Abstract
In search for new biologically active molecules, diversity-oriented synthetic (DOS) strategies break through the limitation of traditional library synthesis by sampling new chemical space. Many natural products can be regarded as intriguing starting points for DOS, wherein stereochemically rich core structures may be reorganized into chemotypes which are distinctly different from the parent structure. Ideally such transformations should be general and involve few steps in order to be suited for library applications. With this objective in mind, the highly oxygenated natural product fumagillol has been successfully remodeled in several ways utilizing a reaction discovery-based approach. In reactions with amines, excellent regiocontrol in a bis-epoxide opening/cyclization sequence can be obtained by size-dependent interaction of an appropriate catalyst with the parent molecule, forming either perhydroisoindole or perhydroisoquinoline products. Perhydroisoindoles can be further remodeled by cascade processes to afford either morpholinone or bridged 4,1-benzoxazepine-containing structures.
In order to identify pharmacological tools for biological processes, compound discovery must expand beyond the sp2-dominated synthetic libraries common in biological screening.1 Diversity-oriented libraries have been demonstrated to occupy areas of chemical space not normally accessed by more traditional planar, heterocyclic libraries.2–4 One approach to access increasingly diverse libraries would employ natural products as starting scaffolds. A number of studies have exploited natural products as starting materials, including use of α-santonin5–7 and (−)-shikimic acid8,9 to identify biologically active molecules including 5-lipoxygenase inhibitors and aurora A kinase ligands. Other methods have focused on altering the core framework of natural products to create small collections of structurally unique compounds. A method involving catalytic, site-selective derivatization of complex natural products (e.g. erythyromycin) has been demonstrated by the Miller group at Yale.10,11 In another study, the macrocyclic diterpenoid lathyrane was converted into a small collection of polycyclic structures using transannular reactions.12 The Miller group from Notre Dame has incorporated oxazine heterocycles into natural products bearing 1,3-butadiene subunits employing iminonitroso Diels-Alder cycloadditions.13,14
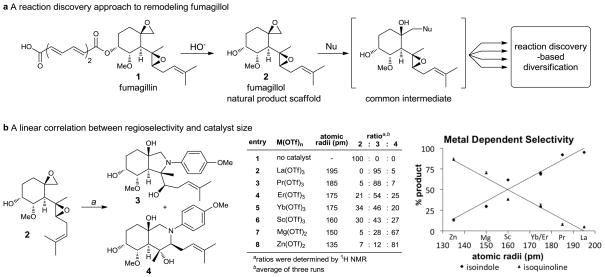
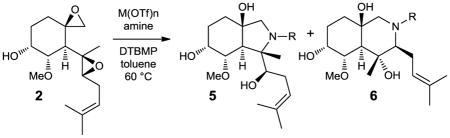
Further evolution of these ideas would involve the creation of a diverse library of remodelled structures derived from a natural product, each one significantly different from the parent compound. Transformations utilized should allow for the incorporation of new functionality and ideally be carried out in a single-step or tandem processes. We therefore initiated a reaction discovery-based approach15–18 that meets these criteria employing a readily available natural product as a starting point for chemically diverse library synthesis (Figure 1a). The highly oxygenated natural product fumagillol (2) was chosen as the reactivity and proximity of the two epoxides present site(s) for potential chemistry, while the hydroxyl and alkene groups offer additional functionality for further diversification. Crude fumagillin (1), a natural product which is readily available from the fermentation broth of Aspergillus fumigates and can be hydrolyzed to fumagillol (2) (see Supplementary Information),19,20, has generated significant interest as both a synthetic target21 and for its anti-angiogenic properties.22–24 We envisioned a series of tandem processes which could remodel fumagillol into novel chemotypes as dictated by either by catalyst or reaction partner choice. Herein, we report our initial studies aimed at remodeling fumagillol through Lewis acid-promoted addition of amines.
Figure 1. Natural Product Remodeling using Fumagillol.
a, Fumagillol can be transformed into multiple chemotypes through a panel of related reaction conditions. Fumagillol was obtained by hydrolysis of crude fumagillin isolated from the fermentation broth of Aspergillus fumigatus. b, a) M(OTf)n (10 mol%), p-anisidine (1.1 equiv.), DTBMP (60 mol%), toluene, 60 °C, 20 h; DTBMP = 2,6-di-tert-butyl-4-methylpyridine.
Results and Discussion
A reaction screen15,16 was first undertaken to explore sequential aminolyses of the 1,4-bis-epoxide. We anticipated that the sequence would be initiated at the spirocyclic epoxide, thereby mimicking the reactivity of fumagillin with aminopeptidase MetAP-2, the putative mode of its antiangiogenic activity.23 An initial reaction screen (see Supporting Figures 1–5) with twelve Lewis acids and four amines resulted in the conversion of the bis-epoxide motif into perhydroisoindole (3)25–27 and/or perhydroisoquinoline (4),28–30 compounds which were identifiable through several characteristic signals in 1H NMR spectra. Best results were obtained using p-anisidine and a metal triflate catalysis. Preliminary optimization of this transformation demonstrated that 2,6-di-tert-butyl-4-methylpyridine (DTBMP) as proton scavenger significantly improved yields, presumably by buffering adventitious triflic acid.31,32 Several metal triflates were subsequently investigated in a second screen and a linear correlation was found between the atomic radius of the metal catalyst and the distribution of isomeric products (Figure 1b). As metal size increased, perhydroisoindole product 3 was increasingly favored. Lanthanum triflate proved to be optimal for production of 3 with >95:5 regioselectivity (entry 2). Conversely, the smaller, bivalent-metal Zn(OTf)2 favored formation of perhydroisoquinoline 4 (entry 8, 13:87), thereby allowing access to either isomer simply by changing the catalyst.33–35 In the absence of a catalyst, no reaction occurred and fumagillol was fully recovered.
Further optimization using La(OTf)3 and Zn(OTf)2 catalysts was next pursued. The transformations were robust and did not require inert atmosphere, nor special precautions for anhydrous solvent. Other nonpolar solvents provided similar regioselectivity, though toluene proved to be optimal, in which case catalyst loading could be reduced to 10 mol% while maintaining reasonable reaction times. Production of 3 was ultimately optimized using La(OTf)3 to 91% isolated yield (91:3 regioselectivity), while 4 could be obtained with Zn(OTf)2 in 76% yield (9:76 regioselectivity).
Bis-epoxide opening, and in particular, catalyst-controlled regioselectivity, proved to be quite general (Table 1). A variety of electron-rich and electron-deficient anilines produced either heterocyclic motif (entries 1 – 5), with La(OTf)3 catalysis forming predominantly perhydroisoindoles 5 and Zn(OTf)2 yielding perhydroisoquinolines 6. In the case of the La(III)-promoted reactions, there was a direct correlation of the nucleophilicity of the aniline with the reaction rate, with electron-rich amines reacting faster. The rate of formation for perhydroisoquinoline 6 under Zn(II)-catalysis was largely unaffected by the electronics of the aniline, until a sufficiently electron deficient analogue (entry 5) was used. Thus, with p-trifluoromethylaniline, the reaction was significantly slower than with the more electron-rich anilines, which all proceeded at approximately the same rate. Heteroaryl amines including 2-aminopyridine and 2-aminothiazole failed to react using either La(OTf)3 or Zn(OTf)2, in which case fumagillol was fully recovered.
Table 1.
Reaction with primary and secondary amines.
| ||||
|---|---|---|---|---|
| entry | amine | M(OTf)n (rxn conditions) | reaction time | % yield 5: 6 |
| 1 |
|
La(OTf)3a | 7 h | 91: 3 |
| Zn(OTf)2b | 29 h | 9: 76 | ||
| 2 |
 (b)
(b)
|
La(OTf)3a | 3 h | 84: 0 |
| Zn(OTf)2b | 24 h | 6: 80 | ||
| 3 |
|
La(OTf)3a | 29 h | 89: 2 |
| Zn(OTf)2b | 29 h | 8: 65 | ||
| 4 |
|
La(OTf)3a | 36 h | 87: 0 |
| Zn(OTf)2b | 29 h | 10: 88 | ||
| 5 |
|
La(OTf)3a | 4 d | 40: 0 |
| Zn(OTf)2b | 48 h | 10: 61 | ||
| 7 |
 (f)
(f)
|
La(OTf)3c | 20 h | 62: 25 |
| Zn(OTf)2b | 60 h | 0: 57 | ||
| Mg(OTf)2d | 16 h | 13: 82 | ||
| 8 |
|
La(OTf)3c | 24 h | 66: 32 |
| Zn(OTf)2b | 60 h | 0: 2 | ||
| Mg(OTf)2d | 36 h | 18: 74 | ||
| 9 |
|
La(OTf)3c | 20 h | 49: 20 |
| Zn(OTf)2b | 60 h | 0: 11 | ||
| Mg(OTf)2d | 20 h | 12: 37 | ||
| 10 |
|
La(OTf)3c |
|
|
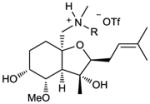
| R = PMP | ||||
| 7 (74%) | ||||
| 11 |
 (j)
(j)
|
La(OTf)3c | ||
| R = Bn | ||||
| 8 (75%) | ||||
La(OTf)3 (10 mol%), DTBMP (60 mol%), amine (1.1 equiv.);
Zn(OTf)2 (10 mol%), DTBMP (60 mol%), amine (1.1 equiv.);
La(OTf)3 (50 mol%), DTBMP (1.5 equiv.), amine (2.0 equiv.);
Mg(OTf)2 (50 mol%), DTBMP (1.5 equiv.), amine (2.0 equiv.); DTBMP = 2,6-di-tert-butyl-4-methylpyridine, PMP = p-methoxyaniline.
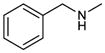
More basic amines were also well tolerated in the reaction (entries 7 – 9), with La(III) again proving to be optimal for perhydroisoindole formation. It was necessary, however, to increase the catalyst loading to 50 mol% in order to obtain reasonable reaction times, presumably due to the greater basicity of these amines leading to tighter interaction with the catalyst. An even greater reduction in reaction rate was observed with Zn(II) catalysis, rendering the reaction unacceptably slow (60 h, approx. 5–10% conversion). Use of Mg(OTf)2 (50 mol%) as the catalyst, however, also led predominantly to the desired perhydroisoquinoline products (6f – 6g) in acceptable reaction times (entries 7 – 9, 16 – 36 hrs). Addition of aromatic and aliphatic secondary amines were also carried out providing highly substituted tetrahydrofuran products 7 and 8, both isolated as triflate salts (Table 1, entries 10 and 11).
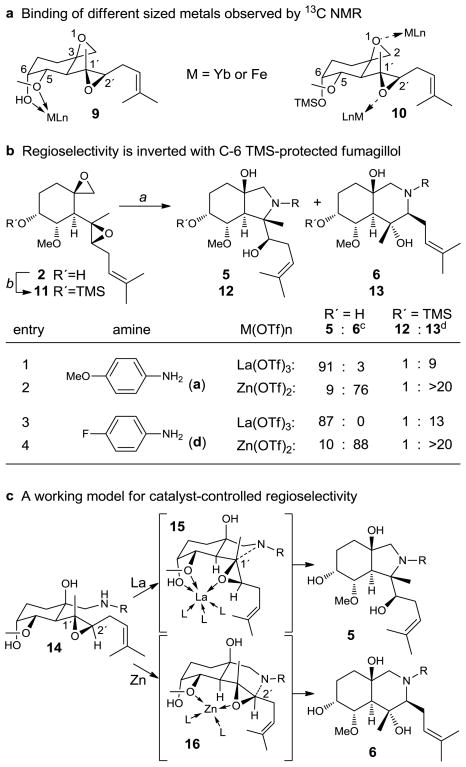
Insights into the interaction of metal catalysts with fumagillol were achieved through a series of 13C NMR experiments (Figure 2, Supplementary Figure 6). In the 13C NMR spectra of fumagillol obtained with 2 mol% of paramagnetic catalysts Yb(OTf)3 (r = 175 pm) or Fe(OTf)2 (r = 140 pm),35,36 broadening of the C5 and C6 resonances was observed, indicating that different sized metals are preferentially bound to the pocket formed by the hydroxyl and methoxy groups of fumagillol (7). The same interaction was not observed in the C6-silylated analogue 10, with only modest broadening of the C2, C1′, and C2′ signals observed.
Figure 2. Mechanistic Studies.
a, Metals of different sizes preferentially coordinate to the C-6 hydroxyl and C-5 methyl ether of fumagillol by 13C NMR. b, Epoxide opening of TMS-protected fumagillol shows an inversion of regioselectivity; a) amine (1.1 equiv.), M(OTf)n (10 mol%), DTBMP (60 mol%), toluene, 60 °C; b) TMSCl, imdazole, DMAP, CH2Cl2, 83%; c) isolated yields; d) ratio by 1H NMR. c, A working model demonstrating the role of the C-6 hydroxyl toward regioselectivity; DMAP = 4-dimethylaminopyridine, DTBMP = 2,6-di-tert-butyl-4-methylpyridine, TMS = trimethylsilyl.
To probe the importance of the interactions observed by 13C NMR, selectivity of silyl ether 11 under the optimized reaction conditions was evaluated. When 11 was reacted with several anilines, the regioselectivity obtained with La(III) catalysis inverted, leading predominantly to perhydroisoquinoline products as originally found in the Zn(II)-catalyzed reactions (Figure 2b). By comparison, reaction of 11 using Zn(II) became more selective to afford perhydroisoquinolines providing greater than 20:1 selectivity. These results suggest a mechanism wherein coordination of the metal to the C6 hydroxyl group of fumagillol with different sized metals greatly affects the regiochemical outcome, either through multidentate ligand effects and/or conformational control.
The observed regioselectivity can be rationalized from a model of metal-coordinated amino alcohol intermediate 14, obtained from opening of the more labile spirocyclic epoxide (Figure 2c).37,38 The larger La(III) catalyst may more easily accommodate the C6 hydroxyl group simultaneously with C1′ epoxide activation (cf. 15), thereby leading to tridentate coordination to the substrate wherein the amine is positioned closer and at a more optimal trajectory for addition to C1′. In contrast, smaller metals such as Zn(II) which cannot as easily accommodate the C6 hydroxyl while activating the second epoxide may adopt a looser bidentate coordination which places C2′ closer the amine (cf. 16). In the case of Zn(II) catalysis, activation of the second epoxide through adoption of 16 may be rate determining, as perhydroisoquinoline formation appeared to be largely independent of aniline nucleophilicity. Further studies to understand the precise mechanism leading to selectivity are currently underway.
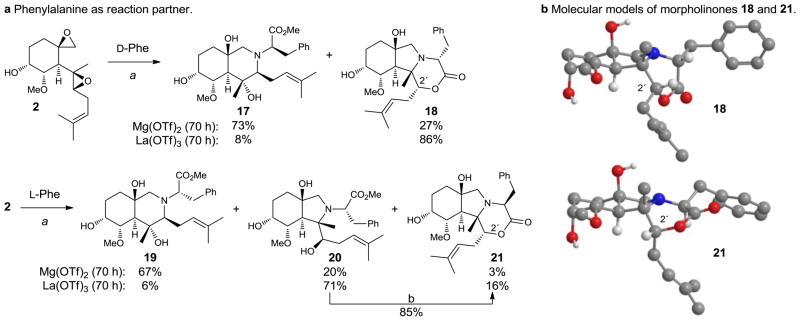
The methodology was further extended by the use of L- and D-phenylalanine methyl esters which underwent lactonization after initial epoxide opening (Figure 3a). With Mg(OTf)2 as catalyst, perhydroisoquinoline products 17 and 19, respectively, were produced in approximately 3:1 regioselectivity relative to the perhydroisoindole- derived products (18, and 20/21, respectively) for each amino acid. Further lactonization of the perhydroisoquinoline analogues was not observed. In comparison, the perhydroisoindole formed with D-phenylalanine (catalyzed by La(OTf)3) lactonized in situ to afford the polycyclic morpholinone derivative 18. Reaction with L-phenylalanine, however, formed isoindole 20 and morpholinone 21 in a 4:1 ratio. The observed resistance to lactonization of 21 can be rationalized from steric congestion caused by the additional pseudoaxial prenyl substituent at C2′ which was calculated to be 3.1 kcal/mol higher in energy relative to diastereomer 18 (Supplementary Figure 7). Lactonization of 20 could eventually be accomplished under basic conditions to yield morpholinone 21 (85%).
Figure 3. Use of amino acid esters as reaction partners.
a, Selective formation of perhydroisoindoles, perhydroisoquinolines, or morpholinones with phenylalanine; a) amine (2.0 equiv.), M(OTf)n (50 mol%), DTBMP (1.5 equiv.), toluene, 60 °C; b) NaOH (2.0 M), THF, rt, 6 h; DTBMP = 2,6-di-tert-butyl-4-methylpyridine, Phe = phenylalanine. b, Molecular models of the phenylalanine-derived morpholinones 18 and 21.
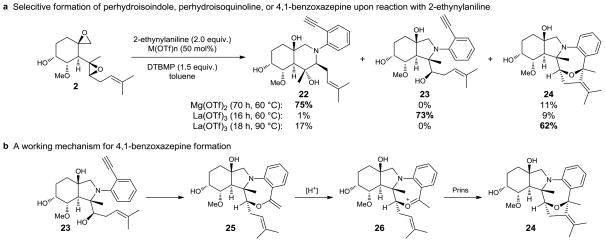
Reaction of 1 with 2-ethynylaniline under Mg(OTf)2 catalysis produced perhydroisoquinoline 22, while La(III) afforded the expected perhydroisoindole product 23 (Figure 4a). Upon extended reaction times (48 h) with La(OTf)3 or at elevated temperature (90 °C), the novel 4,1-benzoxazepine39–41 24 bearing a [4.2.1] ring system was formed from 23 in a highly efficient cascade process.42–45 Benzoxazepine 24 is presumably formed by initial hydroalkoxylation of the alkynyl alcohol of 23 to enol ether 2546,47 followed by protonation to oxonium 26 (Figure 4b). Subsequent Prins cyclization48 forms 4,1-benzoxazepine 24, thereby providing a dramatic example of natural product remodeling via an unanticipated cascade sequence.
Figure 4. Reaction with 2-ethynylaniline.
a, Selective formation of a perhydroisoindole, perhydroisoquinoline, or 4,1-benzoxazepine; DTBMP = 2,6-di-tert-butyl-4-methylpyridine. b, A novel cascade process to form a 4,1-benzoxazepine.
In summary, the natural product fumagillol has been selectively remodeled into a series of perhydroisoindoles and perhydroisoquinolines through sequential ring-opening with amines. Regiocontrol was achieved through choice of metal triflate catalysts, with smaller Zn(II) and Mg(II) catalysts leading to perhydroisoquinolines, while the larger La(III) catalyst favored production of perhydroisoindoles. Addition of secondary amines provided highly substituted tetrahydrofurans. Perhydrosoindole products underwent further reactions, including lactonizations employing amino acid esters as epoxide-opening nucleophiles and bridged 4,1-benzoxazepines from an unexpected cascade sequence with 2-ethynylaniline. Remodeled structures produced in this study are currently being examined in a range of biological screens, including those as part of the Molecular Libraries Probe Production Centers Network (MLPCN, http://mli.nih.gov/mli/) and the NIMH Psychoactive Drug Screening Program (PDSP, http://pdsp.med.unc.edu/indexR.html). These studies should pave the way for work to remodel other natural product scaffolds to access novel chemotypes and pharmacological tools.
Supplementary Material
Acknowledgments
We are grateful to the NIGMS CMLD initiative (P50 GM067041) for financial support, the National Science Foundation for supporting the purchase of NMR (CHE 0619339) and HRMS (CHE 0443618) spectrometers, and the Boston University Undergraduate Research Opportunities Program for support of M.C.M. We are also grateful to Dr. Jia-He Li of Sinova, Inc. for a generous donation of fumagillin.
Footnotes
Author contributions
B.R.B. and M.C.M. carried out the experimental work. A.B.B., J.A.P., Jr., and J.S.K. provided oversight. B.R.B, J.A.P., Jr., and J.S.K. conceived experiments and wrote the manuscript.
Additional information
The authors declare no competing financial interests. Supplementary information and chemical compound information accompany this paper at www.nature.com/naturechemistry. Reprints and permission information is available online at http://npg.nature.com/reprintsandpermissions/. Correspondence and requests for materials should be addressed to J.A.P., Jr.
References
- 1.Lovering F, Bikker J, Humblet C. Escape from flatland: increasing saturation as an approach to improving clinical Success. J Med Chem. 2009;52:6752–6756. doi: 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- 2.Galloway WRJD, Isidro-Llobet A, Spring DR. Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nat Commun 1. 2010;80:1–13. doi: 10.1038/ncomms1081. [DOI] [PubMed] [Google Scholar]
- 3.Clemons PA, Wilson JA, Dančík V, Muller S, Carrinski HA, Wagner BK, Koehler AN, Schreiber SL. Quantifying structure and performance diversity for sets of small molecules comprising small-molecule screening collections. Proc Natl Acad Sci USA. 2011;108:6817–6822. doi: 10.1073/pnas.1015024108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clemons PA, Bodycombe NE, Carrinski HA, Wilson JA, Samji AF, Wanger BK, Koehler AN, Scheiber SL. Small molecules of different origins have distinct distributions of structural complexity that correlate with protein-binding profiles. Proc Natl Acad Sci USA. 2010;107:18787–18792. doi: 10.1073/pnas.1012741107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mang C, Jakupovic S, Schunk S, Ambrosi H-D, Schwarz O, Jakupovic J. Natural products in combinatorial chemistry: an andrographolide-based library. J Comb Chem. 2006;8:268–274. doi: 10.1021/cc050143n. [DOI] [PubMed] [Google Scholar]
- 6.Schwarz O, Jakupovic S, Ambrosi HD, Haustedt LO, Mang C, Müller-Kuhrt L. Natural products in parallel chemistry-novel 5-lipoxygenase inhibitors from BIOS-based libraries starting from α-santonin. J Comb Chem. 2007;9:1104–1113. doi: 10.1021/cc700098t. [DOI] [PubMed] [Google Scholar]
- 7.Frank L, Schwarz O, Müller-Kuhrt L, Hoernig C, Fischer L, George S, Tanrikulu Y, Schneider P, Werz O, Steinhilber D, Schneider G. Identification of natural-product-derived inhibitors of 5-lipoxygenase activity by ligand-based virtual screening. J Med Chem. 2007;50:2640–2646. doi: 10.1021/jm060655w. [DOI] [PubMed] [Google Scholar]
- 8.Tan DS, Foley MA, Shair MD, Schreiber SL. Stereoselective synthesis of over two million compounds having structural features both reminiscent of natural products and compatible with miniaturized cell-based assays. J Am Chem Soc. 1998;120:8565–8566. [Google Scholar]
- 9.Miao H, Tallarico JA, Hayakawa H, Münger K, Duffner JL, Koehler AN, Schreiber SL, Lewis TA. Ring-opening and ring-closing reactions of a shikimic acid-derived substrate leading to diverse small molecules. J Comb Chem. 2007;9:245–253. doi: 10.1021/cc060135m. [DOI] [PubMed] [Google Scholar]
- 10.Lewis CA, Miller SJ. Site-selective derivatization and remodeling of erythromycin A by using simple peptide-based chiral catalysts. Angew Chem Int Ed. 2006;45:5616–5619. doi: 10.1002/anie.200601490. [DOI] [PubMed] [Google Scholar]
- 11.Lewis CA, Longcore KE, Miller SJ, Wender PA. An approach to site-seletive diversification of apoptolidin A with peptide-based catalysts. J Nat Prod. 2009;72:1864–1869. doi: 10.1021/np9004932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Appendino G, Tron GC, Jarevång T, Sterner O. Unnatural natural products from the transannular cyclization of lathyrane diterpenes. Org Lett. 2001;3:1609–1612. doi: 10.1021/ol0155541. [DOI] [PubMed] [Google Scholar]
- 13.Li F, Yang B, Miller MJ, Zajicek J, Noll BC, Möllmann U, Dahse HM, Miller PA. Iminonitroso diels-alder reactions for efficient derivatization and functionalization of complex diene-containing natural products. Org Lett. 2007;15:2923–2926. doi: 10.1021/ol071322b. [DOI] [PubMed] [Google Scholar]
- 14.Krchňák V, Waring KR, Noll BC, Moellmann U, Dahse HM, Miller MJ. Evolution of natural product scaffolds by acyl- arylnitroso hetero-diels-alder reactions: new chemistry on piperine. J Org Chem. 2008;73:4559–4567. doi: 10.1021/jo8004827. [DOI] [PubMed] [Google Scholar]
- 15.Beeler AB, Su S, Singleton CA, Porco JA., Jr Discovery of chemical reactions through multidimensional screening. J Am Chem Soc. 2007;129:1413–1419. doi: 10.1021/ja0674744. [DOI] [PubMed] [Google Scholar]
- 16.Han C, Rangarajan S, Voukides AC, Beeler AB, Johnson R, Porco JA., Jr Reaction discovery employing macrocycles: transannular cyclization of macrocyclic bis-lactams. Org Lett. 2009;11:413–416. doi: 10.1021/ol802729f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones AL, Snyder JK. Synthesis of unique scaffolds via diels-alder cycloadditions of tetrasubstituted cyclohexadienes. Org Lett. 2010;12:1592–1595. doi: 10.1021/ol100318f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medeiros MR, Narayan RS, McDougal NT, Schaus SE, Porco JA., Jr Skeletal diversity via cationic rearrangements of substituted dihydropyrans. Org Lett. 2010;12:3222–3225. doi: 10.1021/ol101144k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanson FR, Eble TE. Upjohn Co; USA: Fumagillin and preparation. 2,652,356. US Patent. 1950 Sep 25;
- 20.Tarbell DS, Carman RM, Chapman DD, Cremer SE, Cross AD, Huffman KR, Kunstmann M, McCorkindale NJ, McNally JG, Jr, Rosowsky A, Varino FHL, West RL. The chemistry of fumagillin. J Am Chem Soc. 1961;83:3096–3113. [Google Scholar]
- 21.Yamaguchi J, Hayashi Y. Syntheses of fumagillin and ovalicin. Chem Eur J. 2010;16:3884–3901. doi: 10.1002/chem.200902433. [DOI] [PubMed] [Google Scholar]
- 22.Ingber D, Fujita T, Kishimoto S, Sudo K, Kanamaru T, Brem H, Folkman J. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumor growth. Nature. 1990;348:555–557. doi: 10.1038/348555a0. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Widom J, Kemp CW, Crews CM, Clardy J. Structure of human methionine aminopeptidase-2 complexed with fumagillin. Science. 1998;282:1324–1327. doi: 10.1126/science.282.5392.1324. [DOI] [PubMed] [Google Scholar]
- 24.Lu J, Chong CR, Hu X, Liu JO. Fumarranol, a rearranged fumagillin analogue that inhibits angiogenesis in vivo. J Med Chem. 2006;49:5645–5648. doi: 10.1021/jm060559v. [DOI] [PubMed] [Google Scholar]
- 25.Lins L, Brasseur R, Malaisse WJ, Biesemans M, Verheyden P, Willem R. Importance of hydrophobic energy: structural determination of a hypoglycemic drug of the meglitinide family by nuclear magnetic resonance and molecular modeling. Biochem Pharmacol. 1996;52:1155–1168. doi: 10.1016/0006-2952(96)00424-8. [DOI] [PubMed] [Google Scholar]
- 26.Giraud E, Luttman C, Lavelle F, Riou JF, Mailliet P, Laoui A. Multivariate data analysis using D-optimal designs, partial least squares, and response surface modeling: a directional approach for the analysis of farnesyltransferase inhibitors. J Med Chem. 2000;43:1807–1816. doi: 10.1021/jm991166h. [DOI] [PubMed] [Google Scholar]
- 27.Jiang J, Bunda JL, Doss GA, Chicchi GG, Kurtz MM, Tsao KLC, Tong X, Zheng S, Upthagrove A, Samuel K, Tschirret-Guth R, Kumar S, Wheeldon A, Carlson EJ, Hargreaves R, Burns D, Hamill T, Ryan C, Krause SM, Eng W, De Vita RJ, Mills SG. Potent, brain-penetrant, hydroisoindoline-based human neurokinin-1 receptor antagonists. J Med Chem. 2009;52:3039–3046. doi: 10.1021/jm8016514. [DOI] [PubMed] [Google Scholar]
- 28.Hansen MM, Bertsch CF, Harkness AR, Huff BE, Hutchison DR, Khau VV, LeTourneau ME, Martinelli MJ, Misner JW, Peterson BC, Reick JA, Sullivan KA, Wright IG. An enantioselective synthesis of cis-perhydroisoquinoline LY235959. J Org Chem. 1998;63:775–785. doi: 10.1021/jo9717649. [DOI] [PubMed] [Google Scholar]
- 29.Rennison D, Neal AP, Cami-Kobeci G, Aceto MD, Martinez-Bermejo F, Lewis JW, Husbands SM. Cinnamoyl derivatives of 7α-aminomethyl-6,14-endo-ethanotetrahydrothebaine and 7α-aminomethyl-6,14-endo- ethanotetrahydrooripavine and related opioid ligands. J Med Chem. 2007;50:5176–5182. doi: 10.1021/jm070255o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frankowski KJ, Ghosh P, Setola V, Tran TB, Roth BL, Aubé J. N-Alkyl-octhydroisoquinoline-1-one-8-carboxamides: selective and nonbasic κ-opioid receptor ligands. ACS Med Chem Lett. 2010;1:189–193. doi: 10.1021/ml100040t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrett AGM, Braddock DC, Henschke JC, Walker ER. Ytterbium(III) triflate-catalyzed preparation of calix[4]resorcinarenes: Lewis-assisted Brønsted acidity. J Chem Soc, Perkin Trans. 1999;1:873–878. [Google Scholar]
- 32.Dumeunier R, Markó IE. On the role of triflic acid in the metal triflate-catalyzed acylation of alcohols. Tetrahedron Lett. 2004;45:825–829. [Google Scholar]
- 33.Fujiwara KT, Tokiwano A. Murai La(OTf)3-catalysed 6-endo epoxide opening of 4,5-epoxy-4-methoxymethyl-1-hexanols. Tetrahedron Lett. 1995;36:8063–8066. [Google Scholar]
- 34.Fujiwara K, Mishima H, Amano A, Tokiwano T, Murai A. La(OTf)3-catalyzed 7-endo and 8-endo selective cyclizations of hydroxy epoxides. Tetrahedron Lett. 1998;39:393–396. [Google Scholar]
- 35.Marson CM. Oxygen-directed carbocyclizations of epoxides. Tetrahedron. 2000;56:8779–8794. [Google Scholar]
- 36.Goodell JR, Leng B, Snyder TK, Beeler AB, Porco JA., Jr Multidimensional screening and methodology development for condensations involving complex 1,2-diketones. Synthesis. 2010:2254–2270. doi: 10.1055/s-0029-1218813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fardis M, Pyun HJ, Tario J, Jin H, Kim CU, Ruckman J, Lin Y, Green L, Hicke B. Design, synthesis and evaluation of a series of novel fumagillin analogues. Bioorg Med Chem. 2003;11:5051–5058. doi: 10.1016/j.bmc.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 38.Pyun HJ, Fardis M, Tario J, Yang CY, Ruckmann J, Henninger D, Jin H, Kim CU. Investigation of novel fumagillin analogues as angiogenesis inhibitors. Bioorg Med Chem Lett. 2004;14:91–94. doi: 10.1016/j.bmcl.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Das J, Kumar MS, Subrahmanyam D, Sastry TVRS, Narasimhulu CP, Rao CVL, Kannan M, Roshaiah M, Awasthi R, Patil SN, Sarnaik HM, Mamidi NVSR, Selvakumar N, Iqbal J. Substituent activity relationship studies on new azolo benzoxazepinyl oxazolidinones. Bioorg Med Chem. 2006;14:8032–8042. doi: 10.1016/j.bmc.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 40.Díaz-Gavilán M, Gómez-Vidal JA, Rodríguez-Serrano F, Marchal JA, Caba O, Aránega A, Gallo MA, Espinosa A, Campos JM. Anticancer activity of (1,2,3,5-tetrahydro-4,1-benzoxazepine-3-yl)-pyrimidines and -purines against the MCF-7 cell line: preliminary cDNA microarray studies. Bioorg Med Chem Lett. 2008;18:1457–1460. doi: 10.1016/j.bmcl.2007.12.070. [DOI] [PubMed] [Google Scholar]
- 41.López-Cara LC, Conejo-García A, Marchal JA, Macchione G, Cruz-López O, Boulaiz H, García MA, Rogdríguez-Serrano F, Ramírez A, Cativiela C, Campos JM. New (RS)-benzoxazepin-purines with antitumor activity: the chiral switch from (RS)-2,6-dichloro-9-[1-(p-nitrobenzenesulfonyl)-1,2,3,5-tetrahydro-4,1-benzoxazepin-3-yl]-9H-purine. Eur J Med Chem. 2011;46:249–258. doi: 10.1016/j.ejmech.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 42.Bhunia S, Wang KC, Liu RS. PtII-Catalyzed synthesis of 9-oxabicyclo[3.3.1]nona-2,6-dienes from 2-alkynyl-1-carbonylbenzenes and allylsilanes by an allylation/annulation cascade. Angew Chem Int Ed. 2008;47:5063–5066. doi: 10.1002/anie.200800826. [DOI] [PubMed] [Google Scholar]
- 43.Barluenga J, Fernández A, Satrústegui A, Diéguez A, Rodríguez F, Fañanás FJ. Tandem intramolecular hydroalkoxylation-hydroarylation reactions: synthesis of enantiopure benzofused cyclic ethers from the chiral pool. Chem Eur J. 2008;14:4153–4156. doi: 10.1002/chem.200800312. [DOI] [PubMed] [Google Scholar]
- 44.Barluenga J, Fernández A, Diéguez A, Rodríguez F, Fañanás FJ. Gold- or platinum-catalyzed cascade processes of alkynol derivatives involving hydroxylation reactions followed by Prins-type cyclizations. Chem Eur J. 2009;15:11660–11667. doi: 10.1002/chem.200900856. [DOI] [PubMed] [Google Scholar]
- 45.Fañanás FJ, Fernández A, Çevic D, Rodríguez F. An expeditious synthesis of bruguierol A. J Org Chem. 2009;74:932–934. doi: 10.1021/jo8021204. [DOI] [PubMed] [Google Scholar]
- 46.Yu X, Seo SY, Marks TJ. Effective, selective hydroalkoxylation/cyclization of alkynyl and allenyl alcohols mediated by lanthanide catalysts. J Am Chem Soc. 2007;129:7244–7245. doi: 10.1021/ja071707p. [DOI] [PubMed] [Google Scholar]
- 47.Motto A, Fragalà IL, Marks TJ. Atom-efficient carbon-oxygen bond formation process. DFT analysis of the intramolecular hydroalkoxylation/cyclization of alkynyl alcohols mediated by lanthanide catalysis. Organometallics. 2010;29:2004–2012. [Google Scholar]
- 48.Olier C, Kaafarani M, Gastaldi S, Bertrand MP. Synthesis of tetrahydropyrans and related heterocycles via Prins cyclization; extension to aza-Prins cyclization. Tetrahedron. 2010;66:413–445. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.