Abstract
Background
Invasive melanoma of the skin is the third most common cancer diagnosed among adolescents and young adults (aged 15-39 years) in the United States. Understanding the burden of melanoma in this age group is important to identifying areas for etiologic research and in developing effective prevention approaches aimed at reducing melanoma risk.
Methods
Melanoma incidence data reported from 38 National Program of Cancer Registries and/or Surveillance Epidemiology and End Results statewide cancer registries covering nearly 67.2% of the US population were used to estimate age-adjusted incidence rates for persons 15-39 years of age. Incidence rate ratios were calculated to compare rates between demographic groups.
Results
Melanoma incidence was higher among females (age-adjusted incidence rates = 9.74; 95% confidence interval 9.62-9.86) compared with males (age-adjusted incidence rates = 5.77; 95% confidence interval 5.68-5.86), increased with age, and was higher in non-Hispanic white compared with Hispanic white and black, American Indians/Alaskan Natives, and Asian and Pacific Islanders populations. Melanoma incidence rates increased with year of diagnosis in females but not males. The majority of melanomas were diagnosed on the trunk in all racial and ethnic groups among males but only in non-Hispanic whites among females. Most melanomas were diagnosed at localized stage, and among those melanomas with known histology, the majority were superficial spreading.
Limitations
Accuracy of melanoma cases reporting was limited because of some incompleteness (delayed reporting) or nonspecific reporting including large proportion of unspecified histology.
Conclusions
Differences in incidence rates by anatomic site, histology, and stage among adolescents and young adults by race, ethnicity, and sex suggest that both host characteristics and behaviors influence risk. These data suggest areas for etiologic research around gene-environment interactions and the need for targeted cancer control activities specific to adolescents and young adult populations.
Keywords: adolescents, cancer, incidence, melanoma, National Program of Cancer Registries, surveillance, Surveillance Epidemiology and End Results, young adults
Cutaneous melanoma (hereafter called melanoma) is the second most commonly diagnosed cancer (after lymphomas) and the most lethal form of skin cancer among adolescents and young adults under the age of 30 years in the United States.1 Melanoma is characterized by the uncontrolled growth of pigment-producing cells, can spread to lymph nodes and, by extension to internal organs, and may result in death.2 However, if detected and treated early, melanoma has a favorable prognosis.3,4
Incidence of melanoma among adolescents and young adults is increasing in the United States,5,6 Canada,7 and in parts of the world in predominantly light-skinned populations.8,9
Primary melanoma evolves from melanocyte transformation directly or in precursor lesions. Melanoma tumori-genesis likely represents a multistep process involving accumulation of sequential genetic alterations.10 The major modifiable risk factor for melanoma is overexposure to ultraviolet (UV) radiation (UVR), particularly intermittent exposure, whether from sunlight or artificial sources.11 Adolescents and young adults appear to be at particular risk for developing melanoma because of these exposures early in life.12,13
Other factors consistently shown to confer an increased risk of developing melanoma include a family history of melanoma, tendency to develop freckles, light hair color, immunosuppression, and a higher number of nevi.14,15 Perhaps the most distinct risk factor for pediatric melanoma is its relationship with pre-existing melanocytic nevi.16,17 Melanoma is rare among darkly pigmented ethnic groups relative to whites. This presumably relates to the higher sensitivity of white skin to UVR exposure. Of all racial groups in the United States, whites have the highest incidence rates of melanoma regardless of age.18
Understanding the burden and risk of melanoma among adolescents and young adults is important to identify areas for etiologic research and to develop effective cancer control activities aimed at reducing melanoma incidence and deaths in this age group. However, incidence patterns by racial and ethnic subgroups within this population have not been well described in the United States because of incomplete surveillance coverage. Through the combined efforts of the two federal cancer surveillance programs, the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) and the National Cancer Institute’s (NCI) Surveillance Epidemiology and End Results (SEER) Program, there are now population-based cancer registries operating in all states and the District of Columbia.19,20 The combined coverage of the two federal programs allows us to better monitor the burden of cancer in racially, ethnically, and geographically diverse populations throughout the United States.18
In this analysis we examined the burden of melanoma in adolescents and young adults between the ages of 15 to 39 years diagnosed between 1999 and 2006 by demographic and tumor characteristics.
METHODS
Detailed descriptions of the data sources and methods used for evaluation and analysis of these data are found in another article in this supplement.21 A brief description of the data sources and methods is included herein.
Cancer cases
Cancer incidence data for the period 1999 through 2006 for individuals aged 15 to 39 years at diagnosis and covering 67.2% of the US population were obtained from 38 statewide cancer registries that participate in either or both NPCR and SEER. Data were collected and reported by use of standards established by the North American Association of Central Cancer Registries.22
Primary site and histology were coded according to the International Classification of Diseases–Oncology (ICD-O) edition in use at the time of diagnosis and converted to the third edition (ICD-O-3).23 Incident cases of invasive melanoma of the skin (defined as ICD-O-3 site codes C440-449 and histology codes 8720-8790) were selected and categorized according to anatomic body sites: face, head, and neck, including skin of lips, eyelids, ears, scalp, and neck (C44.0-C44.4); trunk, including back, abdomen, and chest (C44.5); upper extremities, including shoulders (C44.6); lower extremities, including hips (C44.7); and overlapping sites and not otherwise specified (NOS) (C44.8-C44.9). Among microscopically confirmed melanoma cases, histopathologic types were categorized as follows: superficial spreading (ICD-O-3 code 8743); nodular (ICD-O-3 code 8721); lentigo maligna (ICD-O-3 code 8742); acral lentiginous (ICD-O-3 code 8744); melanoma NOS (Histology NOS) (ICD-O-3 code 8720); and all other specified histologies (other specified) between ICD-O-3 8722 to 8780 and not previously listed. Beginning in 2004, cancer registries throughout the United States began collecting stage data according to the online Collaborative Staging coding instructions.24 For cases diagnosed between 2004 and 2006, the Collaborative Staging data were recoded into Derived SEER Summary Stage 2000: localized, regional, distant, unknown, and NOS.25 Because of the change in staging scheme, stage analyses were limited to diagnosis years 2004 through 2006 to ensure consistency.
Population data
County level population estimates produced by the US Census Bureau and bridged to single-race estimates were used as denominators in the rate calculations. The NCI made further refinements regarding race and county geographic codes and provided public access to these population estimates.26
Hispanic ethnicity is not mutually exclusive from race (Table I). Using race and ethnicity data, the following categories were created: non-Hispanic white (NHW); Hispanic white (HW); black, including non-Hispanic and Hispanic; American Indians/Alaskan Natives (AI/AN) including non-Hispanic and Hispanic; and Asian Pacific Islanders (API) including non-Hispanic and Hispanic. A combined category of black, AI/AN, and API (non-white) was constructed for analyses examining race and ethnicity by site and histology because case counts for populations other than NHW and HW were small. Other unspecified and unknown race categories were combined.
Table I.
Invasive melanoma incident cases among adolescents and young adults (age 15-39 years) by race and ethnicity: United States 1999 through 2006
| Male (n = 15,677) |
Female (n = 26,036) |
|||
|---|---|---|---|---|
| Non-Hispanic | Hispanic | Non-Hispanic | Hispanic | |
| White | 14,191 (93.0%) | 392 (93.8%) | 23,321 (92.8%) | 853 (93.7%) |
| Black | 79 (0.5%) | – | 125 (0.5%) | – |
| AI/AN | 53 (0.4%) | – | 74 (0.3%) | – |
| API | 64 (0.4%) | – | 117 (0.5%) | – |
| Other unspecified and unknown | 872 (5.7%) | 22 (5.3%) | 1489 (5.9%) | 48 (5.3%) |
| All races | 15,259 | 418 | 25,126 | 910 |
Case counts >1 and <16 cases were suppressed.
Data are from population-based cancer registries that participate in National Program of Cancer Registries and/or Surveillance Epidemiology and End Results Program and meet high-quality data criteria. These registries cover 67.2% of population for 1999-2006.
AI/AN, American Indian/Alaskan Natives, API, Asian and Pacific Islander.
Analyses
Frequencies, percent distributions and averaged annual age-adjusted incidence rates per 100,000 population were calculated by use of SEER*Stat software (V6.6.2, National Cancer Institute, Bethesda, Maryland).27 Rates were adjusted to the 2000 US standard population by use of 5-year age groups (15-19, 20-24, 25-29, 30-34, and 35-39 years). Standard errors (SE) and 95% confidence intervals (CI) were constructed by use of the modified gamma method to ensure proper coverage for small case counts, low incidence rates, and populations with age distributions that differ from the standard age distribution.28 Rates based on counts of less than 16 cases for the entire diagnosis period (1999-2006) were suppressed. P values and 95% CI for incidence rate ratios (IRR) were calculated for the purpose of comparing incidence rates between populations.29 Reported IRR are based on findings where the IRR was statistically significantly different from 1 (2-sided P<.05).
Rates were not constructed for other unspecified and unknown racial groups because corresponding population data were not available. Case counts greater than 1 but less than 16 were suppressed for the distribution of incident cases in the tables but were included in the graphical representation of the distribution of incident cases.
RESULTS
Demographic characteristics
A total of 361,394 invasive cancers were diagnosed among adolescents and young adults aged 15 to 39 years during 1999 through 2006. Melanoma was the third most common cancer diagnosed behind lymphoma and breast cancer. Of the 41,715 invasive melanomas reported (Table I); 15,677 were diagnosed in males (37.6%) and 26,036 in females (62.4%). Greater than 92% of cases were diagnosed among NHW and HW males and females. Less than 1% of cases were diagnosed among blacks, AI/AN, or API. Between 5 and 6% of cases were reported with unknown race.
The risk of being given a diagnosis of melanoma was greater in females compared with males (IRR = 1.69; 95% CI 1.65-1.72) (Table II). Age-adjusted incidence rates were higher in females compared with males in every age, diagnosis year, race, and ethnicity group examined. The relative risk of being given a diagnosis of melanoma increased at least 7-fold between the ages of 15 to 19 years and 35 to 39 years among males and females (RR = 7.97; 95% CI 7.40-8.60 and IRR = 7.06; 95% CI 6.65-7.51, respectively). Among females, the incidence rate increased in each subsequent calendar period from 8.85 per 100,000 in 1999 through 2000 to 10.31 per 100,000 in 2004 through 2006. Risk did not increase with calendar period among males. Melanoma incidence was significantly higher among NHW males (7.94/100,000) and females (13.23/100,000) followed in order of magnitude by AI/AN, HW, API, and blacks.
Table II.
Invasive melanoma incidence among adolescents and young adults (age 15-39 years) by demographic characteristics: United States 1999 through 2006
| Sex | No. | Rate | 95% CI | IRR | 95% CI |
|---|---|---|---|---|---|
| Male | 15,677 | 5.77 | 5.68-5.86 | 1.00 | – |
| Female | 26,036 | 9.74 | 9.62-9.86 | 1.69* | 1.65-1.72 |
| Male |
Female |
|||||||
|---|---|---|---|---|---|---|---|---|
| Age, y | No. | Rate (SE) | IRR | 95% CI | No. | Rate (SE) | IRR | 95% CI |
| 15-19 | 783 | 1.39 (0.05) | 1.00 | – | 1191 | 2.23 (0.06) | 1.00 | – |
| 20-24 | 1539 | 2.80 (0.07) | 2.01* | 1.85-2.20 | 3318 | 6.38 (0.11) | 2.86* | 2.68-3.06 |
| 25-29 | 2642 | 5.03 (0.10) | 3.62* | 3.34-3.93 | 5149 | 10.17 (0.14) | 4.56* | 4.28-4.86 |
| 30-34 | 4223 | 7.66 (0.12) | 5.52* | 5.11-5.97 | 7185 | 13.30 (0.16) | 5.97* | 5.61-6.35 |
| 35-39 | 6490 | 11.07 (0.14) | 7.97* | 7.40-8.60 | 9193 | 15.75 (0.16) | 7.06* | 6.65-7.51 |
| Diagnosis year | ||||||||
| 1999-2000 | 3862 | 5.62 (0.09) | 0.94* | 0.90-0.98 | 5993 | 8.85 (0.11) | 0.91* | 0.88-0.94 |
| 2001-2003 | 6121 | 6.00 (0.08) | 1.00 | – | 9813 | 9.77 (0.10) | 1.00 | – |
| 2004-2006 | 5694 | 5.64 (0.07) | 0.94* | 0.91-0.98 | 10,230 | 10.31 (0.10) | 1.06* | 1.03-1.09 |
| Race/ethnicity | ||||||||
| NHW | 14,191 | 7.94 (0.07) | 1.00 | – | 23,321 | 13.23 (0.09) | 1.00 | – |
| HW | 392 | 0.95 (0.05) | 0.12* | 0.11-0.13 | 853 | 2.41 (0.08) | 0.18* | 0.17-0.20 |
| Black | 79 | 0.25 (0.03) | 0.03* | 0.03-0.04 | 125 | 0.37 (0.03) | 0.03* | 0.02-0.03 |
| AI/AN | 53 | 2.22 (0.31) | 0.28* | 0.21-0.37 | 74 | 2.89 (0.34) | 0.22* | 0.17-0.27 |
| API | 64 | 0.42 (0.05) | 0.05* | 0.04-0.07 | 117 | 0.73 (0.07) | 0.05* | 0.05-0.07 |
Data are from population-based cancer registries that participate in National Program of Cancer Registries and/or Surveillance Epidemiology and End Results Program and meet high-quality data criteria. These registries cover 67.2% of population for 1999-2006.
Rates are per 100,000 population and are age-adjusted to 2000 US standard population.
AI/AN, American Indians/Alaska Natives; API, Asian Pacific Islanders; CI, confidence interval; HW, Hispanic white; NHW, non-Hispanic white; –, not applicable.
Rate ratio was statistically significantly different from 1.00 (2-sided P <.05).
Tumor characteristics by sex
The most common site of diagnosis was trunk of body for both males and females (46.7% and 36.8%, respectively) followed by upper extremities in males (18.2%) and lower extremities in females (31.4%) (Table III). For melanomas of the face, head, and neck, males had higher incidence rates compared with females (0.92/100,000 vs 0.78/100,000, respectively) with proportionately twice as many cases diagnosed at this site in males (16.0%) compared with females (8.0%).
Table III.
Invasive melanoma incidence among adolescents and young adults (age 15-39 years) by tumor characteristics and sex: United States 1999-2006
| Male |
Female |
|||||
|---|---|---|---|---|---|---|
| No. | Rate | 95% CI | No. | Rate | 95% CI | |
| Primary site | ||||||
| Trunk | 7317 (46.7%) | 2.70 | 2.63-2.76 | 9570 (36.8%) | 3.57 | 3.50-3.64 |
| Upper extremities | 2857 (18.2%) | 1.05 | 0.02-1.02 | 5618 (21.6%) | 2.11 | 2.05-2.15 |
| Lower extremities | 2296 (14.6%) | 0.85 | 0.81-0.88 | 8181 (31.4%) | 3.07 | 3.00-3.13 |
| Face, head, and neck | 2515 (16.0%) | 0.92 | 0.88-0.96 | 2094 (8.0%) | 0.78 | 0.75-0.82 |
| NOS | 692 (4.4%) | 0.25 | 0.24-0.27 | 573 (2.2%) | 0.21 | 0.20-0.23 |
| Histopathology * | ||||||
| Superficial spreading | 5456 (35.0%) | 2.01 | 1.96-2.06 | 9904 (38.2%) | 3.70 | 3.63-3.78 |
| Nodular | 955 (6.1%) | 0.35 | 0.33-0.37 | 1085 (4.2%) | 0.41 | 0.38-0.43 |
| Lentigo maligna | 133 (0.9%) | 0.05 | 0.04-0.06 | 121 (0.5%) | 0.05 | 0.04-0.05 |
| Acral lentiginous | 87 (0.6%) | 0.03 | 0.0-0.04 | 135 (0.5%) | 0.05 | 0.04-0.06 |
| Other specified | 623 (4.0%) | 0.23 | 0.21-0.25 | 906 (3.5%) | 0.34 | 0.32-0.36 |
| NOS | 8346 (53.5%) | 3.07 | 3.01-3.14 | 13,798 (53.2%) | 5.16 | 5.08-5.25 |
| Stage † | ||||||
| Localized | 4457 (78.3%) | 4.42 | 4.29-4.55 | 8637 (84.4%) | 8.71 | 8.53-8.89 |
| Regional | 639 (11.2%) | 0.63 | 0.58-0.68 | 680 (6.6%) | 0.69 | 0.63-0.74 |
| Distant | 191 (3.4%) | 0.19 | 0.16-0.22 | 134 (1.3%) | 0.14 | 0.11-0.16 |
| NOS | 407 (7.1%) | 0.40 | 0.36-0.44 | 779 (7.6%) | 0.78 | 0.73-0.84 |
Data are from population-based cancer registries that participate in National Program of Cancer Registries and/or Surveillance Epidemiology and End Results Program and meet high-quality data criteria. These registries cover 67.2% of population for 1999-2006.
Rates are per 100,000 population and are age-adjusted to 2000 US standard population.
CI, Confidence interval; NOS, not otherwise specified.
Histologically confirmed cases (n = 41,549).
Cases diagnosed 2004-2006.
Greater than 99% of all melanoma incident cases (n = 41,549) were microscopically confirmed. Among these cases, more than 53% of all cases were reported as melanoma NOS (Histology NOS) among males and females (53.5% and 53.2%, respectively). Among those cases with a specific histologic type reported, superficial spreading melanoma was the most common type reported among males and females (35.0% and 38.2%, respectively) followed by nodular melanoma (6.1% and 4.2%, respectively). Lentigo maligna and acral lentiginous melanoma each accounted for less than 1% of cases among either males or females of all races and ethnicities combined. Other specific histologic types combined accounted for between 3% to 4% all cases combined.
For patients given a diagnosis between 2004 and 2006, the majority of cases were diagnosed at a local stage of disease (78.3% of males and 84.4% of females) with proportionately more males than females given a diagnosis of regional (11.2% vs 6.6%) and distant (3.4% and 1.3%) staged disease.
Tumor characteristics by race and ethnicity
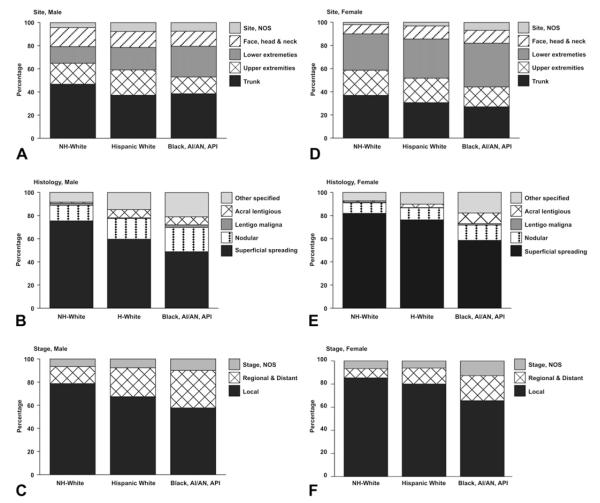
Among adolescents and young adults, incidence rates were highest among NHW compared with HW and non-whites regardless of site, histology, and stage (Table IV). Among males, the most common site of diagnosis was trunk among all racial and ethnic groups, followed by upper extremities in NHW and HW and lower extremities among non-whites (Fig 1, A). Face, head, and neck was the third most common site among NHW males. Among females, trunk of body was the most common site of diagnosis among NHW whereas lower extremities was the most common site among HW and non-whites (Fig 1, D). Conversely, the lower extremities were the second most common site among NHW and the trunk of the body was the second most common site among non-white females. Upper extremities were the third most common site among females of all racial and ethnic groups.
Table IV.
Invasive melanoma incidence among adolescents and young adults (age 15-39 years) by tumor characteristics, race and ethnicity, and sex: United States, 1999 through 2006
| Non-Hispanic white |
Hispanic white |
Non-white (black, AI/AN, API) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Count | Rate | 95% CI | Count | Rate 95% | CI | Count | Rate | 95% CI | |
| Male | |||||||||
| Site | |||||||||
| Trunk | 6627 | 3.71 | 3.62-3.80 | 146 | 0.36 | 0.30-0.42 | 77 | 0.15 | 0.12-0.19 |
| Upper extremities | 2571 | 1.44 | 1.38-1.50 | 86 | 0.21 | 0.16-0.26 | 29 | 0.06 | 0.04-0.08 |
| Lower extremities | 2049 | 1.15 | 1.10-1.20 | 76 | 0.18 | 0.15-0.23 | 53 | 0.10 | 0.08-0.14 |
| Face, head, and neck | 2331 | 1.30 | 1.25-1.35 | 54 | 0.13 | 0.10-0.17 | 26 | 0.05 | 0.03-0.07 |
| NOS | 613 | 0.34 | 0.32-0.37 | 30 | 0.07 | 0.05-0.10 | <16 | – | – |
| Histology | |||||||||
| Superficial spreading | 4998 | 2.80 | 2.72-2.88 | 109 | 0.26 | 0.21-0.32 | 42 | 0.08 | 0.06-0.11 |
| Nodular | 886 | 0.50 | 0.46-0.53 | 33 | 0.08 | 0.05-0.11 | 18 | 0.03 | 0.02-0.05 |
| Lentigo maligna | 123 | 0.07 | 0.06-0.08 | <16 | – | – | <16 | – | – |
| Acral lentiginous | 64 | 0.04 | 0.03-0.05 | <16 | – | – | <16 | – | – |
| Other specified | 555 | 0.31 | 0.28-0.34 | 27 | 0.07 | 0.04-0.10 | 18 | 0.03 | 0.02-0.05 |
| NOS | 7502 | 4.20 | 4.10-4.29 | 205 | 0.50 | 0.43-0.58 | 113 | 0.22 | 0.18-0.26 |
| Stage † | |||||||||
| Localized | 4062 | 6.32 | 6.13-6.52 | 97 | 0.58 | 0.47-0.71 | 41 | 0.25 | 0.19-0.33 |
| Regional and distant | 762 | 1.18 | 1.10-1.27 | 36 | 0.21 | 0.15-0.30 | 23 | 0.13 | 0.08-0.19 |
| NOS | 336 | 0.52 | 0.47-0.58 | <16 | – | – | <16 | – | – |
| Female | |||||||||
| Site | |||||||||
| Trunk | 8611 | 4.88 | 4.78-4.99 | 261 | 0.74 | 0.65-0.83 | 88 | 0.16 | 0.13-0.20 |
| Upper extremities | 5051 | 2.87 | 2.79-2.95 | 181 | 0.51 | 0.44-0.59 | 56 | 0.10 | 0.08-0.13 |
| Lower extremities | 7312 | 4.15 | 4.05-4.24 | 288 | 0.82 | 0.72-0.92 | 123 | 0.22 | 0.19-0.27 |
| Face, head, and neck | 1875 | 1.06 | 1.02-1.11 | 96 | 0.27 | 0.22-0.33 | 36 | 0.06 | 0.04-0.09 |
| NOS | 472 | 0.27 | 0.24-0.29 | 27 | 0.08 | 0.05-0.11 | 22 | 0.04 | 0.02-0.06 |
| Histology * | |||||||||
| Superficial spreading | 8991 | 5.1 | 4.99-5.21 | 286 | 0.81 | 0.72-0.91 | 89 | 0.16 | 0.13-0.20 |
| Nodular | 995 | 0.56 | 0.53-0.60 | 39 | 0.11 | 0.07-0.14 | 20 | 0.04 | 0.02-0.06 |
| Lentigo maligna | 100 | 0.06 | 0.05-0.07 | <16 | – | – | <16 | – | – |
| Acral lentiginous | 106 | 0.06 | 0.05-0.07 | <16 | – | – | <16 | – | – |
| Other specified | 790 | 0.45 | 0.42-0.48 | 38 | 0.11 | 0.08-0.15 | 27 | 0.05 | 0.03-0.07 |
| NOS | 12227 | 6.96 | 6.83-7.08 | 476 | 1.35 | 1.23-1.48 | 171 | 0.31 | 0.26-0.36 |
| Stage † | |||||||||
| Localized | 7788 | 12.21 | 11.94-12.49 | 278 | 1.97 | 1.75-2.22 | 88 | 0.41 | 0.33-0.51 |
| Regional and distant | 726 | 1.14 | 1.06-1.22 | 48 | 0.33 | 0.25-0.44 | 29 | 0.14 | 0.09-0.20 |
| NOS | 608 | 0.95 | 0.88-1.03 | 21 | 0.15 | 0.09-0.23 | 17 | 0.08 | 0.05-0.13 |
Data are from population-based cancer registries that participate in National Program of Cancer Registries and/or Surveillance Epidemiology and End Results Program and meet high-quality data criteria. These registries cover 67.2% of population for 1999-2006.
Rates are per 100,000 population and are age-adjusted to 2000 US standard population. Rates were suppressed if case count was <16. Case counts <16 were suppressed in tables but not in figures.
AI/AN, American Indians/Alaskan Natives; API, Asian Pacific Islanders; CI, confidence interval; NOS, not otherwise specified; –, rate or count suppressed (see footnote).
Histologically confirmed cases (n = 41,549).
Cases diagnosed 2004-2006.
Fig 1.
Distribution of invasive melanoma incident cases among adolescents and young adults (age 15-39 years) by site (A and D), histology (B and E), stage (C and F), race and ethnicity (A to F): United States 1999-2006. Data are from population-based cancer registries that participate in National Program of Cancer Registries and/or Surveillance Epidemiology and End Results Program and meet high-quality data criteria. These registries cover 67.2% of population for 1999-2006. Histology, not otherwise specified (NOS ) not included. Case counts less than 16 are included. API, Asian Pacific Islanders; AI/AN, American Indians/Alaskan Natives.
Histology NOS was the most common histology reporting among males and females of all racial and ethnic groups, accounting for between 52% and 56% of all cases combined (Table IV). Among males and females of all racial and ethnic groups with a specific histologic type reported, superficial spreading was the most common followed by nodular melanoma (Fig 1, B and E ). Lentigo maligna was rare among all racial and ethnic groups accounting for less than 1% of cases among NHW males and females. Acral lentiginous melanomas were rare (0.5%) among NHW males and females, and proportionately more common among HW males and females (3.3% and 1.3%, respectively). Other specified histologic types were reported to occur proportionately more frequently among non-whites followed by HW and NHW.
The majority of melanomas were diagnosed at a localized stage among males and females of all racial and ethnic groups (Fig 1, C and F ). There were proportionately more regional and distant staged cancers diagnosed in HW males and females (25.0% and 13.8%, respectively) and non-white males and females (32.4% and 21.6%, respectively) compared with NHW males and females (14.8% and 8.0%, respectively).
Site and histology among NHW
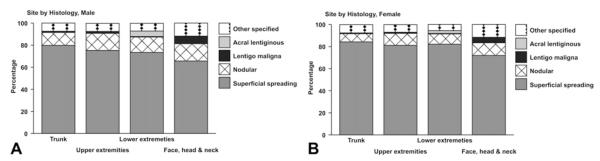
There were sufficient numbers of incident cases to examine incidence patterns by histologic type and site of diagnosis among NHW males and females (Table V). Histology NOS was the most commonly reported histologic type reported at all sites combined in males and females, respectively (data not shown), and occurred at site NOS in 90.3% and 87.3% of cases among males and females, respectively (data not shown). Among cases with a specific histology reported, superficial spreading and nodular melanomas occurred at all body sites: trunk; upper extremities; lower extremities; and face, head, and neck (Fig 2). Lentigo maligna melanoma and other specified histologic types were reported to occur proportionately more often on the face, head, and neck. Acral lentiginous melanoma was reported to occur on the lower extremities of the body.
Table V.
Invasive melanoma incidence among non-Hispanic white adolescents and young adults (age 15-39 years) by site, histology, and sex: United States, 1999 through 2006
| Trunk |
Upper extremities |
Lower extremities |
Face, head, and neck |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Count | Rate | 95% CI | Count | Rate | 95% CI | Count | Rate | 95% CI | Count | Rate | 95% CI | |
| Male | ||||||||||||
| Superficial spreading | 2590 | 1.45 | 1.39-1.51 | 926 | 0.52 | 0.49-0.55 | 717 | 0.40 | 0.37-0.43 | 738 | 0.41 | 0.38-0.44 |
| Nodular | 378 | 0.21 | 0.19-0.23 | 191 | 0.11 | 0.09-0.12 | 136 | 0.08 | 0.06-0.09 | 176 | 0.10 | 0.08-0.11 |
| Lentigo maligna | 28 | 0.02 | 0.01-0.02 | <16 | – | – | <16 | – | – | 76 | 0.04 | 0.03-0.05 |
| Acral lentiginous | <16 | – | – | <16 | – | – | 49 | 0.03 | 0.02-0.04 | <16 | – | – |
| Other specified | 241 | 0.13 | 0.12-0.15 | 90 | 0.05 | 0.04-0.06 | 68 | 0.04 | 0.03-0.05 | 132 | 0.07 | 0.06-0.09 |
| NOS | 3375 | 1.89 | 1.83-1.95 | 1336 | 0.75 | 0.71-0.79 | 1069 | 0.60 | 0.56-0.64 | 1203 | 0.67 | 0.63-0.71 |
| Female | ||||||||||||
| Superficial spreading | 3500 | 1.98 | 1.92-2.05 | 1956 | 1.11 | 1.06-1.16 | 2861 | 1.62 | 1.56-1.68 | 640 | 0.36 | 0.34-0.39 |
| Nodular | 308 | 0.17 | 0.16-0.20 | 260 | 0.15 | 0.13-0.17 | 322 | 0.18 | 0.16-0.20 | 102 | 0.06 | 0.05-0.07 |
| Lentigo maligna | 27 | 0.02 | 0.01-0.02 | <16 | – | – | <16 | – | – | 44 | 0.02 | 0.02-0.03 |
| Acral lentiginous | <16 | – | – | <16 | – | – | 90 | 0.05 | 0.04-0.06 | 0 | 0 | – |
| Other specified | 312 | 0.18 | 0.16-0.20 | 165 | 0.09 | 0.08-0.11 | 192 | 0.11 | 0.09-0.13 | 103 | 0.06 | 0.05-0.07 |
| NOS | 4449 | 2.52 | 2.45-2.60 | 2634 | 1.49 | 1.44-1.55 | 3820 | 2.17 | 2.10-2.24 | 980 | 0.56 | 0.52-0.59 |
Histologically confirmed cases only (n = 41,549).
Data are from population-based cancer registries that participate in National Program of Cancer Registries and/or Surveillance Epidemiology and End Results Program and meet high-quality data criteria. These registries cover 67.2% of population for 1999-2006.
Rates are per 100,000 population and are age-adjusted to 2000 US standard population. Rates and counts were suppressed if case count was <16. Case counts >1 and <16 were suppressed in tables.
CI, Confidence interval; NOS, not otherwise specified; –, rate or count suppressed (see footnote).
Fig 2.
Distribution of histologically confirmed invasive melanoma incident cases among non-Hispanic white adolescents and young adults (age 15-39 years) by site and histology (other than not otherwise specified [NOS]): United States 1999-2006. A, Males. B, Females. Data are from population-based cancer registries that participate in National Program of Cancer Registries and/or Surveillance Epidemiology and End Results Program and meet high-quality data criteria. These registries cover 67.2% of population for 1999-2006. Histology NOS not included. Case counts less than 16 are included.
DISCUSSION
Melanoma is the second most commonly diagnosed cancer among adolescents and young adults in the United States,1 although mortality continues to decline as survival improves, particularly among adolescents and young adults.4 The early age at onset of these cancers suggests that early life factors are important. In NHW in particular, melanomas occurring in this age group may represent gene-environment interactions involving excessive, intermittent UVR exposure among susceptible individuals, whereas late-onset melanomas may more often reflect accumulated, lifelong sun exposures in comparatively less susceptible individuals.30,31
The incidence of melanoma was higher in adolescent and young adult females compared with males in all age subgroups and in all racial and ethnic groups examined. This pattern is consistent with other published reports that have looked primarily at melanoma within the NHW adolescent and young adult populations,1 but differs from the pattern of male excess seen at older ages.32 This reversal in the gender RR between younger and older age groups suggests that there may be differences between etiologic pathways in younger versus older age groups that are mediated by gender.
One factor that may be related to this observed age-specific incidence pattern is female sex hormones. Some studies have found that the use of oral contraceptive or hormonal replacement therapy was associated with an increased risk of melanoma,33,34 whereas other studies have not found an association.35,36 Such inconsistent findings may be related to variations in measurement of estrogen receptor expressions.37 Specific genotypes have been found to be related to increased risk of melanoma among young women and may play a crucial role in the development of melanoma.38 In addition, one case-control study has suggested a link between endogenous female hormones related to pregnancy and increased melanoma risk.35 Additional research is needed to further explore possible etiologic pathways and the relationship between longitudinal trends in incidence and fertility rates (ie, delayed childbearing age at first birth and number of full term pregnancies).39
This study, although limited in years of observation, found evidence that incidence rates increased over time among adolescent and young adult females but not males. This finding is consistent with the results from previous studies based on data from the SEER Program, covering between 10% to 14% of the US population.4-6,32 There was a steeper increase in incidence among young females than males and mortality decreased in both groups. An increase in the incidence of melanoma in the presence of declining mortality could be explained by expanded skin screening and detection of biologically indolent tumors with low metastatic potential. However, an analysis of SEER incidence data over a 10-year period showed that increasing melanoma incidence rates occurred in all socioeconomic groups, histologic subtypes, and tumor thickness. The authors concluded that screening associated diagnoses could not explain the increasing incidence of thicker tumors in lower socioeconomic groups with poorer access to screening.40
Historical data on sun exposure in the United States by age are limited. However, one study reported an increase (although not statistically significant) in prevalence of sunburn in age 16 to 18 years from 1998 to 2004.41 Further, the prevalence of sunburn at all ages has continued to increase in both men and women.42 Sunburn has typically been used as one indicator of high intermittent exposure to UVR, the form of sun exposure most strongly related to melanoma risk.13
In addition, there is increasing evidence that artificial sources of UV exposure, including frequent indoor tanning, are associated with melanoma and other skin cancers.12,43,44 Adolescents, especially white adolescent girls, commonly use indoor tanning: up to 37% of NHW female adolescents and 11% of NHW male adolescents have used tanning booths at least once in their lifetimes45,46 and approximately 11% of adolescents report using a tanning bed in the past year.41,47,48 Certain segments of the population, especially teenagers and young adults, view the purported benefits of UV exposure (eg, protective base tan, appearances, feel healthy, and social interactions with friends) as outweighing the risk for skin cancer and effects on their future appearance (wrinkles).49-54 These attitudes are also associated with sporadic sunscreen use and more frequent sunburns.48
In general, melanoma is uncommon in non-whites and Hispanics when compared with NHW populations of all ages.55 The low incidence of skin cancers in darker-skinned groups is primarily a result of photoprotection provided by increased epidermal melanin, which filters twice as much UVR as does that in the epidermis of whites.56 Hence, UVR, the most important predisposing factor for skin cancer in whites, appears to play a lesser role in darker-skinned individuals.
The body site distribution of melanoma in adolescents and young adults differ somewhat from that of older adults. In both sexes, melanomas of the trunk are overrepresented in adolescents and young adults compared with older adults.55 The preponderance of trunk melanomas in this age group–accounting for nearly half (46.7%) of melanomas in males and 37% in females–suggests the importance of intermittent sun exposure, because the trunk is considered a usually unexposed body site, with most exposure in short, intense bursts. The variation in body site distribution across racial/ethnic groups parallels that for all ages, with melanomas on the lower extremities relatively more common in HW and non-white racial groups.55,57
Among the 4 specified histologic types of melanoma, superficial spreading melanoma and nodular melanoma account for the majority of lesions in adolescents and young adults, and occurred in all sites of the body among NHW. This is consistent with other studies that have reported superficial spreading melanomas to be more common among whites and Hispanics.58 Although the numbers are too small to draw any conclusions from these data about differences in the distribution of histologic types across racial/ethnic groups in adolescents and young adults, there is a suggestion that groups other than NHW have an overrepresentation of acral lentiginous melanomas and other specified histologies. This is consistent with findings for all ages that acral lentiginous melanomas, which usually arise on the palm of the hand or sole of the foot, is more common in people of color, especially blacks, compared with white populations.57,59 This observation probably accounts, at least in part, for the excess of melanomas on the lower extremities observed in these groups. In addition, in whites and to a lesser extent, Hispanics, melanomas predominantly occur in sun-exposed skin, whereas in Asians and blacks, UV does not appear to be a significant risk factor, and the majority occurs in non-sun-exposed skin.60
Strengths and limitations
Understanding the burden and relative risk of melanoma among adolescents and young adults is important to developing effective and targeted approaches to reducing incidence and deaths. This study provides a detailed description of melanoma incidence by demographic and tumor characteristics among patients between the ages of 15 to 39 years using data from 38 population-based cancer registries participating in either or both the NPCR and SEER Program and covering 67.2% of the US population.
Population-based cancer registries provide critical information on the cancer burden and the United States is fortunate to have nearly nationwide cancer surveillance coverage. We used only high-quality registry data to mitigate the influence of the under-reporting of incidence cases and the misclassification of demographic and tumor-related data. However, this study is subject to several limitations.
First, incomplete reporting and misclassification of data remain a potential problem in cancer registries. As this study reported, up to 6% cases had unspecified or unknown race/ethnicity data. And routine misclassification of AI/AN as white has led to the systematic underreporting of cancer in the AI/AN population.61 Routine linkage of cancer registry data with Indian Health Service administrative databases for the purpose of classifying race and improved collection and reporting of race information in population estimates26 has resulted in a more accurate estimate of the cancer burden in the AI/AN population,61 including melanomas in AI/AN adolescents and young adults.62,63 Second, more than 50% of all cases were coded with a Histology NOS, thus limiting our ability to look more closely at histologic subtypes including “other” specific histologic types that may differ by age, race, and ethnicity. Primary data reporters (physicians, and laboratory and medical records staff) play a critical role in the ability of the cancer registry staff to collect, consolidate, and report complete, accurate, and specific incidence data. Improved primary data reporting is needed to increase data specificity in the future.
Public health implications
There are important reasons for examining the cancer burden in adolescents and young adults. An examination of incidence patterns in this age group may foretell the future cancer burden, because changing exposure opportunities of early life that can begin to manifest as cancer during adolescence and young adulthood, and provide insight into etiologic relationships as the time since exposure and diagnosis may be somewhat shorter in young adults than for older adults.7,62 In particular, an analysis of melanoma among the young adult population may provide insight into the current and future burden of melanoma in the US population and serve as a critical first step toward describing, monitoring, and eventually eliminating this cancer.
Public health campaigns to promote awareness of the risk of sun exposure in Australia and Europe may have contributed to stabilizing or declining melanoma incidence rates in young adults in those countries. There is increasing awareness of the potential harmful effects of artificial sources of UV exposure particularly among adolescents and young adults. The World Health Organization has designated tanning equipment to be carcinogenic.44 As of June 2011, 26 states had enacted laws restricting minors access to tanning facilities.63
Promoting awareness of the harmful effects of UV exposure (both artificial and natural) and promoting screening for melanoma remain important strategies for reducing the future burden of melanoma in the adolescent and young adult populations. In addition, additional research is required to understand the role of gene-environment exposure interaction and to be able to identify susceptible individuals in the populations that may be at particular risk of developing melanoma.
CAPSULE SUMMARY.
Melanoma is the third most commonly diagnosed cancer among adolescents and young adults (aged 15-39 years) in the United States.
Understanding the burden of melanoma in this age group is important to identifying areas for etiologic research and in developing effective prevention approaches aimed at reducing melanoma risk.
This study, using National Program of Cancer Registries/Surveillance Epidemiology and End Results combined incidence data, describes the burden of invasive melanoma of the skin in adolescents and young adults in the United States by demographic and tumor characteristics.
Acknowledgments
Publication of this supplement to the JAAD was supported by the Division of Cancer Prevention and Control, Centers for Disease Control and Prevention (CDC).
Abbreviations used
- AI/AN
American Indians/Alaskan Natives
- API:
Asian Pacific Islanders
- CI
confidence interval
- HW
Hispanic white
- ICD-O
International Classification of Diseases–Oncology
- ICD-O-3
International Classification of Diseases–Oncology, Third Edition
- NCI
National Cancer Institute
- NHW
non-Hispanic white
- NOS
not otherwise specified
- NPCR
National Program of Cancer Registries
- IRR
incidence rate ratio
- SEER
Surveillance Epidemiology and End Results
- UV
ultraviolet
- UVR
ultraviolet radiation
Footnotes
Conflicts of interest: None declared.
The opinions or views expressed in this supplement are those of the authors and do not necessarily reflect the opinions, recommendations, or official position of the journal editors or the Centers for Disease Control and Prevention.
REFERENCES
- 1.Bleyer A, Viny A, Barr R. Cancer in 15- to 29-year-olds by primary site. Oncologist. 2006;11:590–601. doi: 10.1634/theoncologist.11-6-590. [DOI] [PubMed] [Google Scholar]
- 2.Gruber SB, Armstrong BK. Cutaneous and ocular melanoma. In: Schottenfeld D, Fraumeni JF, editors. Cancer epidemiology and prevention. 3rd ed Oxford University Press; New York: 2006. pp. 1196–229. [Google Scholar]
- 3.Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J, editors. SEER survival monograph: cancer survival among adults, US, SEER program, 1988-2001, patients and tumor characteristics. National Cancer Institute, SEER Program; Bethesda (MD): 2007. NIH publication No. 07-621556. [Google Scholar]
- 4.Herzog C, Pappo A, Bondy M, Bleyer A, Kirkwood J. Malignant melanoma. In: Bleyer A, O’Leary M, Barr R, Ries LAG, editors. Cancer epidemiology in older adolescents and young adults 15-29 years of age, including SEER incidence and survival 1975-2000. National Cancer Institute; Bethesda (MD): 2006. pp. 53–63. NIH publication No. 06-5767. [Google Scholar]
- 5.Purdue MP, Freeman LE, Anderson WF, Tucker MA. Recent trends in incidence of cutaneous melanoma among US Caucasian young adults. J Invest Dermatol. 2008;128:2905–8. doi: 10.1038/jid.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jemal A, Devesa SS, Hartge P, Tucker MA. Recent trends in cutaneous melanoma incidence among whites in the United States. J Natl Cancer Inst. 2001;93:678–83. doi: 10.1093/jnci/93.9.678. [DOI] [PubMed] [Google Scholar]
- 7.Marrett LD, Frood J, Nishri D, Ugnat AM. Cancer incidence in young adults in Canada: preliminary results of a cancer surveillance project. Chronic Dis Can. 2002;23:58–64. [PubMed] [Google Scholar]
- 8.Alston RD, Rowan S, Eden TO, Moran A, Birch JM. Cancer incidence patterns by region and socioeconomic deprivation in teenagers and young adults in England. Br J Cancer. 2007;96:1760–6. doi: 10.1038/sj.bjc.6603794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrett LD, Nguyen HL, Armstrong BK. Trends in the incidence of cutaneous malignant melanoma in New South Wales, 1983-1996. Int J Cancer. 2001;92:457–62. doi: 10.1002/ijc.1203. [DOI] [PubMed] [Google Scholar]
- 10.Uribe P, Wistuba II, Solar A, Balestrini C, Perez-Cotapos ML, Gonzalez S. Comparative analysis of loss of heterozygosity and microsatellite instability in adult and pediatric melanoma. Am J Dermatopathol. 2005;27:279–85. doi: 10.1097/01.dad.0000171599.40562.7c. [DOI] [PubMed] [Google Scholar]
- 11.Gilchrest BA, Eller MS, Geller AC, Yaar M. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341–8. doi: 10.1056/NEJM199904293401707. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher RP, Spinelli JJ, Lee TK. Tanning beds, sunlamps, and risk of cutaneous malignant melanoma. Cancer Epidemiol Biomarkers Prev. 2005;14:562–6. doi: 10.1158/1055-9965.EPI-04-0564. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong BK, Kricker A, English DR. Sun exposure and skin cancer. Australas J Dermatol. 1997;38(Suppl):S1–6. doi: 10.1111/j.1440-0960.1997.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 14.Lin J, Hocker TL, Singh M, Tsao H. Genetics of melanoma predisposition. Br J Dermatol. 2008;159:286–91. doi: 10.1111/j.1365-2133.2008.08682.x. [DOI] [PubMed] [Google Scholar]
- 15.Bataille V, Winnett A, Sasieni P, Bishop JA Newton, Cuzick J. Exposure to the sun and sunbeds and the risk of cutaneous melanoma in the UK: a case-control study. Eur J Cancer. 2004;40:429–35. doi: 10.1016/j.ejca.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 16.Livestro DP, Kaine EM, Michaelson JS, Mihm MC, Haluska FG, Muzikansky A, et al. Melanoma in the young: differences and similarities with adult melanoma: a case-matched controlled analysis. Cancer. 2007;110:614–24. doi: 10.1002/cncr.22818. [DOI] [PubMed] [Google Scholar]
- 17.Purdue MP, From L, Armstrong BK, Kricker A, Gallagher RP, McLaughlin JR, et al. Etiologic and other factors predicting nevus-associated cutaneous malignant melanoma. Cancer Epidemiol Biomarkers Prev. 2005;14:2015–22. doi: 10.1158/1055-9965.EPI-05-0097. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Cancer Statistics Working Group . United States Cancer Statistics: 1999–2006 Incidence and Mortality Web-based Report. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; Atlanta: [Accessed July 7, 2011]. 2009. Available from: http://www.cdc.gov/uscs. [Google Scholar]
- 19.Wingo PA, Jamison PM, Hiatt RA, Weir HK, Gargiullo PM, Hutton M, et al. Building the infrastructure for nationwide cancer surveillance and controlea comparison between the National Program of Cancer Registries (NPCR) and the Surveillance Epidemiology and End Results (SEER) Program (United States) Cancer Causes Control. 2003;14:175–93. doi: 10.1023/a:1023002322935. [DOI] [PubMed] [Google Scholar]
- 20.Hankey BF, Ries LA, Edwards BK. The Surveillance Epidemiology and End Results program: a national resource. Cancer Epidemiol Biomarkers Prev. 1999;8:1117–21. [PubMed] [Google Scholar]
- 21.Watson M, Johnson CJ, Chen VW, Thomas CC, Weir HK, Sherman R, et al. Melanoma surveillance in the United States: overview of methods. J Am Acad Dermatol. 2011;65:S6–16. doi: 10.1016/j.jaad.2011.04.037. [DOI] [PubMed] [Google Scholar]
- 22.Havener L, Thornton M, editors. Standards for cancer registries volume II: Data standards and data dictionary. Thirteenth edition, Version 11.3 North American Association of Central Cancer Registries; Springfield, IL: Apr, 2008. [Google Scholar]
- 23.Fritz A, Percy C, Jack A. International classification of diseases of oncology. World Health Organization; Geneva (Switzerland): 2000. [Google Scholar]
- 24.Collaborative Stage Data Collection System [Accessed July 7, 2011]; Available from: URL: http://web.facs.org/cstage/schemalist.htm.
- 25.Young JL, Roffers SD, Ries LAG, Fritz AG, Hurlbut AA, editors. SEER summary staging manual–2000: codes and coding instructions. National Cancer Institute; Bethesda (MD): 2001. NIH publication No. 01–4969. [Google Scholar]
- 26.National Cancer Institute. Surveillance Epidemiology and End Results (SEER) program [Accessed July 7, 2011];Statistical resources. US population data 1969-2006. Available from: URL: http://seer.cancer.gov/resources.
- 27.Surveillance Research Program . National Cancer Institute SEER*Stat Software. version 6.6.2. National Cancer Institute; Bethesda, MD: [Accessed July 7, 2011]. 2010. http://www.seer.cancer.gov/seerstat. [Google Scholar]
- 28.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15:547–69. doi: 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]
- 29.Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based on the gamma distribution. Stat Med. 1997;16:791–801. doi: 10.1002/(sici)1097-0258(19970415)16:7<791::aid-sim500>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 30.Anderson WF, Pfeiffer RM, Tucker MA, Rosenberg PS. Divergent cancer pathways for early-onset and late-onset cutaneous malignant melanoma. Cancer. 2009;115:4176–85. doi: 10.1002/cncr.24481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Autier P. Cutaneous malignant melanoma: facts about sunbeds and sunscreen. Expert Rev Anticancer Ther. 2005;5:821–33. doi: 10.1586/14737140.5.5.821. [DOI] [PubMed] [Google Scholar]
- 32.Jemal A, Saraiya M, Patel P, Cherala SS, Barnholtz-Sloan J, Kim J, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992-2006. J Am Acad Dermatol. 2011;65:S17–25. doi: 10.1016/j.jaad.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 33.Feskanich D, Hunter DJ, Willett WC, Spiegelman D, Stampfer MJ, Speizer FE, Colditz GA. Oral contraceptive use and risk of melanoma in premenopausal women. Br J Cancer. 1999;81:918–23. doi: 10.1038/sj.bjc.6690787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holly EA, Cress RD, Ahn DK. Cutaneous melanoma in women: ovulatory life, menopause, and use of exogenous estrogens. Cancer Epidemiol Biomarkers Prev. 1994;3:661–8. [PubMed] [Google Scholar]
- 35.Lea CS, Holly EA, Hartge P, Lee JS, Guerry D, 4th, Elder DE, et al. Reproductive risk factors for cutaneous melanoma in women: a case-control study. Am J Epidemiol. 2007;165:505–13. doi: 10.1093/aje/kwk040. [DOI] [PubMed] [Google Scholar]
- 36.Osterlind A, Tucker MA, Stone BJ, Jensen OM. The Danish case-control study of cutaneous malignant melanoma, III: hormonal and reproductive factors in women. Int J Cancer. 1988;42:821–4. doi: 10.1002/ijc.2910420603. [DOI] [PubMed] [Google Scholar]
- 37.Ohata C, Tadokoro T, Itami S. Expression of estrogen receptor beta in normal skin, melanocytic nevi and malignant melanomas. J Dermatol. 2008;35:215–21. doi: 10.1111/j.1346-8138.2008.00447.x. [DOI] [PubMed] [Google Scholar]
- 38.Firoz EF, Warycha M, Zakrzewski J, Pollens D, Wang G, Shapiro R, et al. Association of MDM2 SNP309, age of onset, and gender in cutaneous melanoma. Clin Cancer Res. 2009;15:2573–80. doi: 10.1158/1078-0432.CCR-08-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matthews TJ, Hamilton BE. Delayed childbearing: more women are having their first child later in life. NCHS Data Brief. 2009;(21):1–8. [PubMed] [Google Scholar]
- 40.Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129:1666–74. doi: 10.1038/jid.2008.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cokkinides V, Weinstock M, Lazovich D, Ward E, Thun M. Indoor tanning use among adolescents in the US, 1998 to 2004. Cancer. 2009;115:190–8. doi: 10.1002/cncr.24010. [DOI] [PubMed] [Google Scholar]
- 42.Robinson JK, Rigel DS, Amonette RA. Trends in sun exposure knowledge, attitudes, and behaviors: 1986 to 1996. J Am Acad Dermatol. 1997;37:179–86. doi: 10.1016/s0190-9622(97)80122-3. [DOI] [PubMed] [Google Scholar]
- 43.Lazovich D, Vogel RI, Berwick M, Weinstock MA, Anderson KE, Warshaw EM. Indoor tanning and risk of melanoma: a case-control study in a highly exposed population. Cancer Epidemiol Biomarkers Prev. 2010;19:1557–68. doi: 10.1158/1055-9965.EPI-09-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.IARC Working Group on Risk of Skin Cancer and Exposure to Artificial Ultraviolet Light . Exposure to artificial UV radiation and skin cancer. World Health Organization; [Access July 7, 2011]. Jun, 2005. Available at: URL: http://whqlibdoc.who.int/iarc/9283224418_eng.pdf. [Google Scholar]
- 45.Balk SJ, Geller AC. Teenagers and artificial tanning. Pediatrics. 2008;121:1040–2. doi: 10.1542/peds.2007-2256. [DOI] [PubMed] [Google Scholar]
- 46.Demko CA, Borawski EA, Debanne SM, Cooper KD, Stange KC. Use of indoor tanning facilities by white adolescents in the United States. Arch Pediatr Adolesc Med. 2003;157:854–60. doi: 10.1001/archpedi.157.9.854. [DOI] [PubMed] [Google Scholar]
- 47.Cokkinides VE, Weinstock MA, O’Connell MC, Thun MJ. Use of indoor tanning sunlamps by US youth, ages 11-18 years, and by their parent or guardian caregivers: prevalence and correlates. Pediatrics. 2002;109:1124–30. doi: 10.1542/peds.109.6.1124. [DOI] [PubMed] [Google Scholar]
- 48.Geller AC, Colditz G, Oliveria S, Emmons K, Jorgensen C, Aweh GN, Frazier AL. Use of sunscreen, sunburning rates, and tanning bed use among more than 10,000 US children and adolescents. Pediatrics. 2002;109:1009–14. doi: 10.1542/peds.109.6.1009. [DOI] [PubMed] [Google Scholar]
- 49.Robinson JK, Kim J, Rosenbaum S, Ortiz S. Indoor tanning knowledge, attitudes, and behavior among young adults from 1988-2007. Arch Dermatol. 2008;144:484–8. doi: 10.1001/archderm.144.4.484. [DOI] [PubMed] [Google Scholar]
- 50.Hillhouse J, Turrisi R. Skin cancer risk behaviors: a conceptual framework for complex behavioral change. Arch Dermatol. 2005;141:1028–31. doi: 10.1001/archderm.141.8.1028. [DOI] [PubMed] [Google Scholar]
- 51.Kolmel KF, Kulle B, Lippold A, Seebacher C. Survival probabilities and hazard functions of malignant melanoma in Germany 1972-1996, an analysis of 10433 patients: evolution of gender differences and malignancy. Eur J Cancer. 2002;38:1388–94. doi: 10.1016/s0959-8049(02)00104-1. [DOI] [PubMed] [Google Scholar]
- 52.Knight JM, Kirincich AN, Farmer ER, Hood AF. Awareness of the risks of tanning lamps does not influence behavior among college students. Arch Dermatol. 2002;138:1311–5. doi: 10.1001/archderm.138.10.1311. [DOI] [PubMed] [Google Scholar]
- 53.Hillhouse JJ, Stair AW, III, Adler CM. Predictors of sunbathing and sunscreen use in college undergraduates. J Behav Med. 1996;19:543–61. doi: 10.1007/BF01904903. [DOI] [PubMed] [Google Scholar]
- 54.Jones JL, Leary MR. Effects of appearance-based admonitions against sun exposure on tanning intentions in young adults. Health Psychol. 1994;13:86–90. doi: 10.1037//0278-6133.13.1.86. [DOI] [PubMed] [Google Scholar]
- 55.Wu X, Eide MJ, King J, Saraiya M, Huang Y, Wiggins C, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65:S26–37. doi: 10.1016/j.jaad.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 56.Brenner M, Hearing VJ. The protective role of melanin against UV damage in human skin. Photochem Photobiol. 2008;84:539–49. doi: 10.1111/j.1751-1097.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246–52. doi: 10.1023/a:1018432632528. [DOI] [PubMed] [Google Scholar]
- 58.Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol. 2007;6:10–6. [PubMed] [Google Scholar]
- 59.Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427–34. doi: 10.1001/archdermatol.2008.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bradford J. Case of the month: diagnostic challenges from your case files. JAAPA. 2005;18:72. doi: 10.1097/01720610-200505000-00012. [DOI] [PubMed] [Google Scholar]
- 61.Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975-2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110:2119–52. doi: 10.1002/cncr.23044. [DOI] [PubMed] [Google Scholar]
- 62.Weir HK, Jim MA, Marrett LD, Fairley T. Cancer in American Indian and Alaska Native young adults (ages 20-44 years): US, 1999-2004. Cancer. 2008;113(Suppl):1153–67. doi: 10.1002/cncr.23731. [DOI] [PubMed] [Google Scholar]
- 63.National Cancer Institute State Cancer Legislative Database Program [Accessed July 7, 2011];States with Laws Addressing Minors’ Access to Tanning Facilities. Available from: URL: http://www.scld-nci.net/linkdocs/products/factsheets93.pdf.