Abstract
Background
Some of the genetic vulnerability for addiction may be mediated by impulsivity. This study investigated relationships among impulsivity, substance use problems and six neurexin-3 (NRXN3) polymorphisms. Neurexins (NRXNs) are presynaptic transmembrane proteins that play a role in the development and function of synapses.
Methods
Impulsivity was assessed with the Barratt Impulsiveness Scale Version 11 (BIS-11), the Boredom Proneness Scale (BPS) and the TIME paradigm; alcohol problems with the Michigan Alcoholism Screening Test (MAST); drug problems with the Drug Abuse Screening Test (DAST-20); and regular tobacco use with a single question. Participants (N = 439 Caucasians, 64.7% female) donated buccal cells for genotyping. Six NRXN3 polymorphisms were genotyped: rs983795, rs11624704, rs917906, rs1004212, rs10146997 and rs8019381. A dual luciferase assay was conducted to determine whether allelic variation at rs917906 regulated gene expression.
Results
In general, impulsivity was significantly higher in those who regularly used tobacco and/or had alcohol or drug problems. In men, there were modest associations between rs11624704 and attentional impulsivity (p = .005) and between rs1004212 and alcohol problems (p = .009). In women, there were weak associations between rs10146997 and TIME estimation (p = .03); and between rs1004212 and drug problems (p = .03). The dual luciferase assay indicated that C and T alleles of rs917906 did not differentially regulate gene expression in vitro.
Conclusions
Associations between impulsivity, substance use problems and polymorphisms in NRXN3 may be gender specific. Impulsivity is associated with substance use problems and may provide a useful intermediate phenotype for addiction.
Keywords: NRXN3, cell adhesion protein, substance use disorder, impulsiveness
1. Introduction
There is abundant evidence that an individual’s vulnerability to addiction is influenced by their genetic makeup but identifying the genes that contribute to that risk has been and continues to be a vexing task (Li and Burmeister, 2009). For a given substance use disorder, there is an expectation that there are genes that affect addiction vulnerability to that particular substance, and that there are also genes that influence traits that increase vulnerability to addiction, in general. For example, polymorphisms in genes that code for enzymes involved in alcohol metabolism (i.e., alcohol dehydrogenase [ADH] and aldehyde dehydrogenase [ALDH]) have been convincingly shown to influence risk for alcohol dependence, and a gene that codes for an enzyme involved in catecholamine metabolism (i.e., catechol-o-methyl transferase [COMT]) appears to influence addiction risk through its influence on behavioral traits such as impulsivity and anxiety (Ducci and Goldman, 2008).
Impulsivity is an interesting trait in the context of addiction vulnerability. The general construct of “impulsivity” represents several independent facets such as response inhibition, resistance to delay of reinforcement, timing, behavioral switching, motor impulsivity, cognitive impulsivity, preparation, execution outcome, premature responding and lack of persistence (Evenden, 1999). Individuals with elevated levels of impulsivity are at increased risk for problems with alcohol (Dick et al., 2009; Lejuez et al., 2010), stimulants (Ersche et al., 2010), and nicotine (Doran et al., 2009; Spillane et al., 2010). In animal models, individual differences in different facets of impulsivity predict drug self-administration and exposure to drugs and increase impulsivity (see Winstanley et al., 2010 for a review). Slow developing behavioral control in children is associated with increased risk for adolescent substance use (Wong et al., 2006) and gender appears to modify the association between different types of impulsivity and alcohol problems (Stoltenberg et al., 2008). The relations among different facets of impulsivity and aspects of substance use or problems are not yet fully characterized, but there is growing appreciation of their complexity (Lejuez et al., 2010). There is some emerging evidence that individual differences in certain facets of impulsivity are influenced by genes in neurotransmitter systems (e.g., Stoltenberg et al., 2006; Walderhaug et al., 2010), but little is known about the underlying genetic architecture of impulsivity.
Recent empirical evidence suggests that the gene that codes for Neurexin-3 (NRXN3) may be a good candidate for general addiction vulnerability. Certain alleles of three single nucleotide polymorphisms (SNPs) within the fifth splice site of the NRXN3 gene were more common in alcohol dependent subjects than in matched controls (Hishimoto et al., 2007). A genome wide association study found suggestive evidence that a NRXN3 SNP (rs2221299) was associated with nicotine dependence (Bierut et al., 2007). Another NRXN3 SNP (rs1004212) was associated with the amount of nicotine consumption in schizophrenia patients (Novak et al., 2009). A genome-wide linkage study indicated an area on chromosome 14q, on which the NRXN3 gene is located, was linked to opioid dependence (Lachman et al., 2007).
Neurexins (NRXNs) are presynaptic transmembrane proteins that function as cell adhesion molecules, binding with neuroligins to stabilize the synapse (Hata and Südhof, 1995). There is growing evidence that neurexins are key elements properly functioning synapses and that NRXN dysfunction may play a role in diseases with a cognitive component (Südhof, 2008). The genes that code for NRXNs are large, contain numerous polymorphisms and are subject to alternative splicing. Regulatory region and splice site variants are likely to have a substantial impact on NRXN expression. NRXN proteins are encoded by three separate, unlinked genes: NRXN1 (2p16.3), NRXN2 (11q13), and NRXN3 (14q31). Each of the three NRXN genes has two promoters from which a longer alpha and shorter beta NRXNs are transcribed (Rowen et al., 2002). The alpha promoters are located at the 5′ end of the genes, while the beta promoters are located between exons 17 and 18 (Rowen et al., 2002). Each of these NRXN genes also has multiple alternative splice sites and thousands of possible isoforms (Tabuchi and Südhof, 2002). The NRXN3 gene is one of the largest genes in the human genome containing 1,826,818 base pairs (Rowen et al., 2002).
Lines of mice with the α-NRXN gene knocked out showed that α-NRXN is responsible for the coupling of Ca2+-channels to synaptic vesicles in preparation for exocytosis, and that they are essential for normal neurotransmitter release (Missler et al., 2003). These α-NRXN knockout mice had low survival rates, and those that did survive had decreased neurotransmitter release at both inhibitory (gamma-amino butyric acid; GABA) and excitatory (glutamate) synapses. GABA is the brain’s major inhibitory neurotransmitter, and alcohol had been shown to mimic its effects on the GABAA receptor (Lovinger and Homanics, 2007). During development, α-NRXNs on pre-synaptic neurons promote post-synaptic specialization of GABAergic neurons by clustering GABAA receptors (Kang et al., 2007). The involvement of NRXNs in normal neurotransmitter release and synaptic integrity suggests that variation in their genes may have widespread and substantial effects on key behavioral phenotypes such as impulsivity. To our knowledge, there have been no studies to date to examine potential associations between NRXN3 polymorphisms and impulsivity.
This study was designed to investigate potential associations among types of impulsivity, substance use problems, and genetic polymorphisms in NRXN3. Our hypothesis is in line with the notion that impulsivity is a key construct in the pathways from genes to risky behaviors, which can lead to behavioral disorders such as addiction.
2. Methods
2.1. Identifying Single Nucleotide Polymorphisms for Genotyping
The SNP@Promoter database was used to identify SNPs in the NRXN3 α-promoter that may affect gene regulation (http://variome.kobic.re.kr/SNPatPromoter/; Kim et al., 2008). We identified 20 SNPs found within the α-promoter, and selected one of these SNPs (rs917906; chromosome 14 position 77,939,227) because of its location in a transcription factor (TF) binding site [the CCAAT/ Enhancer Binding Protein-gamma (C/EBPγ)] 619 base pairs upstream of the start codon. Though termed “enhancer” the C/EBPγ TF can act as both an activator and repressor depending on the cell and promoter context (Parkin et al., 2002).
Recently, a report was published that identified several areas in putative regulatory regions upstream of the NRXN3 structural gene (Pedrosa et al., 2010). Using HapMap, we identified 21 SNPs located in those regions and then used SNP Cutter software (Liu et al, 2009), to identify two (rs983795, position 77,767,309 and rs11624704, position 77,855,830) for which we could design restriction fragment length polymorphism assays.
We also genotyped three other SNPs, using TaqMan SNP Genotyping Assays because they were previously identified as being associated with alcohol dependence (rs8019381, position 79,390,336; Hishimoto et al. 2007), quantity of nicotine intake (rs1004212 position 78,250,979; Novak et al., 2009) and obesity (rs10146997, position 79,014,915; Heard-Costa et al., 2009).
2.2 Participants and Measures
All participants were recruited at a small Midwestern university via posters and in-class presentations about the study, were informed about the particular study and signed informed consent documents. Participants (N = 477) completed multiple questionnaires that assessed impulsivity and involvement in health-risk behaviors, four computer tasks, and donated buccal cells for genotyping. Participation in this study was voluntary, and all participants were compensated $20. The study was approved by the Institutional Review Board (IRB). In this manuscript we will report only analyses focused on testing the associations between impulsivity, NRXN3 polymorphisms, and substance use problems; other findings from this study will be reported elsewhere.
To reduce the risk of population stratification data was analyzed from only the 92% who self-identified as Caucasians (N = 439; 64.7% female). Age ranged from 18 to 67 (Mean= 22.49, SD= 6.12; 81.6% were age 24 or younger). Four individuals did not report age.
The Barratt Impulsiveness Scale (BIS-11) is a 30-item self-report instrument that uses a 4-point Likert scale from Rarely/Never to Almost Always (Patton et al., 1995). A total score is calculated by summing three subscale scores. The subscales are: Motor (“I act on the spur of the moment”), Attentional (“I have outside thoughts when thinking”) and Nonplanning (“I plan trips well ahead of time” reverse scored). The total score is reliable (in terms of internal consistency, Cronbach’s α = .86 in this study).
The Boredom Proneness Scale (BPS) is a 28-item self-report instrument that uses a 5-point Likert scale from “highly disagree” to “highly agree” (Farmer and Sundberg, 1986). A total score is calculated by summing five subscales. The subscales include: External Stimulation (“When I was young, I was often in monotonous and tiresome situations”), Internal Stimulation (“It is easy for me to concentrate on my activities” reverse scored), Affect (“Frequently when I am working I find myself worrying about other things”), Time Perception (“Time always seems to be passing slowly”) and Constraint (“I am good at waiting patiently” reverse scored). The total score has good reliability (Cronbach’s α = .86 in this study).
The The Time Paradigm (Dougherty et al., 2005) was administered on a desk top personal computer with a cathode ray tube display with a refresh rate of 60 Hz. The Time Paradigm is a behavioral assessment of the passage of time. Participants were asked to estimate the passage of 60 s five times. Participants were instructed to press the space bar to start the timer and to press it again when they thought that 60 s had passed. We analyzed the mean of the five estimates. Participants did not receive any feedback regarding the accuracy of their estimates or any payments based on their performance.
We assessed tobacco use with a single yes/no item: “Do you regularly use tobacco (cigarettes, cigars, chewing tobacco)?” We assessed alcohol problems with 22 items from the Michigan Alcohol Screening Test (MAST; Selzer, 1971). It consists of yes or no questions about alcohol consumption and related behaviors that were scored as either 1 (yes) or 0 (no), and are summed for a total score. We assessed drug problems with the Drug Abuse Screening Test (DAST-20), which is a 20 item self-report questionnaire that measures an individual’s involvement with drugs (not alcohol or tobacco) over the preceding 12 months (Skinner, 1982). Responses to the items were scored as either 1 (yes) or 0 (no), and are summed for a total score. For both the MAST and the DAST we used a cutoff score of five to identify those with probable problems (i.e., 0-5 = no problems, 6+ = problems).
2.3. Genotyping
DNA was extracted using the DNeasy Blood and Tissue Kit (Qiagen Inc, Valencia, CA, USA). Twenty μL PCR reactions for rs8019381 (Applied Biosystems, Inc., genotyping assay ID, C_29283249_10), rs917906 (C_2674044_20), rs1004212 (C_16183691_10) and rs10146997 (C_30288512_10), were prepared in 2x ABI TaqMan Universal Master Mix no UNG (Applied Biosystems, Inc., Foster City, CA) adding the corresponding 20x Taqman SNP genotyping assay containing Fam and Vic-labelled allele-specific TaqMan MGB probes and forward and reverse primers. The PCR was performed with the following cycling parameters: 95°C for 10 min, followed by 40 cycles of 92°C for 15 sec and 60°C for 1 min. End point FAM and VIC florescence levels were measured StepOnePlus Real-Time PCR System (Applied Biosystems, Inc., Foster City, CA), and genotype calls were made based on this level of fluorescence signal.
rs983795 was amplified using the primers: forward: 5′-AGG GAT ACC TGT TGG GAG AAC C-3′ and reverse: 5′-TTA GAG CCAA GGC ACT ACA CCC-3′. PCR reactions (25μL) contained 20ng of DNA, 1X GoTaq Green Master Mix (Promega), and 400nM final concentration of each primer. The PCR was performed with the following cycling parameters: 95°C for 6 min, followed by 35 cycles of 95°C for 30 sec, 56.6°C for 30 sec, and 72°C for 1 min, with a final extension at 72°C for 10 min. Ten μL of the PCR product was digested for 1 hour at 37°C with 20 units of NdeI enzyme (New England Biolabs) and run on a 1% agarose gel at 100V for 1.5 hours. Fragments were visualized under UV light with ethidium bromide stain. Fragment sizes for alternative alleles are A= 373bp and G= 259bp and 114bp.
rs11624704 was amplified using the primers: forward: 5′-TTG CCT TAC ACA CTG GTG GTT G-3′ and reverse: 5′-AAT GCA CTT CTG TTC CTC CAG C-3′. PCR reactions (25μL) contained 20ng of DNA, 1X GoTaq Green Master Mix (Promega), and 400nM final concentration of each primer. The PCR was performed with the following cycling parameters: 95°C for 6 min, followed by 35 cycles of 95°C for 30 sec, 55.8°C for 30 sec, and 72°C for 1 min, with a final extension at 72°C for 10 min. Ten μL of the PCR product was digested for 1 hour 37°C with 2.5 units of MseI enzyme (New England Biolabs) and run on a 1% agarose gel at 100V for 1.5 hours. Fragments were visualized under UV light with ethidium bromide stain. Fragment sizes for alternative alleles are C= 276bp and A= 135bp and 141bp.
Genotyping assays were repeated for approximately 10% of samples for all six SNPs. There were no discrepancies among genotype calls.
2.4. Dual Luciferase Assay
To determine whether allelic variation at the rs917906 affected gene expression, a dual luciferase assay was conducted by amplifying the 301bp region from two individual DNA samples (one with a C/C genotype and one with a T/T genotype for the rs917906) as described above with the exception of the final extension being extended to 30 minutes; to ensure addition of the 3′ terminal A by Taq polymerase for use in the T-A cloning. The resulting amplicons were cloned into the TOPO-TA vector (Invitrogen, Carlsbad, CA, USA). Inserts were excised with XhoI and HindIII enzymes to generate constructs in the forward orientation, and cloned into the pGL4.1 vector (Promega, Madison, WI, USA). The constructs were verified by sequencing.
The RN46A raphe-derived neuronal cell line was provided by Scott R. Whittemore (University of Louisville School of Medicine). Cells were cultured in Dulbecco’s Modified Eagles Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) 100 units/mL of penicillin, 100μg/mL of streptomycin, and 250μg/mL G418. Cell culture medium without antibodies included on the DMEM and 10% FBS. RN46A cells were cultured at 33°C in a humidified 5% CO2 atmosphere for two days. The medium was then changed to cell culture medium without antibodies for two days. The transfection then began on the fifth day.
Two clones of each genotype (C/C and T/T) were used. RN46A cells were transfected with 1μg of the rs917906 reporter constructs plus 0.1μg of the Renilla luciferase pRL-SV40 control DNA using Lipofectamine 2000 and incubated for twenty minutes at room temperature. After incubation, 150μL was added to each well which contained the RN46A raphe neurons and medium. The plate was then gently mixed by rocking back and forth, then incubated at 33°C in a humidified 5% CO2 atmosphere for 24 hours. Luciferase activity was then assayed using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Luciferase activity was normalized relative to the activity of the Renilla luciferase produced by the pRL-SV40 control vector and each construct was tested in three independent experiments.
2.5. Statistical Analyses
Multivariate general linear models were used to test associations between impulsivity subscale scores, gender and substance use problems (IBM, SPSS Statistics, 19; Chicago IL). To test associations between NRXN3 SNPs and impulsivity and substance use problems separately for men and women we used basic allele tests in SNP and Variation Suite 7 (Golden Helix, Bozeman MT). In basic allele tests for a given phenotype, individuals are considered to have two identical phenotype scores, one for each allele. That is, each element in an individual’s genotype is considered to correspond to the same phenotypic value. Basic allele tests do not enable testing of additive or dominance models. We chose to conduct basic allele tests rather than examining additive and dominance models to reduce the number of comparisons thereby preserving statistical power. A paired t-test was conducted for the dual luciferase reporter assay to determine whether rs917906 alleles differentially affected transcription.
3. Results
3.1. Descriptive Statistics
Overall descriptive statistics are shown in Table 1. Our sample of men and women did not differ significantly by age. However, men had higher mean scores on Time estimation (p = .038), BIS-11 Total (p = .01), and Motor subscale (p = .001), BPS Total (p = .016) and External Stimulation subscale (p = .000). The percent reporting regular use of tobacco did not differ for men and women. There was a trend for more men to be classified as having alcohol problems (p = .092). More men than women were classified as having drug problems (p = .018). Subsequent analyses of behavioral data were conducted separately for men and women in order to reduce potential confounds and to preserve our capacity to detect differences in patterns of association.
Table 1.
Overall Descriptive Statistics; Mean (S. D.)
| Men (n=155) | Women (N=283) | p-value | |
|---|---|---|---|
| Age | 22.53 (6.28) | 22.46 (6.05) | 0.910 |
| Time Estimation | 63.59 (18.22) | 59.79 (18.28) | 0.038 |
| BIS-11 | |||
| Total | 66.83 (10.64) | 63.96 (11.36) | 0.010 |
| Attentional | 18.07 (4.10) | 17.31 (4.14) | 0.066 |
| Nonplanning | 24.75 (5.03) | 24.10 (5.00) | 0.197 |
| Motor | 24.01 (4.37) | 22.55 (4.68) | 0.001 |
| BPS | |||
| Total | 74.03 (11.03) | 71.13 (12.43) | 0.016 |
| External Stimulation | 22.94 (4.82) | 20.28 (4.85) | 0.000 |
| Internal Stimulation | 19.11 (4.78) | 19.56 (4.63) | 0.336 |
| Affect | 13.84 (2.99) | 13.93 (3.12) | 0.769 |
| Perception of time | 9.83 (2.91) | 9.32 (2.84) | 0.072 |
| Constraint | 5.83 (2.04) | 5.78 (2.08) | 0.838 |
| Tobacco Use (%) | 24.5 | 19.4 | 0.207 |
| Alcohol Problem (%) | 16.1 | 10.6 | 0.092 |
| Drug Problem (%) | 16.8 | 9.2 | 0.018 |
Note: Three men did not report age and one woman did not have scores for BPS. P-values are either from independent sample t-tests or chi-square tests.
Marker statistics for the six NRXN3 SNPs tested are shown in Table 2. Genotype frequencies are shown in Table 3. All markers, but rs917906 were in Hardy-Weinberg equilibrium (p>.05). We genotyped rs917906 using the TaqMan assay and an RFLP method and neither method resulted in genotype calls that were in HW equilibrium. We were unable to determine the cause of this discrepancy and although we are confident with our genotype calls, we acknowledge that the lack of HW equilibrium with this marker should be considered in interpretation. There were no linkage disquilibrium (LD) blocks in our sample or in the combined panel of Utah residents with Northern and Western European ancestry from the CEPH collection and Toscans in Italy (CEU+TSI) sample of HapMap data (see Figure 1). The pattern of LD that we observed in our sample was similar to that seen in the CEU+TSI sample.
Table 2.
NRXN3 Marker Statistics
| SNP | Position on Chr. 14 |
Region | Alleles minor/major |
MAF | HWE P |
|---|---|---|---|---|---|
| rs983795 | 77,767,309 | α-promoter | A/G | 0.133 | 0.900 |
| rs11624704 | 77,855,830 | α-promoter | C/A | 0.134 | 0.459 |
| rs917906 | 77,939,227 | α-promoter | C/T | 0.472 | 0.029 |
| rs1004212 | 78,250,979 | exon 5 | T/C | 0.172 | 0.106 |
| rs10146997 | 79,014,915 | intron | G/A | 0.239 | 0.245 |
| rs8019381 | 79,390,336 | splicing site 5 | T/C | 0.103 | 0.083 |
Note: MAF = Minor Allele Frequency; HWE P = Hardy-Weinberg Equilibrium p-value.
Table 3.
Genotype Frequencies by Gender
| SNP | Genotype | Men (n = 155) |
Women (n = 284) |
Overall (N = 439) |
|---|---|---|---|---|
| rs983795 | A/A | 0 | 8 | 8 |
| A/G | 37 | 71 | 108 | |
| G/G | 118 | 205 | 323 | |
| rs11624704 | C/C | 3 | 3 | 6 |
| C/A | 30 | 75 | 105 | |
| A/A | 118 | 205 | 323 | |
| rs917906 | C/C | 29 | 56 | 85 |
| C/T | 92 | 147 | 239 | |
| T/T | 31 | 79 | 110 | |
| rs1004212 | T/T | 1 | 7 | 8 |
| T/C | 54 | 79 | 133 | |
| C/C | 98 | 197 | 295 | |
| rs10146997 | G/G | 9 | 21 | 30 |
| G/A | 49 | 98 | 147 | |
| A/A | 93 | 162 | 255 | |
| rs8019381 | T/T | 4 | 4 | 8 |
| T/C | 24 | 50 | 74 | |
| C/C | 127 | 229 | 356 |
Note: All call rates >98%.
Figure 1.
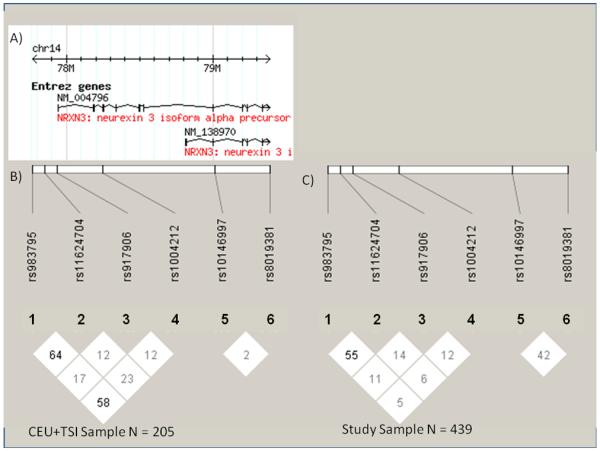
Linkage disequilibrium (LD) data (D’/LOD) for the six NRXN3 SNPs tested. A) The relative location of the two alternatively spliced forms of NRXN3. B) LD data from CEU+TSI samples from HapMap (version 3 release R2). C) LD data from this study.
3.2. Impulsivity and Regular Tobacco Use
For men who indicated regular tobacco use, multivariate general linear models with age as a covariate indicated significantly higher mean scores for the Attentional (p = .005), Nonplanning (p = .000) and Motor (p = .001) subscales of the BIS-11 and for the Perception of time (p = .031) subscale of the BPS (see Figure 2). For women who indicated regular tobacco use, multivariate general linear models with age as a covariate indicated significantly higher mean scores for the Attentional (p = .01), Nonplanning (p = .001) and Motor (p = .001) subscales of the BIS-11 and for the External stimulation (p = .033), Affect (p = .004), Perception of time (p = .028), and Constraint (p = .006) subscales of the BPS. Time estimation was not significantly associated with tobacco use in men or women.
Figure 2.
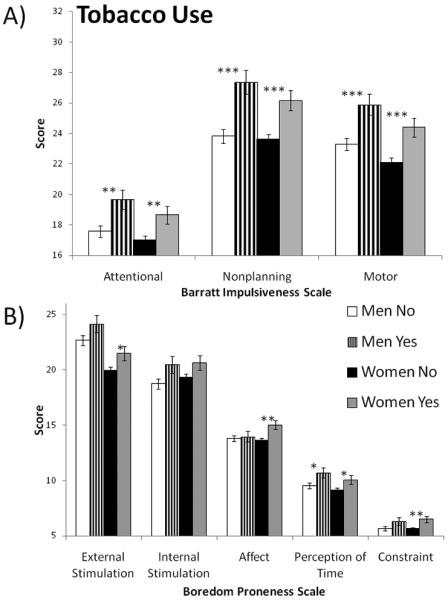
Mean scores on impulsivity subscales for men and women who reported regularly using tobacco. A) Barratt Impulsiveness Scale (version 11). B) Boredom Proneness Scale. *p<.05; **p<.01; ***p<.001
3.3. Impulsivity and Alcohol Problems
For men who had alcohol problems (i.e., MAST > 5), multivariate general linear models with age as a covariate indicated significantly higher mean scores for the Attentional (p = .018), Nonplanning (p = .000) and Motor (p = .000) subscales of the BIS-11 (see Figure 3). None of the mean BPS subscale scores were different for men with and without alcohol problems. For women who had alcohol problems, multivariate general linear models with age as a covariate indicated significantly higher mean scores for the Attentional (p = .005), Nonplanning (p = .011) and Motor (p = .000) subscales of the BIS-11 and for the External stimulation (p = .028), Affect (p = .007), Perception of time (p = .000), and Constraint (p = .035) subscales of the BPS. Time estimation was not significantly associated with alcohol problems in men or women.
Figure 3.
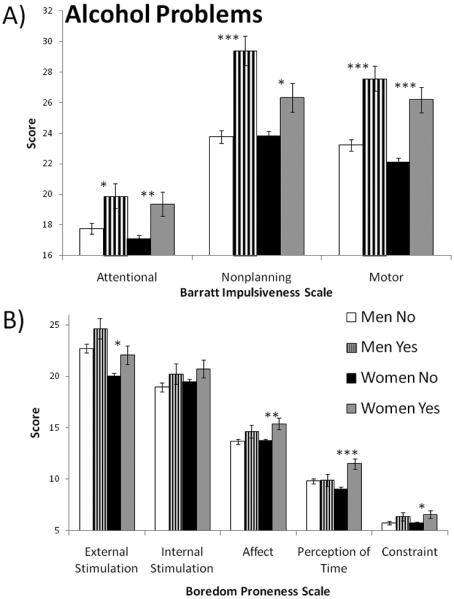
Mean scores on impulsivity subscales for men and women who had alcohol problems (i.e., scoring 6 or more on the Michigan Alcoholism Screening Test). A) Barratt Impulsiveness Scale (version 11). B) Boredom Proneness Scale. *p<.05; **p<.01; ***p<.001
3.4. Impulsivity and Drug Problems
For men who had drug problems (i.e., DAST > 5), multivariate general linear models with age as a covariate indicated significantly higher mean scores for the Nonplanning (p = .002) and Motor (p = .000) subscales of the BIS-11 (see Figure 4). None of the mean BPS subscale scores were different for men with and without drug problems. For women who had drug problems, multivariate general linear models with age as a covariate indicated significantly higher mean scores for the Attentional (p = .000), Nonplanning (p = .000) and Motor (p = .000) subscales of the BIS-11 and for the Perception of time (p = .006), subscale of the BPS. Time estimation was not significantly associated with drug problems in men or women.
Figure 4.
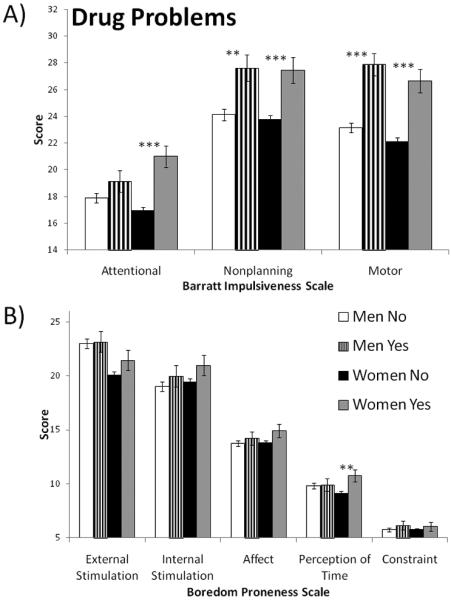
Mean scores on impulsivity subscales for men and women who had drug problems (i.e., scoring 6 or more on the Drug Abuse Screening Test). A) Barratt Impulsiveness Scale (version 11). B) Boredom Proneness Scale. *p<.05; **p<.01; ***p<.001
3.5. NRXN3 Association Tests
Basic allele tests identified a significant association between rs11624704 and BIS-11 Total Score (p = .01; see Table 4). Although this result does not survive Bonferroni correction (i.e., for each phenotype we conducted six independent association tests for each gender; pcrit = .05/6 = .008), it suggests that investigating potential associations between rs11624704 genotype and BIS-11 subscales might identify one or more subscales to be associated with the SNP. Therefore, we then conducted basic allele tests of rs11624704 on the three BIS-11 subscales in men, which indicated a significant association with Attentional impulsivity (p = .005; Bonferroni correction pcrit = .05/3 = .017; see Figure 5). In women, there was evidence of a weak association between Time Estimation and rs10146997 genotype (p = .027) that fails to survive Bonferroni correction (pcrit = .05/6 = .008).
Table 4.
Basic Allele Test p-values.
| SNP | TIME | BIS-11 | BPS | Regular Tobacco use |
Alcohol Problem |
Drug Problem |
|
|---|---|---|---|---|---|---|---|
| Men | rs983795 | 0.67 | 0.42 | 0.20 | 0.84 | 1.00 | 0.16 |
| rs11624704 | 0.60 | 0.01 | 0.43 | 0.23 | 0.63 | 0.48 | |
| rs917906 | 0.12 | 0.45 | 0.74 | 0.90 | 0.35 | 0.36 | |
| rs1004212 | 0.23 | 0.44 | 0.20 | 0.31 | 0.009 | 0.11 | |
| rs10146997 | 0.30 | 0.80 | 0.71 | 1.00 | 1.00 | 0.27 | |
| rs8019381 | 0.12 | 0.51 | 0.42 | 0.83 | 1.00 | 0.62 | |
| Women | rs983795 | 0.16 | 0.95 | 0.91 | 0.22 | 0.84 | 1.00 |
| rs11624704 | 0.75 | 0.85 | 0.07 | 0.17 | 0.33 | 0.40 | |
| rs917906 | 0.42 | 0.40 | 0.27 | 0.67 | 0.50 | 1.00 | |
| rs1004212 | 0.68 | 0.08 | 0.29 | 0.89 | 0.36 | 0.03 | |
| rs10146997 | 0.03 | 0.75 | 0.75 | 0.27 | 0.12 | 0.87 | |
| rs8019381 | 0.97 | 0.72 | 0.75 | 0.73 | 1.00 | 0.64 | |
Note: Uncorrected p-values for Regular Tobacco use, Alcohol Problem, and Drug Problem are from Fisher’s exact tests. Other p-values are from F-tests.
Figure 5.
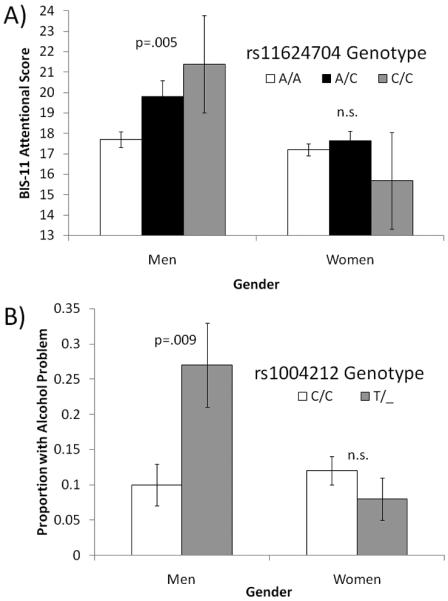
Significant associations between NRXN3 SNPs and behaviors. A) Mean (±s.e.) BIS-11 Attentional score for men and women grouped by rs11624704 genotype. B) Proportion (95% CI) of individuals with alcohol problems in groups defined by rs1004212 genotype and gender. P-values shown are not corrected for multiple comparisons.
Basic allele tests identified a significant association between rs1004212 and alcohol problems in men (p = .009; see Table 4 and Figure 4). Having a copy of the T allele of rs1004212 increased risk for having alcohol problems 2.54 times. Interestingly, in women the same allele decreased risk for having drug problems (p = .03; OR = 0.29). Although neither of these findings survived strict Bonferroni correction, they suggest that further study of this locus is needed.
3.6. Luciferase Reporter Assay
To determine whether alternative alleles at rs917906 regulate gene expression of the luciferase gene in vitro, a ratio of the expression of the luciferase over the expression of the control Renilla luciferase was calculated. Using this ratio a paired t-test was performed. No significant difference was found between the C (Mean expression= 0.0481) and T (Mean expression= 0.0480) alleles: paired t(5) = 0.027, p= 0.97. Therefore, the C and T alleles do not differentially regulate NRXN3 transcription in vitro.
4. Discussion
We found suggestive evidence that NRXN3 polymorphisms are associated with impulsivity (i.e., rs11624704 and Attentional impulsivity) and alcohol problems (rs1004212) in men. To our knowledge, this is the first report of an association between a NRXN3 polymorphism and impulsivity. This finding may prove to be important in understanding the genetic architecture of addiction because impulsivity is an important risk factor for addictions and other behavioral disorders. Evidence is accumulating that NRXN3 may be in a pathway that influences vulnerability to addiction in general. In that sense, our findings add to the growing evidence that implicates polymorphism in NRXN3 in risk for substance use problems and/or dependence (Lachman et al., 2007; Hishimoto et al., 2007; Beirut et al., 2007) and obesity (Heard-Costa et al., 2009). We recognize that the overlap in genetic risk for addiction to different substances and obesity is likely to be quite narrow, but it may be that this overlap includes genetic polymorphisms associated with individual differences in impulsivity. Further investigation with detailed phenotypic assessment that includes patterns of substance use, substance use disorder diagnoses, multiple measures of impulsivity and additional NRXN3 polymorphisms should be conducted.
Novak et al. (2009) reported that in individuals with schizophrenia those with the C/C genotype of rs1004212 smoked more cigarettes per day on average than those with the C/T genotype. We did not find an association between rs1004212 genotype and regular tobacco use, which is consistent with Novak et al. (2009), who did not find an association with rs1004212 and risk for smoking. We did not assess the quantity of tobacco used for those who reported to be regular tobacco users in our study, so we cannot directly test the previously reported association. Our primary finding with substance use problems, however, was that in men, rs1004212 genotype was associated with risk for alcohol problems. We found that men carrying a T allele were at 2.54 times greater risk of having alcohol problems than C/C homozygotes. Interestingly, in women, there is a non-significant trend for the T allele to be associated with reduced risk of having a drug problem by about one third (OR = 0.39). Taken together, these findings suggest that rs1004212 should continue to be a SNP of interest in the context of addiction vulnerability.
Our finding that rs11624704 genotype was associated with a specific facet of impulsivity (BIS-11 Attentional) in men, but not in women, suggests that the influence of NRXN3 polymorphisms may be rather specific and that the use of composite scores or other somewhat gross measures and/or statistically controlling for gender differences may fail to identify potentially interesting associations. At this time, we have no explanation for gender specific effects of NRXN3.
We found strong evidence that higher impulsivity is associated with regular tobacco use and problems with alcohol and drugs. Mean subscale scores from the Barratt Impulsiveness Scale (version 11) were significantly higher for both men and women for regular tobacco users, and for those with alcohol and drug problems with the only exception being Attentional impulsivity in men with drug problems. In this single case, those men with drug problems had higher mean scores than those without, but the difference did not reach significance (p = .17). The Barratt Impulsiveness Scale appears to assess facets of impulsivity that are relevant to addiction similarly in men and women. We observed less consistent patterns with the Boredom Proneness Scale subscales. When there were significant differences between those who used tobacco or had problems with alcohol or drugs, the substance using group had a higher mean score than the comparison group. In men, the mean BPS subscale scores were not different for those with and without substance use problems. Only the perception of time subscale differed for men grouped by regular tobacco use. The BIS-11 appears to better assess substance use relevant aspects of impulsivity. Mean scores for Time estimation were not significantly different for men or women grouped by substance use/problem. However, it should be pointed out that the direction of the effects, while not statistically different, were consistently in the expected direction (i.e., shorter time estimates for substance use/problem groups). We think that behavioral impulsivity measures show promise and we will continue to optimize their parameters (e.g., number of trials and number of seconds estimated) for use in future studies. Dick et al. (2009) present a compelling case that the construct of impulsivity may be best studied by examining its many subfacets. In the present study, we used the well-known BIS-11 subscales to examine Negative Urgency (BIS-11 Attentional), Lack of Planning (all three BIS-11 subscales) and the less well known BPS to examine Lack of Perseverance (boredom proneness). We also used the TIME paradigm to assess Judgment of Time Elapse (see Tables 1 and 2 in Dick et al., 2009 for a discussion of how these measures assess particular impulsivity subfacets). More work to characterize the pattern of association between different subfacets of impulsivity and substance use phenotypes is needed.
Men are consistently found to be more likely than women to have drinking problems (Wilsnack et al., 2000), and generally have more risk factors for alcohol use and problems than women (Nolen-Hoeksema and Hilt, 2006). There is less consistency in findings relating to differences in types of impulsivity based on gender (Reynolds et al., 2006). It does appear however, that higher levels of impulsivity in men, partially mediates their increased risk for alcohol problems (Stoltenberg et al., 2008). The present study appears to be the first report of gender differences in patterns of association with the NRXN3 gene and behavior.
Alternative alleles at rs917906 did not differentially regulate gene expression in vitro. It is possible that rs917906 exerts some regulatory control over NRXN3 expression, but in our hands, the dual luciferase assay did not indicate differential expression. It may be that in vivo rs917906 affects gene regulation in conjunction with other SNPs (i.e., a haplotype) (Glatt et al., 2006), or that it is in linkage disequilibrium (LD) with a functional variant. Another possibility for not observing a difference in transcriptional activity in the dual luciferase assay is that because there are low levels of expression of C/EBPγ in the brain (Kuo et al., 1990) it may not be expressed in raphe neurons or if it is expressed it may be at very low levels. GABAergic Purkinje cells in the cerebellum have higher levels of C/EBPγ expression than cells in other brain areas (Kuo et al., 1990). Given that NRXN proteins have been found to be essential for normal release of neurotransmitters at GABA neurons (Missler et al., 2003) it would be interesting to better characterize the regulatory impact of rs917906 using Purkinje cells and the SHSY57 neuroblastoma cell line used by Pedrosa et al. (2010). Additional NRXN3 regulatory region polymorphisms should also be tested for potential transcriptional regulation.
We recognize that our study has certain limitations and needs to be interpreted with some caution. More detailed assessments of the potential associations between important addiction related phenotypes (e.g., maximum number of drinks, age of onset) and genetic variation across NRXN3 need to be conducted. In addition, other facets of impulsivity such as delayed discounting need to be examined for association with NRXN3 polymorphisms. By current standards, our sample size is considered rather small and the p-values we report are not corrected for multiple comparisons, although we attempted to limit the number of comparisons that we made in order to conserve statistical power. In our study sample, the mean scores for the BIS-11 Total were higher than reported norms for both men and women (Patton and Barratt, 1995). We do not have a ready explanation for this observation, but note that more than a decade separates the two studies. It should be noted that our definitions of alcohol and drug problems were not meant as a diagnosis of alcohol abuse or dependence, but were used as a convenient metric of problematic substance involvement. Subsequent studies to investigate the role of NRXN3 in substance use and problems should employ more sensitive measures.
Our findings suggest that NRXN3 polymorphisms are modestly associated with specific types of impulsivity and substance use problems and that these patterns of association are not the same in men and women. Findings such as these, given their limitations, will be critical as we continue to improve our understanding of the pathways from genes to behavior. Impulsivity increases risk for several behavioral disorders such as addictions and therefore is an outstanding candidate trait for further study. A more complete description of the genetic architecture of impulsivity is likely to have a substantial impact on our understanding of the genetic architecture of a variety of behavioral disorders. NRXN3 is a promising candidate gene for addiction vulnerability and merits further study.
Acknowledgements
Thanks to: Dr. Cynthia Anderson and Dr. Garth Spellman for laboratory advice and manuscript comments, Timea Nelson, MS and Yueshan Hu for assistance with the Dual Luciferase Assay, Joanna Vandever, Natalie Lecy, Krista Highland, Ben Roman, Amber Richter, Hillary Schwab, Heidi Hankerson, Jeanie Stockland, Charity Ward, Brett Montieth, Jim Hellekson, Nathaniel Diede, Ava Sauter, and Matt Luebeck for assistance with data collection, and the participants, without whose efforts this research could not have been conducted. Thanks also to three anonymous reviewers whose comments greatly improved this article.
Role of Funding Source
Funding for this study was provided by NIH Grants 2 P20 RR016479 & R15 MH077654-01A1; the NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors report no financial interests or potential conflicts of interest.
References
- Bierut LJ, Madden PAF, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, Swan GE, Rutter J, Bertelsen S, Fox L, Fugman D, Goate AM, Hinrichs AL, Konvicka K, Martin NG, Montgomery GW, Saccone NL, Saccone SF, Wang JC, Chase GA, Rice JP, Ballinger DG. Novel genes identified in a high density genome wide association study for nicotine dependence. Hum. Mol. Genet. 2007;16:24–35. doi: 10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SO, Sher K. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict. Biol. 2009;15:217–226. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran N, Cook J, McChargue D, Spring B. Impulsivity and cigarette craving: differences across subtypes. Psychopharmacology. 2009;207:365–373. doi: 10.1007/s00213-009-1661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Marsh DM, Jagar AA. Laboratory behavioral measures of impulsivity. Behav. Res. Methods. 2005;37:82–90. doi: 10.3758/bf03206401. [DOI] [PubMed] [Google Scholar]
- Ducci F, Goldman D. Genetic approaches to addiction: genes and alcohol. Addiction. 2008;103:1414–1428. doi: 10.1111/j.1360-0443.2008.02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW. Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biol. Psychiatry. 2010;68:770–773. doi: 10.1016/j.biopsych.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Farmer R, Sundberg ND. Boredom proneness—the development and correlates of a new scale. J. Pers. Assess. 1986;50:4–17. doi: 10.1207/s15327752jpa5001_2. [DOI] [PubMed] [Google Scholar]
- Glatt CE, Wahner AD, Ruiz-Linares A, Ritz B. Gain-of-function haplotypes in the vesicular monoamine transporter promoter are protective for Parkinson disease in women. Hum. Mol. Genet. 2006;15:299–305. doi: 10.1093/hmg/ddi445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata Y, Südhof TC. A novel ubiquitous form of Munc-18 interacts with multiple syntaxins: use of the yeast two-hybrid system to study interactions between proteins involved in membrane traffic. J. Biol. Chem. 1995;270:13022–13028. doi: 10.1074/jbc.270.22.13022. [DOI] [PubMed] [Google Scholar]
- Heard-Costa NL, Zillikens MC, Monda KL, Johansson A, Harris TB, Fu M, Haritunians T, Feitosa MF, Aspelund T, Eiriksdottir G, Garcia M, Launer LJ, Smith AV, Mitchell BD, McArdle PF, Shuldiner AR, Bielinski SJ, Boerwinkle E, Brancati F, Demerath EW, Pankow JS, Arnold AM, Chen Y-DI, Glazer NL, McKnight B, Psaty BM, Rotter JI, Amin N, Campbell H, Gyllensten U, Pattaro C, Pramstaller PP, Rudan I, Struchalin M, Vitart V, Gao X, Kraja A, Province MA, Zhang Q, Atwood LD, Dupuis J, Hirschhorn JN, Jaquish CE, O’Donnell CJ, Vasan RS, White CC, Aulchenko YS, Estrada K, Hofman A, Rivadeneira F, Uitterlinden AG, Witteman JCM, Oostra BA, Kaplan RC, Gudnason V, O’Connelll JR, Borecki IB, van Duijn CM, Cupples LA, Fox CS, North KE. NRXN3 is a novel locus for waist circumference: a genome-wide association study from the CHARGE consortium. PLoS Genet. 2009;5:e1000539. doi: 10.1371/journal.pgen.1000539. doi:10.1371/journal.pgen.1000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersrud SL, Stoltenberg SF. Epistatic interaction between COMT and DAT1 genes in eating behavior: a pilot study. Eat. Behav. 2009;10:131–133. doi: 10.1016/j.eatbeh.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishimoto A, Lui QR, Drgon T, Pletnikova O, Walther D, Zhu XG, Troscoso JC, Uhl GR. Neurexin 3 polymorphisms are associated with alcohol dependence and altered expression of specific isoforms. Hum. Mol. Genet. 2007;16:2880–2891. doi: 10.1093/hmg/ddm247. [DOI] [PubMed] [Google Scholar]
- Kang Y, Zhang XZ, Dobie F, Wu H, Craig AM. Induction of GABAergic postsynaptic differentiation by α-Neurexins. J. Biol. Chem. 2007;238:2323–2334. doi: 10.1074/jbc.M703957200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BC, Kim WY, Park D, Chung WH, Shin K, Bhak J. SNP@Promoter: a database of human SNPs (Single Nucleotide Polymorphism) within the putative promoter regions. BMC Bioinformatics. 2008;9:S2. doi: 10.1186/1471-2105-9-S1-S2. doi:10.1186/1471-2105-9-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CF, Xanthopoulos KG, Darnell JE. Fetal and adult localization of C/EPB: evidence for combinational action of transcription factors in cell-specific gene expression. Development. 1990;109:473–481. doi: 10.1242/dev.109.2.473. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Fann CSJ, Bartzis M, Evgrafov OV, Rosenthal RN, Numes EV, Miner C, Santana M, Gaffney J, Riddick A, Hsu CL, Knowles JA. Genomewide suggestive linkage of opiod dependence to chromosome 14q. Hum. Mol. Genet. 2007;16:1327–1334. doi: 10.1093/hmg/ddm081. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Magidson JF, Mitchell SH, Sinha R, Stevens MC, de Wit H. Behavioral and biological indicators of impulsivity in the development of alcohol use, problems and disorders. Alcohol. Clin. Exp. Res. 2010;34:1334–1345. doi: 10.1111/j.1530-0277.2010.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD, Burmeister M. New insights into the genetics of addiction. Nat. Rev. Genet. 2009;10:225–231. doi: 10.1038/nrg2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Nguyen T, Zhang R, Yao F, Zhu Z, Gershon ES. SNP Information Mining Pipeline (SIMP) for Complex Disease Studies. Am. J. Human Genet. 2009;73:421. [Google Scholar]
- Lovinger DM, Homanics GE. Tonic for what ails us? High-affinity GABAA receptors and alcohol. Alcohol. 2007;41:139–143. doi: 10.1016/j.alcohol.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, Südhof TC. Alpha-Neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;423:939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- Nolan-Hoeksema S, Hilt L. Possible contributors to the gender differences in alcohol use and problems. J. Gen. Psychol. 2006;133:357–374. doi: 10.3200/GENP.133.4.357-374. [DOI] [PubMed] [Google Scholar]
- Novak G, Boukhadra J, Shaikh SA, Kennedy JL, Foll BL. Association of a polymorphism in the NRXN3 gene with the degree of smoking in schizophrenia: a preliminary study. World J. Biol. Psychiatry. 2009;10:929–935. doi: 10.1080/15622970903079499. DOI: 10.1080/15622970903079499. [DOI] [PubMed] [Google Scholar]
- Parkin SE, Baer M, Copeland TD, Schwartz RC, Johnson PF. Regulation of CCAAT/Enhancer-binding protein (C/EBP) activator proteins by heterodimerization with C/EBPgamma. J. Biol. Chem. 2002;277:23563–23572. doi: 10.1074/jbc.M202184200. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pedrosa E, Kaushik S, Lachman HM. ChIP-chip analysis of neurexins and other candidate genes for addiction and neuropsychiatric disorders. J. Neurogenetics. 2010;24:5–17. doi: 10.3109/01677060903305658. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: personality and behavioral measures. Pers. Individ. Dif. 2006;40:305–315. [Google Scholar]
- Rowen L, Young J, Birditt B, Kaur A, Madan A, Philipps D, Qin S, Minx P, Wilson RK, Hood L, Graveley BR. Analysis of the human neurexin genes: alternative splicing and the generation of protein diversity. Genomics. 2002;79:587–597. doi: 10.1006/geno.2002.6734. [DOI] [PubMed] [Google Scholar]
- Selzer ML. Michigan Alcohol Screening Test: the quest for a new diagnostic instrument. Am. J. Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Skinner HA. The drug abuse screening test. Addict. Behav. 1982;7:363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Spillane NS, Smith GT, Kahler CW. Impulsivity-like traits and smoking behavior in college students. Addict. Behav. 2010;35:700–705. doi: 10.1016/j.addbeh.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenberg SF, Batien B, Birgenheir D. Does gender moderate associations among impulsivity and health-risk behaviors? Addict. Behav. 2008;33:252–265. doi: 10.1016/j.addbeh.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenberg SF, Glass JM, Chermack ST, Flynn HA, Weston ME, Burmeister M. Possible association between response inhibition and a variant in the brain-expressed tryptophan hydroxylase-2 gene. Psychiatr. Genet. 2006;16:35–38. doi: 10.1097/01.ypg.0000176528.30362.34. [DOI] [PubMed] [Google Scholar]
- Südhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Südhof TC. Structure and evolution of neurexin genes: insight into the mechanism of alternative splicing. Genomics. 2002;79:849–859. doi: 10.1006/geno.2002.6780. [DOI] [PubMed] [Google Scholar]
- Walderhaug E, Herman AI, Magnusson A, Morgan MJ, Landro NI. The short (S) allele of the serotonin transporter polymorphism and acute tryptophan depletion both increase impulsivity in men. Neurosci. Lett. 2010;473:208–211. doi: 10.1016/j.neulet.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsnack RW, Vogeltanz ND, Wilsnack SC, Harris TR, Ahlström S, Bondy S, Csémy L, Ferrence R, Ferris J, Fleming J, Graham K, Greenfield T, Guyon L, Haavio-Mannila E, Kellner F, Knibbe R, Kubicka L, Loukomskaia M, Mustonen H, Nadeau L, Narusk A, Neve R, Rahav G, Spak F, Teichman M, Trocki K, Webster I, Weiss S. Gender differences in alcohol consumption and adverse drinking consequences: cross-structural patterns. Addiction. 2000;95:251–265. doi: 10.1046/j.1360-0443.2000.95225112.x. [DOI] [PubMed] [Google Scholar]
- Wong MM, Nigg JT, Zucker RA, Puttler LI, Fitzgerald HE, Jester JM, Glass JM, Adams K. Behavioral control and resiliency in the onset of alcohol and illicit drug use: a prospective study from preschool to adolescence. Child Dev. 2006;77:1016–1033. doi: 10.1111/j.1467-8624.2006.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


