Abstract
Objectives
Tuberculosis drug development is hampered by the slow growth of Mycobacterium tuberculosis. Bioluminescence, light produced by an enzymatic reaction, constitutes a rapid and highly sensitive measurement of cell metabolic function that can be used as an indirect marker of cell viability in drug screening assays. The aim of this work was to validate and standardize the use of luminescent M. tuberculosis strains to test the activity of antibacterial drugs in vitro and inside macrophages in a 96-well format.
Methods
We have used strains that express the bacterial lux operon and therefore do not require exogenous substrate to produce light, as well as strains expressing the firefly luciferase that need luciferin substrate. Results were compared with those obtained using the resazurin reduction assay and cfu plating.
Results
Using bioluminescence we were able to reduce the time required to measure the MIC and bactericidal concentrations of antimicrobials to just 3 and 6 days, respectively. Furthermore, antibacterial activity against intracellular mycobacteria was detected within 2 days post-infection. Results were comparable to those obtained by conventional methods.
Conclusions
We have developed a simple and rapid method for screening antimycobacterial drugs in culture and in macrophages. The use of autoluminescent bacteria also facilitates the determination of growth and inhibition kinetics. The method is cost-effective, can easily be adapted to a larger scale and is amenable to automation. Current efforts are directed towards applying this technology to drug screening in vivo.
Keywords: drug screening assay, tuberculosis, bacterial luciferase, firefly luciferase
Introduction
Tuberculosis still constitutes a serious global health threat with 9.4 million new cases and 1.7 million deaths worldwide in 2009.1 There is also a huge reservoir of infection, since an estimated one-third of the world's population is infected with its causative agent, Mycobacterium tuberculosis. The main handicaps in fighting tuberculosis are the BCG vaccine, which works poorly in the most affected populations, and a treatment regimen that takes 6 months with up to four drugs for the active disease and up to 9 months for eradicating latent infection. This is further complicated by multidrug-resistant and extensively drug-resistant strains, requiring even longer treatment times with even less well-tolerated drugs. It is clear then, that new drugs targeting both active and latent infections are urgently needed.
The main steps followed by most tuberculosis whole cell-based drug screening programmes consist of a quick initial screening at one fixed concentration, subsequent MIC determination for the best hits, followed by antimycobacterial activity testing in macrophages for those compounds that have been shown to lack eukaryotic cytotoxicity and, finally, in vivo testing in animal models.2,3 Sometimes, additional assays are carried out, such as determination of the MBC or activity testing against drug-resistant clinical isolates to assess for cross-resistance.3 This already extended protocol becomes even longer due to the slow growth of M. tuberculosis, which divides every 24 h and needs 3–4 weeks to form colonies on solid medium. Therefore, methods able to rapidly detect mycobacterial growth are preferred. The most popular methods for drug testing and MIC determination are redox-based techniques, including the microplate Alamar Blue assay, the resazurin microplate assay (REMA) and assays based on tetrazolium dyes.4–6 These methods have turnaround times of 7–9 days, and their main advantage is that they are colorimetric and therefore results can easily be determined by visual inspection. Besides, a more quantitative and objective result can be obtained by performing colorimetric or fluorometric measurements using a plate reader, which is also more sensitive and can therefore shorten the turnaround time. An alternative microdilution plate assay involves the spectrophotometric measurement of optical density (OD) to assess growth inhibition.7 This requires special equipment, but is faster (3–5 days) and does not require the addition of any reagents. An additional advantage is that measurements are non-destructive and can be taken at various timepoints, producing kinetic data that can be used to plot dose–response curves and calculate effective concentrations more precisely. However, none of these methods is suitable for drug testing inside macrophages, which relies on time-consuming cfu-based methods. In contrast, reporter strains that express either a fluorescent protein or a luciferase can be used for drug testing both in vitro and in cell-based systems, such as macrophages.8–11 This is particularly true for luciferase assays, since luminescence produces a higher signal-to-background ratio than fluorescence measurements. Moreover, luminescent strains can be used for non-invasive drug assessment in animal models by in vivo imaging12–16 or by measuring light production in organ homogenates.17
Two luciferases have been used in mycobacterial research: the firefly luciferase (FFluc) and the bacterial luciferase of Vibrio harveyi (LuxAB). These luciferases produce light in the presence of the substrate/co-factor combinations d-luciferin/ATP and n-decanal/FMNH2, respectively. These co-factors are only found in live cells and so light production provides a sensitive indicator of cell viability. A major advantage of the bacterial luciferase system is that the genes for the synthesis of the substrate have also been described; the expression of the whole operon (luxCDABE) renders the bacteria autoluminescent, i.e. no external addition of substrate is needed for light production. However, until recently, only the bacterial luciferase enzyme (LuxAB) on its own had been successfully used in mycobacteria. Therefore, the drug screening protocols developed so far in mycobacteria, with either of the two luciferases, required the addition of substrate, making them destructive, endpoint measurements. In previous work, we described for the first time the successful expression in mycobacteria of the whole lux operon from Photorhabdus luminescens.18 Furthermore, we optimized the expression of both lux and ffluc, and we proved that these reporters are suitable for in vivo imaging of mycobacteria. In the present study, we have validated and standardized the use of luciferase-expressing M. tuberculosis for drug testing in vitro and inside macrophages. We have used both the Lux operon and FFluc enzyme, since the latter is brighter, even though it requires the addition of substrate. We prove that, using bioluminescence, MICs and MBCs are obtained in just 3 and 6 days, respectively, and that antibacterial activity in macrophages is detected as early as 1–2 days post-infection, with results comparable to those obtained by traditional methods. Furthermore, we show that this method can be applied to clinical isolates and, therefore, could be used to test cross-resistance using drug-resistant isolates.
Materials and methods
Strains and growth conditions
The M. tuberculosis strains used in this study are described in Table 1. All strains were grown at 37°C on Middlebrook 7H11 agar medium (BD Diagnostics) supplemented with 0.5% glycerol and 10% oleic acid-albumin-dextrose-catalase (OADC) (BD Diagnostics) or in Middlebrook 7H9 broth (BD Diagnostics) containing 0.05% Tween 80, 0.2% glycerol and 10% OADC. When required, kanamycin was used at a final concentration of 25 mg/L. For the drug testing assays, Middlebrook 7H9 medium was prepared without Tween or kanamycin. Bacterial strains were kept as frozen stocks at −80°C in 10% glycerol. The stocks were inoculated into 10 mL of medium and incubated for 7–10 days in a shaking incubator. Cultures were then diluted 1/100 into fresh medium and grown to mid-log phase (4–5 days) prior to being used in drug testing or infection assays.
Table 1.
Strains used in this study
| Strain | Description | Reference |
|---|---|---|
| H37Rv hsp | M. tuberculosis strain H37Rv transformed with the integrating expression vector pMV306hsp, Kanr | 18 |
| H37Rv LuxG13 | M. tuberculosis strain H37Rv transformed with the bacterial luciferase-encoding vector pMV306hsp + LuxAB + G13 + CDE, Kanr | 18 |
| H37Rv hspFFluc | M. tuberculosis strain H37Rv transformed with the firefly luciferase-encoding vector pMV306hsp + FFluc, Kanr | 18 |
| 212 hsp | M. tuberculosis Beijing strain 212 transformed with the integrating expression vector pMV306hsp, Kanr | this work |
| 212 LuxG13 | M. tuberculosis Beijing strain 212 transformed with the bacterial luciferase-encoding vector pMV306hsp + LuxAB + G13 + CDE, Kanr | this work |
| 232 hsp | M. tuberculosis clinical isolate 232 from the Indo-Oceanic lineage transformed with the integrating expression vector pMV306hsp, Kanr | this work |
| 232 LuxG13 | M. tuberculosis clinical isolate 232 from the Indo-Oceanic lineage transformed with the bacterial luciferase-encoding vector pMV306hsp + LuxAB + G13 + CDE, Kanr | this work |
| 355 hsp | M. tuberculosis strain 355 belonging to the Euro-American lineage transformed with the integrating expression vector pMV306hsp, Kanr | this work |
| 355 LuxG13 | M. tuberculosis strain 355 belonging to the Euro-American lineage transformed with the bacterial luciferase-encoding vector pMV306hsp + LuxAB + G13 + CDE, Kanr | this work |
Kanr, kanamycin resistant.
Antimicrobial agents
Isoniazid, chloramphenicol, streptomycin, ethambutol, rifampicin and levofloxacin were all from Sigma. Isoniazid, streptomycin, ethambutol and levofloxacin were dissolved in water, whereas chloramphenicol was prepared in ethanol and rifampicin in DMSO. Aliquots of each drug were kept at −20°C and thawed just before use. For the MIC/MBC assays, drugs were diluted to the working concentration in Middlebrook 7H9 supplemented with 0.2% glycerol and 10% OADC. The drug concentrations tested ranged from 0.008 to 0.25 mg/L for isoniazid, 2.5 to 80 mg/L for chloramphenicol, 0.125 to 4 mg/L for streptomycin, 0.5 to 16 mg/L for ethambutol, 0.008 to 0.25 mg/L rifampicin and 0.062 to 2 mg/L for levofloxacin.
MIC determinations
Drug activity was tested by REMA6 and bioluminescence assays.
REMA
Twofold serial dilutions were made in Middlebrook 7H9 medium in 96-well opaque white plates (Corning®) in duplicate. An inoculum at an OD600 of 0.01 was prepared by diluting mid-log cultures into 10 mL of medium and 100 μL was added per well (102–103 cfu). Growth controls containing no drug and a sterile control without bacteria were also prepared for each assay. To prevent sample evaporation during incubation, 200 μL of sterile water was added to all outer perimeter wells. The plates were incubated at 37°C for 7 days before adding 30 μL of sterile 0.01% resazurin to the growth control wells and incubating for a further 24 h. A change in colour from blue (oxidized state) to pink (reduced state) indicated growth of the bacteria and resazurin was added to all remaining wells. After 24 h, the visual MIC was determined as the lowest drug concentration that prevented growth and, therefore, colour change.
Bioluminescence assay
The same plates prepared for REMA were used for the bioluminescence assay. For strains expressing the lux operon, bioluminescence was measured for 10 s using a microplate reader (Luminoskan Ascent, Thermo Scientific). Bioluminescence was expressed as relative light units (RLU). For strains expressing the firefly luciferase, a 10 μL sample from each well was transferred to a new plate containing 40 μL of Middlebrook 7H9 medium and bioluminescence was read for 10 s after injecting 50 μL of 300 mg/L luciferin. Bioluminescence was read after 2, 3, 4, 5, 7 and 8 days of incubation. Cultures of the luciferase-negative M. tuberculosis (hsp) were processed in parallel and the measurements were treated as background luminescence. The MIC was defined as the lowest antibiotic concentration that resulted in a 1 log reduction in bioluminescence compared with no-drug controls (growth controls).
MBC determinations
At days 3 and 8 of incubation, 5 μL from each well of the MIC plates was transferred to a plate containing 195 μL per well of drug-free Middlebrook 7H9 medium. The presence of viable bacteria was then tested by three methods: cfu; bioluminescence; and resazurin. For cfu, 20 μL from each well of the MBC plate was dropped on Middlebrook 7H11 plates that were incubated for up to 4 weeks. For the bioluminescence method, the MBC plates were incubated at 37°C and bioluminescence was read on days 2, 3, 4, 7 and 8, as described for the MIC plates. For the resazurin method, 30 μL of sterile 0.01% resazurin was added to the growth control wells on days 3 or 7 and the plates were incubated for another 24 h; resazurin was then added to the remaining wells if a change in colour had occurred in the control wells. For each of the three methods, the MBC was defined as the lowest antibiotic concentration that resulted in the killing of ≥99% of the bacteria in comparison with the starting inoculum, with a luminescence less than or equal to the background, or which prevented a colour change of the resazurin, respectively. The compound was considered bactericidal when the MBC/MIC ratio was ≤4.
Macrophage assays
The intracellular activity of the drugs was evaluated in J774 cells (ATCC TIB-67™), as reported previously for mycobacteria,19–22 but with some modifications. J774 cells were maintained in Dulbecco's minimal essential medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum. The cells were plated at a concentration of 5 × 104 cells per well in 96-well tissue culture white plates with clear bottoms (Corning®) and allowed to adhere overnight. For the infection, mid-log phase M. tuberculosis were washed twice with PBS + 0.05% Tween, once with Dulbecco's PBS and then sonicated for 2 × 30 s in a bath sonicator. The bacteria were then diluted in DMEM and added to the J774 cells at a concentration of ∼5 × 105 cfu/well. After 4 h of infection at 37°C in 5% CO2, macrophages were treated with 200 mg/L amikacin for 30 min and washed thrice with DMEM to eliminate any extracellular bacteria. Lastly, 200 μL of complete DMEM, with or without antimycobacterial drugs, was added to each well. The concentrations tested for each drug were 0.25×, 1×, 4× and 16× MIC. Each drug concentration was tested in triplicate. Macrophages were observed periodically under the microscope to check for viability and the presence of extracellular bacteria. The medium was changed on day 4.
Bacterial viability was assessed by cfu enumeration and by measuring bioluminescence. Macrophages were lysed with sterile water for 30 min, serially diluted in PBS–Tween and 20 μL was dropped onto Middlebrook 7H11 plates for viable count determination. The cfu were plated just after the infection (day 0) and at the end of the experiment. Bioluminescence from strains expressing the lux operon was measured in whole macrophages for 10 s using a microplate reader (Luminoskan Ascent, Thermo Scientific). For strains expressing the firefly luciferase, a 50 μL sample from the macrophage lysate was transferred to a new plate and bioluminescence was read for 10 s after injecting 50 μL of 300 mg/L luciferin. Bioluminescence for the Lux strains was measured on days 0, 1, 2, 4 and 7 post-infection, whereas for the FFluc strains it was measured on days 0 and 7. Data were expressed as the percentage growth inhibition, i.e.
and was plotted as a function of drug dose. The effective concentration that caused 90% inhibition (EC90) was determined using sigmoidal dose–response (variable slope) non-linear regression, with a special concentration–response function called ‘log (agonist) vs. response—Find ECanything’ in GraphPad Prism 5.02 (GraphPad Software, CA, USA).
Statistical analysis
The results of the macrophage assays are aggregates of two or three independent experiments. To compare the dose–response curves obtained using the cfu method versus the bioluminescence method, GraphPad Prism was used to compare two models. In the first model (null hypothesis), the data were fitted with the assumption that the datasets for the two methods shared the same best-fit value of log EC90. In the second model (alternative hypothesis), the data were fitted with the assumption that the best-fit values of log EC90 were distinct. The models were then compared with the extra sum-of-squares F-test.23 Values of P < 0.05 were considered significant for all comparisons.
Results
Use of bioluminescence to determine MICs in vitro
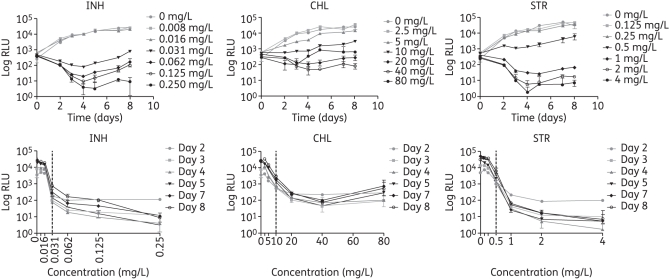
To validate the use of bioluminescence to assess antimycobacterial drug activity, the in vitro activities of six different drugs were evaluated and compared with the MIC data obtained using the published REMA method.6 We chose antibiotics with different mechanisms of action: isoniazid, which affects mycolic acid synthesis and is bactericidal; chloramphenicol, which inhibits protein synthesis by binding to the 50S ribosomal unit and is bacteriostatic; streptomycin, which affects protein synthesis by binding to the 30S ribosomal unit and has a bactericidal effect; ethambutol, which interferes with the synthesis of the arabinogalactan and is bactericidal; rifampicin, which inhibits transcription and is bactericidal; and levofloxacin, which affects DNA synthesis and is bactericidal. The experiments were initially done with the laboratory strain H37Rv using two bioluminescent reporters: Lux and FFluc. The plates were read daily to monitor the kinetics of bioluminescence production by H37Rv LuxG13 in the presence of 2-fold dilutions of antimicrobial agents. Examples of this are shown for isoniazid, chloramphenicol and streptomycin in Figure 1. An effect of the antibiotic could already be observed on day 2 and the dose–response curves were similar for all the timepoints tested (Figure 1). Equivalent results were obtained when using H37Rv hspFFluc, although a higher variability was observed, most likely as a result of the pipetting error introduced while measuring the luminescence of FFluc. For both reporters, the MIC results were available in 2–3 days, compared with 8 days required with REMA, and the median MICs for the six drugs differed by no more than one dilution comparing the bioluminescence and REMA methods (Tables 2 and 3).
Figure 1.
Effect of isoniazid (INH), chloramphenicol (CHL) and streptomycin (STR) on bioluminescence of H37Rv LuxG13 expressed as a function of time for all the concentrations tested (top graphs), and as a dose–response curve after 2–8 days of incubation (bottom graphs). The vertical dotted line in the dose–response curves indicates the MIC measured using the bioluminescence results. Data shown are the mean and standard deviation from one representative experiment.
Table 2.
MICs (mg/L) of six antimicrobial agents against H37Rv hsp and H37Rv LuxG13, as determined with the REMA and bioluminescence methods
| Drug | Bioluminescence |
REMA |
||||||
|---|---|---|---|---|---|---|---|---|
| day 2 | day 3 | day 4 | day 5 | day 7 | day 8 | LuxG13 | hsp | |
| INH | 0.031 (0.031–0.062) | 0.031 (0.031) | 0.031 (0.031) | 0.031 (0.031) | 0.031 (0.031–0.062) | 0.031 (0.031–0.062) | 0.031 (0.031) | 0.031 (0.031) |
| CHL | 10 (5–10) | 10 (5–10) | 10 (5–10) | 10 (10) | 10 (5–10) | 10 (10) | 5 (5) | 5 (5) |
| STR | 0.5 (0.5–1) | 0.5 (0.5) | 0.5 (0.5) | 0.5 (0.5) | 0.5 (0.5) | 0.5 (0.5–1) | 0.5 (0.25–0.5) | 0.5 (0.25–0.5) |
| EMB | 2 (2) | 2 (1–2) | 2 (1–2) | 2 (2) | 2 (1–2) | 2 (2) | 2 (1–2) | 2 (1–2) |
| RIF | 0.062 (0.016–0.062) | 0.031 (0.031–0.062) | 0.031 (0.016–0.031) | ND | 0.062 (0.031–0.062) | 0.062 (0.062) | 0.031 (0.031–0.062) | 0.031 (0.031–0.062) |
| LVX | 1 (0.5–2) | 0.25 (0.125–0.25) | 0.25 (0.125–0.25) | ND | 0.25 (0.125–0.25) | 0.25 (0.125–0.25) | 0.25 (0.125–0.25) | 0.25 (0.125–0.25) |
ND, not determined; INH, isoniazid; CHL, chloramphenicol; STR, streptomycin; EMB, ethambutol; RIF, rifampicin; LVX, levofloxacin.
Results for REMA were read on day 9, whereas bioluminescence results were read at different times from day 2 to day 8.
Results are expressed as the median and range (minimum–maximum) of 3–5 independent experiments.
Table 3.
MICs (mg/L) of six antimicrobial agents against H37Rv hsp and H37Rv hspFFluc, as determined with the REMA and bioluminescence methods
| Drug | Bioluminescence |
REMA |
|||||
|---|---|---|---|---|---|---|---|
| day 2 | day 3 | day 4 | day 7 | day 8 | hspFFluc | hsp | |
| INH | 0.25 (0.25) | 0.062 (0.031–0.062) | 0.031 (0.062) | 0.031 (0.031) | 0.031 (0.031–0.062) | 0.062 (0.062) | 0.062 (0.031–0.062) |
| CHL | 5 (2.5–10) | 5 (2.5–5) | 2.5 (2.5–10) | 5 (2.5–5) | 10 (5–10) | 5 (5) | 5 (5) |
| STR | 0.5 (0.25–0.5) | 0.25 (0.125–0.5) | 0.125 (0.125–0.5) | 0.25 (0.125–0.5) | 0.5 (0.25–0.5) | 0.25, 0.5a | 0.25, 0.5a |
| EMB | 2 (2) | 2 (1–2) | 2 (0.5–2) | 2 (1–2) | 2 (2) | 2 (1–2) | 1.5 (1–2) |
| RIF | 0.031 (0.016–0.062) | 0.016 (0.008–0.031) | 0.016 (0.016–0.062) | 0.031 (0.031) | 0.016 (0.016) | 0.062 (0.031–0.062) | 0.031 (0.031–0.062) |
| LVX | 0.25 (0.125–0.5) | 0.25 (0.125–0.25) | 0.25 (0.125–0.25) | 0.25 (0.125–0.25) | 0.25 (0.25) | 0.25 (0.125–0.25) | 0.25 (0.125–0.25) |
INH, isoniazid; CHL, chloramphenicol; STR, streptomycin; EMB: ethambutol; RIF, rifampicin; LVX, levofloxacin.
Results for REMA were read on day 9, whereas bioluminescence results were read at different times from day 2 to day 8.
Results are expressed as the median and range (minimum–maximum) of 3–5 independent experiments.
aEach value was obtained in two out of four experiments.
To test if this method could also be used with M. tuberculosis strains other than the laboratory strain H37Rv, we assayed the activity of isoniazid and chloramphenicol against three clinical isolates transformed with the lux operon. As for H37Rv, the results for the clinical isolates were obtained in just 2–3 days using the luciferase assay and the median MICs differed by no more than one drug concentration compared with those obtained using the REMA method (Table 4).
Table 4.
MICs (mg/L) of isoniazid and chloramphenicol against M. tuberculosis clinical isolates 212 LuxG13, 212 hsp, 232 LuxG13, 232 hsp, 355 LuxG13 and 355 hsp, as determined with the REMA and bioluminescence methods
| Strain/drug | Bioluminescence |
REMA |
||||||
|---|---|---|---|---|---|---|---|---|
| day 2 | day 3 | day 4 | day 5 | day 7 | day 8 | LuxG13 | hsp | |
| 212INH | 0.062 (0.062) | 0.031, 0.062a | 0.031 (0.031) | 0.031 (0.031) | 0.031 (0.031) | 0.031 (0.031) | 0.062 (0.062) | 0.062 (0.062) |
| 212CHL | 10 (10) | 10 (10) | 10 (10) | 10 (5–10) | 5 (5) | 10 (10) | 5 (5) | 5 (5) |
| 232INH | 0.016 (0.016) | 0.016 (0.016) | 0.016 (0.016) | 0.016, 0.031a | 0.031 (0.031) | 0.031 (0.031) | 0.031 (0.031) | 0.031 (0.031) |
| 232CHL | 10 (10) | 10 (10) | 10 (10) | 10 (5–10) | 10 (10) | 10 (10) | 5 (5–10) | 5 (5–10) |
| 355INH | 0.125 (0.125) | 0.062 (0.062) | 0.062 (0.062) | 0.062 (0.062) | 0.062 (0.062) | 0.062 (0.062) | 0.125 (0.125) | 0.125 (0.125) |
| 355CHL | 10 (10) | 5 (5–10) | 5 (5–10) | 5 (5) | 10 (5–10) | 10 (10) | 2.5 (2.5–5) | 5 (2.5–5) |
INH, isoniazid; CHL, chloramphenicol.
Results for REMA were read on day 9, whereas bioluminescence results were read at different times from day 2 to day 8.
Results are the median and range (minimum–maximum) of 2–3 independent experiments.
aEach value was obtained in one out of two experiments.
As expected, the expression of lux or ffluc did not affect the drug sensitivity of M. tuberculosis, since the MICs for the bioluminescent strains were not significantly different from those for the same strains carrying the empty vector (Tables 2–4).
Use of bioluminescence to determine MBCs in vitro
To our knowledge, the only method reported so far to measure MBCs in M. tuberculosis has been plating samples to count cfu, which can take up to 4 weeks because of the slow growth of this bacterium. Therefore, we wanted to develop a faster method using bioluminescence. We reasoned that if the drugs being tested had killed the bacteria we would not see an increase in bioluminescence upon subsequent transfer of the cells to medium with no drug. Whereas with bacteriostatic drugs, bacterial growth and bioluminescence would recover once the drug was removed.
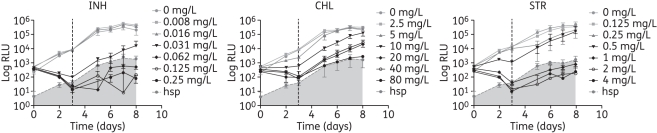
To test this, samples were transferred from the MIC assay to new plates containing antibiotic-free medium and incubated for either 3 or 8 days, leading to a total turnaround (MIC + MBC) of 6 and 16 days, respectively. The results for isoniazid, chloramphenicol and streptomycin for plates incubated for 3 days and using H37Rv LuxG13 can be seen in Figure 2. In the case of isoniazid and streptomycin, upon transferring the cells to drug-free medium, an increase in bioluminescence was observed in the wells that had been inoculated with cells from the lower inhibitory drug concentrations in the MIC plate, whereas luminescence remained below the background in samples coming from higher drug concentration wells. The MBC for these two antibiotics is thus twice the MIC (MBC/MIC ratio = 2) and, therefore, they would be classified as bactericidal drugs, which agrees with the literature.24,25 In the case of chloramphenicol, bioluminescence increased in all samples except those coming from the highest drug concentrations tested (Figure 2), giving an MBC/MIC ratio of 8, which is in agreement with chloramphenicol being a bacteriostatic drug.
Figure 2.
Bioluminescence of H37Rv LuxG13 incubated for 3 days in the presence of the indicated antimicrobial agents (MIC plates), followed by incubation in the absence of antibiotics (MBC plates). The vertical dotted line indicates the time when the antibiotic was removed. Data shown are the mean and standard deviation from one representative experiment. Bioluminescence was corrected for the dilution applied to set up the MBC plates. Readings for the non-luminescent H37Rv hsp strain represent the background bioluminescence, which is indicated as the shaded area. INH, isoniazid; CHL, chloramphenicol; STR, streptomycin.
Because similar results were obtained for plates incubated for 3 days and those incubated for 8 days (data not shown), we decided to perform the rest of the experiments with incubation times of 3 days, since the turnaround was shorter. A summary of the MBC results obtained for H37Rv LuxG13 is shown in Table 5. MBCs were calculated by measuring bioluminescence on days 2–4, or by adding resazurin on days 3–4 and plating for cfu counts. More interexperiment variability was observed for the MBC than for the MIC, regardless of the method employed to determine the MBC. This is probably due to low bacterial numbers and it could be addressed by increasing the initial inoculum. This was particularly problematic when using FFluc as the bioluminescent reporter (data not shown), because of the added variability introduced when taking samples to read the luminescence. Nevertheless, the MBCs obtained by measuring the luminescence from the Lux reporter differed by no more than one drug concentration from those obtained with the traditional cfu method (Table 5). We also tried using resazurin to read the MBCs plates; however, the results were difficult to interpret and the MBCs obtained were much lower than those calculated with any of the other two methods (Table 5), suggesting that resazurin is not sensitive enough to detect low growth.
Table 5.
MBCs (mg/L) of six drugs against H37Rv hsp and H37Rv LuxG13
| Drug | Bioluminescence |
REMA |
cfu |
||||
|---|---|---|---|---|---|---|---|
| day 2 | day 3 | day 4 | LuxG13 | hsp | LuxG13 | hsp | |
| INH | 0.062 (0.031–0.062) | 0.062 (0.031–0.125) | 0.062 (0.031–0.125) | 0.031 (0.031) | 0.031 (0.031) | 0.062 (0.031–0.062) | 0.062 (0.031–0.062) |
| CHL | >80 (10–>80) | 80, >80a | 80, >80a | 10 (5–10) | 10 (5–10) | >80 (>80) | >80 (>80) |
| STR | 0.5 (0.5–1) | 0.5 (0.5–1) | 1 (0.5–1) | 0.25 (0.25) | 0.25 (0.25) | 1 (0.5–2) | 1 (0.5–1) |
| EMB | 4 (4–16) | 8 (8–>16) | 8 (8) | 1.5 (1–2) | 1 (1–2) | 4 (<0.5–16) | 2 (1–8) |
| RIF | 0.062 (0.031–0.062) | 0.062 (0.062–0.125) | 0.062 (0.062–0.125) | 0.008 (0.008) | 0.016 (0.016) | 0.062 (0.016–>0.25) | 0.062 (0.016–>0.25) |
| LVX | 0.5 (0.5–2) | 0.5 (0.5–>2) | 0.5 (0.5–2) | 0.25 (0.25) | 0.25 (0.125–0.25) | 0.25 (0.125–>2) | 0.5 (0.25–2) |
INH, isoniazid; CHL, chloramphenicol; STR, streptomycin; EMB, ethambutol; RIF, rifampicin; LVX, levofloxacin.
Results are the median and range of four independent experiments.
aEach value was obtained in two out of four experiments.
In summary, measuring the luminescence from lux-expressing M. tuberculosis that had been incubated for 3days in the presence of drugs and thereafter transferred to drug-free medium for a further 3days allows the determination of the MBC in a total time of 6 days, with results equivalent to those obtained by the traditional time-consuming cfu plating method.
Drug susceptibility testing inside macrophages
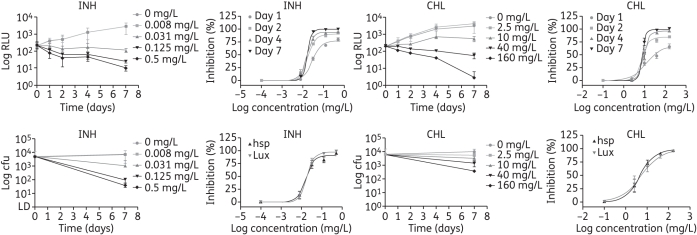
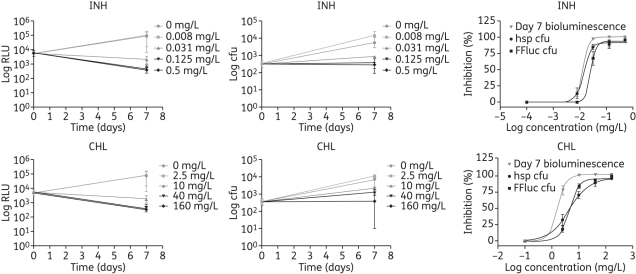
To investigate the feasibility of using bioluminescence to assess intracellular antimycobacterial drug activity, J774 macrophages were infected with luminescent and control M. tuberculosis H37Rv and exposed to various antibiotic concentrations. Bacterial viability was assessed by cfu enumeration and by bioluminescence reading. Four antibiotics were tested: isoniazid; chloramphenicol; streptomycin; and levofloxacin. The cfu and luminescence from H37Rv hspFFluc were determined on days 0 and 7 (end of the experiment) since lysis of the macrophages is required to do such measurements; whereas the bioluminescence produced by the bacterial luciferase was measured throughout the experiment since no lysis is needed.
Antimycobacterial activity, measured as a drop in bioluminescence, for the two highest drug concentrations tested was observed as early as 24 h post-infection when using the Lux reporter (Figure 3) or 7 days post-infection in the case of FFluc (Figure 4). Although a similar trend was obtained with cfu counts (Figures 3 and 4), 4 extra weeks were required for bacterial growth. The dose–response curves that were fitted to the data for isoniazid and chloramphenicol are shown in Figures 3 and 4, and the EC90 values for the four antibiotics tested are shown in Tables 6 and 7. Comparisons of log EC90 showed no significant differences between the results obtained by cfu counts and by bioluminescence reading from H37Rv LuxG13 on days 1, 2 and 4 for isoniazid and chloramphenicol, or between cfu and bioluminescence on day 7 for levofloxacin (Table 6). Although no significant differences were found between results obtained by cfu counts and by luminescence on day 4 for streptomycin, caution should be taken when interpreting these results, since a lot of variation was observed among replicates, which resulted in very wide confidence intervals (Table 6). Similarly, log EC90 calculated using bioluminescence from H37Rv hspFFluc was not significantly different from that calculated using cfu for any of the antibiotics tested (Table 7). Log EC90 values for the vector control strain H37Rv hsp were not significantly different from those obtained for the luminescent strains, with the exception of H37Rv hspFFluc and chloramphenicol, with an EC90 value 2.5 times higher for the control strain. This is most likely due to the high variation observed for the control cfu (Table 7). In fact, in most cases we have observed variability in cfu results, whereas results for bioluminescence were more consistent. This variation could be due to the inherent errors in making dilutions and plating cfu for clumping bacteria, such as mycobacteria, and would account for some of the discrepancies observed between cfu and bioluminescence.
Figure 3.
Effect of isoniazid (INH) and chloramphenicol (CHL) on intracellular H37Rv LuxG13, as determined by bioluminescence reading (top graphs) and by cfu counts (bottom graphs). Data are plotted as a function of time and as dose–response curve, and are the mean and standard deviation of 2–3 independent experiments.
Figure 4.
Effect of isoniazid (INH) and chloramphenicol (CHL) on intracellular H37Rv hspFFluc, as determined by bioluminescence reading and by cfu counts. Data are plotted as a function of time and as dose–response curve, and are the mean and standard deviation of 2–3 independent experiments.
Table 6.
Drug activity against intracellular M. tuberculosis H37Rv LuxG13 and H37Rv hsp, expressed as EC90 (mg/L) and 95% confidence intervals
| Drug | Bioluminescence |
cfu |
||||
|---|---|---|---|---|---|---|
| day 1 | day 2 | day 4 | day 7 | LuxG13 | hsp | |
| INH | 0.087a (0.059–0.128) | 0.046a (0.034–0.063) | 0.035a (0.027–0.046) | 0.026 (0.023–0.029) | 0.042 (0.031–0.056) | 0.044 (0.026–0.076) |
| CHL | 84.11a (19.09–370.6) | 17.02a (11.66–24.85) | 18.36a (6.28–53.66) | 12.22 (6.04–24.73) | 50.42 (8.98–283.1) | 22.95 (8.55–61.57) |
| STR | ambiguousb | ambiguous | 44.82a (2.75–731.4) | 2.89 (2.19–3.79) | 66.54 (0.26–17 003) | 59.2 (0.077–45 514) |
| LVX | ambiguous | 3.099 (1.316–7.297) | 1.547 (1.102–2.17) | 1.023a (0.924–1.132) | 0.878 (0.484–1.593) | 1.708 (0.753–3.876) |
INH, isoniazid; CHL, chloramphenicol; STR, streptomycin; LVX, levofloxacin.
Results represent two or three combined experiments.
aNo significant difference compared with H37Rv LuxG13 cfu (P > 0.05).
bAmbiguous is a term coined by GraphPad meaning that the fit does not allow interpretation of the best-fit values.
Table 7.
Drug activity against intracellular M. tuberculosis H37Rv hspFFluc and H37Rv hsp, expressed as EC90 (mg/L) and 95% confidence intervals
| Drug | Bioluminescence (day 7) | cfu |
|
|---|---|---|---|
| hspFFluc | hsp | ||
| INH | 0.023a (0.017–0.031) | 0.042 (0.017–0.1) | 0.028 (0.016–0.048) |
| CHL | 3.686a (1.264–10.75) | 11.56 (8.589–15.55) | 28.36 (11.08–72.57) |
| STR | 2.035a (0.875–4.734) | 1.304 (0.451–3.774) | 3.305 (0.578–18.91) |
| LVX | 1.424a (0.713–2.845) | 2.498 (2.059–3.03) | 3.417 (1.506–7.752) |
INH, isoniazid; CHL, chloramphenicol; STR, streptomycin; LVX, levofloxacin.
Results represent two or three combined experiments.
aNo significant difference compared with H37Rv hspFFluc cfu (P > 0.05).
Discussion
Tuberculosis drug development is hampered by the slow growth of M. tuberculosis. Bioluminescence, light produced by an enzymatic reaction, constitutes a rapid and highly sensitive measurement of cell metabolic function that can be used as an indirect marker of cell viability in drug screening assays. There have been numerous attempts to develop luciferase assays for antimycobacterial drug testing.26–32 Initially, FFluc was the preferred reporter and the methods developed were able to obtain MIC values similar to those obtained by more traditional methods, but in a shorter time.29–31,33 In two cases, 96-well plate or minitube formats were adapted, but the measurement of bioluminescence still required steps involving transferring samples to a new plate and adding the luciferin substrate.31,33 More recent studies have focused on the use of the bacterial luciferase LuxAB from V. harveyi, since it was found to be brighter in mycobacteria and it uses a cheaper substrate. For example, using LuxAB, Cho et al.27 developed a microplate assay to test antimicrobial agents against non-replicating M. tuberculosis grown under low oxygen conditions. Luminescence was measured after a 28 h recovery step with oxygen and results correlated well with cfu plated before the recovery step. Bioluminescence has also been used for drug testing against Mycobacterium ulcerans, a mycobacterium that requires up to 3 months to form colonies, shortening the assay to only 14 days.32 In spite of the advantages of using LuxAB, the extra steps needed to add the substrate were still a major drawback, and a proper standardization and comparison to more traditional methods was required.
In the present study, we have developed and standardized a 96-well microplate assay for drug testing in vitro using the lux operon, which does not require the addition of substrate, and FFluc, which requires the addition of d-luciferin. Initially, luminescence was read daily and compared with REMA to find the optimal incubation period. Optimal results were obtained in 3 days and the MIC varied by no more than one drug dilution compared with REMA. The luciferase assay here developed is particularly easy when using the lux operon, since luminescence can be read straight from the MIC plate without the need for any extra manipulations. Although FFluc requires the addition of luciferin before reading luminescence, we decided to also validate this reporter, since it is brighter than Lux and can therefore be useful when lower numbers of bacteria are being used, e.g. in a 384-well microplate assay. Moreover, luciferin can be added straight into the MIC plate on day 3, thus limiting the amount of extra steps and avoiding the additional variability introduced by pipetting errors while taking a sample.
We have taken a step further and have also explored the use of bioluminescence to determine whether the mechanism of action of a drug is bacteriostatic or bactericidal. To this end, we measured the luminescence during and after exposing the cells to antibiotics, since we reasoned that removal of the antibiotic would not have an effect on the luminescence for bactericidal drugs, whereas it would result in a recovery of light production if the drug was bacteriostatic. The results obtained with Lux confirmed our hypothesis and the MBC values calculated were very close to those found by the traditional method of cfu plating. However, the assay did not work for FFluc, the results were quite different from those obtained by cfu determination and a large variability was observed. This is likely to be due to the extra errors introduced while taking samples for measuring the luminescence of FFluc and it could be avoided by adding the luciferin straight into the MBC plate, as suggested for the MIC assay.
Lastly, we have also developed a 96-well luciferase assay for testing drugs against intracellular bacteria residing in macrophages. Using a lux-expressing strain to infect macrophages, we have been able to measure the luminescence over time and to plot the growth-inhibition kinetics of the luminescence in the presence of different drug concentrations. This was only possible because of the autoluminescent nature of the Lux-expressing mycobacteria. Moreover, by fitting the data to dose–response curves we were able to calculate the efficacy of the drugs in terms of EC90. We found that the results for the luciferase assay were not statistically different from the results obtained by cfu counts, and that they could be obtained in just 1–4 days for isoniazid, chloramphenicol and streptomycin, and 7 days for levofloxacin. In the case of isoniazid, chloramphenicol and streptomycin, the EC90 values obtained on day 7 were lower than those obtained by cfu counts, which is likely related to a more rapid effect of the drug on bacterial metabolism, and therefore light production, compared with cell viability and colony formation. On the other hand, optimal results as compared with cfu were only obtained on day 7 for levofloxacin. This may be due to the mechanism of action of the quinolone and/or to the poorer uptake of this drug by the macrophages.34
Other authors have also attempted to develop similar assays. For example, Arain et al.11 used M. tuberculosis and Mycobacterium bovis BCG expressing FFluc to infect THP-1 macrophages in 48-well plates. They tested isoniazid and rifampicin at two concentrations, and measured the luminescence at different timepoints, which involved lysis of the macrophages. Although a decrease in luminescence versus time could be observed, they did not calculate the efficacy, nor did they compare the results obtained with the traditional cfu plating method. Deb et al.10 conducted a similar study with Mycobacterium aurum expressing FFluc. In this case a decrease in both cfu and RLU could be observed both in vitro and inside macrophages, but again the efficacy was not calculated. More recently, Eklund et al.26 developed a method to assess M. tuberculosis growth in human monocyte-derived macrophages in 96-well plates using LuxAB. They proved that there was a good correlation between cfu and RLU, and then tested the effect of drug treatment on the bioluminescence production of M. tuberculosis growing intracellularly. However, only one drug concentration was tested, which did not allow dose–response curves to be plotted or efficacy calculated.
We have also optimized the macrophage luciferase assay using FFluc. In this case, the luminescence was only read at the end of the experiment, on day 7, since addition of the substrate was required. Although the macrophages were lysed prior to adding luciferin, lysis is not strictly necessary, since macrophages are permeable to luciferin, although the amount of luminescence obtained is lower than with lysis (data not shown). We have found that permeability, and therefore light production, depends on the buffer used to prepare the luciferin, with the best results obtained when using 0.1 M sodium citrate at pH 5. While this circumvents the need for lysis, it would still make it an endpoint measurement, since the macrophages are adversely affected by exposure to low pH. Nevertheless, using FFluc, the antibiotics' efficacy inside macrophages was determined in just 7 days, with results comparable to those obtained by cfu plating.
In conclusion, we have developed a simple and rapid method for screening antimycobacterial drugs in culture and in macrophages. The results are comparable to those obtained with conventional methods, but with a much shorter turnaround. In addition, the use of autoluminescent bacteria facilitates the determination of growth and inhibition kinetics. The methods presented here are cost-effective, can easily be adapted to a larger scale and are amenable to automation. Current efforts are directed towards applying this technology to drug screening in vivo.
Funding
This work was supported by the Bill and Melinda Gates Foundation TB Drug Accelerator Programme to the Imaging TB consortium (grant number 42786) and the Health Research Council of New Zealand (Sir Charles Hercus Fellowship to S. W. [grant number 09/099]).
Transparency declarations
None to declare.
References
- 1.WHO. Global Tuberculosis Control 2010. Geneva: WHO Press; 2010. [Google Scholar]
- 2.Orme I. Search for new drugs for treatment of tuberculosis. Antimicrob Agents Chemother. 2001;45:1943–6. doi: 10.1128/AAC.45.7.1943-1946.2001. doi:10.1128/AAC.45.7.1943-1946.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Primm TP, Franzblau SG. Recent advances in methodologies for the discovery of antimycobacterial drugs. Curr Bioact Compd. 2007;3:201–8. doi:10.2174/157340707781695550. [Google Scholar]
- 4.Caviedes L, Delgado J, Gilman RH. Tetrazolium microplate assay as a rapid and inexpensive colorimetric method for determination of antibiotic susceptibility of Mycobacterium tuberculosis. J Clin Microbiol. 2002;40:1873–4. doi: 10.1128/JCM.40.5.1873-1874.2002. doi:10.1128/JCM.40.5.1873-1874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins L, Franzblau SG. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother. 1997;41:1004–9. doi: 10.1128/aac.41.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palomino J-C, Martin A, Camacho M, et al. Resazurin microtiter assay plate: simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2002;46:2720–2. doi: 10.1128/AAC.46.8.2720-2722.2002. doi:10.1128/AAC.46.8.2720-2722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gruppo V, Johnson CM, Marietta KS, et al. Rapid microbiologic and pharmacologic evaluation of experimental compounds against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2006;50:1245–50. doi: 10.1128/AAC.50.4.1245-1250.2006. doi:10.1128/AAC.50.4.1245-1250.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Changsen C, Franzblau SG, Palittapongarnpim P. Improved green fluorescent protein reporter gene-based microplate screening for antituberculosis compounds by utilizing an acetamidase promoter. Antimicrob Agents Chemother. 2003;47:3682–7. doi: 10.1128/AAC.47.12.3682-3687.2003. doi:10.1128/AAC.47.12.3682-3687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srivastava R, Deb DK, Srivastava KK, et al. Green fluorescent protein as a reporter in rapid screening of antituberculosis compounds in vitro and in macrophages. Biochem Biophys Res Commun. 1998;253:431–6. doi: 10.1006/bbrc.1998.9147. doi:10.1006/bbrc.1998.9147. [DOI] [PubMed] [Google Scholar]
- 10.Deb DK, Srivastava KK, Srivastava R, et al. Bioluminescent Mycobacterium aurum expressing firefly luciferase for rapid and high throughput screening of antimycobacterial drugs in vitro and in infected macrophages. Biochem Biophys Res Commun. 2000;279:457–61. doi: 10.1006/bbrc.2000.3957. doi:10.1006/bbrc.2000.3957. [DOI] [PubMed] [Google Scholar]
- 11.Arain T, Resconi A, Singh D, et al. Reporter gene technology to assess activity of antimycobacterial agents in macrophages. Antimicrob Agents Chemother. 1996;40:1542–4. doi: 10.1128/aac.40.6.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heuts F, Carow B, Wigzell H, et al. Use of non-invasive bioluminescent imaging to assess mycobacterial dissemination in mice, treatment with bactericidal drugs and protective immunity. Microbes Infect. 2009;11:1114–21. doi: 10.1016/j.micinf.2009.08.005. doi:10.1016/j.micinf.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Mortin LI, Li T, Van Praagh ADG, et al. Rapid bactericidal activity of daptomycin against methicillin-resistant and methicillin-susceptible Staphylococcus aureus peritonitis in mice as measured with bioluminescent bacteria. Antimicrob Agents Chemother. 2007;51:1787–94. doi: 10.1128/AAC.00738-06. doi:10.1128/AAC.00738-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadurugamuwa JL, Modi K, Yu J, et al. Noninvasive biophotonic imaging for monitoring of catheter-associated urinary tract infections and therapy in mice. Infect Immun. 2005;73:3878–87. doi: 10.1128/IAI.73.7.3878-3887.2005. doi:10.1128/IAI.73.7.3878-3887.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadurugamuwa JL, Modi K, Yu J, et al. Noninvasive monitoring of pneumococcal meningitis and evaluation of treatment efficacy in an experimental mouse model. Mol Imaging. 2005;4:137–42. doi: 10.1177/13505068211024891. [DOI] [PubMed] [Google Scholar]
- 16.Cho JS, Zussman J, Donegan NP, et al. Noninvasive in vivo imaging to evaluate immune responses and antimicrobial therapy against Staphylococcus aureus and USA300 MRSA skin infections. J Invest Dermatol. 2011;131:907–15. doi: 10.1038/jid.2010.417. doi:10.1038/jid.2010.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hickey M, Arain T, Shawar R, et al. Luciferase in vivo expression technology: use of recombinant mycobacterial reporter strains to evaluate antimycobacterial activity in mice. Antimicrob Agents Chemother. 1996;40:400–7. doi: 10.1128/aac.40.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andreu N, Zelmer A, Fletcher T, et al. Optimisation of bioluminescent reporters for use with mycobacteria. PLoS ONE. 2010;5:e10777. doi: 10.1371/journal.pone.0010777. doi:10.1371/journal.pone.0010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rastogi N, Labrousse V, Goh KS. In vitro activities of fourteen antimicrobial agents against drug susceptible and resistant clinical isolates of Mycobacterium tuberculosis and comparative intracellular activities against the virulent H37Rv strain in human macrophages. Curr Microbiol. 1996;33:167–75. doi: 10.1007/s002849900095. doi:10.1007/s002849900095. [DOI] [PubMed] [Google Scholar]
- 20.Rastogi N, Potar M-C, David HL. Intracellular growth of pathogenic mycobacteria in the continuous murine macrophage cell line J774: ultrastructure and drug-susceptibility studies. Curr Microbiol. 1987;16:14. [Google Scholar]
- 21.Skinner PS, Furney SK, Jacobs MR, et al. A bone marrow-derived murine macrophage model for evaluating efficacy of antimycobacterial drugs under relevant physiological conditions. Antimicrob Agents Chemother. 1994;38:2557–63. doi: 10.1128/aac.38.11.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright EL, Quenelle DC, Suling WJ, et al. Use of Mono Mac 6 human monocytic cell line and J774 murine macrophage cell line in parallel antimycobacterial drug studies. Antimicrob Agents Chemother. 1996;40:2206–8. doi: 10.1128/aac.40.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motulsky HJ, Christopoulos A. Fitting Models to Biological Data Using Linear and Nonlinear Regression. A Practical Guide to Curve Fitting. San Diego, CA: GraphPad Software Inc.; 2003. [Google Scholar]
- 24.Streptomycin. Tuberculosis (Edinb) 2008;88:162–3. doi: 10.1016/S1472-9792(08)70027-1. doi:10.1016/S1472-9792(08)70027-1. [DOI] [PubMed] [Google Scholar]
- 25.Isoniazid. Tuberculosis (Edinb) 2008;88:112–6. doi: 10.1016/S1472-9792(08)70011-8. doi:10.1016/S1472-9792(08)70011-8. [DOI] [PubMed] [Google Scholar]
- 26.Eklund D, Welin A, Schon T, et al. Validation of a medium-throughput method for evaluation of intracellular growth of Mycobacterium tuberculosis. Clin Vaccine Immunol. 2010;17:513–7. doi: 10.1128/CVI.00446-09. doi:10.1128/CVI.00446-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho SH, Warit S, Wan B, et al. Low-oxygen-recovery assay for high-throughput screening of compounds against nonreplicating Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2007;51:1380–5. doi: 10.1128/AAC.00055-06. doi:10.1128/AAC.00055-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner DJ, Hoyle SL, Snewin VA, et al. An ex vivo culture model for screening drug activity against in vivo phenotypes of Mycobacterium tuberculosis. Microbiology. 2002;148:2929–36. doi: 10.1099/00221287-148-10-2929. [DOI] [PubMed] [Google Scholar]
- 29.Arain TM, Resconi AE, Hickey MJ, et al. Bioluminescence screening in vitro (Bio-Siv) assays for high-volume antimycobacterial drug discovery. Antimicrob Agents Chemother. 1996;40:1536–41. doi: 10.1128/aac.40.6.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooksey RC, Morlock GP, Beggs M, et al. Bioluminescence method to evaluate antimicrobial agents against Mycobacterium avium. Antimicrob Agents Chemother. 1995;39:754–6. doi: 10.1128/AAC.39.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooksey RC, Crawford JT, Jacobs WR, Jr, et al. A rapid method for screening antimicrobial agents for activities against a strain of Mycobacterium tuberculosis expressing firefly luciferase. Antimicrob Agents Chemother. 1993;37:1348–52. doi: 10.1128/aac.37.6.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang T, Bishai WR, Grosset JH, et al. Rapid assessment of antibacterial activity against Mycobacterium ulcerans by using recombinant luminescent strains. Antimicrob Agents Chemother. 2010;54:2806–13. doi: 10.1128/AAC.00400-10. doi:10.1128/AAC.00400-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shawar RM, Humble DJ, Van Dalfsen JM, et al. Rapid screening of natural products for antimycobacterial activity by using luciferase-expressing strains of Mycobacterium bovis BCG and Mycobacterium intracellulare. Antimicrob Agents Chemother. 1997;41:570–4. doi: 10.1128/aac.41.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato K, Tomioka H, Sano C, et al. Comparative antimicrobial activities of gatifloxacin, sitafloxacin and levofloxacin against Mycobacterium tuberculosis replicating within Mono Mac 6 human macrophage and A-549 type II alveolar cell lines. J Antimicrob Chemother. 2003;52:199–203. doi: 10.1093/jac/dkg343. doi:10.1093/jac/dkg343. [DOI] [PubMed] [Google Scholar]