Abstract
Miniature pigs residing in the Ossabaw Island (Ossabaw pigs) exhibit a thrifty genotype, and when fed a high-calorie diet they consistently develop metabolic syndrome defined by obesity, insulin resistance, hypertension, and dyslipidemia. We conducted a study to induce steatohepatitis in Ossabaw pigs by dietary manipulation. Pigs were fed standard chow (controls, n = 15), high-fructose diet (20% kcal from fructose and 10.5% kcal from fat) (fructose group, n = 9), atherogenic diet (20% kcal from fructose and 46% kcal from fat and 2% cholesterol and 0.7% cholate by weight) (atherogenic diet group, n = 13), and modified atherogenic diet (different source of fat and higher protein but lower choline content) (M-Ath diet group, n = 7). All animals were sacrificed at 24 weeks after dietary intervention. The high-fructose group had significant weight gain, hypertension, and insulin resistance but showed normal liver histology. The atherogenic diet group had metabolic syndrome and abnormal liver histology consisting of significant microvesicular steatosis and fatty Kupffer cells but no ballooning or fibrosis. The M-Ath diet group developed severe metabolic syndrome and markedly abnormal liver histology with macrovesicular and microvesicular steatosis, fatty Kupffer cells, extensive hepatocyte ballooning, and pericellular/perisinusoidal fibrosis. Compared with controls, the M-Ath diet group had significantly lower serum adiponectin but higher serum leptin and tumor necrosis factor (TNF) levels and higher hepatic triglyceride and malondialdehyde levels.
Conclusion
Ossabaw pigs fed a modified atherogenic diet develop severe metabolic syndrome and abnormal liver histology with close resemblance to human nonalcoholic steatohepatitis (NASH).
Nonalcoholic fatty liver disease (NAFLD) is one of the most common chronic liver diseases in humans.1-4 Its histology is broadly categorized into simple steatosis and nonalcoholic steatohepatitis (NASH).1-4 Simple steatosis is generally believed to be benign and is histologically characterized by macrovesicular steatosis without additional signs of liver injury. However, NASH is considered to be a progressive condition and can cause advanced fibrosis, cirrhosis, and liver failure.1-4 Histologically, it is characterized by macrovesicular steatosis along with varying degrees of lobular inflammation, balloon degeneration, and pericellular fibrosis.5,6 It remains unclear why some patients exhibit only simple steatosis whereas others with a comparable risk profile exhibit steatohepatitis. A two-hit hypothesis was previously postulated as a conceptual framework for understanding the pathogenesis of NASH,7 in which a first hit leads to the development of steatosis, and subsequently one or more second hits lead to the development of steatohepatitis. However, the validity of this oversimplified concept and the very relevance of macrovesicular steatosis in the development of steatohepatitis have been questioned recently.8,9
Ossabaw Island, off the coast of Georgia near Savannah, is home to a feral breed of swine that were left by Spaniards nearly 500 years ago (Ossabaw pigs).10 In the wild, Ossabaw pigs are typically miniature in size and exhibit a thrifty genotype, which allows them to store large amounts of fat during feasting and to survive long periods of famine.10 To protect the island’s loggerhead turtles, the Georgia Department of Natural Resources had adopted a policy in 2000 to eradicate all of its Ossabaw pigs.10 Recognizing the scientific value of these pigs, one of the authors (M.S.) led an expedition that trapped and exported 26 disease-free animals to the mainland.10 Sturek and his colleagues from Indiana and Purdue Universities have subsequently established a breeding colony and published a number of seminal studies to show that Ossabaw pigs serve as an excellent model for investigating metabolic syndrome, progression to type 2 diabetes, and long-term complications including coronary artery disease.10,11
In general, Ossabaw pigs when fed high-fat and high-calorie diets develop obesity, insulin resistance, glucose intolerance, dyslipidemia, and hypertension—in other words, metabolic syndrome—in a relatively consistent fashion.10,11 Because obesity and insulin resistance are known risk factors for NAFLD, and the entire constellation of components of metabolic syndrome is so closely linked to human NAFLD, we examined the histology of frozen liver samples from 38 pigs that participated in two previous experiments. In these experiments, Ossabaw pigs consumed for 55 weeks either standard chow or an excess calorie atherogenic diet composed of 6% to 8% kcal from protein, 19% kcal from complex carbohydrates, and 46% to 75% kcal from hydrogenated soy bean oil (predominantly trans fatty acids) and 2% cholesterol and 0.7% cholate by weight. All animals were sacrificed at the conclusion of the study, and as published previously, animals receiving atherogenic diet exhibited marked metabolic syndrome.12,13 However, to our surprise, despite these striking metabolic abnormalities, pigs on the atherogenic diet had normal serum liver biochemistries and normal liver histology (data not shown). This indicated that hydrogenated soybean oil–containing atherogenic diets without fructose do not cause liver injury in Ossabaw pigs. Therefore, we conducted a study in which Ossabaw pigs were subjected to different diets for 24 weeks, and our primary objective was to induce liver injury mimicking human NASH in association with metabolic syndrome.
Materials and Methods
All protocols involving animals were approved by an Institutional Animal Care and Use Committee and complied with the recommendations outlined by the National Research Council and the American Veterinary Medical Association Panel on Euthanasia.12,13
Male and female Ossabaw miniature swine, aged 5 to 10 months at the start of the study, were divided into the following four groups for 24 weeks of treatment:
Standard Chow (Control Group, n = 15)
Pigs were fed standard chow consisting of 18.5% calories from protein, 71% calories from carbohydrates, and 10.5% calories from fat for 24 weeks. Hydrogenated soybean oil was the source of the fat calories, and this diet included normal levels of methionine and choline at concentrations of 3500 ppm and 1500 ppm, respectively (Table 1).
Table 1.
Ossabaw-6 Experiment: Study Groups and Diet Composition
| Control Chow (n = 15) |
Fructose Group (n = 9) |
Atherogenic Diet Group (n = 13) |
M-Ath Diet Group (n = 7) |
|
|---|---|---|---|---|
| Average energy intake (kcal/day) | 2500 | 6000 | 6000 | 6000 |
| Carbohydrates (%)* | 71 | 72 | 43 | 40.5 |
| Starch (%) | 41.6 | 51 | 25.2 | 12.4 |
| Sucrose (%) | 1.5 | 1.3 | 0.9 | 1.04 |
| Fructose (%) | 0.5 | 20.0 | 20 | 17.8 |
| Glucose (%) | 0.4 | 0.3 | 0.2 | 4.3 |
| Protein (%)† | 18.5 | 18.5 | 8.0 | 16.5† |
| Fat (%)†fat source | 10.5 | 10.5 | 46.0 | 43.0 |
| Hydrogenated soybean oil | Hydrogenated soybean oil | Hydrogenated soybean oil | Hydrogenated soybean oil, coconut oil, lard | |
| Cholesterol | Nil to negligible | Nil to negligible | 2% by weight | 2% by weight |
| Sodium Cholate | Nil to negligible | Nil to negligible | 0.7% by weight | 0.7% by weight |
| Methionine (ppm) | 3500 | 2800 | 2100 | 3500 |
| Choline (ppm) | 1500 | 1200 | 900 | 700 |
Values represent percent of total daily calories.
M-Ath diet had additional 8% kcal from casein.
Fructose and M-Ath diets were specially formulated by Purina TestDiet, Inc., Richmond, IN, and are available commercially as item numbers 5KA6 and 5B4L, respectively.
Fructose Group (n = 9)
These pigs received high-fructose but normal-fat diet for 24 weeks (5KA6; custom formulated by Purina TestDiet, Inc., Richmond, IN). This diet provided 20% calories from fructose and 10.5% calories from fat derived from hydrogenated soybean oil. It provided methionine and choline at concentrations of 2800 ppm and 1200 ppm, respectively (Table 1).
Atherogenic Diet Group (n = 13)
These pigs received high fructose–containing atherogenic diet for 24 weeks with 18% calories from fructose, 43% calories from fat, and 8% calories from protein. Hydrogenated soybean oil was the source of fat calories. It provided methionine and choline at concentrations of 2100 ppm and 900 ppm, respectively. This diet consisted of 2% cholesterol and 0.7% sodium cholate by weight (Table 1).
Modified Atherogenic Diet Group (M-Ath Group) (n = 7)
These pigs received fructose-based atherogenic diet that was modified in terms of source of fat, protein content, and choline concentration (5B4L; custom formulated by Purina TestDiet, Inc., Richmond, IN). It provided 46% calories from fat, 20% calories from fructose, and 16.5% calories from protein. Fat calories were provided by an admixture of hydrogenated soybean oil, coconut oil and lard, and an additional 8.5% calories from casein, and it provided methionine and choline at 3500 ppm and 700 ppm concentrations, respectively. It consisted of 2% cholesterol and 0.7% sodium cholate by weight (Table 1).
On average, control chow pigs consumed 2500 kcal standard chow per day, whereas pigs belonging to the other three groups consumed 6000 kcal diet daily. All animals enjoyed free access to feed during an approximately 6-hour feeding period each day and unlimited access to water. At the end of the study, all animals were sacrificed by excision of the heart under general anesthesia according to protocol described elsewhere.14,15
Body weights of all animals were obtained at the beginning of the study and thereafter at weekly time intervals until the animals were euthanized. Anatomical measurements were additionally obtained at the end of the dietary treatment period using a previously published method.14,15 Blood pressures were monitored weekly by using tail cuff sphygmomanometer. An intravenous glucose tolerance test was conducted on each pig 1 week before sacrifice. Animals were given an intravenous bolus of 1.0 g glucose/kg body weight, and subsequent samples were obtained at 5, 10, 20, 30, 40, 50, and 60 minutes after injection.
Plasma/Serum Measurements
Plasma samples were analyzed for triglycerides, total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and nonesterified fatty acids using standard methods. Serum liver biochemistries were measured before euthanasia by a local clinical laboratory (Antech Diagnostics, Fishers, IN). Fasting serum levels of leptin and tumor necrosis factor alpha (TNF-α) were performed by a commercial laboratory (Millipore Corp., St. Charles, MO). Serum adiponectin was measured using mass spectrometry by Monarch Laboratories (Indianapolis, IN), and it was expressed as protein intensity.
Determination of Hepatic Triglyceride, Malondialdehyde, and Trolox Equivalent Antioxidant Capacity
As previously described,16 the Infinity Triglyceride Kit (Thermo Electron, Louisville, CO) was used to analyze the homogenates for triglycerides using spectrophotometric assay. The triglyceride content was normalized with the protein content of the liver sample, which was measured by Lowry assay. Sample measurements were carried out in duplicates. Malondialdehyde (MDA) levels in serum and liver tissue homogenates were measured using high-performance liquid chromatography with ultraviolet detection as described previously with some modifications.17 The total antioxidant capacity in serum and liver tissue homogenates were measured using the Trolox Equivalent Antioxidant Capacity described previously, with some modifications.18
Fatty Acid Methyl Esters
The analysis of fatty acids in diets and serum and live tissues was conducted by the gas liquid chromatography. As previously described, lipids in liver, serum, and diet samples were extracted with chloroform:methanol (2:1, vol/vol), saponified, and fatty acid methyl esters prepared using boron trifluoride in methanol (10% wt/vol, Supelco Bellefonte, PA) following the method of Watkins et al. with some modification.19 The fatty acid methyl esters in samples were identified based on the retention time determined from authentic standards (Nu-Chek-Prep Inc., Elysian, MN), and results are presented as area percentages.
Tissue Preparation and Histological Grading
At the time of sacrifice, a portion of the liver left lobe was collected in 2-mL vials, flash-frozen in liquid nitrogen, and stored in −80°C freezers. Frozen liver tissue was fixed in 10% buffered formalin, processed and embedded in paraffin for hematoxylin-eosin (H&E), trichrome, periodic acid-Schiff, oil red-O staining The stains were examined by light microscopy and blindly scored by two expert pathologists (one veterinary and one human hepatopathologist). Macrovesicular and microvesicular steatosis (none [<5%], mild [5%-33%], moderate [34%-66%], severe [>66%]), inflammation (none, mild, moderate, severe), hepatocellular ballooning (none, mild, moderate, severe), fibrosis (none, mild, moderate, severe), Kupffer cell vacuolization (none, mild, moderate, severe), and Kupffer cell fat accumulation (none, mild, moderate, severe) were systematically recorded. Lysosomal staining was conducted to characterize Kupffer cell accumulation. Zonal distribution of each of these variables was also systematically recorded.
Results
Table 2 shows selected characteristics of Ossabaw pigs belonging to different groups. Compared with control pigs, animals in the other three groups had significant weight gain (P < 0.001), although the fructose group gained more weight than the atherogenic and M-Ath diet groups (P < 0.001 for both comparisons). The weight gain between atherogenic and M-Ath diet groups was not significantly different (P = 0.11).
Table 2.
Selected Characteristics at Sacrifice of Pigs Belonging to Different Study Groups
| Control Chow (n = 15) |
Fructose Group (n = 9) |
Atherogenic Diet Group (n = 13) |
M-Ath Diet Group (n = 7) |
|
|---|---|---|---|---|
| Sex (M/F) | 6/9 | 9/0 | 5/8 | 0/7 |
| Weight at sacrifice (kg) | 56.1 ± 2.8 | 97.7 ± 8.6* | 82.5 ± 4.8*,‡ | 87.2 ± 12.6*,‡ |
| Mean weight gain (kg) | 14.7 ± 1.4 | 52.8 ± 7.3* | 36.8 ± 5.3*,‡ | 37.7 ± 11.8*,‡ |
| Body circumference at sacrifice (cm) | 83.9 ± 2.7 | 117.7 ± 5.0* | 112.5 ± 2.2*,‡ | 121.9 ± 9.2*,†,‡ |
| Blood pressure (mmHg) | ||||
| Systolic | 110.4 ± 1.6 | 140.5 ± 2.5 | 147.7 ± 2.7* | 158.2 ± 4.0*,‡ |
| Diastolic | 62.0 ± 2.0 | 91.6 ± 2.3 | 94.2 ± 2.0* | 104.3 ± 3.0*,‡ |
| Serum chemistry profile | ||||
| AST (IU/L) | 33 ± 4 | 30 ± 6 | 32 ± 5 | 100 ± 21*,†,‡ |
| ALT (IU/L) | 40 ± 5 | 18 ± 1* | 29 ± 2‡ | 41 ± 12‡ |
| Alkaline Phosphatase (IU/L) | 71 ± 7 | 60 ± 9 | 112 ± 10*,‡ | 273 ± 110*,†,‡ |
| Bilirubin (mg/dL) | 0.2 ± 0.02 | 0.2 ± 0.03 | 0.2 ± 0.02 | 0.3 ± 0.04* |
| Serum glycemic measures | ||||
| Glucose, fasting(mg/dL) | 77.3 ± 2.4 | 83.5 ± 2.6 | 88.5 ± 5.6* | 87.4 ± 5.9* |
| Insulin, fasting serum (mg/dL) | 10 ± 2 | 15 ± 2 | 14 ± 1 | 18 ± 3* |
| HOMA | 2.0 ± 0.4 | 3.0 ± 0.6 | 2.9 ± 0.3* | 3.9 ± 0.77* |
| Peak insulin (by IVGTT , mg/dL) | 92 ± 11 | 143 ± 27* | 116 ± 15 | 172 ± 36* |
| Plasmalipids | ||||
| Cholesterol (mg/dL) | 72.6 ± 4.5 | 63.0 ± 4.6* | 393.5 ± 30.5*,‡ | 643 ± 67.2*,†,‡ |
| Triglycerides (mg/dL) | 23.8 ± 2.4 | 29.0 ± 2.7 | 44.2 ± 3.0*,‡ | 120 ± 18.3*,†,‡ |
| LDL (mg/dL) | 28.7 ± 3.4 | 25.3 ± 2.8* | 279.3 ± 23.2*,‡ | 533.8 ± 63.9*,†,‡ |
| HDL (mg/dL) | 39.2 ± 3.3 | 31.9 ± 3.0 | 88.1 ± 6.1*,‡ | 85.3 ± 5.5*,‡ |
| NEFA (serum) (mmol/L) | 0.41 ± 0.153 | 0.96 ± 0.20 | 0.92 ± 0.123 | 1.03 ± 0.32 |
| Serum hormones | ||||
| Adiponectin, serum (qauc)§ | 16,801 ± 1138 | Not done | 13,818 ± 807 | 13,296 ± 663* |
| Leptin, serum (ng/dL) | 2 ± 0.2 | 4 ± 1 | 3 ± 1 | 17 ± 5*,†,‡ |
| TNF-α, serum (pg/mL) | 35.4 ± 4.5 | 41.9 ± 11.9 | 41.7 ± 2.4 | 72.9 ± 15.9* |
| Serum MDA (μM) | 1.46 ± 0.08 | 1.75 ± 0.19 | 2.18 ± 0.14 | 3.00 ± 1.35 |
Data shown as mean ± standard error.
P < 0.05 when compared with the control chow group.
P < 0.05 when compared with the atherogenic diet group.
P < 0.05 when compared with the fructose group.
Expressed as protein intensity.
AST, aspartate aminotransferase; ALT, alanine aminotransferase; HOMA, Homeostatic Model Assessment Method; LDL, low-density lipoprotein; HDL, high-density lipoprotein; NEFA, nonesterified fatty acids; TNF-α, tumor necrosis factor-alpha; MDA, malondialdehyde.
Fructose Group
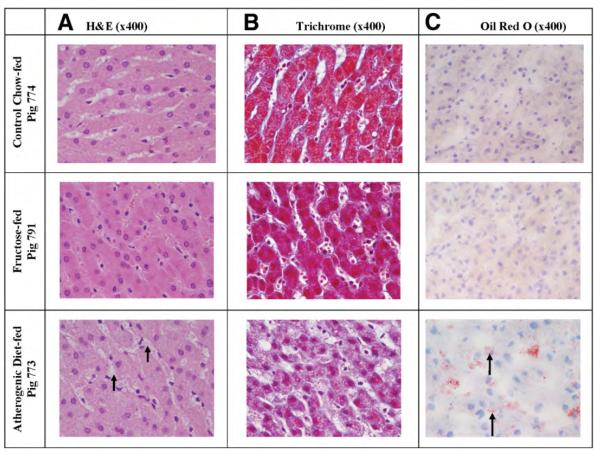
The pigs belonging to this group had the most weight gain (Fig. 1), but compared with the control group their fasting levels of insulin (P = 0.14), glucose (P = 0.1), homeostasis model assessment (HOMA) (P = 0.12), total cholesterol (P = 0.17), LDL (P = 0.5), HDL (P = 0.15), and triglyceride (P = 0.18) were not different. However, these pigs exhibited evidence of insulin resistance on intravenous glucose tolerance test and also had hypertension (Table 2). In essence, pigs in the high-fructose group had limited metabolic syndrome without dyslipidemia. These pigs had significantly higher fasting levels of free fatty acids compared with the control group (0.4 ± 0.15 versus 0.96 ± 0.2 mmol/L, P = 0.04). Compared with controls, these animals had significantly higher levels of serum leptin (3.6 ± 2.8 versus 1.5 ± 0.5 ng/dL, P = 0.03) but not serum TNF-α (P = 0.5) or MDA (P = 0.1) levels. Serum adiponectin levels were not measured in these animals. Serum levels of aspartate aminotransferase (AST), alkaline phosphatase, and total bilirubin were not different between the standard chow and fructose groups, but interestingly, the fructose group had significantly lower levels of alanine aminotransferase (ALT) than the control chow group (18.3 ± 3.6 versus 40 ± 2 IU/L, P = 0.008). Liver histology of pigs in the fructose group was indistinguishable from that of control pigs. Pigs in neither group showed steatosis, ballooning, or fibrosis (Figs. 2, 3).
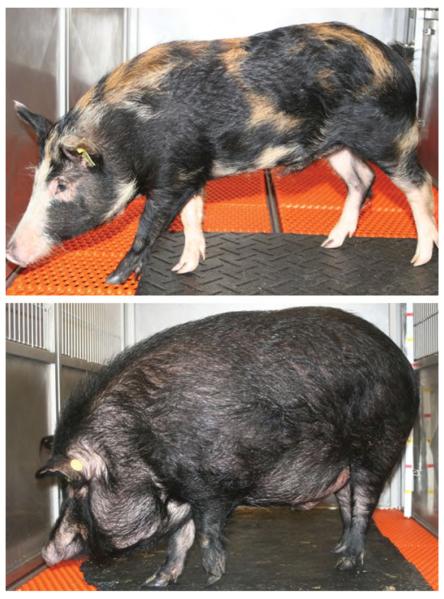
Fig. 1.
(Bottom) M-Ath diet fed Ossabaw pigs are significantly obese in comparison to (top) Ossabaw pigs maintained on a standard chow diet.
Fig. 2.
Standard chow controls (Pig 774). No fat accumulation is seen on H&E (A) or trichrome (B), and oil red O stains (C) show no abnormal findings. Fructose group (Pig 791): No fat accumulation is seen on H&E (A) or trichrome (B), and oil red O stains (C) show no abnormal findings. Atherogenic diet group (Pig 773): (A and B) H&E and trichrome stains show foamy change involving Kupffer cells (short arrows). (C) Oil red O shows fat in Kupffer cells (long arrows) and in hepatocytes. (D) Trichrome stain does not show fibrosis.
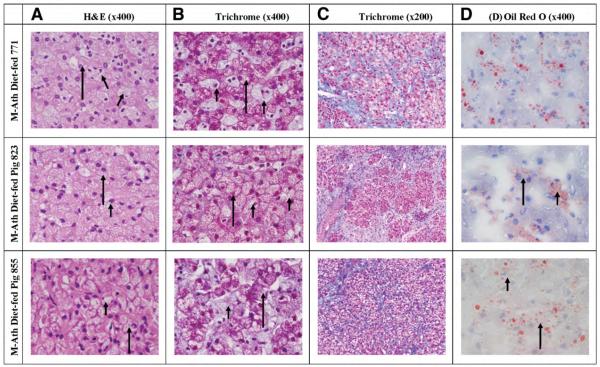
Fig. 3.
Liver histology of Ossabaw Pigs belonging to the modified atherogenic (M-Ath) diet. Pig 771: (A and B) H&E and trichrome stains show extensive foamy change involving both hepatocytes (long arrows) and Kupffer cells (short arrows). (C) Trichrome stain shows fibrous tissue emanating from septa and depositing in a pericellular distribution. (D) Oil red O shows macrovesicular and microvesicular fat, in both hepatocytes and Kupffer cells. Many of the larger droplets are probably in Ito cells. Pig 823: (A and B) H&E and trichrome stains show extensive foamy change involving both hepatocytes (long arrows) and Kupffer cells (short arrows). (C) Trichrome stain shows fibrous tissue emanating from septa and depositing in a pericellular distribution. (D) Oil red O shows fat in both hepatocytes (long arrows) and Kupffer cells (short arrows). Many of the larger droplets are probably in Ito cells. Pig 855: (A and B) H&E and trichrome stains show extensive foamy change involving both hepatocytes (long arrows). (C) Trichrome stain shows fibrous tissue emanating from septa and depositing in a pericellular distribution. (D) Oil red O shows fat in hepatocytes (long arrows) and Kupffer cells (short arrows). Many of the larger droplets are probably in Ito cells.
Atherogenic Diet Group
Pigs belonging to this group had significantly greater weight gain than the control group, but their weight gain was not as pronounced as in the fructose group (Table 2). Compared with controls, pigs in the high-fat group had significantly higher fasting serum levels of insulin (P = 0.048) and HOMA values (P = 0.02) but not glucose (P = 0.07). Not surprisingly, fasting serum levels of total cholesterol, LDL, HDL, and triglycerides of pigs in the atherogenic diet group were dramatically higher than in the control and fructose groups (Table 2). Serum free fatty acid levels were significantly higher than in the control chow group (0.92 ± 0.1 versus 0.41 ± 0.15 mmol/L, respectively; P = 0.018), but not different from the fructose group (0.92 ± 0.1 versus 0.96 ± 0.1 mmol/L, respectively; P = 0.8). Serum leptin levels in the atherogenic diet group were significantly higher than in controls (P = 0.017), but not compared with the fructose group (P = 0.85) (Table 2). Serum adiponectin levels were lower compared with the control chow pigs, but this difference was of borderline statistical significance (P = 0.06). Serum TNF-α levels were not significantly elevated in the atherogenic diet group when compared with the control group or the fructose group. Compared with controls, serum MDA levels of pigs in the atherogenic diet group were significantly higher (P < 0.001), but the difference in serum MDA levels between the atherogenic diet and the fructose groups was of borderline statistical significance (2.2 ± 0.13 versus 1.74 ± 0.2 μM, respectively; P = 0.07). Serum AST, ALT, and total bilirubin levels of pigs in the atherogenic diet group were not higher than pigs in the control and fructose groups, but the alkaline phosphatase level was significantly higher (Table 2).
Liver histology showed that all pigs in the atherogenic diet group had microvesicular steatosis occupying 80% to 100% of hepatocytes and foamy-appearing Kupffer cells with small droplets of fat. One pig showed trivial macrovesicular steatosis (approximately 5%) of hepatocytes, and another pig showed diffuse hepatocyte ballooning. None of the pigs showed inflammation or fibrosis (Fig. 3).
M-Ath Diet Group
Pigs belonging to this group had significantly greater weight gain than the control chow group, but their weight gain was not different from that of pigs in the atherogenic diet group (Table 2). Serum insulin and HOMA values and lipid measures (total cholesterol, LDL, HDL, and triglycerides) were significantly higher in the M-Ath diet group than in controls (Table 2). Serum fasting free fatty acid levels of pigs in the M-Ath diet group was significantly higher than the control group (1.03 ± 0.3 versus 0.41 ± 0.15 mmol/L, respectively; P = 0.02), but not significantly different from the fructose or atherogenic diet groups (Table 2). Compared with the control group, these pigs had significantly lower levels of serum adiponectin levels and higher levels of leptin and TNF-α (Table 2). Serum adiponectin negatively correlated with HOMA in this group, but this was of borderline statistical significance (r = −0.58, P = 0.1). However, the negative association between serum adiponectin and HOMA in all three groups combined (control chow, atherogenic, and M-Ath groups) was statistically significant (r = −0.60, P = 0.01 Spearman correlation). Serum MDA levels in the M-Ath diet group were statistically not higher than in the other three groups, although the difference between the M-Ath diet and the control groups was of borderline statistical significance (P = 0.07). Serum AST, alkaline phosphatase, and total bilirubin levels of the M-Ath diet group were significantly higher than the control chow group, but ALT values were not different (Table 2).
Weight gain or measures of insulin resistance measures were not different between atherogenic and M-Ath groups. Animals in the M-Ath group had significantly higher total cholesterol, LDL, and triglycerides than those in atherogenic diet group, but HDL or free fatty acid levels were not different (Table 2). Compared with the atherogenic diet group, M-Ath diet–fed pigs had significantly higher AST and alkaline phosphatase but not ALT or total bilirubin. Although serum adiponectin was not different, pigs in the M-Ath diet group had significantly higher levels of serum leptin and TNF-α levels than those in the atherogenic diet group (Table 2).
Of seven pigs in the M-Ath diet group, three pigs exhibited macrovesicular steatosis: one pig with 40% and two other pigs with approximately 10% macrovesicular steatosis (Table 3, Fig. 4). All pigs exhibited microvesicular steatosis involving 40% to 80% of hepatocytes. Six pigs showed extensive ballooning changes in most of the hepatocytes, whereas one showed focal ballooning (Table 3, Fig. 4). All pigs showed foamy appearance in all visualized Kupffer cells, whereas microvesicular steatosis in 50% to 80% of visualized Kupffer cells (Fig. 5). Sinusoidal fibrosis was present in all seven pigs, with focal distribution in two pigs and more extensive in the other five pigs. The fibrosis was predominantly in the periportal region originating from the septa that anatomically define pig hepatic lobules. No pigs developed bridging fibrosis or cirrhosis (Table 3, Fig. 4).
Table 3.
Summary of Liver Histology of Ossabaw-6 Pigs at Sacrifice
| Control Chow (n = 15) |
Fructose group (n = 9) |
Atherogenic Diet Group (n = 13) |
M-Ath Diet Group (n = 7) |
|
|---|---|---|---|---|
| Macrovesicular steatosis | – | – | 1 of 13 pigs | 3 of 7 pigs |
| Microvesicular steatosis | – | – | All pigs | All pigs |
| Hepatocyte ballooning | – | – | 1 of 13 pigs | All pigs |
| Kupffer cell vacuoles | – | – | All pigs | All pigs |
| Kupffer cell fat | – | – | All pigs | All pigs |
| Inflammation | – | – | – | 4 of 7 pigs |
| Fibrosis | – | – | – | All pigs |
See text, figures, and supporting material for additional description of liver histology.
Fig. 4.
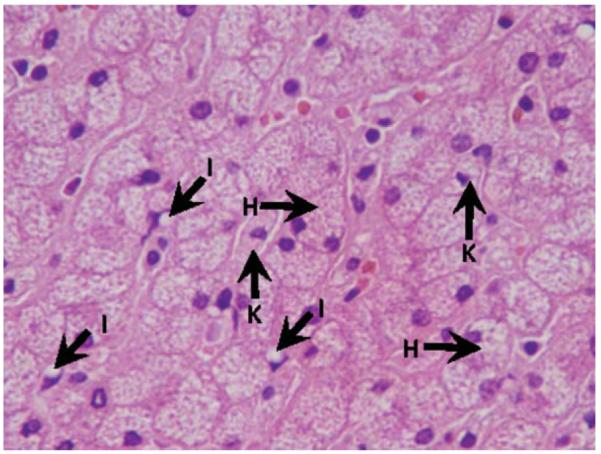
Extensive foamy change in hepatocytes and Kupffer cells induced by modified atherogenic diet (H&E stain). H, hepatocyte; I, Ito cell; K, Kupffer cell.
Fig. 5.
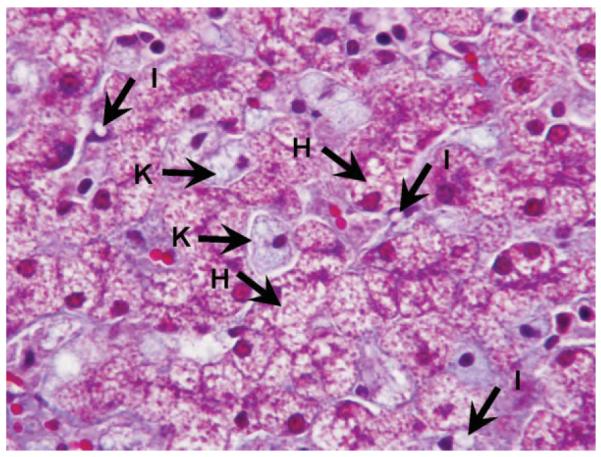
Extensive foamy change in hepatocytes and Kupffer cells induced by modified atherogenic diet (Trichrome stain). H, hepatocyte; I, Ito cell; K, Kupffer cell.
Liver Tissue Analysis
Hepatic triglyceride levels in the atherogenic and M-Ath diet groups were significantly higher than in the other two groups, but they were not different between themselves (Table 4). Hepatic triglyceride levels between the control chow and fructose groups were not statistically different. Hepatic MDA and Trolox Equivalent Antioxidant Capacity levels are shown in Table 4. MDA levels were significantly lower in the control chow group compared with other treatments, but they were not different among fructose, high-fat, and test-diet groups. There was no statistically significant difference in Trolox Equivalent Antioxidant Capacity between any of the groups. Hepatic fatty acid methyl ester data are shown in the following section.
Table 4.
Triglyceride, MDA, and TEAC Measurements in Liver Tissue
| Control Chow (n = 15) |
Fructose Group (n = 9) |
Atherogenic Diet Group (n = 13) |
M-Ath Diet Group (n = 7) |
|
|---|---|---|---|---|
| Triglycerides (μg/mg protein) | 47 ± 3 | 56 ± 9 | 98 ± 19* | 110 ± 19*,† |
| MDA (nmol/pg) | 0.27 ± 0.05 | 0.51 ± 0.11 | 0.48 ± 0.06* | 0.56 ± 0.08* |
| TEAC (μmol/mg protein) | 0.27 ± 0.004 | 0.30 ± 0.01* | 0.31 ± 0.01* | 0.33 ± 0.01* |
| MDA/TEAC | 1.01 ± 0.19 | 1.74 ± 0.41 | 1.55 ± 0.18* | 1.67 ± 0.21* |
Values represent means (standard error).
P < 0.05 when compared with control chow group,
P < 0.05 when compared with fructose group.
MDA, malondialdehyde; TEAC, Trolox equivalent antioxidant capacity.
Fatty Acid Composition
The fatty acid composition of serum from pigs is presented in Table 5. The results in serum generally reflect the fatty acid composition of the dietary lipids fed during the study (data shown in Supporting Table 1). The serum of pigs fed the control chow had the highest amount of 18:2n6, arachidonic acid (20:4n6), total polyunsaturated fatty acids (PUFA), n-6 PUFA, ratio of saturated to monounsaturated fatty acids, and ratio of long chain n-6/n-3 PUFA (Table 5). As expected, the M-Ath diet resulted in the highest amounts of 12:0, 14:0, and 18:1n9 and the highest ratio of Mono/PUFA and ratio of Saturated/PUFA (Table 5). Total PUFA and n-6 PUFA values were lowest in the serum of pigs given the M-ATG diet. Trans 18:1 fatty acids, present in hydrogenated soybean oil used in all diets, resulted in the detection of these isomeric forms in the serum from most of the groups. Most of the fatty acid values in serum of pigs fed on fructose and atherogenic diets were intermediate compared with the amounts found in the control and M-ATG diet groups (Table 5).
Table 5.
Fatty Acid Composition of Serum Lipids from Ossabaw Pigs Fed the Experimental Diets
| Fatty Acids | Control | Fructose Group | Atherogenic Diet | M-Ath Group | ANOVA P Value |
|---|---|---|---|---|---|
| 12:0 | ND* ± 0.01 | 0.01* ± 0.01 | ND* ± ND | 0.15† ± 0.10 | <0.0001 |
| 14:0 | 0.23*‡ ± 0.07 | 0.38* ± 0.08 | 0.18‡ ± 0.05 | 0.86† ± 0.20 | <0.0001 |
| 15:0 | 0.13† ± 0.02 | 0.06* ± 0.02 | 0.04* ± 0.04 | 0.07* ± 0.04 | 0.0007 |
| 16:0 | 13.98† ± 1.33 | 14.20† ± 0.99 | 10.59* ± 0.99 | 14.38† ± 1.90 | 0.0003 |
| 16:1t | 0.45 ± 0.11 | 0.48 ± 0.07 | 0.53 ± 0.10 | 0.57 ± 0.10 | 0.13 |
| 16:1n7 | 0.41‡ ± 0.13 | 1.00* ± 0.17 | 0.75*‡ ± 0.11 | 2.06† ± 0.38 | <0.0001 |
| 17:0 | 0.55† ± 0.08 | 0.30* ± 0.07 | 0.21* ± 0.04 | 0.29* ± 0.08 | <0.0001 |
| 17:1n7 | 0.14* ± 0.05 | 0.19* ± 0.07 | 0.10* ± 0.02 | 0.33† ± 0.08 | <0.0001 |
| 18:0 | 15.07† ± 1.64 | 16.46† ± 1.39 | 12.75* ± 1.14 | 12.45* ± 0.50 | <0.0001 |
| 18:1t | 0.13 ± 0.12 | 0.06 ± 0.18 | 1.54 ± 2.17 | ND ± ND | 0.04 |
| 18:1n9 | 14.57‡ ± 1.85 | 25.66* ± 2.81 | 27.71* ± 2.79 | 32.74† ± 1.94 | <0.0001 |
| 18:1n7 | 1.66d ± 0.22 | 2.13‡ ± 0.19 | 3.87† ± 0.14 | 3.54* ± 0.10 | <0.0001 |
| 18:1 | 0.04‡ ± 0.04 | 0.07‡ ± 0.10 | 2.83† ± 0.23 | 1.62* ± 0.39 | <0.0001 |
| 18:2n6 | 39.23† ± 2.17 | 29.74* ± 1.67 | 27.89* ± 2.58 | 21.22‡ ± 2.50 | <0.0001 |
| 18:3n6 | 0.28† ± 0.07 | 0.12* ± 0.03 | 0.19†* ± 0.05 | 0.19* ± 0.08 | 0.001 |
| 18:3n3 | 0.87† ± 0.07 | 0.80†* ± 0.09 | 0.49‡ ± 0.05 | 0.69* ± 0.11 | <0.0001 |
| 20:0 | 0.03 ± 0.04 | 0.06 ± 0.05 | 0.03 ± 0.03 | 0.08 ± 0.19 | 0.78 |
| 20:1n9 | 0.16†* ± 0.05 | 0.25† ± 0.03 | 0.08* ± 0.05 | 0.13* ± 0.10 | 0.0002 |
| 20:2n6 | 0.66† ± 0.13 | 0.54†* ± 0.14 | 0.32‡ ± 0.06 | 0.43*‡ ± 0.07 | 0.0005 |
| 20:3n6 | 0.40* ± 0.12 | 0.68† ± 0.14 | 0.93† ± 0.23 | 0.71† ± 0.09 | 0.0002 |
| 20:4n6 | 8.12† ± 2.58 | 4.18* ± 1.01 | 2.94* ± 0.48 | 3.25* ± 0.96 | <0.0001 |
| 20:3n3 | 0.12†* ± 0.05 | 0.16† ± 0.04 | 0.01‡ ± 0.03 | 0.07*‡ ± 0.06 | 0.0002 |
| 20:5n3 | 0.25† ± 0.10 | 0.18†* ± 0.07 | 0.15†* ± 0.02 | 0.10* ± 0.06 | 0.01 |
| 22:0 | 0.01 ± 0.03 | 0.02 ± 0.04 | 0.01 ± 0.03 | ND ± ND | 0.55 |
| 22:1n9 | 0.01 ± 0.03 | ND ± 0.01 | ND ± ND | ND ± ND | 0.45 |
| 22:4n6 | 0.28† ± 0.06 | 0.21†* ± 0.09 | 0.14* ± 0.02 | 0.20†* ± 0.06 | 0.036 |
| 22:5n6 | 0.02 ± 0.03 | 0.01 ± 0.02 | ND ± ND | ND ± ND | 0.12 |
| 22:5n3 | 0.83† ± 0.32 | 0.64†* ± 0.28 | 0.41* ± 0.09 | 0.48†* ± 0.20 | 0.045 |
| 22:6n3 | 0.22 ± 0.05 | 0.47 ± 0.29 | 0.38 ± 0.13 | 0.21 ± 0.17 | 0.08 |
| TOTS | 30.02† ± 2.44 | 31.50† ± 1.95 | 23.80* ± 1.83 | 28.27† ± 2.04 | <0.0001 |
| TOTM | 17.59‡ ± 2.04 | 29.84* ± 2.95 | 37.40† ± 1.91 | 41.00† ± 2.06 | <0.0001 |
| PUFA | 51.29† ± 4.03 | 37.73* ± 2.11 | 33.85* ± 2.39 | 27.55‡ ± 2.74 | <0.0001 |
| n-6 PUFA | 48.99† ± 3.95 | 35.48* ± 1.89 | 32.41* ± 2.42 | 26.00‡ ± 2.66 | <0.0001 |
| n-3 PUFA | 2.30† ± 0.39 | 2.25†* ± 0.63 | 1.44‡ ± 0.18 | 1.55*‡ ± 0.25 | 0.004 |
| n-6 LC | 8.42† ± 2.63 | 4.40* ± 1.06 | 3.08* ± 0.49 | 3.45* ± 1.00 | <0.0001 |
| n-3 LC | 1.31 ± 0.43 | 1.29 ± 0.62 | 0.94 ± 0.19 | 0.79 ± 0.28 | 0.14 |
| TOTS/TOTM | 1.72† ± 0.14 | 1.07* ± 0.18 | 0.64‡ ± 0.06 | 0.69‡ ± 0.06 | <0.0001 |
| TOTM/PUFA | 0.35d ± 0.07 | 0.80‡ ± 0.11 | 1.11* ± 0.12 | 1.51† ± 0.21 | <0.0001 |
| TOTS/PUFA | 0.59‡ ± 0.09 | 0.84* ± 0.07 | 0.71*‡ ± 0.09 | 1.04† ± 0.15 | <0.0001 |
| n-6 LC/n-3 LC | 6.60† ± 1.98 | 3.83* ± 1.41 | 3.35* ± 0.52 | 4.49* ± 0.45 | 0.002 |
| n-6/n-3 | 21.75†* ± 3.32 | 16.70* ± 3.86 | 22.80† ± 3.48 | 17.01* ± 2.30 | 0.007 |
n = 9 for the Fructose group, n = 6 for the M-Ath group, n = 5 for Control and Atherogenic groups.
Values in rows with different symbols are significantly different by one-way ANOVA. The superscripts indicate what mean values are different in the rows, and the P values are for all groups.
The fatty acid composition of lipids from liver tissue is shown in Table 6. The patterns of liver fatty acid composition of pigs from different groups are similar to those of serum as described previously. In liver of pigs fed the M-ATG diet, the levels of 12:0 and 14:0 were the highest compared with all the other groups. The amount of 18:1n9 and the essential fatty acid 18:2n6 and totals of PUFA and n-6 PUFA were lowest in the liver lipids of pigs fed the atherogenic and M-ATG diets compared with the control and fructose groups.
Table 6.
Liver Fatty Acid Composition from Ossabaw Pigs Fed the Experimental Diets
| Fatty Acids | Control | Fructose Group | Atherogenic Diet | M-Ath Group | ANOVA P Value |
|---|---|---|---|---|---|
| 12:0 | ND* ± ND | ND* ± ND | ND* ± ND | 0.06† ± 0.10 | <0.0001 |
| 14:0 | 0.16* ± 0.07 | 0.29* ± 0.09 | 0.19* ± 0.05 | 0.83† ± 0.14 | <0.0001 |
| 15:0 | 0.10† ± 0.02 | 0.05†* ± 0.02 | ND‡ ± ND | 0.04*‡ ± 0.05 | 0.0006 |
| 16:0 | 12.35* ± 1.64 | 13.56†* ± 0.75 | 9.71‡ ± 0.47 | 14.11† ± 0.98 | <0.0001 |
| 16:1t | 0.34* ± 0.07 | 0.55† ± 0.10 | 0.53† ± 0.08 | 0.50† ± 0.06 | 0.002 |
| 16:1n7 | 0.26‡ ± 0.10 | 0.66* ± 0.19 | 0.81* ± 0.13 | 1.65† ± 0.39 | <0.0001 |
| 17:0 | 0.76† ± 0.11 | 0.40* ± 0.08 | 0.24‡ ± 0.04 | 0.36*‡ ± 0.10 | <0.0001 |
| 17:1n7 | 0.09* ± 0.03 | 0.10* ± 0.02 | 0.03‡ ± 0.06 | 0.19† ± 0.04 | <0.0001 |
| 18:0 | 27.15† ± 2.02 | 26.37† ± 1.85 | 16.35* ± 0.99 | 16.77* ± 1.14 | <0.0001 |
| 18:1t | 0.25 ± 0.18 | 0.20 ± 0.18 | 1.69 ± 2.32 | ND ± ND | 0.06 |
| 18:1n9 | 8.96‡ ± 0.68 | 14.63* ± 2.62 | 27.12† ± 2.46 | 26.34† ± 1.84 | <0.0001 |
| 18:1n7 | 1.70‡ ± 0.30 | 1.84‡ ± 0.20 | 4.04† ± 0.10 | 3.55* ± 0.17 | <0.0001 |
| 18:1 | 0.08‡ ± 0.08 | 0.07‡ ± 0.10 | 3.63† ± 0.13 | 2.06* ± 0.36 | <0.0001 |
| 18:2n6 | 22.83† ± 2.83 | 20.98† ± 1.04 | 17.12* ± 0.64 | 14.72* ± 0.50 | <0.0001 |
| 18:3n6 | 0.16 ± 0.03 | 0.12 ± 0.03 | 0.07 ± 0.07 | 0.12 ± 0.07 | 0.10 |
| 18:3n3 | 0.41† ± 0.05 | 0.45† ± 0.10 | 0.26* ± 0.04 | 0.46† ± 0.08 | 0.001 |
| 20:0 | 0.10†* ± 0.03 | 0.09* ± 0.01 | 0.15† ± 0.02 | 0.03‡ ± 0.05 | <0.0001 |
| 20:1n9 | 0.24* ± 0.05 | 0.28* ± 0.04 | 0.43† ± 0.09 | 0.41† ± 0.11 | 0.001 |
| 20:2n6 | 0.96† ± 0.32 | 0.87†* ± 0.13 | 0.58* ± 0.15 | 0.89†* ± 0.12 | 0.02 |
| 20:3n6 | 0.81‡ ± 0.25 | 1.19* ± 0.22 | 1.65† ± 0.31 | 1.47†* ± 0.12 | <0.0001 |
| 20:4n6 | 16.29† ± 2.34 | 11.49* ± 1.23 | 7.29‡ ± 0.45 | 8.54‡ ± 1.43 | <0.0001 |
| 20:3n3 | 0.20†* ± 0.05 | 0.23† ± 0.06 | 0.11* ± 0.06 | 0.23† ± 0.04 | 0.004 |
| 20:5n3 | 0.33 ± 0.14 | 0.26 ± 0.11 | 0.22 ± 0.03 | 0.18 ± 0.06 | 0.10 |
| 22:0 | 0.06* ± 0.04 | 0.06* ± 0.03 | 0.14† ± 0.02 | 0.04* ± 0.07 | 0.004 |
| 22:4n6 | 0.71† ± 0.14 | 0.61† ± 0.18 | 0.30* ± 0.03 | 0.62† ± 0.14 | 0.001 |
| 22:5n6 | 0.07 ± 0.06 | 0.09 ± 0.03 | 0.02 ± 0.05 | 0.13 ± 0.08 | 0.07 |
| 22:5n3 | 1.21 ± 0.67 | 1.34 ± 0.44 | 0.73 ± 0.14 | 1.08 ± 0.32 | 0.13 |
| 22:6n3 | 1.28 ± 0.91 | 1.71 ± 0.65 | 1.32 ± 0.30 | 0.89 ± 0.43 | 0.13 |
| TOTS | 40.67† ± 2.47 | 40.82† ± 1.48 | 26.78‡ ± 0.78 | 32.28* ± 1.42 | <0.0001 |
| TOTM | 11.93‡ ± 1.07 | 18.33* ± 3.17 | 38.28† ± 1.06 | 34.71† ± 2.52 | <0.0001 |
| PUFA | 45.25† ± 1.84 | 39.34* ± 1.93 | 29.66‡ ± 1.00 | 29.33‡ ± 1.94 | <0.0001 |
| n-6 PUFA | 41.82† ± 1.99 | 35.35* ± 0.96 | 27.04‡ ± 0.86 | 26.48‡ ± 1.44 | <0.0001 |
| n-3 PUFA | 3.43†* ± 0.43 | 3.99† ± 1.21 | 2.63* ± 0.34 | 2.84†* ± 0.54 | 0.03 |
| n-6 LC | 17.07† ± 2.41 | 12.19* ± 1.33 | 7.62‡ ± 0.47 | 9.29‡ ± 1.57 | <0.0001 |
| n-3 LC | 2.82 ± 0.44 | 3.31 ± 1.17 | 2.26 ± 0.33 | 2.15 ± 0.59 | 0.05 |
| TOTS/TOTM | 3.45† ± 0.50 | 2.31* ± 0.53 | 0.70‡ ± 0.04 | 0.94‡ ± 0.10 | <0.0001 |
| TOTM/PUFA | 0.26‡ ± 0.01 | 0.47‡ ± 0.10 | 1.29† ± 0.08 | 1.19* ± 0.17 | <0.0001 |
| TOTS/PUFA | 0.90* ± 0.09 | 1.04† ± 0.04 | 0.90* ± 0.04 | 1.10† ± 0.06 | <0.0001 |
| n-6 LC/n-3 LC | 6.09† ± 0.66 | 3.99* ± 1.16 | 3.41* ± 0.43 | 4.45* ± 0.62 | 0.0003 |
| n-6/n-3 | 12.39 ± 1.86 | 9.51 ± 2.55 | 10.40 ± 1.15 | 9.55 ± 1.50 | 0.08 |
n = 9 for the fructose group, n = 6 for the M-Ath group, n = 5 for control and atherogenic groups.
Values in rows with different symbols are significantly different by one-way ANOVA. The superscripts indicate what mean values are different in the rows, and the P values are for all groups.
In general, there was a high degree of correlation among diet and serum and hepatic levels of MUFA and PUFA (both n6 and n3) levels; however, the saturated fatty acid and trans-fatty acid levels of diet did not have statistically significant correlation with either serum or hepatic levels of saturated fatty acids or trans-fatty acids (Supporting Table 2). Serum levels of saturated fatty acids, MUFA, and PUFA (both n6 and n3) correlated significantly with their respective levels in the hepatic tissue (Supporting Table 2). Interestingly, trans-fatty acids levels of diet, serum, or hepatic levels did not correlate with each other.
Sex-Matched Subgroup Analyses
In a subset analysis, we compared seven female pigs in the M-Ath diet group with nine pigs in the control chow and eight in the atherogenic diet groups, and selected results are shown in Supporting Tables 3 and 4. The results in this subset analysis are generally consistent with results of analyses conducted on the whole cohort. Because our M-Ath diet group had no male pigs, our study design could not investigate whether the M-Ath diet is able to induce liver lesions in male pigs as it has in female pigs.
Discussion
The pathogenesis of NASH is not well understood, and for obvious ethical and practical reasons, it has been difficult to conduct detailed mechanistic studies in humans. Thus, numerous attempts have been made to develop rodent models of NASH to investigate its pathogenesis.20 However, most of these models have failed to reproduce either characteristic hepatic histology or the features of metabolic syndrome that are nearly universal in human NASH.20 For example, the widely used methionine-deficient and choline-deficient model induces steatohepatitis in mice that histologically has a close resemblance to human histopathology,21,22 but these animals do not typically exhibit obesity or insulin resistance.23 However, some recent rodent models have shown encouraging results.20,24-26 Undoubtedly, these newer models are far more attractive than earlier models, but there are two important shortcomings to any rodent models of NASH: (1) inability to conduct serial histological examination on the same set of animals and (2) general difficulty in inducing steatohepatitis in female rodents.
Our study makes several novel observations. First, pigs in the fructose group, despite having marked weight gain, hypertension, and insulin resistance, had normal liver histology. This argues against a singular role for high-fructose intake in the pathogenesis of NAFLD. This also suggests that metabolic syndrome in the absence of dyslipidemia is not sufficient to cause liver injury. Second, pigs in the atherogenic diet group developed metabolic syndrome and had abnormal liver histology consisting of diffuse microvesicular steatosis and foamy and steatotic Kupffer cells but no ballooning or fibrosis. In our two earlier Ossabaw experiments, pigs fed on atherogenic diet developed no liver injury, but diets in those two experiments consisted of complex carbohydrates rather than fructose as the source for carbohydrate calories. This observation indicates that only fructose-containing atherogenic diets are injurious to liver. Third, pigs fed the M-Ath diet developed severe metabolic syndrome and striking evidence of liver injury, with macrovesicular and microvesicular steatosis, foamy and steatotic Kupffer cells, diffuse ballooning, and importantly, perivenular/perisinusoidal fibrosis. It is not entirely clear why only the animals in the M-Ath group developed steatohepatitis, but it is likely related to its different source of fat (mixed fat) or modestly reduced choline concentration or casein supplementation (or their combination). Recent experimental studies have shown that the source of protein may affect insulin/glucagon secretion from pancreatic islet cells or hepatic gene expression related to lipid metabolism.27,28
An interesting finding in our study is that pigs in our M-Ath diet group exhibited evidence of hepatocyte injury and fibrosis in the absence of massive macrovesicular steatosis. This suggests that significant macrovesicular steatosis is not a prerequisite for developing hepatocyte injury and fibrosis. This observation is supportive of the recent contention that macrovesicular steatosis may in fact represent a protective phenomenon by funneling hepatic free fatty acids into triglycerides, which are generally inert and less toxic than free fatty acids.9.29
It is striking that Ossabaw pigs were resistant to developing significant macrovesicular steatosis despite marked weight gain and metabolic syndrome. Although not specifically documented in the literature (to our knowledge), it appears that pigs generally are resistant to developing macrovesicular steatosis for unclear reasons (personal communication, Ariel Feldstein). Because serum triglyceride levels were not as high as other lipoproteins in the obese Ossabaw pigs, it is unlikely that lack of significant macrovesicular steatosis is attributable to brisk export of triglycerides from the hepatocytes in the form of very-low-density lipoprotein. It is plausible that pig hepatocytes may not have fully developed mechanisms for converting free fatty acids into triglycerides or alternatively may have defective glycerol metabolism.
Another noteworthy finding is the accumulation of fatty Kupffer cells within the liver tissue of Ossabaw pigs fed both types of atherogenic diet. Accumulation of fatty Kupffer cells is not typical of human NAFLD, but it is a common hepatic finding in animals fed an atherogenic diet30-33 and also in some animal models of steatohepatitis.34,35 ApoE2 knock-in mice fed on Western diet developed steatosis and inflammation, and there was early appearance of foamy Kupffer cells in the liver, which subsequently formed larger aggregates with other inflammatory cells. In another study, apolipoprotein E knockout mice fed on cholesterol and fat-enriched diets exhibit steatosis, inflammation, and macrophage accumulation. In that study, administration of cholesterol and palm oil produced a higher accumulation of macrophages than did cholesterol and olive oil. In our study, Kupffer cell accumulation is unlikely to be related to the type of fat consumed because pigs belonging to both types of atherogenic diet exhibited extensive fatty Kupffer cell accumulation.
To our knowledge, this is the first large animal model of steatohepatitis induced by dietary manipulation, and it recapitulates human NAFLD very closely because of histological resemblance and presence of the metabolic syndrome. Admittedly, large animal models are expensive and can be developed and used only by centers with special resources and expertise, but large animal models provide a unique opportunity for serial examination of the same set of animals. Furthermore, large animals such as pigs resemble human anatomy and physiology much closer than rodents. Two limitations of our study design require discussion. First, our groups were not matched numerically or sex-wise because of practical issues such as success of breeding, availability of young animals for experiments, and laboratory space for simultaneous stationing of a large number of pigs. Second, we admit that differences between our atherogenic and M-Ath diets are many, and this is not ideal from a methodological standpoint. However, we would like to point out that our primary objective was to induce NASH phenotype in these animals, rather than testing which dietary variable is more injurious to liver.
In summary, we describe a nutritional model of steatohepatitis in pigs fed a modified atherogenic diet that closely mimics human NASH from a histological as well as a metabolic co-morbidities standpoint. This model will provide a unique opportunity to investigate the pathogenesis of NASH in a longitudinal fashion and also to test the efficacy of novel therapeutic agents.
Supplementary Material
Supplemental Table 1 Fatty acid composition of experimental diets fed to Ossabaw pigs†
Supplemental Table 2 Correlation of fatty acid composition among diet, serum and liver samples
Supplemental Table 3 Selected characteristics at sacrifice of gender–matched pigs belonging to different study groups†
Supplemental Table 4 Triglyceride, MDA and TEAC Measurements in Liver Tissue of gender-matched pigs†
Fig. 6.
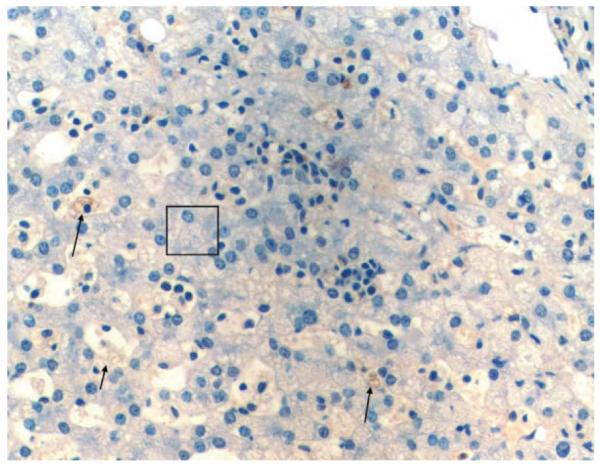
Lysozymal staining of liver tissue of pig fed on modified atherogenic diet. Arrows, Kupffer cells are large and foamy and stain positively for lysozyme. Box, ballooned hepatocytes with markedly vacuolated cytoplasm. This photo also has scattered extramedullary hematopoiesis.
Acknowledgment
The authors thank Drs. Christopher Day, Ariel Feldstein, Scott Friedman, Brent Tetri, and David Crabb for their helpful discussions.
Supported by the Public Health Service Grants RR-013223 and HL-062552 and Purina TestDiet, Inc. to M.S., R01CA100908 to J.K. and the Purdue-Indiana University Comparative Medicine Program.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- HDL
high-density lipoprotein
- HE
hematoxylin-eosin
- HOMA
homeostasis model assessment
- LDL
low-density lipoprotein
- M-Ath
modified atherogenic
- MDA
malondialdehyde
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- PUFA
polyunsaturated fatty acid
- TNF
tumor necrosis factor
Footnotes
Potential conflict of interest: Nothing to report.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 3.Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682–1698. doi: 10.1053/j.gastro.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 4.Vuppalanchi R, Chalasani N. Non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: selected practical issues in their evaluation and management. Hepatology. 2009;49:306–317. doi: 10.1002/hep.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 6.Brunt EM. Pathology of nonalcoholic steatohepatitis. Hepatol Res. 2005;33:68–71. doi: 10.1016/j.hepres.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 8.De Alwis NM, Day CP. Non-alcoholic fatty liver disease: the mist gradually clears. J Hepatol. 2008;48(Suppl):S104–S112. doi: 10.1016/j.jhep.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Jou J, Choi SS, Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:370–379. doi: 10.1055/s-0028-1091981. [DOI] [PubMed] [Google Scholar]
- 10.Sturek M, Alloosh M, Wenzel J, Byrd JP, Edwards JM, Lloyd PG, et al. Ossabaw Island miniature swine: cardiometabolic syndrome assessment. In: Swindle MM, editor. Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques. CRC Press; Boca Raton, FL: 2007. pp. 397–402. [Google Scholar]
- 11.Dyson M, Alloosh M, Vuchetich JP, Mokelke EA, Sturek M. Components of metabolic syndrome and coronary artery disease in female Ossabaw swine fed excess atherogenic diet. Comp Med. 2006;56:35–45. [PubMed] [Google Scholar]
- 12.National Research Council . Guide for the care and use of laboratory animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- 13.Beaver BV, Reed W, Leary S, McKiernan B, Bain F, Schultz R, et al. 2000 Report of the AVMA panel on euthanasia. JAMA. 2001;218:669–696. doi: 10.2460/javma.2001.218.669. [DOI] [PubMed] [Google Scholar]
- 14.Otis CR, Wamhoff BR, Sturek M. Hyperglycemia-induced insulin resistance in diabetic dyslipidemic Yucatan swine. Comp Med. 2003;53:53–64. [PubMed] [Google Scholar]
- 15.Swindle MM. Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques. CRC Press; Boca Raton, FL: 2006. [Google Scholar]
- 16.Vuppalanchi R, Cummings OW, Saxena R, Ulbright TM, Martis N, Jones DR, et al. Relationship among histologic, radiologic, and biochemical assessments of hepatic steatosis: a study of human liver samples. J Clin Gastroenterol. 2007;41:206–210. doi: 10.1097/01.mcg.0000225515.28536.3a. [DOI] [PubMed] [Google Scholar]
- 17.Mateos R, Goya L, Bravo L. Determination of malondialdehyde by liquid chromatography as the 2, 4-dinitrophenylhydrazone derivative: a biomarker for oxidative stress in cell cultures of human hepatoma HepG2. J Chromatogr. 2004;805:33–39. doi: 10.1016/j.jchromb.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 19.Watkins BA, Li Y, Seifert MF. Dietary ratio of n-6/n-3 PUFAs and docosahexaenoic acid: actions on bone mineral and serum biomarkers in ovari-ectomized rats. J Nutrion Biochem. 2006;17:282–289. doi: 10.1016/j.jnutbio.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Larter CZ, Yeh MM. Animal models of NASH: getting both pathology and metaboli context right. J Gastroenterol Hepatol. 2008;23:1635–1648. doi: 10.1111/j.1440-1746.2008.05543.x. [DOI] [PubMed] [Google Scholar]
- 21.Weltman MD, Farrell GC, Liddle C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology. 1996;111:1645–1653. doi: 10.1016/s0016-5085(96)70028-8. [DOI] [PubMed] [Google Scholar]
- 22.Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105:1067–1075. doi: 10.1172/JCI8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rinella ME, Green RM. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J Hepatol. 2004;40:47–51. doi: 10.1016/j.jhep.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Svegliati-Baroni G, Candelaresi C, Saccomanno S, Ferretti G, Bachetti T, Marzioni M, et al. A model of insulin resistance and nonalcoholic steatohepatitis in rats: role of peroxisome proliferator-activated receptor-alpha and n-3 polyunsaturated fatty acid treatment on liver injury. Am J Pathol. 2006;169:846–860. doi: 10.2353/ajpath.2006.050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito M, Suzuki J, Tsujioka S, Sasaki M, Gomori A, Shirakura T, et al. Longitudinal analysis of murine steatohepatitis model induced by chronic exposure to high-fat diet. Hepatol Res. 2007;37:50–57. doi: 10.1111/j.1872-034X.2007.00008.x. [DOI] [PubMed] [Google Scholar]
- 26.Deng QG, She H, Cheng JH, French SW, Koop DR, Xiong S, et al. Steatohepatitis induced by intragastric overfeeding in mice. Hepatology. 2005;42:905–914. doi: 10.1002/hep.20877. [DOI] [PubMed] [Google Scholar]
- 27.Torre-Villalvazo I, Tovar AR, Ramos-Barragan VE, Cerbon-Cervantes MA, Torres N. Soy protein ameriolates metabolic abnormalities in liver and adipose tissue of rats fed a high fat diet. J Nutr. 2008;138:462–468. doi: 10.1093/jn/138.3.462. [DOI] [PubMed] [Google Scholar]
- 28.Aziz A, Ziao CW, Cockell KA, Gilani G Sarward, Cruz-Hernandez C, Ratnayake WM Nimal. Impact of dietary protein on lipid metabolism in hamsters is source-dependent and associated with changes in hepatic gene expression. Br J Nutr. 2008;100:503–511. doi: 10.1017/S0007114508911521. [DOI] [PubMed] [Google Scholar]
- 29.Choi SS, Diehl AM. Hepatic triglyceride synthesis and nonalcoholic fatty liver disease. Curr Opin Lipidol. 2008;19:295–300. doi: 10.1097/MOL.0b013e3282ff5e55. [DOI] [PubMed] [Google Scholar]
- 30.Wanless IR, Belgiorno J, Huet PM. Hepatic sinusoidal fibrosis induced by cholesterol and stibesterol in the rabbit: 1. Morphology and inhibition of fibrogenesis by dipyridamole. Hepatology. 1996;24:855–864. doi: 10.1002/hep.510240417. [DOI] [PubMed] [Google Scholar]
- 31.Buyssens N, Kockx MM, Herman AG, Lazou JM, den Berg KV, Wisse E, et al. Centrilobular liver fibrosis in the hypercholesterolemic rabbit. Hepatology. 1996;24:939–946. doi: 10.1002/hep.510240431. [DOI] [PubMed] [Google Scholar]
- 32.Jeong Won-II, Jeong DH, Do SH, Kim YK, Park HY, Kwon OD, et al. Mild hepatic fibrosis in cholesterol and sodium cholate diet-fed rats. J Vet Med Sci. 2005;67:235–242. doi: 10.1292/jvms.67.235. [DOI] [PubMed] [Google Scholar]
- 33.Vergnes L, Phan J, Strauss M, Tafuri S, Reue K. Cholesterol and cholate components of an atherogenic diet induce distinct stages of hepatic inflammatory gene expression. J Biol Chem. 2003;278:42774–42784. doi: 10.1074/jbc.M306022200. [DOI] [PubMed] [Google Scholar]
- 34.Shiri-Sverdlov R, Wouters K, van Gorp PJ, Gijbels MJ, Noel B, Buffat L, et al. Early diet-induced nonalcoholic steatohepatitis in APOE2 knock-in mice and its prevention by fibrates. J Hepatol. 2006;44:732–741. doi: 10.1016/j.jhep.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 35.Tous M, Ferre N, Camps J, Riu F, Joven J. Feeding apolipoprotein E-knockout mice with cholesterol and fat enriched diets may be a model of non-alcoholic steatohepatitis. Mol Cell Biochem. 2005;268:53–58. doi: 10.1007/s11010-005-2997-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1 Fatty acid composition of experimental diets fed to Ossabaw pigs†
Supplemental Table 2 Correlation of fatty acid composition among diet, serum and liver samples
Supplemental Table 3 Selected characteristics at sacrifice of gender–matched pigs belonging to different study groups†
Supplemental Table 4 Triglyceride, MDA and TEAC Measurements in Liver Tissue of gender-matched pigs†