L., Minelli A., Melone M., Edwards R. H., Defelipe J., Conti F. (2004). Perisomatic glutamatergic axon terminals: a novel feature of cortical synaptology revealed by vesicular glutamate transporter 1 immunostaining. Neuroscience 123, 547–556 10.1016/j.neuroscience.2003.09.033 [DOI] [PubMed] [Google Scholar]
- Bajjalieh S. M., Frantz G. D., Weimann J. M., McConnell S. K., Scheller R. H. (1994). Differential expression of synaptic vesicle protein 2 (SV2) isoforms. J. Neurosci. 14, 5223–5235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajjalieh S. M., Peterson K., Linial M., Scheller R. H. (1993). Brain contains two forms of synaptic vesicle protein 2. Proc. Natl. Acad. Sci. U.S.A. 90, 2150–2154 10.1073/pnas.90.6.2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocchio E. E., Hu H., Pohorille A., Chan J., Pickel V. M., Edwards R. H. (1998). The localization of the brain-specific inorganic phosphate transporter suggests a specific presynaptic role in glutamatergic transmission. J. Neurosci. 18, 8648–8659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P., Gundersen V., Galbete J. L., Seifert G., Steinhauser C., Pilati E., Volterra A. (2004). Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat. Neurosci. 7, 613–620 10.1038/nn1246 [DOI] [PubMed] [Google Scholar]
- Bogen I. L., Boulland J. L., Mariussen E., Wright M. S., Fonnum F., Kao H. T., Walaas S. I. (2006). Absence of synapsin I and II is accompanied by decreases in vesicular transport of specific neurotransmitters. J. Neurochem. 96, 1458–1466 10.1111/j.1471-4159.2005.03636.x [DOI] [PubMed] [Google Scholar]
- Bragina L., Candiracci C., Barbaresi P., Giovedi S., Benfenati F., Conti F. (2007). Heterogeneity of glutamatergic and GABAergic release machinery in cerebral cortex. Neuroscience 146, 1829–1840 10.1016/j.neuroscience.2007.02.060 [DOI] [PubMed] [Google Scholar]
- Bragina L., Giovedi S., Barbaresi P., Benfenati F., Conti F. (2010). Heterogeneity of glutamatergic and GABAergic release machinery in cerebral cortex: analysis of synaptogyrin, vesicle-associated membrane protein, and syntaxin. Neuroscience 165, 934–943 10.1016/j.neuroscience.2009.11.009 [DOI] [PubMed] [Google Scholar]
- Bragina L., Melone M., Fattorini G., Torres-Ramos M., Vallejo-Illarramendi A., Matute C., Conti F. (2006). GLT-1 down-regulation induced by clozapine in rat frontal cortex is associated with synaptophysin up-regulation. J. Neurochem. 99, 134–141 10.1111/j.1471-4159.2006.04030.x [DOI] [PubMed] [Google Scholar]
- Brose N., Petrenko A. G., Sudhof T. C., Jahn R. (1992). Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science 256, 1021–1025 10.1126/science.1589771 [DOI] [PubMed] [Google Scholar]
- Buckley K., Kelly R. B. (1985). Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J. Cell Biol. 100, 1284–1294 10.1083/jcb.100.4.1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger P. M., Hell J., Mehl E., Krasel C., Lottspeich F., Jahn R. (1991). GABA and glycine in synaptic vesicles: storage and transport characteristics. Neuron 7, 287–293 10.1016/0896-6273(91)90267-4 [DOI] [PubMed] [Google Scholar]
- Cai H., Reim K., Varoqueaux F., Tapechum S., Hill K., Sorensen J. B., Brose N., Chow R. H. (2008). Complexin II plays a positive role in Ca2+-triggered exocytosis by facilitating vesicle priming. Proc. Natl. Acad. Sci. U.S.A. 105, 19538–19543 10.1073/pnas.0804608105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry F. A., Reimer R. J., Bellocchio E. E., Danbolt N. C., Osen K. K., Edwards R. H., Storm-Mathisen J. (1998). The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J. Neurosci. 18, 9733–9750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., Candiracci C., Fattorini G. (2005). Heterogeneity of axon terminals expressing VGLUT1 in the cerebral neocortex. Arch. Ital. Biol. 143, 127–132 [PubMed] [Google Scholar]
- Cubelos B., Gimenez C., Zafra F. (2005). Localization of the GLYT1 glycine transporter at glutamatergic synapses in the rat brain. Cereb. Cortex 15, 448–459 10.1093/cercor/bhh147 [DOI] [PubMed] [Google Scholar]
- Fernandez-Chacon R., Konigstorfer A., Gerber S. H., Garcia J., Matos M. F., Stevens C. F., Brose N., Rizo J., Rosenmund C., Sudhof T. C. (2001). Synaptotagmin I functions as a calcium regulator of release probability. Nature 410, 41–49 10.1038/35065004 [DOI] [PubMed] [Google Scholar]
- Fischer von Mollard G., Mignery G. A., Baumert M., Perin M. S., Hanson T. J., Burger P. M., Jahn R., Sudhof T. C. (1990). Rab3 is a small GTP-binding protein exclusively localized to synaptic vesicles. Proc. Natl. Acad. Sci. U.S.A. 87, 1988–1992 10.1073/pnas.87.5.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M. A., Sanes J. R. (2007). Synaptotagmin I and II are present in distinct subsets of central synapses. J. Comp. Neurol. 503, 280–296 10.1002/cne.21381 [DOI] [PubMed] [Google Scholar]
- Fremeau R. T., Jr., Troyer M. D., Pahner I., Nygaard G. O., Tran C. H., Reimer R. J., Bellocchio E. E., Fortin D., Storm-Mathisen J., Edwards R. H. (2001). The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron 31, 247–260 10.1016/S0896-6273(01)00344-0 [DOI] [PubMed] [Google Scholar]
- Galarreta M., Hestrin S. (1998). Frequency-dependent synaptic depression and the balance of excitation and inhibition in the neocortex. Nat. Neurosci. 1, 587–594 10.1038/2882 [DOI] [PubMed] [Google Scholar]
- Geppert M., Bolshakov V. Y., Siegelbaum S. A., Takei K., De Camilli P., Hammer R. E., Sudhof T. C. (1994a). The role of Rab3A in neurotransmitter release. Nature 369, 493–497 10.1038/369493a0 [DOI] [PubMed] [Google Scholar]
- Geppert M., Goda Y., Hammer R. E., Li C., Rosahl T. W., Stevens C. F., Sudhof T. C. (1994b). Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell 79, 717–727 10.1016/0092-8674(94)90556-8 [DOI] [PubMed] [Google Scholar]
- Gil Z., Connors B. W., Amitai Y. (1999). Efficacy of thalamocortical and intracortical synaptic connections: quanta, innervation, and reliability. Neuron 23, 385–397 10.1016/S0896-6273(00)80788-6 [DOI] [PubMed] [Google Scholar]
- Gronborg M., Pavlos N. J., Brunk I., Chua J. J., Munster-Wandowski A., Riedel D., Ahnert-Hilger G., Urlaub H., Jahn R. (2010). Quantitative comparison of glutamatergic and GABAergic synaptic vesicles unveils selectivity for few proteins including MAL2, a novel synaptic vesicle protein. J. Neurosci. 30, 2–12 10.1523/JNEUROSCI.4074-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser C. R., Vaughn D. E., Hendry S. H., Jones E. G., Peters A. (1984). “GABA neurons in the cerebral cortex,” in Cerebral Cortex, eds Jones E. G., Peters A. (New York: Plenum Press; ), 63–89 [Google Scholar]
- Huttner W. B., Schiebler W., Greengard P., De Camilli P. (1983). Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J. Cell Biol. 96, 1374–1388 10.1083/jcb.96.5.1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz R., Sudhof T. C. (1999). SV2C is a synaptic vesicle protein with an unusually restricted localization: anatomy of a synaptic vesicle protein family. Neuroscience 94, 1279–1290 10.1016/S0306-4522(99)00370-X [DOI] [PubMed] [Google Scholar]
- Johnson S. L., Franz C., Kuhn S., Furness D. N., Ruttiger L., Munkner S., Rivolta M. N., Seward E. P., Herschman H. R., Engel J., Knipper M., Marcotti W. (2010). Synaptotagmin IV determines the linear Ca2+ dependence of vesicle fusion at auditory ribbon synapses. Nat. Neurosci. 13, 45–52 10.1038/nn.2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T., Fujiyama F., Hioki H. (2002). Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J. Comp. Neurol. 444, 39–62 10.1002/cne.10129 [DOI] [PubMed] [Google Scholar]
- Liu X. B., Low L. K., Jones E. G., Cheng H. J. (2005). Stereotyped axon pruning via plexin signaling is associated with synaptic complex elimination in the hippocampus. J. Neurosci. 25, 9124–9134 10.1523/JNEUROSCI.2710-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoli M., Takei K., Cameron R., Hurlbut P., Johnston P. A., Sudhof T. C., Jahn R., De Camilli P. (1991). Association of Rab3A with synaptic vesicles at late stages of the secretory pathway. J. Cell Biol. 115, 625–633 10.1083/jcb.115.3.625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melone M., Burette A., Weinberg R. J. (2005). Light microscopic identification and immunocytochemical characterization of glutamatergic synapses in brain sections. J. Comp. Neurol. 492, 495–509 10.1002/cne.20743 [DOI] [PubMed] [Google Scholar]
- Micheva K. D., Busse B., Weiler N. C., O’Rourke N., Smith S. J. (2010). Single-synapse analysis of a diverse synapse population: proteomic imaging methods and markers. Neuron 68, 639–653 10.1016/j.neuron.2010.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minelli A., Alonso-Nanclares L., Edwards R. H., DeFelipe J., Conti F. (2003). Postnatal development of the vesicular GABA transporter in rat cerebral cortex. Neuroscience 117, 337–346 10.1016/S0306-4522(02)00864-3 [DOI] [PubMed] [Google Scholar]
- Montana V., Malarkey E. B., Verderio C., Matteoli M., Parpura V. (2006). Vesicular transmitter release from astrocytes. Glia 54, 700–715 10.1002/glia.20367 [DOI] [PubMed] [Google Scholar]
- Montana V., Ni Y., Sunjara V., Hua X., Parpura V. (2004). Vesicular glutamate transporter-dependent glutamate release from astrocytes. J. Neurosci. 24, 2633–2642 10.1523/JNEUROSCI.3770-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder K. L., Jiang X., Taylor A. A., Shin W., Gillis K. D., Mennerick S. (2007). Vesicle pool heterogeneity at hippocampal glutamate and GABA synapses. J. Neurosci. 27, 9846–9854 10.1523/JNEUROSCI.2803-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya K. L., Tavitian B., Zahraoui A., Tavitian A. (1992). Localization of the ras-like rab3A protein in the adult rat brain. Brain Res. 590, 118–127 10.1016/0006-8993(92)91087-U [DOI] [PubMed] [Google Scholar]
- Pang Z. P., Melicoff E., Padgett D., Liu Y., Teich A. F., Dickey B. F., Lin W., Adachi R., Sudhof T. C. (2006a). Synaptotagmin-2 is essential for survival and contributes to Ca2+ triggering of neurotransmitter release in central and neuromuscular synapses. J. Neurosci. 26, 13493–13504 10.1523/JNEUROSCI.3804-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Z. P., Shin O. H., Meyer A. C., Rosenmund C., Sudhof T. C. (2006b). A gain-of-function mutation in synaptotagmin-1 reveals a critical role of Ca2+-dependent soluble N-ethylmaleimide-sensitive factor attachment protein receptor complex binding in synaptic exocytosis. J. Neurosci. 26, 12556–12565 10.1523/JNEUROSCI.3804-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A., Palay S. L., Webster H. (1991). The Fine Structure of the Nervous System: Neurons and their Supporting Cells. New York: Oxford University Press [Google Scholar]
- Pfeffer S., Aivazian D. (2004). Targeting Rab GTPases to distinct membrane compartments. Nat. Rev. Mol. Cell Biol. 5, 886–896 10.1038/nrm1500 [DOI] [PubMed] [Google Scholar]
- Ribak C. E. (1978). Aspinous and sparsely-spinous stellate neurons in the visual cortex of rats contain glutamic acid decarboxylase. J. Neurocytol. 7, 461–478 10.1007/BF01173991 [DOI] [PubMed] [Google Scholar]
- Schluter O. M., Basu J., Sudhof T. C., Rosenmund C. (2006). Rab3 superprimes synaptic vesicles for release: implications for short-term synaptic plasticity. J. Neurosci. 26, 1239–1246 10.1523/JNEUROSCI.3553-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter O. M., Khvotchev M., Jahn R., Sudhof T. C. (2002). Localization versus function of Rab3 proteins. Evidence for a common regulatory role in controlling fusion. J. Biol. Chem. 277, 40919–40929 10.1074/jbc.M203704200 [DOI] [PubMed] [Google Scholar]
- Schluter O. M., Schmitz F., Jahn R., Rosenmund C., Sudhof T. C. (2004). A complete genetic analysis of neuronal Rab3 function. J. Neurosci. 24, 6629–6637 10.1523/JNEUROSCI.1610-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staple J. K., Morgenthaler F., Catsicas S. (2000). Presynaptic heterogeneity: Vive la difference. News Physiol. Sci. 15, 45–49 [DOI] [PubMed] [Google Scholar]
- Staple J. K., Osen-Sand A., Benfenati F., Pich E. M., Catsicas S. (1997). Molecular and functional diversity at synapses of individual neurons in vitro. Eur. J. Neurosci. 9, 721–731 10.1111/j.1460-9568.1997.tb01420.x [DOI] [PubMed] [Google Scholar]
- Sudhof T. C. (2002). Synaptotagmins: why so many? J. Biol. Chem. 277, 7629–7632 10.1074/jbc.R100052200 [DOI] [PubMed] [Google Scholar]
- Sugino K., Hempel C. M., Miller M. N., Hattox A. M., Shapiro P., Wu C., Huang Z. J., Nelson S. B. (2006). Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nat. Neurosci. 9, 99–107 10.1038/nn0206-292b [DOI] [PubMed] [Google Scholar]
- Sun J., Pang Z. P., Qin D., Fahim A. T., Adachi R., Sudhof T. C. (2007). A dual-Ca2+-sensor model for neurotransmitter release in a central synapse. Nature 450, 676–682 10.1038/nature06308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafoya L. C., Mameli M., Miyashita T., Guzowski J. F., Valenzuela C. F., Wilson M. C. (2006). Expression and function of SNAP-25 as a universal SNARE component in GABAergic neurons. J. Neurosci. 26, 7826–7838 10.1523/JNEUROSCI.1866-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S., Rhee J. S., Rosenmund C., Jahn R. (2001). Identification of differentiation-associated brain-specific phosphate transporter as a second vesicular glutamate transporter (VGLUT2). J. Neurosci. 21, RC182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S., Riedel D., Jahn R. (2000). Immunoisolation of GABA-specific synaptic vesicles defines a functionally distinct subset of synaptic vesicles. J. Neurosci. 20, 4904–4911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. M. (2000). Molecular frequency filters at central synapses. Prog. Neurobiol. 62, 159–196 10.1016/S0301-0082(00)00008-3 [DOI] [PubMed] [Google Scholar]
- Ullrich B., Li C., Zhang J. Z., McMahon H., Anderson R. G., Geppert M., Sudhof T. C. (1994). Functional properties of multiple synaptotagmins in brain. Neuron 13, 1281–1291 10.1016/0896-6273(94)90415-4 [DOI] [PubMed] [Google Scholar]
- Varela J. A., Song S., Turrigiano G. G., Nelson S. B. (1999). Differential depression at excitatory and inhibitory synapses in visual cortex. J. Neurosci. 19, 4293–4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqui H., Schafer M. K., Zhu H., Weihe E., Erickson J. D. (2002). Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J. Neurosci. 22, 142–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Kriegstein K., Schmitz F., Link E., Sudhof T. C. (1999). Distribution of synaptic vesicle proteins in the mammalian retina identifies obligatory and facultative components of ribbon synapses. Eur. J. Neurosci. 11, 1335–1348 10.1046/j.1460-9568.1999.00542.x [DOI] [PubMed] [Google Scholar]
- Weston M. C., Nehring R. B., Wojcik S. M., Rosenmund C. (2011). Interplay between VGLUT isoforms and endophilin A1 regulates neurotransmitter release and short-term plasticity. Neuron 69, 1147–1159 10.1016/j.neuron.2011.02.002 [DOI] [PubMed] [Google Scholar]
- Xu J., Mashimo T., Sudhof T. C. (2007). Synaptotagmin-1, -2, and -9: Ca(2+) sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron 54, 567–581 10.1016/j.neuron.2007.05.004 [DOI] [PubMed] [Google Scholar]
- Zerial M., McBride H. (2001). Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2, 107–117 10.1038/35052055 [DOI] [PubMed] [Google Scholar]