Abstract
The process of sprouting angiogenesis involves activating endothelial cells in a quiescent monolayer of an existing vessel to degrade and migrate into the underlying matrix to form new blood vessels. While the roles of biochemical factors in angiogenic sprouting have been well characterized, the roles of fluid forces have received much less attention. This review summarizes results that support a role for wall shear stress in post-capillary venules as a mechanical factor capable of synergizing with biochemical factors to stimulate pro-angiogenic signaling in endothelial cells and promote sprout formation.
Keywords: angiogenesis, extracellular matrix, mechanotransduction, sphingosine-1-phosphate, sprout formation
INTRODUCTION
The growth of new blood vessels from pre-existing vessels is necessary to support the perfusion of blood into new tissues during formation of the vascular system in the developing fetus. In adults, angiogenesis also occurs during menstruation, ovulation, wound healing, and in certain diseases (e.g. tumor growth, diabetic retinopathy). The viability of engineered tissues depends on the ability to transport nutrients and oxygen to cells that cannot be accessed through simple diffusion, thus providing a strong motivation for understanding the mechanisms involved in controlling angiogenesis.
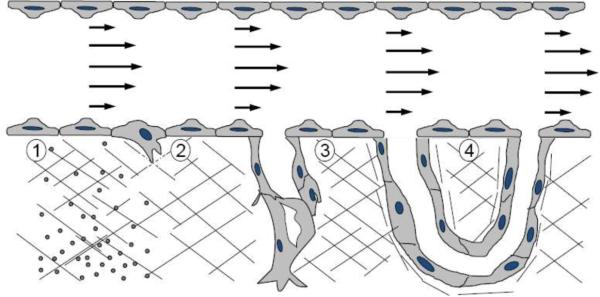
As depicted in Figure 1, sprouting angiogenesis is a complex, multi-step process (reviewed in 32). First, one or more cells in the endothelium of a pre-existing blood vessel must be induced to enzymatically digest the underlying extracellular matrix to generate a space for subsequent migration into the matrix. These tip cells are then followed by other endothelial cells (ECs) to form the stalk of the nascent sprout (9). A lumen is created through formation and fusion of vacuoles within the sprouting structures (53). Tip cells from neighboring sprouts eventually join to form a continuous conduit for blood flow that is eventually stabilized by pericytes and smooth muscle cells.
Figure 1.
The process of angiogenesis occurs as an orderly series of events. 1) Fluid WSS and a chemotactic concentration gradient of biochemical agonists synergistically activate ECs lining a pre-existing blood vessel. 2) Activated ECs begin to cleave the underlying extracellular matrix through enzymatic proteolysis and migrate into newly-formed passages. 3) A lumen forms while additional ECs migrate and proliferate behind the tip cell to form the stalk. 4) Sprouts join at their tips, anastomosing with existing vessels, to form a new path for blood flow.
Sprouting angiogenesis is stimulated by growth factors (32). In addition, ECs are exquisitely sensitive to fluid wall shear stress (WSS). Much of the EC flow mechanotransduction literature has been motivated by a need to understand the role of fluid forces in atherosclerosis. These studies largely indicate that arterial levels of WSS (>15 dyn/cm2) promote a quiescent cell phenotype, while low and/or reversing WSS stimulates a pro-atherogenic phenotype (69). Fluid forces also play a role in sprouting angiogenesis, as was first reported 80 years ago (21). Sprouting primarily occurs in the post-capillary venules, where WSS is estimated to range from 1 to 8 dyn/cm2 (51, 56, 58). In this review, we shall briefly review studies that have investigated the role of flow in sprouting angiogenesis and associated signal transduction. These studies provide evidence indicating that blood flow within venules provides an optimal WSS stimulus for sprouting angiogenesis.
Roles of Biochemical Factors and Fluid Forces
Of the many extracellular signals that are capable of inducing the transition of a quiescent EC into an invading phenotype, biochemical signals have been the most studied by far. Vascular endothelial growth factor A (VEGF-A) is generally regarded as the master regulator of angiogenesis. Secretion of VEGF-A from cells in an avascular region creates a concentration gradient that provides a chemoattractive cue for stimulating and directing angiogenic sprouting from nearby blood vessels. Other growth factors, including acidic-and basic-fibroblast growth factors (aFGF and bFGF), platelet-derived growth factor (PDGF), stromal-derived factor-1α (SDF-1α), and placental growth factor (PlGF) are also potent pro-angiogenic factors with similar influences on sprouting. With the exception of SDF-1α, which signals through a G-protein coupled receptor, CXCR4, these growth factors bind to their cognate tyrosine kinase receptors to elicit effects on EC function via intracellular signals. Sphingosine 1-phosphate (S1P) is a lysosphingolipid that acts as both an intracellular signaling molecule and an extracellular factor deposited by activated platelets during wound healing (17, 47). Sphingosine kinase 1 (SPHK1) and SPHK2 phosphorylate the sphingosine backbone to produce S1P. S1P binds one of five G-protein coupled receptors (S1P1-S1P5). ECs express S1P1 and S1P3 receptors and activate phosphatidylinositol 3-kinase (PI3K). Exogenous S1P administration or endogenous S1P production by SPHK1 over-expression promotes post-ischemic angiogenesis and blood flow recovery in mouse ischemic hind limb models (82).
Only a few studies have directly evaluated the role of WSS in angiogenesis in vivo. Zhou et al. (118) reported angiogenesis occurring through internal capillary division in response to elevated flow in rat skeletal muscle in response to chronic vasodilation with the alpha1-antagonist prazosin. Ichioka et al. (51) reported that increasing WSS from 3.7 to 5.3 dyn/cm2 using prazosin increased the rate of microvascular ingrowth through sprout formation in a rabbit ear chamber. While enhanced flow due to prazosin treatment may have additional pro-angiogenic effects other than changes in WSS, these studies suggest that flow promotes angiogenesis in vivo. Dickinson and colleagues (66) demonstrated that shear forces stimulate endothelial nitric oxide synthase (eNOS) expression and are crucial for vascular remodeling during development in the murine yolk sac. Altogether, these studies indicate mechanical forces contribute to proper vascular remodeling and angiogenesis.
In Vitro Systems for Studying Sprouting Angiogenesis
Multiple approaches have been developed that recapitulate the process of angiogenesis in cell culture. These approaches can be separately categorized as 2-D and 3-D systems.
2-D systems
Unlike the EC monolayers formed on tissue culture plastic, ECs cultured on surfaces coated with Matrigel create cord-like networks of cells (Fig. 2A). Although widely-used to test activity of many pro-and anti-angiogenic compounds, some caution should be used in interpreting those results, because many non-vascular cell types such as fibroblasts, tumor cells and kidney cells also form cords when cultured on Matrigel (11, 31, 33, 108). Cord formation is inhibited by reducing the thickness of the matrix, by including fibrillar type I collagen in the matrix, or by disrupting cytoskeletal microfilaments and microtubules (108). These results suggest that cord formation on Matrigel may be caused by intracellular tension generation rather than by specific differentiation of ECs into capillary-like structures.
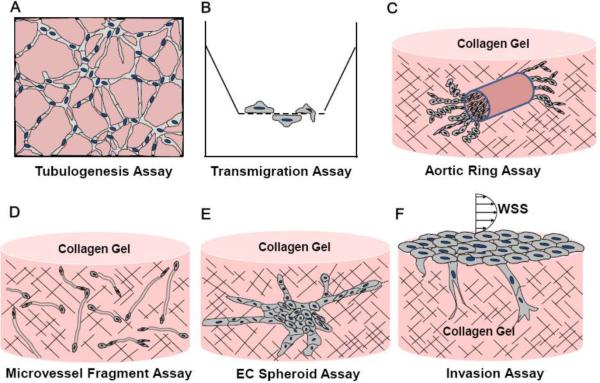
Figure 2.
Experimental models for studying angiogenic events.
The effects of pro-angiogenic stimuli on cell migration have been characterized by quantifying cell motility along a 2-D surface (3). A drawback to this assay is that the cells do not need to circumnavigate a dense matrix. Boyden chambers or Transwell migration assays (Fig. 2B) are a convenient method for quantifying migration orthogonal to the cell monolayer. In this assay, cells in a monolayer on the top face of a porous substrate are driven to migrate through the pores to the opposite face due to the presence of a chemotactic factor (e.g. VEGF-A) in the lower media chamber. The substrate can be coated with matrix proteins to occlude the pores, thus requiring the cells to degrade the matrix to transmigrate.
3-D systems
Standard 2-D cell culture systems cannot replicate all the steps involved in sprouting angiogenesis, including EC activation, basement membrane degradation, invasion and proliferation of ECs, lumen formation, and stabilization. Three-dimensional extracellular matrices (ECM), typically composed of fibrillar collagen I, fibrin, or basement membrane components, provide an environment in which some or all of these steps can be observed. As summarized in Figure 2 and described below, there are a number of ways to introduce ECs into these matrices.
Vasculogenesis model
During vasculogenesis, individual foci of ECs assemble into vascular structures, thus distinguishing this process from angiogenesis, where new blood vessels originate from pre-existing vessels. Individual ECs can be suspended in collagen and fibrin matrices prior to polymerization (7, 26). In these systems, ECs interconnect to form tubes through sprout extension and the formation and coalescence of vacuoles and lumens. Davis and colleagues have utilized this model extensively to study molecular signals that drive lumen formation events (27). These studies illustrate the utility of 3-D systems for better understanding various steps of vasculogenesis, and angiogenesis, many of which are likely to overlap.
Blood vessel explant assays
Another commonly used assay to quantify angiogenesis is the ex vivo aortic ring assay (Fig. 2C), where a segment of rat or mouse aorta is embedded within an ECM. ECs, primarily from the vessel intima, sprout into the matrix. In this system, key steps of angiogenesis (cf. Fig. 1) are represented, and native cells (including pericytes, fibroblasts and monocytes) are available to contribute to stimulate growth and stabilize the sprouts. A disadvantage of this system is the variability introduced by incomplete removal of connective tissue surrounding the aortas and variability in the aorta sources themselves.
Angiogenesis typically occurs in microvessels, but not major vessels such as the aorta. Microvascular fragments isolated from adipose tissue can be implanted in collagen matrices (59) to provide ECs for sprouting angiogenesis (Fig. 2D). These sprouts are then able to intersect and fuse with other nascent microvessels, yielding complex 3-D networks. Like the aortic ring preparation, this model incorporates multiple cell types. Disadvantages are the heterogeneity in microvessel fragments and the lack of a homogenous imaging plane to perform quantification studies.
EC spheroids and EC-coated microbeads
ECs cultured with water-soluble methylcellulose polymers spontaneously form cell spheroids (Fig. 2E). These spheroids have been implanted into collagen matrices to induce sprouting (57). In separate assays, microbeads coated with ECs prior to embedding in fibrin matrices recapitulates EC sprouting, migration, proliferation, lumen formation and anastomosis (77, 79). In these experiments, fibroblasts are cultured on the surface of the fibrin matrix and secrete factors that complement exogenous VEGF and bFGF. This model has the advantage of studying the contribution of fibroblasts, but does not incorporate a polarized layer of ECs forming an interface between the underlying ECM and luminal compartment.
EC monolayer invasion assay
Bayless and Davis (5) developed a model of sprouting angiogenesis where an EC monolayer is seeded on the surface of a 3-D collagen matrix and stimulated to invade into the underlying matrix (Fig. 2F). Unlike other 3-D systems described above, this is the only system which begins with a monolayer of ECs seeded on a basal ECM with an apical fluid-filled compartment. EC sprouting responses stimulated by S1P, VEGF, and bFGF occur overnight in the absence of serum and are readily quantifiable (6).
Systems for studying the role of WSS on angiogenesis
The assays above are generally used to study the effects of biochemicals (e.g. growth factors) on angiogenic processes in static cultures. Many of these assays are not amenable to studying the role of fluid forces. Initial approaches involved first applying WSS to cell monolayers and then performing angiogenic assays afterwards using the presheared cells. Gloe et al. (38) presheared ECs on laminin-coated plates at 16 dyn/cm2 for 6h, then further cultivated the cells under static conditions to observe tubulogenesis. Preshearing caused cord formation in a manner dependent on WSS-induced bFGF secretion. After applying WSS to ECs for 16h, Cullen et al. (22) trypsinized and transferred presheared cells to new chambers to quantify cord formation on Matrigel and migration through Transwell filters. Both migration and cord network formation increased monotonically with increasing preshearing magnitude from 1 to 20 dyn/cm2 in a manner dependent on G-protein signaling. Using a similar approach, Tressel et al. (105) compared the effects of preshearing cells with either steady (5 and 15 dyn/cm2) or oscillatory WSS (0±5 and 0±15 dyn/cm2) on cord formation on Matrigel. They found that steady, but not oscillatory, WSS significantly inhibited network formation (relative to static cells) and this correlated with downregulation of Ang-2 production by steady WSS. Though the results of these studies are not consistent with each other, the results do indicate that angiogenic responses are highly dependent on the WSS magnitude and pattern. A drawback to these approaches is that the effects of WSS must be “remembered” by the cells during the angiogenesis assay.
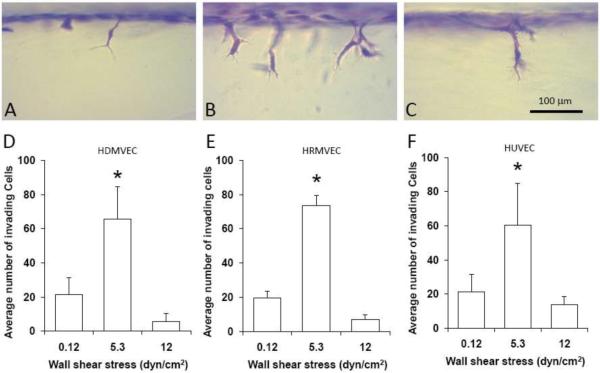
We and others (54, 106) have applied WSS to endothelial monolayers in parallel-plate flow chambers while the ECs simultaneously invaded into the underlying 3-D collagen matrix. These two studies demonstrated that 3 dyn/cm2 WSS promoted sprouting. By performing our experiment using defined culture media, we found that S1P must be present within the collagen matrix for WSS to induce sprouting into the matrix (54). Apparently, the S1P serves as a chemoattractant to stimulate the cells to move deeper into the matrix. The synergy between WSS and S1P is similar to that observed between VEGF/bFGF and S1P (54, 98). Furthermore, the number of sprouts was highly dependent on WSS magnitude, with the greatest number of sprouts occurring at an intermediate WSS of 5.3 dyn/cm2 (54). As illustrated in Figures 3A–C for human dermal microvascular ECs, the 3-D collagen invasion assay provides information about the morphological characteristics of the resulting sprouts using primary ECs. At 0.12 dyn/cm2 WSS, some cell invasion was observed (Figure 3A), while at 5.3 dyn/cm2 WSS, many more cells invaded (Figure 3B). At 12 dyn/cm2 WSS, very few cells invaded (Figure 3C). Quantification of human dermal (Figure 3D) and retinal (Figure 3E) microvascular EC, and HUVEC (Figure 3F) invasion reveals the same dependence on WSS magnitude we previously reported (54).
Figure 3.
EC invasion depends on the magnitude of WSS. Human dermal microvascular ECs (HDMVECs) on 3-D collagen matrices containing 1μM S1P were subjected to 24h of 0.12 (A), 5.3 (B) and 12 dyn/cm2 WSS (C). The cultures were fixed and stained with toluidine blue to identify invading cells. Scale bar, 100μm. Quantification of HDMVECs (D), human retinal MVECs (E) and HUVECs (F) invasion into 3-D collagen matrices containing 1μM S1P subjected to 22h of WSS ranging from 0.12 to 12 dyn/cm2. Cultures were fixed, stained with toluidine blue for morphometric analysis and analyzed for invasion density. The density of invading ECs are plotted as a function of WSS magnitude (mean ±SD). * indicates the invasion density at 5.3 dyn/cm2 is significantly different from the values at the other WSS magnitudes (ANOVA followed by Student-Newman-Keuls post-hoc test; P<0.01).
While WSS experiments with 2-D EC monolayers are comparatively simple, a number of unique issues must be considered when performing WSS experiments on a 3-D collagen matrix. Wells containing collagen matrices must be fabricated within one of the plates of a parallel-plate system so that the gels are flush with the plate surface. Our system involves a simple modification of a typical parallel-plate flow chamber with the addition of a customized silicone rubber gasket to form the wells. The collagen density must be sufficiently high to provide the mechanical resilience required to keep the collagen matrix flat during exposure to WSS. In our experience, WSS alone does not induce invasion; hence, a chemoattractive agent (e.g. S1P) must also be incorporated into the collagen matrix. After gaining experience with this system, we have found that EC invasion in our system is reproducible. Under these conditions, we have documented a role for activated Akt and metalloproteinases in sprouting responses induced by WSS and S1P (54). In addition, we have recently shown calpains regulate localization of a key metalloproteinase to facilitate sprouting events (Kang et al., in revision). Thus, this system incorporates application of controlled levels of WSS to an endothelial monolayer, which is seeded on an underlying 3-D matrix. This design is amenable to varying WSS and S1P levels, as well as separation of biochemical extracts to better understand how S1P and WSS combine to direct sprouting behavior.
Angiogenic Signaling Regulated by Biochemical Signals and Fluid Forces
In addition to the handful of studies that have directly quantified the effects of WSS on angiogenic events, a number of studies have been performed using ECs cultured on 2-D substrates to investigate flow mechanotransduction relevant to angiogenesis. Several of these studies have reported changes in angiogenic signaling induced by flow in a manner that is highly dependent on WSS magnitude. These findings are summarized in Table 1 and discussed below.
Table 1.
Effects of WSS magnitude on angiogenic events and signaling. Experiments were performed using HUVECs unless otherwise stated.
| <2 dyn/cm2 | 3–8 dyn/cm2 | >10 dyn/cm2 | References | |
|---|---|---|---|---|
|
| ||||
| 3-D invasion assay | ||||
| invasion density | ↓ | ↑ | ↓ | (54) |
| invasion depth | ↑ | ↑ | (54) | |
|
| ||||
| 2-D Matrigel tubule formation assay | ↑ | ↑ ↑ | ↑ ↑ ↑ | (22) |
|
| ||||
| Transwell cell migration rate | - | ↑ | ↑ ↑ | (22) |
|
| ||||
| Ang-2 expression | ↑ | ↓ | ↓ | (40) |
| ↓ | (19)* | |||
|
| ||||
| TIE-2 expression | ↑ | ↑ | (19)* | |
| - | ↑ | ↑ | (40) | |
|
| ||||
| VEGF-A expression (in skeletal muscle microvascular EC) | ↑ | ↑ ↑ | (40) | |
| ↑ | (71)* | |||
|
| ||||
| Ephrin-B2 expression | ↑ | ↓ | ↓ | (39) |
| Ephrin-B2 expression (in VEGFR+ ESC) | ↑ | ↑ | (70) | |
| Ephrin-B4 expression (in VEGFR+ ESC) | ↓ | (70) | ||
|
| ||||
| Notch | ↑ | (111) | ||
| Notch1 and Notch 4 expression (in VEGFR2+ ESC) | ↓ | ↑ | (70) | |
|
| ||||
| Akt Phosphorylation | ↓ | ↑ | ↓ | (54) |
| ↑ | (19)* | |||
| ↑ | ↑ ↑ | (29) | ||
|
| ||||
| eNOS phosphorylation | ↑ | (29) | ||
|
| ||||
| HIF-1α, HIF-2α expression (in skeletal muscle microvascular EC) | ↓ | (71)* | ||
|
| ||||
| Foxo-1 binding activity | - | ↓ | (40) | |
| Foxo-1 expression | ↓ | (19)* | ||
| Foxo-1 phosphorylation and nuclear exclusion | ↑ | (19)* | ||
|
| ||||
| MMP-2 secretion | ↓ | ↑ | ↓ | (54) |
| - | ↓ | (72) | ||
| ↓ | (116) | |||
| ↑ | (99)* | |||
|
| ||||
| MMP-9 secretion | ↑ | (100)* | ||
| ↑ | (99)* | |||
|
| ||||
| ADAMTS1 expression | - | ↑ | ↑ ↑ | (48) |
vs. static condition
Cell surface receptors
Like VEGF receptors, the Tie receptors (Tyr kinase with Ig and EGF homology domains) represent a class of receptor tyrosine kinases specific to vascular cells that are important contributors to angiogenesis (101). The two known Tie receptors, TIE1 and TIE2, bind to angiopoietins, Ang-1 and Ang-2, and phosphorylate tyrosine residues to mediate intracellular signaling. Ang-1 and Ang-2 are agonistic and antagonistic TIE2 ligands, respectively. Ang-TIE signaling is closely associated with the stabilization of new microvessels. Mice deficient in Ang-1 or TIE2 expression fail to develop their cardiovascular system beyond the primary capillary plexus (101). Overexpression of Ang-2, on the other hand, disrupts blood vessel formation (68). In contrast to TIE2, TIE1 signal transduction is poorly understood. Goettsch et al. (40) demonstrated that Ang-2 mRNA, protein expression and release from HUVECs was upregulated by 1 dyn/cm2 WSS, but downregulated by 30 dyn/cm2 WSS. VEGF-A expression was upregulated at both levels of WSS, while vascular endothelial growth factor 2 (VEGFR-2) inhibition attenuated the effects of low WSS on Ang-2. Interestingly, high WSS blocked the ability of VEGF to upregulate Ang-2 expression. Thus, VEGF and WSS have a complex interactive effect on signaling via the Tie receptors.
Ephrin is a transmembrane signaling molecule involved in cell-cell communication during angiogenesis. Ephrin-B2 is capable of binding to several different transmembrane Eph receptor tyrosine kinases. While the Ephrin-B2 receptor EphB4 is primarily expressed in veins, it is also observed in retinal capillaries and sprouts. Ephrin-B2 is essential for angiogenesis and is upregulated during both physiological and pathological angiogenesis in adults (93). Recently, Ephrin-B2 has been reported to regulate sprouting through regulation of VEGF internalization and signaling (92, 112). HUVECs and human coronary arterial ECs each express Ephrin-B2 in static culture, but the level of expression decreases significantly in response to 24h of high WSS (30–50 dyn/cm2), but not for low WSS (1–10 dyn/cm2) (39). Hu and Chien (50) reported that 20 dyn/cm2 WSS induced increased Protein Kinase C immunostaining in HUVECs. Consistent with a role for WSS induction of PKC in mediating Ephrin-B2 suppression, the downregulation of Ephrin-B2 expression by high WSS was blocked by an inhibitor of Protein Kinase C (39). The expression of the receptor EphB4 was not significantly changed by any WSS in the range of 1–50 dyn/cm2 (39). Since Ephrin-B2 is critical to angiogenesis, these results are consistent with high WSS having a negative impact on cell invasion.
Only a fraction of the cells in the endothelium of a vessel are induced to invade, and this decision is regulated by the Notch pathway. Delta-like 4 (Dll4) and Jagged1 are each proangiogenic transmembrane ligands for the Notch receptor (8). Tip cells express relatively high levels of Dll4, which binds Notch receptors on adjacent stalk cells leading to downregulation of VEGF receptors. With relatively high levels of VEGF receptors, tip cells are thought to be more responsive to VEGF gradients to induce their migration. Stalk cells primarily express Jagged1, which antagonizes Dll4-Notch signaling and thereby upregulate the number of tip cells and sprouts. Inhibition of Notch signaling leads to enhanced sprouting through increased tip cell proliferation in 3-D fibrin (89) and collagen (98) matrices. The effects of WSS on Notch signaling has not been investigated in ECs, though experiments performed using murine embryonic stem cells (mESC) positively expressing VEGF receptor 2 (VEGFR2) may provide insight (70). WSS induced an increase in Ephrin-B2 expression in VEGFR2-positive mESCs in a magnitude-dependent manner for WSS magnitudes of 5 to 20 dyn/cm2, while 10 dyn/cm2 WSS decreased EphB4 expression. The levels of Notch ligand cleavage and Ephrin-B2 expression induced by 10 dyn/cm2 WSS was equivalent to that induced by VEGF. The expression of the Notch signaling proteins Notch1, Notch4, Dll4 and Jagged1 were each increased in a WSS magnitude-dependent manner for WSS applied over a range of 1.5 to 10 dyn/cm2. Further, the upregulation of Ephrin-B2 by WSS was abolished upon inhibition of Notch signaling using inhibitors of γ-secretase, as well as by a VEGF receptor tyrosine kinase inhibitor. There is still much to learn regarding the role of WSS on Notch signaling.
Intracellular signaling
Akt is a serine/threonine kinase that plays a major role in EC invasion and sprouting. Akt1 knockout mice exhibit a 40% reduction in the average length of sprouts compared to wild-type mice in ex vivo aortic ring assays (115), which is consistent with the proposed role of Akt in vascular maturation and angiogenesis during wound healing (97). Multiple angiogenic events are mediated by Akt, including EC migration, survival, and tube formation (95). WSS, growth factors (VEGF-A and bFGF), and S1P induce dose-dependent Akt phosphorylation and correlate with tubulogenesis and endothelial sprouting (29–30, 52, 54, 60, 75). Dimmler et al. (28) demonstrated that Akt phosphorylation increases monotonically with increasing WSS ranging from 0 to 45 dyn/cm2 in 2-D cultures. We found S1P and WSS-induced Akt phosphorylation at Ser473 in ECs cultured on 3-D collagen matrices was maximal at 5.3 dyn/cm2 WSS and decreased at 12 dyn/cm2 WSS (54). Furthermore, Akt inhibition diminished EC invasion responses induced by the combined application of WSS and S1P (54). S1P1 receptor activation in ECs stimulates Akt activation via pertussis toxin-sensitive Gi proteins (41, 64), and S1P-induced migration (64) and 3-D invasion (5) are blocked by pertussis toxin. Interestingly, EC migration and tubulogenesis following preshearing are also attenuated by pertussis toxin (22). Akt has several downstream targets implicated in angiogenesis, including endothelial nitric oxide synthase (eNOS) (29), hypoxia-inducible factor (42), and the transcription factor FOXO (19). WSS regulation of eNOS activity has an important role in the regulation of vascular remodeling and angiogenesis. Dickinson and colleagues (66) reported that WSS is critical for vessel remodeling and eNOS expression in mouse embryos. WSS induces Akt phosphorylation at Ser473, which then mediates eNOS activation and subsequent NO production (29). S1P and VEGF are also reported to stimulate the phosphoinositide 3-kinase (PI3K)/Akt pathway to induce eNOS activation (19, 29). WSS (12 dyn/cm2) induces tyrosine phosphorylation of the junctional protein PECAM-1 in ECs, and PECAM-1 expression is necessary for shear-induced Akt and eNOS phosphorylation, suggesting that PECAM-1 may act as the mechanosensor for this pathway (35).
Transcriptional regulation
An important component of homeostatic mechanisms linking vascular oxygen supply with demand is the regulation of angiogenesis by hypoxia. Hypoxia increases the expression of VEGF and PDGF in ECs and this is mediated by activation of hypoxia-inducible transcription factors (HIF) (19, 29). HIF is a heterodimer composed of α and β subunits. HIF-1α is stabilized by hypoxia, while HIF-1β is constitutively expressed in the nucleus. Activated HIF-1α and HIF-2α bind to hypoxia response elements (HREs) to increase promoter activity, while HIF-3α appears to negatively regulate transcription. After 24 h of 16 dyn/cm2 WSS, HIF-1α and HIF-2α expression were reduced significantly relative to static controls and HIF-α proteins were undetectable (71). Consistent with the regulation of VEGF-A by both HIF isoforms, VEGF-A expression was significantly reduced by 16 dyn/cm2 WSS (71). These results suggest long-term application of laminar WSS promotes vascular stabilization by downregulating HIF and VEGF production. Interestingly, NO can either drive HIF-1α stabilization under normoxia or degradation under hypoxia (4).
The O subgroup of forkhead transcription factor (FoxO) proteins are involved in vascular development and maturation. Loss of FoxO1 expression results in embryonic lethality caused by severe defects in vascular development (37). The FoxO protein family is regulated primarily by posttranslational modifications, including phosphorylation, acetylation, and ubiquitination, which control protein levels, subcellular localization, and transcriptional activity (25, 90). FoxO factors are negatively regulated by Akt; dephosphorylated FoxO1 localizes to the nucleus and exhibits transcriptional activity, whereas Akt-dependent phosphorylation of FoxO1 induces nuclear export and allows proteasomal degradation (2, 10, 16). Activation of the FoxO pathway increases expression of Angiopoietin-1, which promotes vascular stability, and (although it is not well-understood) also increases expression of its antagonist, angiopoietin-2 (24, 40). FoxO1 and FoxO3a may elicit anti-angiogenic effects in part by repressing eNOS (84). Haas and colleagues demonstrated that chronic ischemia reduced Akt signaling, and enhanced FoxO1 levels, which ultimately suppressed angiogenic responses (73). FoxO1 binding activity and nuclear translocation were also reduced by 30 dyn/cm2 WSS, but were not affected by 1 dyn/cm2 WSS. Chlench et al. (19) reported that 6 dyn/cm2 WSS downregulates FoxO1 expression and increases Foxo-1 exclusion from the nucleus. Meanwhile, WSS at 6 dyn/cm2 (vs. static) had no effect on Ang-1 expression. Together, these studies indicate that Ang-2 induction via Foxo-1 is inversely related to the magnitude of applied WSS. Whether or not these signals are affected by the S1P signaling pathway in either 2-D or 3-D settings remains to be investigated.
Matrix proteolysis
Matrix metalloproteinases (MMPs) are zinc-dependent enzymes that can cleave extracellular matrix proteins including collagen type I and fibrin to control tissue morphogenesis (76, 113) and vascularization (62) by regulating many biological processes including liberation of growth factors (55, 76). A link between mechanotransduction and MMPs has been extensively reviewed (23). Various studies have linked MMPs and their endogenous inhibitors to regulating angiogenic sprouting and invasion events (1, 62, 102–103, 110). The membrane-type matrix metalloproteinases (MT-MMPs) have been shown to play a critical role in invasion of 3-D matrices by degrading ECM proteins at the cell surface-ECM interface (5, 91). MT-MMPs allow highly-regulated proteolysis to occur at the cell surface, while maintaining the integrity of the supporting ECM scaffold (46). MT-MMPs coordinate with integrins and growth factors to direct angiogenic sprouting and lumen formation (5, 88), and mice lacking MT1-MMP, (but not MMP-2 or MMP-9), exhibit defective sprouting responses (20). Thus, membrane-associated metalloproteinases are critical in mediating successful angiogenic responses. Using gelatin zymography, we measured increases in pro and active forms of MMP-2 in the perfusate that was highest at 5.3 dyn/cm2 WSS and significantly lower when WSS was either 0.12 or 12 dyn/cm2 (54). Active MT1-MMP cleaves pro-MMP-2, and in HUVEC, MMP-2 activation is a reliable indicator of MT1-MMP activity (63). In addition, a number of studies have investigated the effects of WSS on MMP activity and expression. Microvascular ECs produced more MMP-2 mRNA and protein levels when subjected to 5 dyn/cm2 WSS than to 16 dyn/cm2 WSS (72). MMP-2 secretion is downregulated by 30 dyn/cm2 WSS in bovine aortic ECs (BAECs) (116), while MMP-2 secretion was upregulated by 8 dyn/cm2 WSS in human saphenous vein ECs (HSVEC) (99). Furthermore, MMP-9 expression and activation increased in HSVEC and HUVEC subjected to WSS in the range of 4 to 8 dyn/cm2 (99). These results are consistent with MMP activity being increased by WSS at intermediate, but not high, magnitudes.
A Disintegrin and Metalloproteinases (ADAMs) are membrane-associated proteinases that contain N-terminal zinc-dependent metalloproteinase, disintegrin, transmembrane and cytoplasmic tail domains. Like MT-MMPs, anchoring at the plasma membrane renders these molecules perfectly poised to mediate shedding events. ADAM17 was originally identified as tumor necrosis factor (TNF)-alpha-converting-enzyme, or TACE, because of its ability to mediate TNF-α release (12). Other substrates for ADAMs include adhesion molecules, surface receptors and members of the epidermal growth factor family (13–14). Like MT1-MMP, ADAMs also co-localize with integrins (34). Functional ADAM17 appears to be required for normal embryonic development and most transgenic animals lacking the ADAM17 metalloproteinase domain die between embryonic day 17.5 and birth (83). Regarding angiogenesis, pathological retinal neovascularization and growth of heterotopically injected tumor cells was reduced in mice lacking ADAM17 expression in ECs. Moreover, silencing of ADAM17 in ECs decreased endothelial sprouting responses (61) and cord formation on Matrigel (114). Mice lacking expression of ADAM15, ADAM8 and ADAM9 exhibit reduced neovascularization responses in a model of retinopathy of prematurity (43–44, 49), and ADAM10 null mice die at day 9.5 of embryogenesis, a critical time at which the vasculature is forming (45). Recently, ADAM17 has been implicated in mechanotransduction by compressive stress in airway epithelial cells (96). Whether the ADAMs family of metalloproteinases is modulated by mechanical signals remains to be demonstrated. Clearly, ADAM proteinases regulate vasculogenesis and angiogenesis in vivo and in vitro. Recombinant, soluble ADAMTS1 inhibited angiogenesis in corneal pocket and chick chorioallantoic angiogenic assays (107). In rat mesentery, Hohberg et al. (48) demonstrated positive immunostaining for ADAMTS1 in microvessels experiencing flow, while there was a lack of ADAMTS1 staining in unperfused sprouts. Hohberg et al. also reported that ADAMTS1 expression and liberation of TSP fragments in HUVECs increased monotonically with WSS in the range of 1 to 20 dyn/cm2 (28). These results are consistent with an anti-angiogenic role for ADAMTS1, where ADAMTS1expression is high in stabilized, non-angiogenic vessels experiencing high WSS, but is downregulated in sprouting structures where WSS is low. In contrast, ADAMTS1 is expressed in the highly vascularized tissue during ovulation (15, 74, 86), suggesting a positive effect on angiogenesis. In our 3-D EC invasion assay, increased expression of full length, cell-associated ADAMTS1 correlated with endothelial sprouting responses, and ADAMTS1 silencing inhibited sprout formation (94). ADAMTS1 can degrade collagen (85), consistent with our data showing that decreasing ADAMTS1 expression levels resulted in decreased endothelial sprout density and length (94). ADAMTS1 can cleave and release anti-angiogenic polypeptides from matrix-bound Thrombospondin 1 and 2 (TSP1 and TSP2), and TSP1 is critical for the anti-angiogenic response mediated by soluble ADAMTS1 (65). The C-terminus of ADAMTS1 can be cleaved to release the last two TSP domains and liberate ADAMTS1 from the cell surface. The cleavage event requires metalloproteinases, which may include MMP-2, MMP-8, and MMP-15 (87). In our 3-D invasion system, ADAMTS1 remains associated with the endothelial surface (98), suggesting a more limited ability to release the TSP domains within ADAMTS1. Altogether, these data indicate that the ability of ADAMTS1 to promote angiogenesis depends on the solubility state of ADAMTS1, as well as other local factors, including TSP and MMPs, which must be tested and considered carefully.
Clinical Relevance of WSS Effects on Angiogenesis
Angiogenesis plays a critical role in wound healing and tumor vascularization (36, 104). Experimental and computational studies indicate that WSS and S1P modulate microvascular growth during these pathogenic events. During wound healing, the levels of blood flow and cytokines such as S1P provided by activated platelets are increased in the wound area (47). Local hemodynamics have been implicated in microvascular growth within tumors (78). In silico models incorporating WSS-dependent microvascular growth rules have been used to describe angiogenic network formation during tumor growth and wound healing (18, 67, 81). Quantitative relationships between WSS and angiogenic sprouting responses can be used to inform such models and may potentially lead to a more accurate description of angiogenic network formation.
CONCLUSIONS
Post-capillary venules have relatively thin walls that are particularly permissive to angiogenic sprouting. The WSS at these locations appears to also prime ECs to respond to pro-angiogenic signals such as S1P. It remains unclear how cells perceive the magnitude of WSS to regulate an angiogenic response. While it is tempting to draw upon the vast literature of WSS mechanotransduction studies, results obtained from flow experiments performed on 2-D substrates do not always translate directly to endothelial mechanoresponses on 3-D matrices. For example, Akt activation increases monotonically in response to WSS for ECs on 2-D substrates (28), but Akt shows maximal activation at an intermediate WSS of 5.3 dyn/cm2 for ECs on 3-D collagen matrices (54). ECs are sensitive to matrix stiffness (117), so it is very possible that the endothelial response to WSS on pliable collagen matrices may differ significantly from that of ECs on stiff matrix-coated glass slides.
This review focused on the effects of luminal WSS on angiogenesis, but ECs are also subjected to fluid forces generated by fluid flow through the matrix, i.e. interstitial flow. There is strong evidence that interstitial flow has profound effects on endothelial network morphology in 3-D matrices (80, 109). Cell culture systems capable of controlling both tangential WSS and interstitial flow are expected to provide even greater insight into the roles of fluid forces on sprouting angiogenesis.
ACKNOWLEDGEMENTS
This work was supported by American Heart Association Scientist Development Grant #0730238N (RRK) and NIH R01HL09576 (KJB).
REFERENCES
- 1.Anand-Apte B, Pepper MS, Voest E, Montesano R, Olsen B, Murphy G, Apte SS, Zetter B. Inhibition of angiogenesis by tissue inhibitor of metalloproteinase-3. Invest Ophthalmol Vis Sci. 1997;38:817–823. [PubMed] [Google Scholar]
- 2.Aoki M, Jiang H, Vogt PK. Proteasomal degradation of the FoxO1 transcriptional regulator in cells transformed by the P3k and Akt oncoproteins. Proc Natl Acad Sci U S A. 2004;101:13613–13617. doi: 10.1073/pnas.0405454101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augustin-Voss HG, Pauli BU. Quantitative analysis of autocrine-regulated, matrix-induced, and tumor cell-stimulated endothelial cell migration using a silicon template compartmentalization technique. Exp Cell Res. 1992;198:221–227. doi: 10.1016/0014-4827(92)90374-h. [DOI] [PubMed] [Google Scholar]
- 4.Balligand JL, Feron O, Dessy C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol Rev. 2009;89:481–534. doi: 10.1152/physrev.00042.2007. [DOI] [PubMed] [Google Scholar]
- 5.Bayless KJ, Davis GE. Sphingosine-1-phosphate markedly induces matrix metalloproteinase and integrin-dependent human endothelial cell invasion and lumen formation in three-dimensional collagen and fibrin matrices. Biochem Biophys Res Commun. 2003;312:903–913. doi: 10.1016/j.bbrc.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Bayless KJ, Kwak HI, Su SC. Investigating endothelial invasion and sprouting behavior in three-dimensional collagen matrices. Nat Protoc. 2009;4:1888–1898. doi: 10.1038/nprot.2009.221. [DOI] [PubMed] [Google Scholar]
- 7.Bayless KJ, Salazar R, Davis GE. RGD-dependent vacuolation and lumen formation observed during endothelial cell morphogenesis in three-dimensional fibrin matrices involves the alpha(v)beta(3) and alpha(5)beta(1) integrins. Am J Pathol. 2000;156:1673–1683. doi: 10.1016/s0002-9440(10)65038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 9.Bentley K, Mariggi G, Gerhardt H, Bates PA. Tipping the balance: robustness of tip cell selection, migration and fusion in angiogenesis. PLoS Comput Biol. 2009;5:e1000549. doi: 10.1371/journal.pcbi.1000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biggs WH, 3rd, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci U S A. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bikfalvi A, Cramer EM, Tenza D, Tobelem G. Phenotypic modulations of human umbilical vein endothelial cells and human dermal fibroblasts using two angiogenic assays. Biol Cell. 1991;72:275–278. doi: 10.1016/0248-4900(91)90298-2. [DOI] [PubMed] [Google Scholar]
- 12.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 13.Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 14.Blobel CP. Remarkable roles of proteolysis on and beyond the cell surface. Curr Opin Cell Biol. 2000;12:606–612. doi: 10.1016/s0955-0674(00)00139-3. [DOI] [PubMed] [Google Scholar]
- 15.Brown HM, Dunning KR, Robker RL, Pritchard M, Russell DL. Requirement for ADAMTS-1 in extracellular matrix remodeling during ovarian folliculogenesis and lymphangiogenesis. Dev Biol. 2006;300:699–709. doi: 10.1016/j.ydbio.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 17.Chalfant CE, Spiegel S. Sphingosine 1-phosphate and ceramide 1-phosphate: expanding roles in cell signaling. J Cell Sci. 2005;118:4605–4612. doi: 10.1242/jcs.02637. [DOI] [PubMed] [Google Scholar]
- 18.Chaplain MA, McDougall SR, Anderson AR. Mathematical modeling of tumor-induced angiogenesis. Annu Rev Biomed Eng. 2006;8:233–257. doi: 10.1146/annurev.bioeng.8.061505.095807. [DOI] [PubMed] [Google Scholar]
- 19.Chlench S, Mecha Disassa N, Hohberg M, Hoffmann C, Pohlkamp T, Beyer G, Bongrazio M, Da Silva-Azevedo L, Baum O, Pries AR, Zakrzewicz A. Regulation of Foxo-1 and the angiopoietin-2/Tie2 system by shear stress. FEBS Lett. 2007;581:673–680. doi: 10.1016/j.febslet.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 20.Chun TH, Sabeh F, Ota I, Murphy H, McDonagh KT, Holmbeck K, Birkedal-Hansen H, Allen ED, Weiss SJ. MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J Cell Biol. 2004;167:757–767. doi: 10.1083/jcb.200405001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark ER, Hitschler WJ, Kirby-Smith HT, Rex RO, Smith JH. General observations on the ingrowth of new blood vessels into standardized chambers in the rabbit's ear, and the subsequent changes in the newly grown vessels over a period of months. Anat Rec. 1931;50:129–168. [Google Scholar]
- 22.Cullen JP, Sayeed S, Sawai RS, Theodorakis NG, Cahill PA, Sitzmann JV, Redmond EM. Pulsatile flow-induced angiogenesis: role of G(i) subunits. Arterioscler Thromb Vasc Biol. 2002;22:1610–1616. doi: 10.1161/01.atv.0000034470.37007.58. [DOI] [PubMed] [Google Scholar]
- 23.Cummins PM, von Offenberg Sweeney N, Killeen MT, Birney YA, Redmond EM, Cahill PA. Cyclic strain-mediated matrix metalloproteinase regulation within the vascular endothelium: a force to be reckoned with. Am J Physiol Heart Circ Physiol. 2007;292:H28–42. doi: 10.1152/ajpheart.00304.2006. [DOI] [PubMed] [Google Scholar]
- 24.Daly C, Wong V, Burova E, Wei Y, Zabski S, Griffiths J, Lai KM, Lin HC, Ioffe E, Yancopoulos GD, Rudge JS. Angiopoietin-1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1) Genes Dev. 2004;18:1060–1071. doi: 10.1101/gad.1189704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dansen TB, Burgering BM. Unravelling the tumor-suppressive functions of FOXO proteins. Trends Cell Biol. 2008;18:421–429. doi: 10.1016/j.tcb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Davis GE, Camarillo CW. An alpha 2 beta 1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp Cell Res. 1996;224:39–51. doi: 10.1006/excr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 27.Davis GE, Stratman AN, Sacharidou A, Koh W. Molecular basis for endothelial lumen formation and tubulogenesis during vasculogenesis and angiogenic sprouting. Int Rev Cell Mol Biol. 2011;288:101–165. doi: 10.1016/B978-0-12-386041-5.00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dimmeler S, Assmus B, Hermann C, Haendeler J, Zeiher AM. Fluid shear stress stimulates phosphorylation of Akt in human endothelial cells: involvement in suppression of apoptosis. Circ Res. 1998;83:334–341. doi: 10.1161/01.res.83.3.334. [DOI] [PubMed] [Google Scholar]
- 29.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 30.Dimmeler S, Zeiher AM. Akt takes center stage in angiogenesis signaling. Circ Res. 2000;86:4–5. doi: 10.1161/01.res.86.1.4. [DOI] [PubMed] [Google Scholar]
- 31.Donovan D, Brown NJ, Bishop ET, Lewis CE. Comparison of three in vitro human 'angiogenesis' assays with capillaries formed in vivo. Angiogenesis. 2001;4:113–121. doi: 10.1023/a:1012218401036. [DOI] [PubMed] [Google Scholar]
- 32.Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol. 2010;22:617–625. doi: 10.1016/j.ceb.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Emonard H, Calle A, Grimaud JA, Peyrol S, Castronovo V, Noel A, Lapiere CM, Kleinman HK, Foidart JM. Interactions between fibroblasts and a reconstituted basement membrane matrix. J Invest Dermatol. 1987;89:156–163. doi: 10.1111/1523-1747.ep12470552. [DOI] [PubMed] [Google Scholar]
- 34.Evans JP. Fertilin beta and other ADAMs as integrin ligands: insights into cell adhesion and fertilization. Bioessays. 2001;23:628–639. doi: 10.1002/bies.1088. [DOI] [PubMed] [Google Scholar]
- 35.Fleming I, Fisslthaler B, Dixit M, Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J Cell Sci. 2005;118:4103–4111. doi: 10.1242/jcs.02541. [DOI] [PubMed] [Google Scholar]
- 36.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 37.Furuyama T, Kitayama K, Shimoda Y, Ogawa M, Sone K, Yoshida-Araki K, Hisatsune H, Nishikawa S, Nakayama K, Ikeda K, Motoyama N, Mori N. Abnormal angiogenesis in Foxo1 (Fkhr)-deficient mice. J Biol Chem. 2004;279:34741–34749. doi: 10.1074/jbc.M314214200. [DOI] [PubMed] [Google Scholar]
- 38.Gloe T, Sohn HY, Meininger GA, Pohl U. Shear stress-induced release of basic fibroblast growth factor from endothelial cells is mediated by matrix interaction via integrin alpha(v)beta3. J Biol Chem. 2002;277:23453–23458. doi: 10.1074/jbc.M203889200. [DOI] [PubMed] [Google Scholar]
- 39.Goettsch W, Augustin HG, Morawietz H. Down-regulation of endothelial ephrinB2 expression by laminar shear stress. Endothelium. 2004;11:259–265. doi: 10.1080/10623320490904151. [DOI] [PubMed] [Google Scholar]
- 40.Goettsch W, Gryczka C, Korff T, Ernst E, Goettsch C, Seebach J, Schnittler HJ, Augustin HG, Morawietz H. Flow-dependent regulation of angiopoietin-2. J Cell Physiol. 2008;214:491–503. doi: 10.1002/jcp.21229. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez E, Kou R, Michel T. Rac1 modulates sphingosine 1-phosphate-mediated activation of phosphoinositide 3-kinase/Akt signaling pathways in vascular endothelial cells. J Biol Chem. 2006;281:3210–3216. doi: 10.1074/jbc.M510434200. [DOI] [PubMed] [Google Scholar]
- 42.Gordan JD, Simon MC. Hypoxia-inducible factors: central regulators of the tumor phenotype. Curr Opin Genet Dev. 2007;17:71–77. doi: 10.1016/j.gde.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guaiquil V, Swendeman S, Yoshida T, Chavala S, Campochiaro PA, Blobel CP. ADAM9 is involved in pathological retinal neovascularization. Mol Cell Biol. 2009;29:2694–2703. doi: 10.1128/MCB.01460-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guaiquil VH, Swendeman S, Zhou W, Guaiquil P, Weskamp G, Bartsch JW, Blobel CP. ADAM8 is a negative regulator of retinal neovascularization and of the growth of heterotopically injected tumor cells in mice. Journal of molecular medicine (Berlin, Germany) 88:497–505. doi: 10.1007/s00109-010-0591-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hartmann D, de Strooper B, Serneels L, Craessaerts K, Herreman A, Annaert W, Umans L, Lubke T, Lena Illert A, von Figura K, Saftig P. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alpha-secretase activity in fibroblasts. Hum Mol Genet. 2002;11:2615–2624. doi: 10.1093/hmg/11.21.2615. [DOI] [PubMed] [Google Scholar]
- 46.Hiraoka N, Allen E, Apel IJ, Gyetko MR, Weiss SJ. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell. 1998;95:365–377. doi: 10.1016/s0092-8674(00)81768-7. [DOI] [PubMed] [Google Scholar]
- 47.Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Semin Cell Dev Biol. 2004;15:513–520. doi: 10.1016/j.semcdb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Hohberg M, Knochel J, Hoffmann CJ, Chlench S, Wunderlich W, Alter A, Maroski J, Vorderwulbecke BJ, Da Silva-Azevedo L, Knudsen R, Lehmann R, Fiedorowicz K, Bongrazio M, Nitsche B, Hoepfner M, Styp-Rekowska B, Pries AR, Zakrzewicz A. Expression of ADAMTS1 in endothelial cells is induced by shear stress and suppressed in sprouting capillaries. J Cell Physiol. 2011;226:350–361. doi: 10.1002/jcp.22340. [DOI] [PubMed] [Google Scholar]
- 49.Horiuchi K, Weskamp G, Lum L, Hammes HP, Cai H, Brodie TA, Ludwig T, Chiusaroli R, Baron R, Preissner KT, Manova K, Blobel CP. Potential role for ADAM15 in pathological neovascularization in mice. Mol Cell Biol. 2003;23:5614–5624. doi: 10.1128/MCB.23.16.5614-5624.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu YL, Chien S. Effects of shear stress on protein kinase C distribution in endothelial cells. J Histochem Cytochem. 1997;45:237–249. doi: 10.1177/002215549704500209. [DOI] [PubMed] [Google Scholar]
- 51.Ichioka S, Shibata M, Kosaki K, Sato Y, Harii K, Kamiya A. Effects of shear stress on wound-healing angiogenesis in the rabbit ear chamber. J Surg Res. 1997;72:29–35. doi: 10.1006/jsre.1997.5170. [DOI] [PubMed] [Google Scholar]
- 52.Igarashi J, Bernier SG, Michel T. Sphingosine 1-phosphate and activation of endothelial nitric-oxide synthase. differential regulation of Akt and MAP kinase pathways by EDG and bradykinin receptors in vascular endothelial cells. J Biol Chem. 2001;276:12420–12426. doi: 10.1074/jbc.M008375200. [DOI] [PubMed] [Google Scholar]
- 53.Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006;442:453–456. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- 54.Kang H, Bayless KJ, Kaunas R. Fluid shear stress modulates endothelial cell invasion into three-dimensional collagen matrices. Am J Physiol Heart Circ Physiol. 2008;295:H2087–2097. doi: 10.1152/ajpheart.00281.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kheradmand F, Werb Z. Shedding light on sheddases: role in growth and development. Bioessays. 2002;24:8–12. doi: 10.1002/bies.10037. [DOI] [PubMed] [Google Scholar]
- 56.Kim MB, Sarelius IH. Distributions of wall shear stress in venular convergences of mouse cremaster muscle. Microcirculation. 2003;10:167–178. doi: 10.1038/sj.mn.7800182. [DOI] [PubMed] [Google Scholar]
- 57.Korff T, Augustin HG. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J Cell Biol. 1998;143:1341–1352. doi: 10.1083/jcb.143.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koutsiaris AG, Tachmitzi SV, Batis N, Kotoula MG, Karabatsas CH, Tsironi E, Chatzoulis DZ. Volume flow and wall shear stress quantification in the human conjunctival capillaries and post-capillary venules in vivo. Biorheology. 2007;44:375–386. [PubMed] [Google Scholar]
- 59.Krishnan L, Underwood CJ, Maas S, Ellis BJ, Kode TC, Hoying JB, Weiss JA. Effect of mechanical boundary conditions on orientation of angiogenic microvessels. Cardiovasc Res. 2008;78:324–332. doi: 10.1093/cvr/cvn055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, Sessa WC, Walsh K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kwak HI, Mendoza EA, Bayless KJ. ADAM17 co-purifies with TIMP-3 and modulates endothelial invasion responses in three-dimensional collagen matrices. Matrix Biol. 2009 doi: 10.1016/j.matbio.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 62.Lafleur MA, Handsley MM, Edwards DR. Metalloproteinases and their inhibitors in angiogenesis. Expert Rev Mol Med. 2003;5:1–39. doi: 10.1017/S1462399403006628. [DOI] [PubMed] [Google Scholar]
- 63.Lafleur MA, Handsley MM, Edwards DR. Metalloproteinases and their inhibitors in angiogenesis. Expert Rev Mol Med. 2003;5:1–39. doi: 10.1017/S1462399403006628. [DOI] [PubMed] [Google Scholar]
- 64.Lee MJ, Evans M, Hla T. The inducible G protein-coupled receptor edg-1 signals via the G(i)/mitogen-activated protein kinase pathway. J Biol Chem. 1996;271:11272–11279. doi: 10.1074/jbc.271.19.11272. [DOI] [PubMed] [Google Scholar]
- 65.Lee NV, Sato M, Annis DS, Loo JA, Wu L, Mosher DF, Iruela-Arispe ML. ADAMTS1 mediates the release of antiangiogenic polypeptides from TSP1 and 2. EMBO J. 2006;25:5270–5283. doi: 10.1038/sj.emboj.7601400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134:3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Machado MJ, Watson MG, Devlin AH, Chaplain MA, McDougall SR, Mitchell CA. Dynamics of angiogenesis during wound healing: a coupled in vivo and in silico study. Microcirculation. 2011;18:183–197. doi: 10.1111/j.1549-8719.2010.00076.x. [DOI] [PubMed] [Google Scholar]
- 68.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 69.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. Jama. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 70.Masumura T, Yamamoto K, Shimizu N, Obi S, Ando J. Shear stress increases expression of the arterial endothelial marker ephrinB2 in murine ES cells via the VEGF-Notch signaling pathways. Arterioscler Thromb Vasc Biol. 2009;29:2125–2131. doi: 10.1161/ATVBAHA.109.193185. [DOI] [PubMed] [Google Scholar]
- 71.Milkiewicz M, Doyle JL, Fudalewski T, Ispanovic E, Aghasi M, Haas TL. HIF-1alpha and HIF-2alpha play a central role in stretch-induced but not shear-stress-induced angiogenesis in rat skeletal muscle. J Physiol. 2007;583:753–766. doi: 10.1113/jphysiol.2007.136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Milkiewicz M, Kelland C, Colgan S, Haas TL. Nitric oxide and p38 MAP kinase mediate shear stress-dependent inhibition of MMP-2 production in microvascular endothelial cells. J Cell Physiol. 2006;208:229–237. doi: 10.1002/jcp.20658. [DOI] [PubMed] [Google Scholar]
- 73.Milkiewicz M, Roudier E, Doyle JL, Trifonova A, Birot O, Haas TL. Identification of a mechanism underlying regulation of the anti-angiogenic forkhead transcription factor FoxO1 in cultured endothelial cells and ischemic muscle. Am J Pathol. 2011;178:935–944. doi: 10.1016/j.ajpath.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mittaz L, Russell DL, Wilson T, Brasted M, Tkalcevic J, Salamonsen LA, Hertzog PJ, Pritchard MA. Adamts-1 is essential for the development and function of the urogenital system. Biol Reprod. 2004;70:1096–1105. doi: 10.1095/biolreprod.103.023911. [DOI] [PubMed] [Google Scholar]
- 75.Morales-Ruiz M, Lee MJ, Zollner S, Gratton JP, Scotland R, Shiojima I, Walsh K, Hla T, Sessa WC. Sphingosine 1-phosphate activates Akt, nitric oxide production, and chemotaxis through a Gi protein/phosphoinositide 3-kinase pathway in endothelial cells. J Biol Chem. 2001;276:19672–19677. doi: 10.1074/jbc.M009993200. [DOI] [PubMed] [Google Scholar]
- 76.Nagase H, Woessner JF., Jr. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 77.Nakatsu MN, Hughes CC. An optimized three-dimensional in vitro model for the analysis of angiogenesis. Methods Enzymol. 2008;443:65–82. doi: 10.1016/S0076-6879(08)02004-1. [DOI] [PubMed] [Google Scholar]
- 78.Nasu R, Kimura H, Akagi K, Murata T, Tanaka Y. Blood flow influences vascular growth during tumour angiogenesis. Br J Cancer. 1999;79:780–786. doi: 10.1038/sj.bjc.6690125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nehls V, Drenckhahn D. A novel, microcarrier-based in vitro assay for rapid and reliable quantification of three-dimensional cell migration and angiogenesis. Microvasc Res. 1995;50:311–322. doi: 10.1006/mvre.1995.1061. [DOI] [PubMed] [Google Scholar]
- 80.Ng CP, Helm CL, Swartz MA. Interstitial flow differentially stimulates blood and lymphatic endothelial cell morphogenesis in vitro. Microvasc Res. 2004;68:258–264. doi: 10.1016/j.mvr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 81.Owen MR, Alarcon T, Maini PK, Byrne HM. Angiogenesis and vascular remodelling in normal and cancerous tissues. J Math Biol. 2009;58:689–721. doi: 10.1007/s00285-008-0213-z. [DOI] [PubMed] [Google Scholar]
- 82.Oyama O, Sugimoto N, Qi X, Takuwa N, Mizugishi K, Koizumi J, Takuwa Y. The lysophospholipid mediator sphingosine-1-phosphate promotes angiogenesis in vivo in ischaemic hindlimbs of mice. Cardiovasc Res. 2008 doi: 10.1093/cvr/cvn002. [DOI] [PubMed] [Google Scholar]
- 83.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 84.Potente M, Urbich C, Sasaki K, Hofmann WK, Heeschen C, Aicher A, Kollipara R, DePinho RA, Zeiher AM, Dimmeler S. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest. 2005;115:2382–2392. doi: 10.1172/JCI23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rehn AP, Birch MA, Karlstrom E, Wendel M, Lind T. ADAMTS-1 increases the three-dimensional growth of osteoblasts through type I collagen processing. Bone. 2007;41:231–238. doi: 10.1016/j.bone.2007.04.187. [DOI] [PubMed] [Google Scholar]
- 86.Richards JS, Hernandez-Gonzalez I, Gonzalez-Robayna I, Teuling E, Lo Y, Boerboom D, Falender AE, Doyle KH, LeBaron RG, Thompson V, Sandy JD. Regulated expression of ADAMTS family members in follicles and cumulus oocyte complexes: evidence for specific and redundant patterns during ovulation. Biol Reprod. 2005;72:1241–1255. doi: 10.1095/biolreprod.104.038083. [DOI] [PubMed] [Google Scholar]
- 87.Rodriguez-Manzaneque JC, Westling J, Thai SN, Luque A, Knauper V, Murphy G, Sandy JD, Iruela-Arispe ML. ADAMTS1 cleaves aggrecan at multiple sites and is differentially inhibited by metalloproteinase inhibitors. Biochem Biophys Res Commun. 2002;293:501–508. doi: 10.1016/S0006-291X(02)00254-1. [DOI] [PubMed] [Google Scholar]
- 88.Sacharidou A, Koh W, Stratman AN, Mayo AM, Fisher KE, Davis GE. Endothelial lumen signaling complexes control 3D matrix-specific tubulogenesis through interdependent Cdc42- and MT1-MMP-mediated events. Blood. 2010;115:5259–5269. doi: 10.1182/blood-2009-11-252692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sainson RC, Aoto J, Nakatsu MN, Holderfield M, Conn E, Koller E, Hughes CC. Cell-autonomous notch signaling regulates endothelial cell branching and proliferation during vascular tubulogenesis. FASEB J. 2005;19:1027–1029. doi: 10.1096/fj.04-3172fje. [DOI] [PubMed] [Google Scholar]
- 90.Salih DA, Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol. 2008;20:126–136. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saunders WB, Bohnsack BL, Faske JB, Anthis NJ, Bayless KJ, Hirschi KK, Davis GE. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol. 2006;175:179–191. doi: 10.1083/jcb.200603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sawamiphak S, Seidel S, Essmann CL, Wilkinson GA, Pitulescu ME, Acker T, Acker-Palmer A. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature. 2010;465:487–491. doi: 10.1038/nature08995. [DOI] [PubMed] [Google Scholar]
- 93.Shin D, Garcia-Cardena G, Hayashi S, Gerety S, Asahara T, Stavrakis G, Isner J, Folkman J, Gimbrone MA, Jr., Anderson DJ. Expression of ephrinB2 identifies a stable genetic difference between arterial and venous vascular smooth muscle as well as endothelial cells, and marks subsets of microvessels at sites of adult neovascularization. Dev Biol. 2001;230:139–150. doi: 10.1006/dbio.2000.9957. [DOI] [PubMed] [Google Scholar]
- 94.Shindo T, Kurihara H, Kuno K, Yokoyama H, Wada T, Kurihara Y, Imai T, Wang Y, Ogata M, Nishimatsu H, Moriyama N, Oh-hashi Y, Morita H, Ishikawa T, Nagai R, Yazaki Y, Matsushima K. ADAMTS-1: a metalloproteinase-disintegrin essential for normal growth, fertility, and organ morphology and function. J Clin Invest. 2000;105:1345–1352. doi: 10.1172/JCI8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243–1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 96.Shiomi T, Tschumperlin DJ, Park JA, Sunnarborg SW, Horiuchi K, Blobel CP, Drazen JM. TACE/ADAM17 Mediates Mechanotransduction in Murine Tracheal Epithelial Cells. American journal of respiratory cell and molecular biology. doi: 10.1165/rcmb.2010-0234OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Somanath PR, Chen J, Byzova TV. Akt1 is necessary for the vascular maturation and angiogenesis during cutaneous wound healing. Angiogenesis. 2008;11:277–288. doi: 10.1007/s10456-008-9111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Su SC, Mendoza EA, Kwak HI, Bayless KJ. Molecular profile of endothelial invasion of three-dimensional collagen matrices: insights into angiogenic sprout induction in wound healing. Am J Physiol Cell Physiol. 2008;295:C1215–1229. doi: 10.1152/ajpcell.00336.2008. [DOI] [PubMed] [Google Scholar]
- 99.Sultan S, Gosling M, Nagase H, Powell JT. Shear stress-induced shedding of soluble intercellular adhesion molecule-1 from saphenous vein endothelium. FEBS Lett. 2004;564:161–165. doi: 10.1016/S0014-5793(04)00337-0. [DOI] [PubMed] [Google Scholar]
- 100.Sun HW, Li CJ, Chen HQ, Lin HL, Lv HX, Zhang Y, Zhang M. Involvement of integrins, MAPK, and NF-kappaB in regulation of the shear stress-induced MMP-9 expression in endothelial cells. Biochem Biophys Res Commun. 2007;353:152–158. doi: 10.1016/j.bbrc.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 101.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 102.Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM, Bissell MJ, Werb Z. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J Cell Biol. 1994;125:681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Talhouk RS, Bissell MJ, Werb Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J Cell Biol. 1992;118:1271–1282. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000;5:40–46. doi: 10.1046/j.1087-0024.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 105.Tressel SL, Huang RP, Tomsen N, Jo H. Laminar shear inhibits tubule formation and migration of endothelial cells by an angiopoietin-2 dependent mechanism. Arterioscler Thromb Vasc Biol. 2007;27:2150–2156. doi: 10.1161/ATVBAHA.107.150920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ueda A, Koga M, Ikeda M, Kudo S, Tanishita K. Effect of shear stress on microvessel network formation of endothelial cells with in vitro three-dimensional model. Am J Physiol Heart Circ Physiol. 2004;287:H994–1002. doi: 10.1152/ajpheart.00400.2003. [DOI] [PubMed] [Google Scholar]
- 107.Vazquez F, Hastings G, Ortega MA, Lane TF, Oikemus S, Lombardo M, Iruela-Arispe ML. METH-1, a human ortholog of ADAMTS-1, and METH-2 are members of a new family of proteins with angio-inhibitory activity. J Biol Chem. 1999;274:23349–23357. doi: 10.1074/jbc.274.33.23349. [DOI] [PubMed] [Google Scholar]
- 108.Vernon RB, Angello JC, Iruela-Arispe ML, Lane TF, Sage EH. Reorganization of basement membrane matrices by cellular traction promotes the formation of cellular networks in vitro. Lab Invest. 1992;66:536–547. [PubMed] [Google Scholar]
- 109.Vickerman V, Blundo J, Chung S, Kamm R. Design, fabrication and implementation of a novel multi-parameter control microfluidic platform for three-dimensional cell culture and real-time imaging. Lab Chip. 2008;8:1468–1477. doi: 10.1039/b802395f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang XL, Fu A, Raghavakaimal S, Lee HC. Proteomic analysis of vascular endothelial cells in response to laminar shear stress. Proteomics. 2007;7:588–596. doi: 10.1002/pmic.200600568. [DOI] [PubMed] [Google Scholar]
- 112.Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Luthi U, Barberis A, Benjamin LE, Makinen T, Nobes CD, Adams RH. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 113.Werb Z, Sympson CJ, Alexander CM, Thomasset N, Lund LR, MacAuley A, Ashkenas J, Bissell MJ. Extracellular matrix remodeling and the regulation of epithelial-stromal interactions during differentiation and involution. Kidney Int Suppl. 1996;54:S68–74. [PMC free article] [PubMed] [Google Scholar]
- 114.Weskamp G, Mendelson K, Swendeman S, Le Gall S, Ma Y, Lyman S, Hinoki A, Eguchi S, Guaiquil V, Horiuchi K, Blobel CP. Pathological neovascularization is reduced by inactivation of ADAM17 in endothelial cells but not in pericytes. Circ Res. 106:932–940. doi: 10.1161/CIRCRESAHA.109.207415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu H, Riha GM, Yang H, Li M, Yao Q, Chen C. Differentiation and proliferation of endothelial progenitor cells from canine peripheral blood mononuclear cells. J Surg Res. 2005;126:193–198. doi: 10.1016/j.jss.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 116.Yamane T, Mitsumata M, Yamaguchi N, Nakazawa T, Mochizuki K, Kondo T, Kawasaki T, Murata S, Yoshida Y, Katoh R. Laminar high shear stress up-regulates type IV collagen synthesis and down-regulates MMP-2 secretion in endothelium. A quantitative analysis. Cell Tissue Res. 2010;340:471–479. doi: 10.1007/s00441-010-0968-6. [DOI] [PubMed] [Google Scholar]
- 117.Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 118.Zhou A, Egginton S, Hudlicka O, Brown MD. Internal division of capillaries in rat skeletal muscle in response to chronic vasodilator treatment with alpha1-antagonist prazosin. Cell Tissue Res. 1998;293:293–303. doi: 10.1007/s004410051121. [DOI] [PubMed] [Google Scholar]