Abstract
Accurate localization of epileptogenic brain is critical for successful epilepsy surgery. Recent research using wide bandwidth intracranial EEG has demonstrated that interictal high-frequency oscillations are preferentially localized to the brain region generating spontaneous seizures, and are a potential biomarker of epileptogenic brain. The existence of an interictal, electrophysiological biomarker of epileptogenic brain has the potential to significantly advance epilepsy surgery by improving outcomes through improved localization and potentially eliminating the reliance on chronic intracranial EEG monitoring.
Keywords: epilepsy surgery, epileptogenic zone, fast ripple, high-frequency oscillation, ripple, seizure onset zone, wide bandwidth EEG
Neuronal oscillations recorded from human brain span a wide range of spatiotemporal scales that extend well beyond traditional clinical EEG [1,2]. Extracellular local field potentials (LFPs) recorded from human brain range from direct current shifts to high-frequency oscillations (HFOs; ~0–1000 Hz). The mechanisms underlying these activities are varied [1], making the direct association of LFP characteristics (e.g., frequency, amplitude, spectral pattern, waveform morphology) with physiology or pathology difficult. Despite this challenge, neurophysiologists have had significant success associating physiological function and pathology with EEG activity [1].
Historically, neurophysiologists largely focused on activity in the Berger bands (1–25 Hz) [3]. Recent studies, however, report that γ-frequency oscillations and synchrony (γ: 25–80 Hz) are involved in cognitive function and pathology (for a recent review see [4,5]). Beyond the γ-frequency range, hippocampal ripple frequency oscillations (ripple: 80–200 Hz) are believed to be important for memory [6–8].
Ictal EEG is a powerful tool for diagnosis and classification of seizures and epilepsy. It was recognized early on that epileptogenic brain capable of generating spontaneous seizures also generated interictal epileptiform spikes and sharp waves [9]. These interictal EEG signatures of epileptogenic brain are generated by the paroxysmal discharge of large neuronal populations and are highly specific for epilepsy [10]. In addition to interictal epileptiform spikes, recent studies suggest that HFOs are an interictal signature of epileptogenic networks [11–19] involved in seizure generation [20–25] and even epileptogenesis [14,26,27].
While epileptiform spikes are highly specific for epileptic brain, they are not a particularly good biomarker because they are only loosely related to disease activity: they are not a good indicator of the likelihood of seizure occurrence and they do not fluctuate as seizures do in relation to antiepileptic drug treatment (see ‘Association of HFOs with disease activity’ section). The specificity of HFOs to epilepsy has proven difficult to assess because it is not clear how to separate pathological HFOs from normal physiological oscillations that occur in the same frequency range [14,28]. In addition, HFOs are brief low-amplitude transients that have almost exclusively been described in intracranial EEG (iEEG) recordings of patients with epilepsy. Lastly, because these studies are limited to patients with medically resistant partial epilepsy, the specificity of HFOs as a biomarker for epileptogenic brain remains open. Whether HFOs recorded in epileptic brain are generated by unique pathological mechanism(s) or represent an aberration of normal physiological oscillations is not clear. There are currently no established criteria for distinguishing physiological from pathological HFOs.
In this article we review the clinical evidence supporting that HFOs are electrophysiological biomarkers of epileptogenic brain.
Ictal HFOs
High-frequency oscillations at the onset of human seizures were initially described in iEEG recordings of patients undergoing evaluation for epilepsy surgery. These early observations showed that partial seizures originating in hippocampus and neocortex often begin with low-amplitude HFOs (Figure 1). The range of frequencies that have been reported vary, but are in the γ-, ripple and fast ripple (FR) frequency range: 30–500 Hz [29], 40–120 Hz [21], 60–100 Hz [24], 70–90 Hz [23], 80–110 Hz [20] and 100–500 Hz [25]. Focal low-voltage fast oscillations (>20–100 Hz) at seizure onset have been demonstrated to be associated with good epilepsy surgery outcomes if the seizure onset zone (SOZ) is completely resected [30,31].
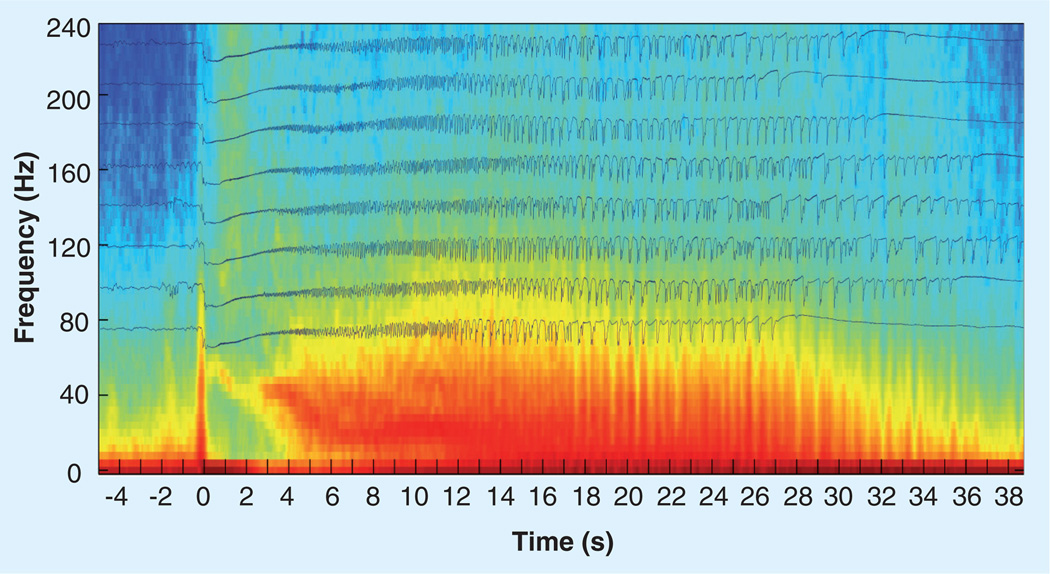
Figure 1. Average of eight seizures recorded from the hippocampus of a patient with medically resistant partial epilepsy.
The time traces of eight of the patient’s habitual seizures aligned so that the seizure onset is at 0 s. The average time frequency spectrogram is shown in the background. The seizures all show a characteristic slow-wave transient that is associated with a high-frequency oscillation (~110 Hz) at seizure onset.
Interictal HFOs
Ripple frequency oscillations (80–200 Hz) were first described in hippocampus of freely behaving rats, and hypothesized to be associated with memory [6,7]. Later, the same group described FR oscillations (FR: 250–500 Hz) in a rat model of epilepsy that were not observed in control animals [13,32]. In the epileptogenic hippocampus of epileptic rats Bragin et al. observed that FR oscillations were localized to submillimeter scale volumes [13] and increased [32]. In addition, physiological ripple frequency oscillations were decreased in the epileptogenic hippocampus [32]. These seminal observations led to the first detailed analysis of wide bandwidth electrophysiology recordings from human subjects. Similar to the epileptic rat models, both ripple and FR oscillations were identified in epileptogenic human hippocampus (Figure 2) [11,12]. These early studies with microelectrodes made a number of important observations:
-
▪
Ripple and FR oscillations were increased in slow-wave sleep compared with the waking period;
-
▪
FR oscillations were increased in the epileptogenic hippocampus;
-
▪
FR oscillations were localized to submillimeter scale volumes of tissue;
-
▪
Ripples were decreased in the epileptogenic hippocampus.
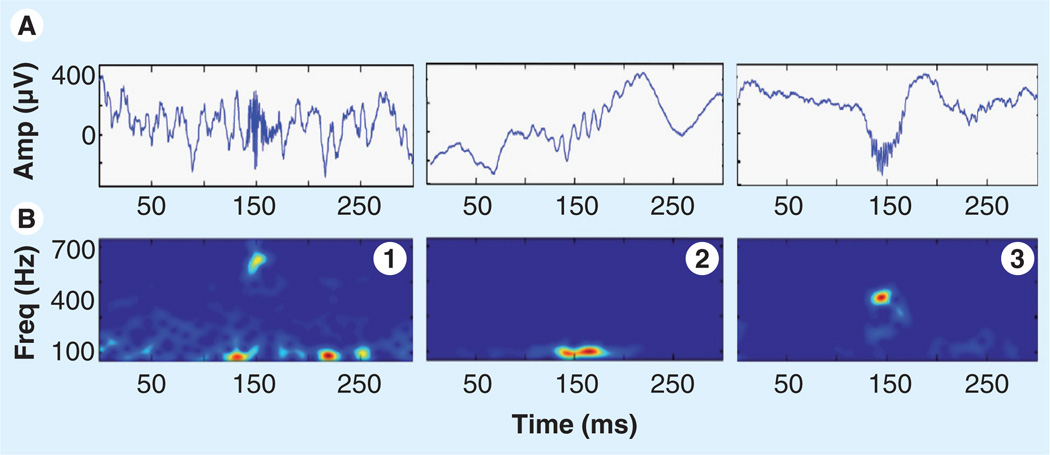
Figure 2. Representative high-frequency oscillations.
Each plot shows two views of high-frequency oscillation activity over a 300 ms epoch: (A) unfiltered EEG with a high-frequency oscillation event centered at 150 ms, (B) spectrogram (2.6 ms window). Note that high-frequency oscillations are primarily characterized by a sharp spectral mode in the fast ripple (1 & 3) or ripple frequency range (2).
Amp: Amplification; Freq: Frequency.
Subsequent studies have partially confirmed these initial results in human hippocampus. Discrepancies could originate from the fact that many human studies have employed macroelectrode recordings rather than the microelectrodes used in rats and in some human studies.
For instance, FR HFOs can be spatially more widespread than predicted from microelectrode studies [33], and multiple studies have clearly demonstrated that ripples and FRs are reliably recorded using clinical macroelectrodes (Figure 3) [17,19]. In addition, recent studies report ripple HFOs are often increased in the epileptogenic brain (Figure 3) [19,34]. The fact that ripple HFOs are increased in the SOZ, rather than decreased, is consistent with reports from animals describing an increase in ripples prior to seizures [22,35], and supports the hypothesis that ripple-frequency HFOs are involved in the generation of seizures [36]. These macroelectrode-recorded ripples may also be independent of the physiological HFOs recorded with microelectrodes and may be more similar to FRs, from the point of view of being potential biomarkers of epilepsy. Indirect evidence that macroelectrode recordings of ripples most likely represent a pathological phenomenon is that it is common to find several regions in a patient in which there are no HFOs. If they were in part a physiological phenomenon, one would expect to record them often in regions devoid of epileptic activity.
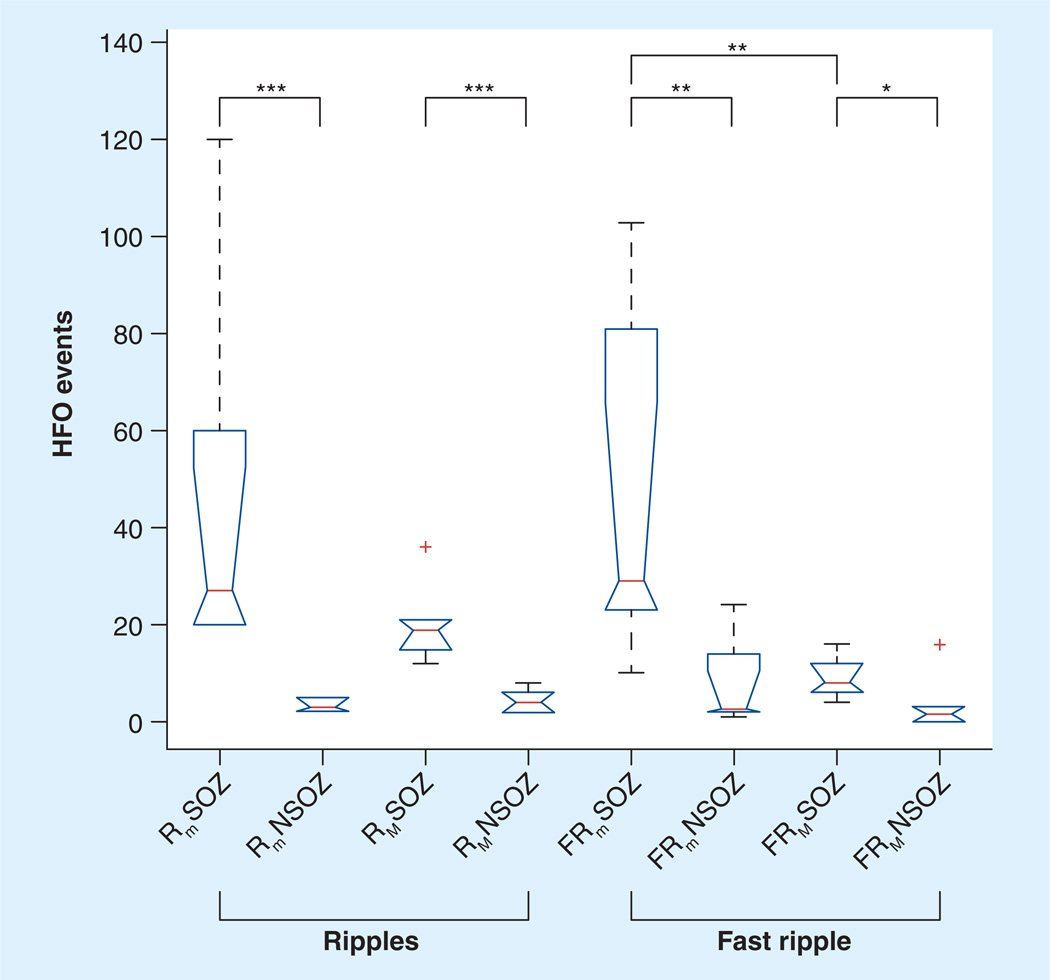
Figure 3. Kurskal–Wallis applied to high-frequency oscillations (ripple/fast ripple), electrode type (microwire/macroelectrode), and brain region (seizure onset zone/nonseizure onset zone).
Box-plots and the results from post hoc analysis using Wilcoxon rank sum. The number of Rm and FRm HFOs are increased in the SOZ compared with NSOZ. The number of RM and FRM HFOs were increased in the SOZ compared with NSOZ.
*p < 0.05
**p < 0.01
***p < 0.002
FRM: Macroelectrode fast ripple; FRm: Microwire fast ripple; HFO: High-frequency oscillation; NSOZ: Nonseizure onset zone; RM: Macroelectrode ripple; Rm: Microwire ripple; SOZ: Seizure onset zone.
Reproduced with permission from [24].
Emerging areas of clinical relevance
The fact that HFOs can be recorded with clinical macroelectrodes is of significant clinical importance, since high-impedance microwires introduce a number of additional recording challenges. At this time, however, the optimal electrode cross-section and spacing for mapping epileptic brain is not known. While studies have previously demonstrated that FRs are often localized to submillimeter scales [13], recent studies with larger electrodes did not find a correlation with a range of larger electrode sizes [37].
Digital electronics and computing have revolutionized clinical iEEG and wide bandwidth electrophysiology recordings from hundreds of electrodes are now common. These studies have redefined the spatial and temporal bandwidth of human brain activity [2,11,12,19,38–40]. There remain many questions regarding clinical utility, but this is now receiving significant attention (for reviews see [14,15]). In the following sections we discuss recent developments of potential clinical importance.
Interictal to ictal transitions
A question of fundamental importance to epilepsy is understanding the transition from interictal state to seizure. Because interictal HFOs localize epileptogenic brain regions and seizures often show HFOs at onset, multiple groups have investigated whether HFOs precede seizures. In an in vitro model of epilepsy, ripple FR activity increased prior to the onset of seizures [35]. In a series of elegant in vivo experiments by Greiner et al. in a model of generalized or widespread seizures induced by anesthesia [22,41], ripple frequency oscillations were associated with seizure generation, and an increase in ripple oscillation amplitude preceded onset. The increase in ripple HFO amplitude was shown to directly affect the transmembrane potential of local neurons. The possibility that local neurons might be synchronously brought to threshold with the ripple frequency LFPs generating a positive feedback loop for the initiation and spread of seizures was later developed [36]. They propose that beyond a certain threshold of ripple HFO amplitude local neurons are entrained into the ripple frequency seizure discharge [36].
Currently, there are no conclusive studies showing that HFOs are precursor events in human partial epilepsy, but similar to the above mentioned studies in an in vitro model [35] γ-frequency HFOs are increased in some patients prior to seizure [24]. Khosravani et al. found that HFO activity often increased in the seconds immediately preceding a seizure, but the timing of seizure onset is not always easy to define within a period of a few seconds [42]. Examining fluctuations in HFOs in the 15-, 5-, and 1-min intervals preceding seizure occurrence, however, Jacobs et al. did not find any consistent pattern or pre-ictal change [43].
Association of HFOs with seizure outcome
The current conceptual model for epilepsy surgery defines the epileptogenic zone (EZ) as the brain region necessary and sufficient to generate seizures and therefore the minimum region that must be resected for seizure freedom [44,45]. Unfortunately, it is not possible to define this zone in practice because the EZ does not have a biomarker. The SOZ is often used as an indicator for the EZ but the relationships between the SOZ, EZ and regions generating interictal spikes remain poorly defined. In practice, the tissue involved in the SOZ, early seizure propagation and adjacent tissue with active interictal spiking are often targeted for surgical resection, also taking into consideration the possible epileptogenic lesion.
The existence of a reliable interictal marker of the EZ could transform the practice of epilepsy surgery by removing the reliance on chronic iEEG. Pathological HFOs may provide a means for interictal EZ localization [14,15]. The spatial distribution of interictal spikes is typically more widespread than the SOZ and while they typically overlap, the relationship can be complex. In a study investigating the spatial relation between HFOs and interictal spiking the HFOs had a tighter correlation with the SOZ [16]. Additionally, HFOs were more closely coupled with the region of seizure onset than an epileptogenic structural lesion on MRI [46]. HFOs, therefore, appear to be a more reliable biomarker of ictogenesis than interictal spikes and MRI structural lesions.
Two recent papers have extended the clinical investigation of HFOs to address their association with epilepsy surgery outcomes. While previous studies had demonstrated an association with the SOZ, currently the primary signal-guiding epilepsy surgery, they had not directly investigated the association with surgery outcome. By correlating the resection of HFO generating tissue with epilepsy surgery outcome it is possible to directly probe whether HFOs are an electrophysiological biomarker of the EZ. Jacobs et al. demonstrated that resection of tissue generating ripple and FR HFOs was associated with a more favorable epilepsy surgery outcome (Figure 4) [34]. In another study by Wu et al. an average of 11.8 min of intraoperative iEEG was used to identify FR HFOs (>250 Hz) [47]. Remarkably, based on similarly short recordings they found that all patients who had tissue with HFOs resected (19/19) were seizure free. By contrast, none of the patients (5/5) who did not have all FR HFOs tissue resected were seizure free.
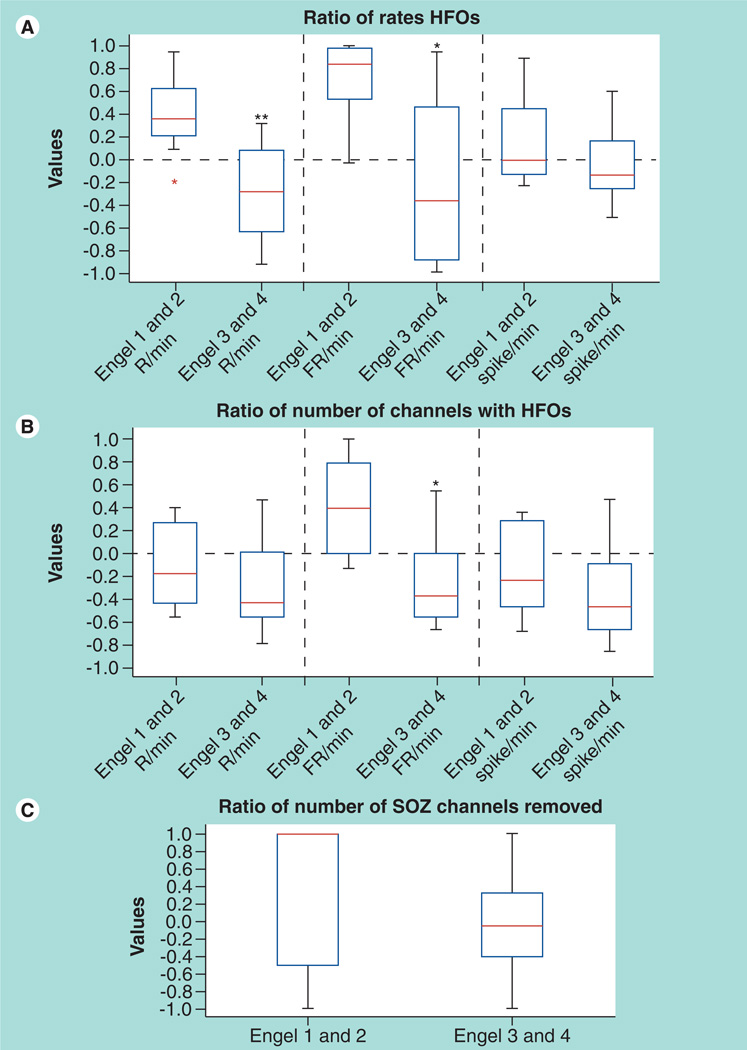
Figure 4. Removal of fast ripple and ripple high-frequency oscillation are associated with a good surgical outcome.
(A) Ratio of event rates in the removed areas to rates in the nonremoved areas for patients with good versus bad outcome. Regions generating high rates of R and FR were more likely to have been removed in patients with good outcome. (B) Ratio of the number of contacts with events to the number of contacts without any event in patients with good versus poor surgical outcomes. Only the number of contacts carrying FR showed a significant difference. (C) The ratio of removed versus nonremoved SOZ contacts is compared for the two outcome groups. No significant difference was seen.
*Significantly different from Engel classes 1 and 2 (p < 0.04).
**Significantly different from Engel classes 1 and 2 (p < 0.008).
FR: Fast ripples; HFO: High-frequency oscillation; R: Ripples; SOZ: Seizure onset zone.
Reproduced with permission from [34].
Association between HFOs & disease activity in patients
High-frequency oscillations have until now been discussed in the context of localization of the epileptic focus and as a possible mechanism for seizure generation. In the context of a possible biomarker of epileptic activity, it is also interesting to evaluate if HFOs are a good indicator of disease activity, as measured by the frequency of seizures. Interictal spikes have long been discussed in this context, but there is not much evidence that they are a faithful representation of disease activity. It has, for instance, been demonstrated that spiking rate is not a good predictor of the probability of seizure occurrence in the short term (during epilepsy monitoring) [48–50]. The same studies have shown that spikes increase after seizures and that spikes do not increase when antiepileptic medication is reduced, as seizures do. In addition, the presence of spikes has long been recognized as an ambiguous predictor of successful surgical treatment and as an unreliable indicator of potential successful withdrawal of antiepileptic medication.
Similarly to spikes, HFOs are more abundant during slow-wave sleep than during paradoxical sleep and wakefulness [51,52]. This does not reflect the distribution of seizures across the stages and from this point of view both spikes and HFOs do not parallel seizures. One study has evaluated in patients the variations of HFO rates with changing medication and with seizure occurrence [53]. Results indicated that HFOs increased when antiepileptic medication was reduced, in parallel with seizure occurrence but differently from spikes; and that HFOs did not increase following seizures, here again differently from spikes. These changes seem to indicate that HFOs fluctuate like seizures in the context of variable antiepileptic drug levels, and may thus be a better marker of disease activity than spikes. Another aspect of the relationship of HFOs to epilepsy is their relationship to excitability, as measured by brain responsiveness to electrical stimulation. A study showed that HFO rates were negatively correlated with thresholds for responses to electrical stimulation [54]. Especially in neocortical regions, areas with low threshold and high HFO rates were colocalized even outside the SOZ.
Automated detection & mining of HFOs
Much of the research to date has used retrospective visual review and analysis of relatively limited EEG data sets. Of course, the fact that these positive results come from limited recordings suggest that the signal (pathological HFOs) is robust. The fact that the detection of HFOs was based on subjective visual review remains, however, a methodological weakness. Multiple groups are currently working on the development of automated detectors for HFOs and their translation to advance epilepsy surgery.
Automated analysis applied to EEG event detection include techniques from many areas of signal processing and machine learning. There is considerable literature that makes clear how difficult it is to reliably detect interictal spikes and seizure [55,56]. However, unlike interictal spikes that are generally of high amplitude compared with the background recording, HFOs are low-voltage events. The detection and labeling of iEEG recording HFOs is technically challenging [57] and agreement between reviewers can be poor [58].
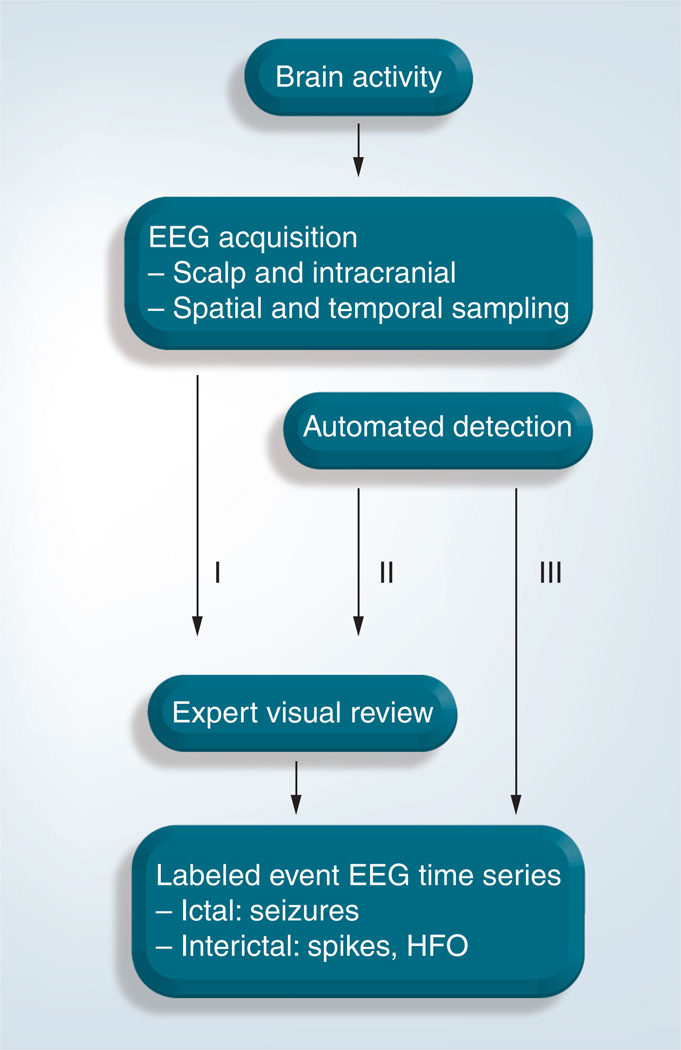
The detection and labeling of interictal HFOs can be broadly categorized into three different approaches (Figure 5):
-
▪
Expert manual EEG review is considered the gold standard, but is only feasible for small data sets. Although there is often poor interviewer concordance, it is possible to improve the situation by measuring this concordance between reviewers on short sections and discussing divergent cases [59];
-
▪
Automated detection combined with expert review is the primary approach for automated analysis and can significantly reduce the data volume to review. However, the approach generally does not provide detector specificity [52,58]. An extension of this approach is to set the parameters of the detector so that there is very high sensitivity at the expense of specificity. While this will generate significant numbers of false-positive detections the approach can still massively reduce the data volume for review. It may decrease the concordance problem, although different reviewers may accept different events among those detected. In addition, different detectors perform differently because they have usually been optimized on different types of data (e.g., micro- or macro-electrodes, types of patients) [60];
-
▪
Fully automated detection and labeling of epileptiform events requires high specificity and sensitivity detectors. The approach is highly efficient if detectors can be realized, but this remains dependent on the existence of a yet elusive unambiguous definition for the different HFO types.
Figure 5. Approaches for detection of high-frequency oscillations used to date.
HFO: High-frequency oscillation.
Most methods for automated HFO detection implicitly model the events as short-duration, high-frequency transients added to background iEEG [52,58]. The detection of HFO events can generally be separated into two stages, preprocessing and event detection. During preprocessing, data are typically band pass-filtered to restrict the range of frequencies under consideration. Additional preprocessing steps include spectral equalization and artifact removal. The detection of HFOs usually consists of calculating a signal feature (e.g., band spectral energy or line-length) and applying a threshold to select statistically significant events. Not surprisingly, thresholds based on global statistics perform poorly (often even on short data sets) because iEEG can be highly nonstationary and exhibit significant fluctuations. Further refinement in classification of HFO events can be made by sequentially applying additional classifiers, for example HFO duration or number of oscillations cycles [19,52,58,60].
Blanco et al. recently introduced an algorithm for automatically classifying HFOs, and demonstrated the tractability of analyzing 31,000 channel-hours of iEEG [61]. Using an unsupervised clustering approach that does not specify the number of clusters three distinct physiological classes of transient oscillations within the 100–500 Hz frequency range were identified. Two of the classes are consistent with ripple and FR oscillations, and a third consisted of mixed-frequency events. The performance of this approach compared with expert review was not determined.
The development of reliable HFO detectors should have a significant impact on the clinical application of wide bandwidth recordings. Given the huge number of iEEG data that are collected from patients undergoing evaluation for epilepsy surgery it is likely untenable to rely on visual review if the complete recordings are to be analyzed. However, studies to date have indicated that it may only be necessary to analyze a few minutes of EEG to obtain reliable information on HFO distribution. If this is confirmed, automatic analysis would be very helpful but may not be indispensable.
Noninvasive modalities & HFOs
Studies using combined scalp and iEEG have previously reported that an epileptiform sharp wave can be detected on surface EEG if at least approximately 7 cm2 of cortex is involved. Given that HFOs can be spatially limited, this may represent a significant challenge to the use of noninvasive electrophysiology. HFOs can be more widely distributed than initially thought [33,62], but nonetheless tend to occur on submillimeter and millimeter scales so likely represent a significant challenge for noninvasive electrophysiology. As discussed below, however, spontaneous γ- and ripple band activity have been recorded on the scalp in epileptic patients and this requirement of 7 cm2 of cortical involvement may have to be revised.
There has been intense interest in γ-band oscillations for over a decade, and multiple scalp EEG [63–65] and magnetoencephalography (MEG) [66,67] studies report on the importance of γ-band activity. Unlike the epileptic HFOs discussed above, this activity has always been measured in a context of repeated stimuli, thus allowing a considerable improvement in signal-to-noise ratio by averaging. This remains a technically challenging field [68,69] as scalp EEG and MEG oscillations above 30 Hz are low-amplitude signals and difficult to distinguish from artifacts. There are some examples in the literature in which noncerebral activity (artifact) was initially attributed to cerebral generators. For example, induced γ-band activity on scalp EEG during presentation of a visual stimulus was ultimately shown to be artifact associated with microsaccade eye movements [69]. Saccades are accompanied by a spike potential of muscle origin, which have a broad high-frequency spectrum that could be misinterpreted as having a cerebral origin. Thus, some of the induced γ-band power recorded from scalp EEG that was thought to be of cerebral origin is likely of muscular origin (eye movement related) [69].
In a recent study using simultaneous MEG and iEEG recordings from four patients during cognitive task time–frequency analysis of iEEG revealed attention-modulated high γ-band (50–150 Hz) power increases and α/β (9–25 Hz) suppressions in a reading task [70]. At the MEG sensor level analysis was performed using a beam former technique. α and β suppression were correlated with the iEEG. However, the MEG high γ-band enhancement was more difficult and unclear in two of the four patients studied.
A recent study using wide bandwidth EEG recordings from ten children with continuous spike and wave during slow-wave sleep reported HFOs on scalp EEG. The scalp EEG showed HFOs (ripples) concurrent with spikes. The peak HFO power ranged from 97.7 to 140.6 Hz. These data look convincing and show brief HFO events visible in the raw data associated with the EEG large amplitude spike [71]. Similarly, in patients with focal epilepsy, γ- and ripple-band activity has been reported on scalp EEG, most often around the time of spikes, and this activity was better correlated with the SOZ then interictal spikes [72]. The distinction between brain-generated activity and short electromyography discharges is not simple but is possible.
The electrophysiological correlates of the functional MRI (fMRI) blood oxygenation level dependent (BOLD) is an active area of research [73]. The BOLD signal reflects the neural responses elicited by a stimulus, and appears to be best correlated with the LFPs [74]. Simultaneous fMRI and electrophysiological recordings show that the BOLD signal is coupled with task-related power increases in the high-frequency range (broad-band γ, 50–250 Hz) [75–79].
The BOLD correlates of epileptiform spikes have been extensively investigated. The role of fMRI in epilepsy surgery was recently evaluated [80–86]. However, whether pathological HFOs can be localized with fMRI has not been reported to date.
Conclusion & future perspective
Over the past decade there has been significant progress in mining the electrophysiology of human epileptogenic brain. The evidence for interictal HFOs as a possible biomarker of human epileptogenic brain tissue comes from independent groups, in experimental animals and patients, in relation to seizures, interictal activity, antiepileptic medication, surgical outcome and electrical stimulation.
One of the most exciting aspects of future work will be to fully exploit these advances for clinical practice. In the future it may be possible to reliably map epileptogenic brain using only interictal recordings. This would open the door to intraoperative mapping of epileptogenic brain and could eliminate the need for chronic iEEG and its associated discomfort, morbidity and cost. It is also possible that noninvasive investigations will allow us to follow disease activity by following fluctuations in HFOs. The feasibility of noninvasively recording HFOs using scalp EEG has been reported now by two independent groups [71,72], and determining if increases in HFOs track with disease activity, that is, seizure frequency, can now be tested.
Executive summary.
Ictal high-frequency oscillations
High-frequency oscillations (HFOs) at the onset of human seizures were initially described in intracranial EEG (iEEG) recordings of patients undergoing evaluation for epilepsy surgery.
Partial seizures originating in hippocampus and neocortex began in the γ-, ripple, and fast ripple (FR) frequency range: 30–500 Hz, 40–120 Hz, 60–100 Hz, 70–90 Hz, 80–110 Hz and 100–500 Hz.
Removal of focal fast oscillations at seizure onset is associated with good surgical outcomes.
Interictal HFOs
Ripples (80–200 Hz) were first described in hippocampus of freely behaving rats, and hypothesized to be associated with memory.
Fast ripples (FR: 250–500 Hz) were first described in a rat model of epilepsy and not observed in control animals.
FRs can be spatially more widespread than predicted from microelectrode studies [33], and are reliably recorded using clinical macroelectrodes.
Ripples and FRs are increased in the epileptic brain.
Interictal to ictal transition
There are no conclusive studies showing that HFOs are precursors to seizures in human partial epilepsy.
High-frequency oscillation activity may be increased in the seconds immediately preceding a seizure, but the exact timing of seizure onset is not always easy to define within a period of a few seconds.
Association of HFOs with seizure outcome
Jacobs et al. recently demonstrated that resection of tissue generating ripple and FR HFOs was associated with a more favorable epilepsy surgery outcome. In another study by Wu et al. all patients who had tissue with HFOs resected (19/19) were seizure free. In contrast, none of the patients (5/5) that did not have all FR HFOs tissue resected were seizure free.
Association between HFOs & disease activity in patients
High-frequency oscillations are a possible biomarker of epileptic activity and an indicator of disease activity,
Similar to spikes, HFOs are more abundant during slow-wave sleep than during paradoxical sleep and wakefulness.
High-frequency oscillations increase when antiepileptic medication are reduced, in parallel with seizure occurrence.
Automated detection & mining of HFOs
The detection and labeling of HFOs can be categorized into three approaches: expert manual EEG review is considered the gold standard; automated detection combined with expert review can significantly reduce the data volume to review; and fully automated detection and labeling of epileptiform events requires high specificity and sensitivity.
The development of reliable HFO detectors should have a significant impact on the clinical application of wide bandwidth recordings.
Noninvasive modalities & HFOs
EEG recordings from ten children with continuous spike and wave during slow-wave sleep reported HFOs on scalp EEG. The scalp EEG showed HFOs (ripples) concurrent with spikes.
In patients with focal epilepsy, γ- and ripple-band activity has been reported on scalp EEG, most often around the time of spikes, and this activity was better correlated with the SOZ then interictal spikes.
Conclusion
Evidence for interictal HFOs as a possible biomarker of human epileptogenic brain tissue comes from independent groups and in experimental animals and patients.
In the future it may be possible to reliably map epileptogenic brain using only interictal recordings.
Noninvasive investigations may allow tracking disease activity by following fluctuations in HFOs.
Acknowledgments
G Worrell is funded by NIH grant RO1NS063039 and J Gotman by grant MOP-102710 from the Canadian Institutes of Health Research.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
- 1.Neidermeyer E, Lopes da Silva F. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 2.Vanhatalo S, Voipio J, Kaila K. Full-band EEG (FbEEG): an emerging standard in electroencephalography. Clin. Neurophysiol. 2005;116:1–8. doi: 10.1016/j.clinph.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Gloor P. Contributions of electroencephalography and electrocorticography to the neurosurgical treatment of the epilepsies. Adv. Neurol. 1975;8:59–105. [PubMed] [Google Scholar]
- 4.Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- 5.Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 6. Buzsaki G, Horvath Z, Urioste R, Hetke J, Wise K. High-frequency network oscillaiton in hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. ▪ Initial description of ripple frequency oscillations in rats.
- 7.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 8.Lisman JE, Idiart MA. Storage of 7 ± 2 short-term memories in oscillatory subcycles. Science. 1995;267 doi: 10.1126/science.7878473. 1512-1155. [DOI] [PubMed] [Google Scholar]
- 9.Gloor P. Contributions of electroencephalography and electrocorticography to the neurosurgical treatment of the epilepsies. Adv. Neurol. 1975;8:59–105. [PubMed] [Google Scholar]
- 10.Ayala GF, Dichter M, Gumnit RJ, Matsumoto H, Spencer WA. Genesis of epileptic interictal spikes. New knowledge of cortical feedback systems suggests a neurophysiological explanation of brief paroxysms. Brain Res. 1973;52:1–17. doi: 10.1016/0006-8993(73)90647-1. [DOI] [PubMed] [Google Scholar]
- 11. Bragin A, Engel J, Jr, Wilson CL, Fried I, Buzsaki G. High-frequency oscillations in human brain. Hippocampus. 1999;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. ▪ Initial description of interictal high-frequency oscillations in humans.
- 12.Bragin A, Wilson CL, Staba RJ, Reddick M, Fried I, Engel J., Jr Interictal high-frequency oscillations (80–500 Hz) in the human epileptic brain: entorhinal cortex. Ann. Neurol. 2002;52:407–415. doi: 10.1002/ana.10291. [DOI] [PubMed] [Google Scholar]
- 13. Bragin A, Mody I, Wilson CL, Engel J., Jr Local generation of fast ripples in epileptic brain. J. Neurosci. 2002;22:2012–2221. doi: 10.1523/JNEUROSCI.22-05-02012.2002. ▪ Seminal article describing ripple and fast ripple oscillations in epileptic rat model.
- 14.Engel J, Bragin A, Staba R, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- 15.Gotman J. High frequency oscillations: the new EEG frontier? Epilepsia. 2010;51 Suppl. 1:63–65. doi: 10.1111/j.1528-1167.2009.02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs J, Levan P, Chander R, Hall J, Dubeau F, Gotman J. Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008;49:1893–1907. doi: 10.1111/j.1528-1167.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Urrestarazu E, Jirsch JD, LeVan P, et al. High-frequency intracerebral EEG activity (100–500 Hz) following interictal spikes. Epilepsia. 2006;47:1465–1476. doi: 10.1111/j.1528-1167.2006.00618.x. ▪ Initial description of interictal high-frequency oscillations recorded using clinical macroelectrodes.
- 18.Urrestarazu E, Chander R, Dubeau F, Gotman J. Interictal high-frequency oscillations (100–500 Hz) in the intracerebral EEG of epileptic patients. Brain. 2007;130:2354–2366. doi: 10.1093/brain/awm149. [DOI] [PubMed] [Google Scholar]
- 19. Worrell GA, Gardner AB, Stead SM, et al. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131:928–937. doi: 10.1093/brain/awn006. ▪ Initial description of increased ripple frequency oscillations in human epileptic hippocampus.
- 20.Allen PJ, Fish DR, Smith SJ. Very high-frequency rhythmic activity during SEEG suppression in frontal lobe epilepsy. Electroencephalogr. Clin. Neurophysiol. 1992;82 doi: 10.1016/0013-4694(92)90160-j. 155-119. [DOI] [PubMed] [Google Scholar]
- 21.Fisher RS, Webber WR, Lesser RP, Arroyo S, Uematsu S. High-frequency EEG activity at the start of seizures. J. Clin. Neurophysiol. 1992;9:441–448. doi: 10.1097/00004691-199207010-00012. [DOI] [PubMed] [Google Scholar]
- 22.Grenier F, Timofeev I, Steriade M. Neocortical very fast oscillations (ripples, 80–200 Hz) during seizures: intracellular correlates. J. Neurophysiol. 2003;89:841–852. doi: 10.1152/jn.00420.2002. [DOI] [PubMed] [Google Scholar]
- 23.Traub RD, Whittington MA, Buhl EH, et al. A possible role for gap junctions in generation of very fast EEG oscillations preceding the onset of, and perhaps initiating, seizures. Epilepsia. 2001;42:153–170. doi: 10.1046/j.1528-1157.2001.26900.x. [DOI] [PubMed] [Google Scholar]
- 24.Worrell GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, Litt B. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain. 2004;127:1496–1506. doi: 10.1093/brain/awh149. [DOI] [PubMed] [Google Scholar]
- 25.Jirsch JD, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain. 2006;129:1593–1608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- 26.Bragin A, Wilson CL, Engel J., Jr Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: a hypothesis. Epilepsia. 2000;41 Suppl. 6:S144–S152. doi: 10.1111/j.1528-1157.2000.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 27.Bragin A, Wilson CL, Engel J. Rate of interictal events and spontaneous seizures in epileptic rats after electrical stimulation of hippocampus and its afferents. Epilepsia. 2002;43:81–85. doi: 10.1046/j.1528-1157.43.s.5.22.x. [DOI] [PubMed] [Google Scholar]
- 28.Le Van Quyen M, Khalilov I, Ben-Ari Y. The dark side of high-frequency oscillations in the developing brain. Trends Neurosci. 2006;29:419–427. doi: 10.1016/j.tins.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Alarcon G, Binnie CD, Elwes RD, Polkey CE. Power spectrum and intracranial EEG patterns at seizure onset in partial epilepsy. Electroencephalogr. Clin. Neurophysiol. 1995;94:326–337. doi: 10.1016/0013-4694(94)00286-t. [DOI] [PubMed] [Google Scholar]
- 30.Lee SA, Spencer DD, Spencer SS. Intracranial EEG seizure-onset patterns in neocortical epilepsy. Epilepsia. 2000;41:297–307. doi: 10.1111/j.1528-1157.2000.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 31.Wetjen NM, Marsh WR, Meyer FB, et al. Intracranial electroencephalography seizure onset patterns and surgical outcomes in nonlesional extratemporal epilepsy. J. Neurosurg. 2009;110:1147–1152. doi: 10.3171/2008.8.JNS17643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bragin A, Engel J, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia. 1999;40:127–137. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- 33.Crépon B, Navarro V, Hasboun D, et al. Mapping interictal oscillations greater than 200 Hz recorded with intracranial macroelectrodes in human epilepsy. Brain. 2010;133:33–45. doi: 10.1093/brain/awp277. [DOI] [PubMed] [Google Scholar]
- 34. Jacobs J, Zijlmans M, Zelmann R, et al. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann. Neurol. 2010;67:209–220. doi: 10.1002/ana.21847. ▪ Description of association of high-frequency oscillations with seizure outcome in epilepsy surgery.
- 35.Dzhala VI, Staley KJ. Transition from interictal to ictal activity in limbic networks in vitro. J. Neurosci. 2003;23:7873–7880. doi: 10.1523/JNEUROSCI.23-21-07873.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Timofeev I, Steriade M. Neocortical seizures: initiation, development and cessation. Neuroscience. 2004;123:299–336. doi: 10.1016/j.neuroscience.2003.08.051. [DOI] [PubMed] [Google Scholar]
- 37.Châtillon CE, Zelmann R, Bortel A, Avoli M, Gotman J. Contact size does not affect high frequency oscillation detection in intracerebral EEG recordings in a rat epilepsy model. Clin. Neurophysiol. 2011;122:1701–1705. doi: 10.1016/j.clinph.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ren L, Terada K, Baba K, et al. Ictal very low frequency oscillation in human epilepsy patients. Ann. Neurol. 2011;69:201–206. doi: 10.1002/ana.22158. [DOI] [PubMed] [Google Scholar]
- 39.Schevon CA, Trevelyan AJ, Schroeder CE, Goodman RR, McKhann G, Emerson RG. Spatial characterization of interictal high frequency oscillations in epileptic neocortex. Brain. 2009;132(Pt 11):3047–3059. doi: 10.1093/brain/awp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stead M, Bower M, Brinkmann BH, et al. Microseizures and the spatiotemporal scales of human partial epilepsy. Brain. 2010;133:2789–2797. doi: 10.1093/brain/awq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grenier F, Timofeev I, Steriade M. Focal synchronization of ripples (80–200 Hz) in neocortex and their neuronal correlates. J. Neurophysiol. 2001;86 doi: 10.1152/jn.2001.86.4.1884. 1884-1198. [DOI] [PubMed] [Google Scholar]
- 42.Khosravani H, Mehrotra N, Rigby M, et al. Spatial localization and time-dependant changes of electrographic high frequency oscillations in human temporal lobe epilepsy. Epilepsia. 2009;50:605–616. doi: 10.1111/j.1528-1167.2008.01761.x. [DOI] [PubMed] [Google Scholar]
- 43.Jacobs J, Zelmann R, Jirsch J, Chander R, Dubeau CE, Gotman J. High frequency oscillations (80–500 Hz) in the preictal period in patients with focal seizures. Epilepsia. 2009;50:1780–1792. doi: 10.1111/j.1528-1167.2009.02067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engel J, Pedley TA, Aicardi J, Dichter MA, Moshé S. Epilepsy: a Comprehensive Textbook. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 45.Luders JJ, Comair Y. Epilepsy Surgery. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 46.Jacobs J, Levan P, Châtillon CE, Olivier A, Dubeau F, Gotman J. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain. 2009;132:1022–1037. doi: 10.1093/brain/awn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu JY, Sankar R, Lerner JT, Matsumoto JH, Vinters HV, Mathern GW. Removing interictal fast ripples on electrocorticography linked with seizure freedom in children. Neurology. 2010;75:1686–1694. doi: 10.1212/WNL.0b013e3181fc27d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gotman J, Marciani MG. Electroencephalographic spiking activity, drug levels, and seizure occurrence in epileptic patients. Ann. Neurol. 1985;17:597–603. doi: 10.1002/ana.410170612. [DOI] [PubMed] [Google Scholar]
- 49.Gotman J, Koffler DJ. Interictal spiking increases after seizures but does not after decrease in medication. Electroencephalogr. Clin. Neurophysiol. 1989;72:7–15. doi: 10.1016/0013-4694(89)90026-6. [DOI] [PubMed] [Google Scholar]
- 50.Spencer SS, Goncharova II, Duckrow RB, Novotny EJ, Zaveri HP. Interictal spikes on intracranial recording: behavior, physiology, and implications. Epilepsia. 2008;49:1881–1892. doi: 10.1111/j.1528-1167.2008.01641.x. [DOI] [PubMed] [Google Scholar]
- 51.Bagshaw AP, Jacobs J, LeVan P, Dubeau F, Gotman J. Effect of sleep stage on interictal high-frequency oscillations recorded from depth macroelectrodes in patients with focal epilepsy. Epilepsia. 2009;50:617–628. doi: 10.1111/j.1528-1167.2008.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Staba RJ, Wilson CL, Bragin A, Fried I, Engel J., Jr Sleep states differentiate single neuron activity recorded from human epileptic hippocampus, entorhinal cortex, and subiculum. J. Neurosci. 2002;22:5694–5704. doi: 10.1523/JNEUROSCI.22-13-05694.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zijlmans M, Jacobs J, Zelmann R, Dubeau F, Gotman J. High-frequency oscillations mirror disease activity in patients with epilepsy. Neurology. 2009;72:979–986. doi: 10.1212/01.wnl.0000344402.20334.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacobs J, Zijlmans M, Zelmann R, et al. Value of electrical stimulation and high frequency oscillations (80–500 Hz) in identifying epileptogenic areas during intracranial EEG recordings. Epilepsia. 2010;51:573–582. doi: 10.1111/j.1528-1167.2009.02389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saab ME, Gotman J. A system to detect the onset of epileptic seizures in scalp EEG. Clin. Neurophysiol. 2005;116:427–442. doi: 10.1016/j.clinph.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 56.Wilson SB, Emerson R. Spike detection: a review and comparison of algorithms. Clin. Neurophysiol. 2002;113:1873–1881. doi: 10.1016/s1388-2457(02)00297-3. [DOI] [PubMed] [Google Scholar]
- 57.Bénar CG, Chauvière L, Bartolomei F, Wendling F. Pitfalls of high-pass filtering for detecting epileptic oscillations: a technical note on ‘false’ ripples. Clin. Neurophysiol. 2010;121:301–310. doi: 10.1016/j.clinph.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 58.Gardner AB, Worrell GA, Marsh E, Dlugos D, Litt B. Human and automated detection of high-frequency oscillations in clinical intracranial EEG recordings. Clin. Neurophysiol. 2007;118:1134–1143. doi: 10.1016/j.clinph.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zelmann R, Zijlmans M, Jacobs J, Châtillon CE, Gotman J. Improving the identification of high frequency oscillations. Clin. Neurophysiol. 2009;120:1457–1464. doi: 10.1016/j.clinph.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zelmann R, Mari F, Jacobs J, Zijlmans M, Dubeau F, Gotman J. A comparison between detectors of high frequency oscillations. Clin. Neurophysiol. 2011 doi: 10.1016/j.clinph.2011.06.006. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blanco JA, Stead M, Krieger A, et al. Unsupervised classification of high-frequency oscillations in human neocortical epilepsy and control patients. J. Neurophysiol. 2010;104:2900–2912. doi: 10.1152/jn.01082.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tao JX, Ray A, Hawes-Ebersole S, Ebersole JS. Intracranial EEG substrates of scalp EEG interictal spikes. Epilepsia. 2005;46:669–676. doi: 10.1111/j.1528-1167.2005.11404.x. [DOI] [PubMed] [Google Scholar]
- 63.Rodriguez E, George N, Lachaux JP, Martinerie J, Renault B, Varela FJ. Perception’s shadow: long-distance synchronization of human brain activity. Nature. 1999;397:430–433. doi: 10.1038/17120. [DOI] [PubMed] [Google Scholar]
- 64.Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J. Neurosci. 1996;16:4240–4249. doi: 10.1523/JNEUROSCI.16-13-04240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tallon-Baudry C, Bertrand O. Oscillatory γ-activity in humans and its role in object representation. Trends Cogn. Sci. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- 66.Hoogenboom N, Schoffelen JM, Oostenveld R, Parkes LM, Fries P. Localizing human visual γ-band activity in frequency, time and space. Neuroimage. 2006;29:764–773. doi: 10.1016/j.neuroimage.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 67.Ribary U, Ioannides AA, Singh KD, et al. Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proc. Natl Acad. Sci. USA. 1991;88:11037–11041. doi: 10.1073/pnas.88.24.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trujillo LT, Peterson MA, Kaszniak AW, Allen JJ. EEG phase synchrony differences across visual perception conditions may depend on recording and analysis methods. Clin. Neurophysiol. 2005;116:172–189. doi: 10.1016/j.clinph.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 69.Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY. Transient induced γ-band response in EEG as a manifestation of miniature saccades. Neuron. 2008;58:429–441. doi: 10.1016/j.neuron.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 70.Dalal SS, Baillet S, Adam C, et al. Simultaneous MEG and intracranial EEG recordings during attentive reading. Neuroimage. 2009;45:1289–1304. doi: 10.1016/j.neuroimage.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 71.Kobayashi K, Watanabe Y, Inoue T, Oka M, Yoshinaga H, Ohtsuka Y. Scalp-recorded high-frequency oscillations in childhood sleep-induced electrical status epilepticus. Epilepsia. 2010;51:2190–2194. doi: 10.1111/j.1528-1167.2010.02565.x. [DOI] [PubMed] [Google Scholar]
- 72.Andrade-Valenca LP, Dubeau F, Mari F, Zelmann R, Gotman J. Interictal scalp fast oscillations as a marker of the seizure onset zone. Neurology. 2011;77:524–531. doi: 10.1212/WNL.0b013e318228bee2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 74.Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J. Neurosci. 2003;23:3963–3971. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lachaux JP, Fonlupt P, Kahane P, et al. Relationship between task-related γ-oscillations and BOLD signal: new insights from combined fMRI and intracranial EEG. Hum. Brain Mapp. 2007;28:1368–1375. doi: 10.1002/hbm.20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, Malach R. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309:951–954. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- 77.Nir Y, Fisch L, Mukamel R, et al. Coupling between neuronal firing rate, γ-LFP, and BOLD fMRI is related to interneuronal correlations. Curr. Biol. 2007;17:1275–1285. doi: 10.1016/j.cub.2007.06.066. [DOI] [PubMed] [Google Scholar]
- 78.Ojemann GA, Corina DP, Corrigan N, et al. Neuronal correlates of functional magnetic resonance imaging in human temporal cortex. Brain. 2010;133:46–59. doi: 10.1093/brain/awp227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shmuel A, Leopold DA. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: implications for functional connectivity at rest. Hum. Brain Mapp. 2008;29:751–761. doi: 10.1002/hbm.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bénar CG, Grova C, Kobayashi E, et al. EEG-fMRI of epileptic spikes: concordance with EEG source localization and intracranial EEG. Neuroimage. 2006;30:1161–1170. doi: 10.1016/j.neuroimage.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 81.Krakow K, Woermann FG, Symms MR, et al. EEG-triggered functional MRI of interictal epileptiform activity in patients with partial seizures. Brain. 1999;122(Pt 9):1679–1688. doi: 10.1093/brain/122.9.1679. [DOI] [PubMed] [Google Scholar]
- 82.Krakow K, Messina D, Lemieux L, Duncan JS, Fish DR. Functional MRI activation of individual interictal epileptiform spikes. Neuroimage. 2001;13:502–505. doi: 10.1006/nimg.2000.0708. [DOI] [PubMed] [Google Scholar]
- 83.Lemieux L, Krakow K, Fish DR. Comparison of spike-triggered functional MRI BOLD activation and EEG dipole model localization. Neuroimage. 2001;14:1097–1104. doi: 10.1006/nimg.2001.0896. [DOI] [PubMed] [Google Scholar]
- 84.Gotman J. Epileptic networks studied with EEG-fMRI. Epilepsia. 2008;49 Suppl. 3:42–51. doi: 10.1111/j.1528-1167.2008.01509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moeller F, Tyvaert L, Nguyen DK, et al. EEG-fMRI: adding to standard evaluations of patients with nonlesional frontal lobe epilepsy. Neurology. 2009;73:2023–2030. doi: 10.1212/WNL.0b013e3181c55d17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zijlmans M, Huiskamp G, Hersevoort M, Seppenwoolde JH, van Huffelen AC, Leijten FS. EEG-fMRI in the preoperative work-up for epilepsy surgery. Brain. 2007;130:2343–2353. doi: 10.1093/brain/awm141. [DOI] [PubMed] [Google Scholar]