Abstract
The host’s inflammatory response to sepsis can be divided into two phases, the initial detection and response to the pathogen initiated by the innate immune response, and the persistent inflammatory state characterized by multiple organ dysfunction syndrome (MODS). New therapies aimed at pathogen recognition receptors (PRRs) particularly the TLRs and the NOD-like receptors offer hope to suppress the initial inflammatory response in early sepsis and to bolster this response in late sepsis. The persistence of MODS after the initial inflammatory surge can also be a determining factor to host survival. MODS is due to the cellular damage and death induced by sepsis. The mechanism of this cell death depends in part upon mitochondrial dysfunction. Damaged mitochondria have increased membrane permeability prompting their autophagic removal if few mitochondria are involved but apoptotic cell death may occur if the mitochondrial losses are more extensive. In addition. severe loss of mitochondria results in low cell energy stores, necrotic cell death, and increased inflammation driven by the release of cell components such as HMGB1. Therapies, which aim at improving cellular energy reserves such as the promotion of mitochondrial biogenesis by insulin, may have a role in future sepsis therapies. Finally, both the inflammatory responses and the susceptibility to organ failure may be modulated by nutritional status and micronutrients, such as zinc, Therapies aimed at micronutrient repletion may further augment approaches targeting PRR function and mitochondrial viability.
1. INTRODUCTION
How and why humans respond to sepsis (pathogen invasion of the blood stream) with such devastating consequences as shock and organ failure remains a mystery. Is the host response excessive in some cases, leading to collateral damage to organs and tissue or, on the contrary, is the invasive nature of the pathogen challenge such that this seemingly excessive “septic” response is critical for survival? Of course, insight into the host response must be considered from an evolutionary perspective, i.e. from a pre-antibiotic era. How does the septic response improve the host’s chance of surviving an infectious challenge? From a therapeutics perspective, improved knowledge of the details of this complex response is likely to be required in order to develop comprehensive and definitive therapeutic approaches that extend beyond anti-infectives and supportive care.
It is in this context that we have chosen to focus on two different aspects of the host response to sepsis that we consider to be linearly connected albeit not all-inclusive. First, we direct our attention to the ability of the host to recognize and sense pathogens and to discriminate friend from foe. Much has been learned in the past decade about pathogen sensing. Importantly, pathogen sensing has important effects on the sensor cells that dramatically modulate their function with an attention to suppressing pathogen growth. This concept of sensing-induced changes in host metabolism directs us to the final and most intriguing aspect of our considerations here.
Second, we delve into fundamental aspects of the host response that highlight the role of host cell metabolism and the recently recognized secondary phase of inflammation due to release of constitutive/ components from damaged cells. The focus is on mitochondria and regulation of energy conservation in cells emphasizing non-specific stress on the host (oxidant stress caused by neutrophils, shock, intracellular free iron, and mitochondrial damage). These consequences are dictated by factors such as age (metabolic reserve) and nutrition (e.g., antioxidant status). Related to this, we then single out a vital nutritional factor, zinc, that we believe may predispose the host to the septic insult. How does nutritional status, with an emphasis upon a micronutrient such as zinc, impact the host’s ability to mount an appropriate response to the challenge ? Emerging evidence is provided in support that predisposing factors linked to nutritional status may provide the impetus for the persistence of the inflammatory response and progressive loss of organ function even after eradication of the offending organisms
2. PATHOGEN SENSING IN SEPSIS RESPONSE
The traditional view of sepsis is one of infection and a disproportionate and overwhelming inflammatory response to the pathogen resulting in organ dysfunction and death [1]. However, therapies aimed at inhibiting the inflammatory cascade have largely proven ineffective in larger clinical studies in either blunting the host’s inflammatory response or improving survival [2, 3]. Specifically, these studies have included evaluating ubiquitous, downstream inflammatory cytokines such as TNFα [4, 5] and IL-1β [6] as well as general anti-inflammatory therapy with corticosteroids [7, 8]. Indeed, the only immunosuppressive therapy to have shown benefit in large randomized controlled trials is drotrecogin α or activated protein C [9].
The failure of immunosuppressive therapy in sepsis to demonstrate benefit is likely due to several issues. First, the production of these inflammatory cytokines is a relatively early step in the pathophysiology of sepsis, patients presenting with established sepsis are unlikely to benefit from anti-TNF or IL-1β therapy [2]. Second, our understanding of the pathophysiology of sepsis has changed from overwhelming inflammation to a secondary immunosuppressed state with persistent organ failure and an inability to respond to secondary infections [10]. This aspect of sepsis will be discussed in more detail elsewhere in this Special Issue. Lastly, the redundancy of the inflammatory cascade in response to infection precludes blockade of a single cytokine or mediator to arrest the inflammatory response of the host. This realization has driven investigators to evaluate the initial innate immune response to pathogens as a potential therapeutic target, either as an anti-inflammatory therapy in early sepsis or to bolster the immune response during late sepsis. The chief targets of this early innate immune response are the pathogen sensing proteins, particularly the Toll-like receptors (TLRs) and the NOD-like receptors (NLRs), which regulate downstream effector enzymes, including inflammatory caspases.
Toll-Like Receptors
Toll was initially described in a Drosophila mutant [11] and later, homologs in mammals were identified. In 1998 toll-like receptor 4 (TLR4) was identified as the receptor responsible for endotoxin (LPS) responsiveness [12, 13] and a TLR4 mutation in mouse strains C3H/HeJ and C57BL/10ScCr was identified as being responsible the resistance these animals showed to endotoxemia. TLR4 was one of the first recognized pathogen sensing or pathogen recognition receptors (PRR). Each PRR identifies a specific pathogen-associated molecular pattern (PAMP) [14]. The PRR are highly conserved across species and represent the hosts initial defense against infection [15]. Since the identification of TLR4, ten TLRs have been identified in humans each with a different PAMP that is recognized including: peptidoglycan and atypical LPS by TLR2, LPS by TLR4, flagellin by TLR5, and bacterial DNA (CpG DNA) by TLR9 [16]. TLRs are type 1 membrane receptors with a N-terminal leucine-rich repeat (LRR) domain, a transmembrane domain, and a toll-IL-1 receptor (TIR) cytoplasmic domain [14]. The LRR domain is thought to act as the sensing region for PAMP.
The mechanism by which TLR proteins detect their respective PAMP is unclear. It appears that the PAMP is brought in proximity to the TLR by an membrane associated adapter molecules for example CD14 and MD2 for TLR4 or CD36 for TLR2 [17]. Once activated TLR initiate the inflammatory response via adapter molecules, MyD88 or TRIF. These adaptors then initiate a signal cascade which results in the initiation of the innate immune response: interferon and TNF release, nitric oxide synthase production, and activation of NFκB [18].
Toll-like receptors are essential to the initiation of the host immune response as evidenced by the finding that the expression at both a message and protein level for TLR 2 and 4 correlated with survival in a mouse model of sepsis [19]. Deficiency of TLR4 can render the host resistant to endotoxemia [12], however its role in true infection and sepsis is less clear. Original work using TLR4 knockout mice indicated that the lack of LPS responsiveness in TLR4 knockouts increased susceptibility to Gram negative infections such as Salmonella [20, 21], E. coli [22], and Neisseria [23]. In polymicrobial sepsis the picture is less clear with some investigators reporting decreased survival in mice lacking the Toll signal adaptor protein MyD88 [24], others reporting no significant difference [25], and others reporting a survival benefit [26]. Alves-Fiho et al. in 2006 reported that TLR4 knockout mice had impaired clearance and decreased survival when given Gram-negative Salmonella intraperitoneally, had no survival advantage to a sublethal polymicrobial, cecal ligation and perforation (CLP) sepsis model and had improved survival with a lethal CLP [20]. They hypothesized that this difference was accounted for by an impairment of neutrophil migration that was associated with the lethal CLP model in the wild-type mouse, thus the severity and type of infection may determine whether Toll-like receptors represent an adaptive response to sepsis.
Work to investigate pharmacologic blockade of TLR4 is ongoing. TAK-242 a signal transduction inhibitor of TLR4 has demonstrated improved survival in murine models of endotoxemia [27] and another signal transduction inhibitor M62812 has shown improved survival in a murine CLP model of sepsis [28]. Other investigators have looked at monoclonal antibodies directed at the TLR4/MD-2 complex, which, have improved sepsis outcomes in a rat endotoxemia and CLP model of sepsis [29].
Human trials of TLR4 inhibition in sepsis are ongoing, although findings from these trial have not yet been published. However, there is some data that the prior use of HMG-COA reductase inhibitors (statins) by patients decreased the rate of severe sepsis [30, 31]. Recent work has demonstrated that pretreatment with simvastatin resulted in a blunted upregulation of TLR2 and TLR4 mRNA in monocytes during an endotoxemia in healthy hosts [32]. From this we may infer that inhibition of TLR transcription is a possible mechanism for the protective effects of HMG-COA reductase inhibitors during sepsis, but further study needs to be done in this area.
Genetic evaluation of TLR4 mutations in sepsis show mixed results, with some series showing that inhibition of TLR4 signaling is beneficial and others showing that inhibition of TLR4 is deleterious in sepsis. In a study of 91 septic shock patients and 73 healthy controls, individuals with a mutation in the TLR4 receptor that renders them hyporesponsive to endotoxin were shown to have an increased incidence of septic shock and an increased incidence of Gram-negative infection [33]. Furthermore, burn victims at high risk to develop sepsis had a further increased risk of severe sepsis if they had the TLR +896G allele which renders them hyporesponsive to endotoxin [34].
In contrast, other studies have demonstrated a beneficial effect to TLR4 mutations resulting in inhibition of TLR4 downstream signaling. A function variant allele for the adapter protein Mal, which is downstream of both TLR2 and TLR4, shows that attenuation of TLR signal transduction was protective from invasive pneumococcal disease, bacteremia, malaria, and tuberculosis [35]. In addition, a variant haplotype of the NFkB inhibitor IRAK-1 which results in a gain of function in the NFkB signal transduction secondary to toll signaling shows an increase risk of organ failure and death [36]. Evaluating the early animal work and genetic studies in human sepsis together, it is too soon to tell if blockade of toll signaling will be beneficial in sepsis if administered in a clinically relevant time frame. The potential role of therapy aimed at toll signaling will likely also depend on the pathogens involved. Further study is needed to determine the therapeutic potential of these agents.
NLR Proteins (NODs and NALPs)
The NLR proteins, also known as CATERPILLAR proteins, NODs or NALPs, are a family of PRR proteins that are intracellular as opposed to the extracellular TLR family. Much like the TLRs, these proteins consist of a LRR region which is responsible for PAMP binding, but have a centrally located nucleotide-binding oligomerization domain (NOD) that mediates self-oligomerization and an amino-terminal effector domain [37]. The effector domain can be a caspase-recruitment domain in the case of the NODs or a pyrin domain in the case of the NALPs.
The NOD Proteins
The NOD class of proteins includes NOD1 and NOD2. Both are thought to operate intracellularly to detect bacterial cell wall components, diaminopimelic acid generally found in Gram negative bacteria in the case of NOD1 and muramyldipeptide (MDP), a peptidoglycan found in most bacteria in the case of NOD2 [38]. Once their associated ligand is detected, the NODs activate the inflammatory cascade via NFkB using receptor-interacting protein 2 (RIP-2) as an adapter protein [39].
Current interest in NOD1 in human disease centers on its role in asthma and eczema [40]. Its role in inflammatory bowel disease is controversial. NOD1 may also play an important role in maintain mucosal barriers to infection as it represents the primary sensing system for epithelial cells for Gram negative bacteria [41]. During sepsis, NOD1 in vascular smooth muscle may potentiate the development of refractory shock through its induction of nitric oxide synthase-II in response to NOD1 specific agonists and it is surmised in response to diaminopimelic acid from Gram negative bacteria as well [42].
Better known for its role in human disease is NOD2. NOD2 was initially described in the context of familial Crohn’s disease [43] where truncated mutant alleles (Arg702Trp, Gly908Arg, or Leu1007, fsinsC) were demonstrated to reduce NFkB activation secondary to LPS. Subsequently, NOD2 mutant alleles have also been shown to predispose to Blau syndrome, early-onset granulomatous arthritis, uveitis and skin rash with camptodactyly [44]. NOD2 knockout mice have been shown to be deficient in the anti-bacterial peptide cryptdins which renders them more susceptible to an enteral bacterial challenge, but not to an intravenous bacterial challenge [45]. There is some evidence that the Crohn’s disease mutation in NOD2 results in a paradoxical inflammatory state secondary to persistence of pathogenic intestinal flora [46].
It has been observed that NOD2 mutant alleles are associated with increased graft versus host disease during stem cell transplant and increased mortality [47]. This increased transplant related mortality was largely due to pulmonary and gastrointestinal complications, leading Brenmoehl et al. to speculate that it was an increase in bacterial translocation in the epithelial layer of these patients that was responsible [48]. An investigation into the NOD2 variant alleles during sepsis revealed that the Leu1007fsinsC mutant, which results in a severe reduction in MDP-induced NFkB signal transduction compared with wild-type, is an independent predictor of sepsis mortality [48-50].
To date, there is very little data on the potential of NOD1 or NOD2 manipulation during sepsis. Studies competed so far show that upregulation of NOD1 and NOD2 may improve mucosal integrity and perhaps prevent secondary bacterial translocation during sepsis. However, NOD2 knockout mice do show attenuation of neuronal dysfunction during sepsis [51] so further studies into the role of NOD1 and NOD2 during sepsis are needed to fully assess their potential as therapeutic targets
The NALP Proteins and the Inflammasome
The NALPS are a family of 14 intracellular PRR proteins comprised of an effecter pyrin domain, a NACHT domain, and the LRR domain thought to be responsible for pathogen recognition [52]. Little is know about the function of many of the NALPs however NALP1 (as known as DEFCAP) and NALP3 (also known as PY-PAF1 or cryopyrin) have been more extensively studied and appear to be important in human disease and especially to the innate immune response during infection [53].
The function of the NALP family of PRRs is to act as a molecular scaffold for the inflammasome, a molecular platform for the activation of the inflammatory caspase, caspase-1 [54]. The activation of caspase-1 depends in part on the NALP scaffold protein, but requires the apoptosis-associated speck-like protein containing a CARD (ASC) and another inflammatory caspase, caspase-5 for assembly [55]. Thus far inflammasomes for several of the NALP proteins have been identified and are thought to assemble in response to different PAMPs. The structure of the inflammasome is shown in Fig. (1).
Fig. (1). Inflammasome Structure.
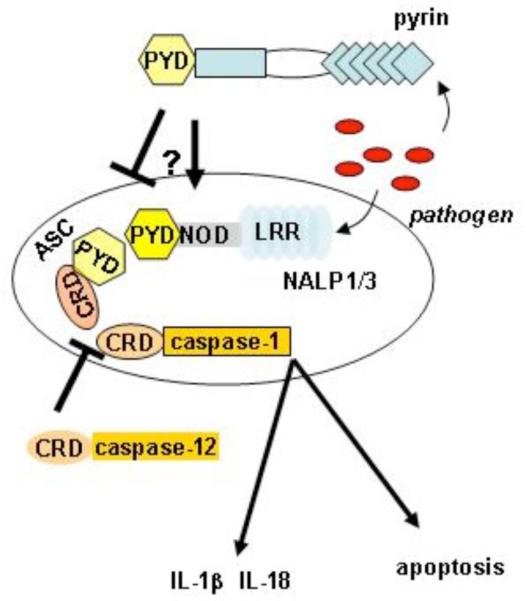
Pathogens can be recognized intracellularly by NALPs (e.g. NALP1 [54] and NALP3 [61]) via their leucine rich repeats (LRR) and possibly by pyrin [70] to induce the assembly of a protein complex termed an inflammasome. This interaction involves the electrostatic interaction of CARD (CRD) domains and pyrin domains (PYD) via the adaptor protein ASC. An assembled inflammasome can drive cell apoptosis and the generation of “danger” signals such as IL-1β and IL-18 by the activation of the cysteine protease, caspase-1. Caspase-12 a homologue of caspase-1 may downregulate the inflammasome [80] as may pyrin in certain situations .
The NALP1 inflammasome was the first to be described. It is thought to assemble in response to LPS [54] or anthrax toxin [56] and induce the activation of caspase-1 which subsequently triggers the processing and release of IL-1β and IL-18. In addition, activation of caspase-1 may be an integral contributor to apoptosis seen in sepsis (see discussion below). The importance of NALP1 in inflammation was recently demonstrated when SNPs in the NALP1 gene were found in patients with generalized vitiligo, autoimmune thyroid disease, latent autoimmune diabetes in adults, rheumatoid arthritis, psoriasis, pernicious anemia, systemic lupus erythematosus, and Addison’s disease [57-59]. The role of NALP1 in human sepsis is not entirely clear; however, our group has recently found that monocytes from patients with septic shock had decreased mRNA for NALP1 and that the suppression of NALP1 was associated with increased severity of illness and mortality during septic shock [60].
The NALP3 inflammasome is better understood as it has been described in several human disease states. NALP3 inflammasome assembly is thought to occur in response to bacterial MDP (similar to NOD2) [61]. Mutations in the CIAS1 encoding for NALP3 result in an exaggerated production of IL-1β in response to MDP and clinically these patients present with Muckle-Wells syndrome [62]. Other familial disorders associated with NALP3 mutations include familial cold urticaria [63], AA amyloidosis [64], and neonatalonset multisystem inflammatory disease (NOMID or chronic infantile neurologic, cutaneous, articular CINCA syndrome) [65, 66]. All of these conditions result in periodic inflammatory changes thought to result from the dysregulation of caspase-1 activation by NALP3. Another potentially important molecule in sepsis response is the protein pyrin discovered as the molecule responsible for familial Mediterranean fever [67, 68], a disease characterized by excessive IL-1β production and relapsing fevers. Pyrin provided the prototype for the pyrin domain (PYD) structure which is present in all NALPs. Pyrin has been shown to variably enhance or suppress inflammasome function [69, 70].
The role of NALP3 and pyrin during sepsis is only beginning to be studied. In NALP3 knock out mice there was only partial protection from endotoxemia [71]. We have recently found an interesting relationship with pyrin expression in critically ill children, i.e., enhanced survival in those who downregulated monocyte pyrin mRNA levels [72], presumably secondary to pyrin’s suppression of NALP3 and caspase-1 activation. In contrast, genetic studies have demonstrated a higher prevalence of mutations in the pyrin gene, not associated with symptomatic FMF, in individuals with infections or sepsis [73]. This increased prevalence was attributed to an increased inflammatory state of individuals with the pyrin mutation. Thus, the role of NALP1 and NALP3 during sepsis is likely related to the etiology and time course of the infection and further study into the NALPs in human sepsis are needed to assess their potential as therapeutic targets. In total the data on NALPs in sepsis support the central role of caspase-1 in sepsis as discussed below.
Inflammatory Caspases
The inflammatory caspases (cysteinyl aspartic acid protease) including principally caspase-1, also referred to as interleukin-1 converting enzyme (ICE), play an integral role in the severity of the host response to sepsis (Fig. 1). Caspase-1 is constituently expressed in monocytes, macrophages, and neutrophils [74]. As the downstream target of the NALP proteins and as the processor of IL-1β and IL-18, caspase-1 is necessary for the initial attempt at bacterial clearance by the innate immune system [75]. Caspase-1 has been implicated as both pro-inflammatory and pro-apoptotic [76] and contributes to the apoptosis of the immune system associated with sepsis [77].
The full role of the caspase-1 during sepsis is unclear, but the extent of caspase-1 activity may correlated with the severity of septic injury. In a murine model of intra-abdominal sepsis, caspase-1 messenger RNA correlates with the degree of apoptosis of the gut epithelium [78] and in murine endotoxemia, caspase-1 activity in the spleen was increased in mice receiving lethal injury compared with sub-lethal endotoxin doses [79].
Insight into the importance of caspase-1 in sepsis can be seen in the recently described caspase-12 sepsis connection. Wild-type caspase-12 (the long form) is believed to inhibit caspase-1. It confers hyporesponsiveness to LPS and is associated with an increased risk of severe sepsis in human population studies [80]. The wild-type gene is less frequent than the mutant allele which is found worldwide except in some populations of African descent. It has been speculated that the mutated caspase-12 gene which has a premature stop codon resulting in a truncated protein was evolutionarily selected as population densities and thus risk of infectious disease and sepsis increased [81]. Mice that are caspase-12 deficient show a resistance to intraabdominal sepsis and enhanced bacterial clearance [82]. The contribution of caspase-12 in sepsis may be linked to regulatory effects on caspase-1, as caspase-1 activation is important to the initial phase of bacterial clearance during sepsis. It’s role in late sepsis is less clear
Caspase-1 knockout mice are resistant to endotoxemia [83] and initial work using synthetic generalized caspase inhibitors demonstrated improved mortality in mouse CLP models of sepsis [84, 85]. Our group showed that mice deficient in caspase-1 are protected from sepsis induced apoptosis and death due to intraperitoneal E.coli [77] and that this protection was independent of its role as an activator of IL-1 and IL-18 and thought to be secondary to a reduction in lymphocyte apoptosis. Caspase-1 knockout mice were also protected from enteral salmonella infections without any difference compared with wild-types when challenged with intravenous Salmonella [86]. Notably, inhibition of caspase-1 also protected against endotoxin induced acute renal failure [87] and acute pancreatitis associated acute lung injury [88].
However, controversy as to the beneficial effects of caspase-1 inhibition exists, as caspase-1 knockout animals have also been shown to be susceptible to Shigella when given intranasally [89], and to pathogenic stains of E.coli given intraperitoneally [79]. Taken together, it may be that the benefits of caspase-1 inhibition depends of the extent of inhibition and the timing of drug delivery as therapy with caspase-1 inhibitors results in improved survival in a wide variety of murine sepsis models, even when given hours post infectious insult [77, 84, 85, 90]. Thus caspase-1 inhibitors may be a promising new therapy in sepsis and further study to define the risks and benefits of these agents are needed.
Other Potential Functions of Pathogen Sensing
Although therapeutic approaches attempting to modulate the host response to pathogen sensing have traditionally focused on blocking the effects of secreted cytokines (as highlighted above), increased understanding of the downstream consequences of TLR and NLR signaling events will be crucial to the development of novel therapeutics. Responses to pathogens may also modulate more fundamental functions of the host cell. As the studies that have focused on caspase-1 regulation highlight, early receptor mediated activation of NFκB and caspases can have profound effects on many other functions including regulation of essential nutrients (e.g. Fe++ and Zn++) and upregulation of antimicrobial peptides [91-99]. Furthermore, it is becoming abundantly clear that the host’s baseline tissue and organ energy reserve is a critical predictor of outcome. Although pathogen challenges may acutely modulate tissue energy reserves, constitutive factors such as age, baseline nutritional status, and antioxidant stores are likely important contributing factors that determine the overall effectiveness of the host response. Furthermore, when host cells do succumb to these added pressures, their death may produce a second wave of cell stress via the release of cell components (e.g. HMGB1) that are also inflammatory. These fundamental but often overlooked aspects of the sepsis response are reviewed in the last section of this review.
3. THE MECHANISTIC LINK BETWEEN ORGAN DAMAGE AND INFLAMMATION
Recent advances in our understanding of the mechanistic link between early organ injury, resulting from inflammation and/or tissue hypoperfusion (shock), and delayed organ failures, commonly referred to as multiple organ dysfunction syndrome (MODS), may provide novel therapeutic opportunities. The risk of sepsis-related mortality increases significantly as the number of organ failures increases [100], with most deaths occurring days to weeks after the onset of sepsis. In some cases death occurs after the primary infection has been controlled through the innate immune response and clinical interventions, including surgical drainage and antimicrobial treatments [101]. While certain components of the immune system are compromised following the acute phase of sepsis, others remain hyperactive, as reflected by ongoing inflammation at sites remote from the source of infection [102] and high levels of circulating pro-inflammatory cytokines [103]. Until recently it was unclear why certain aspects of the immune system remain active even after the resolution of the acute infection.
The potent immune response required to effectively eradicate infection produces significant collateral damage, wherein the toxic enzymes and oxidant species released from activated immune cells are capable of destroying regional parenchymal cells. Furthermore, in some cases the hemodynamic manifestations of sepsis (i.e., shock) serve to amplify cell and organ damage. In the case of pneumonia or other localized infections, the normal tissue architecture may be almost entirely replaced by inflammatory cells and damaged cellular debris. Cell damage is initially restricted to the area of infection; however, a delocalization of the inflammatory response can occur in the setting of severe sepsis causing wide-spread cell and tissue damage remote from the site of infection. The putative mechanism of MODS in the context of severe localized infection or other types of focal tissue damage (e.g., burns, pancreatitis) is the systemic release of pro-inflammatory cytokines resulting in the expression of endothelial receptors for neutrophil adherence [104] and stimulation of large reservoirs of macrophages in the liver spleen and lungs [105, 106], a phenomenon that has been dubbed the “systemic inflammatory response syndrome” or SIRS [107]. However, it is becoming increasingly apparent that other signals, including the release of components of damaged cells that are recognized by the immune system, contribute significantly to MODS.
The Mechanism of Cell Injury Dictates the Immune Response
Upon resolution of the infection, inflammatory cells and damaged parenchymal cells are removed through programmed cell death (apoptosis) and/or ingested by regional macrophages. Apoptosis is considered to be the preferred mode of cell death, as this process is not associated with induction of innate immune cell activation. In fact, it has recently been shown that recognition of specific apoptotic cell-associated antigens by CD8 alpha(+) dendritic cells results in suppression of immune responses to cell-associated antigens [108]. In contrast, during “accidental” or necrotic cell death potent immune cells are mobilized and activated. In this way, acute cell and tissue damage can provoke secondary activation of the immune system.
In contrast to apoptotic cell death wherein potentially immunogenic components, such as DNA and proteins, are enzymatically degraded, necrotic cell death leads to the release of intact proinflammatory antigens that are normally sequestered within the cell. This is an emerging area of research and, as expected, some controversy surrounds the mechanism by which necrotic cells promote the activation of immune cells. In this regard, in 1982 Carp demonstrated that mitochondrial DNA-encoded peptides, which are similar to bacterial peptides in that they possess N-formyl peptide sequences, are potent chemoattractants for neutrophils [109]. More recently, Scaffidi et al. reported that monocytes respond specifically to a nuclear chromatin binding protein, high mobility group box 1 (HMGB1), through the activation of receptors for advanced glycation end products [110]. However, subsequent studies have suggested that highly purified (uncontaminated) HMGB1 has no intrinsic pro-inflammatory action [111], but acts in synergy with other antigens, particularly CpG dinucleotides, to promote innate immune responses [112]. In eukaryotes, CpG dinucleotides are restricted to the mitochondrial compartment where they are associated with mitochondria-specific chromatin binding proteins [113]. Thus, it is unclear how HMGB1 would become associated with mitochondrial DNA in the context of cell necrosis. Nonetheless, it would appear that CpG DNA and HMGB1 can contribute to “sterile” inflammation in the context of autoimmune disease [114], and HMGB1 has been shown to prime the immune system to bacterial antigens responses [115]. However, recent investigations clearly demonstrate that HMGB1 is not primarily responsible for the induction of innate immune responses to necrotic cells in vivo [116]. Thus, other intracellular antigens are incriminated. Interestingly, using cell fractionation techniques, unpublished data from our laboratories show that the mitochondrial fraction of necrotic cells is by far the most potent in terms of inducing innate immune responses. Moreover, the mitochondrial homologue of HMGB1, mitochondrial transcription factor A (TFAM), can act synergistically with other mitochondrial antigens to promote immune cell activation. Thus, more work is needed to identify the specific antigens and the immune cell receptors responsible for inducing inflammatory responses to necrotic cells and damaged tissues in the context of sepsis-induced organ injury.
Despite the controversy currently surrounding the specific cell components responsible, there is evidence that interruption of the cycle of tissue damage and inflammation is possible even after sepsis is well established. To the extent that damaged parenchymal cells and inflammatory cells committed to death during their activation by LPS and other bacterial components [117] promote delayed systemic inflammation through the release of HMGB1 and other immunogenic factors, it follows that treatments designed to interrupt this inflammatory response would be effective in the late phase of sepsis. In this regard, a recent study showed that a blocking antibody against HMGB1 effectively reduces mortality in mice, even when administered 24 hrs after the onset of sepsis [118]. Similar results were obtained with a soluble inhibitor of the receptor for advanced glycation end products (RAGE), a putative receptor for HMGB1 [119]. The potential benefits of inhibiting TREM-1/TLR4 [115], TLR9 [112] and other receptors known to promote inflammatory responses to self-antigens remain to be determined in the context of sepsis and other conditions associated with acute tissue damage (pancreatitis, trauma, burns, etc.). However, attenuation of innate immune responses consequent to tissue damage holds promise for the treatment of patients with signs of ongoing inflammation in the absence of identifiable infection, which commonly occurs in the late phase of sepsis during the evolution of MODS.
Cytoprotective Measures as a Strategy to Interrupt the Cycle of SIRS and MODS
The mechanisms regulating cell apoptosis are discussed in detail elsewhere in this issue. However, several observations are worthy of discussion in the context of inflammation. The response of a cell to stress, such as occurs in areas of inflammation or ischemia caused by shock, varies according to the nature of the cell and other host factors, including age, nutritional status, and genetics. These factors dictate how effectively the cell will fend itself from oxidative stress (e.g., antioxidants), how quickly oxidant stress will be resolved (e.g., DNA repair enzymes, enzymatic removal of irreversibly altered lipids and proteins), and how effectively the damaged components of the cell are replaced. In this regard, sublethal cell injury is associated with the autodigestion of irreversibly damaged cell organelles, such as damaged mitochondria [120-122]. The mechanisms regulating mitochondrial autophagy also dictate cell life-death decisions [122]. In particular, the open/closed status of the mitochondrial permeability transition pore (MPTP), a high conductance pore that is permeable to molecules and solutes up to 1.5 kDa in size is highly relevant. Sustained opening of the MPTP, commonly referred to as the mitochondrial permeability transition (MPT), leads to dissolution of the chemiosmotic gradient generated across the inner mitochondrial membrane by proton pumping (the result of electron transport) and the inability to produce ATP. The de-energization of the mitochondrion causes activation of endogenous enzymes (autolysis) and eventual lysosomal removal (autophagy) of individual mitochondrion [120]. When a critical number of mitochondria become damaged/deenergized, mitochondrial apoptosis is triggered [122]. All of these cell-repair processes are energy-requiring, and the condition of sepsis is further complicated by compromise of the bioenergetic pathways, most notably altered mitochondrial respiration [123-125]. Thus, it becomes necessary for a compromised cell to “shut down” in order to facilitate its own repair, thus superseding its contribution to organ function. Otherwise, the energy status of the cell, as reflected by ATP levels, would reach critically low values, leading to necrotic cell death [126]. Indeed, in the first human study of its kind, Brealey et al. showed that tissue ATP levels, and attendant decreases in mitochondrial respiratory capacity, more accurately predicted sepsis mortality than did conventional severity of illness metrics [124].
Studies using animal sepsis models show that mechanisms contributing to oxidant stress can be targeted to significantly attenuate organ damage and dysfunction in the context of sepsis. The situation with oxidant stress is quite complicated, because certain cell compartments are particularly susceptible to oxidative damage and have adapted unique antioxidant defenses. For instance, mitochondria, the largest producers of oxidants, are equipped with a series of enzymes that catalyze the detoxification of superoxide radicals, the exhaust produced during oxidative phosphorylation, to produce water. In this regard, sepsis induces an imbalance in the expression of catalase relative to superoxide dismutase resulting in a buildup of hydrogen peroxide (H2O2), which explains the encouraging results of intracellular catalase mimetics [127] and H2O2 scavengers [128] in reducing organ damage and mortality in animal models of sepsis.
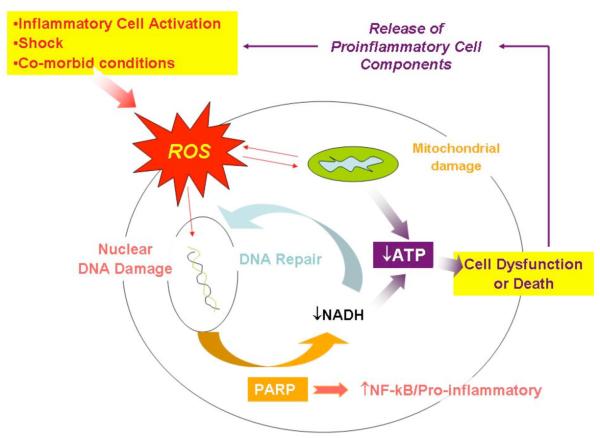
DNA, both mitochondrial and nuclear, is another important target of oxidative stress, and DNA damage has important implications for cell and organ function during sepsis. DNA damage leads to the activation of the DNA repair protein poly(ADP-ribose) polymerase (PARP), which has a number of important implications in terms of fate of the cell (see Pacher and Szabó for a comprehensive review [129]). For instance, PARP activation consumes large amounts of α-nicotinamide adenine dinucleotide (NAD), which, along with oxygen, is a substrate for mitochondrial ATP production. Consequently, highly aerobic cells that sustain significant DNA damage undergo rapid ATP depletion and death in response to excessive PARP activation. Interestingly, supplementation of substrate for mitochondrial ATP production (i.e., pyruvate) protects against cell death in the setting of acute DNA damage [130]. The situation during sepsis is further complicated by altered pyruvate [131] and mitochondrial metabolism [132]. Thus, energy metabolism and DNA repair are intimately related. Moreover, a direct link between PARP activation and NFκB-regulated pro-inflammatory gene transcription has also been observed [133]. As shown schematically in Fig. (2), PARP is centrally positioned to influence cell and organ function and inflammatory responses in the setting of acute illnesses, such as sepsis.
Fig. (2). The putative role of PARP in the regulation of cell viability and inflammation.
Sepsis presents a number of challenges to host cells, including exposure to oxidants produced by immune cells (e.g., PMNs), tissue hypoxia, and direct effects of cytokines (e.g., TNFα) on the function of mitochondria [177]. PARP plays a central role in the repair of oxidant-induced DNA damage, which comes at the expense of ATP substrate (NADH). Thus, the combination of mitochondrial dysfunction (↓ capacity of aerobic ATP synthesis) and PARP activation (↓ ATP substrate) results in compromised cell function or even necrotic cell death. The latter, together with activation of NF-κB by PARP, can promote a second wave of inflammation (see text).
The relationship between PARP activation and sepsis-induced MODS appears to be significant. In humans who died of sepsis, the activity of PARP was shown to correlate with impaired cardiac performance and acute myocardial injury [134]. Furthermore, transgenic mice deficient in PARP [135] or animals treated with PARP inhibitors [133, 136] demonstrated less severe organ damage and improved mortality during conditions modeling sepsis. The encouraging results of animal studies, wherein manipulation of PARP is performed in advance of sepsis onset, must be viewed with caution pending the results of trials wherein the intervention is rendered after the onset of sepsis.
Rather than attempting to limit ATP-consuming pathways, such as the DNA and protein repair mechanisms described above, it may also be beneficial to enhance ATP production. This approach would presumably allow the cells and organs to maintain function, even in the face of ATP-dependent cell repair. However, ATP and other high energy phosphates are unable to penetrate cell membranes and extracellular ATP possesses various pro-inflammatory actions [137, 138]. Thus, optimization of the ATP-producing mitochondria, which account for more than 90% of the ATP production of the most cell types, may be the most effective approach. In this context, measures designed to interrupt mitochondrial turnover and mitochondria-dependent cell death (apoptosis) related to induction of the MPT have shown dramatic benefit in animal models. For instance, pharmacological inhibitors of the MPT, including cyclosporin A and related drugs, are shown to mitigate mitochondrial ultrastructural damage [139], preserve mitochondrial respiratory function [139, 140], improve organ function [121] and reduce mortality [140]. In keeping with these findings, over-expression of endogenous MPT inhibitors (e.g., Bcl-2) is shown to improve vital organ function and survival in a murine model of sepsis [140]. Melatonin, a pleiotropic hormone that is shown to protect mitochondria during sepsis [141], also improves sepsis mortality in rodents [142]; however, the timing and duration of melatonin administration appears to be critical [143]. As with most other treatments that have demonstrated benefit in animals, it remains unclear if these promising new therapies are efficacious after the onset of severe sepsis or if the findings are relevant to humans.
Mitochondrial Biogenesis: The Role of Insulinomimetic Agents
Typically, the diagnosis and treatment of severe sepsis is significantly delayed such that some degree of organ damage is already present, which obviates the utility of preventive measures, such as bolstering of anti-oxidant defenses and inhibition of mitochondrial autophagy/apoptosis. However, recent advances in our understanding of endogenous regulators of mitochondrial biogenesis may be of use in terms of repopulating energy-strapped organs with new mitochondria. Insulin is a potent mitochondrial biogenesis factor which is shown to preserve mitochondrial integrity [144] and improve clinical outcomes in patients with more severe sepsis [145]. Like insulin, peroxisome proliferators-activated receptor-γ (PPARγ) agonists promote mitochondrial function [146], and are shown to improve organ function during sepsis. However, the mechanism of organ protection by the PPARγ agonists may relate more to anti-inflammatory effects rather than direct mitochondrial effects [147]. The role of other mitochondrial biogenesis factors, such as nitric oxide derived from the endothelial nitric oxide synthase isoform (eNOS) [148], or other insulinomimetics, such as zinc complexes [149, 150], have not yet been explored. Considered together, agents designed to mitigate mitochondrial damage and to augment mitochondrial biogenesis deserve further consideration as potential treatments for sepsis-induced organ failures.
4. NUTRITION, IMMUNE FUNCTION AND SEPSIS
Poor nutrition is a significant host factor that causes immune suppression and increases susceptibility to developing sepsis [151, 152]. Likewise, septic patients have increased energy expenditure and catabolic needs [153, 154]. Trace elements that support anti-oxidant function have been recommended as adjuncts for use in critically ill patients yet we do not know which patients may benefit from trace mineral supplementation. The remainder of this review will consider the example of a single trace metal, zinc, an essential nutrient that is intimately connected to pathogen sensing, immune function, oxidative balance, and the inflammatory response in the setting of sepsis. From this discussion it will become evident that nutritional variables are likely to contribute significantly to the outcome of sepsis.
Relationship between Zinc and Sepsis
Humans, in response to sepsis or endotoxin administration, experience an acute decrease in plasma zinc that occurs without a concomitant loss in whole body content [155]. Alterations in plasma metal content result from bio-redistribution into cellular and tissue compartments [156]. This redistribution likely reflects the innate host response to redirect trace metals intracellularly for cyto-protective functions that involve protein synthesis, neutralization of reactive oxygen species, and prevention of microbial invasion [157]. In addition, enzymes that regulate oxidant defense including superoxide dismutase, catalase and glutathione reductase, depend on zinc for normal function. This is particularly relevant since sepsis patients have a reduced capacity to detoxify reactive oxygen species (ROS)[158, 159]. Moreover, the more severe the insult, the larger is the depletion of antioxidant function [160]. The consequences of defective antioxidant defense during sepsis are significant and can result in augmentation of the systemic inflammatory response and increased cell injury [161].
Our group has recently observed that zinc deficiency in adult mice, at a level sufficient to mimic moderate zinc deficiency in humans, significantly augments inflammation, organ injury, and mortality in response to polymicrobial sepsis (unpublished observation). These findings are in agreement with other published findings using similar animal models. Therefore, it is reasonable to postulate that individuals who are nutritionally deficient in trace metals, in comparison to sufficient individuals, may be at a disadvantage in their ability to respond to microbial challenges as well as to mount a systemic response during sepsis. Although zinc nutriture can be determined to some extent, for practical reasons it is not routinely done. This is further complicated by a lack of specific indices or markers to accurately determine body content over an extended time frame, prior to sepsis. Considering the fundamental role that zinc play in so many biologic pathways, we propose that nutritional zinc status has the potential to significantly influence clinical outcomes in the setting of sepsis. In particular, zinc deficiency is capable of amplifying the immune response and increasing host susceptibility to cellular injury and multiple organ dysfunction. If correct, then the ability to prospectively identify nutritional deficiencies with greater precision, zinc being one example, may provide opportunities to prevent or treat disease.
Metal Homeostasis, Human Disease and Immune Function
Zinc is a divalent metal cation and micronutrient that is required to sustain life. Maintenance of total zinc body composition and cellular content in humans, defined as zinc homeostasis, is tightly controlled with approximately 1% of total body zinc content replenished daily by dietary intake [157]. Metabolism is tightly regulated in mammals by zinc transporters, a family of multiple transmembrane spanning domain proteins that are encoded by two solute-linked carrier (SLC) gene families: SLC30 (a.k.a. ZnT) and SLC39 (a.k.a. Zip). In collaboration with transporters, zinc plays a fundamental housekeeping role in physiology, cellular metabolism and gene expression and serves as a catalytic cofactor for over 300 enzymes in addition to stabilizing the structure of literally thousands of protein domains [162]. When dietary intake is low for sustained periods of time, a negative zinc balance occurs affecting multiple tissue compartments [163]. At the cellular level, deficits in zinc content result in diminished cytoprotection, wound healing, tissue repair and modulation of the inflammatory response [164]. We and others have shown that zinc deficiency enhances apoptosis in response to relevant inflammatory stimuli and ROS [165-167]. Zinc has also been proven to play a substantial role in maintenance of host immune function.
Zinc deficiency rapidly diminishes antibody- and cell-mediated responses in both humans and animals resulting in increased risk of infection. In particular, thymic atrophy, lymphopenia, and compromised cell- and antibody-mediated responses are immunologic hallmarks of zinc deficiency in humans [168]. The features of zinc-mediated immunoparalysis are remarkably similar to the features of immunoparalysis that occur in sepsis patients [169].
Molecular Targets of Trace Metals
The consequences of zinc deficiency may also be manifest by increasing inflammation, oxidant stress, tissue dysfunction and in extreme situations, mortality. Since many proteins utilize zinc as part of their structure or function (greater than 3% of the human genome) [157] we anticipate that multiple signaling networks are modified by zinc status in the setting of sepsis. Work by Kitamura and colleagues was the first to reveal a direct connection between zinc metabolism and pathogen recognition [96]. Nuclear Factor (NF)-κB is a transcription factor central to many of the signaling networks involved in sepsis and its function may be adversely affected by zinc deficiency [170]. NF-κB is activated by most pathogens commonly associated with sepsis and its activity is markedly elevated in every organ studied in septic patients. Moreover, higher levels of activity are associated with a more robust pro-inflammatory response and higher mortality rates in sepsis patients [171]. Zinc has a concentration-dependent effect on cytokine production. Low physiologic concentrations of zinc potentiate NF-κB activity causing enhanced cytokine and chemokine production (both proinflammatory and anti-inflammatory) whereas supraphysiologic concentrations of zinc attenuate cytokine production in response to LPS [172, 173] by interacting with proximal signaling intermediates thereby suppressing NF-κB activation [174]. NF-κB activation is also directly linked to SOD function and regulation of ROS. Inflammation leads to the generation of ROS. Previous reports and our own observations demonstrate that zinc deficiency leads to attenuation of SOD activity thereby favoring an increase in the formation of ROS. In fact, zinc itself has the potential to act indirectly as an antioxidant by virtue of its interaction with sulfur (Zn-S). Within the cell the reversible Zn-S interaction regulates mechanisms of enzyme catalysis, allows zinc to be tightly bound and yet to be available, and importantly, generates redox-active coordination environments for the redox-inert zinc ion thereby allowing zinc to assist in “buffering” oxidant environments (as reviewed by Maret [175]). Therefore, zinc deficiency can further decrease the intracellular capacity to tolerate oxidant environments.
In summary, selected nutritional deficiencies of metals (e.g., zinc) and other nutrients may have profound effects upon the outcome of sepsis. The example of zinc deficiency highlights the role of vital nutrients in the regulation of immune responses and cellular defenses in the context of severe infections. Although the role of zinc deficiency is emphasized herein (Table 1), there are many other potential predisposing conditions that could influence clinical outcomes during sepsis. As depicted in Fig. (3), various environmental factors, including factors that have not yet been considered, are likely to increase one’s predilection for sepsis-induced organ failure. Additional compounding genetic and acquired factors undoubtedly are important, with age and history of alcoholism being particularly recognized. Indeed, bacterial sensing itself may induce compounding effects on the availability of metals. This has been well known for iron regulation in response to LPS and inflammation whereby activation of TLRs drives iron regulation [93, 95]. In this context, pathogen-driven signaling events that drive the metal “hoarding” process are integral predisposing, as well as modifying, components of the sepsis syndrome.
Table 1.
Links Between Zinc Deficiency and Stages of Sepsis.*
| Stages of Sepsis |
ZN++ Metabolic Status | |
|---|---|---|
| Normal | Deficient [155,156,164] (Diet, Genetic, Disease, Age) |
|
| Pathogen Recognition |
++ | +++ (Increased Response) [168] |
| Pro-Inflammatory Response |
+ | +++ (Increased Cyto- and Chemokine Production) [96,170,172,174] |
| Oxidant Production |
+ | +++ (Increased ROS) [175] |
| Anti-Inflammatory Response |
+ | +++ (Immunosuppressed) [168] |
| Organ Injury | + | +++ (Increased Cell Deah) [164-168] |
| Outcome | Increased Survival |
Decreased Survival [32-36] |
numbers refer to published references for each component.
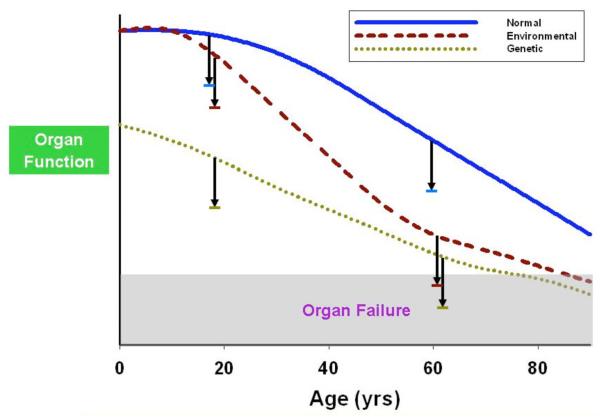
Fig. (3). The “threshold” hypothesis of organ failure during sepsis.
Assuming that a nominal level of metabolic activity is required to sustain the function of any given cell or vital organ, certain preexisting conditions could predispose to organ failures during sepsis. Genetic defects of cell metabolism are not rare [176], and given a the same amount of “collateral damage” resulting from sepsis, represented by the downward arrow, these patients are expected to be at increased risk for organ failure. Likewise, those with malnutrition, deconditioning, or co-morbid conditions (e.g., advanced age) would be susceptible to organ failure in the context of a similar infectious challenge.
CONCLUSIONS
Host responses to pathogenic challenges represent some of the earliest and most fundamental functions of living organisms. Beginning with the development of divergent single cell organisms, it became important for organisms to distinguish self from non-self. This competition for limited resources between organisms has driven the evolution of increasingly more complex mechanisms for distinguishing self from non-self and for distinguishing friend from foe. These mechanisms are undoubtedly hard-wired into our cellular frameworks and yet we are only now beginning to understand some of the intricacies and nuances of this struggle. In this review, we have highlighted selected components of this struggle. Recognition of pathogen challenges by a host of extracellular (e.g. TLR) and intracellular (e.g. NLR) sensors drive not only alarm signals such as cytokine release but probably also drive fundamental changes in energy metabolism and decisions about individual cell survival that have complex effects on organ function and overall host survival. The ultimate changes within the challenged cell involve important modifications of mitochondrial function, activation of ROS and a recently described positive inflammatory feedback loop driven by necrotic cell components like HMGB1 and CpG DNA. Importantly, the overall functional reserve of host cells and organ reserve at the time of a septic challenge determine overall susceptibility to the detrimental effects of the hard-wired host response. In short, the interconnections between pathogen sensing, the resultant cell injury and competition for nutrients demonstrate the complexity of the sepsis response. It is therefore important that future novel therapeutics take a broad view on the impact that specific targeted therapies may have upon the interplay among individual components of the host response.
REFERENCES
- [1].Thomas L. Germs. N Engl J Med. 1972;287:553–5. doi: 10.1056/NEJM197209142871109. [DOI] [PubMed] [Google Scholar]
- [2].Vincent JL, Sun Q, Dubois MJ. Clinical trials of immunomodulatory therapies in severe sepsis and septic shock. Clin Infect Dis. 2002;34:1084–93. doi: 10.1086/339549. [DOI] [PubMed] [Google Scholar]
- [3].Riedemann NC, Guo RF, Ward PA. The enigma of sepsis. J Clin Invest. 2003;112:460–7. doi: 10.1172/JCI19523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Abraham E, Anzueto A, Gutierrez G, Tessler S, Pedro G San, Wunderink R, et al. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet. 1998;351:929–33. [PubMed] [Google Scholar]
- [5].Cohen J, Carlet J. INTERSEPT: an international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor-alpha in patients with sepsis. International Sepsis Trial Study Group. Crit Care Med. 1996;24:1431–40. doi: 10.1097/00003246-199609000-00002. [DOI] [PubMed] [Google Scholar]
- [6].Opal SM, Fisher CJ, Jr, Dhainaut JF, Vincent JL, Brase R, Lowry SF, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med. 1997;25:1115–24. doi: 10.1097/00003246-199707000-00010. [DOI] [PubMed] [Google Scholar]
- [7].Cronin L, Cook DJ, Carlet J, Heyland DK, King D, Lansang MA, et al. Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med. 1995;23:1430–9. doi: 10.1097/00003246-199508000-00019. [DOI] [PubMed] [Google Scholar]
- [8].Lefering R, Neugebauer EA. Steroid controversy in sepsis and septic shock: a meta-analysis. Crit Care Med. 1995;23:1294–303. doi: 10.1097/00003246-199507000-00021. [DOI] [PubMed] [Google Scholar]
- [9].Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- [10].Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- [11].Mitchell JA, Fitzgerald KA, Coyle A, Silverman N, Cartwright N. TOLLing away in Brazil. Nat Immunol. 2006;7:675–9. doi: 10.1038/ni0706-675. [DOI] [PubMed] [Google Scholar]
- [12].Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- [13].Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- [14].Mitchell JA, Paul-Clark MJ, Clarke GW, McMaster SK, Cartwright N. Critical role of toll-like receptors and nucleotide oligomerisation domain in the regulation of health and disease. J Endocrinol. 2007;193:323–30. doi: 10.1677/JOE-07-0067. [DOI] [PubMed] [Google Scholar]
- [15].Girardin SE, Sansonetti PJ, Philpott DJ. Intracellular vs extracellular recognition of pathogens--common concepts in mammals and flies. Trends Microbiol. 2002;10:193–9. doi: 10.1016/s0966-842x(02)02334-x. [DOI] [PubMed] [Google Scholar]
- [16].West AP, Koblansky AA, Ghosh S. Recognition and signaling by toll-like receptors. Annu Rev Cell Dev Biol. 2006;22:409–37. doi: 10.1146/annurev.cellbio.21.122303.115827. [DOI] [PubMed] [Google Scholar]
- [17].Miyake K. Roles for accessory molecules in microbial recognition by Toll-like receptors. J Endotoxin Res. 2006;12:195–204. doi: 10.1179/096805106X118807. [DOI] [PubMed] [Google Scholar]
- [18].Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- [19].Williams DL, Ha T, Li C, Kalbfleisch JH, Schweitzer J, Vogt W, et al. Modulation of tissue Toll-like receptor 2 and 4 during the early phases of polymicrobial sepsis correlates with mortality. Crit Care Med. 2003;31:1808–18. doi: 10.1097/01.CCM.0000069343.27691.F3. [DOI] [PubMed] [Google Scholar]
- [20].Alves-Filho JC, de Freitas A, Russo M, Cunha FQ. Toll-like receptor 4 signaling leads to neutrophil migration impairment in polymicrobial sepsis. Crit Care Med. 2006;34:461–70. doi: 10.1097/01.ccm.0000198527.71819.e1. [DOI] [PubMed] [Google Scholar]
- [21].O’Brien AD, Rosenstreich DL, Scher I, Campbell GH, MacDermott RP, Formal SB. Genetic control of susceptibility to Salmonella typhimurium in mice: role of the LPS gene. J Immunol. 1980;124:20–4. [PubMed] [Google Scholar]
- [22].Hagberg L, Hull R, Hull S, McGhee JR, Michalek SM, Svanborg Eden C. Difference in susceptibility to gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect Immun. 1984;46:839–44. doi: 10.1128/iai.46.3.839-844.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Woods JP, Frelinger JA, Warrack G, Cannon JG. Mouse genetic locus Lps influences susceptibility to Neisseria meningitidis infection. Infect Immun. 1988;56:1950–5. doi: 10.1128/iai.56.8.1950-1955.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Peck-Palmer OM, Unsinger J, Chang KC, Davis CG, McDunn JE, Hotchkiss RS. Deletion of MyD88 markedly attenuates sepsis-induced T and B lymphocyte apoptosis but worsens survival. J Leukoc Biol. 2008 doi: 10.1189/jlb.0807528. [DOI] [PubMed] [Google Scholar]
- [25].Echtenacher B, Freudenberg MA, Jack RS, Mannel DN. Differences in innate defense mechanisms in endotoxemia and polymicrobial septic peritonitis. Infect Immun. 2001;69:7271–6. doi: 10.1128/IAI.69.12.7271-7276.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Baker CC, Niven-Fairchild T, Caragnano C, Kupper TS. Outcome following femur fracture and subsequent cecal ligation and puncture in endotoxin-sensitive (C3H/HeN) and endotoxin-resistant (C3H/HeJ) mice. J Surg Res. 1991;50:170–4. doi: 10.1016/0022-4804(91)90242-e. [DOI] [PubMed] [Google Scholar]
- [27].Sha T, Sunamoto M, Kitazaki T, Sato J, Ii M, Iizawa Y. Therapeutic effects of TAK-242, a novel selective Toll-like receptor 4 signal transduction inhibitor, in mouse endotoxin shock model. Eur J Pharmacol. 2007;571:231–9. doi: 10.1016/j.ejphar.2007.06.027. [DOI] [PubMed] [Google Scholar]
- [28].Nakamura M, Shimizu Y, Sato Y, Miyazaki Y, Satoh T, Mizuno M, et al. Toll-like receptor 4 signal transduction inhibitor, M62812, suppresses endothelial cell and leukocyte activation and prevents lethal septic shock in mice. Eur J Pharmacol. 2007;569:237–43. doi: 10.1016/j.ejphar.2007.05.013. [DOI] [PubMed] [Google Scholar]
- [29].Daubeuf B, Mathison J, Spiller S, Hugues S, Herren S, Ferlin W, et al. TLR4/MD-2 monoclonal antibody therapy affords protection in experimental models of septic shock. J Immunol. 2007;179:6107–14. doi: 10.4049/jimmunol.179.9.6107. [DOI] [PubMed] [Google Scholar]
- [30].Almog Y, Shefer A, Novack V, Maimon N, Barski L, Eizinger M, et al. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation. 2004;110:880–5. doi: 10.1161/01.CIR.0000138932.17956.F1. [DOI] [PubMed] [Google Scholar]
- [31].Liappis AP, Kan VL, Rochester CG, Simon GL. The effect of statins on mortality in patients with bacteremia. Clin Infect Dis. 2001;33:1352–7. doi: 10.1086/323334. [DOI] [PubMed] [Google Scholar]
- [32].Niessner A, Steiner S, Speidl WS, Pleiner J, Seidinger D, Maurer G, et al. Simvastatin suppresses endotoxin-induced upregulation of toll-like receptors 4 and 2 in vivo. Atherosclerosis. 2006;189:408–13. doi: 10.1016/j.atherosclerosis.2005.12.022. [DOI] [PubMed] [Google Scholar]
- [33].Lorenz E, Mira JP, Frees KL, Schwartz DA. Relevance of mutations in the TLR4 receptor in patients with gram-negative septic shock. Arch Intern Med. 2002;162:1028–32. doi: 10.1001/archinte.162.9.1028. [DOI] [PubMed] [Google Scholar]
- [34].Barber RC, Chang LY, Arnoldo BD, Purdue GF, Hunt JL, Horton JW, et al. Innate immunity SNPs are associated with risk for severe sepsis after burn injury. Clin Med Res. 2006;4:250–5. doi: 10.3121/cmr.4.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Khor CC, Chapman SJ, Vannberg FO, Dunne A, Murphy C, Ling EY, et al. A Mal functional variant is associated with protection against invasive pneumococcal disease, bacteremia, malaria and tuberculosis. Nat Genet. 2007;39:523–8. doi: 10.1038/ng1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Arcaroli J, Silva E, Maloney JP, He Q, Svetkauskaite D, Murphy JR, et al. Variant IRAK-1 haplotype is associated with increased nuclear factor-kappaB activation and worse outcomes in sepsis. Am J Respir Crit Care Med. 2006;173:1335–41. doi: 10.1164/rccm.200603-341OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Inohara N, Nunez G. NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol. 2003;3:371–82. doi: 10.1038/nri1086. [DOI] [PubMed] [Google Scholar]
- [38].Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4:702–7. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- [39].Chin AI, Dempsey PW, Bruhn K, Miller JF, Xu Y, Cheng G. Involvement of receptor-interacting protein 2 in innate and adaptive immune responses. Nature. 2002;416:190–4. doi: 10.1038/416190a. [DOI] [PubMed] [Google Scholar]
- [40].Hysi P, Kabesch M, Moffatt MF, Schedel M, Carr D, Zhang Y, et al. NOD1 variation, immunoglobulin E and asthma. Hum Mol Genet. 2005;14:935–41. doi: 10.1093/hmg/ddi087. [DOI] [PubMed] [Google Scholar]
- [41].Chamaillard M, Girardin SE, Viala J, Philpott DJ. Nods Nalps and Naip: intracellular regulators of bacterial-induced inflammation. Cell Microbiol. 2003;5:581–92. doi: 10.1046/j.1462-5822.2003.00304.x. [DOI] [PubMed] [Google Scholar]
- [42].Cartwright N, Murch O, McMaster SK, Paul-Clark MJ, van Heel DA, Ryffel B, et al. Selective NOD1 agonists cause shock and organ injury/dysfunction in vivo. Am J Respir Crit Care Med. 2007;175:595–603. doi: 10.1164/rccm.200608-1103OC. [DOI] [PubMed] [Google Scholar]
- [43].Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. NOD2 expressed in macrophages and dendritic cells. Nature. 2001;411:603–6. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- [44].Miceli-Richard C, Lesage S, Rybojad M, Prieur AM, Manouvrier-Hanu S, Hafner R, et al. CARD15 mutations in Blau syndrome. Nat Genet. 2001;29:19–20. doi: 10.1038/ng720. [DOI] [PubMed] [Google Scholar]
- [45].Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–4. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- [46].Maeda S, Hsu LC, Liu H, Bankston LA, Iimura M, Kagnoff MF, et al. Nod2 mutation in Crohn’s disease potentiates NF-kappaB activity and IL-1beta processing. Science. 2005;307:734–8. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- [47].Holler E, Rogler G, Herfarth H, Brenmoehl J, Wild PJ, Hahn J, et al. Both donor and recipient NOD2/CARD15 mutations associate with transplant-related mortality and GvHD following allogeneic stem cell transplantation. Blood. 2004;104:889–94. doi: 10.1182/blood-2003-10-3543. [DOI] [PubMed] [Google Scholar]
- [48].Brenmoehl J, Herfarth H, Gluck T, Audebert F, Barlage S, Schmitz G, et al. Genetic variants in the NOD2/CARD15 gene are associated with early mortality in sepsis patients. Intensive Care Med. 2007;33:1541–8. doi: 10.1007/s00134-007-0722-z. [DOI] [PubMed] [Google Scholar]
- [49].Ahrens P, Kattner E, Kohler B, Hartel C, Seidenberg J, Segerer H, et al. Mutations of genes involved in the innate immune system as predictors of sepsis in very low birth weight infants. Pediatr Res. 2004;55:652–6. doi: 10.1203/01.PDR.0000112100.61253.85. [DOI] [PubMed] [Google Scholar]
- [50].Sgambato E. Nosocomial sepsis and polymorphism of the NOD-2 gene. Clin Ter. 2005;156:7–10. [PubMed] [Google Scholar]
- [51].Orihuela CJ, Fillon S, Smith-Sielicki SH, El Kasmi KC, Gao G, Soulis K, et al. Cell wall-mediated neuronal damage in early sepsis. Infect Immun. 2006;74:3783–9. doi: 10.1128/IAI.00022-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tschopp J, Martinon F, Burns K. NALPs: a novel protein family involved in inflammation. Nat Rev Mol Cell Biol. 2003;4:95–104. doi: 10.1038/nrm1019. [DOI] [PubMed] [Google Scholar]
- [53].Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2006 doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- [54].Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–26. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- [55].Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–8. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- [56].Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–4. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- [57].Jin Y, Birlea SA, Fain PR, Spritz RA. Genetic variations in NALP1 are associated with generalized vitiligo in a Romanian population. J Invest Dermatol. 2007;127:2558–62. doi: 10.1038/sj.jid.5700953. [DOI] [PubMed] [Google Scholar]
- [58].Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC, et al. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. 2007;356:1216–25. doi: 10.1056/NEJMoa061592. [DOI] [PubMed] [Google Scholar]
- [59].Taieb A. NALP1 and the inflammasomes: challenging our perception of vitiligo and vitiligo-related autoimmune disorders. Pigment Cell Res. 2007;20:260–2. doi: 10.1111/j.1600-0749.2007.00393.x. [DOI] [PubMed] [Google Scholar]
- [60].Fahy RJ, Exline MC, Gavrilin MA, Bhatt NY, Besecker BY, Sarkar A, et al. Inflammasome mRNA expression in human monocytes during early septic shock. American Journal of Respiratory and Critical Care Medicine. 2008 doi: 10.1164/rccm.200703-418OC. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Martinon F, Agostini L, Meylan E, Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol. 2004;14:1929–34. doi: 10.1016/j.cub.2004.10.027. [DOI] [PubMed] [Google Scholar]
- [62].Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–25. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- [63].Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–5. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Aganna E, Martinon F, Hawkins PN, Ross JB, Swan DC, Booth DR, et al. Association of mutations in the NALP3/CIAS1/PYPAF1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural deafness, and AA amyloidosis. Arthritis Rheum. 2002;46:2445–52. doi: 10.1002/art.10509. [DOI] [PubMed] [Google Scholar]
- [65].Aksentijevich I, Nowak M, Mallah M, Chae JJ, Watford WT, Hofmann SR, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002;46:3340–8. doi: 10.1002/art.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Feldmann J, Prieur AM, Quartier P, Berquin P, Certain S, Cortis E, et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet. 2002;71:198–203. doi: 10.1086/341357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].A candidate gene for familial Mediterranean fever. The French FMF Consortium. Nat Genet. 1997;17:25–31. doi: 10.1038/ng0997-25. [DOI] [PubMed] [Google Scholar]
- [68].Kastner DL. Familial Mediterranean fever: the genetics of inflammation. Hosp Pract (Minneap) 1998;33:131–4. 139–40, 143–6 assim. doi: 10.3810/hp.1998.04.90. [DOI] [PubMed] [Google Scholar]
- [69].Chae JJ, Komarow HD, Cheng J, Wood G, Raben N, Liu PP, et al. Targeted disruption of pyrin, the FMF protein, causes heightened sensitivity to endotoxin and a defect in macrophage apoptosis. Mol Cell. 2003;11:591–604. doi: 10.1016/s1097-2765(03)00056-x. [DOI] [PubMed] [Google Scholar]
- [70].Yu JW, Fernandes-Alnemri T, Datta P, Wu J, Juliana C, Solorzano L, et al. Pyrin activates the ASC pyroptosome in response to engagement by autoinflammatory PSTPIP1 mutants. Mol Cell. 2007;28:214–27. doi: 10.1016/j.molcel.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–27. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- [72].Hall MW, Gavrilin MA, Knatz NL, Duncan MD, Fernandez SA, Wewers MD. Monocyte mRNA phenotype and adverse outcomes from pediatric multiple organ dysfunction syndrome. Pediatr Res. 2007;62:597–603. doi: 10.1203/PDR.0b013e3181559774. [DOI] [PubMed] [Google Scholar]
- [73].Koc B, Oktenli C, Bulucu F, Karadurmus N, Sanisoglu SY, Gul D. The rate of pyrin mutations in critically ill patients with systemic inflammatory response syndrome and sepsis: a pilot study. J Rheumatol. 2007;34:2070–5. [PubMed] [Google Scholar]
- [74].Watson RW, Rotstein OD, Parodo J, Bitar R, Marshall JC. The IL-1 beta-converting enzyme (caspase-1) inhibits apoptosis of inflammatory neutrophils through activation of IL-1 beta. J Immunol. 1998;161:957–62. [PubMed] [Google Scholar]
- [75].Scott AM, Saleh M. The inflammatory caspases: guardians against infections and sepsis. Cell Death Differ. 2006 doi: 10.1038/sj.cdd.4402026. [DOI] [PubMed] [Google Scholar]
- [76].Joshi VD, Kalvakolanu DV, Cross AS. Simultaneous activation of apoptosis and inflammation in pathogenesis of septic shock: a hypothesis. FEBS Lett. 2003;555:180–4. doi: 10.1016/s0014-5793(03)01271-7. [DOI] [PubMed] [Google Scholar]
- [77].Sarkar A, Hall MW, Exline M, Hart J, Knatz N, Gatson NT, et al. Caspase-1 Regulates Escherichia coli Sepsis and Splenic B Cell Apoptosis Independently of Interleukin-1beta and Interleukin-18. Am J Respir Crit Care Med. 2006;174:1003–10. doi: 10.1164/rccm.200604-546OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Chen L, Song X, Meng X. Correlation of interleukin 1 beta-converting enzyme(ICE) gene expression with gut epithelial cell apoptosis in septic mice. Zhonghua Yi Xue Za Zhi. 1998;78:544–6. [PubMed] [Google Scholar]
- [79].Joshi VD, Kalvakolanu DV, Hebel JR, Hasday JD, Cross AS. Role of caspase 1 in murine antibacterial host defenses and lethal endotoxemia. Infect Immun. 2002;70:6896–903. doi: 10.1128/IAI.70.12.6896-6903.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Saleh M, Vaillancourt JP, Graham RK, Huyck M, Srinivasula SM, Alnemri ES, et al. Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature. 2004;429:75–9. doi: 10.1038/nature02451. [DOI] [PubMed] [Google Scholar]
- [81].Xue Y, Daly A, Yngvadottir B, Liu M, Coop G, Kim Y, et al. Spread of an inactive form of caspase-12 in humans is due to recent positive selection. Am J Hum Genet. 2006;78:659–70. doi: 10.1086/503116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Saleh M, Mathison JC, Wolinski MK, Bensinger SJ, Fitzgerald P, Droin N, et al. Enhanced bacterial clearance and sepsis resistance in caspase-12-deficient mice. Nature. 2006;440:1064–8. doi: 10.1038/nature04656. [DOI] [PubMed] [Google Scholar]
- [83].Li P, Allen H, Banerjee S, Seshadri T. Characterization of mice deficient in interleukin-1 beta converting enzyme. J Cell Biochem. 1997;64:27–32. doi: 10.1002/(sici)1097-4644(199701)64:1<27::aid-jcb5>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- [84].Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, et al. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol. 2000;1:496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- [85].Hotchkiss RS, Tinsley KW, Swanson PE, Chang KC, Cobb JP, Buchman TG, et al. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci U S A. 1999;96:14541–6. doi: 10.1073/pnas.96.25.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Monack DM, Navarre WW, Falkow S. Salmonella-induced macrophage death: the role of caspase-1 in death and inflammation. Microbes Infect. 2001;3:1201–12. doi: 10.1016/s1286-4579(01)01480-0. [DOI] [PubMed] [Google Scholar]
- [87].Wang W, Faubel S, Ljubanovic D, Mitra A, Falk SA, Kim J, et al. Endotoxemic acute renal failure is attenuated in caspase-1-deficient mice. Am J Physiol Renal Physiol. 2005;288:F997–1004. doi: 10.1152/ajprenal.00130.2004. [DOI] [PubMed] [Google Scholar]
- [88].Zhang XH, Zhu RM, Xu WA, Wan HJ, Lu H. Therapeutic effects of caspase-1 inhibitors on acute lung injury in experimental severe acute pancreatitis. World J Gastroenterol. 2007;13:623–7. doi: 10.3748/wjg.v13.i4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Sansonetti PJ, Phalipon A, Arondel J, Thirumalai K, Banerjee S, Akira S, et al. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity. 2000;12:581–90. doi: 10.1016/s1074-7613(00)80209-5. [DOI] [PubMed] [Google Scholar]
- [90].Grobmyer SR, Armstrong RC, Nicholson SC, Gabay C, Arend WP, Potter SH, et al. Peptidomimetic fluoromethylketone rescues mice from lethal endotoxic shock. Mol Med. 1999;5:585–94. [PMC free article] [PubMed] [Google Scholar]
- [91].Aydemir TB, Blanchard RK, Cousins RJ. Zinc supplementation of young men alters metallothionein, zinc transporter, and cytokine gene expression in leukocyte populations. Proc Natl Acad Sci U S A. 2006;103:1699–704. doi: 10.1073/pnas.0510407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Book M, Chen Q, Lehmann LE, Klaschik S, Weber S, Schewe JC, et al. Inducibility of the endogenous antibiotic peptide beta-defensin 2 is impaired in patients with severe sepsis. Crit Care. 2007;11:R19. doi: 10.1186/cc5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Ganz THepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102:783–8. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- [94].Ho LH, Ruffin RE, Murgia C, Li L, Krilis SA, Zalewski PD. Labile zinc and zinc transporter ZnT4 in mast cell granules: role in regulation of caspase activation and NF-kappaB translocation. J Immunol. 2004;172:7750–60. doi: 10.4049/jimmunol.172.12.7750. [DOI] [PubMed] [Google Scholar]
- [95].Kemna E, Pickkers P, Nemeth E, van der Hoeven H, Swinkels D. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood. 2005;106:1864–6. doi: 10.1182/blood-2005-03-1159. [DOI] [PubMed] [Google Scholar]
- [96].Kitamura H, Morikawa H, Kamon H, Iguchi M, Hojyo S, Fukada T, et al. Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat Immunol. 2006;7:971–7. doi: 10.1038/ni1373. [DOI] [PubMed] [Google Scholar]
- [97].Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- [98].Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, et al. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci U S A. 2005;102:6843–8. doi: 10.1073/pnas.0502257102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Voss E, Wehkamp J, Wehkamp K, Stange EF, Schroder JM, Harder J. NOD2/CARD15 mediates induction of the antimicrobial peptide human beta-defensin-2. J Biol Chem. 2006;281:2005–11. doi: 10.1074/jbc.M511044200. [DOI] [PubMed] [Google Scholar]
- [100].Vincent JL, Ferreira F, Moreno R. Scoring systems for assessing organ dysfunction and survival. Crit Care Clin. 2000;16:353–66. doi: 10.1016/s0749-0704(05)70114-7. [DOI] [PubMed] [Google Scholar]
- [101].Deitch EA. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg. 1992;216:117–34. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Weiss YG, Maloyan A, Tazelaar J, Raj N, Deutschman CS. Adenoviral transfer of HSP-70 into pulmonary epithelium ameliorates experimental acute respiratory distress syndrome. J Clin Invest. 2002;110:801–6. doi: 10.1172/JCI15888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Pettila V, Hynninen M, Takkunen O, Kuusela P, Valtonen M. Predictive value of procalcitonin and interleukin 6 in critically ill patients with suspected sepsis. Intensive Care Med. 2002;28:1220–5. doi: 10.1007/s00134-002-1416-1. [DOI] [PubMed] [Google Scholar]
- [104].Muller AM, Cronen C, Muller KM, Kirkpatrick CJ. Heterogeneous expression of cell adhesion molecules by endothelial cells in ARDS. J Pathol. 2002;198:270–5. doi: 10.1002/path.1186. [DOI] [PubMed] [Google Scholar]
- [105].Huston JM, Gallowitsch-Puerta M, Ochani M, Ochani K, Yuan R, Rosas-Ballina M, et al. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit Care Med. 2007;35:2762–8. doi: 10.1097/01.CCM.0000288102.15975.BA. [DOI] [PubMed] [Google Scholar]
- [106].Sakamori R, Takehara T, Ohnishi C, Tatsumi T, Ohkawa K, Takeda K, et al. Signal transducer and activator of transcription 3 signaling within hepatocytes attenuates systemic inflammatory response and lethality in septic mice. Hepatology. 2007;46:1564–73. doi: 10.1002/hep.21837. [DOI] [PubMed] [Google Scholar]
- [107].Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530–8. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- [108].Miyake Y, Asano K, Kaise H, Uemura M, Nakayama M, Tanaka M. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J Clin Invest. 2007;117:2268–78. doi: 10.1172/JCI31990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Carp, HMitochondrial N-formylmethionyl proteins as chemoattractants for neutrophils. J Exp Med. 1982;155:264–75. doi: 10.1084/jem.155.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- [111].Rouhiainen A, Tumova S, Valmu L, Kalkkinen N, Rauvala H. Pivotal advance: analysis of proinflammatory activity of highly purified eukaryotic recombinant HMGB1 (amphoterin) J Leukoc Biol. 2007;81:49–58. doi: 10.1189/jlb.0306200. [DOI] [PubMed] [Google Scholar]
- [112].Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–96. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- [113].Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, et al. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet. 1998;18:231–6. doi: 10.1038/ng0398-231. [DOI] [PubMed] [Google Scholar]
- [114].Collins LV, Hajizadeh S, Holme E, Jonsson IM, Tarkowski A. Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. J Leukoc Biol. 2004;75:995–1000. doi: 10.1189/jlb.0703328. [DOI] [PubMed] [Google Scholar]
- [115].El Mezayen R, El Gazzar M, Seeds MC, McCall CE, Dreskin SC, Nicolls MR. Endogenous signals released from necrotic cells augment inflammatory responses to bacterial endotoxin. Immunol Lett. 2007;111:36–44. doi: 10.1016/j.imlet.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–6. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- [117].Jiang W, Bell CW, Pisetsky DS. The relationship between apoptosis and high-mobility group protein 1 release from murine macrophages stimulated with lipopolysaccharide or polyinosinic-polycytidylic acid. J Immunol. 2007;178:6495–503. doi: 10.4049/jimmunol.178.10.6495. [DOI] [PubMed] [Google Scholar]
- [118].Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Lutterloh EC, Opal SM, Pittman DD, Keith JC, Jr, Tan XY, Clancy BM, et al. Inhibition of the RAGE products increases survival in experimental models of severe sepsis and systemic infection. Crit Care. 2007;11:R122. doi: 10.1186/cc6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Broekemeier KM, Iben JR, LeVan EG, Crouser ED, Pfeiffer DR. Pore formation and uncoupling initiate a Ca2+-independent degradation of mitochondrial phospholipids. Biochemistry. 2002;41:7771–80. doi: 10.1021/bi020157z. [DOI] [PubMed] [Google Scholar]
- [121].Crouser ED, Julian MW, Huff JE, Struck J, Cook CH. Carbamoyl phosphate synthase-1: a marker of mitochondrial damage and depletion in the liver during sepsis. Crit Care Med. 2006;34:2439–46. doi: 10.1097/01.CCM.0000230240.02216.21. [DOI] [PubMed] [Google Scholar]
- [122].Kundu M, Thompson CB. Macroautophagy versus mitochondrial autophagy: a question of fate? Cell Death Differ. 2005;12(Suppl 2):1484–9. doi: 10.1038/sj.cdd.4401780. [DOI] [PubMed] [Google Scholar]
- [123].Belikova I, Lukaszewicz AC, Faivre V, Damoisel C, Singer M, Payen D. Oxygen consumption of human peripheral blood mononuclear cells in severe human sepsis. Crit Care Med. 2007;35:2702–8. doi: 10.1097/01.ccm.0000295593.25106.c4. [DOI] [PubMed] [Google Scholar]
- [124].Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–23. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- [125].Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, et al. A network-based analysis of systemic inflammation in humans. Nature. 2005;437:1032–7. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- [126].Miyoshi N, Oubrahim H, Chock PB, Stadtman ER. Age-dependent cell death and the role of ATP in hydrogen peroxide-induced apoptosis and necrosis. Proc Natl Acad Sci U S A. 2006;103:1727–31. doi: 10.1073/pnas.0510346103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].McDonald MC, d’Emmanuele di Villa Bianca R, Wayman NS, Pinto A, Sharpe MA, Cuzzocrea S, et al. A superoxide dismutase mimetic with catalase activity (EUK-8) reduces the organ injury in endotoxic shock. Eur J Pharmacol. 2003;466:181–9. doi: 10.1016/s0014-2999(03)01538-3. [DOI] [PubMed] [Google Scholar]
- [128].Ritter C, Andrades ME, Reinke A, Menna-Barreto S, Moreira JC, Dal-Pizzol F. Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit Care Med. 2004;32:342–9. doi: 10.1097/01.CCM.0000109454.13145.CA. [DOI] [PubMed] [Google Scholar]
- [129].Pacher P, Szabo C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors. Cardiovasc Drug Rev. 2007;25:235–60. doi: 10.1111/j.1527-3466.2007.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Zong WX, Ditsworth D, Bauer DE, Wang ZQ, Thompson CB. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 2004;18:1272–82. doi: 10.1101/gad.1199904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Levy B, Sadoune LO, Gelot AM, Bollaert PE, Nabet P, Larcan A. Evolution of lactate/pyruvate and arterial ketone body ratios in the early course of catecholamine-treated septic shock. Crit Care Med. 2000;28:114–9. doi: 10.1097/00003246-200001000-00019. [DOI] [PubMed] [Google Scholar]
- [132].Fredriksson K, Hammarqvist F, Strigard K, Hultenby K, Ljungqvist O, Wernerman J, et al. Derangements in mitochondrial metabolism in intercostal and leg muscle of critically ill patients with sepsis-induced multiple organ failure. Am J Physiol Endocrinol Metab. 2006;291:E1044–50. doi: 10.1152/ajpendo.00218.2006. [DOI] [PubMed] [Google Scholar]
- [133].Petrilli V, Herceg Z, Hassa PO, Patel NS, Di Paola R, Cortes U, et al. Noncleavable poly(ADP-ribose) polymerase-1 regulates the inflammation response in mice. J Clin Invest. 2004;114:1072–81. doi: 10.1172/JCI21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Soriano FG, Nogueira AC, Caldini EG, Lins MH, Teixeira AC, Cappi SB, et al. Potential role of poly(adenosine 5′-diphosphate-ribose) polymerase activation in the pathogenesis of myocardial contractile dysfunction associated with human septic shock. Crit Care Med. 2006;34:1073–9. doi: 10.1097/01.CCM.0000206470.47721.8D. [DOI] [PubMed] [Google Scholar]
- [135].Soriano FG, Liaudet L, Szabo E, Virag L, Mabley JG, Pacher P, et al. Resistance to acute septic peritonitis in poly(ADP-ribose) polymerase-1-deficient mice. Shock. 2002;17:286–92. doi: 10.1097/00024382-200204000-00008. [DOI] [PubMed] [Google Scholar]
- [136].Murakami K, Enkhbaatar P, Shimoda K, Cox RA, Burke AS, Hawkins HK, et al. Inhibition of poly (ADP-ribose) polymerase attenuates acute lung injury in an ovine model of sepsis. Shock. 2004;21:126–33. doi: 10.1097/01.shk.0000108397.56565.4a. [DOI] [PubMed] [Google Scholar]
- [137].Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282:2871–9. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007;13:913–9. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- [139].Crouser ED, Julian MW, Huff JE, Joshi MS, Bauer JA, Gadd ME, et al. Abnormal permeability of inner and outer mitochondrial membranes contributes independently to mitochondrial dysfunction in the liver during acute endotoxemia. Crit Care Med. 2004;32:478–88. doi: 10.1097/01.CCM.0000109449.99160.81. [DOI] [PubMed] [Google Scholar]
- [140].Larche J, Lancel S, Hassoun SM, Favory R, Decoster B, Marchetti P, et al. Inhibition of mitochondrial permeability transition prevents sepsis-induced myocardial dysfunction and mortality. J Am Coll Cardiol. 2006;48:377–85. doi: 10.1016/j.jacc.2006.02.069. [DOI] [PubMed] [Google Scholar]
- [141].Lopez LC, Escames G, Ortiz F, Ros E, Acuna-Castroviejo D. Melatonin restores the mitochondrial production of ATP in septic mice. Neuro Endocrinol Lett. 2006;27:623–30. [PubMed] [Google Scholar]
- [142].Reynolds FD, Dauchy R, Blask D, Dietz PA, Lynch D, Zuckerman R. The pineal gland hormone melatonin improves survival in a rat model of sepsis/shock induced by zymosan A. Surgery. 2003;134:474–9. doi: 10.1067/s0039-6060(03)00253-8. [DOI] [PubMed] [Google Scholar]
- [143].Wichmann MW, Haisken JM, Ayala A. Chaudry, IHMelatonin administration following hemorrhagic shock decreases mortality from subsequent septic challenge. J Surg Res. 1996;65:109–14. doi: 10.1006/jsre.1996.0351. [DOI] [PubMed] [Google Scholar]
- [144].Vanhorebeek I, De Vos R, Mesotten D, Wouters PJ, De Wolf-Peeters C, Van den Berghe G. Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients. Lancet. 2005;365:53–9. doi: 10.1016/S0140-6736(04)17665-4. [DOI] [PubMed] [Google Scholar]
- [145].Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–61. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- [146].Dello Russo C, Gavrilyuk V, Weinberg G, Almeida A, Bolanos JP, Palmer J, et al. Peroxisome proliferator-activated receptor gamma thiazolidinedione agonists increase glucose metabolism in astrocytes. J Biol Chem. 2003;278:5828–36. doi: 10.1074/jbc.M208132200. [DOI] [PubMed] [Google Scholar]
- [147].Lee S, Kim W, Kang KP, Moon SO, Sung MJ, Kim DH, et al. Agonist of peroxisome proliferator-activated receptor-gamma, rosiglitazone, reduces renal injury and dysfunction in a murine sepsis model. Nephrol Dial Transplant. 2005;20:1057–65. doi: 10.1093/ndt/gfh705. [DOI] [PubMed] [Google Scholar]
- [148].Le Gouill E, Jimenez M, Binnert C, Jayet PY, Thalmann S, Nicod P, et al. Endothelial nitric oxide synthase (eNOS) knockout mice have defective mitochondrial beta-oxidation. Diabetes. 2007;56:2690–6. doi: 10.2337/db06-1228. [DOI] [PubMed] [Google Scholar]
- [149].Fugono J, Fujimoto K, Yasui H, Kawabe K, Yoshikawa Y, Kojima Y, et al. Metallokinetic study of zinc in the blood of normal rats given insulinomimetic zinc(II) complexes and improvement of diabetes mellitus in type 2 diabetic GK rats by their oral administration. Drug Metab Pharmacokinet. 2002;17:340–7. doi: 10.2133/dmpk.17.340. [DOI] [PubMed] [Google Scholar]
- [150].Sakurai H, Adachi Y. The pharmacology of the insulinomimetic effect of zinc complexes. Biometals. 2005;18:319–23. doi: 10.1007/s10534-005-3688-8. [DOI] [PubMed] [Google Scholar]
- [151].Guidet B, Aegerter P, Gauzit R, Meshaka P, Dreyfuss D. Incidence and impact of organ dysfunctions associated with sepsis. Chest. 2005;127:942–51. doi: 10.1378/chest.127.3.942. [DOI] [PubMed] [Google Scholar]
- [152].Moss M. Epidemiology of sepsis: race, sex, and chronic alcohol abuse. Clin Infect Dis. 2005;41(Suppl 7):S490–7. doi: 10.1086/432003. [DOI] [PubMed] [Google Scholar]
- [153].Bartlett RH, Dechert RE, Mault JR, Ferguson SK, Kaiser AM, Erlandson EE. Measurement of metabolism in multiple organ failure. Surgery. 1982;92:771–9. [PubMed] [Google Scholar]
- [154].Cerra FB, Siegel JH, Coleman B, Border JR, McMenamy RR. Septic autocannibalism. A failure of exogenous nutritional support. Ann Surg. 1980;192:570–80. doi: 10.1097/00000658-198010000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Gaetke LM, McClain CJ, Talwalkar RT, Shedlofsky SI. Effects of endotoxin on zinc metabolism in human volunteers. Am J Physiol. 1997;272:E952–6. doi: 10.1152/ajpendo.1997.272.6.E952. [DOI] [PubMed] [Google Scholar]
- [156].Moshage H. Cytokines and the hepatic acute phase response. J Pathol. 1997;181:257–66. doi: 10.1002/(SICI)1096-9896(199703)181:3<257::AID-PATH756>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- [157].Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem. 2006;281:24085–9. doi: 10.1074/jbc.R600011200. [DOI] [PubMed] [Google Scholar]
- [158].Metnitz PG, Bartens C, Fischer M, Fridrich P, Steltzer H, Druml W. Antioxidant status in patients with acute respiratory distress syndrome. Intensive Care Med. 1999;25:180–5. doi: 10.1007/s001340050813. [DOI] [PubMed] [Google Scholar]
- [159].Therond P, Bonnefont-Rousselot D, Davit-Spraul A, Conti M, Legrand A. Biomarkers of oxidative stress: an analytical approach. Curr Opin Clin Nutr Metab Care. 2000;3:373–84. doi: 10.1097/00075197-200009000-00009. [DOI] [PubMed] [Google Scholar]
- [160].Motoyama T, Okamoto K, Kukita I, Hamaguchi M, Kinoshita Y, Ogawa H. Possible role of increased oxidant stress in multiple organ failure after systemic inflammatory response syndrome. Crit Care Med. 2003;31:1048–52. doi: 10.1097/01.CCM.0000055371.27268.36. [DOI] [PubMed] [Google Scholar]
- [161].Goode HF, Cowley HC, Walker BE, Howdle PD, Webster NR. Decreased antioxidant status and increased lipid peroxidation in patients with septic shock and secondary organ dysfunction. Crit Care Med. 1995;23:646–51. doi: 10.1097/00003246-199504000-00011. [DOI] [PubMed] [Google Scholar]
- [162].Devirgiliis C, Zalewski PD, Perozzi G, Murgia C. Zinc fluxes and zinc transporter genes in chronic diseases. Mutat Res. 2007;622:84–93. doi: 10.1016/j.mrfmmm.2007.01.013. [DOI] [PubMed] [Google Scholar]
- [163].King JC, Shames DM, Woodhouse LR. Zinc homeostasis in humans. J Nutr. 2000;130:1360S–6S. doi: 10.1093/jn/130.5.1360S. [DOI] [PubMed] [Google Scholar]
- [164].Walsh CT, Sandstead HH, Prasad AS, Newberne PM, Fraker PJ. Zinc: health effects and research priorities for the 1990s. Environ Health Perspect. 1994;102(Suppl 2):5–46. doi: 10.1289/ehp.941025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Bao S, Knoell DL. Zinc modulates airway epithelium susceptibility to death receptor-mediated apoptosis. Am J Physiol Lung Cell Mol Physiol. 2006;290:L433–41. doi: 10.1152/ajplung.00341.2005. [DOI] [PubMed] [Google Scholar]
- [166].Bao S, Knoell DL. Zinc modulates cytokine-induced lung epithelial cell barrier permeability. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1132–41. doi: 10.1152/ajplung.00207.2006. [DOI] [PubMed] [Google Scholar]
- [167].Zalewski PD, Truong-Tran AQ, Grosser D, Jayaram L, Murgia C, Ruffin RE. Zinc metabolism in airway epithelium and airway inflammation: basic mechanisms and clinical targets. A review. Pharmacol Ther. 2005;105:127–49. doi: 10.1016/j.pharmthera.2004.09.004. [DOI] [PubMed] [Google Scholar]
- [168].Fraker PJ, King LE. Reprogramming of the immune system during zinc deficiency. Annu Rev Nutr. 2004;24:277–98. doi: 10.1146/annurev.nutr.24.012003.132454. [DOI] [PubMed] [Google Scholar]
- [169].Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–22. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- [170].Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290:L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- [171].Abraham E. Alterations in cell signaling in sepsis. Clin Infect Dis. 2005;41(Suppl 7):S459–64. doi: 10.1086/431997. [DOI] [PubMed] [Google Scholar]
- [172].Haase H, Rink L. Signal transduction in monocytes: the role of zinc ions. Biometals. 2007;20:579–85. doi: 10.1007/s10534-006-9029-8. [DOI] [PubMed] [Google Scholar]
- [173].Rink L, Haase H. Zinc homeostasis and immunity. Trends Immunol. 2007;28:1–4. doi: 10.1016/j.it.2006.11.005. [DOI] [PubMed] [Google Scholar]
- [174].von Bulow V, Dubben S, Engelhardt G, Hebel S, Plumakers B, Heine H, et al. Zinc-dependent suppression of TNF-alpha production is mediated by protein kinase A-induced inhibition of Raf-1, I kappa B kinase beta, and NF-kappa B. J Immunol. 2007;179:4180–6. doi: 10.4049/jimmunol.179.6.4180. [DOI] [PubMed] [Google Scholar]
- [175].Maret W. Zinc and sulfur: a critical biological partnership. Biochemistry. 2004;43:3301–9. doi: 10.1021/bi036340p. [DOI] [PubMed] [Google Scholar]
- [176].Haas RH, Parikh S, Falk MJ, Saneto RP, Wolf NI, Darin N, et al. Mitochondrial disease: a practical approach for primary care physicians. Pediatrics. 2007;120:1326–33. doi: 10.1542/peds.2007-0391. [DOI] [PubMed] [Google Scholar]
- [177].Pastorino JG, Simbula G, Yamamoto K, Glascott PA, Jr, Rothman RJ, Farber JL. The cytotoxicity of tumor necrosis factor depends on induction of the mitochondrial permeability transition. J Biol Chem. 1996;271:29792–8. doi: 10.1074/jbc.271.47.29792. [DOI] [PubMed] [Google Scholar]