Summary
Background
After breast-conserving surgery, radiotherapy reduces recurrence and breast cancer death, but it may do so more for some groups of women than for others. We describe the absolute magnitude of these reductions according to various prognostic and other patient characteristics, and relate the absolute reduction in 15-year risk of breast cancer death to the absolute reduction in 10-year recurrence risk.
Methods
We undertook a meta-analysis of individual patient data for 10 801 women in 17 randomised trials of radiotherapy versus no radiotherapy after breast-conserving surgery, 8337 of whom had pathologically confirmed node-negative (pN0) or node-positive (pN+) disease.
Findings
Overall, radiotherapy reduced the 10-year risk of any (ie, locoregional or distant) first recurrence from 35·0% to 19·3% (absolute reduction 15·7%, 95% CI 13·7–17·7, 2p<0·00001) and reduced the 15-year risk of breast cancer death from 25·2% to 21·4% (absolute reduction 3·8%, 1·6–6·0, 2p=0·00005). In women with pN0 disease (n=7287), radiotherapy reduced these risks from 31·0% to 15·6% (absolute recurrence reduction 15·4%, 13·2–17·6, 2p<0·00001) and from 20·5% to 17·2% (absolute mortality reduction 3·3%, 0·8–5·8, 2p=0·005), respectively. In these women with pN0 disease, the absolute recurrence reduction varied according to age, grade, oestrogen-receptor status, tamoxifen use, and extent of surgery, and these characteristics were used to predict large (≥20%), intermediate (10–19%), or lower (<10%) absolute reductions in the 10-year recurrence risk. Absolute reductions in 15-year risk of breast cancer death in these three prediction categories were 7·8% (95% CI 3·1–12·5), 1·1% (–2·0 to 4·2), and 0·1% (–7·5 to 7·7) respectively (trend in absolute mortality reduction 2p=0·03). In the few women with pN+ disease (n=1050), radiotherapy reduced the 10-year recurrence risk from 63·7% to 42·5% (absolute reduction 21·2%, 95% CI 14·5–27·9, 2p<0·00001) and the 15-year risk of breast cancer death from 51·3% to 42·8% (absolute reduction 8·5%, 1·8–15·2, 2p=0·01). Overall, about one breast cancer death was avoided by year 15 for every four recurrences avoided by year 10, and the mortality reduction did not differ significantly from this overall relationship in any of the three prediction categories for pN0 disease or for pN+ disease.
Interpretation
After breast-conserving surgery, radiotherapy to the conserved breast halves the rate at which the disease recurs and reduces the breast cancer death rate by about a sixth. These proportional benefits vary little between different groups of women. By contrast, the absolute benefits from radiotherapy vary substantially according to the characteristics of the patient and they can be predicted at the time when treatment decisions need to be made.
Funding
Cancer Research UK, British Heart Foundation, and UK Medical Research Council.
Introduction
For many women with early-stage breast cancer, breast-conserving surgery can remove any macroscopic disease that has been detected; however, some microscopic tumour foci might remain in the conserved breast that could, if untreated, lead to locoregional recurrence or life-threatening distant metastases, or both. This report updates previous analyses from the Early Breast Cancer Trialists' Collaborative Group (EBCTCG) of individual patient data from the randomised trials of radiotherapy after breast-conserving surgery.1–5 It includes further follow-up for nine of the ten trials analysed previously;5 adds data from seven new trials, six of which were in low-risk women; and increases the total number of women analysed by nearly 50%. The report focuses mainly on women for whom pathological axillary lymph node status is known (negative [pN0] or positive [pN+]), because such information is usually available nowadays. It assesses the extent to which the radiotherapy-related absolute reduction in 10-year risk of first recurrence at any site (locoregional or distant) varies for women with different prognostic and other factors. It then relates the absolute reduction in the 15-year risk of breast cancer death to the absolute reduction in the 10-year risk of a recurrence.
Methods
Study design
Eligible trials were those beginning before the year 2000 of adjuvant radiotherapy versus no radiotherapy following breast-conserving surgery for invasive cancer. Trial identification and data handling were as previously reported.5 For every woman, information was sought regarding initial characteristics, allocated treatment, time to first recurrence, whether the first recurrence was locoregional or distant (excluding contralateral breast cancer), and date last known alive or date and underlying cause of death. When no recurrence was reported before breast cancer death, distant recurrence was assumed to have just preceded it. If contralateral breast cancer occurred before any other recurrence, follow-up was censored on that date in recurrence analyses.
Statistical analysis
The method used in this study differs from that used previously.5 First, to avoid assumptions about how locoregional and distant recurrence relate to each other, the main emphasis is on analyses of any first recurrence rather than, as before, of time to locoregional recurrence as a first event. Second, most analyses of recurrence present data for only 10 years, because many of these trials did not follow up women beyond this time for recurrence. This absence of follow-up should not bias the estimated proportional effect on recurrence after year 10, but could affect estimates of the absolute recurrence risk after year 10 substantially. Third, deaths of unknown cause before recurrence are no longer attributed to breast cancer, because most occurred many years after trial entry, by which time non-breast-cancer mortality predominated. Other aspects of the methods used are as before.5 Log-rank analyses are stratified by trial, individual follow-up year, and nodal status. They are also stratified by age, either in five groups (<40, 40–49, 50–59, 60–69, 70+ years) or, when data are also subdivided by other factors, in two groups (<50 and 50+ years).
Analyses of the dependence of absolute risk, and absolute risk reduction, on several factors simultaneously use Poisson regression fitted by maximum likelihood (webappendix pp 20–25). In these analyses absolute risk of any first recurrence is adjusted for trial, individual follow-up year, nodal status, and age (five groups), and also for tumour grade, tumour size, oestrogen-receptor (ER) status, and whether or not tamoxifen had been used in both randomised groups in the trial.
Preliminary results were presented to collaborators in September, 2010. A report was circulated to collaborators for comment in January, 2011, and then revised centrally.
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The Secretariat had full access to all data and analyses. Submission for publication was decided only by the writing committee.
Results
Information was available for 10 801 women in 17 trials (table 1). Six trials were of radiotherapy after lumpectomy and included both low-risk and high-risk women (category A, 4398 women), four were of radiotherapy after sector resection or quadrantectomy (category B, 2399 women), and seven more recent trials were of radiotherapy after lumpectomy in low-risk women (category C, 4004 women). In most of the trials radiotherapy was to the conserved breast only (webappendix p 4). Median follow-up was 9·5 years at risk per woman and 25% of women were followed up for more than a decade. 3143 (29%) had died by the final follow-up date of Sept 30, 2006.
Table 1.
Availability of data from randomised trials of radiotherapy after breast-conserving surgery for invasive cancer that began before the year 2000
| Number of trials available* | Years trials began | Women | Deaths |
Woman-years at risk |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median/woman | Total (thousands) |
Distribution by years since diagnosis (thousands) |
||||||||||
| <5 | 5–9 | 10–14 | 15–19 | 20+ | ||||||||
| Trial category† | ||||||||||||
| (A) Lumpectomy, original trials6–11 | 6 | 1976–86 | 4398 | 1982 | 11·8 | 52·9 | 20·3 | 16·0 | 10·1 | 4·8 | 1·7 | |
| (B) Sector resection or quadrantectomy12–15 | 4 | 1981–91 | 2399 | 708 | 12·4 | 29·4 | 11·6 | 10·3 | 6·0 | 1·4 | 0·1 | |
| (C) Lumpectomy in low-risk women16–22 | 7 | 1989–99 | 4004 | 453 | 6·6 | 26·9 | 17·9 | 7·9 | 1·1 | 0·0 | 0·0 | |
| Pathological nodal status | ||||||||||||
| Negative (pN0) | .. | .. | 7287 | 1801 | 9·7 | 73·7 | 34·0 | 23·3 | 11·3 | 3·9 | 1·2 | |
| Positive (pN+) | .. | .. | 1050 | 585 | 10·3 | 11·8 | 4·6 | 3·2 | 2·2 | 1·3 | 0·5 | |
| Unknown | .. | .. | 2464 | 757 | 8·8 | 23·6 | 11·3 | 7·6 | 3·7 | 1·0 | 0·0 | |
| All women | 17 | 1976–99 | 10 801 | 3143 | 9·5 | 109·1 | 49·8 | 34·1 | 17·2 | 6·3 | 1·7 | |
Only unconfounded trials are considered—ie, trials in which there was no difference between the treatment groups in the type or extent of surgery or in the use of systemic therapy. Two further eligible trials,23,24 both category A with a total of 133 women, were identified but data were unavailable. Details of the 17 available trials are given in webappendix pp 4, 45–47.
Elsewhere, these trial categories are abbreviated to: (A) Lumpectomy: original; (B) >Lumpectomy; (C) Lumpectomy: low risk. In category A, 55% were pathologically node negative, 5% were aged 70+ years, 10% had low-grade tumours, 54% had T1 tumours (1–20 mm), 81% had oestrogen-receptor (ER)-positive disease or unknown status, and 44% were in trials in which tamoxifen was used in both trial groups. In category B, 81% were pathologically node negative, 10% were aged 70+ years, 9% had low-grade tumours, 89% had T1 tumours, 86% had ER positive disease or unknown status, and 6% were in trials in which tamoxifen was used in both trial groups. In category C, 73% were pathologically node negative, 40% were aged 70+ years, 33% had low-grade tumours, 90% had T1 tumours, 98% had ER positive disease or unknown status, and 88% were in trials in which tamoxifen was used in both trial groups.
The 10-year risk of any (locoregional or distant) first recurrence was 19·3% in women allocated to radiotherapy and 35·0% in women allocated to breast-conserving surgery only, corresponding to an absolute risk reduction of 15·7% (95% CI 13·7–17·7, 2p<0·00001; figure 1). Nearly three-quarters of first recurrences in the no radiotherapy group were locoregional (25% locoregional first, 10% distant first), compared with fewer than half of those in the radiotherapy group (8% locoregional first, 12% distant first; webappendix p 9). In addition to reducing recurrence substantially, radiotherapy also reduced breast cancer death by a moderate amount: the 15-year absolute risk reduction was 3·8% (95% CI 1·6–6·0, 2p=0·00005), suggesting on average about one breast cancer death avoided for every four recurrences avoided by radiotherapy.
Figure 1.
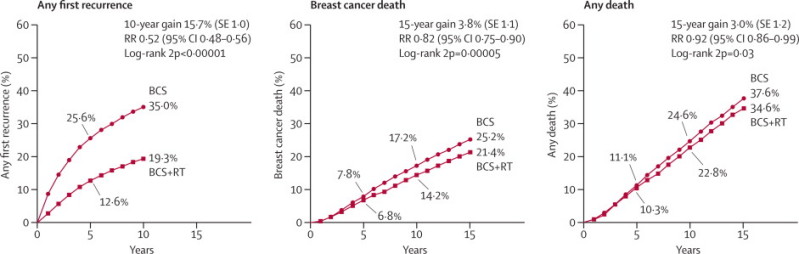
Effect of radiotherapy (RT) after breast-conserving surgery (BCS) on 10-year risk of any (locoregional or distant) first recurrence and on 15-year risks of breast cancer death and death from any cause in 10 801 women (67% with pathologically node-negative disease) in 17 trials
Further details are in webappendix p 5. RR=rate ratio. Rate ratios in this figure include all available years of follow-up.
Allocation to radiotherapy halved the average annual rate of any first recurrence (rate ratio [RR] 0·52, 95% CI 0·48–0·56, figure 1). The proportional reduction was greatest in the first year (0·31, 0·26–0·37), but was still substantial during years 5–9 (0·59, 0·50–0·70; webappendix p 13). Beyond year 10 there was no evidence of any further effect on the first recurrence rate, but information about recurrence in this period was incomplete so the number of events was small and the CI wide (figure 1). Radiotherapy reduced the annual breast cancer death rate by a sixth (RR 0·82, 0·75–0·90). The timing of breast cancer death differed from that of first recurrence, with few events during the first year (figure 1), and there were substantial numbers of breast cancer deaths after year 10.
Mortality without recurrence from non-breast-cancer causes was slightly higher in women allocated to radiotherapy than in women allocated to breast-conserving surgery only, but the excess was not significant (RR 1·09, 95% CI 0·97–1·22, 2p=0·14). If the mortality rate from non-breast-cancer causes in women allocated to radiotherapy had been identical to that in women allocated to breast-conserving surgery only, then the 15-year absolute risk reduction in all-cause mortality would have been 3·2%. In fact, the 15-year absolute risk reduction in all-cause mortality was 3·0% (95% CI 0·6–5·4, 2p=0·03; figure 1).
Most women (n=7287) had pN0 disease. In this group, allocation to radiotherapy halved the average annual recurrence rate during the first decade (RR 0·46, 95% CI 0·41–0·51), reducing the 10-year risk of any first recurrence from 31·0% to 15·6%, an absolute reduction of 15.4% (95% CI 13·2–17·6, 2p<0.00001 figure 2). For these women, radiotherapy reduced breast cancer death by about a sixth (RR 0·83, 95% CI 0·73–0·95), and reduced the 15-year risk of breast cancer death from 20·5% to 17·2%, an absolute reduction of 3·3% (95% CI 0·8–5·8, 2p=0.005; figure 2).
Figure 2.
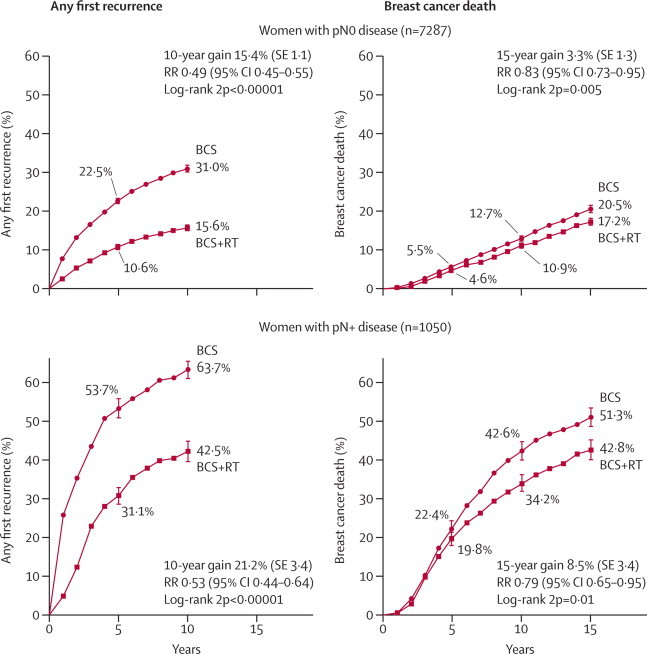
Effect of radiotherapy (RT) after breast-conserving surgery (BCS) on 10-year risk of any (locoregional or distant) first recurrence and on 15-year risk of breast cancer death in women with pathologically verified nodal status
Vertical lines indicate 1 SE above or below the 5, 10, and 15 year percentages. Further details are in webappendix pp 6–7. pN0=pathologically node-negative. pN+=pathologically node-positive. RR=rate ratio. Rate ratios in this figure include all available years of follow-up.
For the 1050 women with pN+ disease, allocation to radiotherapy reduced the 1-year recurrence risk from 26·0% to 5·1%, which is a five-fold reduction (RR 0·20, 95% CI 0·14–0·29). There was a moderate additional effect over the next few years but little further effect after year 5 (figure 2). When these very different proportional effects in different periods since treatment were combined, the mean annual recurrence rate during the whole of the first decade was halved in pN+ disease (RR 0·50, 95% CI 0·41–0·61). Although the proportional reductions in the mean annual recurrence rate were similar in pN0 and pN+ disease, the absolute 10-year recurrence reduction seemed to be somewhat larger in pN+ disease at 21·2% (95% CI 14·5–27·9, 2p<0·00001, 42·5% vs 63·7%). Radiotherapy also reduced breast cancer death in pN+ disease (RR 0·79, 95% CI 0·65–0·95, 2p=0·01), with a 15-year absolute reduction of 8·5% (95% CI 1·8–15·2; 42·8% vs 51·3%).
In both pN0 and pN+ disease, the first recurrence was locoregional for a higher proportion of women allocated to breast-conserving surgery only than of women allocated to breast-conserving surgery plus radiotherapy (webappendix p 10).
Analyses of the effects of treatment in various subgroups of women with pN0 disease are much more likely to identify differences reliably if they are based on recurrence than on mortality, because the significance level of the effect of radiotherapy is much more extreme for recurrence than for mortality (2p<0·00001 for recurrence [χ21=209·0], 2p=0·005 for breast cancer death [χ21=7·8]).
In a series of analyses which focus first on proportional and then on absolute reductions in recurrence, women were subdivided into different groups to ascertain whether some groups benefit more than others from radiotherapy. In the first analysis, the proportional effect of radiotherapy on the rate of any first recurrence in pN0 disease during the first 10 years was estimated for each of several factors considered separately (figure 3). In most subgroups, radiotherapy roughly halved the annual recurrence rate (except that among women who had been given lumpectomy the proportional recurrence reduction was somewhat less extreme in ER-poor disease [2p=0·01, section (d) of figure 3] and somewhat more extreme in the low-risk [category C] trials [2p=0·009, section (f) of figure 3]).
Figure 3.
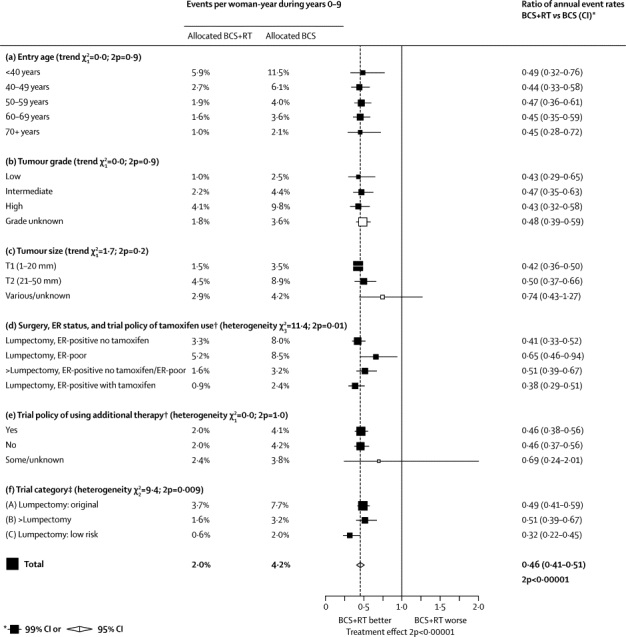
Event rates for any (locoregional or distant) first recurrence (% per year) and recurrence rate ratios for various factors, considered separately, during years 0–9 in women with pathologically node-negative disease (n=7287)
BCS=breast-conserving surgery. RT=radiotherapy. ER=oestrogen receptor. Categories including unknowns excluded from tests for trend and heterogeneity. †A trial policy of tamoxifen use gives tamoxifen to both treatment groups if the disease is ER positive (or ER unknown, here counted with ER positive); additional therapy could be chemotherapy (usually cyclophosphamide, methotrexate, fluorouracil [CMF]) for both treatment groups, or additional RT (nodal RT or a boost or both) for those allocated BCS+RT. ‡Definitions of trial categories A, B, and C are in table 1. Further details are in webappendix p 14.
Halving a big risk produces a bigger absolute benefit than halving a small risk. In women with pN0 disease, the annual recurrence rate without radiotherapy was strongly correlated with age (inversely), tumour grade, tumour size, ER status (especially when tamoxifen was used in ER-positive disease), and extent of surgery (inversely). The absolute recurrence reduction produced by radiotherapy also depended strongly on these factors (table 2). In the second analysis, the way in which the absolute reduction in 10-year recurrence risk produced by radiotherapy depended on all the potential explanatory factors listed in table 2 considered together was modelled. The modelling was carried out by considering information both for women allocated to radiotherapy and for those allocated to no radiotherapy (webappendix pp 20–25 shows methodological details).
Table 2.
Effect of radiotherapy (RT) after breast-conserving surgery (BCS) on 10-year risk of any (locoregional or distant) first recurrence in women with pathologically node-negative disease (n=7287), subdivided by patient and trial characteristics
| Number allocated BCS+RT/BCS |
10-year risk of a locoregional or distant recurrence (%) |
Test for trend/heterogeneity in absolute reduction |
|||||
|---|---|---|---|---|---|---|---|
| BCS+RT | BCS | Absolute reduction with RT (95% CI) | 2p unadjusted* | 2p adjusted* | |||
| (a) Entry age (years) | <0·00001 | 0·0002 | |||||
| <40 | 189/174 | 36·1 | 60·7 | 24·6 (13·2 to 36·0) | |||
| 40–49 | 576/582 | 20·8 | 41·4 | 20·6 (15·1 to 26·1) | |||
| 50–59 | 1093/1028 | 15·0 | 29·7 | 14·7 (10·8 to 18·6) | |||
| 60–69 | 1138/1167 | 14·2 | 28·3 | 14·1 (10·4 to 17·8) | |||
| 70+ | 679/661 | 8·8 | 17·7 | 8·9 (4·0 to 13·8) | |||
| (b) Tumour grade | <0·00001 | <0·00001 | |||||
| Low | 750/757 | 11·0 | 22·4 | 11·4 (6·3 to 16·5) | |||
| Intermediate | 816/843 | 16·4 | 31·6 | 15·3 (10·4 to 20·2) | |||
| High | 448/431 | 28·6 | 53·3 | 24·7 (17·6 to 31·8) | |||
| Grade unknown | 1661/1581 | 14·7 | 28·2 | 13·5 (10·4 to 16·6) | |||
| (c) Tumour size | 0·02 | 0·06 | |||||
| T1 (1–20 mm) | 2942/2920 | 12·4 | 27·5 | 15·1 (12·7 to 17·5) | |||
| T2 (21–50 mm) | 513/487 | 30·7 | 50·0 | 19·3 (12·6 to 26·0) | |||
| Various/unknown | 220/205 | 24·9 | 32·6 | 7·6 (−1·8 to 17·0) | |||
| (d) ER status and trial policy of tamoxifen use† | <0·00001 | 0·003 | |||||
| ER-poor | 448/427 | 28·9 | 43·8 | 14·9 (8·0 to 21·8) | |||
| ER-positive no tamoxifen | 1686/1626 | 18·6 | 36·0 | 17·4 (14·3 to 20·5) | |||
| ER-positive with tamoxifen | 1541/1559 | 8·7 | 22·0 | 13·3 (10·0 to 16·6) | |||
| (e) Trial policy of using additional therapy† | 0·06 | 0·45 | |||||
| No | 1498/1471 | 15·8 | 31·6 | 15·8 (12·7 to 18·9) | |||
| Yes | 2127/2085 | 16·1 | 31·8 | 15·6 (12·3 to 18·9) | |||
| Some/unknown | 50/56 | .. | .. | .. | |||
| (f) Trial category‡ | <0·00001 (A vs C); 0·90 (A+C vs B) | 0·16 (A vs C); 0·00003 (A+C vs B) | |||||
| (A) Lumpectomy: original | 1223/1197 | 27·8 | 47·9 | 20·1 (16·0 to 24·2) | |||
| (B) >Lumpectomy | 986/970 | 14·3 | 25·9 | 11·6 (7·9 to 15·3) | |||
| (C) Lumpectomy: low risk | 1466/1445 | 6·3 | 19·9 | 13·6 (9·7 to 17·5) | |||
| Total | 3675/3612 | 15·6 | 31·0 | 15·4 (13·2 to 17·6) | |||
Information about numbers of events and woman-years is in webappendix p 26. Results for 5-year risks are in webappendix p 31. ER=oestrogen receptor.
Unadjusted: each factor alone. Adjusted: each factor adjusted for all other factors by means of regression modelling. Categories including unknowns excluded from test for trend or heterogeneity.
A trial policy of tamoxifen use gives tamoxifen to both treatment groups if the disease is ER positive (or ER unknown, here counted with ER positive); additional therapy could be chemotherapy (usually cyclophosphamide, methotrexate, fluorouracil [CMF]) for both treatment groups, or additional RT (nodal RT or a boost or both) for those allocated BCS+RT.
Definitions of trial categories A, B, and C are in table 1.
The absolute recurrence reduction produced by radiotherapy and the absolute recurrence risk remaining even with radiotherapy varied significantly with age, tumour grade, ER status, and tamoxifen use, even after adjustment for all other factors (table 2). Tumour size was independently predictive of absolute recurrence risk although not of the absolute risk reduction.
When the original trials with lumpectomy (category A) and the later trials with lumpectomy in low-risk women (category C) were compared, there was a substantial difference in the absolute recurrence risk without radiotherapy and in the absolute reduction in this risk produced by radiotherapy, but these differences were largely accounted for by the other recorded factors (table 2; webappendix pp 15–17, 19).
Surgery that is more extensive than lumpectomy (as in the category B trials) reduced the absolute recurrence risk without radiotherapy and reduced the absolute reduction in this risk produced by radiotherapy. However, because the category B trials did not give tamoxifen, this effect of the extent of surgery on the absolute effect of radiotherapy was apparent only after adjustment for tamoxifen use (and the other recorded factors), but not before (table 2; webappendix p 18).
The characteristics that were independently predictive of the absolute risk of recurrence, or of the absolute risk reduction with radiotherapy, were included in a model to show how 10-year recurrence risks with and without radiotherapy depended in these trials on age, grade, ER status, tamoxifen (which was given much more often in the recent category C trials of low-risk patients than in the original category A trials), and extent of surgery. Figure 4 shows estimates based on this model. Within each section of the figure, younger women and those with high-grade tumours had substantially larger absolute recurrence risks without radiotherapy and substantially larger absolute risk reductions with radiotherapy than did older women and those with low-grade tumours (figure 4). Among women given lumpectomy but not radiotherapy, for a specific age and grade, the highest risks and largest absolute reductions with radiotherapy were for women with ER-positive disease not given tamoxifen; however, even with tamoxifen the additional effects of radiotherapy were substantial for women with high-grade tumours and younger women with intermediate-grade tumours (figure 4).
Figure 4.
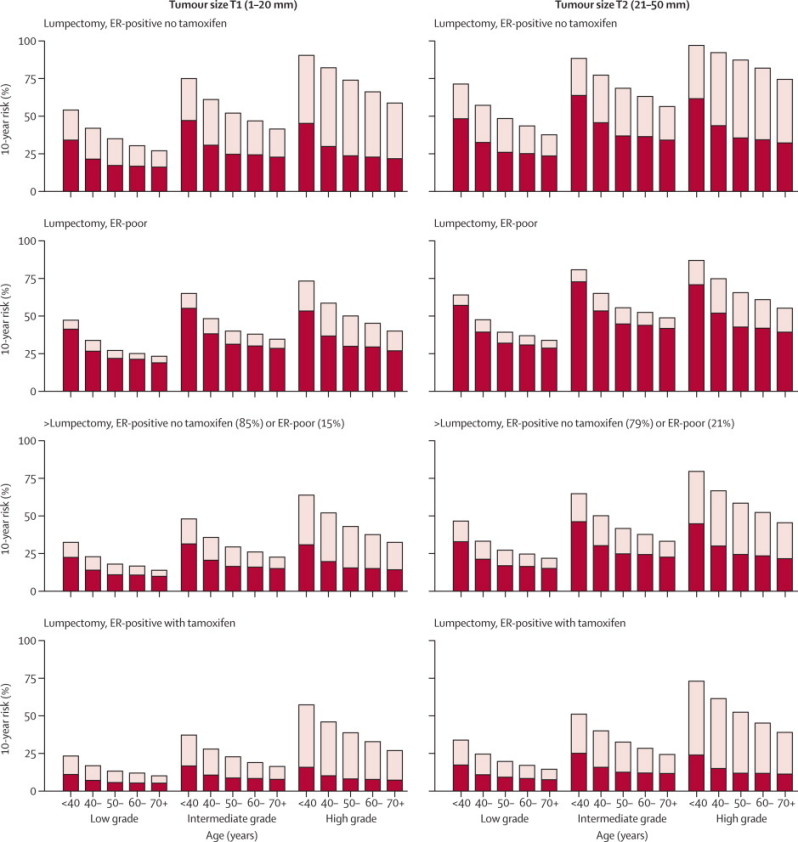
Absolute 10-year risks (%) of any (locoregional or distant) first recurrence with and without radiotherapy (RT) following breast-conserving surgery (BCS) in pathologically node-negative women by patient and trial characteristics, as estimated by regression modelling of data for 7287 women
Further details are in webappendix pp 27–30. Results for 5-year risks are in webappendix pp 31–34. Bars show 10-year risks in women allocated to BCS only, dark sections show 10-year risks in women allocated to BCS plus RT, light sections show absolute reduction with RT. ER=oestrogen receptor.
Each woman with pN0 disease was assigned a predicted absolute reduction in 10-year recurrence risk from radiotherapy on the basis of her individual characteristics, the characteristics of the trial that she was in, and the model-based estimates in figure 4. The absolute reduction in 10-year recurrence risk was large (≥20%) for 1924 women (56% of women in trial category A, 16% of trial category B, and 9% of trial category C), intermediate (10–19%) for 3763 women (32%, 74%, and 53% of trial categories A, B, and C respectively), and lower (<10%) for 1600 women (12%, 10%, and 38% of trial categories A, B, and C respectively). For these three groups (pN0-lower, pN0-intermediate, and pN0-large), the observed 10-year recurrence risks with and without radiotherapy, calculated directly from data for individual women, were 26.0% versus 50.3% (absolute reduction of 24.3%, 95% CI 19.6–29.0), 12.4% versus 24.8% (absolute reduction of 12.4%, 9.7–15.1), and 12.0% versus 18.9% (absolute reduction of 6.9%, 2.2–11.6), respectively. The corresponding absolute reductions in 15-year risk of breast cancer death in the three groups were 7·8% (95% CI 3·1–12·5), 1·1% (–2·0 to 4·2), and 0·1% (–7·5 to 7·7), respectively (trend in absolute mortality reduction: 2p=0·03; figure 5, webappendix pp 35–37). For all three groups, the first recurrence was locoregional for a much larger proportion of women allocated to breast-conserving surgery only than for those allocated to breast-conserving surgery plus radiotherapy (webappendix pp 38–39). Because only 1050 women had pN+ disease in these trials, the relevance of prognostic factors and other characteristics could not be explored reliably in this group (webappendix pp 40–44).
Figure 5.
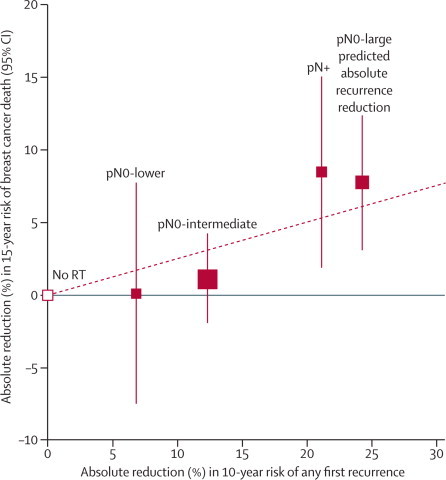
Absolute reduction in 15-year risk of breast cancer death with radiotherapy (RT) after breast-conserving surgery versus absolute reduction in 10-year risk of any (locoregional or distant) recurrence
Women with pN0 disease are subdivided by the predicted absolute reduction in 10-year risk of any recurrence suggested by regression modelling (pN0-large ≥20%, pN0-intermediate 10–19%, pN0-lower <10%; further details are in webappendix pp 35–39). Vertical lines are 95% CIs. Sizes of dark boxes are proportional to amount of information. Dashed line: one death from breast cancer avoided for every four recurrences avoided. pN0=pathologically node-negative. pN+=pathologically node-positive.
On average, in all the women in these trials, about one breast cancer death was avoided by year 15 for every four recurrences avoided by year 10 (figure 1). For pN+ disease and for pN0 disease with large predicted absolute recurrence benefit the observed ratio was slightly larger, whereas for pN0 disease with intermediate or lower predicted absolute benefit it was somewhat smaller (figure 5). However, the departure from linearity was not statistically significant (2p=0·11).
Discussion
The overall findings from these trials show that radiotherapy after breast-conserving surgery not only substantially reduces the risk of recurrence but also moderately reduces the risk of death from breast cancer. These results suggest that killing microscopic tumour foci in the conserved breast with radiotherapy reduces the potential for both local recurrence and distant metastasis. Both proportional and absolute reductions in the annual recurrence rate are largest in the first year but the recurrence rate continues to be somewhat lower throughout the first decade, whereas the reduction in breast cancer death rate becomes definite only after the first few years and appears to continue into the second decade. Non-breast-cancer mortality and the incidence of contralateral and other second cancers in these and other trials of radiotherapy in early breast cancer will be reported elsewhere, but there appeared to be little adverse effect on 15-year mortality from the aggregate of all causes other than breast cancer, so 15-year all-cause mortality was reduced by almost as much as would be expected from the reduction in breast cancer mortality.
Previous analyses had shown that in pN0 disease young age, large tumour size, and high grade were each strongly predictive of the risk of locoregional recurrence and of the absolute reduction in that risk with radiotherapy.5 This report combines the predictive value of these and other factors, such as wider tamoxifen use in recent trials. Taken together, they account for the fact that the absolute recurrence reduction with radiotherapy is lower in the more recent postlumpectomy trials (ie, category C trials) than in the original postlumpectomy trials (ie, category A trials, table 2). However, even for women with pN0 disease in the recent low-risk trials the predicted absolute 10-year recurrence reduction with radiotherapy exceeded 10% in most women and exceeded 20% in some women.
Almost a quarter of women with pN0 disease were in trials in which sector resection or quadrantectomy, rather than lumpectomy, was undertaken and tamoxifen was not trial policy for pN0 disease in any of these trials. Recurrence rates were, however, lower than those for women with similar characteristics in trials in which lumpectomy was performed (figure 4). This difference accords with the findings of the one randomised trial of quadrantectomy versus lumpectomy, both with radiotherapy, in 700 women, two-thirds of whom were node negative.25 Most of the trials of radiotherapy after lumpectomy required negative surgical margins for invasive cancer (although not for ductal carcinoma in situ). However, pathological techniques have become more sensitive since the women entered these trials, and some would probably have been positive with modern techniques. Therefore, whether the lower recurrence rates recorded in trials of radiotherapy in women given sector resection or quadrantectomy are simply the result of a reduction in the proportion of women with positive margins, or whether there is further benefit to be derived from more extensive surgery, is unknown.
The classification of pN0 disease into three groups with large, intermediate, and lower predicted absolute recurrence benefit from radiotherapy was derived from the recurrence data in these trials. The classification cannot be validated externally and so should not be overinterpreted. Nevertheless, the size of the difference in the absolute recurrence reduction between the three groups is striking. Furthermore, the 15-year reduction in risk of breast cancer death was about as big in pN0 disease with large predicted absolute recurrence benefit as it was in pN+ disease, whereas in pN0 disease with intermediate or lower predicted recurrence benefit the mortality reduction was smaller and neither differed significantly from zero. Therefore the number of breast cancer deaths avoided per recurrence avoided might be more than one in four in pN+ disease and in pN0 disease with large predicted absolute recurrence benefit, and less than one in four for women with intermediate or lower predicted absolute benefit (figure 5). However, the present data do not depart significantly from the one-in-four relationship.
The main analyses presented are of first recurrence of any type rather than, as previously, locoregional recurrence as a first event. This difference is partly because it is now clear that radiotherapy after breast-conserving surgery reduces breast cancer death, so it must also reduce distant recurrence. It is also because women with higher risk of locoregional recurrence have higher risk of distant recurrence (ie, the probabilities of locoregional and of distant recurrence are not statistically independent), so valid estimates of the separate effects of radiotherapy on local and distant recurrence cannot be obtained.26,27 These issues are more important at 10 years than at 5 years (as presented previously), because more distant recurrences occur by 10 years than by 5 years. The fact that the proportion of first recurrences that were locoregional was much lower in irradiated women than in controls does, however, suggest that the main effect of radiotherapy was to reduce locoregional recurrence, whereas the reduction of the breast cancer death rate by a sixth suggests that the distant recurrence rate was reduced by at least as much. No analyses have been undertaken of recurrences after the first, because they are affected by treatment policies for the first recurrence, and many trials did not record further recurrences.
Screening, surgery, pathology, radiotherapy, and systemic therapy28,29 have all changed substantially since most of the women entered these trials, so the absolute recurrence reduction with radiotherapy in future patients might differ greatly from that recorded in these trials. Moreover, information about additional risk factors will often be available (eg, HER2, gene expression profile, margin status) and a radiotherapy boost may be given.30,31 Nevertheless, the finding that radiotherapy roughly halved the recurrence rate after breast-conserving surgery in a wide range of patients with very different absolute risks (figure 3) suggests that it might also roughly halve the recurrence rate in future patients given breast-conserving surgery but who are not comparable with the women included in the trials analysed here. If so, then in these future patients, a reasonable way to predict the absolute recurrence benefit of radiotherapy would be to construct contemporary estimates of the absolute risk of first recurrence of any type and to assume that radiotherapy will approximately halve it.
Correspondence to: EBCTCG Secretariat, Clinical Trial Service Unit (CTSU), Richard Doll Building, Oxford OX3 7LF, UK bc.overview@ctsu.ox.ac.uk
Acknowledgments
Acknowledgments
The main acknowledgments are to the thousands of women who took part in the trials, to the staff who treated and cared for them and who undertook the trials and shared their data with the Secretariat, and to the CTSU which hosts this collaboration. DC was supported by the British Heart Foundation Centre for Research Excellence (RE/08/04).
Contributors
SD, PMcG, and CC designed and undertook the analyses with DC, RP, and CT as internal advisers and TW, ME, LP, and RA as external advisers. All writing committee members contributed to the report. The EBCTCG secretariat, including SD, PMcG, CC, CT, MC, DC, CD, JG, RG, YW, and RP, identified trials, and received, collated, and checked datasets.
Writing committee
S Darby, P McGale, C Correa, C Taylor, R Arriagada, M Clarke, D Cutter, C Davies, M Ewertz, J Godwin, R Gray, L Pierce, T Whelan, Y Wang, R Peto.
Attendees at EBCTCG Steering Committee meetings
K Albain, S Anderson, R Arriagada, W Barlow, J Bergh, J Bliss, *M Buyse, D Cameron, E Carrasco, *†M Clarke, C Correa, A Coates, *†R Collins, J Costantino, †D Cutter, J Cuzick, *†S Darby, N Davidson, *†C Davies, †K Davies, †A Delmestri, A Di Leo, M Dowsett, †P Elphinstone, †V Evans, *M Ewertz, R Gelber, †L Gettins, C Geyer, A Goldhirsch, †J Godwin, †R Gray, †C Gregory, D Hayes, C Hill, J Ingle, R Jakesz, †S James, M Kaufmann, †A Kerr, †E MacKinnon, †P McGale, †T McHugh, L Norton, Y Ohashi, S Paik, †H C Pan, E Perez, *†R Peto, *M Piccart (co-chair), L Pierce, *K Pritchard (co-chair), G Pruneri, V Raina, P Ravdin, J Robertson, E Rutgers, Y F Shao, S Swain, †C Taylor, P Valagussa, G Viale, T Whelan, *E Winer, †Y Wang, *W Wood. *Executive Group. †Secretariat.
EBCTCG collaborators are listed in webappendix p 48 and in reference 29.
Conflicts of interest
Any interests of trialists in treatments or diagnostics and company support of trials (see trial publications) have not affected data availability or EBCTCG meta-analyses of these datasets. EBCTCG is funded from the general long-term financial support from the Cancer Research UK, the British Heart Foundation, and the UK Medical Research Council to the CTSU, University of Oxford (http://www.ctsu.ox.ac.uk/about/). CTSU also performs trials funded by industry grants to the University of Oxford that are initiated, conducted, and interpreted independently of study funders. CTSU staff policy excludes honoraria or consultancy fees. All secretariat and writing committee members declare that they have no conflicts of interest.
Web Extra Material
References
- 1.Cuzick J, Stewart H, Peto R. Overview of randomized trials of postoperative adjuvant radiotherapy in breast cancer. Cancer Treat Rep. 1987;71:15–29. [PubMed] [Google Scholar]
- 2.Cuzick J, Stewart H, Rutqvist L. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol. 1994;12:447–453. doi: 10.1200/JCO.1994.12.3.447. [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of radiotherapy and surgery in early breast cancer: an overview of the randomized trials. N Engl J Med. 1995;333:1444–1455. doi: 10.1056/NEJM199511303332202. [DOI] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Lancet. 2000;355:1757–1770. [PubMed] [Google Scholar]
- 5.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 6.Fisher B, Anderson S, Fisher ER. Significance of ipsilateral breast tumour recurrence after lumpectomy. Lancet. 1991;338:327–331. doi: 10.1016/0140-6736(91)90475-5. [DOI] [PubMed] [Google Scholar]
- 7.Ford HT, Coombes RC, Gazet JC. Long-term follow-up of a randomised trial designed to determine the need for irradiation following conservative surgery for the treatment of invasive breast cancer. Ann Oncol. 2006;17:401–408. doi: 10.1093/annonc/mdj080. [DOI] [PubMed] [Google Scholar]
- 8.Whelan T, Clark R, Roberts R, Levine M, Foster G, Investigators of the Ontario Clinical Oncology Group Ipsilateral breast tumor recurrence postlumpectomy is predictive of subsequent mortality: Results from a randomized trial. Int J Radiat Oncol Biol Phys. 1994;30:11–16. doi: 10.1016/0360-3016(94)90513-4. [DOI] [PubMed] [Google Scholar]
- 9.Forrest AP, Stewart HJ, Everington D, Scottish Cancer Trials Breast Group Randomised controlled trial of conservation therapy for breast cancer: 6-year analysis of the Scottish trial. Lancet. 1996;348:708–713. doi: 10.1016/s0140-6736(96)02133-2. [DOI] [PubMed] [Google Scholar]
- 10.Spooner D, Stocken DD, Jordan S. A randomised controlled trial to evaluate both the role and optimal fractionation of radiotherapy in the conservative management of early breast cancer. Cancer Res. 2009;69(suppl):A5125. doi: 10.1016/j.clon.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Houghton J, Potyka I, Tobias J, Baum M, Odling-Smee W. Prophylactic radiotherapy following surgery for early breast cancer—is the benefit mainly to patients with involved margins? Results from a Cancer Research Campaign trial. Proc Ann Meet Am Soc Clin Oncol. 2001;20:31a. A122. [Google Scholar]
- 12.Liljegren G, Holmberg L, Adami HO, Westman G, Graffman S, Bergh J, for the Uppsala-Örebro Breast Cancer Study Group Sector resection with or without postoperative radiotherapy for stage I breast cancer: five year results of a randomized trial. J Natl Cancer Inst. 1994;86:717–722. doi: 10.1093/jnci/86.9.717. [DOI] [PubMed] [Google Scholar]
- 13.Veronesi U, Luini A, del Vecchio M. Radiotherapy after breast-preserving surgery in women with localized cancer of the breast. N Engl J Med. 1993;328:1587–1591. doi: 10.1056/NEJM199306033282202. [DOI] [PubMed] [Google Scholar]
- 14.Holli K, Hietanen P, Saaristo R. Radiotherapy after segmental resection of breast cancer with favorable prognostic features: 12-year follow-up results of a randomized trial. J Clin Oncol. 2009;27:927–932. doi: 10.1200/JCO.2008.19.7129. [DOI] [PubMed] [Google Scholar]
- 15.Malmström P, Holmberg L, Anderson H. Breast conservation surgery, with and without radiotherapy, in women with lymph node-negative breast cancer: a randomised clinical trial in a population with access to public mammography screening. Eur J Cancer. 2003;39:1690–1697. doi: 10.1016/s0959-8049(03)00324-1. [DOI] [PubMed] [Google Scholar]
- 16.Fisher B, Bryant J, Dignam JJ. Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol. 2002;20:4141–4149. doi: 10.1200/JCO.2002.11.101. [DOI] [PubMed] [Google Scholar]
- 17.Winzer KJ, Sauerbrei W, Braun M. Radiation therapy and tamoxifen after breast-conserving surgery; updated results of a 2×2 randomised clinical trial in patients with low risk of recurrence. Eur J Cancer. 2010;46:95–101. doi: 10.1016/j.ejca.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Fyles AW, McCready DR, Manchul LA. Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med. 2004;351:963–970. doi: 10.1056/NEJMoa040595. [DOI] [PubMed] [Google Scholar]
- 19.Blamey RW, Chetty U, Bates T. Radiotherapy and/or tamoxifen after conserving surgery for breast cancers of excellent prognosis: BASO II TRIAL. Eur J Cancer Suppl. 2007;5 doi: 10.1016/j.ejca.2013.02.031. O-11. [DOI] [PubMed] [Google Scholar]
- 20.Hughes KS, Schnaper LA, Berry D. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351:971–977. doi: 10.1056/NEJMoa040587. [DOI] [PubMed] [Google Scholar]
- 21.Potter R, Gnant M, Kwasny W. Lumpectomy plus tamoxifen or anastrozole with or without whole breast irradiation in women with favorable early breast cancer. Int J Radiat Oncol Biol Phys. 2007;68:334–340. doi: 10.1016/j.ijrobp.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 22.Prescott RJ, Kunkler IH, Williams LJ. A randomised controlled trial of postoperative radiotherapy following breast-conserving surgery in a minimum-risk older population. The PRIME trial. Health Technol Assess. 2007;11:1–170. doi: 10.3310/hta11310. i–x. [DOI] [PubMed] [Google Scholar]
- 23.Inoue M, Tanaka I, Masuda R, Furuhata Y. Local control and cosmetic outcome after sector resection with or without radiation therapy for early breast cancer. Breast Cancer. 1996;3:39–46. doi: 10.1007/BF02966961. [DOI] [PubMed] [Google Scholar]
- 24.National Cancer Institute Phase III randomized study of breast irradiation in women aged 50 years and over after breast-conserving surgery for early stage, low-risk breast cancer. August, 1997. http://www.cancer.gov/search/ViewClinicalTrials.aspx?cdrid=64259&version=HealthProfessional&protocolsearchid=8745051 (accessed April 13, 2011).
- 25.Veronesi U, Volterrani F, Luini A. Quadrantectomy versus lumpectomy for small size breast cancer. Eur J Cancer. 1990;26:671–673. doi: 10.1016/0277-5379(90)90114-9. [DOI] [PubMed] [Google Scholar]
- 26.Gelman R, Gelber R, Henderson IC, Coleman CN, Harris JR. Improved methodology for analyzing local and distant recurrence. J Clin Oncol. 1990;8:548–555. doi: 10.1200/JCO.1990.8.3.548. [DOI] [PubMed] [Google Scholar]
- 27.Tsiatis A. A nonidentifiability aspect of the problem of competing risks. Proc Nat Acad Sci. 1975;72:20–22. doi: 10.1073/pnas.72.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 29.Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome in 100 000 randomised women. Lancet (in press). [DOI] [PMC free article] [PubMed]
- 30.Bartelink H, Horiot JC, Poortmans PM. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol. 2007;25:3259–3265. doi: 10.1200/JCO.2007.11.4991. [DOI] [PubMed] [Google Scholar]
- 31.Romestaing P, Lehingue Y, Carrie C. Role of a 10-Gy boost in the conservative treatment of early breast cancer: results of a randomized clinical trial in Lyon, France. J Clin Oncol. 1997;15:963–968. doi: 10.1200/JCO.1997.15.3.963. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


