Abstract
By mimicking embryonic development of the hematopoietic system, we have developed an optimized in vitro differentiation protocol for the generation of precursors of hematopoietic lineages and primitive hematopoietic cells from human embryonic stem cells (ESC) and induced pluripotent stem cells (iPSCs). Factors such as cytokines, extra cellular matrix components, and small molecules as well as the temporal association and concentration of these factors were tested on seven different human ESC and iPSC lines. We report the differentiation of up to 84% human CD45+ cells (average 41% ± 16%, from seven pluripotent lines) from the differentiation culture, including significant numbers of primitive CD45+/CD341 and CD45+/CD341/CD38− hematopoietic progenitors. Moreover, the numbers of hematopoietic progenitor cells generated, as measured by colony forming unit assays, were comparable to numbers obtained from fresh umbilical cord blood mononuclear cell isolates on a per CD45+ cell basis. Our approach demonstrates highly efficient generation of multipotent hematopoietic progenitors with among the highest efficiencies reported to date (CD45+/CD341) using a single standardized differentiation protocol on several human ESC and iPSC lines. Our data add to the cumulating evidence for the existence of an in vitro derived precursor to the hematopoietic stem cell (HSC) with limited engrafting ability in transplanted mice but with multipotent hematopoietic potential. Because this protocol efficiently expands the preblood precursors and hematopoietic progenitors, it is ideal for testing novel factors for the generation and expansion of definitive HSCs with long-term repopulating ability.
Keywords: Differentiation, Hematopoiesis, Hematopoietic progenitors, Pluripotent stem cells
Introduction
Induced pluripotent stem cells (iPSC) hold enormous potential for the treatment of diseases due to their molecular and functional similarity to embryonic stem cells (ESC) and patient specificity [1, 2]. The ability to generate hematopoietic cells, including hematopoietic progenitor and stem cells from patient derived iPSCs, would enable the generation of an unlimited supply of human leukocyte antigen matched transplantable cells for the treatment of both hematological disorders and malignancies. Multipotent hematopoietic progenitors or their mature progeny, such as erythrocytes, macrophages, granulocytes, B-cells, T-cells, and natural killer cells [3-20], have been derived from ESC/iPSCs in in vitro cultures. The majority of these studies report generation of only low numbers of mature hematopoietic cells, which likely results from inefficient expansion of pre-blood precursors and hematopoietic progenitor cells. None of these studies have shown robust generation of transplantable hematopoietic stem cells (HSCs).
In this study, we report the optimization of culture conditions and show an increase in the efficiency of differentiating human ESC and iPSCs to the hematopoietic lineage. Efficient generation of these cells is a requirement to further study the signaling pathways involved in generating and expanding bona fide HSCs with repopulating potential.
Materials and Methods
ESC Culture
The pluripotent human ESC and iPSC lines used in this study were grown as described previously [21-23]. We used the HUES 3, WA01 (H1), WA09 (H9), FA404-KiPS, RB9-CBiPS2 (CB2p2), 2937-iPS, BJ1-iPS cell lines. All cell lines were determined to be karyotypically normal by cytogenetic analysis and shown to be pluripotent by in vivo teratoma histological assays and polymerase chain reaction (PCR). The pluripotent cell lines tested were cultured and expanded using either Matrigel (BD Biosciences, San Diego, CA, www.bdbiosciences.com) or murine embryonic fibroblast feeder cells to maintain pluripotency. No differences in blood cell differentiation efficiency were noted between lines cultured under these conditions.
EB Generation
Embryoid bodies (EBs) were generated from ESCs/iPSCs grown on mouse embryonic fibroblasts or Matrigel coated 10-cm dishes for 5 or 6 days, so that the colonies were large but still independent. Colonies were separated from the plate with 4 ml of dispase (0.5 mg/ml, Invitrogen, Carlsbad, CA, www.invitrogen.com) for 30–45 minutes. Colonies were collected in EB medium (Iscove’s modified Dulbecco’s medium supplemented with 15% fetal bovine serum [FBS] [Thermo Scientific Hyclone, Rockford, IL, www.thermoscientific.com], 1% nonessential amino acids [Invitrogen], and 1% GlutaMax [Invitrogen]), allowed to settle at the bottom of a 15-ml conical tube, rinsed twice with EB medium, and placed in a nonadherent T-25 flask (Corning, Corning, NY, www.corning.com) in EB medium overnight.
Mesoderm Generation
Newly generated EB colonies (at 24 hours) were then cultured in suspension flasks containing mesoderm specifying medium (MesoTotal) (Dulbecco’s modified Eagle medium/F12 [Invitrogen] supplemented with 15% FBS [Hyclone], 10 ng ml−1 bone morphogenetic protein 4 [BMP4] [Humanzyme, Chicago, IL, www.humanzyme.com], 5 ng ml−1 transforming growth factor beta 1 [TGFβ1] [Humanzyme], 1 ng ml−1 vascular endothelial growth factor [VEGF] [Humanzyme], 20 ng ml−1 thrombopoietin [TPO] [R&D Systems, Minneapolis, MN, www.rndsystems.com], 20 ng ml−1 erythropoietin [EPO] [Humanzyme], 20 ng ml−1 stem cell factor [SCF] [Human-zyme], 20 ng ml−1 FMS-like tyrosine kinase 3 ligand [FLT3L] [Humanzyme], 200 μg ml−1 holotransferrin [Sigma–Aldrich, St. Louis, MO, www.sigmaaldrich.com], 2 μM prostaglandin E2 [PGE2] [Cayman Chemical, Ann Arbor, MI, www.caymanchem.com], and 50 μg ml−1 ascorbic acid [Sigma]) for 6 additional days with partial medium changes made every other day. Addition of activin to media cultures during this stage did not yield increases in blood cell generation and thus was not used in subsequent experiments.
Plating on OP9 and Hematopoietic Expansion
Day 7 whole and dissociated EBs were then plated on 5,000 rad irradiated OP9 feeder cells (ATCC, Manassas, VA, www.atcc.org) grown on growth factor reduced Matrigel (BD Biosciences) coated six-well plates. The mesoderm-specifying medium (MesoTotal) was changed every other day for 7 days.
Medium on the 2nd week of OP9 coculture was changed to serum-free expansion medium (StemCell Technologies, Vancouver, Canada, www.stemcell.com) supplemented with 20 ng ml−1 TPO (R&D Systems), 20 ng ml−1 EPO (Human-zyme), 20 ng ml−1 SCF (Humanzyme), 20 ng ml−1 FLT3L (Humanzyme), and 2 μM PGE2 (Cayman Chemical).
Analysis by Flow Cytometry
Cells from the differentiation cultures were collected at time points indicated and washed in phosphate-buffered saline (PBS) supplemented with 2% FBS. Adherent cells were individualized using TrypLE (Invitrogen), passed through a 27.5-gauge needle and filtered through a 40–70 μm cell strainer (BD Falcon, San Diego, CA, www.bdbiosciences.com). Cells were treated with 7-aminoactinomycin D before analysis and positive cells were gated out of results. Cells were stained using fluorescein isothiocyanate-conjugated CD34, CD235a, phycoerythrin-conjugated CD45, allophycocyanin-conjugated CD11b, CD15, CD33, CD38, and CD133 (AC133), and Alexa-647-conjugated CD31 (all from BD). Cells were analyzed on an LSR (BD, Franklin Lakes, NJ, www.bd.com) using CellQuest v3.3 (BD).
Hematopoietic Colony Formation in Methyl Cellulose
Single-cell suspensions of differentiated EBs on OP9 were plated in triplicate at approximately 200,000 cells per well in 2 ml of MethoCult H4435 (StemCell Technologies) in six-well plates. Cells were incubated for 14 days in a humidified incubator at 37°C with 5% CO2 and evaluated for colonies by bright-field microscopy (Zeiss [Oberkochen, Germany, www.zeiss.com] Axiovert 200 with a Zeiss Axiocam). Cells were resuspended in PBS supplemented with 2% FBS and analyzed by flow cytometry for the presence of erythroid and myeloid lineages as described above.
Reverse Transcriptase PCR of Human Globin Genes
Colonies from methylcellulose were lysed using Trizol (Invitrogen) and RNA was isolated according to manufacturer’s specifications. A cDNA library was reverse transcribed by the High Capacity RNA-to-cDNA Kit from Applied Biosystems. PCR products were amplified using primers previously described in [24] for 40 cycles.
Quantitative Analysis of Cell Numbers and Statistical Analyses
Cells were counted by hemacytometer under a bright-field microscope to obtain absolute cell numbers. We calculated the total numbers of cells of each subpopulation by multiplying the number of cells by the proportion of cells of that line-age as determined by flow cytometry. Statisitical analyses of data points and error bars of this study show mean and standard deviation by Student’s t-test.
Mouse Transplantations
Single-cell suspensions of differentiated EBs on OP9 were generated as described above and injected into sublethally irradiated (325 rad, Cobalt-60 source) NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice (JAX, Bar Harbor, ME, www.jax.org) via tail vein injections. Blood was harvested at times indicated via tail or retro-orbital eye bleed. Erythrocytes were lysed using ammonium chloride solution (0.8% NH4Cl with 0.1 mM EDTA) (Stem Cell Technologies), spun, and stained flow cytometric analysis as described above. All animal experiments were conducted in accordance with Institutional Animal Care and Use Committee and Association for Assessment and Accreditation of Laboratory Animal Care International policies.
Results and Discussion
Hematopoietic Cell Generation
The in vitro blood cell differentiation process can be divided into key stages where specific signals are provided for mesodermal, hemangioblast/hemogenic-endothelium, and hematopoietic stem/progenitor cell specification, maturation, and expansion (Fig. 1A). In agreement with the findings of Hong et al. [19], we obtained higher yields of blood (CD45+ cells) by generating specialized EBs grown in mesodermal stimulating conditions with BMP4. The addition of TGFβ1 to the media appeared to marginally increase hematopoietic cell output (Fig. 2A). We were surprised by an additive role of the factors for blood generation when combined in the medium given that the factors compete for the shared downstream transcription factor SMAD4.
Figure 1.
Schematic representation of the optimized differentiation protocol used to generate hematopoietic precursors and progenitors from embryonic stem cell (ESC) and induced pluripotent stem cell (iPSC) lines. (A): Developmental stages of differentiation of ESCs/iPSCs in culture showing the precursors of the hematopoietic lineage targeted for cellular expansion to putative predefinitive hematopoietic stem cells (HSCs) and hematopoietic progenitor cells. (B): Optimized differentiation protocol showing conditions including media cytokine cocktails (see Materials and Methods for details) and timing of applied factors for more than 3 weeks for the efficient differentiation of ESCs/iPSCs through three distinct stages of development. A representative meso-/hemo-embryoid body (EB) is shown with large cystic growths after 7 days in culture indicates efficient mesoderm differentiation and eventually blood lineage generation. The whole meso-/hemo-EB is then plated onto OP9 feeder layer in the same medium cocktail for 1 week before a hematopoietic cocktail is applied for the final week. Assays used to determine efficiency of generation of hematopoietic progenitors and predefinitive HSCs at the 3 week time point are shown (NSG mouse transplant model). Abbreviations: CFU, colony forming unit; EB, Embryoid body; FACS, flow cytometry; hESC, human embryonic stem cell; HSC, hematopoietic stem cell; iPSC, induced pluripotent stem cell; NSG, NOD/SCID/il2rg−/−; SFEM HC, serum-free expansion media–hematopoietic cell.
Figure 2.
Efficient generation of hematopoietic cells correlates with higher yields of upstream precursors. Panel (A) shows the percentage CD45+ hematopoietic cells from BJ1-induced pluripotent stem cell (iPSC) differentiated hematopoietic cells at 3 weeks using MesoTotal media containing 10 ng ml−1 bone morphogenetic protein 4 (BMP4) and 5 ng ml−1 transforming growth factor beta 1 (TGFβ1) (BMP4+TGF-β1), 5 ng ml−1 TGF-β1 alone and 10 ng ml−1 BMP4 alone, as determined by flow cytometry. Panels (B) and (C) show data from representative wells of iPSCs differentiated for 21 days generate more hematopoietic cells (CD45+) and clonogenic progenitors than cultures of 18 days. Following differentiation of iPSC lines FANC 404-KiPSC, total wells were harvested and analyzed for CD45 expression by flow cytometry (panel B) or colony forming unit (CFU) progenitor assay (panel C). Highest CD45 counts and CFU progenitor counts were obtained from the 21-day cultures in the experiments shown. Panels (D–I) show flow cytometric analysis of human embryonic stem cell line Hues3 cells undergoing differentiation with MesoTotal medium supplemented with 20 ng ml−1 vascular endothelial growth factor (VEGF) (high-dose VEGF) (D, E) or with 0.5 ng ml−1 VEGF (low-dose VEGF) (F, G), showing an inhibitory effect of the high-dose of VEGF on the generation of both CD45+ hematopoietic (left panels) and CD45− CD34+ CD31+ endothelial lineages (right panels). Low-dose VEGF results in higher proportions of both hematopoietic cells and endothelial cells. Average data of both hematopoietic (H) and endothelial cell (I) output from the Hues3 cell line (n = 3). The correlation between higher endothelial cell yield and hematopoietic cell yield suggests a common hemangioblast/hemogenic endothelial cell origin of the hematopoietic and endothelial cell lineages in vitro. Abbreviations: BMP4, bone morphogenetic protein 4; CFU, colony forming unit; TGFβ1, transforming growth factor beta 1; KiPSC, keratinocyte-induced pluripotent stem cell; VEGF, vascular endothelial growth factor.
Time course flow cytometry analysis of the expression of pan-hematopoietic cell marker, CD45, the ontologically early hematopoietic cell marker in ESC derived cultures, CD43, and the progenitor cell marker, CD34, during the differentiation process (at days 14, 17, and 21) shows that the peak number of hematopoietic progenitor CD45+/CD34+ cells occurs at the 3-week time point with undiminished counts of earlier CD43+/CD34+ blood progenitors (supporting information Fig. 1). Differentiation efficiencies and colony forming unit (CFU) counts on average appeared to be highest at day 21, suggesting that continuous expansion of the progenitor and CFU pool occurred, as shown in representative wells (Fig. 2B, 2C). While these results were not statistically significant, day 21 was selected as the optimal point to harvest and analyze for hematopoietic stem/progenitor output.
Yields of Hemogenic Endothelial Cells and Hematopoietic Cells: Role of VEGF
Following mesoderm lineage specification, we focused on the presence of endothelial lineages as possible indicators of hematopoietic potential via the hemangioblast or hemogenic endothelium using endothelial lineage markers CD34+/CD31+ in CD45− (nonhematopoietic) cells. As VEGF receptor 1 (VEGFR1) is present on endothelial cells, including hemogenic endothelial cells [25], we hypothesized that adding VEGF to the differentiation cultures would increase hemogenic-endothelial cell proliferation and hematopoietic cell output. There was a high correlation between blood cell output and the presence of endothelial cells as measured by CD45−/CD34+/CD31+ cells, when comparing Figure 2D, 2E with Figure 2F, 2G. Interestingly, we found that low levels of VEGF significantly increased the amount of blood produced when compared with high levels. It is possible that the high levels of VEGF yielded very few hematopoietic cells due to an effect at the initial ESC/iPSC to mesodermal differentiation stage, whereby cells moving toward the hematopoietic/endothelial cell lineage may be hampered by a negative feedback loop at saturating levels.
Efficient Differentiation of ESC/iPSC Lines to Hematopoietic Progenitors
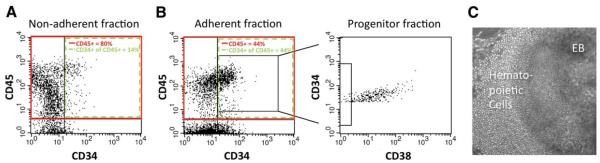
Using conditions that generated the highest levels of blood cells (CD45+) (see Materials and Methods for complete details), we assayed multiple ESC and iPSC lines differentiated to the blood lineage for efficiency. Figure 3(A, B) shows representative flow cytometry analyses for a single well of an in vitro culture of a genetically corrected keratinocyte-iPSC (KiPSC) line from a Fanconi anemia patient [15]. Isotype controls are shown in supporting information Figure 2. Using phase-contrast microscopy, we observed significant numbers of hematopoietic cells present in suspension in the media as well as attached to the plate adjacent to the EB (Fig. 3C). These adherent cells are similar in appearance to cobblestone area forming cells from an adult hematopoietic source such as bone marrow. Further analysis of these two populations of cells revealed that the majority of hematopoietic progenitors (CD45+/CD34+ cells) were present in the adherent fraction of cells rather than the nonadherent fraction (30,900 ± 13,000 vs. 8,000 ± 3,100, respectively).
Figure 3.
Efficient generation of hematopoietic progenitor cells from embryonic stem cell/induced pluripotent stem cells (iPSCs). Representative flow cytometric analysis data of adherent (A) and nonadherent (B) in vitro culture fractions, from a genetically corrected Fanconi anemia patient-derived iPSC line (FA404-KiPSC). Staining for the Pan-hematopoietic marker CD45+ (solid box) and the hematopoietic progenitor markers CD45+/CD34+ (dashed box) was done in the iPSC line culture at 3 weeks using the optimized differentiation protocol. The adherent fraction, while lower in the proportion of hematopoietic progenitors, contains significantly greater numbers of these progenitors. In addition, a more primitive population of hematopoietic progenitor cells (lowest 5% of CD45+/CD34+/CD38−), which represents the putative repopulating fraction in cord blood, is also present in the adherent fraction. These data in combination with higher total cell yield of the adherent fraction when compared with the nonadherent fraction (as described in the Results section) demonstrate that the majority of hematopoietic primitive progenitor cells are located in the adherent fraction. Panel (C) shows bright-field microscopy of meso-/hemo-embryoid bodies following plating onto an OP9 feeder layer after 14 days (3 weeks of differentiation using the optimized protocol). Large areas of cells show round, semiadherent, hematopoietic progenitor-like morphology. Abbreviation: EB, embryoid body.
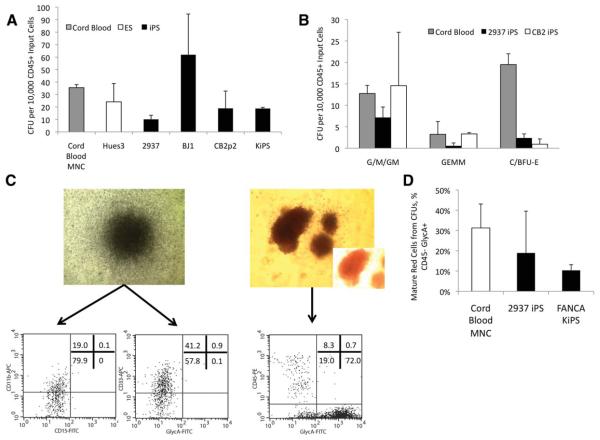
Using the optimized 3-week protocol, we have tested seven different human ESC and iPSC lines to determine the reproducibility of our protocol for the generation of hematopoietic progenitors. The four iPSC lines that have been tested are two fibroblast-derived cell lines 2937 and BJ1, a Fanconi disease derived KiPSC line [15], and a human umbilical cord blood cell-derived iPSC line. We have also tested the HUES3 and H1 human ESC lines. All of these lines are capable of producing high yields of hematopoietic cells (CD45+) and hematopoietic progenitors (CD45+/CD34+: Fig. 4A, 4B and summarized in supporting information Table 1). In addition to cell surface marker expression, we functionally assayed for progenitors, using the CFU assay. Figure 5A compares the number of CFUs generated per 10,000 CD45+ cells from multiple ESC/iPSC lines and human cord blood mononuclear cells. Further characterization of the cells revealed CFU-granulocytes, CFU-macrophages, and burst forming unit erythroid colonies, including more primitive CFU-granulocytes, erythrocytes, monocytes, and megakaryocytes colonies (Fig. 5B). In addition to having significant numbers of CD45+, CD11b+ , and CD33+ cells in CFU–granulocyte, monocyte type colonies, the erythroid colonies showed a high proportion of CD45−/glycophorinA+ cells, indicating erythropoiesis [8] (Fig. 5C). When tested by reverse transcriptase PCR, these cells express alpha, gamma, epsilon, and zeta-globins (supporting information Fig. 3).
Figure 4.
Multiple human embryonic stem cell (ESC) and induced pluripotent stem cell (iPSC) lines efficiently differentiate to hematopoietic cells and progenitors using our optimal protocol. Bar graphs show average percentages of CD45+ hematopoietic cells (A) and CD45+CD34+ hematopoietic progenitors (B) from the pluripotent cell lines indicated. White bars indicate human ESC lines and black bars indicate human iPSC lines. The iPSC lines used in this study were derived from various starting material cell sources: the 2937, BJ1 lines were derived from fibroblasts, keratinocyte-iPSC from hair follicle keratinocyte, and CB2p2 from cord blood cells. These data demonstrate that both human ESC and iPSC from various starting material sources can be efficiently differentiated into hematopoietic lineages. Abbreviations: ESC, embryonic stem cell; iPSC, induced pluripotent stem cell; KiPSC, keratinocyte-induced pluripotent stem cell.
Figure 5.
Multiple human embryonic stem cell (ESC) and induced pluripotent stem cell (iPSC) lines yield multipotent myeloid progenitors at frequencies comparable to those of umbilical cord blood mononuclear cells (UCBMNC). Panel (A) shows the number of colony forming unit (CFU) from both cord blood mononuclear cell (UBMNC) isolates and iPSC-derived hematopoietic cells per 10,000 CD45+ input cells. Panel (B) shows the numbers of generated CFUs categorized by colony type from UBMNC, the 2937 iPSC line and CB with CFU-G/M/GM, more primitive CFU-granulocytes, erythrocytes, monocytes, and megakaryocytes colonies as well as erythroid B/CFU-E colonies. (C): Bright-field microscopy and flow cytometric analysis showing common myeloid cell markers (CD11b, CD15, and CD33) from a representative CFU-GM colony (left), and the mature erythroid cell markers (CD45−/GlycophorinA+) from a representative burst forming unit erythroid colony derived from the FA404-KiPSC line (right). The flow cytometry plot demonstrates a high proportion of cells being CD45− and GlycophorinA+, indicative of a committed erythroid cell. (D): Summary histogram plots of numbers of CD45−/GlycophorinA+ mature erythrocytes for the iPSC lines 2937 and FA404-KiPSC in comparison with UCBMNC isolates. White bars indicate hematopoietic cells derived from human ESC lines, black bars from human iPSC lines, and the gray bar from human UCBMNC isolates. Together, these data show the ability for multiple ESC/iPSC lines to generate high numbers of progenitors of both the myeloid and erythroid cell lineages at frequencies comparable to UCBMNC isolates. Abbreviations: APC, allophycocyanin; BFU-E, burst forming unit erythroid; CFU, colony forming unit; ESC, embryonic stem cell; FITC, fluorescein isothiocyanate; GEMM, granulocytes, erythrocytes, monocytes, and megakaryocytes, G/M/GM, granulocyte/monocyte/granulocyte, monocyte; iPSC, induced pluripotent stem cell; KiPSC, keratinocyte-induced pluripotent stem cell; MNC, mononuclear cell; PE, R-phycoerythrin.
Similar results were also seen for the highly proliferative erythroid progenitors, as measured by flow cytometry for CD45−/GlycophorinA+ cells in the CFU-C assay with comparably high yields of erythrocytes (Fig. 5D). To our knowledge, this is the first time high numbers of progenitors that approach the levels seen in cord blood have been achieved.
While we were able to generate high levels of hematopoietic progenitors, the NOD/SCID/il2rg−/− transplant assay for HSC revealed no repopulating lymphoid/myeloid HSCs. Although we saw engraftment significantly above nontransplant controls in two of 35 mice transplanted over eight separate experiments, the level of engraftment (human CD45+ cells) diminished from a peak of 2.1%–0% by 10 weeks post-transplant (N.-B.W and A.S.P., unpublished data). This suggests that while short term repopulating cells can be generated using this protocol, the key factors needed for generating de novo HSCs remain to be elucidated.
In addition, several novel factors have recently been identified as being able to expand adult hematopoietic progenitors and/or stem cells from human umbilical cord blood or murine bone marrow in vitro and in vivo. These include PGE2 [26, 27] or a combination of angiopoietin-like-5 and insulin growth factor binding protein 2 [28]). Interestingly, no individual factor, nor a combination of all three factors, could generate repopulating HSCs in our culture system as assayed in NOD/SCID/il2rg−/− mice (data not shown). These results suggest that different factors are required for generating de novo HSCs and expanding an already existing pool of mature HSCs. The inability to generate stable repopulating HSCs, while simultaneously generating high amounts of hematopoietic progenitors, suggests that we are either (a) bypassing the HSCs entirely and proceeding directly from a hemogenic endothelial cell to a hematopoietic progenitor or (b) rapidly passing through the HSCs transiently to a hematopoietic progenitor. Both of these proposed differentiation scenarios require the existence of a precursor cell that is immediately upstream of the transient HSC. Matsuoka et al. [29] demonstrated that murine yolk-sac blood island cells, which have no repopulating ability, can be converted to a repopulating cell if given the correct signals. This may suggest that there is a cell type immediately upstream of the HSC that can become an HSC when given the correct signals. We hypothesize that this type of cell is possibly being generated in our cultures and may be defined as a predefinitive HSC precursor.
In summary, we demonstrate a robust and reproducible method for efficient generation of multipotent hematopoietic progenitor cells from ESCs and iPSCs. We have achieved these levels by breaking down the differentiation into a multistep process that attempts to mimic in utero development. Because this method enables generation of higher amounts of hematopoietic progenitors, it may facilitate the testing of hematopoietic progenitor based cell therapeutics and allow for assessment of novel factors for the generation and expansion of the repopulating HSCs.
Supplementary Material
Acknowledgments
We thank Oded Singer, Quan Zhu, Joseph Chambers, Morgan Allen, Amanda Chambers, Roger Rönn, and Anurag Kashyap for technical assistance. This work was supported in part by grants from the NIH, California Institute for Regenerative Medicine, Leducq Foundation, Merieux Foundation, Ellison Medical Foundation, Ipsen/Biomeasure, Sanofi Aventis, Prostate Cancer Foundation, and the H.N. and Frances C. Berger Foundation. N.B.W. and his laboratory were supported by The Royal Physiographic Society of Sweden project grant, Hemato-Linné Program Grant Sweden, the Lund University Medical Faculty, The Lars Hiertas Minne Stiftelse Project Grant, and the Lund University Medical Faculty Grant for Pre-Clinical research. A.S.P. was supported by the UCSD Cellular and Molecular Genetics Training Grant and the H.A. and Mary K. Chapman Charitable Trust. I.M.V. is an American Cancer Society Professor of Molecular Biology, and holds the Irwin and Joan Jacobs Chair in Exemplary Life Sciences.
Footnotes
Author contributions: N.-B.W.: conception and design, financial support, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; A.S.P.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; R.M.: conception and design, collection and/or assembly of data, data analysis and interpretation; M.K.L., K.J.B., W.T.B., and J.C.I.B.: conception and design, provision of study material or patients; A.L.F.: conception and design, collection and/or assembly of data; A.R.: collection and/or assembly of data, provision of study materials or patients; F.H.G: financial support, provision of study material or patients; I.M.V.: conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
N.-B.W. and A.S.P. contributed equally to this article.
Disclosure of Potential Conflicts of Interest
The other authors indicate no potential conflicts of interest.
See www.StemCells.com for supporting information available online.
References
- 1.Daley GQ. Stem cells: Roadmap to the clinic. J Clin Invest. 2010;120:8–10. doi: 10.1172/JCI41801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiskinis E, Eggan K. Progress toward the clinical application of patient-specific pluripotent stem cells. J Clin Invest. 2010;120:51–59. doi: 10.1172/JCI40553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufman DS, Hanson ET, Lewis RL, et al. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2001;98:10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zambidis ET, Peault B, Park TS, et al. Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development. Blood. 2005;106:860–870. doi: 10.1182/blood-2004-11-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vodyanik MA, Bork JA, Thomson JA, et al. Human embryonic stem cell-derived CD34+ cells: Efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005;105:617–626. doi: 10.1182/blood-2004-04-1649. [DOI] [PubMed] [Google Scholar]
- 6.Tian X, Woll PS, Morris JK, et al. Hematopoietic engraftment of human embryonic stem cell-derived cells is regulated by recipient innate immunity. Stem Cells. 2006;24:1370–1380. doi: 10.1634/stemcells.2005-0340. [DOI] [PubMed] [Google Scholar]
- 7.Narayan AD, Chase JL, Lewis RL, et al. Human embryonic stem cell-derived hematopoietic cells are capable of engrafting primary as well as secondary fetal sheep recipients. Blood. 2006;107:2180–2183. doi: 10.1182/blood-2005-05-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang KH, Nelson AM, Cao H, et al. Definitive-like erythroid cells derived from human embryonic stem cells coexpress high levels of embryonic and fetal globins with little or no adult globin. Blood. 2006;108:1515–1523. doi: 10.1182/blood-2005-11-011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galic Z, Kitchen SG, Kacena A, et al. T lineage differentiation from human embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:11742–11747. doi: 10.1073/pnas.0604244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ledran MH, Krassowska A, Armstrong L, et al. Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell. 2008;3:85–98. doi: 10.1016/j.stem.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Lu SJ, Feng Q, Park JS, et al. Biologic properties and enucleation of red blood cells from human embryonic stem cells. Blood. 2008;112:4475–4484. doi: 10.1182/blood-2008-05-157198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takayama N, Nishikii H, Usui J, et al. Generation of functional platelets from human embryonic stem cells in vitro via ES-sacs, VEGF-promoted structures that concentrate hematopoietic progenitors. Blood. 2008;111:5298–5306. doi: 10.1182/blood-2007-10-117622. [DOI] [PubMed] [Google Scholar]
- 13.Choi KD, Yu J, Smuga-Otto K, et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27:559–567. doi: 10.1634/stemcells.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eilken HM, Nishikawa S, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457:896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- 15.Raya A, Rodrigez-Piza I, Guenechea G, et al. Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature. 2009;460:53–59. doi: 10.1038/nature08129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi KD, Vodyanik MA, Slukvin II. Generation of mature human myelomonocytic cells through expansion and differentiation of pluripotent stem cell-derived lin-CD34+CD43+CD45+ progenitors. J Clin Invest. 2009;119:2818–2829. doi: 10.1172/JCI38591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatzistavrou T, Micallef SJ, Ng ES, et al. ErythRED, a hESC line enabling identification of erythroid cells. Nat Methods. 2009;6:659–662. doi: 10.1038/nmeth.1364. [DOI] [PubMed] [Google Scholar]
- 18.Galic Z, Kitchen SG, Subramanian A, et al. Generation of T lineage cells from human embryonic stem cells in a feeder free system. Stem Cells. 2009;27:100–107. doi: 10.1634/stemcells.2008-0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong SH, Werbowetski-Ogilvie T, Ramos-Mejia V, et al. Multiparameter comparisons of embryoid body differentiation toward human stem cell applications. Stem Cell Res. 2010;5:120–130. doi: 10.1016/j.scr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Ramos-Mejia V, Melen GJ, Sanchez L, et al. nodal/activin signaling predicts human pluripotent stem cell lines prone to differentiate toward the hematopoietic lineage. Mol Ther. 2010;18:2173–2181. doi: 10.1038/mt.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludwig TE, Levenstein ME, Jones JM, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig TE, Bergendahl V, Levenstein ME, et al. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006;3:637–646. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- 23.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 24.Qiu C, Olivier EN, Velho M, et al. Globin switches in yolk sac-like primitive and fetal-like definitive red blood cells produced from human embryonic stem cells. Blood. 2008;111:2400–2408. doi: 10.1182/blood-2007-07-102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertrand JY, Chi NC, Santoso B, et al. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goessling W, North TE, Loewer S, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136:1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.North TE, Goessling W, Walkley CR, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang CC, Kaba M, Iizuka S, et al. Angiopoietin-like 5 and IGFBP2 stimulate ex vivo expansion of human cord blood hematopoietic stem cells as assayed by NOD/SCID transplantation. Blood. 2008;111:3415–3423. doi: 10.1182/blood-2007-11-122119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuoka S, Tsuji K, Hisakawa H, et al. Generation of definitive hematopoietic stem cells from murine early yolk sac and paraaortic splanchnopleures by aorta-gonad-mesonephros region-derived stromal cells. Blood. 2001;98:6–12. doi: 10.1182/blood.v98.1.6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.