Abstract
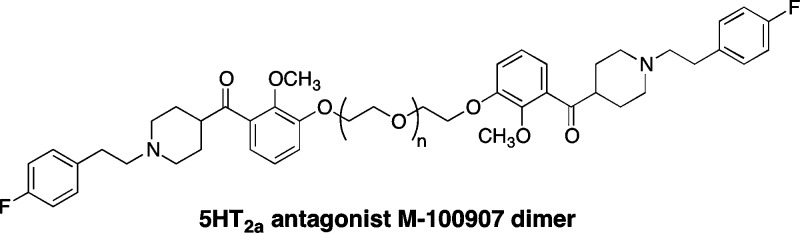
It is now well accepted that at least some serotonin receptors exist in dimeric and oligmeric forms. The linking of receptor ligands has been shown to have potential in the development of selective agonists and antagonists for traditionally refractive receptors. Here we report the development of a dimeric version of the known 5-HT2AR antagonist, M-100907. Derivatives of M-100907 were synthesized to determine an appropriate site for the linker connection. Then, homodimers with polyether linkers of different lengths were functionally tested in a bioassay to determine the optimal linker length. Attachment at the catechol of M-100907 with linkers between 12 and 18 atoms in length proved to be optimal.
Keywords: Serotonin, 5-HT2AR, antagonist, dimers, addiction
Serotonin (5-hydroxytryptamine, 5-HT) and its receptors (particularly the 5-HT2 subgroup) are implicated in a variety of different functions in the CNS ranging from appetite control, learning and memory, depression, and anxiety, to playing a role in impulsivity.1 A significant amount of work has been carried out in an effort to develop ligands that exhibit selectivity for one of the many different subtypes of 5-HT receptors.1,2 Given that all these receptor subtypes have evolved to bind the same agonist and that 5-HT2 receptors share a high degree of homology, this has been a daunting task.3 Recent evidence indicates that at least some 5-HT receptors exist as dimers, and possibly oligomers.4 Consequently, one approach to improve affinity and selectivity of ligands for a given receptor would be the development of multivalent ligands.5 The synthesis of dimeric ligands has been used in a number of G protein-coupled receptors to probe the role of receptor dimerization as well as to improve binding selectivity.6 Additionally, there have been reports of differences between the functional properties of bivalent ligands versus monomeric ligands.5a This paper reports the synthesis of dimeric 5-HT2AR antagonists and the optimization of such molecules.
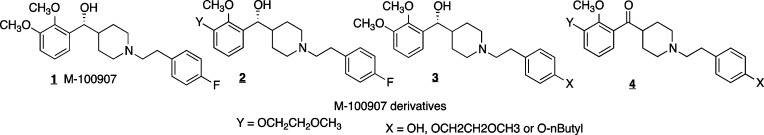
The design of a bivalent ligand begins with the selection of an appropriate monomeric ligand. The piperidine M-100907 (1) has been shown to bind to 5-HT2AR with high affinity (IC50 = 3.3 nM) and is greater than 100 times more selective for the 5-HT2AR over either the 5-HT2CR or 5-HT2BR (Figure 1).7 M-100907 has been used as a selective 5-HT2AR antagonist in a wide variety of in vitro and in vivo studies.8 Despite this work, few studies have examined the structure–activity relationships with analogues of this molecule to optimizing the attachment of fluorescent tags, bioaffinity labels, or other molecules to the basic M-100907 structure.9 Such derivatives would offer the potential to develop molecular probes with a variety of moieties attached, thus allowing imaging or the delivery of other molecules.10 We sought to determine the proper location for attachment of a tether, without significantly decreasing functional activity, by synthesizing derivatives with alkyl chains or ethylene glycol groups attached at opposite ends of the parent molecule (2, 3). We additionally examined a highly active precursor of M-100907, in which the secondary alcohol has been replaced as a ketone. Relative to M-100907, this molecule lacks a chiral center, thus eliminating the diastereomers obtained when linking a racemic mixture of monomers.
Figure 1.
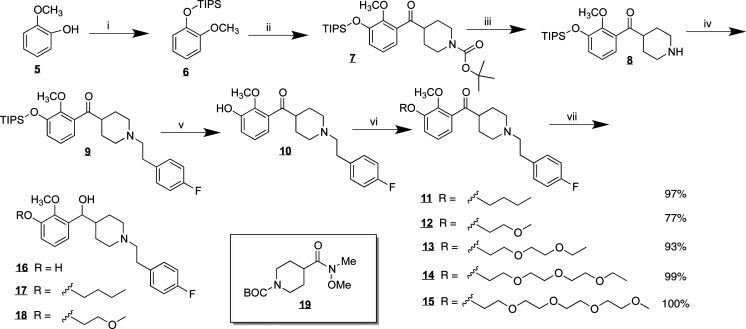
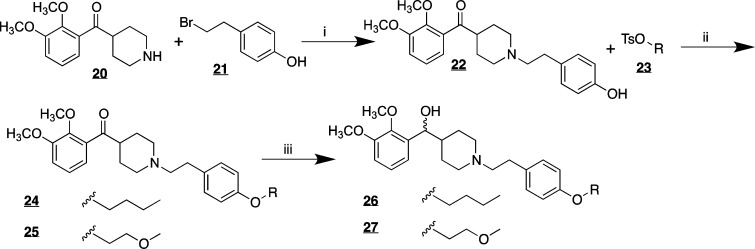
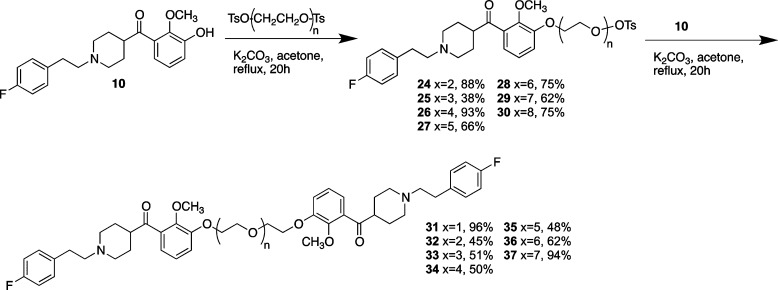
Variations of M-100907 were synthesized (Scheme 1) in which one of the methoxy groups on the catechol was replaced (2) or in which the fluoro group on the opposite end of the molecule was modified (3). The derivatives were synthesized by a route similar to the approach of Rice and Ullrich.11 Metalation of the triisopropylsilyl protected catechol 6 followed by reaction with Weinreb amide 19 provided the necessary ketone 7. Removal of the Boc protecting group followed by alkylation with the tosylate of p-fluorophenethanol yielded the basic structure 9. Deprotection of the triisopropylsilyl protecting group gave the free phenol 10 that could then be used to add the desired linker. In the case illustrated in Scheme 1, the alkylation proceeded with 1-bromobutane (11) or a 1-bromo-ether (12–15). In the case of 11 and 12, the ketone was reduced with sodium borohydride to yield the racemic alcohols 17 and 18. The derivatives in which the fluorophenyl group has been modified (Scheme 2) were accessed by alkylation of intermediate 20 with the bromide of p-hydroxyphenethanol and then alkylation of the phenol with the appropriate alkyl or ether tosylate (23).
Scheme 1.
Reagents and conditions: (i) TIPSCl, imidazole, DMF, rt, 24 h, 63%; (ii) nBuLi, −78 °C, reflux, 5 h, 19 rt, 18 h, 44%; (iii) TFA, rt, 2 h, 96%; (iv) (4-FPh) C2H4OTs, DIEA, CH3CN, reflux, 24 h, 70%; (v) TBAF, THF, rt, 4.5 h, 60%; (vi) TsOR, K2CO3, acetone, reflux, 24 h; (vii) NaBH4, EtOH, rt, 24 h, K2CO3, acetone, reflux, 2 h, 17, 77% 18, 74%.
Scheme 2.
Reagents and conditions: (i) NaHCO3, DMF, 110 °C, 48 h 53%; (ii) K2CO3, acetone, reflux, 18 h, (20, 75% 21, 68%); (iii) NaBH4, EtOH, rt, 1 h, (26, 77%, 27, 74%).
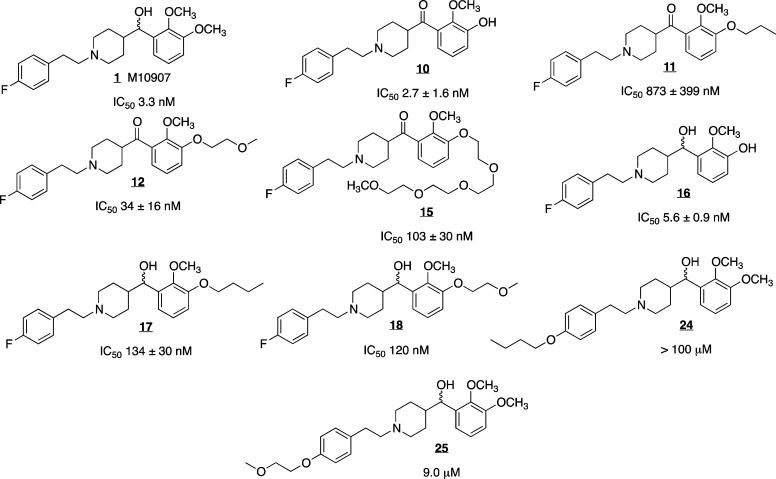
Inhibition of 5-HT2AR activity was determined by measuring the reduction in 5-HT (1 μM) stimulated intracellular calcium (Ca2+) release in a line of CHO cells expressing 5-HT2AR.12 Replacement of fluorine with an ether (24, 25) resulted in significant loss of antagonist potency (Figure 2; see IC50s below respective structures). Attachment of an aliphatic chain to the catechol (11, 17) reduced the derivative’s potency, while placement of an ethylene glycol group at that position (12, 15, 18) was less deleterious Inhibition of 5-HT-induced intracellular Ca2+ release was maintained even with a 13-atom chain attached (15), indicating that this location is a potentially useful site for attachment of the necessary tether for the development of M-100907 derivatives.
Figure 2.
Because our initial synthesis of M-100907 is racemic, linking racemic versions would result in the formation of diastereomers, complicating both purification and biological testing. Accordingly, the decision was made to use versions of the ketone intermediate (4) which was shown to be a reasonably potent (IC50 = 34 nM) inhibitor of 5-HT2AR in the initial Ca2+ bioassays (12). Utilizing the benign tether location we had identified, a series of homodimers was synthesized. These dimers were synthesized by reaction of ketone 10 with an excess of the bis-tosylate of the appropriate polyethylene glycol. The monotosylate products (24–30) were then reacted with excess ketone 10 to provide homodimers 31–37 (Scheme 3).
Scheme 3.
The homodimeric derivatives were tested in the same Ca2+ release bioassay described earlier to determine whether they retained, or possibly had increased, potency. The ability of each derivative to inhibit 5-HT-induced intracellular Ca2+ release was determined in three separate assays for each compound and reported in Table 1 as an IC50. These data indicate that antagonist potency increased as tether length increased, with 12–18 atoms showing comparable inhibition, but fell off sharply by 24 atoms. While the potency of the effective dimers is not greater than the monomeric molecules, it is clear that increasing or reducing the length of the tether beyond the optimal range reduces antagonism. Additionally, compound 15 (Figure 2) demonstrated that the polyether did not affect the activity in a nonspecific manner by interaction with the membrane in which the receptor resides.
Table 1. Dimer Potency as a Function of Length.
| compd | atoms in linker | IC50 |
|---|---|---|
| 31 | 6 | 181 ± 71 nM |
| 32 | 8 | 56 ± 14 nM |
| 33 | 12 | 28 ± 16 nM |
| 34 | 14 | 32 ± 6 nM |
| 35 | 18 | 34 ± 10 nM |
| 36 | 21 | 154 ± 25 nM |
| 37 | 24 | 373 ± 153 nM |
We have identified an appropriate site for dimerization of M-100907 analogues and have demonstrated that the dimeric molecules are antagonists at the 5-HT2AR receptor. This work is the first step in the development of other homodimeric 5-HT ligands that will be evaluated as selective ligands for both homo- and heterodimeric receptor complexes. Molecules of this type have potential as probes for receptor dimerization as well as selective activation of dimeric ligands over their monomeric versions.
This research was supported by the National Institute on Drug Abuse, P20 DA024157, KO5 DA020087 (K.A.C.), T32 DA007287 (M.J.S.), and the M. D. Anderson Foundation (S.R.G.).
Supporting Information Available
Experimental section and NMR spectra of compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The design and synthesis of the molecules described in this paper was carried out by M.J.S. and S.R.G. K.A.C., P.K.S., A.M., T.S., and C.S.W. were responsible for assay development and testing.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Bockaert J.; Claeysen S.; Bécamel C.; Dumuis A.; Marin P. (2006) Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 326(2), 553–572. [DOI] [PubMed] [Google Scholar]
- Hoyer D.; Hannon J. P.; Martin G. R. (2002) Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol., Biochem. Behav. 71, 533–554. [DOI] [PubMed] [Google Scholar]
- a Monck N. J. T.; Kennett G. A. (2008) 5-HT2C ligands: recent progress. Prog. Med. Chem. 46, 281–390. [DOI] [PubMed] [Google Scholar]; b Kitson S. L. (2007) 5-Hydroxytryptamine (5-HT) receptor ligands. Curr. Pharm. Des. 13(25), 2621–2637. [DOI] [PubMed] [Google Scholar]
- a Herrick-Davis K.; Grinde E.; Mazurkiewicz J. E. (2004) Biochemical and Biophysical Characterization of Serotonin 5-HT 2CReceptor Homodimers on the Plasma Membrane of Living Cells. Biochemistry 43(44), 13963–13971. [DOI] [PubMed] [Google Scholar]; b Herrick-Davis K.; Grinde E.; Harrigan T. J.; Mazurkiewicz J. E. (2005) Inhibition of serotonin 5-hydroxytryptamine2c receptor function through heterodimerization: receptor dimers bind two molecules of ligand and one G-protein. J. Biol. Chem. 280(48), 40144–40151. [DOI] [PubMed] [Google Scholar]; c Pellissier L. P.; Barthet G.; Gaven F.; Cassier E.; Trinquet E.; Pin J. P.; Marin P.; Dumuis A.; Bockaert J.; Baneres J. L.; Claeysen S. (2011) G Protein Activation by Serotonin Type 4 Receptor Dimers: Evidence That Turning on Two Protomers is More Efficient. J. Biol. Chem. 286(12), 9985–9997. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Raymond J. R.; Mukhin Y. V.; Gelasco A.; Turner J.; Collinsworth G.; Gettys T. W.; Grewal J. S.; Garnovskaya M. N. (2001) Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol. Ther. 92, 179–212. [DOI] [PubMed] [Google Scholar]
- a Lezoualc’h F.; Jockers R.; Berque-Bestel I. (2009) Multivalent-based drug design applied to serotonin 5-HT4 receptor oligomers. Curr. Pharm. Des. 15(6), 719–729. [DOI] [PubMed] [Google Scholar]; b Munoz-Torrero D. (2009) Exploiting multivalency in drug design. Curr. Pharm. Des. 15(6), 585–586. [DOI] [PubMed] [Google Scholar]; c Krishnamurthy V. M.; Estroff L. A.; Whitesides G. M. (2006) Multivalency in ligand design. Methods Princ. Med. Chem. 34, 11–53 (Fragment-Based Approaches in Drug Discovery). [Google Scholar]
- a Vagner J.; Handl H. L.; Gillies R. J.; Hruby V. J. (2004) Novel targeting strategy based on multimeric ligands for drug delivery and molecular imaging: homooligomers of alpha-MSH. Bioorg. Med. Chem. Lett. 14(1), 211–215. [DOI] [PubMed] [Google Scholar]; b Xu L.; Vagner J.; Josan J.; Lynch R. M.; Morse D. L.; Baggett B.; Han H.; Mash E. A.; Hruby V. J.; Gillies R. J. (2009) Enhanced targeting with heterobivalent ligands. Mol. Cancer Ther. 8(8), 2356–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Choi S.-K.; Green D.; Ho A.; Klein U.; Marquess D.; Taylor R.; Turner S. D. (2008) Designing Selective, High Affinity Ligands of 5-HT 1DReceptor by Covalent Dimerization of 5-HT 1FLigands Derived From 4-Fluoro-N-[3-(1-methyl-4-piperidinyl)-1H-indol-5-yl]benzamide. J. Med. Chem. 51(12), 3609–3616. [DOI] [PubMed] [Google Scholar]; d Bruno A.; Guadix A. E.; Costantino G. (2009) Molecular Dynamics Simulation of the Heterodimeric mGluR2/5HT 2AComplex. An Atomistic Resolution Study of a Potential New Target in Psychiatric Conditions. J. Chem. Inf. Model. 49(6), 1602–1616. [DOI] [PubMed] [Google Scholar]
- Herth M. M.; Kramer V.; Piel M.; Palner M.; Riss P. J.; Knudsen G. M.; Rösch F. (2009) Synthesis and in vitro affinities of various MDL 100907 derivatives as potential 18F-radioligands for 5-HT2A receptor imaging with PET. Bioorg. Med. Chem. 17(8), 2989–3002. [DOI] [PubMed] [Google Scholar]
- a Meltzer H. Y.; Horiguchi M.; Massey B. W. (2011) The role of serotonin in the NMDA receptor antagonist models of psychosis and cognitive impairment. Psychopharmacology (Heidelberg, Ger.) 213(2–3), 289–305. [DOI] [PubMed] [Google Scholar]; b Pockros L. A.; Pentkowski N. S.; Swinford S. E.; Neisewander J. L. (2011) Blockade of 5-HT2A receptors in the medial prefrontal cortex attenuates reinstatement of cue-elicited cocaine-seeking behavior in rats. Psychopharmacology (Heidelberg, Ger.) 213(2–3), 307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Semenova S.; Markou A. (2010) The alpha 2 adrenergic receptor antagonist idazoxan, but not the serotonin-2A receptor antagonist M100907, partially attenuated reward deficits associated with nicotine, but not amphetamine, withdrawal in rats. Eur. Neuropsychopharmacol. 20(10), 731–746. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Zaniewska M.; McCreary A. C.; Wydra K.; Filip M. (2010) Differential effects of serotonin (5-HT)2 receptor-targeting ligands on locomotor responses to nicotine-repeated treatment. Synapse (Hoboken, NJ, U.S.) 64(7), 511–519. [DOI] [PubMed] [Google Scholar]; e Dhonnchadha B. A. N.; Fox R. G.; Stutz S. J.; Rice K. C.; Cunningham K. A. (2009) Blockade of the serotonin 5-HT2A receptor suppresses cue-evoked reinstatement of cocaine-seeking behavior in a rat self-administration model. Behav. Neurosci. 123(2), 382–396. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Zaniewska M.; McCreary A. C.; Wydra K.; Filip M. (2010) Effects of serotonin (5-HT)2 receptor ligands on depression-like behavior during nicotine withdrawal. Neuropharmacology 58(7), 1140–1146. [DOI] [PubMed] [Google Scholar]
- Heinrich T.; Boettcher H.; Pruecher H.; Gottschlich R.; Ackermann K.-A.; van Amsterdam C. (2006) 1-(1-Phenethylpiperidin-4-yl)-1-phenylethanols as potent and highly selective 5-HT2A antagonists. ChemMedChem 1(2), 245–255. [DOI] [PubMed] [Google Scholar]
- a Herth M. M.; Piel M.; Debus F.; Schmitt U.; Lueddens H.; Roesch F. (2009) Preliminary in vivo and ex vivo evaluation of the 5-HT2A imaging probe [18F]MH.MZ. Nucl. Med. Biol. 36(4), 447–454. [DOI] [PubMed] [Google Scholar]; b Herth M. M.; Kramer V.; Piel M.; Palner M.; Riss P. J.; Knudsen G. M.; Roesch F. (2009) Synthesis and in vitro affinities of various MDL 100907 derivatives as potential 18F-radioligands for 5-HT2A receptor imaging with PET. Bioorg. Med. Chem. 17(8), 2989–3002. [DOI] [PubMed] [Google Scholar]
- Ullrich T.; Rice K. C. (2000) A Practical Synthesis of the Serotonin 5-HT2A Receptor Antagonist MDL 100907, its Enantiomer and their 3-Phenolic Derivatives as Precursors for [11C]Labeled PET Ligands. Bioorg. Med. Chem. 8(10), 2427–2432. [DOI] [PubMed] [Google Scholar]
- a Anasatsio N. C., Witkin B. M., Seitz P. K., Watson C. S., and Cunningham K. A. (2009) Novel medium-throughput 96-well plate assay to immunohistochemicallly detect key brain proteins in the serotonin 5-HT2C receptor-ERK pathway. Abstract 787.2. Society of Neuroscience Meeting; October 17–21, 2009.; b Berg K. A.; Clarke W. P.; Sailstad C.; Saltzman A.; Maayani S. (1994) Signal transduction differences between 5-hydroxytryptamine type 2A and type 2C receptor systems. Mol. Pharmacol. 46, 477–484. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.