Abstract
Some ions exhibit “ion fragility” in quadrupole ion trap mass spectrometry (QIT-MS) during mass analysis with resonance ejection. In many cases, different ions generated from the same compound exhibit different degrees of ion fragility, with some ions (e.g., the [M+H]+ ion) stable and other ions (e.g., the [M+Na]+ ion) fragile. The ion fragility for quadrupole ion trap (QIT) mass spectrometry (MS) for protonated and sodiated ions of three phospholipids, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine, PC (16:0/16:0), 1,2-dipalmitoyl-sn-glycero-3-phophoethanolamine, PE (16:0/16:0), and N-palmitoyl-D-erythro-sphingosylphosphorylcholine, SM (d18:1/16:0), was determined using three previously developed experiments: 1) the peak width using a slow scan speed, 2) the width of the isolation window for efficient isolation, and 3) the energy required for collision-induced dissociation. In addition, ion fragility studies were designed and performed to explore a correlation between ion fragility in QIT mass analysis and ion fragility during transport between the ion source and the ion trap. These experiments were: 1) evaluating the amount of thermal-induced dissociation as a function of heated capillary temperature, and 2) determining the extent of fragmentation occurring with increasing tube lens voltage. All phospholipid species studied exhibited greater ion fragility as protonated species in ion trap mass analysis than as sodiated species. In addition, the protonated species of both SM (d18:0/16:0) and PC (16:0/16:0) exhibited greater tendencies to fragment at higher heated capillary temperatures and high tube lens voltages, whereas the PE (16:0/16:0) ions did not appear to exhibit fragility during ion transport.
Keywords: ion fragility, quadrupole ion trap, phospholipid, thermal-induced dissociation, collision-induced dissociation
1. Introduction
Glycerophospholipids (GPLs) serve one primary function in biology, as structural components of cell membranes.[1] GPLs typically consist of a glycerol backbone with a polar head group and two non-polar fatty acid tails. GPLs with this basic structure are phosphatidylcholines (PCs), phosphatidlyethanolamines (PEs), phosphatidylinositols (PIs), and phosphatidylserines (PSs), with the classes determined by the composition of the head group.
Because of their biological abundance and importance, GPLs have been studied by mass spectrometry for many years, from electron ionization (EI) coupled to gas chromatography/mass spectrometry (GC/MS)[2] (not as intact ions, but as derivatized species where the head group and tails are removed from the glycerol backbone) to more recently matrix-assisted laser desorption/ionization (MALDI)[3–6] and electrospray ionization (ESI)[7–10]. Under appropriate experimental conditions, the latter ionization techniques produce a predominant ion ([M+H]+ or [M+cation]+) corresponding to the molecular weight of the intact molecule M, whereas EI causes a high degree of fragmentation, and thus requires derivitization and only analyzes the fatty acid tails. In ESI, relying solely on the molecular weight for compound identification is typically inadequate because of the wide variety of GPLs present (varying fatty acyl chains and head groups) and the possibility of isomers; thus compound identification is typically performed by tandem MS with triple quadrupole or ion trap mass spectrometers.[7, 9, 11, 12]
Many previous studies have focused on differences in fragmentation between protonated and cationized GPLs.[8, 11–14] For positive ions, experiments have shown that protonated and sodiated PCs produce very different fragmentation patterns, with sodiated PCs providing a more informative fragmentation (identification of fatty acyl substituents) pattern.[9] Upon collision-induced dissociation (CID), protonated PCs produce primarily one fragment ion, m/z 184, corresponding to the polar head group, indicating that the charge is retained on the head group. In contrast, CID of sodiated PCs produces fragment ions that correspond to losses of the head group with retention of the charge on the glycerol backbone. The most abundant fragment ion in MS/MS shows a neutral loss of 59, which corresponds to the loss of trimethylamine, -N(CH3)3.[9]
The difference in fragmentation of protonated and cationized GPLs has been of considerable interest, particularly in the study of different metal ions for cationization. Due to the limitation of only a single stage of tandem MS (MS/MS or MS2) provided by a triple quadrupole instrument, more informative fragment ions after MS/MS is desired. The use of lithiated adducts was shown to provide many structurally informative fragment ions for PCs[12] and PEs[13] after a single stage of tandem MS, and many other cations have been evaluated for other GPLs.[11] A structurally significant fragment ion in the analysis of lipids allows for the correct identification of the fatty acid tails and their location on the glycerol backbone. A novel adduction with trifluoroacetic acid (TFA)/K+ for PCs was determined to provide an abundance of structurally informative fragment ions as well.[15] The resonating theme of most positive ion studies was that the cationized GPLs, or adducts with other complexes, should be preferred over protonated GPLs for structural identification of fatty acyl substituents.
This difference in fragmentation behavior under CID is also of concern in ion trap mass spectrometry for both ion production and ion fragility. In previous studies from our laboratory,[16] the fragility of an ion within the ion trap was quantified and results showed that different ions formed from the same molecule can exhibit varying degrees of fragility. For example, cationized oleandomycin is a stable ion, whereas its protonated counterpart is fragile; in contrast, acylcarnitines are more stable protonated ions. A consequence of fragility is an inability to efficiently isolate a fragile ion without widening the isolation notch, as well as mass shifts and reduced mass resolution, particularly at slow scan speeds.[16] Thus far, a relationship between differences seen in fragmentation behavior between different ions of the same compound in tandem MS experiments and differences in ion fragility of those ions has not been evaluated; we explore this relationship using GPLs as a test case in this paper. In addition, we expand the concept of ion fragility in the ion trap to fragility in ion transport.
It has already been shown that cationized and protonated species of the same molecule can exhibit varying degrees of ion fragility in ion trap analysis, but the effect of ion fragility on transport from atmosphere to vacuum and the accompanying desolvation process has not be evaluated. The temperature of the heated capillary, and thus the effective temperature of an ion, has been shown to affect the onset of source fragmentation.[17] In MALDI mass spectrometry, source fragmentation of GPLs has been demonstrated to arise from gas-phase reactions rather than from laser-induced photodissociation.[3] This is an important factor, since gas-phase reactions occur in electrospray as well.
Fragmentation during ion transport has been studied previously. Early studies in electrospray showed the possibility of performing thermally-induced dissociation (TID) of highly-charged protein ions.[18] A heated capillary was used for those studies, and the results indicated that the higher charged ions (+6, +5, and +4) were more susceptible to TID due to increased coulombic repulsions. Source collision-induced dissociation (SCID) has been used for many years for controllable dissociation of complexes and to provide fragment ions that can be further fragmented in a tandem mass spectrometer.
In this paper, the concept of ion fragility in ion trap mass analysis is examined for GPLs (and sphingolipids) and the effects on fragmentation during ion transport are explored by comparing two different GPLs and one sphingolipid as protonated and sodiated species, namely PC (16:0/16:0), PE (16:0/16:0), and sphingomyelin, SM, (d18:0/16:0) (a sphingolipid, but still containing a phosphate group).
2. Experimental
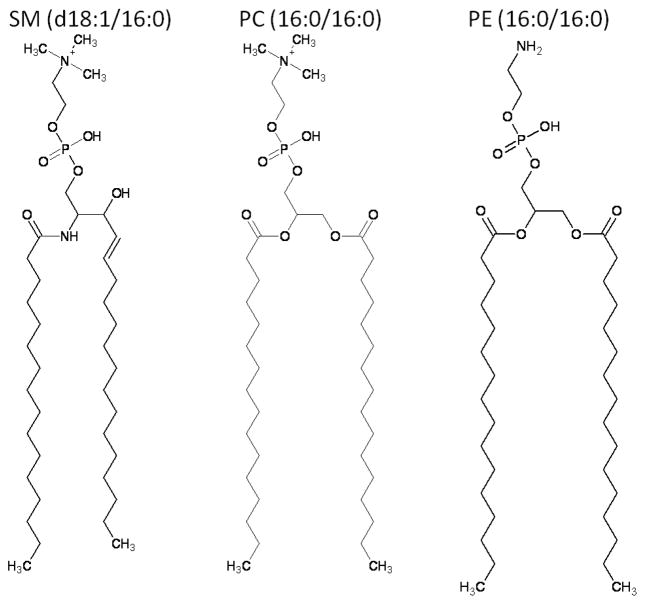
All experiments were performed in the positive ion mode on the Finnigan LCQ instrument (San Jose, CA), an electrospray ionization quadrupole ion trap (ESI-QIT) mass spectrometer. Analyte solutions were directly infused at a flow rate of 1 μL/min using a syringe pump, 4.5 kV applied to the electrospray needle, and nitrogen sheath gas set to 30 arbitrary units. For evaluating differences in ion fragility of protonated and cationized molecules, three related phospholipids were chosen: PC (16:0/16:0), SM (d18:1/16:0), and PE (16:0/16:0). The PC (16:0/16:0) and SM (d18:1/16:0) were chosen because they share the same head group and exhibit the same differences in fragmentation of the protonated and sodiated species upon collision-induced dissociation (CID). The main difference is in their fatty acid tail arrangement: SM (d18:1/16)) has one amide-linked fatty acyl chain, palmitoyl, as it is derived from the sphingosine base, whereas PC (16:0/16:0) has two ester-linked fatty acyl chains as it is derived from glycerol. PE (16:0/16:0) was chosen because it has the same fatty acyl chain arrangement as PC (16:0/16:0), but a slightly different head group; it also exhibits a different fragmentation pathway between protonated and cationized species. The difference in the head group is the replacement of trimethylamine (-N(CH3)3) with amine (-NH3), (Figure 1). For this discussion, these three species will be referred to as phospholipids because of the presence of phosphate in the head group.
Figure 1.
Structures of the phospholipids used in studying ion fragility and source fragmentation. The protonated species are shown. Abbreviations are as follows: SM (d18:1/16:0), N-palmitoyl-D-erythro-sphingosylphosphorylcholine, PC (16:0/16:0), 1,2-di-palmitoyl-sn-glycero-phosphocholine, and PE (16:0/16:0), 1,2-dipalmitoyl-sn-glycerol-phosphoethanolamine. SM and PC share the same head group, but differ in the fatty acid tails, whereas PC and PE share the same fatty acid tails, but have slightly different head groups.
Standards of PE and PC with the desired fixed fatty acid substituents of palmitoyl (16:0) were obtained from Avanti Polar Lipids (Birmingham, AL); SM was purchased from Avanti as a chicken egg extract, but with palmitoyl (16:0) as the predominant acyl chain (80%). All phospholipids were obtained as powders and prepared to the desired concentrations. PC (16:0/16:0) and SM (d18:1/16:0) were made as stock solutions of 1000 ppm (~1.4 μM) in 50:50 isopropanol:methanol and PE was made as a stock solution of 500 ppm (~0.7 μM) in 75:25 choloroform:methanol. For ESI QIT-MS analysis, the stock solutions were diluted to 10 ppm (~14 nM) in methanol.
Due to the presence of unwanted source fragmentation and to uncover the origin of those fragment ions, solutions were prepared to ensure that either the [M+H]+ ion or the [M+Na]+ ion was the most abundant ion formed during ESI. Even with careful cleaning of all fittings and connectors, it was difficult to remove the presence of the [M+Na]+ ions in all experiments, so we settled on ensuring that the protonated species was the most abundant for these studies. For production of protonated species, formic acid was added to a final concentration of 0.1%; for the production of sodiated species, sodium acetate was added to a concentration of 100 μM. All solvents were HPLC grade and were obtained from Fisher Scientific (Pittsburgh, PA).
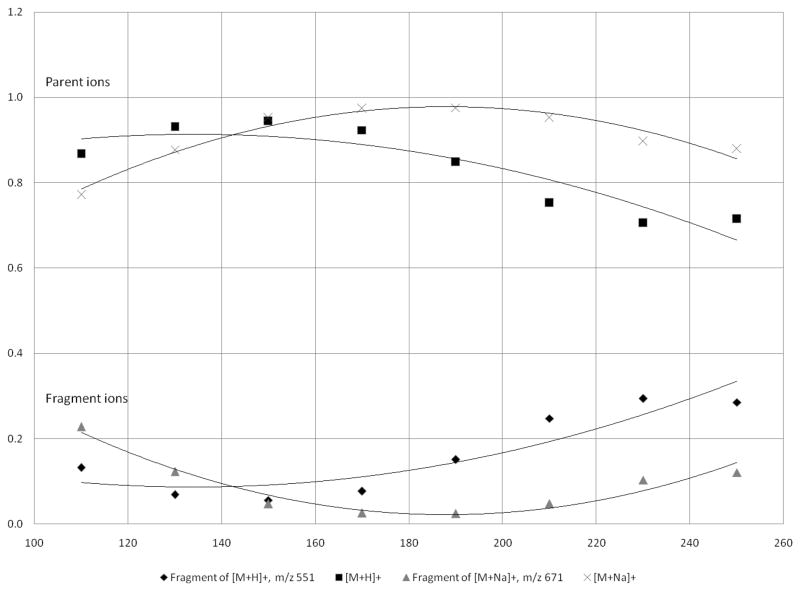
Ion fragility in the ion trap was determined using three experiments described previously.[16] These experiments were 1) measuring the peak width of the parent ion at 10% peak height (PW10%) using a slow scan speed (called zoom scan), 2) finding the isolation width required for isolating the desired ion, and 3) determining the amount of energy required for CID. Typically, a fragile ion will exhibit a wider peak width at a slower scan rate, will need a wider isolation window, and will require less energy to fragment under CID. These experiments were set up using Xcalibur software. Data for the zoom scan were averaged over 2 minutes. The isolation width was determined by changing the width from 1 to 4 amu wide in 1 amu increments, and collecting data for 1 minute at each interval. In studying the amount of energy required for CID, all experiments were conducted at a heated capillary temperature of 250°C and a tube lens offset of 30 V, while changing the percent CID energy and collecting data for 1 minute at each level. Values for percent CID energy were 15%, 16%, 17%, 18%, 19%, 20%, 21%, 25%, and 30%.
Ion fragility during ion transport, or the susceptibility of ions to source fragmentation, was also studied using two injection parameters on the LCQ, the temperature of the heated capillary and the voltage applied to the tube lens (tube lens offset). These two parameters assist in desolvating and declustering ions during ion transport from atmosphere to vacuum. Typical operation involves a high capillary temperature, 250°C, and a low voltage for the tube lens, <+30V. The sensitivity of analysis is typically increased with a more positive tube lens offset for positive ions and a more negative value for negative ions; even higher voltages may result in ion fragmentation. To determine the effect of temperature on fragmentation of lipids, an Xcalibur instrument control file was created that recalled different tune files, allowing the capillary temperature to be changed at 20-degree intervals from 250°C to 50°C. Prior to setting up the temperature profile scan, it was determined that a temperature change took 8 minutes before equilibration; therefore, the instrument file collected data for 10 minutes at each temperature level, but only the last two minutes of each temperature change were used for comparisons between protonated and sodiated species. The tube lens was maintained at 0 V for these experiments. The final parameter used to understand the susceptibility of the phospholipid ions to source fragmentation was the tube lens voltage. For this experiment, the heated capillary was held at 250°C and the tube lens voltage was changed; spectra were collected for two minutes at 4 voltage levels (0, 10, 20, and 30 V) and saved as separate files.
3. Results and Discussion
The three phospholipids studied can form many different ions under ESI conditions. For example, the PC (16:0/16:0), a synthetic GPL, if ionized without control of the counter ions in solution, is detected as [M+H]+, [M+Na]+, and [M+K]+ ions. These reflect the primary cations present in solution, as are often seen in direct tissue analysis for imaging mass spectrometry.[19] Determining which ion is the most stable for ion trap mass analysis is of importance to ensure good mass accuracy and lower detection limits (if the more fragile ion readily fragments during ion transport, there will ultimately be less of it left for mass analysis).
3.1. Fragility in the ion trap
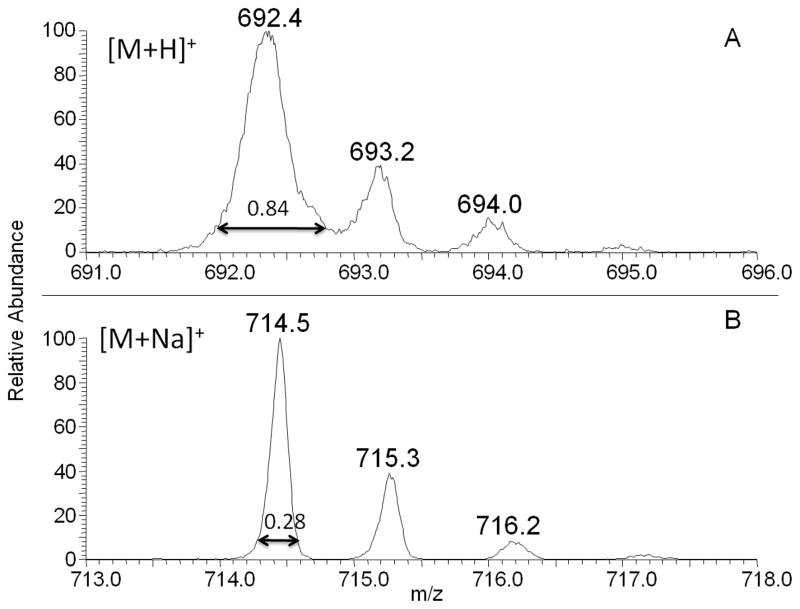
The LCQ zoom scan feature allows for a higher resolution spectrum over a narrower mass range, 10 amu, by using a 20x slower scan rate.[16] A fragile ion will have a broader PW10% than a more stable ion.[16] The value of 10% for PW was chosen because both peak fronting and broadening are most evident at this level. Examples of the zoom scan experimental data are shown in Figure 2 for the analysis of PE 16:0/16:0 as [M+H]+ (A) and [M+Na]+ (B). Protonated PE 16:0/16:0 has a wider PW10% than the sodiated PE ion for the monoisotopic peak. Zoom scan spectra were collected and PW10% values were determined for each phospholipid studied (Table 1); protonated phospholipids exhibited wider peaks widths than their sodiated counterpart, indicating for this experiment that the sodiated species is the more stable ion. PE (16:0/16:0) showed the largest difference in peak width between the [M+H]+ and [M+Na]+ ions, and the [M+H]+ ion was the only ion with a peak width so broad that isotopic resolution was not obtained in a zoom scan (Figure 2). Previous studies determined that a PW10% less than or equal to 0.30 amu was indicative of a stable ion in QIT analysis.[16] Using this value, both the sodiated and protonated species of SM (d18:1/16:0) and PC (16:0/16:0) could be considered fragile ions, with the sodiated version exhibiting less fragility, while the sodiated species of PE (16:0/16:0) would be considered stable.
Figure 2.
Spectrum A, above, is a zoom scan of protonated phosphatidylethanolamine (PE) 16:0/16:0. The peak width at 10% peak height (PW10%) was determined to be 0.84±0.05. The peak width for the sodiated counterpart, measured from the zoom scan in spectrum B, was 0.28±0.03. A narrower isolation width indicates that the sodiated species is less fragile than the protonated species in ion trap mass analysis.
Table 1.
Zoom scan results for peak width at 10% peak height, isolation width required for precursor ion isolation, and percent collision-induced dissociation required for fragmentation for each of the GPLs studied as both protonated and sodiated species.
| PW10% | Isolation Width at 50% | %CID at 50% | ||||
|---|---|---|---|---|---|---|
| Lipid studied | [M+H]+ | [M+Na]+ | [M+H]+ | [M+Na]+ | [M+H]+ | [M+Na]+ |
| SM (d18:1/16:0) | 0.45 ± 0.05 | 0.34 ± 0.03 | 3.2 | 1.8 | 20.0 | 24.5 |
| PE (16:0/16:0) | 0.84 ± 0.05 | 0.28 ± 0.02 | 2.8 | 2.5 | 21.5 | 24.5 |
| PC (16:0/16:0) | 0.46 ± 0.04 | 0.36 ± 0.03 | 2.4 | 1.6 | 21.0 | 23.5 |
For secondary confirmation that the sodiated species is more stable than the protonated phospholipid species, data were collected to determine the isolation width needed to efficiently isolate the parent ion before CID. In this experiment, an efficient isolation width was defined as the width needed to reach 50% of the intensity at an isolation width of 4.0 (Table 1). It should be noted that in the previous publication[16] the isolation width was determined using 90% of the parent ion; it was observed that values at 50% and 25% were equally accurate in predicting ion fragility, so 50% was chosen here. All the sodiated species permitted narrower isolation widths than the protonated species (Table 1); however, the difference for PE (16:0/16:0) was not as great as that for PC (16:0/16:0) and SM (d18:1/16:0), indicating both protonated and sodiated PE(16:0/16:0) are fragile ions during isolation. This result is contrary to the findings from the zoom scan experiment. Both PC (16:0/16:0) and SM (d18:1/16:0) share the same head group, phosphocholine, whereas in PE (16:0/16:0) the head group is phosphoethanolamine, (-NH3, rather than trimethylamine, -N(CH3) at the head). This structural difference may cause a dramatic change in inter- and intra-molecular bonding for PE (16:0/16:0) ions in the gas-phase as well as differences in the gas-phase basicity. An interesting observation was that even the more stable sodiated species for all phospholipids studied required an isolation width of greater than 1 amu, implying that these ions are still fragile (previous results indicated that a stable ion such as caffeine [M+H]+ could be isolated with a 1 amu wide window[16]).
Finally, the %CID energy, given as a percent of the maximum 5 Vp-p resonance excitation waveform, required to reduce the precursor ion signal by 50%, was determined for each ion (Table 1). These results also indicated that the protonated phospholipids are more fragile than the sodiated, because less energy is required for 50% fragmentation of the precursor. The major fragment ions for CID of the [M+Na]+ ions result from partial losses of the head groups, –N(CH3)3 (NL of 59) for PC (16:0/16:0) and SM (d18:1/16:0) and –C2H5N (NL of 43) for PE (16:0/16:0). On the other hand, CID of the [M+H]+ ion for PC (16:0/16:0) and SM (d18:1/16:0) produced one dominant fragment ion, m/z 184, corresponding to the polar head group phosphocholine. In contrast, CID of the [M+H]+ ion for PE (16:0/16:0) produced an ion corresponding to a neutral loss of 141, loss of the phosphoethanolamine head group, with the charge retained by the glycerol backbone. PE(16:0/16:0) is differentiated from PC (16:0/16:0) by the presence of amine instead of trimethylamine (Figure 1), which likely changes the gas-phase basicity and the ability to stabilize the phosphate enough to alter the fragmentation pathway under CID so that the head group does not retain the charge during CID.[20]
3.2. Fragility during ion transport
Fragmentation during ion transport could occur in the source region at atmospheric pressure, during transmission through the heated capillary, or in the ion optics after the capillary, where the pressure is reduced. If an ion is thermally unstable, the heat the ion absorbs in the heated capillary could also cause fragmentation, termed thermally-induced dissociation (TID). TID has been studied previously for protein ions, but the temperatures required for dissociation were from 250°C to 400°C.[18] The heated capillary temperatures used in these experiments were lower than that, but still showed the effect of TID.
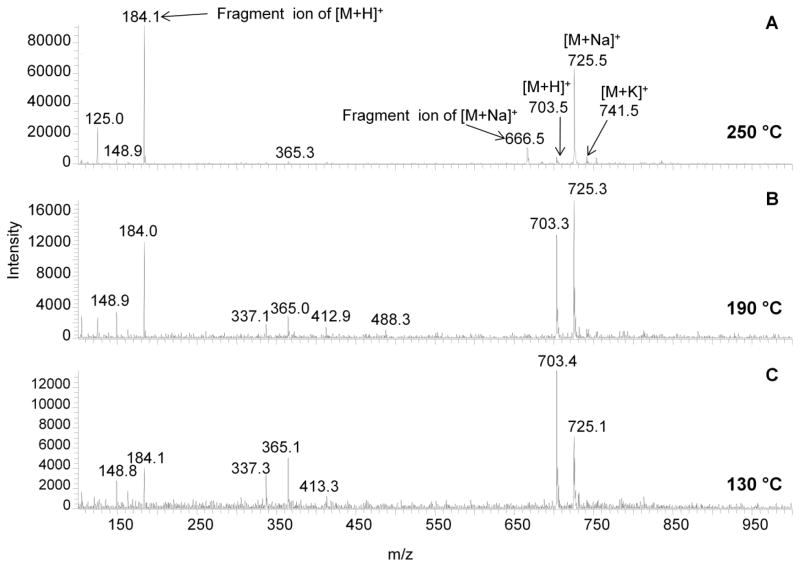
Mass spectra collected at three different capillary temperatures (250°C, 190°C, and 130°C) for SM (d18:0/16:0) show a decrease in the fragment ions associated with each parent ion with a decrease in temperature (Figure 3A–C). Because there were other cations present in the solvent (even though care was taken to remove them), the protonated, sodiated, and potassiated species were all detected at a capillary temperature of 250°C. With both the protonated and sodiated species present in the spectra, it is easy to follow the effect of TID on each ion as long as the fragment ions related to TID can be identified. Since TID experiments have been shown to cause similar fragmentation pathways to CID, comparison of the MS2 spectra with these spectra permits the identification of the fragment ions associated with TID.[18] The major fragment ion from the protonated species, m/z 184, is detected at all three capillary temperatures, but shows a marked decrease in intensity at the lowest capillary temperature of 130°C (Figure 3C) where it is finally less abundant than the parent ion. Note that the major fragment ion from the sodiated species, m/z 666, is only detected at the highest capillary temperature tested, 250°C (Figure 3A). Analyzing SM (d18:1/16:0) at a capillary temperature of 130°C reduces fragmentation for the [M+H]+ ion, but also increases the signal for solvent cluster ions (increased noise), as would be expected because desolvation is not as effective at lower capillary temperatures. Operating this instrument at a low capillary temperature is not recommended because of this desolvation problem. However, at 250°C, the intact [M+H]+ ion is almost completely fragmented, resulting in a very low intensity peak for the intact ion, thus it does appear that when operating at a low ESI flow rate (1 μL/min) the temperature of the heated capillary should be adjusted to reduce this thermal dissociation.
Figure 3.
Mass spectra collected for SM (d18:1/16:0) at three different capillary temperatures (A=250°C, B=190°C and C=130°C). A total of 100 spectra were averaged for each temperature from a 10 ppm (~14 nM) solution infused at 1 μL/min. The fragment ions for the [M+H]+ and [M+Na]+ ions are indicated and were determined by CID experiments. The [M+H]+ ion shows a high degree of fragmentation at the highest temperature tested, where the parent ion is ~10% relative intensity.
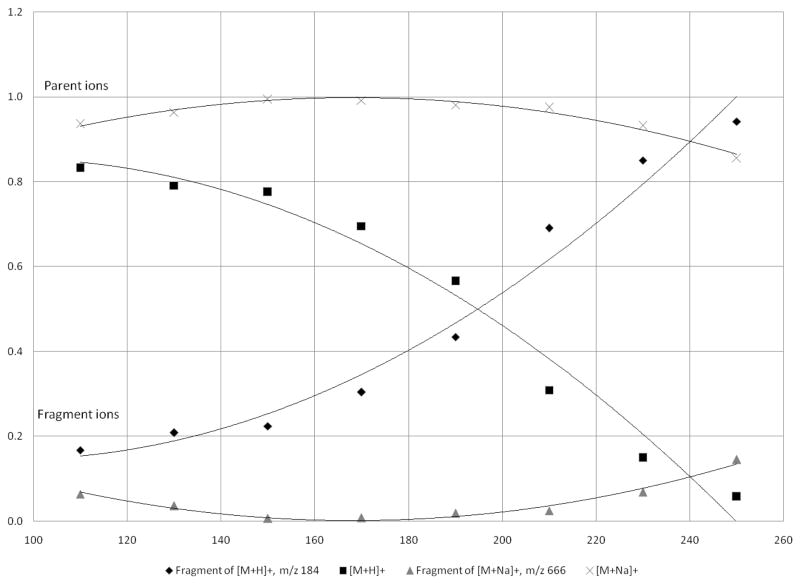
In order to determine the optimum capillary temperature for each phospholipid, a complete temperature profile was obtained by collecting spectra with a tube lens offset of 30V and adjusting the capillary temperature from 250 °C to 50 °C at 20 °C intervals. This complete investigation of the heated capillary temperature showed that protonated SM was greatly affected by the temperature of the heated capillary, as is evident from the increased fragmentation at a higher temperature (Figure 4). The graph was cut off at a temperature of 110 °C because the signal for all ions decreased as a result of poor desolvation. Fragmentation of the [M+H]+ ion of SM (d18:1/16:0) occurs during transport into the vacuum chamber, but can be minimized by operating at lower capillary temperatures. When operating the heated capillary at 250°C, a typical operating temperature for most ESI analyses, the fragmentation of the [M+H]+ ion was nearly 90%, whereas the fragmentation of the [M+Na]+ ion was only around 20%. Based on this experiment, the fragmentation for the SM [M+H]+ ion can be minimized, but not eliminated, when operating with a capillary temperature below 150°C. On the other hand, the fragmentation of the [M+Na]+ ion is minimal even at 250 °C and is reduced to almost zero at a capillary temperature of 160 °C. The signal for the fragment ion of [M+Na]+ at the highest capillary temperature, 250°C, was less than 20% of the parent ion.
Figure 4.
The effect of changing the capillary temperature (°C) on the [M+H]+ and [M+Na]+ ions for SM (d18:1/16:0). The temperature was adjusted at 20° intervals from 250°C to 50°C (graph only shows data down to 110°C). From the graph, it is evident that the protonated species is more susceptible to TID than the sodiated species.
This temperature profile experiment shows a different fragmentation behavior for the sodiated species and the protonated species of the same phospholipid, as was also observed in the ion trap fragility experiments. If a correlation exists between fragility inside the trap and fragility outside the ion trap, the protonated species would be considered fragile, or in other terms more labile, as was the case for ion trap fragility. This fragility makes the protonated species more susceptible to TID, and thus care must be taken when analyzing lipid solutions for protonated species at the flow rate studied here. However, because of this extensive fragmentation, it would be beneficial to induce cationization instead of protonation.
The same temperature profile experiment was performed on PC (16:0/16:0) and PE (16:0/16:0). The effect of temperature on fragmentation for PC (16:0/16:0) was very similar to that of SM (d18:1/16:0), which was anticipated due to the presence of the same head group and similar fragmentation pathways. However, the crossover point, at which the intensity of the TID fragment ion equals the intensity of the parent ion, was higher for the [M+H]+ ion of PC (16:0/16:0) (225 °C) than for the [M+H]+ ion of SM (d18:1/16:0) (190 °C) (Table 2), perhaps indicating a gas-phase interaction between the fatty acid tails and the head group or a stabilization of the phosphate. A future study on this aspect should be conducted to determine how fatty acid chain length and saturation affect ion fragility for these two classes of compounds. In contrast to the high degree of TID observed for the protonated species of both SM (d18:1/16:0) and PC (16:0/16:0), the TID for protonated PE (16:0/16:0) was not as intense (Figure 5). The protonated species of PE (16:0/16:0) did show a greater tendency to fragment under increased capillary temperatures; however, a crossover point never occurred and the TID fragment ion was always less intense than the parent ion. For both protonated and sodiated species, the dominant ion at all temperatures was the parent ion. For PE (16:0/16:0), the head group does not retain the charge under any conditions employed. The extent of ion transfer fragility in phospholipids may be more associated with the head group than with the fatty acid tails; however, a complete study of the phenomenon has not yet been attempted.
Table 2.
A summary of the results from each experiment performed in the studies of ion fragility during ion transport. From these results, it was determined that all sodiated species studied were less susceptible to fragmentation that can occur before mass analysis. However, in the analysis of protonated PE (16:0/16:0), a high degree of fragmentation was not observed, as was the case for both PC (16:0/16:0) and SM (d18:1/16:0).
| Lipid Ion | Capillary Temp crossover (°C) | Max Intensity at Capillary T of (°C) | Tube lens offset crossover (V) |
|---|---|---|---|
| SM (d18:1/16:0) [M+H]+ | 190 | 110 | 1.0 |
| SM (d18:1/16:0) [M+Na]+ | Did not occur | 170 | Did not occur |
| PE (16:0/16:0) [M+H]+ | Did not occur | 150 | Did not occur |
| PE (16:0/16:0) [M+Na]+ | Did not occur | 200 | Did not occur |
| PC (16:0/16:0) [M+H]+ | 230 | 150 | 18.0 |
| PC (16:0/16:0) [M+Na]+ | Did not occur | 190 | Did not occur |
Figure 5.
The effect of changing the capillary temperature on the [M+H]+ and [M+Na]+ ions for PE (16:0/16:0). The protonated species showed a greater tendency to fragment upon increasing the capillary temperature, but the parent ion signal was still more intense than the fragment ion even at the highest temperature. This is in contrast to the other two phospholipids studied.
3.3. Tube lens offset studies
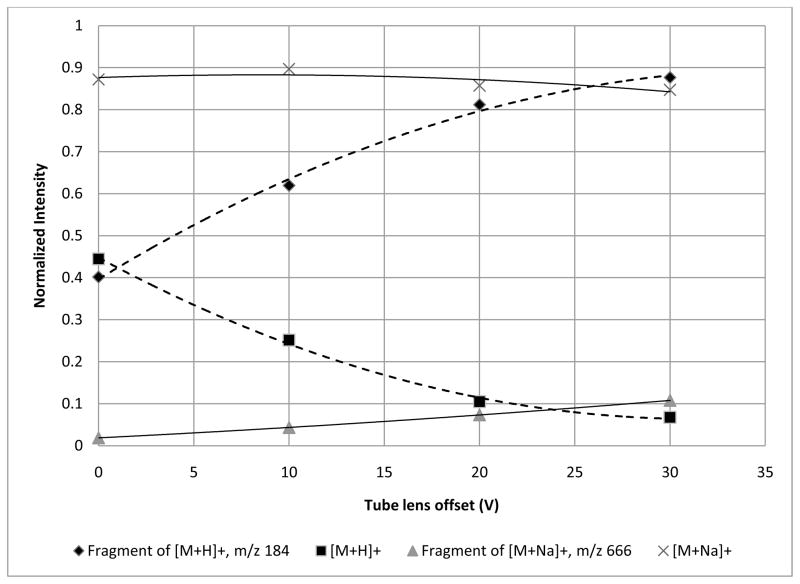
A final test of ion transport fragility involved changing the voltage of the tube lens. To evaluate the effect of the tube lens offset, the capillary temperature was maintained at 250°C while varying the tube lens voltage. For the [M+H]+ ion of SM (d18:1/16:0), as the tube lens voltage was increased from 0 V to 30 V, the fragment ion at m/z 184 was the major ion detected (Figure 6). However, fragmentation was only reduced by 50% when running at 0 V, showing that by adjusting the tube lens offset alone without lowering the capillary temperature, fragmentation cannot be completely eliminated for the [M+H]+ ion of SM (d18:1/16:0). It is possible to operate the tube lens at negative voltages to further reduce fragmentation, but this was not evaluatecd here. A future study will be performed to include negative tube lens values. Again, fragmentation was seen to only a small degree for sodiated SM, indicating a more stable ion. Data were also collected for PE (16:0/16:0) and PC (16:0/16:0) and are summarized in Table 2. As far as fragility during ion transport to the ion trap, protonated SM (d18:1/16:0) appears to be the most fragile ion, requiring the lowest tube lens offset and the lowest capillary temperature to reduce fragmentation by 50%. Apparently, the [M+H]+ ion of PE (16:0/16:0) is not appreciably fragmented by conditions outside the trap. This may be related to the location of the charge on protonated PE (16:0/16:0) and its gas-phase basicity.
Figure 6.
The effect of changing the tube lens offset from 0V to 30V on the [M+H]+ and [M+Na]+ ions for SM (d18:1/16:0). The capillary temperature was held at 250°C for each voltage level. At the lowest tube lens voltage, fragmentation of the parent ion is still around 50%. Adjusting only the tube lens offset is not enough to limit the fragmentation. The sodiated species appears to be unaffected by a change in the tube lens offset.
The source fragment ions identified for each ionized species are consistent with the most abundant CID fragments of the same parent ion. In matrix-assisted laser desorption/ionization (MALDI) of GPLs, Al-Saad et al.[21] described these fragment ions as “prompt” and considered them distinct from post-source decay (PSD), but used PSD as a way to identify the source of those fragment ions. In that study, PC and PE species were detected as [M+H]+ and [M+Na]+ ions. The susceptibility of each ion to produce prompt fragments was discussed as well, and the protonated species tended to fragment more readily, possibly due to the differences between ionic binding and proton binding. [21] In our studies on source pressure for phospholipid analysis by MALDI, [19] we observed a decrease in source fragmentation for SM and PC species when the pressure in the source region was increased to 170 mTorr. This result likely arose from collisional-cooling of the analyte ions with the N2 gas in the source region, and has been observed for other fragile species in MALDI analysis.[19, 22, 23] Even though those studies were from MALDI generated ions, the same effect is evident in the ESI studies performed here, although the term prompt may not be appropriate in ESI because the fragmentation appears to be partially dependent on the temperature of ion transfer and the energy needed to remove clusters (set by the tube lens offset) which are parameters that can be adjusted to remove the fragmentation.
4. Conclusions
The fragility of the ions formed by ESI from model compounds of three phospholipid classes was studied, and results showed that all three classes are fragile within the ion trap as [M+H]+ ions, although PE (16:0/16:0) showed fragility as both the [M+H]+ and the [M+Na]+ ions. From prior experiments,[16] a fragile ion exhibits a PW10% of greater than or equal to 0.31 amu and requires a minimum of isolation width of 2.0 to isolate 90% of the parent ion signal. According to these results, both the sodiated and protonated species of PC (16:0/16:0) and SM (d18:1/16:0) exhibited a degree of fragility, but the protonated species was determined to be more fragile because it exhibited a wider PW10% and a required a wider isolation width. The fact that both ions of the same molecule exhibit a degree of ion fragility when compared to very stable ions may indicate that there is a structural characteristic of PC and SM; computer modeling of the structure of both the [M+H]+ and the [M+Na]+ ions of these and other compounds may aid in determining if there is a structural correlation to ion fragility. This result also suggests that the presence of certain functional groups, such as phosphate, can affect the fragility of the ion.
The fragility in the ion trap for PE (16:0/16:0) was very interesting because it showed the widest peak width in zoom scan for the protonated species, but did not exhibit a large difference in %CID to fragment either the protonated or sodiated species. Perhaps PE ions do not fully desolvate until after they are stored in the ion trap.
Two experiments studying fragility during ion transport were developed and showed that protonated SM (d18:1/16:0) and PC (16:0/16:0) were also fragile, with decreased parent ion signals resulting from source fragmentation at high capillary temperatures and high tube lens offsets. Protonated SM (d18:1/16:0) was nearly undetectable at a capillary temperature of 250°C and tube lens offset of 30 V. Thus, SM species need to be analyzed under 200°C and at lower offsets if protonated in solution. For sodiated SMs, this is not the case, and thus it may be beneficial to use cationization when studying phospholipids. Fragility with other cations besides sodium should also be evaluated, especially adduction with lithium, because it is often used for structural characterization of lipids. However, fragmentation caused by these ion transport parameters was minimal for protonated and sodiated PE (16:0/16:0), even though there is only a small change in the head group between PE and PC (or SM). Clearly, the gas-phase conformation (or site of protonation) or desolvation conditions are very different for PE than for PC and SM. Future studies on the effect of fatty acid chain length, arrangement, and unsaturation would aid in understanding how these structural changes can alter ion trap analysis and fragility. In addition, the analysis of PSs and PIs would help to elucidate how changes in the head group affect both aspects of ion fragility.
Acknowledgments
The authors wish to acknowledge the National Science Foundation’s Research Experience for Undergraduates (REU) program for funding to Matthew Merves. The NIH (R01 ES007355) is also acknowledged for support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References cited
- 1.Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Molecular Biology of the Cell Garland. New York: 1994. Membrane Structure; pp. 477–506. [Google Scholar]
- 2.Murphy RC. Mass Spectrometry of Lipids. Plenum Press; New York: 1993. [Google Scholar]
- 3.Harvey DJ. Matrix-assisted laser desorption/ionization mass spectrometry of phospholipids. J Mass Spectrom. 1995;30:1333–1346. [Google Scholar]
- 4.Harvey DJ. Matrix-assisted laser desorption/ionization mass spectrometry of sphingo- and glycoshingo-lipids. J Mass Spectrom. 1995;30:1311–1324. [Google Scholar]
- 5.Schiller J, Arnhold J, Benard S, Muller M, Reichl S, Arnold K. Lipid analysis by matrix-assisted laser desorption/ionization mass spectrometry: A methodological approach. Anal Biochem. 1999;267:46–56. doi: 10.1006/abio.1998.3001. [DOI] [PubMed] [Google Scholar]
- 6.Guittard J, Hronowski XL, Costello CE. Direct matrix-assisted laser desorption/ionization mass spectrometric analysis of glycoshingolipids on thin layer chromatographic plates and transfer membranes. Rapid Commun Mass Spectrom. 1999;13:1838–1849. doi: 10.1002/(SICI)1097-0231(19990930)13:18<1838::AID-RCM726>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Pulfer M, Murphy RC. Electrospray mass spectrometry of phospholipids. Mass Spectrom Rev. 2003;22:332–364. doi: 10.1002/mas.10061. [DOI] [PubMed] [Google Scholar]
- 8.Han X, Gross RW. Electrospray ionization mass spectroscopic analysis of human erythrocyte plasma membrane phospholipids. Proc Natl Acad Sci. 1994;91:10635–10639. doi: 10.1073/pnas.91.22.10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han X, Gross RW. Structural determination of picomole amounts of phospholipids via electrospray ionization tandem mass spectrometry. J Am Soc Mass Spectrom. 1995;6:1201–1210. doi: 10.1016/1044-0305(95)00568-4. [DOI] [PubMed] [Google Scholar]
- 10.Larsen A, Uran S, Jacobsen PB, Skotland T. Collision-induced dissociation of glycero phospholipids using electrospray ion-trap mass spectrometry. Rapid Commun Mass Spectrom. 2001;15:2393–2398. doi: 10.1002/rcm.520. [DOI] [PubMed] [Google Scholar]
- 11.Ho YP, Huang PC, Deng KH. Metal ion complexes in the structural analysis of phospholipids by electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2003;17:114–121. doi: 10.1002/rcm.880. [DOI] [PubMed] [Google Scholar]
- 12.Hsu FF, Bohrer A, Turk J. Formation of lithiated adducts of glycerophosphocholine lipids facilitates their identification by electrospray ionization tandem mass spectrometry. J Am Soc Mass Spectrom. 1997;9:516–526. doi: 10.1016/S1044-0305(98)00012-9. [DOI] [PubMed] [Google Scholar]
- 13.Hsu FF, Turk J. Characterization of phosphatidylethanolamine as a lithiated adduct by triple quadrupole tandem mass spectometry with electrospray ionization. J Mass Spectrom. 2000;35:596–606. doi: 10.1002/(SICI)1096-9888(200005)35:5<595::AID-JMS965>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 14.Hsu FF, Turk J. Studies on phosphatidylserine by tandem quadrupole and multiple stage quadrupole ion-trap mass spectrometry with electrospray ionization: structural characterization and the fragmentation processes. J Am Soc Mass Spectrom. 2005;16:1510–1522. doi: 10.1016/j.jasms.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Ho YP, Huang PC. A novel structural analysis of glycerophosphocholines as TFA/K+ adducts by electrospray ionization ion trap tandem mass spectrometry. Rapid Commun Mass Spectrom. 2002;16:1582–1589. doi: 10.1002/rcm.751. [DOI] [PubMed] [Google Scholar]
- 16.McClellan JE, Murphy I, James P, Mulholland JJ, Yost RA. Effects of fragile ions on mass resolution and on isolation for tandem mass spectrometry in the quadrupole ion trap mass spectrometer. Anal Chem. 2002;74:402–412. doi: 10.1021/ac015610b. [DOI] [PubMed] [Google Scholar]
- 17.Gabelica V, De Pauw E, Karas M. Influence of the capillary temperature and the source pressure on the internal energy distribution of electrosprayed ions. Int J Mass Spectrom. 2004;231:189–195. [Google Scholar]
- 18.Rockwood AL, Busman M, Udseth HR, Smith RD. Thermally induced dissociation of ions from electrospray mass spectrometry. Rapid Commun Mass Spectrom. 1991;5:582–585. [Google Scholar]
- 19.Garrett TJ, Prieto-Conaway MC, Kovtoun V, Bui H, Izgarian N, Stafford GC, Yost RA. Imaging of small molecules in tissue sections with a new intermediate-pressure MALDI linear ion trap mass spectrometer. Int J Mass Spectrom. 2007;260:166–176. [Google Scholar]
- 20.Thomas MC, Mitchell TW, Blanksby SJ. A comparison of the gas phase acidities of phospholipid headgroups. 2005 doi: 10.1016/j.jasms.2005.02.019. http://ro.uow.edu.au/scipapers/9. [DOI] [PubMed]
- 21.Al-Saad KA, Zabrouskov V, Siems WF, Knowles NR, Hannan RM, Hill J, Herbert H. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of lipids: ionization and prompt fragmentation patterns. Rapid Commun Mass Spectrom. 2003;17:87–96. doi: 10.1002/rcm.858. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor PB, Costello CE. A high pressure matrix-assisted laser desorption/ionization Fourier transform mass spectrometry ion source for thermal stabilization of labile molecules. Rapid Commun Mass Spectrom. 2001;15:1862–1868. doi: 10.1002/rcm.447. [DOI] [PubMed] [Google Scholar]
- 23.O’Connor PB, Mirgorodskaya E, Costello CE. High pressure matrix-assisted laser desorption/ionization Fourier transform mass spectrometry for minimization of ganglioside fragmentation. J Am Soc Mass Spectrom. 2002;13:402–407. doi: 10.1016/S1044-0305(02)00351-3. [DOI] [PubMed] [Google Scholar]