Abstract
Many studies that found associations between depression and nicotine dependence have ignored possible shared genetic influences associated with antisocial traits. The present study examined the contribution of genetic and environmental effects associated with conduct disorder (CD) and antisocial personality disorder (ASPD) to the comorbidity of major depression (MD) and nicotine dependence (ND). A telephone diagnostic interview, the Diagnostic Interview Schedule-III-R, was administered to eligible twins from the Vietnam Era Twin (VET) Registry in 1992. Multivariate genetic models were fitted to 3360 middle-aged and predominantly white twin pairs (1868 monozygotic, 1492 dizygotic pairs) of which both members completed the pertinent diagnostic interview sections. Genetic influences on CD accounted for 100%, 68%, and 50% of the total genetic variance in risk for ASPD, MD and ND, respectively. After controlling for genetic influences on CD, the partial genetic correlation between MD and ND was no longer statistically significant. Nonshared environmental contributions to the comorbidity among these disorders were not significant. This study not only demonstrates that the comorbidity between ND and MD is influenced by common genetic risk factors, but also further suggests that the common genetic risk factors overlapped with those for antisocial traits such as CD and ASPD in men.
Research has shown a significant association between smoking and depression (Covey et al., 1998; Glassman, 1993; Glassman et al., 1990; Hughes, 1999; Paperwalla et al., 2004; Williams & Ziedonis, 2004). Data from the National Comorbidity Study (NCS) in the United States conducted in early 1990s showed that the prevalence of lifetime and current smokers was 59% and 37%, respectively, among persons with lifetime major depression (MD) and 60% and 45%, respectively, among persons who reported MD in the past month compared to 39% and 23% in those with no history of mental illness (Lasser et al., 2000). The NCS data also suggest that preexisting MD that has not remitted increases risk of progression to nicotine dependence (ND) by 1.2 times (Breslau et al., 2004a). The National Epidemiologic Survey on Alcohol and Related Conditions reveals that the 12-month prevalence of ND is 30% among persons who reported MD in the past 12 month compared to 13% in the United States general population during this period (Grant et al., 2004). A significant association between MD and ND was also found in male and female adolescents (Fergusson et al., 1996). Although an understanding of risk factors that underlie the association between the two conditions has important implications for clinical diagnosis and treatment, the mechanism underlying the observed comorbidity is not clear.
Both causal and noncausal hypotheses have been proposed to explain the comorbidity between smoking and depression. Each type of hypotheses has found support from some, but not all, prospective studies. In support of the causal hypothesis, a bidirectional relationship between depression and smoking has been proposed. MD and depression symptoms are found to be associated with subsequent smoking and ND in teens and young adults in some longitudinal studies (Breslau et al., 1993, 1998; Escobedo et al., 1998; Fergusson et al., 2003; Kandel & Davies, 1986; Killen et al., 1997; Patton et al., 1998), but not in others (Dierker et al., 2001; Goodman & Capitman, 2000). Depression also decreases the likelihood of quitting tobacco use (Anda et al., 1990). Conversely, smoking has been shown to predict later depressed mood and symptoms in adolescents and young adults in some studies (Choi et al., 1997; Windle & Windle, 2001; Wu & Anthony, 1999), but not in the other prospective epidemiological studies (Brook et al., 2004). An association between daily smoking and subsequent onset of MD was reported in the NCS (Breslau et al., 2004b).
Evidence regarding factors that influence both smoking and depression has emerged to support the noncausal hypothesis for the explanation of the association between the two conditions. A prospective study of young adults found that after controlling for conduct disorder (CD), smoking did not predict the later onset of MD, and MD was not associated with subsequent onset of smoking (Breslau et al., 1998). A study of an adolescent cohort found that the strength of the correlation between ND and MD was reduced by half, after controlling for factors (e.g., conduct problems, delinquent peer affiliation and other variables) correlated with both conditions (Fergusson et al., 1996). This common etiologic hypothesis has been further examined from a genetic perspective. Twin research has found that the association between cigarette smoking and MD was accounted for by correlated genetic factors (rA = .56) in women (Kendler et al., 1993) and a genetic correlation (rA = .17) in men (McCaffery et al., 2003). In a pediatric sample, early experimental smoking was reported to be genetically correlated with depression in girls and environmentally correlated in boys (Silberg et al., 2003). A family study reported that heavy smoking and dysthymia, but not MD, co-aggregated between probands and their relatives (Dierker et al., 2002).
Previous studies on shared genetic risk of MD and ND have ignored the potential contribution from antisocial traits such as CD and antisocial personality disorder (ASPD). We have previously reported genetic covariance between ASPD and MD in this sample (Fu et al., 2002). It is plausible to hypothesize that genetic factors associated with antisocial traits may be attributable to the genetic association between ND and MD.
The current study used a large population-based male twin cohort selected from the Vietnam Era Twin (VET) Registry to address the following questions: (1) Are there common factors underlying the comorbidity between ND and MD? (2) If common factors are found, are they genetic or environmental? and (3) Do those common factors overlap with those affecting conduct disorder (CD) and ASPD?
Materials and Methods
Participants
The VET Registry is a general population registry of male twins constructed in the mid 1980s from computerized Department of Defense files and other sources. Twins who were born between 1939 and 1957 and served on active military duty during the Vietnam era (1965–1975) were included in the registry. Zygosity was assessed by a series of questions about sibling similarity and supplemented with limited blood group data obtained from military records. Zygosity determination by such methods has been shown to have 95% accuracy (Eisen et al., 1989). The development and characteristics of the Registry have been published elsewhere (Eisen et al., 1987, 1989; Henderson et al., 1990). Registry members participating in research studies have been found to be representative of twins who served in the military during the Vietnam era on a variety of sociodemographic and other variables (Goldberg et al., 1987; Henderson et al., 1990).
The data reported here are from structured diagnostic telephone interviews administered to the VET panel in 1992 and 1993 (Lyons et al., 1995; Tsuang et al., 1996). Of 10,300 eligible individuals, after accepting informed consent, 79.7% completed the interview. The overall pairwise response rate was 66% (3372 complete pairs). A total of 3360 pairs (1868 monozygotic [MZ], 1492 dizygotic [DZ] pairs) in which both members completed the pertinent diagnostic interview sections are included in the present report. The mean age at interview of respondents was 42.0 years (SD ± 2.7, range 33–52 years); 93.8% were non-Hispanic white, 5.8% were African American, less than 1% were Hispanic, and 0.3% were of other ethnicity; 33.3% were high school graduates and 38.7% college graduates; and 92.6% were employed full-time, 1.8% part-time, and 5.6% unemployed. The sociodemographic profile of the VET panel has been reviewed in detail elsewhere (Goldberg et al., 1987; Tsuang et al., 2001).
Measures
A computerized telephone version of the Diagnostic Interview Schedule, Version 3, Revised (DIS-III-R; Robins et al., 1988) was used to assess common axis I psychiatric disorders. The research protocol was approved by the institutional review boards. Experienced interviewers from the Institute for Survey Research at Temple University were trained by one of the project investigators to administer the telephone interview. The interview was conducted after the respondent had given verbal informed consent. Lifetime diagnoses of CD, ASPD, MD, and ND were determined according to Diagnostic and Statistical Manual of Mental Disorders (3rd ed., rev.; DSM-III-R; American Psychiatric Association, 1987) criteria. All diagnostic variables were coded dichotomously. An ordinal variable of CD symptom counts (from no symptom, coded as 0, to having five or more symptoms, coded as 6) was created for the biometric modeling analysis, based on the number of symptoms before age 15. Earlier analyses of the VET data reported good test–retest reliability of diagnostic measures (Slutske et al., 1997; True et al., 1999).
Statistical Analysis
Logistic regression analyses were performed to analyze the associations of ND with CD (without ASPD), ASPD, and MD, respectively. Each twin member was treated as an observation in a general population sample. Odds ratios (OR) and 95% confidence intervals (CI) were estimated using the Huber-White robust variance estimator implemented in Stata (StataCorp, 1999) to adjust for the nonindependence of observations in the twin sample (which would otherwise lead to underestimation of 95% CIs for the ORs).
Because MZ pairs are genetically identical, whereas DZ pairs are genetically no more alike than ordinary full siblings, comparing MZ and DZ twin intrapair correlations provides a test for genetic and environmental effects. The intrapair correlations matrix includes cross-twin within-variable, within-twin cross-variable, and cross-twin cross-variable polychoric correlations. We assumed that for each disorder there is a continuous and approximately normally distributed liability in the general population, which is determined by the combined effects of multiple genetic and environmental risk factors (Falconer, 1965). With twin data, the total phenotypic variance of ND can be decomposed into genetic, shared environmental and nonshared environmental components that overlap with CD, ASPD, and MD, and specific to ND, using a Cholesky factor model (Neale et al., 1999). A detailed description of this model can be found elsewhere (Neale et al., 1999). MZ and DZ pairs are not assumed to differ in their concordance for pertinent shared environmental risk factors. In fact, little evidence has been found that this equal environment assumption is violated in this VET panel (Xian et al., 2000). This result is consistent with findings in other twin samples (Kendler & Gardner, 1998).
According to the DSM-III-R, the diagnosis of ASPD requires a history of CD with onset before age 15 (Robins et al., 1998). Missing values were assigned on ASPD for the CD diagnosis negative group (i.e., fewer than 3 CD symptoms) because a negative CD diagnosis precludes a diagnosis of ASPD. To obtain unbiased genetic and environmental estimates of parameters for ASPD, the CD symptoms and ASPD were jointly analyzed in a model where the CD symptom variable had two or more categories that were cross-classified with the ASPD diagnosis (e.g., number of CD symptoms above the minimum of three required for a CD diagnosis). More technical details about analyzing this type of hierarchical data can be found elsewhere (Heath et al., 2002).
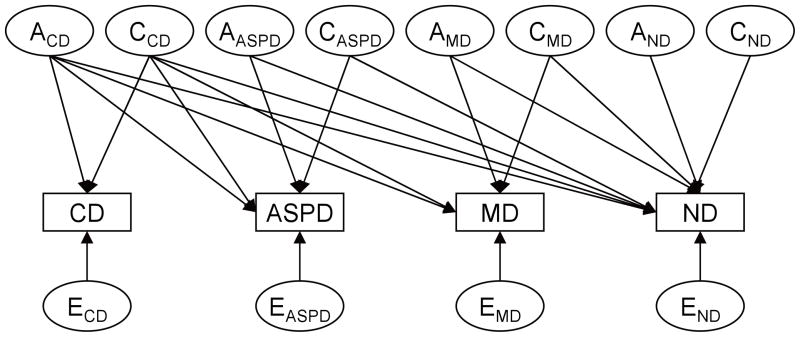
A quadravariate model including CD symptoms, ASPD, MD, and ND was fitted to the raw data using the full information maximum likelihood method implemented in Mx (Neale et al., 1999). A schematic path diagram of the full model is illustrated in Figure 1. Then, we tested a series of submodels nested within the full model by constraining to zero the insignificant genetic or environmental parameter estimates. The fit of each submodel was evaluated using a likelihood ratio chi-squared test. An insignificant chi-squared test result (p > .05) suggests the submodel with fewer parameter estimates gives an equal good fit to the data compared to the more comprehensive one that the submodel was nested. For the nonnested model, Akaike Information Criterion (AIC), defined as −2 (maximum log-likelihood) +2 (number of parameters) was used to compare different models. The best fitting model was determined by a balance of parsimony and goodness-of-fit according to the smallest AIC.
Figure 1.
Schematic path diagram of a Cholesky decomposition model for genetic and environmental influences on CD, ASPD, MD, and ND. The variance in liability to each disorder was decomposed into additive genetic (A), shared environmental (C), and nonshared environmental components (represented by latent factors) that overlap with other disorders and specific to the disorder. One-way arrows represent standardized factor loadings to be estimated.
Results
Lifetime prevalence of DSM-III-R CD, ASPD, MD, and ND in the VET sample was 10%, 2.7%, 9.2%, and 47.8%, respectively. Thirty-six per cent of respondents with ASPD met lifetime criteria for MD, compared to only 8% of those without a history of ASPD. Prevalence of co-occurring ND and MD was 6.5%; co-occurring ND and ASPD 2.3%; and co-occurring ND and MD and ASPD 0.9%. The estimated odds of ND for respondents with a history of CD only, ASPD or MD were 1.95, 5.4, and 2.88 times the estimated odds for those without any of those disorders, respectively. When all of these variables were included in a regression model, the estimated odds of ND for respondents with a history of CD only, ASPD, or MD were equal to 2.01, 4.63, and 2.63 times the estimated odds for those without these disorders, respectively. The results from logistic regression indicated that CD, ASPD, MD and ND were associated with one another.
Table 1 presents intrapair polychoric correlations across disorders by zygosity. All MZ correlations were greater than corresponding DZ correlations across the four disorders, suggesting that genetic influences partially caused the comorbidity among those disorders.
Table 1.
Polychoric Correlations among Conduct Disorder (CD), Antisocial Personality Disorder (ASPD), Major Depression (MD), and Nicotine Dependence (ND) by Zygosity
| MZ Co-twin
|
DZ Co-twin
|
|||||||
|---|---|---|---|---|---|---|---|---|
| CD | ASPD | MD | ND | CD | ASPD | MD | ND | |
| CD | .41 | .53 | .23 | .23 | .33 | .37 | .10 | .17 |
| ASPD | .43 | .70 | .27 | .34 | .31 | .22 | .23 | .22 |
| MD | .25 | .19 | .42 | .18 | .10 | .18 | .13 | .13 |
| ND | .24 | .37 | .32 | .60 | .20 | .28 | .12 | .31 |
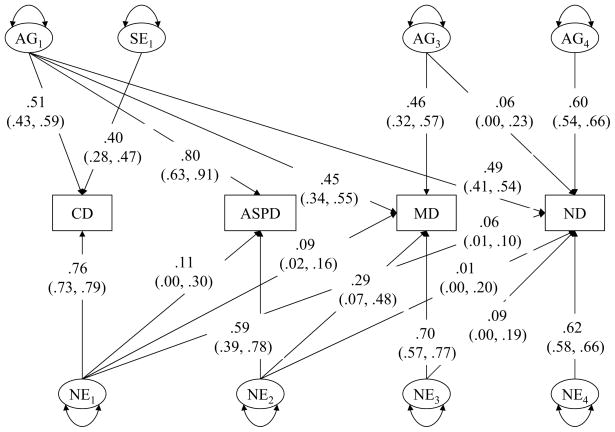
The results of fitting a Cholesky model to the raw data are summarized in Table 2. When compared to the full model, Model 2, which excluded genetic effects on all disorders, was rejected by the likelihood ratio chi-squared test (χ2 = 64.67, df = 10, p < .001, AIC = 44.67). Model 3, which did not contain shared environmental parameters on any disorder, gave a marginally good fit (χ2 = 17.99, df = 10, p = .06, AIC = −2.00). However, Model 4, which retained a single shared environmental parameter for a shared environmental effect specific to CD, gave a substantial improvement in fit (χ2 = 7.69, df = 9, p = .56, AIC = −10.31). When a genetic effect specific to ASPD was removed, Model 5 gave a better fit to the data (χ2 = 7.88, df = 12, p = .82, AIC = −16.10), suggesting that genetic correlation between CD and ASPD did not differ significantly from unity. We further tested an alternative model, Model 6, which assumed no significant residual genetic covariance between CD and ND after controlling for genetic influences on MD. The model fit index for this model indicated a substantially worse fit to the data than Model 5. Thus Model 5 was the best fitting model. Standardized path coefficients and 95% CIs under this model are presented in Figure 2.
Table 2.
Model Fit Indices and Goodness-of-Fit for Full and Submodels
| Model | −2LL | df | Δχ2 | Δdf | p value | AIC |
|---|---|---|---|---|---|---|
| 1 (full) | 28887.80 | 3393 | ||||
| 2 (no AG) | 28952.47 | 3403 | 64.67 | 10 | < 0.01 | 44.67 |
| 3 (no SE) | 28905.79 | 3403 | 17.99 | 10 | 0.06 | −2.0 |
| 4 (retaining SE on CD only) | 28895.49 | 3402 | 7.69 | 9 | 0.56 | −10.3 |
| 5 (no AG specific to ASPD) | 28895.68 | 3405 | 7.88 | 12 | 0.82 | −16.1 |
| 6 (alternative model)a | 28904.57 | 3405 | 16.77 | 12 | 0.16 | −7.23 |
Note: Abbreviations: −2LL = −2 log-likelihood; df: degrees of freedom; χ2 = chi-squared test comparing a nested submodel to the full model; df = difference of degrees of freedom between a specific model and the full model; AIC = Akaike’s Information Criterion; AG = additive genetic effect; SE = shared environmental effect.
The model reordered the observed variables as major depression (MD), conduct disorder (CD), antisocial personality disorder (ASPD) and nicotine dependence (ND) to test whether MD genetic factor explain genetic covariance between CD and ND.
Figure 2.
Factor loadings (95% confidence intervals) of CD, ASPD, MD, and ND on genetic (AGi), shared environmental (SEi), and nonshared environmental (NEi) factors in Model 5.
Heritability estimates for lifetime DSM-III-R CD, ASPD, MD, and ND were 26%, 63%, 41% and 61%, respectively. Shared environmental variance was 16% for CD. Genetic influences on CD accounted for 20% of the total phenotypic variance for MD and 24% for ND. Furthermore, of the total genetic variances in risk for MD and ND, 49% (.452/[.452 + .462]) and 40% (.492/[.492 + .602]) were explained by the genetic factor for CD. Although the genetic covariance between MD and ND was not significant after controlling for the genetic factor of CD, we examined this covariance to avoid an overestimation of genetic effects associated with CD on the comordity of MD and ND. We found that 90.3% (95% CI 67.5–100%) of the total genetic correlation between MD and ND could be explained by the CD genetic factor, and only 9.7% (95% CI 0.0–33.4%) by the genetic factor for MD. This supports the original hypothesis that the genetic risk factors for CD largely accounted for the genetic correlation between MD and ND.
Discussion
The purpose of the present study was to examine genetic and environmental factors underlying the association between MD and ND. Our data showed that both MD and ND were highly genetically correlated with CD. The causes of the comorbidity of the two conditions were largely genetically rather than environmentally determined. The residual genetic correlation between ND and MD was no longer statistically significant after controlling for those genetic effects on CD and ASPD. Thus, the genetic determinants of this comorbidity were genes that increased the risk for CD and ASPD. In these analyses, we made an allowance for the diagnostic hierarchical relationship of ASPD and CD. An additional finding was the complete genetic correlation of risk of CD and risk of ASPD in those who develop CD (having three or more CD symptoms). CD and ASPD were influenced by different nonshared environmental determinants (including measurement error). Shared environmental effects were significant for CD, but not for ASPD.
Our data support the common etiological hypothesis for the association between MD and ND; that is, there is a genetic predisposition to impulsive traits (Slutske et al., 1997, 2002), which in turn increases risk for MD and ND. The present study results are supported by findings that CD and MD are often comorbid (Marmorstein & Iacono, 2003). Or, more broadly defined, externalizing and internalizing disorders are positively correlated (Krueger, 1999) and that familial aggregation exists among ASPD, MD, and substance use disorders (Kendler et al., 1997). Our conclusion is consistent with substance abuse or dependence literature that shows the genetic association between juvenile and adult antisocial personality disorders and substance abuse or dependence (Krueger et al., 2002). For example, genetic risk for behavior under-control accounts for most common genetic risk for alcohol dependence and CD reported by an Australian twin study (Slutske et al., 2002). An adolescent twin-family study showed that familial transmission of CD/ASPD and substance (i.e., alcohol and drug) dependence was mainly due to common genetic risk factors (Hicks et al., 2004). A recent genome-wide screen study found that the risks for CD and alcohol dependence were linked in the same region on chromosome 2 (Dick et al., 2004).
With respect to genetic risk factors associated with CD, the heritability estimate of CD derived from this male twin sample is greater than that from the Virginia male twin sample (26% vs. 13%) and smaller than that from the Virginia female twin sample (26% vs. 38%; Goldstein et al., 2001; Jacobson et al., 2000). Greater shared environmental influences were found to contribute to the total variance of CD in this male twin sample, consistent with the findings from the Virginia male twin (Jacobson et al., 2000). The familial (genetic plus shared environmental ) effects (26% + 16% = 42%) estimated in the present study were slightly greater than the genetic estimate of CD reported in the Virginia female twin sample, which did not find significant contributions from shared environmental effects (Goldstein et al., 2001). Compared to an earlier report based on the same data set, we found somewhat stronger evidence for a genetic effect (26% vs. 7%), most likely because of the increased precision of a multivariate genetic analysis which includes ASPD. However, the estimates of familial effects in the two reports are relatively similar.
Some limitations of this study should be noted. The VET sample was composed of a relatively homogenous group of middle-aged and predominantly white male United States military veterans, precluding generalization to women and other ethnic groups. Previous examinations of this twin panel (perhaps because of readily available tobacco products during military service) showed a higher prevalence of ND and comparable figures for MD than was obtained from nonveteran males (Jordan et al., 1991; Lyons et al., 1998; Slutske et al., 1997; Tsuang et al., 1996, 1998). Entry into military service most likely excluded individuals with the most severe, early onset antisocial behaviors. The much lower prevalence of DSM-III-R ASPD in this twin sample (2.7%) compared to the Untied States general population prevalence in men (5.8%) supports this hypothesis (Kessler et al., 1994). This sample selection may have led to the disproportionate inclusion of individuals with ASPD who have mild to moderate antisocial behaviors, but would be expected to attenuate rather than exaggerate associations between ASPD and ND. Heritability estimates of MD and ND found in this twin panel are similar to results from other nonveteran samples (Kendler, 1999; Slutske et al., 1998). Thus, it seems implausible that our findings are based on an artefactual contribution of CD to the genetic correlations of MD with ND.
Our study not only demonstrates that comorbidity between ND and MD is influenced by common genetic risk factors, but also further explicitly shows that the common genetic risk is accounted for by genes associated with CD and ASPD. These results have implications for studies which search genes that might increase risk for ND. The comorbidity of CD and ND instead of that of MD and ND may be a promising phenotype to identify predisposing genes for ND. Our results also have implications for attempts to understand the etiology of ND, which is a common cause of failure of smoking cessation. Although clinicians have long observed that ND is comorbid with MD and CD, our data suggests that greater attention should be paid to the genetic risk associated with impulsive or antisocial traits that underlie the observed comorbidity in men.
Acknowledgments
The authors are grateful for support from NIH grants: K07CA104119, DA04604, DA07261, AA07788. The United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Numerous organizations have provided invaluable assistance in the conduct of this study, including: Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University. Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Without their contribution this research would not have been possible.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: Author; 1987. rev. [Google Scholar]
- Anda RF, Williamson DF, Escobedo LG, Mast EE, Giovino GA, Remington PL. Depression and the dynamics of smoking. A national perspective. Journal of the American Medical Association. 1990;264:1541–1545. [PubMed] [Google Scholar]
- Breslau N, Kilbey MM, Andreski P. Nicotine dependence and major depression. New evidence from a prospective investigation. Archives of General Psychiatry. 1993;50:31–35. doi: 10.1001/archpsyc.1993.01820130033006. [DOI] [PubMed] [Google Scholar]
- Breslau N, Novak SP, Kessler RC. Psychiatric disorders and stages of smoking. Biological Psychiatry. 2004a;55:69–76. doi: 10.1016/s0006-3223(03)00317-2. [DOI] [PubMed] [Google Scholar]
- Breslau N, Novak SP, Kessler RC. Daily smoking and the subsequent onset of psychiatric disorders. Psychological Medicine. 2004b;34:323–333. doi: 10.1017/s0033291703008869. [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson EL, Schultz LR, Chilcoat HD, Andreski P. Major depression and stages of smoking: A longitudinal investigation. Archives of General Psychiatry. 1998;55:161–166. doi: 10.1001/archpsyc.55.2.161. [DOI] [PubMed] [Google Scholar]
- Brook JS, Schuster E, Zhang C. Cigarette smoking and depressive symptoms: A longitudinal study of adolescents and young adults. Psychological Report. 2004;95:159–166. doi: 10.2466/pr0.95.1.159-166. [DOI] [PubMed] [Google Scholar]
- Choi WS, Patten CA, Gillin JC, Kaplan RM, Pierce JP. Cigarette smoking predicts development of depressive symptoms among U.S. adolescents. Annals of Behavior Medicine. 1997;19:42–50. doi: 10.1007/BF02883426. [DOI] [PubMed] [Google Scholar]
- Covey LS, Glassman AH, Stetner F. Cigarette smoking and major depression. Journal of Addictive Disorder. 1998;17:35–46. doi: 10.1300/J069v17n01_04. [DOI] [PubMed] [Google Scholar]
- Dick DM, Li TK, Edenberg HJ, Hesselbrock V, Kramer J, Kuperman S, Porjesz B, Bucholz K, Goate A, Nurnberger J, Foroud T. A genome-wide screen for genes influencing conduct disorder. Molecular Psychiatry. 2004;9:81–86. doi: 10.1038/sj.mp.4001368. [DOI] [PubMed] [Google Scholar]
- Dierker LC, Avenevoli S, Merikangas KR, Flaherty BP, Stolar M. Association between psychiatric disorders and the progression of tobacco use behaviors. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:1159–1167. doi: 10.1097/00004583-200110000-00009. [DOI] [PubMed] [Google Scholar]
- Dierker LC, Avenevoli S, Stolar M, Merikangas KR. Smoking and depression: An examination of mechanisms of comorbidity. American Journal of Psychiatry. 2002;159:947–953. doi: 10.1176/appi.ajp.159.6.947. [DOI] [PubMed] [Google Scholar]
- Eisen S, Newman R, Goldberg J, Rice J, True W. Determining zygosity in the Vietnam Era Twin Registry: An approach using questionnaires. Clinical Genetics. 1989;35:423–432. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Eisen SA, Ture W, Goldberg J, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: Method of construction. Acta Geneticae Medicae et Gemellologae. 1987;36:61–66. doi: 10.1017/s0001566000004591. [DOI] [PubMed] [Google Scholar]
- Escobedo LG, Reddy M, Giovino GA. The relationship between depressive symptoms and cigarette smoking in US adolescents. Addiction. 1998;93:433–440. doi: 10.1046/j.1360-0443.1998.93343311.x. [DOI] [PubMed] [Google Scholar]
- Falconer DS. The inheritance of liability to certain disease, estimated from the incidence among relatives. Annals of Human Genetics. 1965;29:51–76. [Google Scholar]
- Fergusson DM, Goodwin RD, Horwood LJ. Major depression and cigarette smoking: results of a 21-year longitudinal study. Psychological Medicine. 2003;33:1357–1367. doi: 10.1017/s0033291703008596. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Lynskey MT, Horwood LJ. Comorbidity between depressive disorders and nicotine dependence in a cohort of 16-year-olds. Archives of General Psychiatry. 1996;53:1043–1047. doi: 10.1001/archpsyc.1996.01830110081010. [DOI] [PubMed] [Google Scholar]
- Fu Q, Heath AC, Bucholz KK, Nelson E, Goldberg J, Lyons MJ, True WR, Jacob T, Tsuang MT, Eisen SA. Shared genetic risk of major depression, alcohol dependence, and marijuana dependence: Contribution of antisocial personality disorder in men. Archives of General Psychiatry. 2002;59:1125–1132. doi: 10.1001/archpsyc.59.12.1125. [DOI] [PubMed] [Google Scholar]
- Glassman AH. Cigarette smoking: Implications for psychiatric illness. American Journal of Psychiatry. 1993;150:546–553. doi: 10.1176/ajp.150.4.546. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, Johnson J. Smoking, smoking cessation, and major depression. Journal of American Medical Association. 1990;264:1546–1549. [PubMed] [Google Scholar]
- Goldberg J, True W, Eisen SA, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: Ascertainment bias. Acta Geneticae Medicae et Gemellologae. 1987;36:67–78. doi: 10.1017/s0001566000004608. [DOI] [PubMed] [Google Scholar]
- Goldstein RB, Prescott CA, Kendler KS. Genetic and environmental factors in conduct problems and adult antisocial behavior among adult female twins. Journal of Nervous and Mental Disease. 2001;189:201–209. doi: 10.1097/00005053-200104000-00001. [DOI] [PubMed] [Google Scholar]
- Goodman E, Capitman J. Depressive symptoms and cigarette smoking among teens. Pediatrics. 2000;106:748–755. doi: 10.1542/peds.106.4.748. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: Results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Heath AC, Martin NG, Lynskey MT, Todorov AA, Madden PA. Estimating two-stage models for genetic influences on alcohol, tobacco or drug use initiation and dependence vulnerability in twin and family data. Twin Research. 2002;5:113–124. doi: 10.1375/1369052022983. [DOI] [PubMed] [Google Scholar]
- Henderson GH, Eisen SA, Goldberg J, True WR, Barnes JE, Vitek ME. The Vietnam Era Twin Registry: A resource for medical research. Public Health Reports. 1990;105:368–373. [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. Family transmission and heritability of externalizing disorders: A twin-family study. Archives of General Psychiatry. 2004;61:922–928. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Comorbidity and smoking. Nicotine and Tobacco Research. 1999;1:S149–152. doi: 10.1080/14622299050011981. [DOI] [PubMed] [Google Scholar]
- Jacobson KC, Neale CA, Prescott MC, Kendler KS. Cohort differences in genetic and environmental influences on retrospective reports of conduct disorder among adult male twins. Psychological Medicine. 2000;30:775–787. doi: 10.1017/s0033291799002561. [DOI] [PubMed] [Google Scholar]
- Jordan BK, Schlenger WE, Hough R, Kulka RA, Weiss D, Fairbank JA, Marmar CR. Lifetime and current prevalence of specific psychiatric disorders among Vietnam veterans and controls. Archives of General Psychiatry. 1991;48:207–215. doi: 10.1001/archpsyc.1991.01810270019002. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Davies M. Adult sequelae of adolescent depressive symptoms. Archives of General Psychiatry. 1986;43:255–262. doi: 10.1001/archpsyc.1986.01800030073007. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Davis CG, Kessler RC. The familial aggregation of common psychiatric and substance use disorders in the National Comorbidity Survey: A family history study. British Journal of Psychiatry. 1997;170:541–548. doi: 10.1192/bjp.170.6.541. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO., Jr Twin studies of adult psychiatric and substance dependence disorders: Are they biased by differences in the environmental experiences of monozygotic and dizygotic twins in childhood and adolescence? Psychological Medicine. 1998;28:625–633. doi: 10.1017/s0033291798006643. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A population-based twin study of major depression in women: The impact of varying definitions of illness. Archives of General Psychiatry. 1992;49:257–266. doi: 10.1001/archpsyc.1992.01820040009001. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, MacLean CJ, Heath AC, Eaves LJ, Kessler RC. Smoking and major depression. A causal analysis. Archives of General Psychiatry. 1993;50:36–43. doi: 10.1001/archpsyc.1993.01820130038007. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Killen JD, Robinson TN, Haydel KF, Hayward C, Wilson DM, Hammer LD, Litt IF, Taylor CB. Prospective study of risk factors for the initiation of cigarette smoking. Journal of Consulting and Clinical Psychology. 1997;65:1011–1016. doi: 10.1037//0022-006x.65.6.1011. [DOI] [PubMed] [Google Scholar]
- Krueger RF. The structure of common mental disorders. Archives of General Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. Journal of American Medical Association. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, Meyer JM, Toomey R, Faraone SV, Merla-Ramos M, Tsuang MT. A twin registry-based twin study of depression in men. Archives of General Psychiatry. 1998;55:468–472. doi: 10.1001/archpsyc.55.5.468. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, True WR, Eisen SA, Goldberg J, Meyer J, Faraone SV, Eaves LJ, Tsuang MT. Differential heritability of adult and juvenile antisocial traits. Archives of General Psychiatry. 1995;52:906–915. doi: 10.1001/archpsyc.1995.03950230020005. [DOI] [PubMed] [Google Scholar]
- Marmorstein NR, Iacono WG. Major depression and conduct disorder in a twin sample: Gender, functioning, and risk for future psychopathology. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:225–233. doi: 10.1097/00004583-200302000-00017. [DOI] [PubMed] [Google Scholar]
- McCaffery JM, Niaura R, Swan GE, Carmelli D. A study of depressive symptoms and smoking behavior in adult male twins from the NHLBI twin study. Nicotine and Tobacco Research. 2003;5:77–83. doi: 10.1080/14622200307259. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical modeling. 5. Richmond, VA: Department of Psychiatry, Virginia Commonwealth University; 1999. [Google Scholar]
- Paperwalla KN, Levin TT, Weiner J, Saravay SM. Smoking and depression. Medical Clinics of North America. 2004;88:1483–1494. doi: 10.1016/j.mcna.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Patton CG, Carlin JB, Koffey C, Wolfe R, Hibbert M, Bowes G. Depression, anxiety, and smoking initiation: A prospective study over 3 years. American Journal of Public Health. 1998;88:1518–1522. doi: 10.2105/ajph.88.10.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Cottler L, Goldring E. National Institute of Mental Health diagnostic interview schedule Version III Revised. St. Louis, MO: Department of Psychiatry, Washington University; 1988. [Google Scholar]
- Silberg J, Rutter M, D’Onofrio B, Eaves L. Genetic and environmental risk factors in adolescent substance use. Journal of Child Psychology and Psychiatry. 2003;44:664–676. doi: 10.1111/1469-7610.00153. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Dinwiddie SH, Madden PA, Bucholz KK, Dunne MP, Statham DJ, Martin NG. Modeling genetic and environmental influences in the etiology of conduct disorder: A study of 2,682 adult twin pairs. Journal of Abnormal Psychology. 1997;106:266–279. doi: 10.1037//0021-843x.106.2.266. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Madden PA, Bucholz KK, Statham DJ, Martin NG. Personality and the genetic risk for alcohol dependence. Journal of Abnormal Psychology. 2002;111:124–133. [PubMed] [Google Scholar]
- Slutske WS, True WR, Scherrer JF, Bucholz KK, Heath AC, Eisen SA, Goldberg J, Lyons MJ, Tsuang MT. Long-term reliability and validity of alcoholism diagnoses and symptoms in a large national telephone interview survey. Alcoholism Clinical and Experimental Research. 1998;54:1126–1128. doi: 10.1111/j.1530-0277.1998.tb04292.x. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata statistical software (Release 6.0) [Computer software] College Station, TX: Stata Corporation; 1999. [Google Scholar]
- True WR, Xian H, Scherrer JF, Madden PAF, Bucholz KK, Heath AC, Eisen SA, Lyons MJ, Goldberg J, Tsuang MT. Common genetic vulnerability for nicotine and alcohol dependence in men. Archives of General Psychiatry. 1999;56:655–661. doi: 10.1001/archpsyc.56.7.655. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Bar JL, Harley RM, Lyons MJ. The Harvard Twin Study of Substance Abuse: What we have learned. Harvard Review of Psychiatry. 2001;9:267–279. [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, Meyer JM, Toomy R, Faraone SV, Eaves L. Genetic influences on DSM-III-R drug abuse and dependence: A study of 3,372 twin pairs. American Journal of Medical Genetics (Neuropsychiatric Genetics) 1996;67:473–477. doi: 10.1002/(SICI)1096-8628(19960920)67:5<473::AID-AJMG6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, True W, Lin N, Toomy R, Eaves L. Co-occurrence of abuse of different drugs in men. Archives of General Psychiatry. 1998;55:967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- Williams JM, Ziedonis D. Addressing tobacco among individuals with a mental illness or an addiction. Addictive Behavior. 2004;29:1067–1083. doi: 10.1016/j.addbeh.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Windle M, Windle RC. Depressive symptoms and cigarette smoking among middle adolescents: Prospective associations and intrapersonal and interpersonal influences. Journal of Consulting and Clinical Psychology. 2001;69:215–226. [PubMed] [Google Scholar]
- Wu LT, Anthony JC. Tobacco smoking and depressed mood in late childhood and early adolescence. American Journal of Public Health. 1999;89:1837–1840. doi: 10.2105/ajph.89.12.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian H, Scherrer J, Eisen S, True W, Heath AC, Goldberg J, Lyons M, Tsuang MT. Self-reported zygosity and the equal environmental assumption for psychiatric disorders in the Vietnam Era Twin Registry. Behavior Genetics. 2000;30:303–310. doi: 10.1023/a:1026549417364. [DOI] [PubMed] [Google Scholar]